

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50200642 ((S)-4-(6-(2-(2-methylpyrrolidin-1-yl)ethyl)naphtha...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]-N-alpha-methylhistamine from human cloned histamine H3 receptor expressed in C6 cells | Bioorg Med Chem Lett 17: 1443-6 (2007) Article DOI: 10.1016/j.bmcl.2006.11.073 BindingDB Entry DOI: 10.7270/Q2416WR3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50200641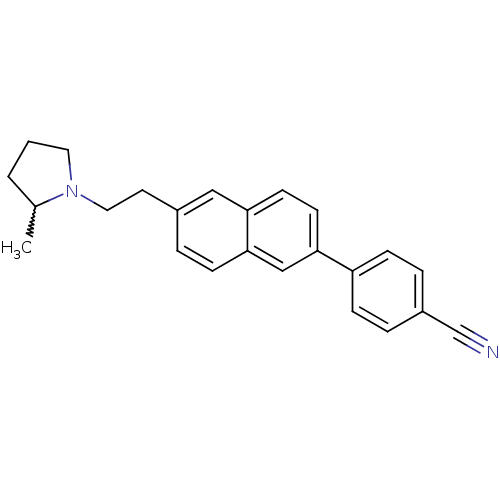 (4-(6-(2-(2-methylpyrrolidin-1-yl)ethyl)naphthalen-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]-N-alpha-methylhistamine from human cloned histamine H3 receptor expressed in C6 cells | Bioorg Med Chem Lett 17: 1443-6 (2007) Article DOI: 10.1016/j.bmcl.2006.11.073 BindingDB Entry DOI: 10.7270/Q2416WR3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50200646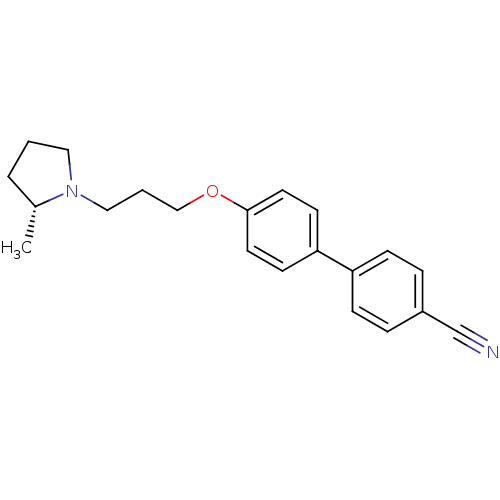 (4'-[3-((R)-2-methyl-pyrrolidin-1-yl)-propoxy]-biph...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]-N-alpha-methylhistamine from human cloned histamine H3 receptor expressed in C6 cells | Bioorg Med Chem Lett 17: 1443-6 (2007) Article DOI: 10.1016/j.bmcl.2006.11.073 BindingDB Entry DOI: 10.7270/Q2416WR3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50200647 ((R)-4-(6-(2-(2-methylpyrrolidin-1-yl)ethyl)naphtha...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]-N-alpha-methylhistamine from human cloned histamine H3 receptor expressed in C6 cells | Bioorg Med Chem Lett 17: 1443-6 (2007) Article DOI: 10.1016/j.bmcl.2006.11.073 BindingDB Entry DOI: 10.7270/Q2416WR3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50200639 ((S)-4-(6-(2-(2-(hydroxymethyl)pyrrolidin-1-yl)ethy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]-N-alpha-methylhistamine from human cloned histamine H3 receptor expressed in C6 cells | Bioorg Med Chem Lett 17: 1443-6 (2007) Article DOI: 10.1016/j.bmcl.2006.11.073 BindingDB Entry DOI: 10.7270/Q2416WR3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50139391 ((R)-4-(2-(2-(2-methylpyrrolidin-1-yl)ethyl)benzofu...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]-N-alpha-methylhistamine from human cloned histamine H3 receptor expressed in C6 cells | Bioorg Med Chem Lett 17: 1443-6 (2007) Article DOI: 10.1016/j.bmcl.2006.11.073 BindingDB Entry DOI: 10.7270/Q2416WR3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50200644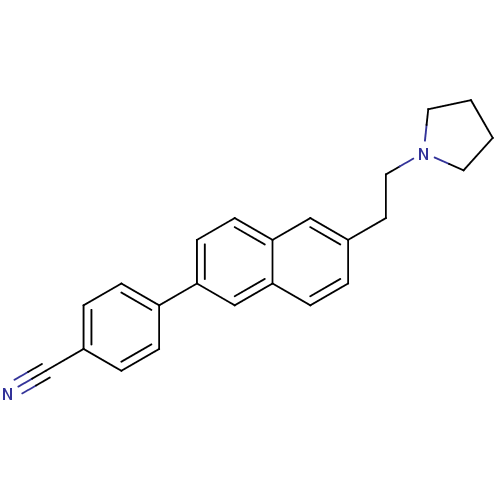 (4-(6-(2-(pyrrolidin-1-yl)ethyl)naphthalen-2-yl)ben...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]-N-alpha-methylhistamine from human cloned histamine H3 receptor expressed in C6 cells | Bioorg Med Chem Lett 17: 1443-6 (2007) Article DOI: 10.1016/j.bmcl.2006.11.073 BindingDB Entry DOI: 10.7270/Q2416WR3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50159110 (1-(3-(4-(piperidin-1-ylmethyl)phenoxy)propyl)piper...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]-N-alpha-methylhistamine from human cloned histamine H3 receptor expressed in C6 cells | Bioorg Med Chem Lett 17: 1443-6 (2007) Article DOI: 10.1016/j.bmcl.2006.11.073 BindingDB Entry DOI: 10.7270/Q2416WR3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium- and chloride-dependent glycine transporter 1 (Homo sapiens (Human)) | BDBM50368698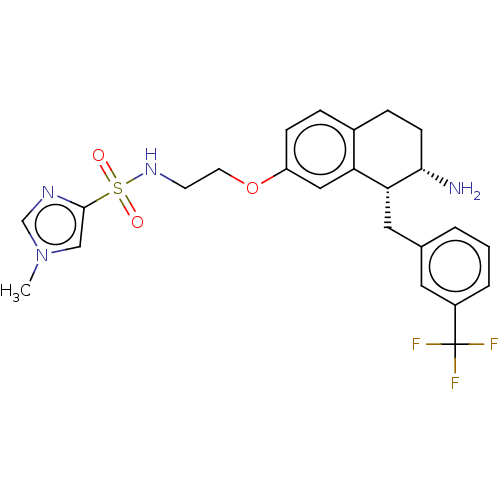 (CHEMBL4161906) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Deutschland GmbH & Co. KG Curated by ChEMBL | Assay Description Displacement of [3H]N-Methyl-SSR504734 from human GlyT1c expressed in cell membranes incubated for 1 hr by liquid scintillation spectrometry | J Med Chem 61: 7503-7524 (2018) Article DOI: 10.1021/acs.jmedchem.8b00300 BindingDB Entry DOI: 10.7270/Q2W66P9B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium- and chloride-dependent glycine transporter 1 (Homo sapiens (Human)) | BDBM50368757 (CHEMBL4169829) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Deutschland GmbH & Co. KG Curated by ChEMBL | Assay Description Displacement of [3H]N-Methyl-SSR504734 from human GlyT1c expressed in cell membranes incubated for 1 hr by liquid scintillation spectrometry | J Med Chem 61: 7503-7524 (2018) Article DOI: 10.1021/acs.jmedchem.8b00300 BindingDB Entry DOI: 10.7270/Q2W66P9B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50200629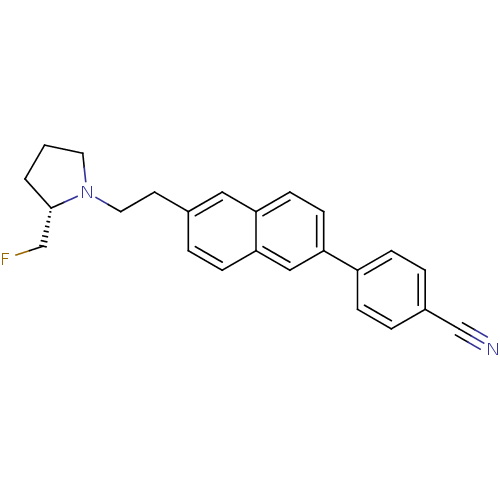 ((S)-4-(6-(2-(2-(fluoromethyl)pyrrolidin-1-yl)ethyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.810 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]-N-alpha-methylhistamine from human cloned histamine H3 receptor expressed in C6 cells | Bioorg Med Chem Lett 17: 1443-6 (2007) Article DOI: 10.1016/j.bmcl.2006.11.073 BindingDB Entry DOI: 10.7270/Q2416WR3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50200627 (4-(6-(2-(diethylamino)ethyl)naphthalen-2-yl)benzon...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.840 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]-N-alpha-methylhistamine from human cloned histamine H3 receptor expressed in C6 cells | Bioorg Med Chem Lett 17: 1443-6 (2007) Article DOI: 10.1016/j.bmcl.2006.11.073 BindingDB Entry DOI: 10.7270/Q2416WR3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium- and chloride-dependent glycine transporter 1 (Homo sapiens (Human)) | BDBM50368693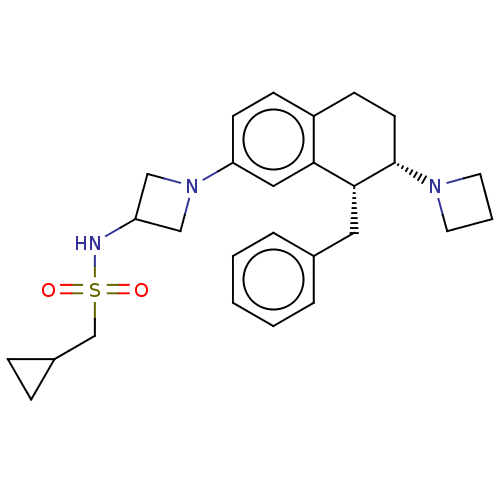 (CHEMBL4171521) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Deutschland GmbH & Co. KG Curated by ChEMBL | Assay Description Displacement of [3H]N-Methyl-SSR504734 from human GlyT1c expressed in cell membranes incubated for 1 hr by liquid scintillation spectrometry | J Med Chem 61: 7503-7524 (2018) Article DOI: 10.1021/acs.jmedchem.8b00300 BindingDB Entry DOI: 10.7270/Q2W66P9B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50200628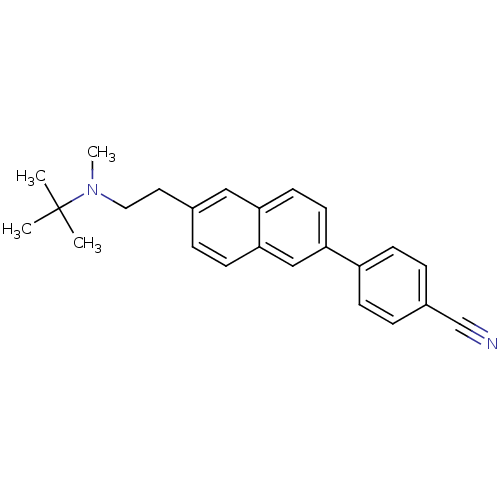 (4-(6-(2-(tert-butyl(methyl)amino)ethyl)naphthalen-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.09 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]-N-alpha-methylhistamine from human cloned histamine H3 receptor expressed in C6 cells | Bioorg Med Chem Lett 17: 1443-6 (2007) Article DOI: 10.1016/j.bmcl.2006.11.073 BindingDB Entry DOI: 10.7270/Q2416WR3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium- and chloride-dependent glycine transporter 1 (Homo sapiens (Human)) | BDBM50368701 (CHEMBL4173657) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Deutschland GmbH & Co. KG Curated by ChEMBL | Assay Description Displacement of [3H]N-Methyl-SSR504734 from human GlyT1c expressed in cell membranes incubated for 1 hr by liquid scintillation spectrometry | J Med Chem 61: 7503-7524 (2018) Article DOI: 10.1021/acs.jmedchem.8b00300 BindingDB Entry DOI: 10.7270/Q2W66P9B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium- and chloride-dependent glycine transporter 1 (Homo sapiens (Human)) | BDBM50368752 (CHEMBL4166406) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Deutschland GmbH & Co. KG Curated by ChEMBL | Assay Description Displacement of [3H]N-Methyl-SSR504734 from human GlyT1c expressed in cell membranes incubated for 1 hr by liquid scintillation spectrometry | J Med Chem 61: 7503-7524 (2018) Article DOI: 10.1021/acs.jmedchem.8b00300 BindingDB Entry DOI: 10.7270/Q2W66P9B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium- and chloride-dependent glycine transporter 1 (Homo sapiens (Human)) | BDBM50368702 (CHEMBL4161781) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Deutschland GmbH & Co. KG Curated by ChEMBL | Assay Description Displacement of [3H]N-Methyl-SSR504734 from human GlyT1c expressed in cell membranes incubated for 1 hr by liquid scintillation spectrometry | J Med Chem 61: 7503-7524 (2018) Article DOI: 10.1021/acs.jmedchem.8b00300 BindingDB Entry DOI: 10.7270/Q2W66P9B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium- and chloride-dependent glycine transporter 1 (Homo sapiens (Human)) | BDBM50368692 (CHEMBL4165340) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Deutschland GmbH & Co. KG Curated by ChEMBL | Assay Description Displacement of [3H]N-Methyl-SSR504734 from human GlyT1c expressed in cell membranes incubated for 1 hr by liquid scintillation spectrometry | J Med Chem 61: 7503-7524 (2018) Article DOI: 10.1021/acs.jmedchem.8b00300 BindingDB Entry DOI: 10.7270/Q2W66P9B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium- and chloride-dependent glycine transporter 1 (Homo sapiens (Human)) | BDBM50368759 (CHEMBL4159998) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Deutschland GmbH & Co. KG Curated by ChEMBL | Assay Description Displacement of [3H]N-Methyl-SSR504734 from human GlyT1c expressed in cell membranes incubated for 1 hr by liquid scintillation spectrometry | J Med Chem 61: 7503-7524 (2018) Article DOI: 10.1021/acs.jmedchem.8b00300 BindingDB Entry DOI: 10.7270/Q2W66P9B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50200641 (4-(6-(2-(2-methylpyrrolidin-1-yl)ethyl)naphthalen-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]-N-alpha-methylhistamine from histamine H3 receptor in rat cortical membranes | Bioorg Med Chem Lett 17: 1443-6 (2007) Article DOI: 10.1016/j.bmcl.2006.11.073 BindingDB Entry DOI: 10.7270/Q2416WR3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium- and chloride-dependent glycine transporter 1 (Homo sapiens (Human)) | BDBM50368767 (CHEMBL4163697) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Deutschland GmbH & Co. KG Curated by ChEMBL | Assay Description Displacement of [3H]N-Methyl-SSR504734 from human GlyT1c expressed in cell membranes incubated for 1 hr by liquid scintillation spectrometry | J Med Chem 61: 7503-7524 (2018) Article DOI: 10.1021/acs.jmedchem.8b00300 BindingDB Entry DOI: 10.7270/Q2W66P9B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50200648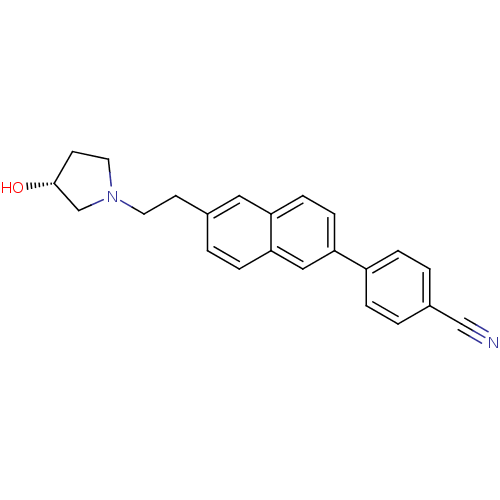 ((R)-4-(6-(2-(3-hydroxypyrrolidin-1-yl)ethyl)naphth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.56 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]-N-alpha-methylhistamine from human cloned histamine H3 receptor expressed in C6 cells | Bioorg Med Chem Lett 17: 1443-6 (2007) Article DOI: 10.1016/j.bmcl.2006.11.073 BindingDB Entry DOI: 10.7270/Q2416WR3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50200636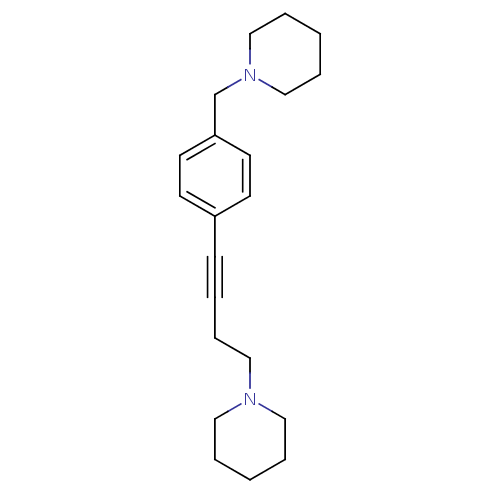 (1-(4-(4-(piperidin-1-ylmethyl)phenyl)but-3-ynyl)pi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]-N-alpha-methylhistamine from human cloned histamine H3 receptor expressed in C6 cells | Bioorg Med Chem Lett 17: 1443-6 (2007) Article DOI: 10.1016/j.bmcl.2006.11.073 BindingDB Entry DOI: 10.7270/Q2416WR3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50200646 (4'-[3-((R)-2-methyl-pyrrolidin-1-yl)-propoxy]-biph...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.96 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]-N-alpha-methylhistamine from histamine H3 receptor in rat cortical membranes | Bioorg Med Chem Lett 17: 1443-6 (2007) Article DOI: 10.1016/j.bmcl.2006.11.073 BindingDB Entry DOI: 10.7270/Q2416WR3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50200631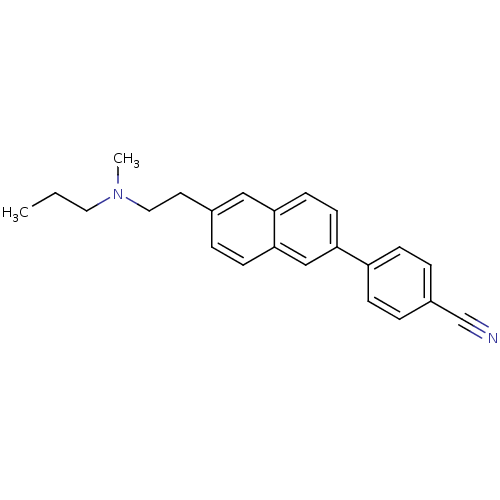 (4-(6-(2-(methyl(propyl)amino)ethyl)naphthalen-2-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.09 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]-N-alpha-methylhistamine from human cloned histamine H3 receptor expressed in C6 cells | Bioorg Med Chem Lett 17: 1443-6 (2007) Article DOI: 10.1016/j.bmcl.2006.11.073 BindingDB Entry DOI: 10.7270/Q2416WR3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50200647 ((R)-4-(6-(2-(2-methylpyrrolidin-1-yl)ethyl)naphtha...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.48 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]-N-alpha-methylhistamine from histamine H3 receptor in rat cortical membranes | Bioorg Med Chem Lett 17: 1443-6 (2007) Article DOI: 10.1016/j.bmcl.2006.11.073 BindingDB Entry DOI: 10.7270/Q2416WR3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium- and chloride-dependent glycine transporter 1 (Homo sapiens (Human)) | BDBM294231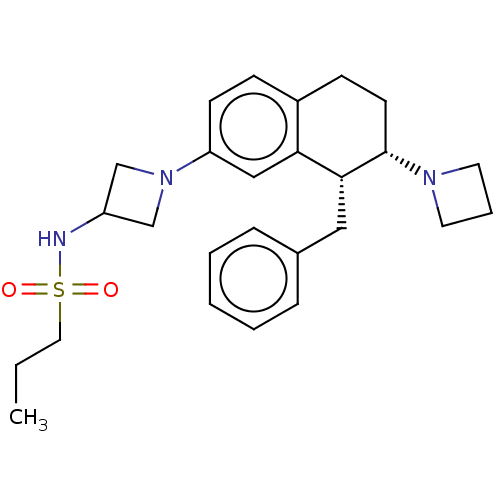 (US9586942, 5*) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Deutschland GmbH & Co. KG Curated by ChEMBL | Assay Description Displacement of [3H]N-Methyl-SSR504734 from human GlyT1c expressed in cell membranes incubated for 1 hr by liquid scintillation spectrometry | J Med Chem 61: 7503-7524 (2018) Article DOI: 10.1021/acs.jmedchem.8b00300 BindingDB Entry DOI: 10.7270/Q2W66P9B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium- and chloride-dependent glycine transporter 1 (Homo sapiens (Human)) | BDBM50368758 (CHEMBL4173253) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Deutschland GmbH & Co. KG Curated by ChEMBL | Assay Description Displacement of [3H]N-Methyl-SSR504734 from human GlyT1c expressed in cell membranes incubated for 1 hr by liquid scintillation spectrometry | J Med Chem 61: 7503-7524 (2018) Article DOI: 10.1021/acs.jmedchem.8b00300 BindingDB Entry DOI: 10.7270/Q2W66P9B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium- and chloride-dependent glycine transporter 1 (Homo sapiens (Human)) | BDBM50368771 (CHEMBL4166296) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Deutschland GmbH & Co. KG Curated by ChEMBL | Assay Description Displacement of [3H]N-Methyl-SSR504734 from human GlyT1c expressed in cell membranes incubated for 1 hr by liquid scintillation spectrometry | J Med Chem 61: 7503-7524 (2018) Article DOI: 10.1021/acs.jmedchem.8b00300 BindingDB Entry DOI: 10.7270/Q2W66P9B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50200638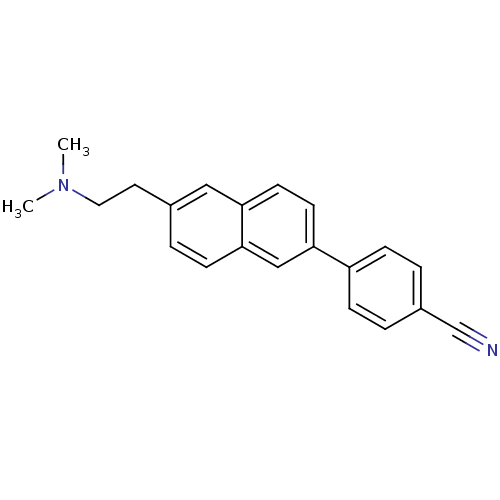 (4-(6-(2-(dimethylamino)ethyl)naphthalen-2-yl)benzo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.65 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]-N-alpha-methylhistamine from human cloned histamine H3 receptor expressed in C6 cells | Bioorg Med Chem Lett 17: 1443-6 (2007) Article DOI: 10.1016/j.bmcl.2006.11.073 BindingDB Entry DOI: 10.7270/Q2416WR3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50200640 ((R)-4-(6-(2-(2-(hydroxymethyl)pyrrolidin-1-yl)ethy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.89 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]-N-alpha-methylhistamine from human cloned histamine H3 receptor expressed in C6 cells | Bioorg Med Chem Lett 17: 1443-6 (2007) Article DOI: 10.1016/j.bmcl.2006.11.073 BindingDB Entry DOI: 10.7270/Q2416WR3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium- and chloride-dependent glycine transporter 1 (Homo sapiens (Human)) | BDBM50368699 (CHEMBL4167917) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Deutschland GmbH & Co. KG Curated by ChEMBL | Assay Description Displacement of [3H]N-Methyl-SSR504734 from human GlyT1c expressed in cell membranes incubated for 1 hr by liquid scintillation spectrometry | J Med Chem 61: 7503-7524 (2018) Article DOI: 10.1021/acs.jmedchem.8b00300 BindingDB Entry DOI: 10.7270/Q2W66P9B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium- and chloride-dependent glycine transporter 1 (Homo sapiens (Human)) | BDBM141044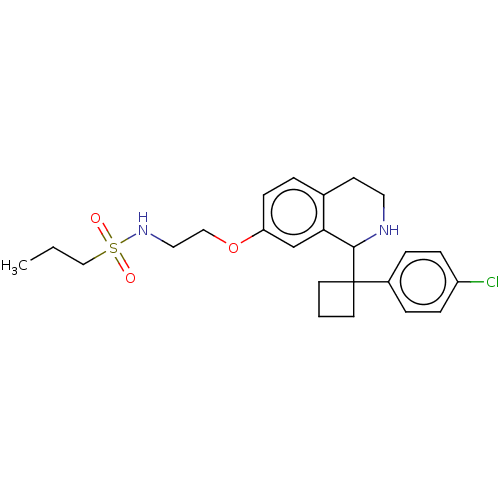 (US8653100, 4) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Deutschland GmbH & Co. KG Curated by ChEMBL | Assay Description Displacement of [3H]N-Methyl-SSR504734 from human GlyT1c expressed in cell membranes incubated for 1 hr by liquid scintillation spectrometry | J Med Chem 61: 7503-7524 (2018) Article DOI: 10.1021/acs.jmedchem.8b00300 BindingDB Entry DOI: 10.7270/Q2W66P9B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50139391 ((R)-4-(2-(2-(2-methylpyrrolidin-1-yl)ethyl)benzofu...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 3.22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]-N-alpha-methylhistamine from histamine H3 receptor in rat cortical membranes | Bioorg Med Chem Lett 17: 1443-6 (2007) Article DOI: 10.1016/j.bmcl.2006.11.073 BindingDB Entry DOI: 10.7270/Q2416WR3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium- and chloride-dependent glycine transporter 1 (Homo sapiens (Human)) | BDBM50368760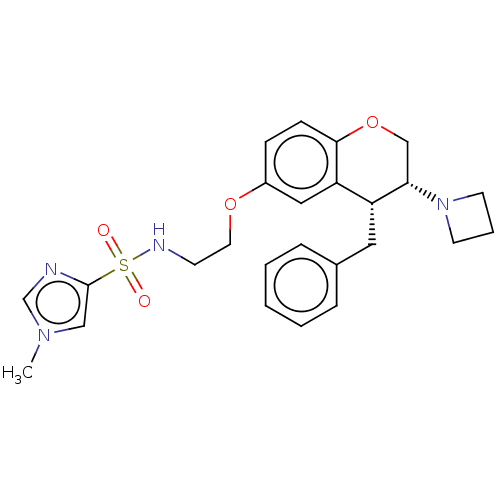 (CHEMBL4164744) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Deutschland GmbH & Co. KG Curated by ChEMBL | Assay Description Displacement of [3H]N-Methyl-SSR504734 from human GlyT1c expressed in cell membranes incubated for 1 hr by liquid scintillation spectrometry | J Med Chem 61: 7503-7524 (2018) Article DOI: 10.1021/acs.jmedchem.8b00300 BindingDB Entry DOI: 10.7270/Q2W66P9B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50200633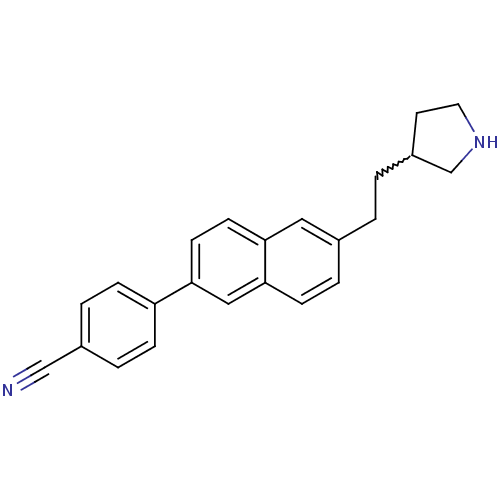 (4-(6-(2-(pyrrolidin-3-yl)ethyl)naphthalen-2-yl)ben...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 3.31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]-N-alpha-methylhistamine from human cloned histamine H3 receptor expressed in C6 cells | Bioorg Med Chem Lett 17: 1443-6 (2007) Article DOI: 10.1016/j.bmcl.2006.11.073 BindingDB Entry DOI: 10.7270/Q2416WR3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50200642 ((S)-4-(6-(2-(2-methylpyrrolidin-1-yl)ethyl)naphtha...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.47 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]-N-alpha-methylhistamine from histamine H3 receptor in rat cortical membranes | Bioorg Med Chem Lett 17: 1443-6 (2007) Article DOI: 10.1016/j.bmcl.2006.11.073 BindingDB Entry DOI: 10.7270/Q2416WR3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium- and chloride-dependent glycine transporter 1 (Homo sapiens (Human)) | BDBM50368697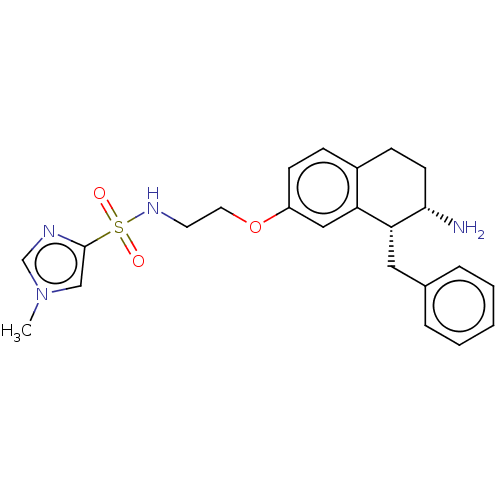 (CHEMBL4162953) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Deutschland GmbH & Co. KG Curated by ChEMBL | Assay Description Displacement of [3H]N-Methyl-SSR504734 from human GlyT1c expressed in cell membranes incubated for 1 hr by liquid scintillation spectrometry | J Med Chem 61: 7503-7524 (2018) Article DOI: 10.1021/acs.jmedchem.8b00300 BindingDB Entry DOI: 10.7270/Q2W66P9B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50200632 (4-(6-(2-(ethyl(isopropyl)amino)ethyl)naphthalen-2-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.62 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]-N-alpha-methylhistamine from human cloned histamine H3 receptor expressed in C6 cells | Bioorg Med Chem Lett 17: 1443-6 (2007) Article DOI: 10.1016/j.bmcl.2006.11.073 BindingDB Entry DOI: 10.7270/Q2416WR3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium- and chloride-dependent glycine transporter 1 (Homo sapiens (Human)) | BDBM50368783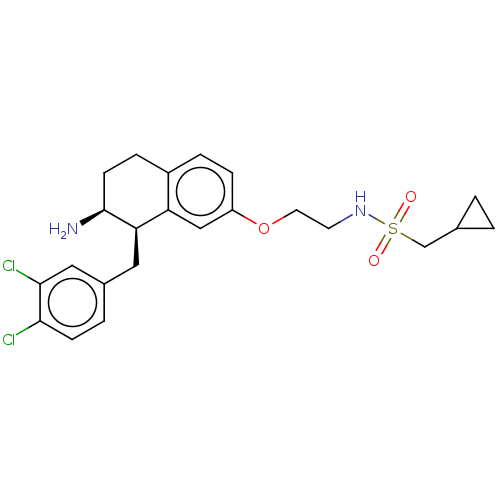 (CHEMBL4165214) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Deutschland GmbH & Co. KG Curated by ChEMBL | Assay Description Displacement of [3H]N-Methyl-SSR504734 from human GlyT1c expressed in cell membranes incubated for 1 hr by liquid scintillation spectrometry | J Med Chem 61: 7503-7524 (2018) Article DOI: 10.1021/acs.jmedchem.8b00300 BindingDB Entry DOI: 10.7270/Q2W66P9B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50200644 (4-(6-(2-(pyrrolidin-1-yl)ethyl)naphthalen-2-yl)ben...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.42 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]-N-alpha-methylhistamine from histamine H3 receptor in rat cortical membranes | Bioorg Med Chem Lett 17: 1443-6 (2007) Article DOI: 10.1016/j.bmcl.2006.11.073 BindingDB Entry DOI: 10.7270/Q2416WR3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50200634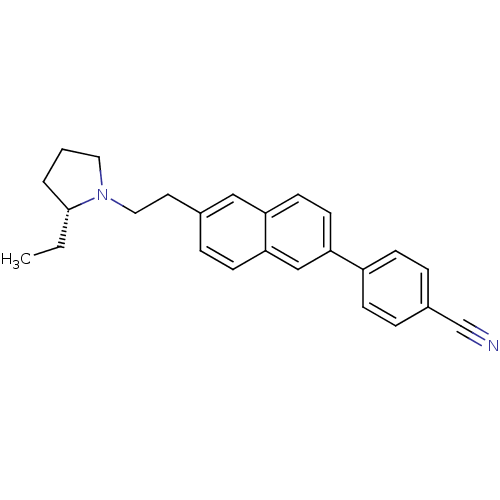 ((R)-4-(6-(2-(2-ethylpyrrolidin-1-yl)ethyl)naphthal...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8.61 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]-N-alpha-methylhistamine from human cloned histamine H3 receptor expressed in C6 cells | Bioorg Med Chem Lett 17: 1443-6 (2007) Article DOI: 10.1016/j.bmcl.2006.11.073 BindingDB Entry DOI: 10.7270/Q2416WR3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50200637 ((R)-4-(6-(2-(2-methylpiperidin-1-yl)ethyl)naphthal...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8.71 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]-N-alpha-methylhistamine from human cloned histamine H3 receptor expressed in C6 cells | Bioorg Med Chem Lett 17: 1443-6 (2007) Article DOI: 10.1016/j.bmcl.2006.11.073 BindingDB Entry DOI: 10.7270/Q2416WR3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium- and chloride-dependent glycine transporter 1 (Homo sapiens (Human)) | BDBM50368695 (CHEMBL4172640) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 9.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Deutschland GmbH & Co. KG Curated by ChEMBL | Assay Description Displacement of [3H]N-Methyl-SSR504734 from human GlyT1c expressed in cell membranes incubated for 1 hr by liquid scintillation spectrometry | J Med Chem 61: 7503-7524 (2018) Article DOI: 10.1021/acs.jmedchem.8b00300 BindingDB Entry DOI: 10.7270/Q2W66P9B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50200629 ((S)-4-(6-(2-(2-(fluoromethyl)pyrrolidin-1-yl)ethyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 9.77 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]-N-alpha-methylhistamine from histamine H3 receptor in rat cortical membranes | Bioorg Med Chem Lett 17: 1443-6 (2007) Article DOI: 10.1016/j.bmcl.2006.11.073 BindingDB Entry DOI: 10.7270/Q2416WR3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50200635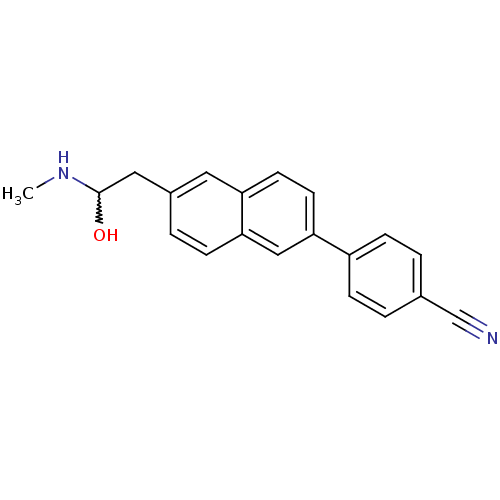 (4-(6-(2-hydroxy-2-(methylamino)ethyl)naphthalen-2-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 10.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]-N-alpha-methylhistamine from human cloned histamine H3 receptor expressed in C6 cells | Bioorg Med Chem Lett 17: 1443-6 (2007) Article DOI: 10.1016/j.bmcl.2006.11.073 BindingDB Entry DOI: 10.7270/Q2416WR3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50200639 ((S)-4-(6-(2-(2-(hydroxymethyl)pyrrolidin-1-yl)ethy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]-N-alpha-methylhistamine from histamine H3 receptor in rat cortical membranes | Bioorg Med Chem Lett 17: 1443-6 (2007) Article DOI: 10.1016/j.bmcl.2006.11.073 BindingDB Entry DOI: 10.7270/Q2416WR3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50200627 (4-(6-(2-(diethylamino)ethyl)naphthalen-2-yl)benzon...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 12.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]-N-alpha-methylhistamine from histamine H3 receptor in rat cortical membranes | Bioorg Med Chem Lett 17: 1443-6 (2007) Article DOI: 10.1016/j.bmcl.2006.11.073 BindingDB Entry DOI: 10.7270/Q2416WR3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium- and chloride-dependent glycine transporter 1 (Homo sapiens (Human)) | BDBM50368766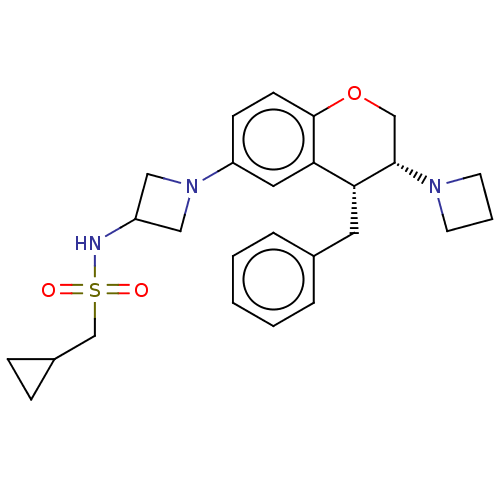 (CHEMBL4164312) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Deutschland GmbH & Co. KG Curated by ChEMBL | Assay Description Displacement of [3H]N-Methyl-SSR504734 from human GlyT1c expressed in cell membranes incubated for 1 hr by liquid scintillation spectrometry | J Med Chem 61: 7503-7524 (2018) Article DOI: 10.1021/acs.jmedchem.8b00300 BindingDB Entry DOI: 10.7270/Q2W66P9B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50200630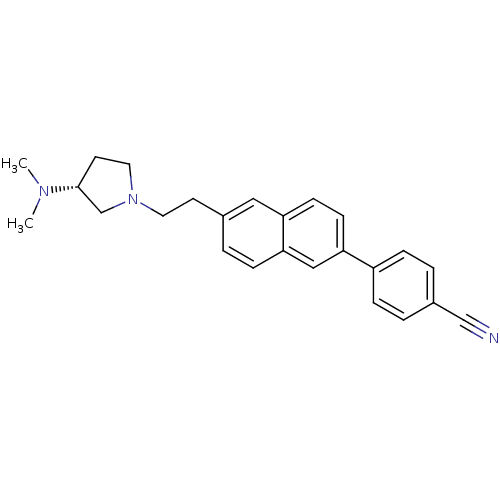 ((R)-4-(6-(2-(3-(dimethylamino)pyrrolidin-1-yl)ethy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 20.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]-N-alpha-methylhistamine from human cloned histamine H3 receptor expressed in C6 cells | Bioorg Med Chem Lett 17: 1443-6 (2007) Article DOI: 10.1016/j.bmcl.2006.11.073 BindingDB Entry DOI: 10.7270/Q2416WR3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 88 total ) | Next | Last >> |