

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Neuraminidase (Influenza B virus (B/Lee/40)) | BDBM4707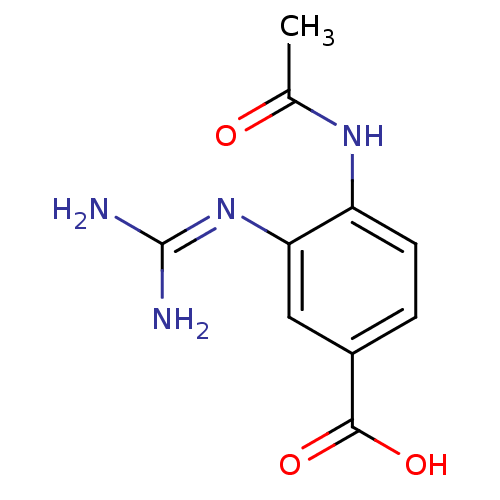 (3-(2,2-diaminoimino)-4-methylcarboxamidobenzoate |...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Alabama at Birmingham Curated by ChEMBL | Assay Description In vitro inhibitory activity of the compound against B/Lee/40 Influenza B Neuraminidase. | J Med Chem 42: 2332-43 (1999) Article DOI: 10.1021/jm980707k BindingDB Entry DOI: 10.7270/Q25B01NJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (A/Singapore/1/57(H2N2))) | BDBM5266 ((2S,3S,4R)-4-carbamimidamido-2-(dipropylcarbamoyl)...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 6.5 | 37 |
University of Alabama at Birmingham | Assay Description A standard fluorimetric assay was used to measure influenza virus neuraminidase activity. The substrate 2 -(4-methylumbelliferyl)-alpha-D-acetylneura... | Bioorg Med Chem 7: 2487-97 (1999) Article DOI: 10.1016/s0968-0896(99)00197-2 BindingDB Entry DOI: 10.7270/Q2Z036CV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza B virus (B/Lee/40)) | BDBM5265 ((2S,3S,4R)-4-carbamimidamido-3-acetamido-2-[(1R,2S...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | 6.5 | 37 |
University of Alabama at Birmingham | Assay Description A standard fluorimetric assay was used to measure influenza virus neuraminidase activity. The substrate 2 -(4-methylumbelliferyl)-alpha-D-acetylneura... | Bioorg Med Chem 7: 2487-97 (1999) Article DOI: 10.1016/s0968-0896(99)00197-2 BindingDB Entry DOI: 10.7270/Q2Z036CV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (A/Singapore/1/57(H2N2))) | BDBM5265 ((2S,3S,4R)-4-carbamimidamido-3-acetamido-2-[(1R,2S...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | 6.5 | 37 |
University of Alabama at Birmingham | Assay Description A standard fluorimetric assay was used to measure influenza virus neuraminidase activity. The substrate 2 -(4-methylumbelliferyl)-alpha-D-acetylneura... | Bioorg Med Chem 7: 2487-97 (1999) Article DOI: 10.1016/s0968-0896(99)00197-2 BindingDB Entry DOI: 10.7270/Q2Z036CV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4A (Homo sapiens (Human)) | BDBM50393511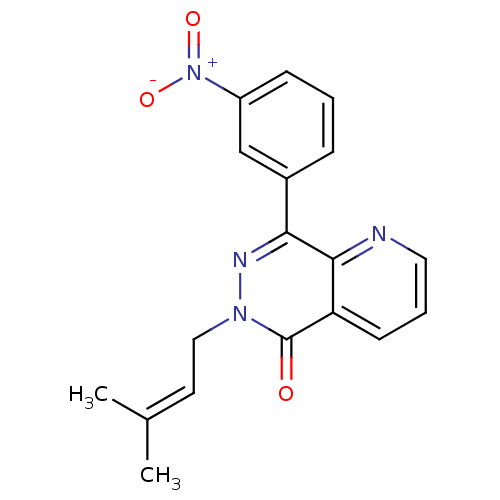 (CHEMBL2158056) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Glasgow Curated by ChEMBL | Assay Description Inhibition of full-length PDE4A4 using cAMP as substrate by two-step radiochemical assay | J Med Chem 54: 3331-47 (2011) Article DOI: 10.1021/jm200070e BindingDB Entry DOI: 10.7270/Q2CF9R75 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4A (Homo sapiens (Human)) | BDBM50393510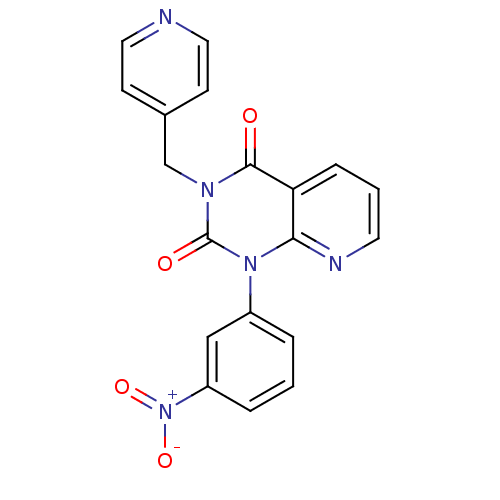 (CHEMBL1232082) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Glasgow Curated by ChEMBL | Assay Description Inhibition of full-length PDE4A4 using cAMP as substrate by two-step radiochemical assay | J Med Chem 54: 3331-47 (2011) Article DOI: 10.1021/jm200070e BindingDB Entry DOI: 10.7270/Q2CF9R75 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4A (Homo sapiens (Human)) | BDBM50085135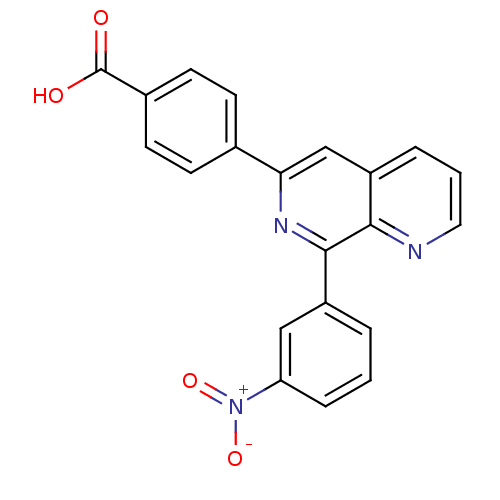 (4-(8-(3-nitrophenyl)-1,7-naphthyridin-6-yl)benzoic...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Glasgow Curated by ChEMBL | Assay Description Inhibition of full-length PDE4A4 using cAMP as substrate by two-step radiochemical assay | J Med Chem 54: 3331-47 (2011) Article DOI: 10.1021/jm200070e BindingDB Entry DOI: 10.7270/Q2CF9R75 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Neuraminidase (Influenza A virus (A/Puerto Rico/8/34/Mount Sinai(...) | BDBM50078329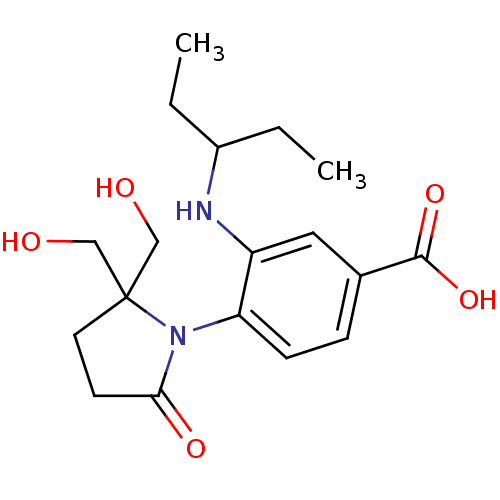 (1-[4-CARBOXY-2-(3-PENTYLAMINO)PHENYL]-5,5'-DI(HYDR...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Alabama at Birmingham Curated by ChEMBL | Assay Description In vitro inhibitory activity of the compound against H1N9 Influenza A Neuraminidase. | J Med Chem 42: 2332-43 (1999) Article DOI: 10.1021/jm980707k BindingDB Entry DOI: 10.7270/Q25B01NJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B (Homo sapiens (Human)) | BDBM50344327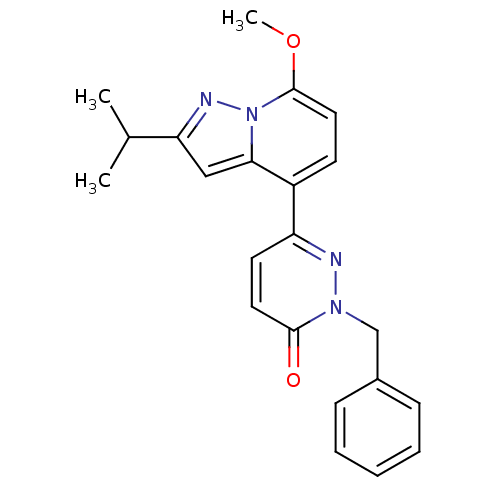 (2-benzyl-6-(2-isopropyl-7-methoxypyrazolo[1,5-a]py...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
Heriot-Watt University Curated by ChEMBL | Assay Description Inhibition of human PDE4B catalytic domain using cAMP/cGMP substrate | Bioorg Med Chem Lett 21: 3307-12 (2011) Article DOI: 10.1016/j.bmcl.2011.04.021 BindingDB Entry DOI: 10.7270/Q2X067C3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B (Homo sapiens (Human)) | BDBM50344329 (2-benzyl-6-(2-isopropyl-7-methoxypyrazolo[1,5-a]py...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Heriot-Watt University Curated by ChEMBL | Assay Description Inhibition of human PDE4B catalytic domain using cAMP/cGMP substrate | Bioorg Med Chem Lett 21: 3307-12 (2011) Article DOI: 10.1016/j.bmcl.2011.04.021 BindingDB Entry DOI: 10.7270/Q2X067C3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4A (Homo sapiens (Human)) | BDBM50108504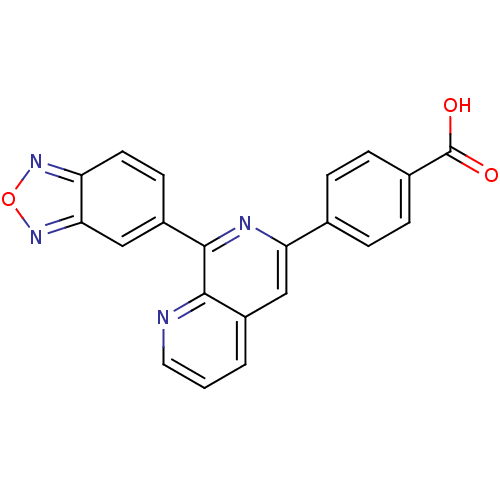 (4-(8-(benzo[c][1,2,5]oxadiazol-5-yl)-1,7-naphthyri...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 290 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Glasgow Curated by ChEMBL | Assay Description Inhibition of full-length PDE4A4 using cAMP as substrate by two-step radiochemical assay | J Med Chem 54: 3331-47 (2011) Article DOI: 10.1021/jm200070e BindingDB Entry DOI: 10.7270/Q2CF9R75 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4A (Homo sapiens (Human)) | BDBM50393512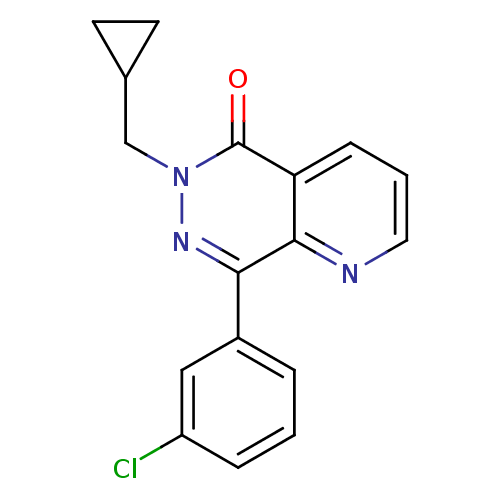 (CHEMBL2158057) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 340 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Glasgow Curated by ChEMBL | Assay Description Inhibition of full-length PDE4A4 using cAMP as substrate by two-step radiochemical assay | J Med Chem 54: 3331-47 (2011) Article DOI: 10.1021/jm200070e BindingDB Entry DOI: 10.7270/Q2CF9R75 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4A (Homo sapiens (Human)) | BDBM50393509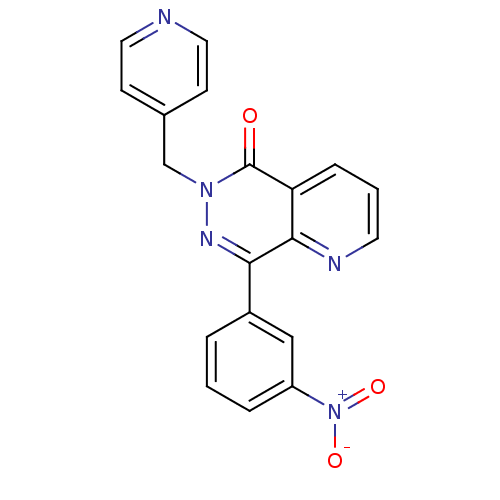 (PYRIDOPYRIDAZINONE) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 340 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Glasgow Curated by ChEMBL | Assay Description Inhibition of full-length PDE4A4 using cAMP as substrate by two-step radiochemical assay | J Med Chem 54: 3331-47 (2011) Article DOI: 10.1021/jm200070e BindingDB Entry DOI: 10.7270/Q2CF9R75 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (strain A/Udorn/307/1972 H3N2)) | BDBM50078329 (1-[4-CARBOXY-2-(3-PENTYLAMINO)PHENYL]-5,5'-DI(HYDR...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 490 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Alabama at Birmingham Curated by ChEMBL | Assay Description Inhibition of Influenza A virus (A/udorn/1972(H3N2)) neuraminidase using fluorogenic substrate 2'-(4-methylumbelliferyl)-alpha-D-N-acetylneuraminic a... | Bioorg Med Chem 20: 4582-9 (2012) Article DOI: 10.1016/j.bmc.2012.05.001 BindingDB Entry DOI: 10.7270/Q28S4QZS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza B virus (B/Lee/40)) | BDBM5266 ((2S,3S,4R)-4-carbamimidamido-2-(dipropylcarbamoyl)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | 6.5 | 37 |
University of Alabama at Birmingham | Assay Description A standard fluorimetric assay was used to measure influenza virus neuraminidase activity. The substrate 2 -(4-methylumbelliferyl)-alpha-D-acetylneura... | Bioorg Med Chem 7: 2487-97 (1999) Article DOI: 10.1016/s0968-0896(99)00197-2 BindingDB Entry DOI: 10.7270/Q2Z036CV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4A (Homo sapiens (Human)) | BDBM50042058 ((-)-rolipram | (4R)-4-[3-(cyclopentyloxy)-4-methox...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 550 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Glasgow Curated by ChEMBL | Assay Description Inhibition of full-length PDE4A4 using cAMP as substrate by two-step radiochemical assay | J Med Chem 54: 3331-47 (2011) Article DOI: 10.1021/jm200070e BindingDB Entry DOI: 10.7270/Q2CF9R75 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4A (Homo sapiens (Human)) | BDBM50393518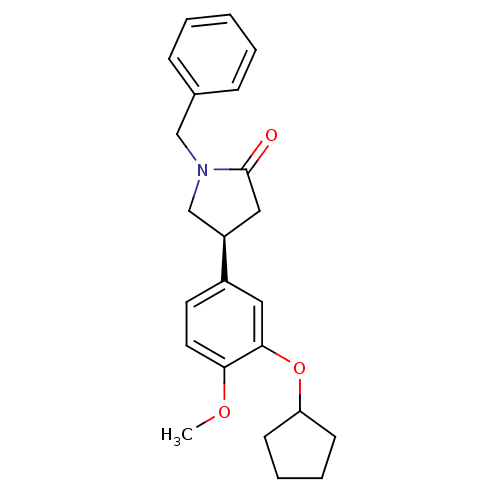 (CHEMBL2158064) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 730 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Glasgow Curated by ChEMBL | Assay Description Inhibition of full-length PDE4A4 using cAMP as substrate by two-step radiochemical assay | J Med Chem 54: 3331-47 (2011) Article DOI: 10.1021/jm200070e BindingDB Entry DOI: 10.7270/Q2CF9R75 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4A (Homo sapiens (Human)) | BDBM14361 ((R,S)-Rolipram | 4-(3-cyclopentyloxy-4-methoxy-phe...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 830 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Glasgow Curated by ChEMBL | Assay Description Inhibition of full-length PDE4A4 using cAMP as substrate by two-step radiochemical assay | J Med Chem 54: 3331-47 (2011) Article DOI: 10.1021/jm200070e BindingDB Entry DOI: 10.7270/Q2CF9R75 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4A (Homo sapiens (Human)) | BDBM50393515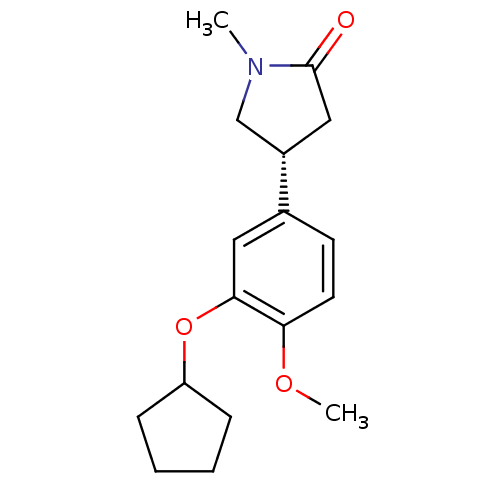 (CHEMBL2158061) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Glasgow Curated by ChEMBL | Assay Description Inhibition of full-length PDE4A4 using cAMP as substrate by two-step radiochemical assay | J Med Chem 54: 3331-47 (2011) Article DOI: 10.1021/jm200070e BindingDB Entry DOI: 10.7270/Q2CF9R75 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (A/Singapore/1/57(H2N2))) | BDBM5272 (3-carbamimidamido-4-acetamido-5-(pentan-3-yloxy)be...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | 6.5 | 37 |
University of Alabama at Birmingham | Assay Description A standard fluorimetric assay was used to measure influenza virus neuraminidase activity. The substrate 2 -(4-methylumbelliferyl)-alpha-D-acetylneura... | Bioorg Med Chem 7: 2487-97 (1999) Article DOI: 10.1016/s0968-0896(99)00197-2 BindingDB Entry DOI: 10.7270/Q2Z036CV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4A (Homo sapiens (Human)) | BDBM50240404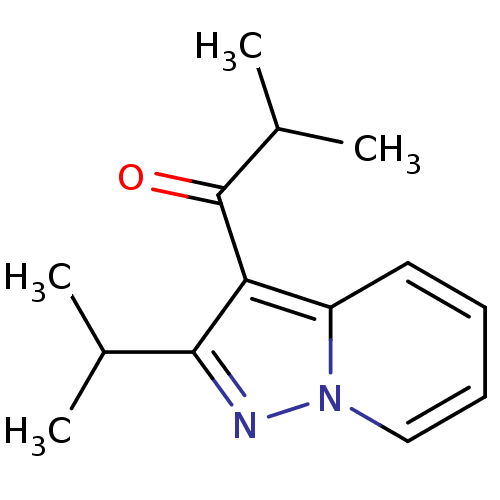 ((Ibudilast)1-(2-Isopropyl-pyrazolo[1,5-a]pyridin-3...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents | DrugBank Article PubMed | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Glasgow Curated by ChEMBL | Assay Description Inhibition of full-length PDE4A4 using cAMP as substrate by two-step radiochemical assay | J Med Chem 54: 3331-47 (2011) Article DOI: 10.1021/jm200070e BindingDB Entry DOI: 10.7270/Q2CF9R75 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B (Homo sapiens (Human)) | BDBM50344325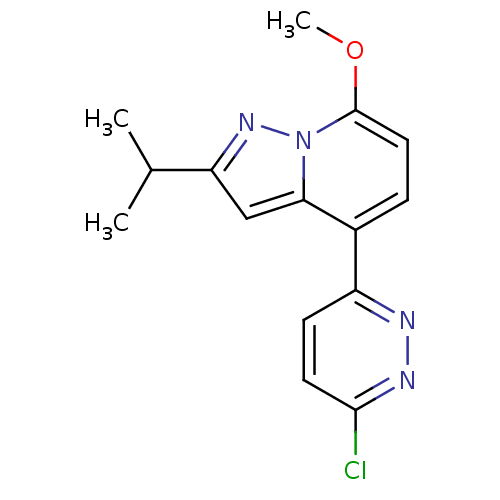 (4-(6-chloropyridazin-3-yl)-2-isopropyl-7-methoxypy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.38E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Heriot-Watt University Curated by ChEMBL | Assay Description Inhibition of human PDE4B catalytic domain using cAMP/cGMP substrate | Bioorg Med Chem Lett 21: 3307-12 (2011) Article DOI: 10.1016/j.bmcl.2011.04.021 BindingDB Entry DOI: 10.7270/Q2X067C3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4A (Homo sapiens (Human)) | BDBM50040348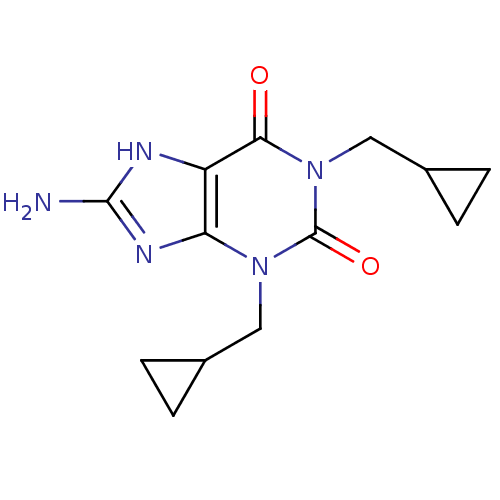 (8-Amino-1,3-bis-cyclopropylmethyl-3,7-dihydro-puri...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Glasgow Curated by ChEMBL | Assay Description Inhibition of full-length PDE4A4 using cAMP as substrate by two-step radiochemical assay | J Med Chem 54: 3331-47 (2011) Article DOI: 10.1021/jm200070e BindingDB Entry DOI: 10.7270/Q2CF9R75 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B (Homo sapiens (Human)) | BDBM50344321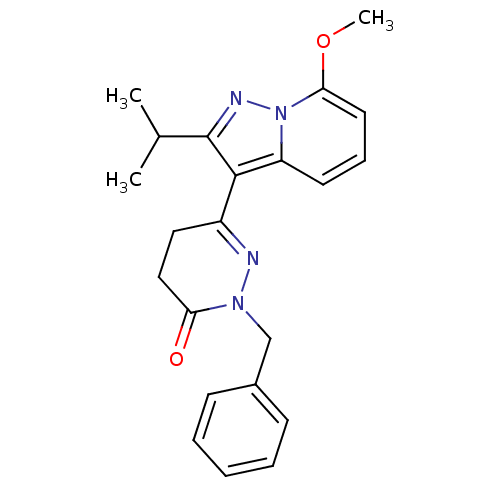 (2-benzyl-6-(2-isopropyl-7-methoxypyrazolo[1,5-a]py...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Heriot-Watt University Curated by ChEMBL | Assay Description Inhibition of human PDE4B catalytic domain using cAMP/cGMP substrate | Bioorg Med Chem Lett 21: 3307-12 (2011) Article DOI: 10.1016/j.bmcl.2011.04.021 BindingDB Entry DOI: 10.7270/Q2X067C3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B (Homo sapiens (Human)) | BDBM50344314 (CHEMBL1779432 | rac-2-Benzyl-4a-methyl-4,4a,5,6-te...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Heriot-Watt University Curated by ChEMBL | Assay Description Inhibition of human PDE4B catalytic domain using cAMP/cGMP substrate | Bioorg Med Chem Lett 21: 3307-12 (2011) Article DOI: 10.1016/j.bmcl.2011.04.021 BindingDB Entry DOI: 10.7270/Q2X067C3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4A (Homo sapiens (Human)) | BDBM50393516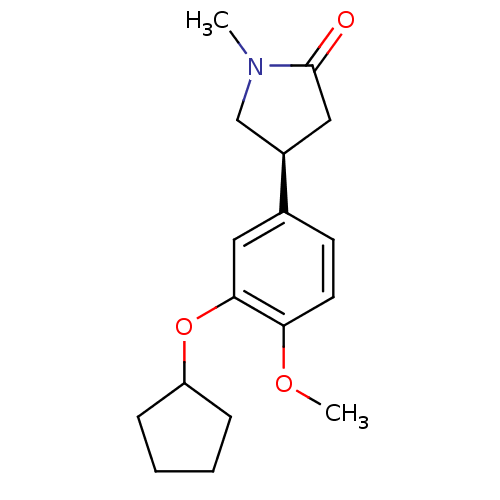 (CHEMBL2158062) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Glasgow Curated by ChEMBL | Assay Description Inhibition of full-length PDE4A4 using cAMP as substrate by two-step radiochemical assay | J Med Chem 54: 3331-47 (2011) Article DOI: 10.1021/jm200070e BindingDB Entry DOI: 10.7270/Q2CF9R75 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B (Homo sapiens (Human)) | BDBM50344318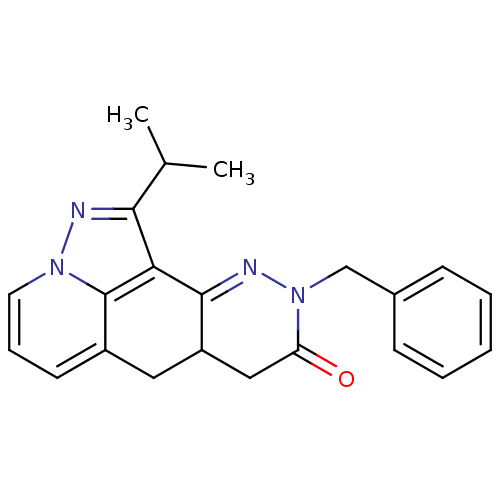 (CHEMBL1779436 | rac-9-Benzyl-1-isopropyl-6a,9,10b,...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Heriot-Watt University Curated by ChEMBL | Assay Description Inhibition of human PDE4B catalytic domain using cAMP/cGMP substrate | Bioorg Med Chem Lett 21: 3307-12 (2011) Article DOI: 10.1016/j.bmcl.2011.04.021 BindingDB Entry DOI: 10.7270/Q2X067C3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B (Homo sapiens (Human)) | BDBM50344326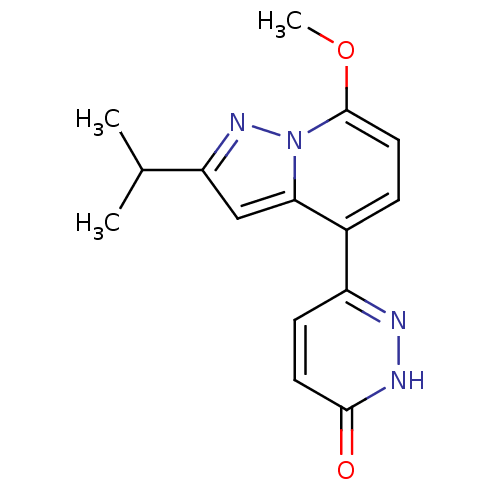 (6-(2-isopropyl-7-methoxypyrazolo[1,5-a]pyridin-4-y...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.58E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Heriot-Watt University Curated by ChEMBL | Assay Description Inhibition of human PDE4B catalytic domain using cAMP/cGMP substrate | Bioorg Med Chem Lett 21: 3307-12 (2011) Article DOI: 10.1016/j.bmcl.2011.04.021 BindingDB Entry DOI: 10.7270/Q2X067C3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B (Homo sapiens (Human)) | BDBM50344328 (6-(2-isopropyl-7-methoxypyrazolo[1,5-a]pyridin-4-y...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.61E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Heriot-Watt University Curated by ChEMBL | Assay Description Inhibition of human PDE4B catalytic domain using cAMP/cGMP substrate | Bioorg Med Chem Lett 21: 3307-12 (2011) Article DOI: 10.1016/j.bmcl.2011.04.021 BindingDB Entry DOI: 10.7270/Q2X067C3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-inhibited 3',5'-cyclic phosphodiesterase 3A (Homo sapiens (Human)) | BDBM50344326 (6-(2-isopropyl-7-methoxypyrazolo[1,5-a]pyridin-4-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.75E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Heriot-Watt University Curated by ChEMBL | Assay Description Inhibition of human PDE3A catalytic domain using cAMP/cGMP substrate | Bioorg Med Chem Lett 21: 3307-12 (2011) Article DOI: 10.1016/j.bmcl.2011.04.021 BindingDB Entry DOI: 10.7270/Q2X067C3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (N9)) | BDBM5272 (3-carbamimidamido-4-acetamido-5-(pentan-3-yloxy)be...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | 6.5 | 37 |
University of Alabama at Birmingham | Assay Description A standard fluorimetric assay was used to measure influenza virus neuraminidase activity. The substrate 2 -(4-methylumbelliferyl)-alpha-D-acetylneura... | Bioorg Med Chem 7: 2487-97 (1999) Article DOI: 10.1016/s0968-0896(99)00197-2 BindingDB Entry DOI: 10.7270/Q2Z036CV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-inhibited 3',5'-cyclic phosphodiesterase 3A (Homo sapiens (Human)) | BDBM50344315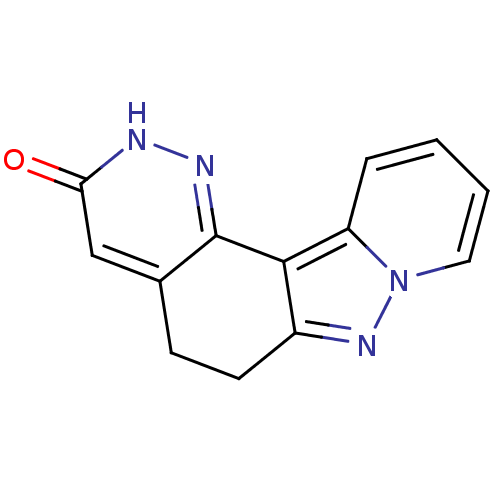 (5,6-Dihydro-2H-1,2,7,7a-tetraaza-benzo[c]fluoren-3...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Heriot-Watt University Curated by ChEMBL | Assay Description Inhibition of human PDE3A catalytic domain using cAMP/cGMP substrate | Bioorg Med Chem Lett 21: 3307-12 (2011) Article DOI: 10.1016/j.bmcl.2011.04.021 BindingDB Entry DOI: 10.7270/Q2X067C3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-inhibited 3',5'-cyclic phosphodiesterase 3A (Homo sapiens (Human)) | BDBM50344306 ((R)-6-(2-isopropylpyrazolo[1,5-a]pyridin-3-yl)-5-m...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Heriot-Watt University Curated by ChEMBL | Assay Description Inhibition of human PDE3A catalytic domain using cAMP/cGMP substrate | Bioorg Med Chem Lett 21: 3307-12 (2011) Article DOI: 10.1016/j.bmcl.2011.04.021 BindingDB Entry DOI: 10.7270/Q2X067C3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4A (Homo sapiens (Human)) | BDBM50042056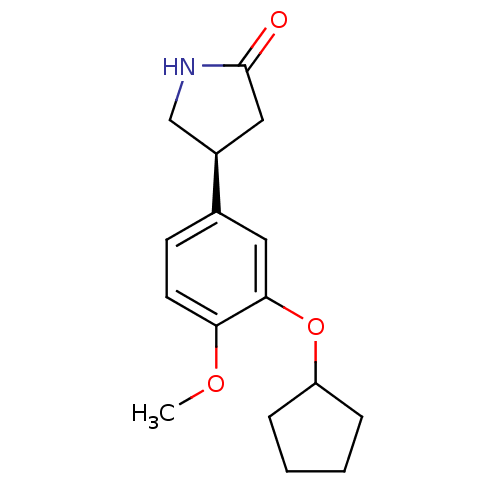 ((R)-4-(3-Cyclopentyloxy-4-methoxy-phenyl)-pyrrolid...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Glasgow Curated by ChEMBL | Assay Description Inhibition of full-length PDE4A4 using cAMP as substrate by two-step radiochemical assay | J Med Chem 54: 3331-47 (2011) Article DOI: 10.1021/jm200070e BindingDB Entry DOI: 10.7270/Q2CF9R75 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4A (Homo sapiens (Human)) | BDBM50393517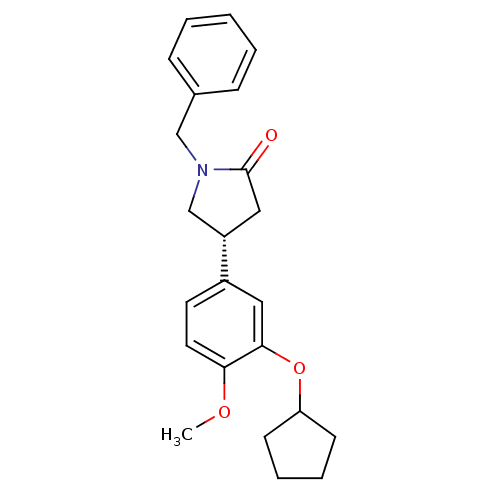 (CHEMBL2158063) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Glasgow Curated by ChEMBL | Assay Description Inhibition of full-length PDE4A4 using cAMP as substrate by two-step radiochemical assay | J Med Chem 54: 3331-47 (2011) Article DOI: 10.1021/jm200070e BindingDB Entry DOI: 10.7270/Q2CF9R75 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4A (Homo sapiens (Human)) | BDBM50010981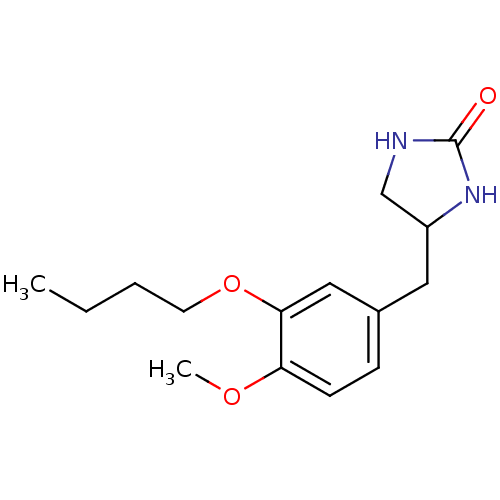 ((Ro 20-1724)4-(3-Butoxy-4-methoxy-benzyl)-imidazol...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 4.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Glasgow Curated by ChEMBL | Assay Description Inhibition of full-length PDE4A4 using cAMP as substrate by two-step radiochemical assay | J Med Chem 54: 3331-47 (2011) Article DOI: 10.1021/jm200070e BindingDB Entry DOI: 10.7270/Q2CF9R75 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Neuraminidase (Influenza A virus (A/Puerto Rico/8/34/Mount Sinai(...) | BDBM50078330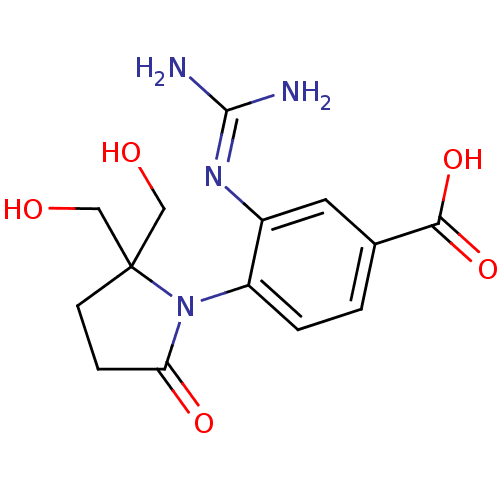 (1-(4-CARBOXY-2-GUANIDINOPENTYL)-5,5'-DI(HYDROXYMET...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Alabama at Birmingham Curated by ChEMBL | Assay Description In vitro inhibitory activity of the compound against H1N9 Influenza A Neuraminidase. | J Med Chem 42: 2332-43 (1999) Article DOI: 10.1021/jm980707k BindingDB Entry DOI: 10.7270/Q25B01NJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B (Homo sapiens (Human)) | BDBM50240404 ((Ibudilast)1-(2-Isopropyl-pyrazolo[1,5-a]pyridin-3...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents | Article PubMed | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Heriot-Watt University Curated by ChEMBL | Assay Description Inhibition of human PDE4B catalytic domain using cAMP/cGMP substrate | Bioorg Med Chem Lett 21: 3307-12 (2011) Article DOI: 10.1016/j.bmcl.2011.04.021 BindingDB Entry DOI: 10.7270/Q2X067C3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4A (Homo sapiens (Human)) | BDBM50393525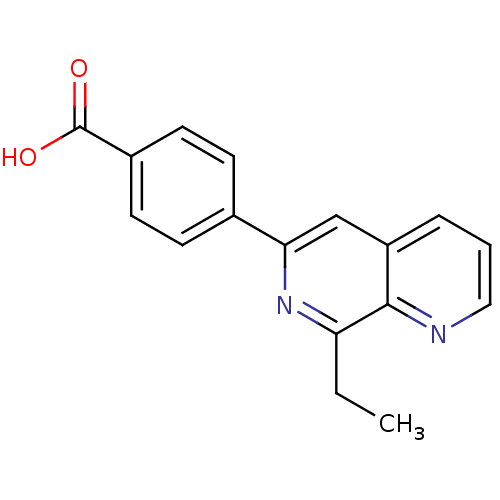 (CHEMBL2158070) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Glasgow Curated by ChEMBL | Assay Description Inhibition of full-length PDE4A4 using cAMP as substrate by two-step radiochemical assay | J Med Chem 54: 3331-47 (2011) Article DOI: 10.1021/jm200070e BindingDB Entry DOI: 10.7270/Q2CF9R75 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4A (Homo sapiens (Human)) | BDBM50393523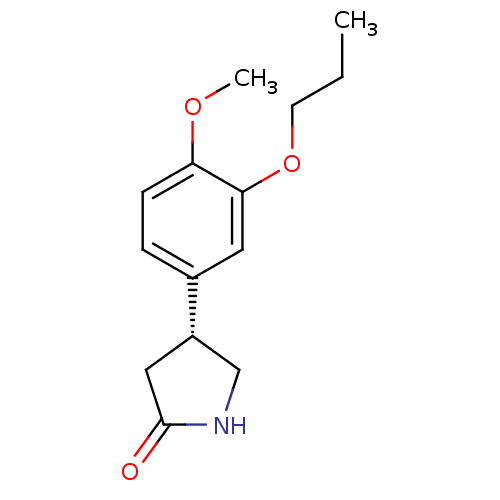 (CHEMBL2158068) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Glasgow Curated by ChEMBL | Assay Description Inhibition of full-length PDE4A4 using cAMP as substrate by two-step radiochemical assay | J Med Chem 54: 3331-47 (2011) Article DOI: 10.1021/jm200070e BindingDB Entry DOI: 10.7270/Q2CF9R75 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4A (Homo sapiens (Human)) | BDBM50393522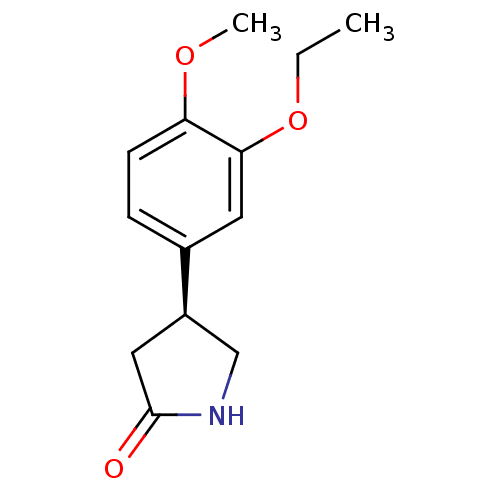 (CHEMBL2158003) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Glasgow Curated by ChEMBL | Assay Description Inhibition of full-length PDE4A4 using cAMP as substrate by two-step radiochemical assay | J Med Chem 54: 3331-47 (2011) Article DOI: 10.1021/jm200070e BindingDB Entry DOI: 10.7270/Q2CF9R75 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50344327 (2-benzyl-6-(2-isopropyl-7-methoxypyrazolo[1,5-a]py...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Heriot-Watt University Curated by ChEMBL | Assay Description Inhibition of human PDE5A catalytic domain using cAMP/cGMP substrate | Bioorg Med Chem Lett 21: 3307-12 (2011) Article DOI: 10.1016/j.bmcl.2011.04.021 BindingDB Entry DOI: 10.7270/Q2X067C3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4A (Homo sapiens (Human)) | BDBM50393521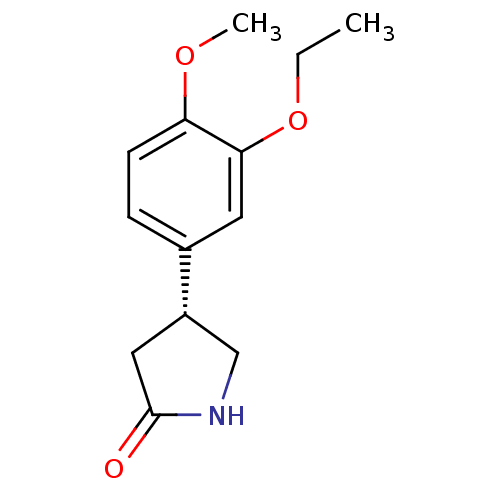 (CHEMBL2158067) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Glasgow Curated by ChEMBL | Assay Description Inhibition of full-length PDE4A4 using cAMP as substrate by two-step radiochemical assay | J Med Chem 54: 3331-47 (2011) Article DOI: 10.1021/jm200070e BindingDB Entry DOI: 10.7270/Q2CF9R75 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-inhibited 3',5'-cyclic phosphodiesterase 3A (Homo sapiens (Human)) | BDBM50344311 (CHEMBL1779429 | rac-4,4a,5,6-Tetrahydro-2H-1,2,7,7...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Heriot-Watt University Curated by ChEMBL | Assay Description Inhibition of human PDE3A catalytic domain using cAMP/cGMP substrate | Bioorg Med Chem Lett 21: 3307-12 (2011) Article DOI: 10.1016/j.bmcl.2011.04.021 BindingDB Entry DOI: 10.7270/Q2X067C3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza B virus (B/Lee/40)) | BDBM50078330 (1-(4-CARBOXY-2-GUANIDINOPENTYL)-5,5'-DI(HYDROXYMET...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB Article PubMed | n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Alabama at Birmingham Curated by ChEMBL | Assay Description In vitro inhibitory activity of the compound against B/Lee/40 Influenza B Neuraminidase. | J Med Chem 42: 2332-43 (1999) Article DOI: 10.1021/jm980707k BindingDB Entry DOI: 10.7270/Q25B01NJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (A/Singapore/1/57(H2N2))) | BDBM5271 (3-amino-4-acetamido-5-(pentan-3-yloxy)benzoic acid...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | MMDB PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a | 6.5 | 37 |
University of Alabama at Birmingham | Assay Description A standard fluorimetric assay was used to measure influenza virus neuraminidase activity. The substrate 2 -(4-methylumbelliferyl)-alpha-D-acetylneura... | Bioorg Med Chem 7: 2487-97 (1999) Article DOI: 10.1016/s0968-0896(99)00197-2 BindingDB Entry DOI: 10.7270/Q2Z036CV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4A (Homo sapiens (Human)) | BDBM50393524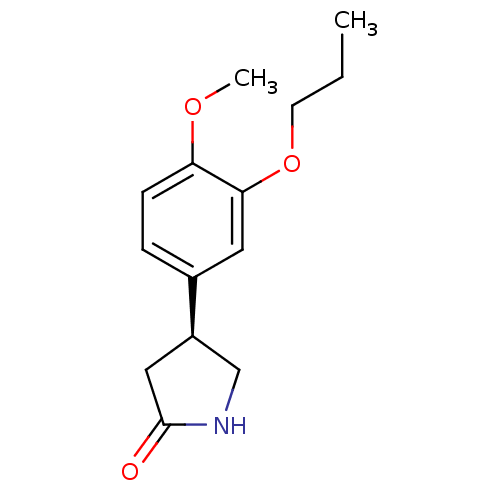 (CHEMBL2158069) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Glasgow Curated by ChEMBL | Assay Description Inhibition of full-length PDE4A4 using cAMP as substrate by two-step radiochemical assay | J Med Chem 54: 3331-47 (2011) Article DOI: 10.1021/jm200070e BindingDB Entry DOI: 10.7270/Q2CF9R75 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-inhibited 3',5'-cyclic phosphodiesterase 3A (Homo sapiens (Human)) | BDBM50344314 (CHEMBL1779432 | rac-2-Benzyl-4a-methyl-4,4a,5,6-te...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Heriot-Watt University Curated by ChEMBL | Assay Description Inhibition of human PDE3A catalytic domain using cAMP/cGMP substrate | Bioorg Med Chem Lett 21: 3307-12 (2011) Article DOI: 10.1016/j.bmcl.2011.04.021 BindingDB Entry DOI: 10.7270/Q2X067C3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B (Homo sapiens (Human)) | BDBM50344305 (CHEMBL1779335 | rac-6-(2-isopropylpyrazolo[1,5-a]p...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 9.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Heriot-Watt University Curated by ChEMBL | Assay Description Inhibition of human PDE4B catalytic domain using cAMP/cGMP substrate | Bioorg Med Chem Lett 21: 3307-12 (2011) Article DOI: 10.1016/j.bmcl.2011.04.021 BindingDB Entry DOI: 10.7270/Q2X067C3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza B virus (B/Lee/40)) | BDBM5279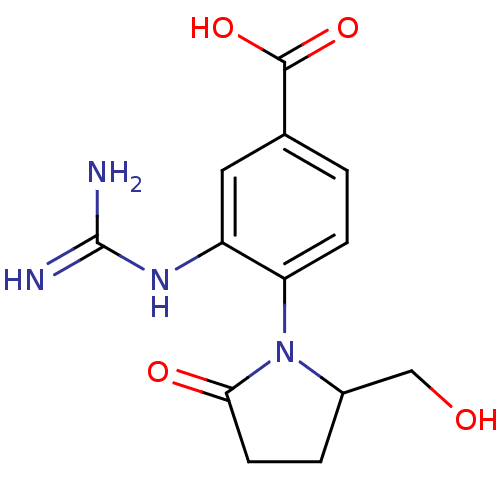 (3-carbamimidamido-4-[2-(hydroxymethyl)-5-oxopyrrol...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | 6.5 | 37 |
University of Alabama at Birmingham | Assay Description A standard fluorimetric assay was used to measure influenza virus neuraminidase activity. The substrate 2 -(4-methylumbelliferyl)-alpha-D-acetylneura... | Bioorg Med Chem Lett 9: 1901-6 (1999) Article DOI: 10.1016/s0960-894x(99)00318-2 BindingDB Entry DOI: 10.7270/Q2T72FN4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 168 total ) | Next | Last >> |