

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Apoptosis regulator Bcl-2 (Homo sapiens (Human)) | BDBM50162774 (ABT-199 | US11420968, Example ABT-199 | Venetoclax) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase KEGG PC cid PC sid UniChem Similars | <0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50375695 (CHEMBL270984) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Stanford University School of Medicine Curated by ChEMBL | Assay Description Displacement of [3H]deltorphin2 from delta opioid receptor in Sprague-Dawley rat brain P2 synaptosomal membrane | J Med Chem 51: 1817-23 (2008) Article DOI: 10.1021/jm7014765 BindingDB Entry DOI: 10.7270/Q24T6K7K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B1 bradykinin receptor (Homo sapiens (Human)) | BDBM50362366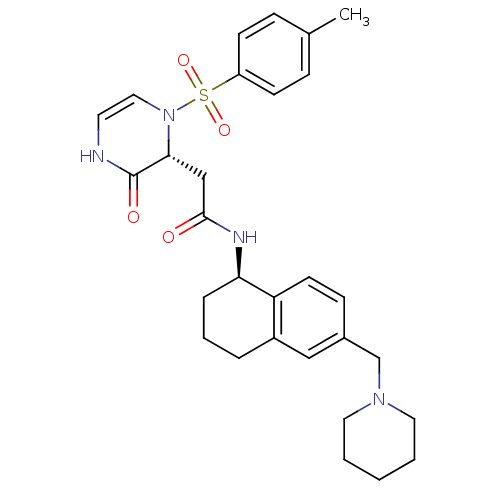 (CHEMBL1939755) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-DAK from human bradykinin B1 receptor expressed in CHO-D-/aequorin cells membrane after 90 mins by scintillation counting | Bioorg Med Chem Lett 22: 1061-7 (2012) Article DOI: 10.1016/j.bmcl.2011.11.112 BindingDB Entry DOI: 10.7270/Q22J6CB2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bcl-2-like protein 1 (Homo sapiens (Human)) | BDBM50558853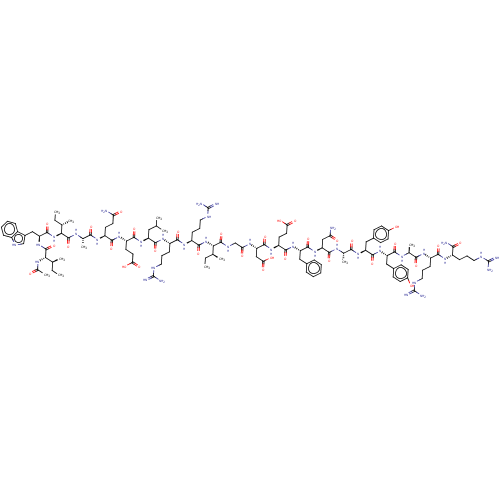 (CHEMBL4465826) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B1 bradykinin receptor (Homo sapiens (Human)) | BDBM50344100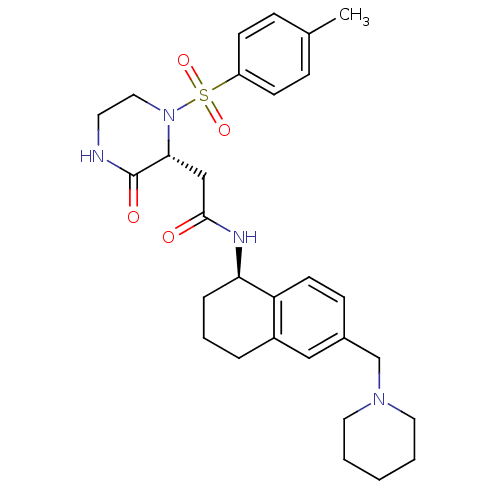 (2-((2R)-1-((4-methylphenyl)sulfonyl)-3-oxo-2-piper...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-DAK from human bradykinin B1 receptor expressed in CHO-D-/aequorin cells membrane after 90 mins by scintillation counting | Bioorg Med Chem Lett 22: 1061-7 (2012) Article DOI: 10.1016/j.bmcl.2011.11.112 BindingDB Entry DOI: 10.7270/Q22J6CB2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50375694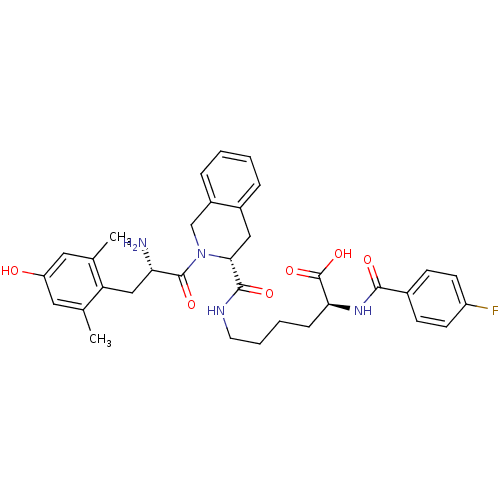 (CHEMBL244265) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.172 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Stanford University School of Medicine Curated by ChEMBL | Assay Description Displacement of [3H]deltorphin2 from delta opioid receptor in Sprague-Dawley rat brain P2 synaptosomal membrane | J Med Chem 51: 1817-23 (2008) Article DOI: 10.1021/jm7014765 BindingDB Entry DOI: 10.7270/Q24T6K7K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B1 bradykinin receptor (Homo sapiens (Human)) | BDBM50362363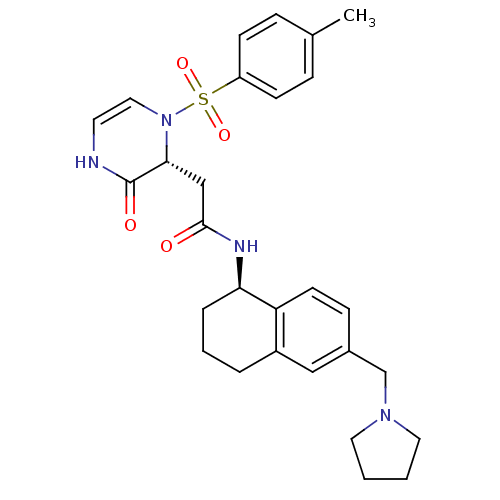 (CHEMBL1939758) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.197 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-DAK from human bradykinin B1 receptor expressed in CHO-D-/aequorin cells membrane after 90 mins by scintillation counting | Bioorg Med Chem Lett 22: 1061-7 (2012) Article DOI: 10.1016/j.bmcl.2011.11.112 BindingDB Entry DOI: 10.7270/Q22J6CB2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B1 bradykinin receptor (Homo sapiens (Human)) | BDBM50272456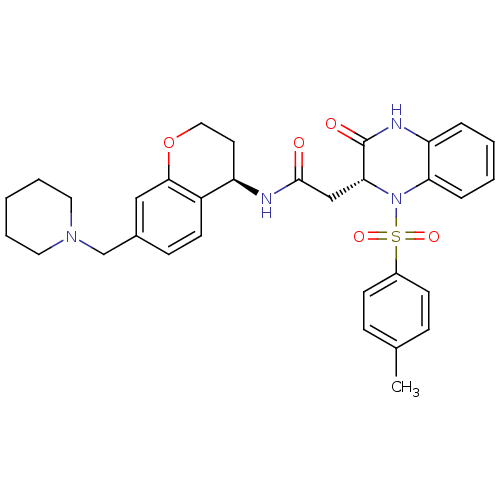 (2-((R)-3-oxo-1-tosyl-1,2,3,4-tetrahydroquinoxalin-...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-DAK from human bradykinin B1 receptor expressed in CHO-D-/aequorin cells membrane after 90 mins by scintillation counting | Bioorg Med Chem Lett 22: 1061-7 (2012) Article DOI: 10.1016/j.bmcl.2011.11.112 BindingDB Entry DOI: 10.7270/Q22J6CB2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50375696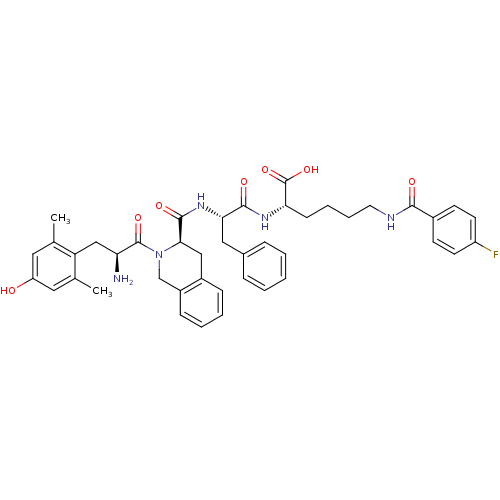 (CHEMBL269842) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.248 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Stanford University School of Medicine Curated by ChEMBL | Assay Description Displacement of [3H]deltorphin2 from delta opioid receptor in Sprague-Dawley rat brain P2 synaptosomal membrane | J Med Chem 51: 1817-23 (2008) Article DOI: 10.1021/jm7014765 BindingDB Entry DOI: 10.7270/Q24T6K7K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Rattus norvegicus (Rat)) | BDBM18161 ((1S,2S,7S,10R,11S,14S,15S)-14-hydroxy-2,15-dimethy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.270 | -50.8 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
The Ohio State University | Assay Description The Ki values were determined by the application of the Cheng-Prusoff equation: Ki = (IC50 x Kd)/(Kd+[L]) where [L] is the concentration of [3H]MIB (... | Bioorg Med Chem Lett 18: 5567-70 (2008) Article DOI: 10.1016/j.bmcl.2008.09.002 BindingDB Entry DOI: 10.7270/Q2W0948B | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| B1 bradykinin receptor (Homo sapiens (Human)) | BDBM50362361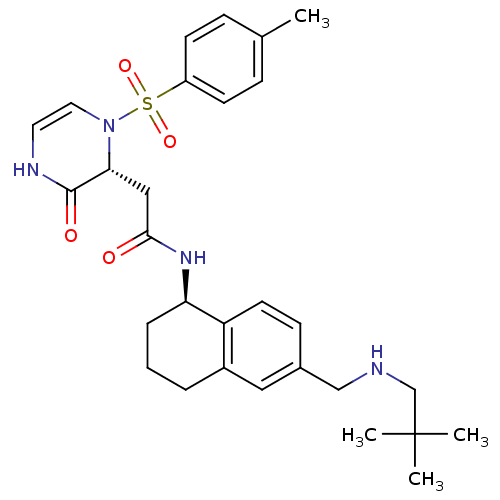 (CHEMBL1939760) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-DAK from human bradykinin B1 receptor expressed in CHO-D-/aequorin cells membrane after 90 mins by scintillation counting | Bioorg Med Chem Lett 22: 1061-7 (2012) Article DOI: 10.1016/j.bmcl.2011.11.112 BindingDB Entry DOI: 10.7270/Q22J6CB2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50347782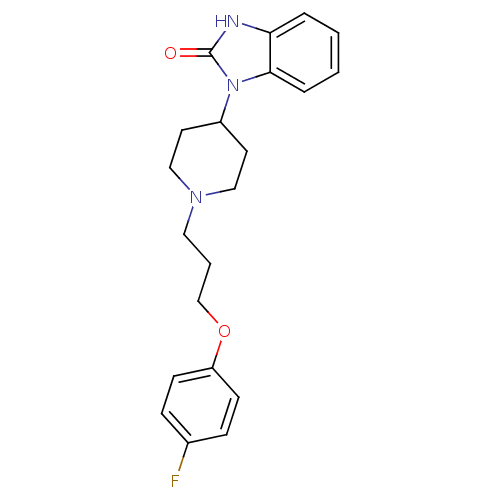 (CHEMBL1802360) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity to dopamine D2 receptor | ACS Med Chem Lett 1: 244-248 (2010) Article DOI: 10.1021/ml100105x BindingDB Entry DOI: 10.7270/Q20R9QGK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Rattus norvegicus (Rat)) | BDBM26260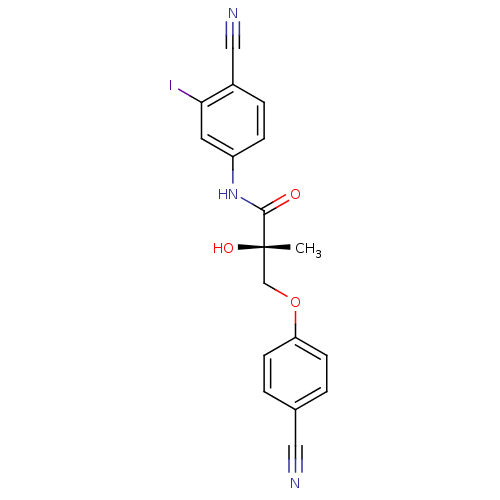 ((2S)-N-(4-cyano-3-iodophenyl)-3-(4-cyanophenoxy)-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem | MMDB PDB Article PubMed | 0.540 | -49.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
The Ohio State University | Assay Description The Ki values were determined by the application of the Cheng-Prusoff equation: Ki = (IC50 x Kd)/(Kd+[L]) where [L] is the concentration of [3H]MIB (... | Bioorg Med Chem Lett 18: 5567-70 (2008) Article DOI: 10.1016/j.bmcl.2008.09.002 BindingDB Entry DOI: 10.7270/Q2W0948B | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| B1 bradykinin receptor (Homo sapiens (Human)) | BDBM50362362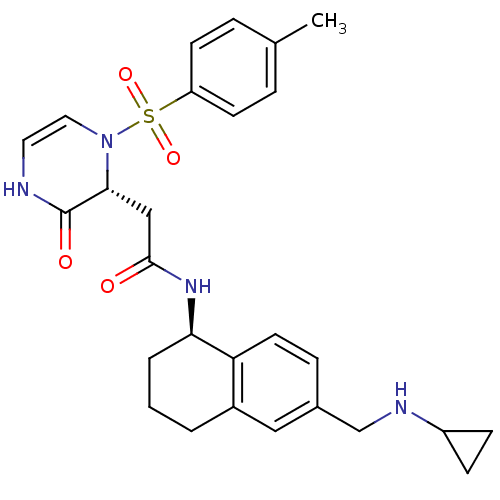 (CHEMBL1939759) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-DAK from human bradykinin B1 receptor expressed in CHO-D-/aequorin cells membrane after 90 mins by scintillation counting | Bioorg Med Chem Lett 22: 1061-7 (2012) Article DOI: 10.1016/j.bmcl.2011.11.112 BindingDB Entry DOI: 10.7270/Q22J6CB2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 3 (Homo sapiens (Human)) | BDBM50369890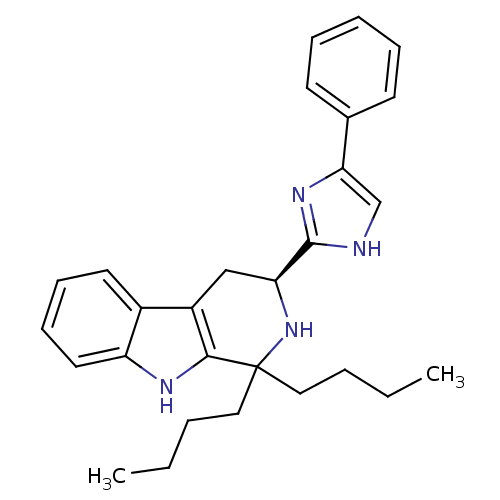 (CHEMBL1237140 | CHEMBL1788167) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.640 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]SS14 from human SST3 expressed in CHO membrane after 60 to 90 mins by scintillation counting | ACS Med Chem Lett 5: 690-5 (2014) Article DOI: 10.1021/ml500079u BindingDB Entry DOI: 10.7270/Q22N53V4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 3 (Homo sapiens (Human)) | BDBM50021074 (CHEMBL3287628) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.815 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]SS14 from human SST3 expressed in CHO membrane after 60 to 90 mins by scintillation counting | ACS Med Chem Lett 5: 690-5 (2014) Article DOI: 10.1021/ml500079u BindingDB Entry DOI: 10.7270/Q22N53V4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50192752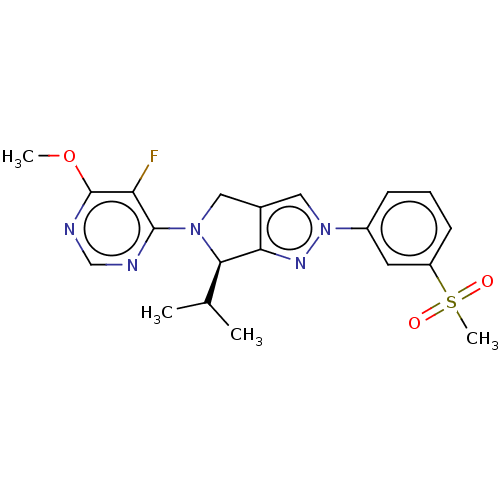 (CHEMBL3905741) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of radiolabeled T0901317 from LXRbeta LBD (unknown origin) | Bioorg Med Chem Lett 26: 5044-5050 (2016) Article DOI: 10.1016/j.bmcl.2016.08.089 BindingDB Entry DOI: 10.7270/Q28P62GC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptosis regulator Bcl-2 (Homo sapiens (Human)) | BDBM21447 (4-(4-{[2-(4-chlorophenyl)phenyl]methyl}piperazin-1...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dalian University of Technology Curated by ChEMBL | Assay Description Binding affinity to Bcl-2 (unknown origin) fluorescence polarization assay | Bioorg Med Chem 21: 11-20 (2012) Article DOI: 10.1016/j.bmc.2012.11.008 BindingDB Entry DOI: 10.7270/Q25X2B7H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50177012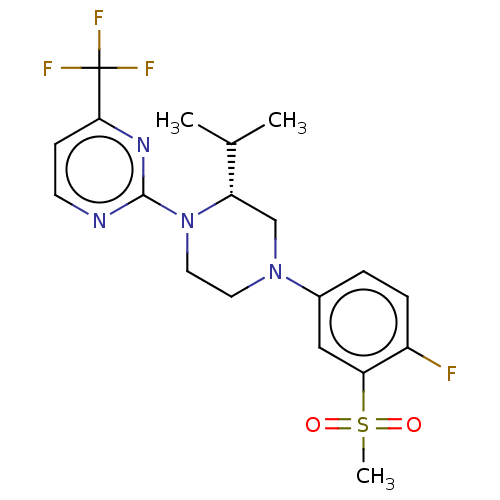 (CHEMBL3814153 | US10144715, Compound 7-32) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]TO901317 from LXRbeta ligand binding domain (unknown origin) after 30 mins by liquid scintillation counting | J Med Chem 59: 3264-71 (2016) Article DOI: 10.1021/acs.jmedchem.5b02029 BindingDB Entry DOI: 10.7270/Q2XP76V7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50177016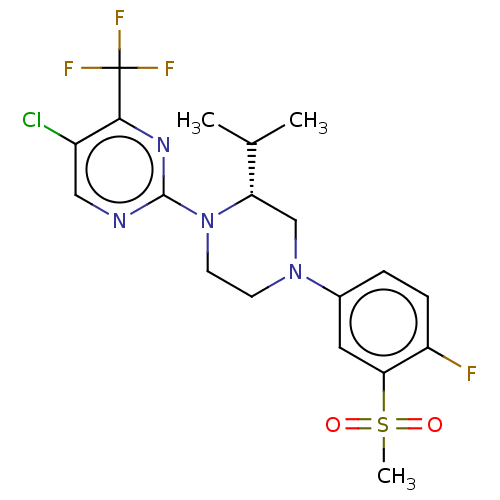 (CHEMBL3814501) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]TO901317 from LXRbeta ligand binding domain (unknown origin) after 30 mins by liquid scintillation counting | J Med Chem 59: 3264-71 (2016) Article DOI: 10.1021/acs.jmedchem.5b02029 BindingDB Entry DOI: 10.7270/Q2XP76V7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuromedin-K receptor (Homo sapiens (Human)) | BDBM50412939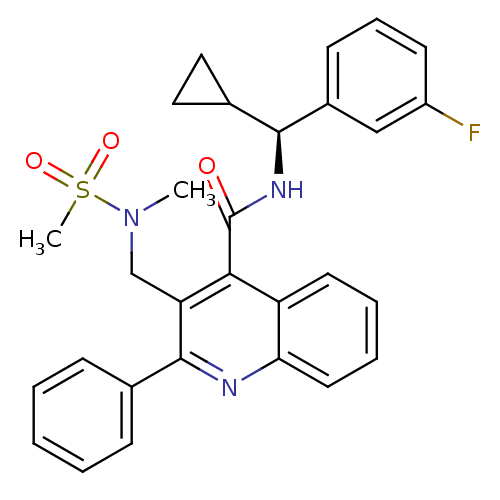 (CHEMBL479463 | GSK-256471) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neuroscience CEDD GlaxoSmithKline Research& Development Curated by ChEMBL | Assay Description Binding affinity to NK3 receptor | Bioorg Med Chem Lett 19: 837-40 (2009) Article DOI: 10.1016/j.bmcl.2008.12.005 BindingDB Entry DOI: 10.7270/Q20K29S8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM252994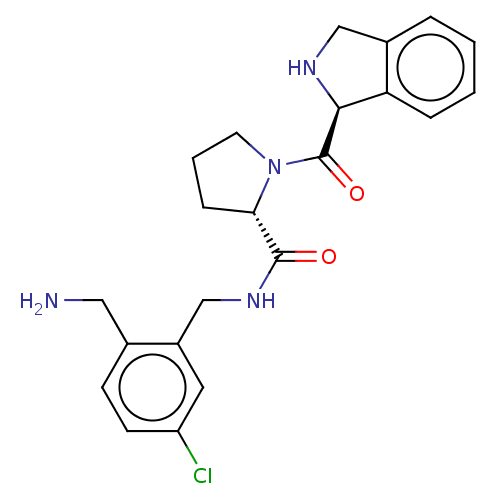 (US9469608, 23-4b) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Relevant in vitro assays are referenced in Morrissette, et al., Bioorg. Med. Chem. Lett. 2004, 14, 4161-4164 and described in Lewis, et al. Thromb. R... | US Patent US9469608 (2016) BindingDB Entry DOI: 10.7270/Q2F18XNQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B1 bradykinin receptor (Homo sapiens (Human)) | BDBM50362364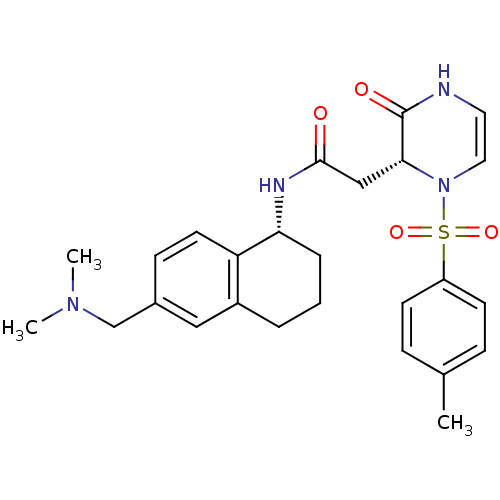 (CHEMBL1939757) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-DAK from human bradykinin B1 receptor expressed in CHO-D-/aequorin cells membrane after 90 mins by scintillation counting | Bioorg Med Chem Lett 22: 1061-7 (2012) Article DOI: 10.1016/j.bmcl.2011.11.112 BindingDB Entry DOI: 10.7270/Q22J6CB2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50347782 (CHEMBL1802360) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity to dopamine D3 receptor | ACS Med Chem Lett 1: 244-248 (2010) Article DOI: 10.1021/ml100105x BindingDB Entry DOI: 10.7270/Q20R9QGK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuromedin-K receptor (Homo sapiens (Human)) | BDBM50412940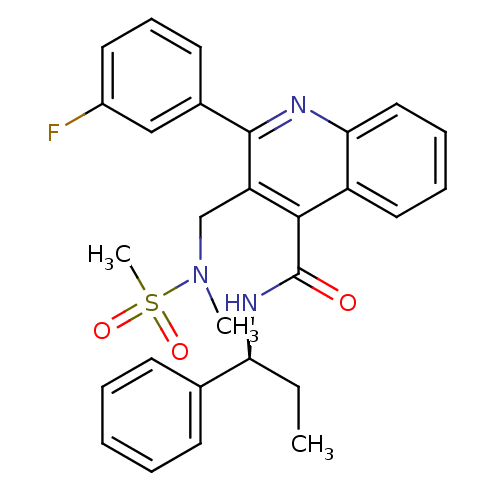 (CHEMBL519770) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neuroscience CEDD GlaxoSmithKline Research& Development Curated by ChEMBL | Assay Description Binding affinity to NK3 receptor | Bioorg Med Chem Lett 19: 837-40 (2009) Article DOI: 10.1016/j.bmcl.2008.12.005 BindingDB Entry DOI: 10.7270/Q20K29S8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuromedin-K receptor (Homo sapiens (Human)) | BDBM50412941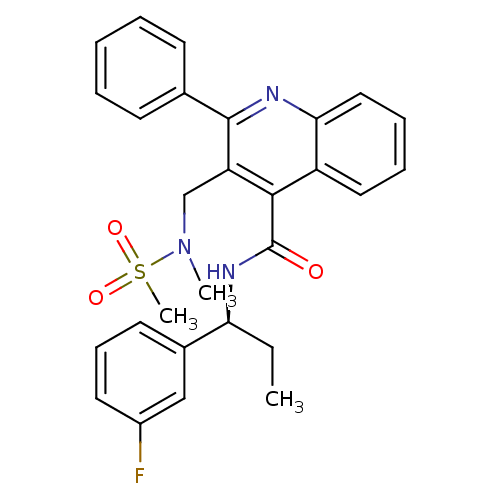 (CHEMBL521416) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neuroscience CEDD GlaxoSmithKline Research& Development Curated by ChEMBL | Assay Description Binding affinity to NK3 receptor | Bioorg Med Chem Lett 19: 837-40 (2009) Article DOI: 10.1016/j.bmcl.2008.12.005 BindingDB Entry DOI: 10.7270/Q20K29S8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 3 (Homo sapiens (Human)) | BDBM50110470 (CHEMBL3605798) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]SS-14 from human SSTR3 expressed in CHO cells after 60 to 90 mins | Bioorg Med Chem Lett 25: 3520-5 (2015) Article DOI: 10.1016/j.bmcl.2015.06.087 BindingDB Entry DOI: 10.7270/Q29W0H9M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50177010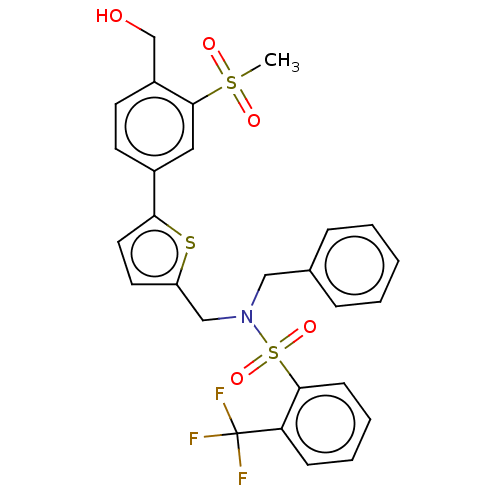 (CHEMBL3814006) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]TO901317 from LXRbeta ligand binding domain (unknown origin) after 30 mins by liquid scintillation counting | J Med Chem 59: 3264-71 (2016) Article DOI: 10.1021/acs.jmedchem.5b02029 BindingDB Entry DOI: 10.7270/Q2XP76V7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Rattus norvegicus (Rat)) | BDBM26262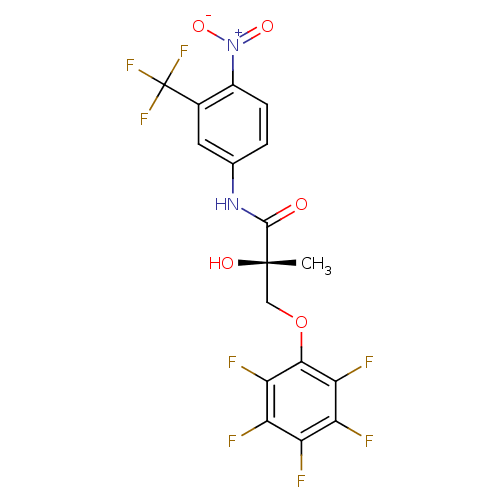 ((2S)-2-hydroxy-2-methyl-N-[4-nitro-3-(trifluoromet...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 1.40 | -47.0 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
The Ohio State University | Assay Description The Ki values were determined by the application of the Cheng-Prusoff equation: Ki = (IC50 x Kd)/(Kd+[L]) where [L] is the concentration of [3H]MIB (... | Bioorg Med Chem Lett 18: 5567-70 (2008) Article DOI: 10.1016/j.bmcl.2008.09.002 BindingDB Entry DOI: 10.7270/Q2W0948B | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Neuromedin-K receptor (Homo sapiens (Human)) | BDBM50412942 (CHEMBL480249) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.58 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neuroscience CEDD GlaxoSmithKline Research& Development Curated by ChEMBL | Assay Description Binding affinity to NK3 receptor | Bioorg Med Chem Lett 19: 837-40 (2009) Article DOI: 10.1016/j.bmcl.2008.12.005 BindingDB Entry DOI: 10.7270/Q20K29S8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B1 bradykinin receptor (Homo sapiens (Human)) | BDBM50362365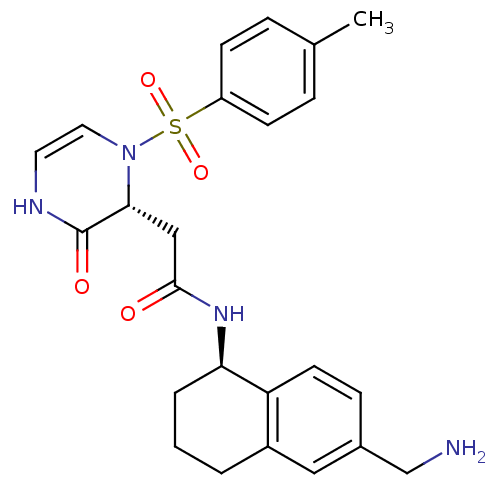 (CHEMBL1939756) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.64 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-DAK from human bradykinin B1 receptor expressed in CHO-D-/aequorin cells membrane after 90 mins by scintillation counting | Bioorg Med Chem Lett 22: 1061-7 (2012) Article DOI: 10.1016/j.bmcl.2011.11.112 BindingDB Entry DOI: 10.7270/Q22J6CB2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Rattus norvegicus (Rat)) | BDBM26261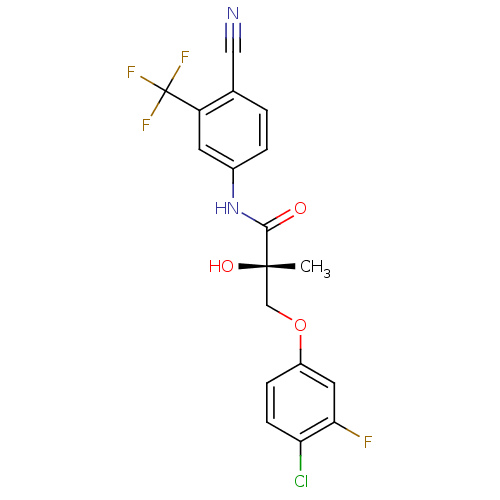 ((2S)-3-(4-chloro-3-fluorophenoxy)-N-[4-cyano-3-(tr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | 1.70 | -46.5 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
The Ohio State University | Assay Description The Ki values were determined by the application of the Cheng-Prusoff equation: Ki = (IC50 x Kd)/(Kd+[L]) where [L] is the concentration of [3H]MIB (... | Bioorg Med Chem Lett 18: 5567-70 (2008) Article DOI: 10.1016/j.bmcl.2008.09.002 BindingDB Entry DOI: 10.7270/Q2W0948B | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Somatostatin receptor type 3 (Homo sapiens (Human)) | BDBM50110461 (CHEMBL3605789) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]SS-14 from human SSTR3 expressed in CHO cells after 60 to 90 mins | Bioorg Med Chem Lett 25: 3520-5 (2015) Article DOI: 10.1016/j.bmcl.2015.06.087 BindingDB Entry DOI: 10.7270/Q29W0H9M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuromedin-K receptor (Homo sapiens (Human)) | BDBM50412943 (CHEMBL480818) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neuroscience CEDD GlaxoSmithKline Research& Development Curated by ChEMBL | Assay Description Binding affinity to NK3 receptor | Bioorg Med Chem Lett 19: 837-40 (2009) Article DOI: 10.1016/j.bmcl.2008.12.005 BindingDB Entry DOI: 10.7270/Q20K29S8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50005429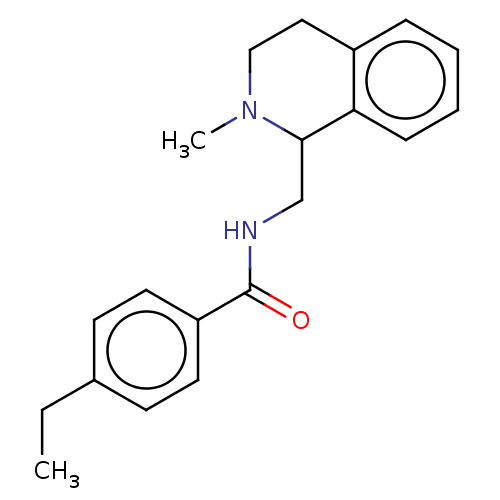 (CHEMBL4070288) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Displacement of [3H]naloxone from recombinant human MOR expressed in HEK cell membranes incubated for 60 mins | Eur J Med Chem 126: 202-217 (2017) Article DOI: 10.1016/j.ejmech.2016.09.003 BindingDB Entry DOI: 10.7270/Q2RB76TS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM253008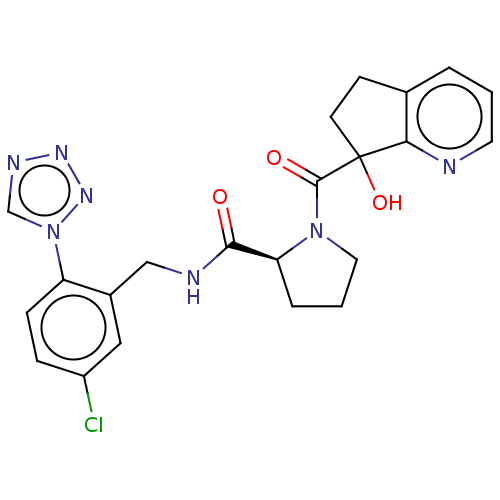 (US9469608, 36-3) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Relevant in vitro assays are referenced in Morrissette, et al., Bioorg. Med. Chem. Lett. 2004, 14, 4161-4164 and described in Lewis, et al. Thromb. R... | US Patent US9469608 (2016) BindingDB Entry DOI: 10.7270/Q2F18XNQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM253006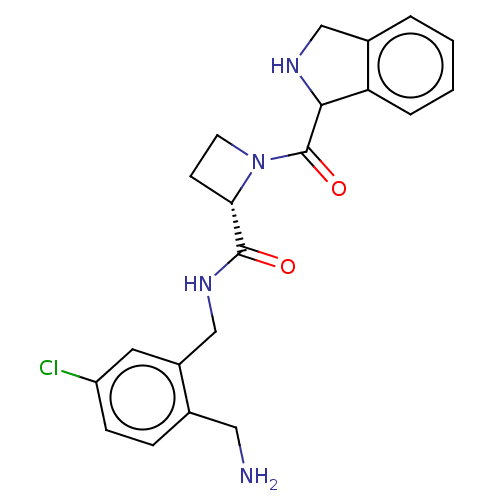 (US9469608, 34-7) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Relevant in vitro assays are referenced in Morrissette, et al., Bioorg. Med. Chem. Lett. 2004, 14, 4161-4164 and described in Lewis, et al. Thromb. R... | US Patent US9469608 (2016) BindingDB Entry DOI: 10.7270/Q2F18XNQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Rattus norvegicus (Rat)) | BDBM26259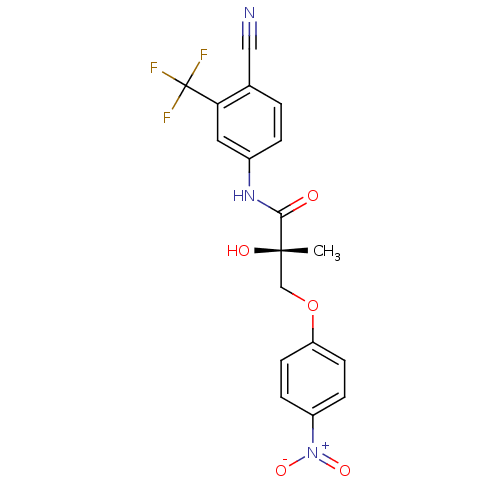 ((2S)-N-[4-cyano-3-(trifluoromethyl)phenyl]-2-hydro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | MMDB PC cid PC sid UniChem Similars | MMDB Article PubMed | 2.5 | -45.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
The Ohio State University | Assay Description The Ki values were determined by the application of the Cheng-Prusoff equation: Ki = (IC50 x Kd)/(Kd+[L]) where [L] is the concentration of [3H]MIB (... | Bioorg Med Chem Lett 18: 5567-70 (2008) Article DOI: 10.1016/j.bmcl.2008.09.002 BindingDB Entry DOI: 10.7270/Q2W0948B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50375695 (CHEMBL270984) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Stanford University School of Medicine Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from mu opioid receptor in Sprague-Dawley rat brain P2 synaptosomal membrane | J Med Chem 51: 1817-23 (2008) Article DOI: 10.1021/jm7014765 BindingDB Entry DOI: 10.7270/Q24T6K7K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 1 (Homo sapiens (Human)) | BDBM230664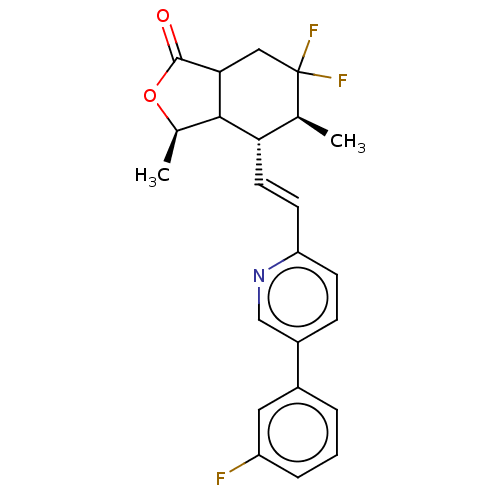 (US9340530, 2) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 3 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Thrombin receptor antagonists were screened using a modification of the thrombin receptor radioligand binding assay of Ahn et al. (Ahn et al., Mol. P... | US Patent US9340530 (2016) BindingDB Entry DOI: 10.7270/Q2K35SJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 1 (Homo sapiens (Human)) | BDBM230675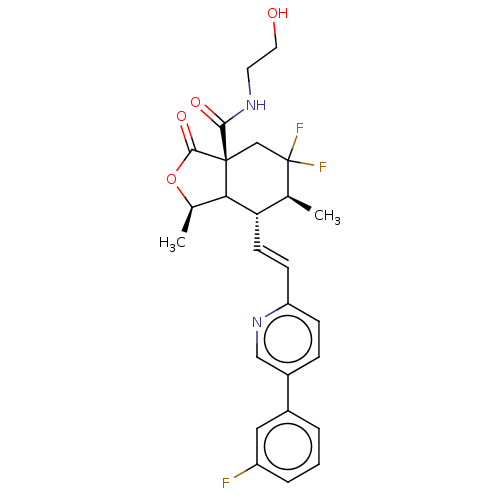 (US9340530, 13) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | US Patent | 3 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Thrombin receptor antagonists were screened using a modification of the thrombin receptor radioligand binding assay of Ahn et al. (Ahn et al., Mol. P... | US Patent US9340530 (2016) BindingDB Entry DOI: 10.7270/Q2K35SJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 1 (Homo sapiens (Human)) | BDBM230672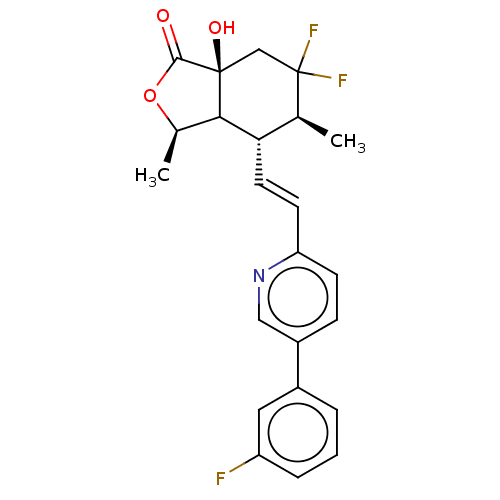 (US9340530, 10) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 3 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Thrombin receptor antagonists were screened using a modification of the thrombin receptor radioligand binding assay of Ahn et al. (Ahn et al., Mol. P... | US Patent US9340530 (2016) BindingDB Entry DOI: 10.7270/Q2K35SJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50192753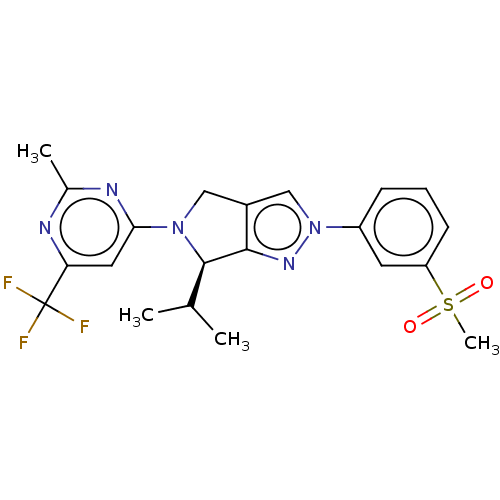 (CHEMBL3985591) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of radiolabeled T0901317 from LXRbeta LBD (unknown origin) | Bioorg Med Chem Lett 26: 5044-5050 (2016) Article DOI: 10.1016/j.bmcl.2016.08.089 BindingDB Entry DOI: 10.7270/Q28P62GC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50177015 (CHEMBL3814206 | US10144715, Compound 19-1) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of radiolabeled T0901317 from LXRbeta LBD (unknown origin) | Bioorg Med Chem Lett 26: 5044-5050 (2016) Article DOI: 10.1016/j.bmcl.2016.08.089 BindingDB Entry DOI: 10.7270/Q28P62GC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Glutamate receptor 2 (Homo sapiens (Human)) | BDBM50494356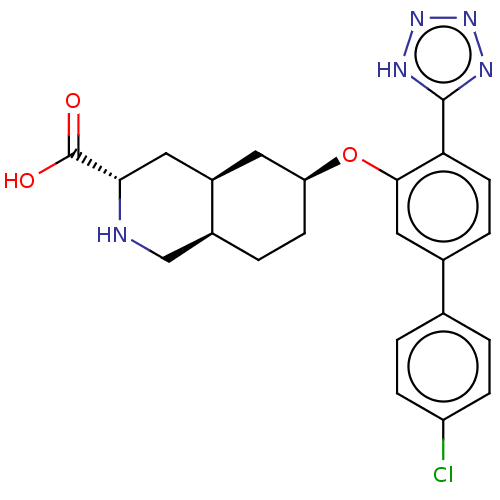 (CHEMBL3088070) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centro de Investigaci�n Lilly Curated by ChEMBL | Assay Description Displacement of [3H]AMPA from homomeric recombinant GluA2 receptor (unknown origin) | Bioorg Med Chem Lett 23: 6463-6 (2013) Article DOI: 10.1016/j.bmcl.2013.09.045 BindingDB Entry DOI: 10.7270/Q2XS5ZBW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50177015 (CHEMBL3814206 | US10144715, Compound 19-1) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]TO901317 from LXRbeta ligand binding domain (unknown origin) after 30 mins by liquid scintillation counting | J Med Chem 59: 3264-71 (2016) Article DOI: 10.1021/acs.jmedchem.5b02029 BindingDB Entry DOI: 10.7270/Q2XP76V7 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM50177016 (CHEMBL3814501) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]TO901317 from LXRalpha ligand binding domain (unknown origin) after 30 mins by liquid scintillation counting | J Med Chem 59: 3264-71 (2016) Article DOI: 10.1021/acs.jmedchem.5b02029 BindingDB Entry DOI: 10.7270/Q2XP76V7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 3 (Homo sapiens (Human)) | BDBM50021075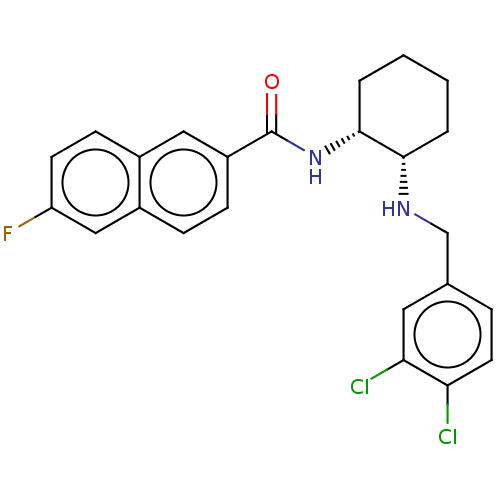 (CHEMBL3287629) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]SS14 from human SST3 expressed in CHO membrane after 60 to 90 mins by scintillation counting | ACS Med Chem Lett 5: 690-5 (2014) Article DOI: 10.1021/ml500079u BindingDB Entry DOI: 10.7270/Q22N53V4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50347782 (CHEMBL1802360) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity to 5HT2A receptor | ACS Med Chem Lett 1: 244-248 (2010) Article DOI: 10.1021/ml100105x BindingDB Entry DOI: 10.7270/Q20R9QGK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuromedin-K receptor (Homo sapiens (Human)) | BDBM50412944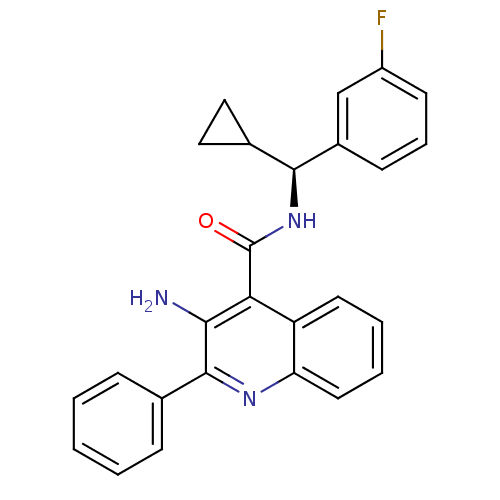 (CHEMBL479652) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neuroscience CEDD GlaxoSmithKline Research& Development Curated by ChEMBL | Assay Description Binding affinity to NK3 receptor | Bioorg Med Chem Lett 19: 837-40 (2009) Article DOI: 10.1016/j.bmcl.2008.12.005 BindingDB Entry DOI: 10.7270/Q20K29S8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 9114 total ) | Next | Last >> |