 Found 859 hits with Last Name = 'bain' and Initial = 'g'
Found 859 hits with Last Name = 'bain' and Initial = 'g' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
3-hydroxy-3-methylglutaryl-coenzyme A reductase
(Homo sapiens (Human)) | BDBM50215692

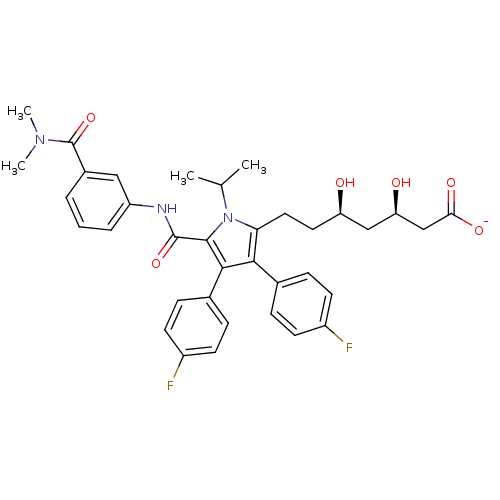
(CHEMBL400874 | sodium (3R,5R)-7-(5-((4-cyanobenzyl...)Show SMILES CC(C)n1c(CC[C@@H](O)C[C@@H](O)CC([O-])=O)c(c(c1C(=O)NCc1ccc(cc1)C#N)-c1ccccc1)-c1ccc(F)cc1 Show InChI InChI=1S/C35H36FN3O5/c1-22(2)39-30(17-16-28(40)18-29(41)19-31(42)43)32(26-12-14-27(36)15-13-26)33(25-6-4-3-5-7-25)34(39)35(44)38-21-24-10-8-23(20-37)9-11-24/h3-15,22,28-29,40-41H,16-19,21H2,1-2H3,(H,38,44)(H,42,43)/p-1/t28-,29-/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of HMG-CoA reductase |
Bioorg Med Chem Lett 17: 4538-44 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.096
BindingDB Entry DOI: 10.7270/Q2SJ1K9Q |
More data for this
Ligand-Target Pair | |
3-hydroxy-3-methylglutaryl-coenzyme A reductase
(Homo sapiens (Human)) | BDBM50215688
(CHEMBL399773 | sodium (3R,5R)-7-(5-((3-(dimethylca...)Show SMILES CC(C)n1c(CC[C@@H](O)C[C@@H](O)CC([O-])=O)c(c(c1C(=O)Nc1cccc(c1)C(=O)N(C)C)-c1ccc(F)cc1)-c1ccc(F)cc1 Show InChI InChI=1S/C36H39F2N3O6/c1-21(2)41-30(17-16-28(42)19-29(43)20-31(44)45)32(22-8-12-25(37)13-9-22)33(23-10-14-26(38)15-11-23)34(41)35(46)39-27-7-5-6-24(18-27)36(47)40(3)4/h5-15,18,21,28-29,42-43H,16-17,19-20H2,1-4H3,(H,39,46)(H,44,45)/p-1/t28-,29-/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of HMG-CoA reductase |
Bioorg Med Chem Lett 17: 4538-44 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.096
BindingDB Entry DOI: 10.7270/Q2SJ1K9Q |
More data for this
Ligand-Target Pair | |
3-hydroxy-3-methylglutaryl-coenzyme A reductase
(Homo sapiens (Human)) | BDBM50215689

(CHEMBL250317 | sodium (3R,5R)-7-(5-((4-carbamoylph...)Show SMILES CC(C)n1c(CC[C@@H](O)C[C@@H](O)CC([O-])=O)c(c(c1C(=O)Nc1ccc(cc1)C(N)=O)-c1ccccc1)-c1ccc(F)cc1 Show InChI InChI=1S/C34H36FN3O6/c1-20(2)38-28(17-16-26(39)18-27(40)19-29(41)42)30(22-8-12-24(35)13-9-22)31(21-6-4-3-5-7-21)32(38)34(44)37-25-14-10-23(11-15-25)33(36)43/h3-15,20,26-27,39-40H,16-19H2,1-2H3,(H2,36,43)(H,37,44)(H,41,42)/p-1/t26-,27-/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of HMG-CoA reductase |
Bioorg Med Chem Lett 17: 4538-44 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.096
BindingDB Entry DOI: 10.7270/Q2SJ1K9Q |
More data for this
Ligand-Target Pair | |
3-hydroxy-3-methylglutaryl-coenzyme A reductase
(Homo sapiens (Human)) | BDBM50215676
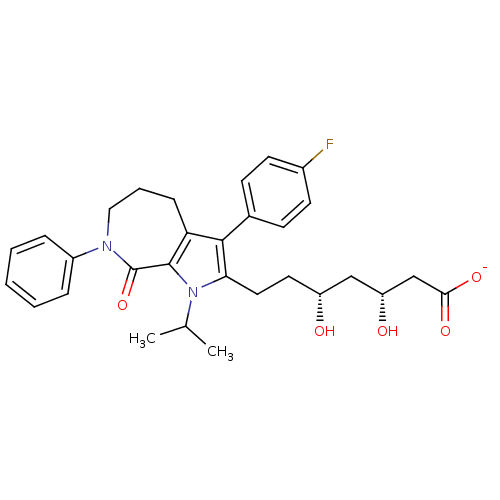
(CHEMBL228955 | sodium (3R,5R)-7-(3-(4-fluorophenyl...)Show SMILES CC(C)n1c(CC[C@@H](O)C[C@@H](O)CC([O-])=O)c(c2CCCN(c3ccccc3)C(=O)c12)-c1ccc(F)cc1 Show InChI InChI=1S/C30H35FN2O5/c1-19(2)33-26(15-14-23(34)17-24(35)18-27(36)37)28(20-10-12-21(31)13-11-20)25-9-6-16-32(30(38)29(25)33)22-7-4-3-5-8-22/h3-5,7-8,10-13,19,23-24,34-35H,6,9,14-18H2,1-2H3,(H,36,37)/p-1/t23-,24-/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of HMGCoA reductase |
Bioorg Med Chem Lett 17: 4531-7 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.097
BindingDB Entry DOI: 10.7270/Q2X929Z4 |
More data for this
Ligand-Target Pair | |
3-hydroxy-3-methylglutaryl-coenzyme A reductase
(Homo sapiens (Human)) | BDBM50215693
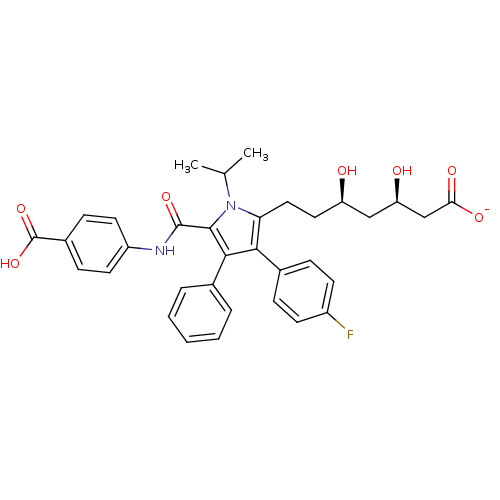
(CHEMBL437774 | sodium (3R,5R)-7-(5-((4-carboxyphen...)Show SMILES CC(C)n1c(CC[C@@H](O)C[C@@H](O)CC([O-])=O)c(c(c1C(=O)Nc1ccc(cc1)C(O)=O)-c1ccccc1)-c1ccc(F)cc1 Show InChI InChI=1S/C34H35FN2O7/c1-20(2)37-28(17-16-26(38)18-27(39)19-29(40)41)30(22-8-12-24(35)13-9-22)31(21-6-4-3-5-7-21)32(37)33(42)36-25-14-10-23(11-15-25)34(43)44/h3-15,20,26-27,38-39H,16-19H2,1-2H3,(H,36,42)(H,40,41)(H,43,44)/p-1/t26-,27-/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of HMG-CoA reductase |
Bioorg Med Chem Lett 17: 4538-44 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.096
BindingDB Entry DOI: 10.7270/Q2SJ1K9Q |
More data for this
Ligand-Target Pair | |
Arachidonate 5-lipoxygenase-activating protein
(Homo sapiens (Human)) | BDBM50359073
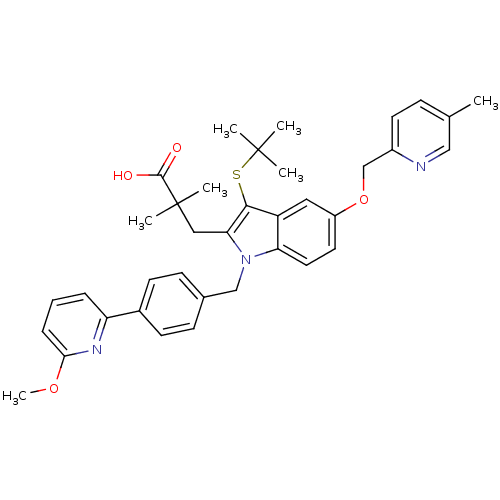
(CHEMBL1922653)Show SMILES COc1cccc(n1)-c1ccc(Cn2c(CC(C)(C)C(O)=O)c(SC(C)(C)C)c3cc(OCc4ccc(C)cn4)ccc23)cc1 Show InChI InChI=1S/C37H41N3O4S/c1-24-11-16-27(38-21-24)23-44-28-17-18-31-29(19-28)34(45-36(2,3)4)32(20-37(5,6)35(41)42)40(31)22-25-12-14-26(15-13-25)30-9-8-10-33(39-30)43-7/h8-19,21H,20,22-23H2,1-7H3,(H,41,42) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Amira Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of FLAP in human peripheral leukocytes assessed as inhibition of calcium ionophore A23187-induced LTB4 production after 10 mins by ELISA |
J Med Chem 54: 8013-29 (2011)
Article DOI: 10.1021/jm2008369
BindingDB Entry DOI: 10.7270/Q269740W |
More data for this
Ligand-Target Pair | |
Arachidonate 5-lipoxygenase-activating protein
(Homo sapiens (Human)) | BDBM50359083

(CHEMBL1922663)Show SMILES Cc1ccc(COc2ccc3n(Cc4ccc(cc4)-c4ccc(F)cn4)c(CC(C)(C)C(O)=O)c(SC(C)(C)C)c3c2)nc1 Show InChI InChI=1S/C36H38FN3O3S/c1-23-7-13-27(38-19-23)22-43-28-14-16-31-29(17-28)33(44-35(2,3)4)32(18-36(5,6)34(41)42)40(31)21-24-8-10-25(11-9-24)30-15-12-26(37)20-39-30/h7-17,19-20H,18,21-22H2,1-6H3,(H,41,42) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Amira Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of FLAP in human peripheral leukocytes assessed as inhibition of calcium ionophore A23187-induced LTB4 production after 10 mins by ELISA |
J Med Chem 54: 8013-29 (2011)
Article DOI: 10.1021/jm2008369
BindingDB Entry DOI: 10.7270/Q269740W |
More data for this
Ligand-Target Pair | |
Arachidonate 5-lipoxygenase-activating protein
(Homo sapiens (Human)) | BDBM50359077

(CHEMBL1922657)Show SMILES Cc1ccc(COc2ccc3n(Cc4ccc(cc4)-c4ccc(nc4)C(F)(F)F)c(CC(C)(C)C(O)=O)c(SC(C)(C)C)c3c2)nc1 Show InChI InChI=1S/C37H38F3N3O3S/c1-23-7-13-27(41-19-23)22-46-28-14-15-30-29(17-28)33(47-35(2,3)4)31(18-36(5,6)34(44)45)43(30)21-24-8-10-25(11-9-24)26-12-16-32(42-20-26)37(38,39)40/h7-17,19-20H,18,21-22H2,1-6H3,(H,44,45) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Amira Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of FLAP in human peripheral leukocytes assessed as inhibition of calcium ionophore A23187-induced LTB4 production after 10 mins by ELISA |
J Med Chem 54: 8013-29 (2011)
Article DOI: 10.1021/jm2008369
BindingDB Entry DOI: 10.7270/Q269740W |
More data for this
Ligand-Target Pair | |
3-hydroxy-3-methylglutaryl-coenzyme A reductase
(Homo sapiens (Human)) | BDBM50215700

(CHEMBL400973 | sodium (3R,5R)-7-(3-(4-fluorophenyl...)Show SMILES CC(C)n1c(CC[C@@H](O)C[C@@H](O)CC([O-])=O)c(c(c1C(=O)Nc1ccc(O)cc1)-c1ccccc1)-c1ccc(F)cc1 Show InChI InChI=1S/C33H35FN2O6/c1-20(2)36-28(17-16-26(38)18-27(39)19-29(40)41)30(22-8-10-23(34)11-9-22)31(21-6-4-3-5-7-21)32(36)33(42)35-24-12-14-25(37)15-13-24/h3-15,20,26-27,37-39H,16-19H2,1-2H3,(H,35,42)(H,40,41)/p-1/t26-,27-/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of HMG-CoA reductase |
Bioorg Med Chem Lett 17: 4538-44 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.096
BindingDB Entry DOI: 10.7270/Q2SJ1K9Q |
More data for this
Ligand-Target Pair | |
Arachidonate 5-lipoxygenase-activating protein
(Homo sapiens (Human)) | BDBM50359075
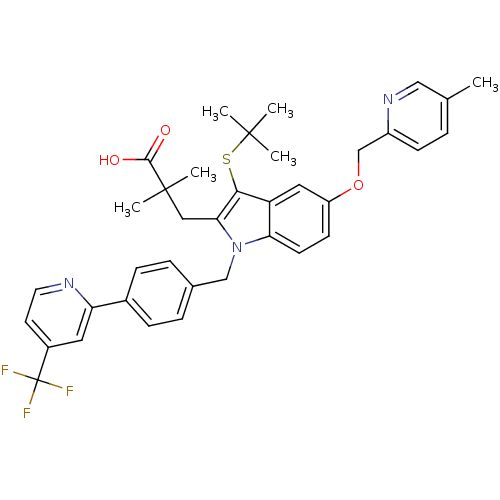
(CHEMBL1922655)Show SMILES Cc1ccc(COc2ccc3n(Cc4ccc(cc4)-c4cc(ccn4)C(F)(F)F)c(CC(C)(C)C(O)=O)c(SC(C)(C)C)c3c2)nc1 Show InChI InChI=1S/C37H38F3N3O3S/c1-23-7-12-27(42-20-23)22-46-28-13-14-31-29(18-28)33(47-35(2,3)4)32(19-36(5,6)34(44)45)43(31)21-24-8-10-25(11-9-24)30-17-26(15-16-41-30)37(38,39)40/h7-18,20H,19,21-22H2,1-6H3,(H,44,45) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Amira Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of FLAP in human peripheral leukocytes assessed as inhibition of calcium ionophore A23187-induced LTB4 production after 10 mins by ELISA |
J Med Chem 54: 8013-29 (2011)
Article DOI: 10.1021/jm2008369
BindingDB Entry DOI: 10.7270/Q269740W |
More data for this
Ligand-Target Pair | |
Arachidonate 5-lipoxygenase-activating protein
(Homo sapiens (Human)) | BDBM50359076

(CHEMBL1922656)Show SMILES CCOc1nc(cs1)-c1ccc(Cn2c(CC(C)(C)C(O)=O)c(SC(C)(C)C)c3cc(OCc4ccc(C)cn4)ccc23)cc1 Show InChI InChI=1S/C36H41N3O4S2/c1-8-42-34-38-29(22-44-34)25-12-10-24(11-13-25)20-39-30-16-15-27(43-21-26-14-9-23(2)19-37-26)17-28(30)32(45-35(3,4)5)31(39)18-36(6,7)33(40)41/h9-17,19,22H,8,18,20-21H2,1-7H3,(H,40,41) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Amira Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of FLAP in human peripheral leukocytes assessed as inhibition of calcium ionophore A23187-induced LTB4 production after 10 mins by ELISA |
J Med Chem 54: 8013-29 (2011)
Article DOI: 10.1021/jm2008369
BindingDB Entry DOI: 10.7270/Q269740W |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50029559
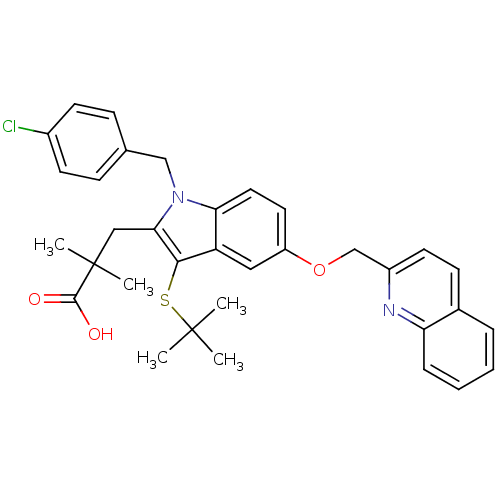
(2-[3-tert-Butylsulfanyl-1-(4-chloro-benzyl)-5-(qui...)Show SMILES CC(C)(C)Sc1c(CC(C)(C)C(O)=O)n(Cc2ccc(Cl)cc2)c2ccc(OCc3ccc4ccccc4n3)cc12 Show InChI InChI=1S/C34H35ClN2O3S/c1-33(2,3)41-31-27-18-26(40-21-25-15-12-23-8-6-7-9-28(23)36-25)16-17-29(27)37(20-22-10-13-24(35)14-11-22)30(31)19-34(4,5)32(38)39/h6-18H,19-21H2,1-5H3,(H,38,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Amira Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 |
J Med Chem 52: 5803-15 (2009)
Article DOI: 10.1021/jm900945d
BindingDB Entry DOI: 10.7270/Q2G44QB1 |
More data for this
Ligand-Target Pair | |
Arachidonate 5-lipoxygenase-activating protein
(Homo sapiens (Human)) | BDBM50359062
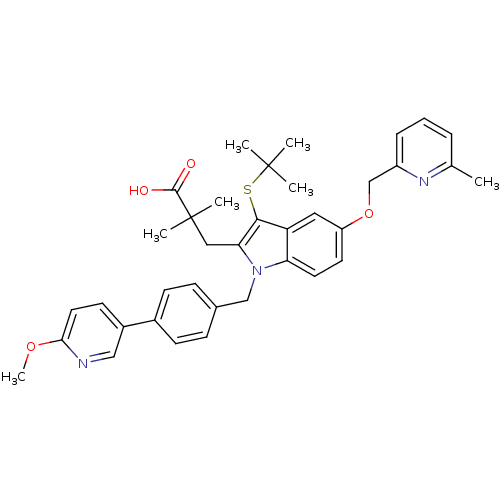
(CHEMBL1922642)Show SMILES COc1ccc(cn1)-c1ccc(Cn2c(CC(C)(C)C(O)=O)c(SC(C)(C)C)c3cc(OCc4cccc(C)n4)ccc23)cc1 Show InChI InChI=1S/C37H41N3O4S/c1-24-9-8-10-28(39-24)23-44-29-16-17-31-30(19-29)34(45-36(2,3)4)32(20-37(5,6)35(41)42)40(31)22-25-11-13-26(14-12-25)27-15-18-33(43-7)38-21-27/h8-19,21H,20,22-23H2,1-7H3,(H,41,42) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Amira Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of FLAP in human peripheral leukocytes assessed as inhibition of calcium ionophore A23187-induced LTB4 production after 10 mins by ELISA |
J Med Chem 54: 8013-29 (2011)
Article DOI: 10.1021/jm2008369
BindingDB Entry DOI: 10.7270/Q269740W |
More data for this
Ligand-Target Pair | |
Arachidonate 5-lipoxygenase-activating protein
(Homo sapiens (Human)) | BDBM50052018
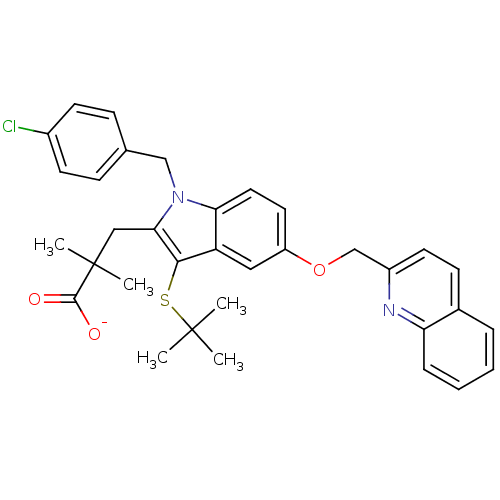
(3-[3-tert-Butylsulfanyl-1-(4-chloro-benzyl)-5-(qui...)Show SMILES CC(C)(C)Sc1c(CC(C)(C)C([O-])=O)n(Cc2ccc(Cl)cc2)c2ccc(OCc3ccc4ccccc4n3)cc12 Show InChI InChI=1S/C34H35ClN2O3S/c1-33(2,3)41-31-27-18-26(40-21-25-15-12-23-8-6-7-9-28(23)36-25)16-17-29(27)37(20-22-10-13-24(35)14-11-22)30(31)19-34(4,5)32(38)39/h6-18H,19-21H2,1-5H3,(H,38,39)/p-1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Amira Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of FLAP in human peripheral leukocytes assessed as inhibition of calcium ionophore A23187-induced LTB4 production after 10 mins by ELISA |
J Med Chem 54: 8013-29 (2011)
Article DOI: 10.1021/jm2008369
BindingDB Entry DOI: 10.7270/Q269740W |
More data for this
Ligand-Target Pair | |
Arachidonate 5-lipoxygenase-activating protein
(Homo sapiens (Human)) | BDBM50359061
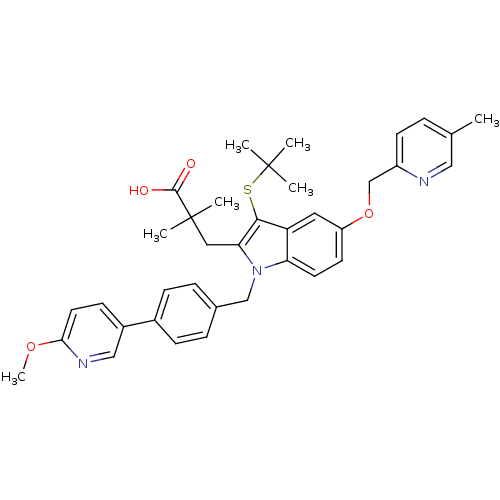
(CHEMBL1922532)Show SMILES COc1ccc(cn1)-c1ccc(Cn2c(CC(C)(C)C(O)=O)c(SC(C)(C)C)c3cc(OCc4ccc(C)cn4)ccc23)cc1 Show InChI InChI=1S/C37H41N3O4S/c1-24-8-14-28(38-20-24)23-44-29-15-16-31-30(18-29)34(45-36(2,3)4)32(19-37(5,6)35(41)42)40(31)22-25-9-11-26(12-10-25)27-13-17-33(43-7)39-21-27/h8-18,20-21H,19,22-23H2,1-7H3,(H,41,42) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Amira Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of FLAP in human peripheral leukocytes assessed as inhibition of calcium ionophore A23187-induced LTB4 production after 10 mins by ELISA |
J Med Chem 54: 8013-29 (2011)
Article DOI: 10.1021/jm2008369
BindingDB Entry DOI: 10.7270/Q269740W |
More data for this
Ligand-Target Pair | |
Arachidonate 5-lipoxygenase-activating protein
(Homo sapiens (Human)) | BDBM50359082

(CHEMBL1922662)Show SMILES Cc1ccc(COc2ccc3n(Cc4ccc(cc4)-c4ccc(C)cn4)c(CC(C)(C)C(O)=O)c(SC(C)(C)C)c3c2)nc1 Show InChI InChI=1S/C37H41N3O3S/c1-24-8-14-28(38-20-24)23-43-29-15-17-32-30(18-29)34(44-36(3,4)5)33(19-37(6,7)35(41)42)40(32)22-26-10-12-27(13-11-26)31-16-9-25(2)21-39-31/h8-18,20-21H,19,22-23H2,1-7H3,(H,41,42) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Amira Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of FLAP in human peripheral leukocytes assessed as inhibition of calcium ionophore A23187-induced LTB4 production after 10 mins by ELISA |
J Med Chem 54: 8013-29 (2011)
Article DOI: 10.1021/jm2008369
BindingDB Entry DOI: 10.7270/Q269740W |
More data for this
Ligand-Target Pair | |
Arachidonate 5-lipoxygenase-activating protein
(Homo sapiens (Human)) | BDBM50359078

(CHEMBL1922658)Show SMILES Cc1ccc(COc2ccc3n(Cc4ccc(cc4)-c4ccc(cn4)C(F)(F)F)c(CC(C)(C)C(O)=O)c(SC(C)(C)C)c3c2)nc1 Show InChI InChI=1S/C37H38F3N3O3S/c1-23-7-13-27(41-19-23)22-46-28-14-16-31-29(17-28)33(47-35(2,3)4)32(18-36(5,6)34(44)45)43(31)21-24-8-10-25(11-9-24)30-15-12-26(20-42-30)37(38,39)40/h7-17,19-20H,18,21-22H2,1-6H3,(H,44,45) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Amira Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of FLAP in human peripheral leukocytes assessed as inhibition of calcium ionophore A23187-induced LTB4 production after 10 mins by ELISA |
J Med Chem 54: 8013-29 (2011)
Article DOI: 10.1021/jm2008369
BindingDB Entry DOI: 10.7270/Q269740W |
More data for this
Ligand-Target Pair | |
Arachidonate 5-lipoxygenase-activating protein
(Homo sapiens (Human)) | BDBM50359079
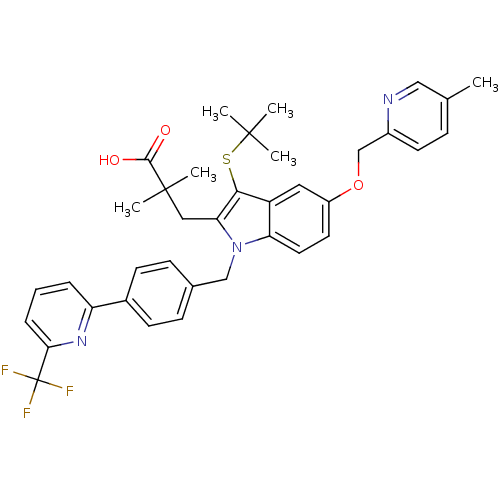
(CHEMBL1922659)Show SMILES Cc1ccc(COc2ccc3n(Cc4ccc(cc4)-c4cccc(n4)C(F)(F)F)c(CC(C)(C)C(O)=O)c(SC(C)(C)C)c3c2)nc1 Show InChI InChI=1S/C37H38F3N3O3S/c1-23-10-15-26(41-20-23)22-46-27-16-17-30-28(18-27)33(47-35(2,3)4)31(19-36(5,6)34(44)45)43(30)21-24-11-13-25(14-12-24)29-8-7-9-32(42-29)37(38,39)40/h7-18,20H,19,21-22H2,1-6H3,(H,44,45) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Amira Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of FLAP in human peripheral leukocytes assessed as inhibition of calcium ionophore A23187-induced LTB4 production after 10 mins by ELISA |
J Med Chem 54: 8013-29 (2011)
Article DOI: 10.1021/jm2008369
BindingDB Entry DOI: 10.7270/Q269740W |
More data for this
Ligand-Target Pair | |
Arachidonate 5-lipoxygenase-activating protein
(Homo sapiens (Human)) | BDBM50297385
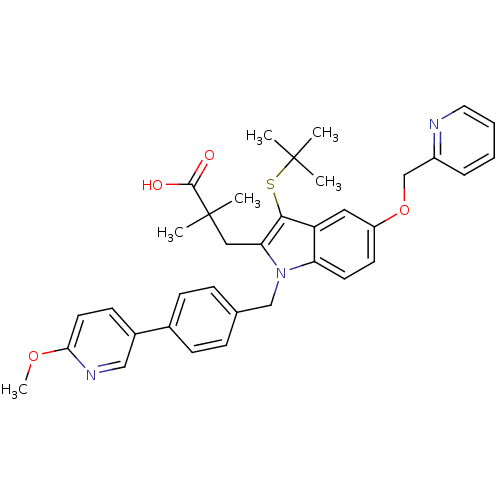
(3-[3-tert-Butylsulfanyl-1-[4-(6-methoxy-pyridin-3-...)Show SMILES COc1ccc(cn1)-c1ccc(Cn2c(CC(C)(C)C(O)=O)c(SC(C)(C)C)c3cc(OCc4ccccn4)ccc23)cc1 Show InChI InChI=1S/C36H39N3O4S/c1-35(2,3)44-33-29-19-28(43-23-27-9-7-8-18-37-27)15-16-30(29)39(31(33)20-36(4,5)34(40)41)22-24-10-12-25(13-11-24)26-14-17-32(42-6)38-21-26/h7-19,21H,20,22-23H2,1-6H3,(H,40,41) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Amira Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of FLAP in human peripheral leukocytes assessed as inhibition of calcium ionophore A23187-induced LTB4 production after 10 mins by ELISA |
J Med Chem 54: 8013-29 (2011)
Article DOI: 10.1021/jm2008369
BindingDB Entry DOI: 10.7270/Q269740W |
More data for this
Ligand-Target Pair | |
Arachidonate 5-lipoxygenase-activating protein
(Homo sapiens (Human)) | BDBM50359085
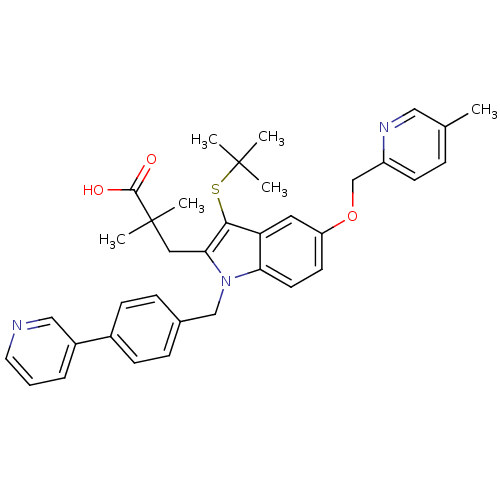
(CHEMBL1922665)Show SMILES Cc1ccc(COc2ccc3n(Cc4ccc(cc4)-c4cccnc4)c(CC(C)(C)C(O)=O)c(SC(C)(C)C)c3c2)nc1 Show InChI InChI=1S/C36H39N3O3S/c1-24-9-14-28(38-20-24)23-42-29-15-16-31-30(18-29)33(43-35(2,3)4)32(19-36(5,6)34(40)41)39(31)22-25-10-12-26(13-11-25)27-8-7-17-37-21-27/h7-18,20-21H,19,22-23H2,1-6H3,(H,40,41) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Amira Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]-3-[5-(pyrid-2-ylmethoxy)-3-tert-butylthio-1-benzylindol-2-yl]-2,2-dimethylpropionic acid from FLAP in human polymorphonuclear ce... |
J Med Chem 54: 8013-29 (2011)
Article DOI: 10.1021/jm2008369
BindingDB Entry DOI: 10.7270/Q269740W |
More data for this
Ligand-Target Pair | |
Arachidonate 5-lipoxygenase-activating protein
(Homo sapiens (Human)) | BDBM50359063
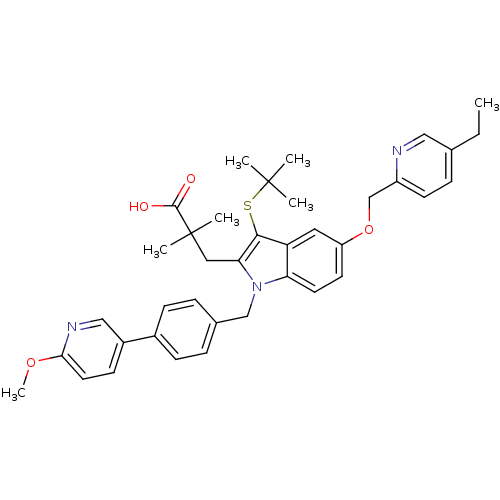
(CHEMBL1922643)Show SMILES CCc1ccc(COc2ccc3n(Cc4ccc(cc4)-c4ccc(OC)nc4)c(CC(C)(C)C(O)=O)c(SC(C)(C)C)c3c2)nc1 Show InChI InChI=1S/C38H43N3O4S/c1-8-25-11-15-29(39-21-25)24-45-30-16-17-32-31(19-30)35(46-37(2,3)4)33(20-38(5,6)36(42)43)41(32)23-26-9-12-27(13-10-26)28-14-18-34(44-7)40-22-28/h9-19,21-22H,8,20,23-24H2,1-7H3,(H,42,43) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Amira Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of FLAP in human peripheral leukocytes assessed as inhibition of calcium ionophore A23187-induced LTB4 production after 10 mins by ELISA |
J Med Chem 54: 8013-29 (2011)
Article DOI: 10.1021/jm2008369
BindingDB Entry DOI: 10.7270/Q269740W |
More data for this
Ligand-Target Pair | |
Arachidonate 5-lipoxygenase-activating protein
(Homo sapiens (Human)) | BDBM50359065
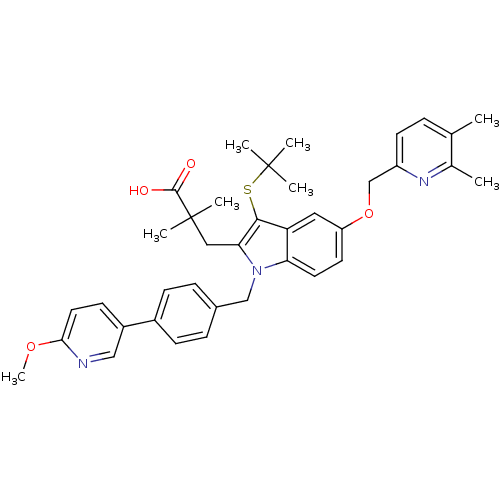
(CHEMBL1922645)Show SMILES COc1ccc(cn1)-c1ccc(Cn2c(CC(C)(C)C(O)=O)c(SC(C)(C)C)c3cc(OCc4ccc(C)c(C)n4)ccc23)cc1 Show InChI InChI=1S/C38H43N3O4S/c1-24-9-15-29(40-25(24)2)23-45-30-16-17-32-31(19-30)35(46-37(3,4)5)33(20-38(6,7)36(42)43)41(32)22-26-10-12-27(13-11-26)28-14-18-34(44-8)39-21-28/h9-19,21H,20,22-23H2,1-8H3,(H,42,43) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Amira Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of FLAP in human peripheral leukocytes assessed as inhibition of calcium ionophore A23187-induced LTB4 production after 10 mins by ELISA |
J Med Chem 54: 8013-29 (2011)
Article DOI: 10.1021/jm2008369
BindingDB Entry DOI: 10.7270/Q269740W |
More data for this
Ligand-Target Pair | |
Arachidonate 5-lipoxygenase-activating protein
(Homo sapiens (Human)) | BDBM50359088

(CHEMBL1229205)Show SMILES COc1cnc(nc1)-c1ccc(Cn2c(CC(C)(C)C(O)=O)c(SC(C)(C)C)c3cc(OCc4ccc(C)cn4)ccc23)cc1 Show InChI InChI=1S/C36H40N4O4S/c1-23-8-13-26(37-18-23)22-44-27-14-15-30-29(16-27)32(45-35(2,3)4)31(17-36(5,6)34(41)42)40(30)21-24-9-11-25(12-10-24)33-38-19-28(43-7)20-39-33/h8-16,18-20H,17,21-22H2,1-7H3,(H,41,42) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Amira Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of FLAP in human peripheral leukocytes assessed as inhibition of calcium ionophore A23187-induced LTB4 production after 10 mins by ELISA |
J Med Chem 54: 8013-29 (2011)
Article DOI: 10.1021/jm2008369
BindingDB Entry DOI: 10.7270/Q269740W |
More data for this
Ligand-Target Pair | |
Arachidonate 5-lipoxygenase-activating protein
(Homo sapiens (Human)) | BDBM50359081

(CHEMBL1922661)Show SMILES CCOc1cccc(n1)-c1ccc(Cn2c(CC(C)(C)C(O)=O)c(SC(C)(C)C)c3cc(OCc4ccc(C)cn4)ccc23)cc1 Show InChI InChI=1S/C38H43N3O4S/c1-8-44-34-11-9-10-31(40-34)27-15-13-26(14-16-27)23-41-32-19-18-29(45-24-28-17-12-25(2)22-39-28)20-30(32)35(46-37(3,4)5)33(41)21-38(6,7)36(42)43/h9-20,22H,8,21,23-24H2,1-7H3,(H,42,43) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Amira Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of FLAP in human peripheral leukocytes assessed as inhibition of calcium ionophore A23187-induced LTB4 production after 10 mins by ELISA |
J Med Chem 54: 8013-29 (2011)
Article DOI: 10.1021/jm2008369
BindingDB Entry DOI: 10.7270/Q269740W |
More data for this
Ligand-Target Pair | |
Arachidonate 5-lipoxygenase-activating protein
(Homo sapiens (Human)) | BDBM50359086
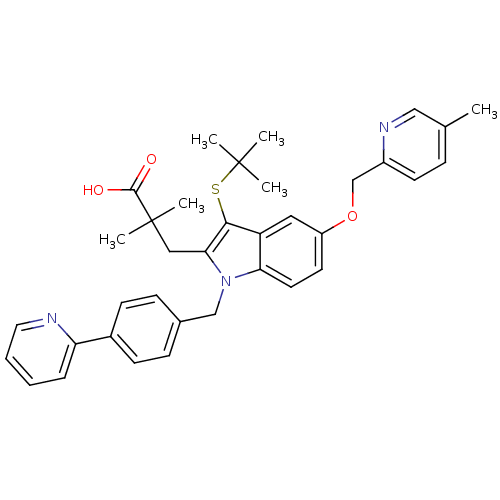
(CHEMBL1922666)Show SMILES Cc1ccc(COc2ccc3n(Cc4ccc(cc4)-c4ccccn4)c(CC(C)(C)C(O)=O)c(SC(C)(C)C)c3c2)nc1 Show InChI InChI=1S/C36H39N3O3S/c1-24-10-15-27(38-21-24)23-42-28-16-17-31-29(19-28)33(43-35(2,3)4)32(20-36(5,6)34(40)41)39(31)22-25-11-13-26(14-12-25)30-9-7-8-18-37-30/h7-19,21H,20,22-23H2,1-6H3,(H,40,41) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Amira Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]-3-[5-(pyrid-2-ylmethoxy)-3-tert-butylthio-1-benzylindol-2-yl]-2,2-dimethylpropionic acid from FLAP in human polymorphonuclear ce... |
J Med Chem 54: 8013-29 (2011)
Article DOI: 10.1021/jm2008369
BindingDB Entry DOI: 10.7270/Q269740W |
More data for this
Ligand-Target Pair | |
Arachidonate 5-lipoxygenase-activating protein
(Homo sapiens (Human)) | BDBM50359084
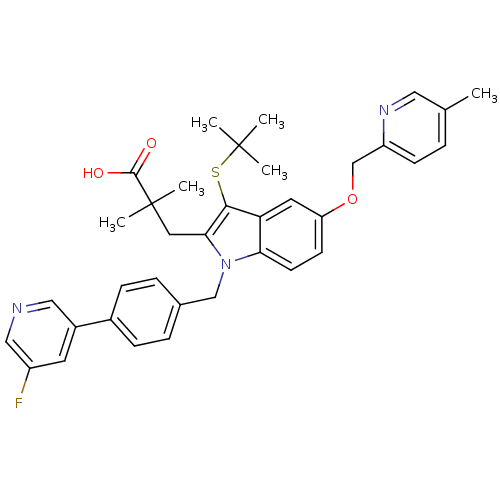
(CHEMBL1922664)Show SMILES Cc1ccc(COc2ccc3n(Cc4ccc(cc4)-c4cncc(F)c4)c(CC(C)(C)C(O)=O)c(SC(C)(C)C)c3c2)nc1 Show InChI InChI=1S/C36H38FN3O3S/c1-23-7-12-28(39-18-23)22-43-29-13-14-31-30(16-29)33(44-35(2,3)4)32(17-36(5,6)34(41)42)40(31)21-24-8-10-25(11-9-24)26-15-27(37)20-38-19-26/h7-16,18-20H,17,21-22H2,1-6H3,(H,41,42) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Amira Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]-3-[5-(pyrid-2-ylmethoxy)-3-tert-butylthio-1-benzylindol-2-yl]-2,2-dimethylpropionic acid from FLAP in human polymorphonuclear ce... |
J Med Chem 54: 8013-29 (2011)
Article DOI: 10.1021/jm2008369
BindingDB Entry DOI: 10.7270/Q269740W |
More data for this
Ligand-Target Pair | |
3-hydroxy-3-methylglutaryl-coenzyme A reductase
(Homo sapiens (Human)) | BDBM50215704

(CHEMBL398551 | sodium (3R,5R)-7-(5-(((1,5-dimethyl...)Show SMILES CC(C)n1c(CC[C@@H](O)C[C@@H](O)CC([O-])=O)c(c(c1C(=O)NCc1cc(C)n(C)n1)-c1ccccc1)-c1ccc(F)cc1 Show InChI InChI=1S/C33H39FN4O5/c1-20(2)38-28(15-14-26(39)17-27(40)18-29(41)42)30(23-10-12-24(34)13-11-23)31(22-8-6-5-7-9-22)32(38)33(43)35-19-25-16-21(3)37(4)36-25/h5-13,16,20,26-27,39-40H,14-15,17-19H2,1-4H3,(H,35,43)(H,41,42)/p-1/t26-,27-/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of HMG-CoA reductase |
Bioorg Med Chem Lett 17: 4538-44 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.096
BindingDB Entry DOI: 10.7270/Q2SJ1K9Q |
More data for this
Ligand-Target Pair | |
3-hydroxy-3-methylglutaryl-coenzyme A reductase
(Homo sapiens (Human)) | BDBM50215699

(CHEMBL400747 | sodium (3R,5R)-7-(3,4-bis(4-fluorop...)Show SMILES CC(C)n1c(CC[C@@H](O)C[C@@H](O)CC([O-])=O)c(c(c1C(=O)Nc1cccc(O)c1)-c1ccc(F)cc1)-c1ccc(F)cc1 Show InChI InChI=1S/C33H34F2N2O6/c1-19(2)37-28(15-14-26(39)17-27(40)18-29(41)42)30(20-6-10-22(34)11-7-20)31(21-8-12-23(35)13-9-21)32(37)33(43)36-24-4-3-5-25(38)16-24/h3-13,16,19,26-27,38-40H,14-15,17-18H2,1-2H3,(H,36,43)(H,41,42)/p-1/t26-,27-/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of HMG-CoA reductase |
Bioorg Med Chem Lett 17: 4538-44 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.096
BindingDB Entry DOI: 10.7270/Q2SJ1K9Q |
More data for this
Ligand-Target Pair | |
Arachidonate 5-lipoxygenase-activating protein
(Homo sapiens (Human)) | BDBM50359096

(CHEMBL1922525)Show SMILES COc1ccc(cn1)-c1ccc(Cn2c(CC(C)(C)C(O)=O)c(SC(C)(C)C)c3cc(OCc4ccc5cc(F)ccc5n4)ccc23)cc1 Show InChI InChI=1S/C40H40FN3O4S/c1-39(2,3)49-37-32-20-31(48-24-30-14-11-27-19-29(41)13-16-33(27)43-30)15-17-34(32)44(35(37)21-40(4,5)38(45)46)23-25-7-9-26(10-8-25)28-12-18-36(47-6)42-22-28/h7-20,22H,21,23-24H2,1-6H3,(H,45,46) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Amira Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of FLAP in human peripheral leukocytes assessed as inhibition of calcium ionophore A23187-induced LTB4 production after 10 mins by ELISA |
J Med Chem 54: 8013-29 (2011)
Article DOI: 10.1021/jm2008369
BindingDB Entry DOI: 10.7270/Q269740W |
More data for this
Ligand-Target Pair | |
Arachidonate 5-lipoxygenase-activating protein
(Homo sapiens (Human)) | BDBM50359080
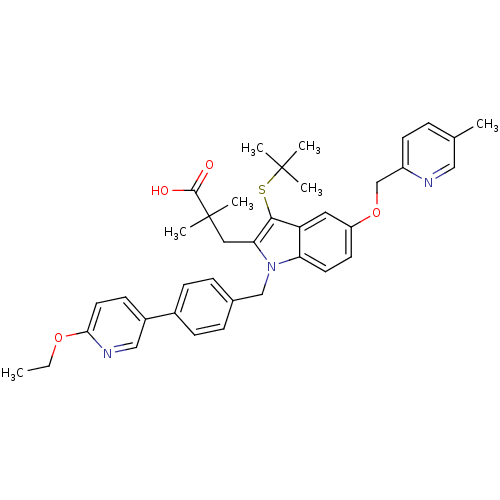
(CHEMBL1922660)Show SMILES CCOc1ccc(cn1)-c1ccc(Cn2c(CC(C)(C)C(O)=O)c(SC(C)(C)C)c3cc(OCc4ccc(C)cn4)ccc23)cc1 Show InChI InChI=1S/C38H43N3O4S/c1-8-44-34-18-14-28(22-40-34)27-12-10-26(11-13-27)23-41-32-17-16-30(45-24-29-15-9-25(2)21-39-29)19-31(32)35(46-37(3,4)5)33(41)20-38(6,7)36(42)43/h9-19,21-22H,8,20,23-24H2,1-7H3,(H,42,43) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Amira Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of FLAP in human peripheral leukocytes assessed as inhibition of calcium ionophore A23187-induced LTB4 production after 10 mins by ELISA |
J Med Chem 54: 8013-29 (2011)
Article DOI: 10.1021/jm2008369
BindingDB Entry DOI: 10.7270/Q269740W |
More data for this
Ligand-Target Pair | |
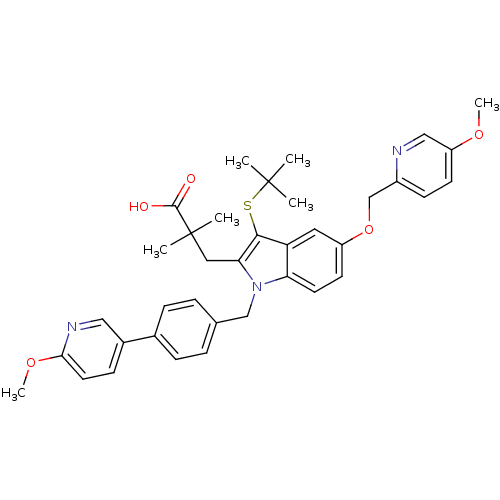
Arachidonate 5-lipoxygenase-activating protein
(Homo sapiens (Human)) | BDBM50359067
(CHEMBL1922647)Show SMILES COc1ccc(COc2ccc3n(Cc4ccc(cc4)-c4ccc(OC)nc4)c(CC(C)(C)C(O)=O)c(SC(C)(C)C)c3c2)nc1 Show InChI InChI=1S/C37H41N3O5S/c1-36(2,3)46-34-30-18-28(45-23-27-13-14-29(43-6)21-38-27)15-16-31(30)40(32(34)19-37(4,5)35(41)42)22-24-8-10-25(11-9-24)26-12-17-33(44-7)39-20-26/h8-18,20-21H,19,22-23H2,1-7H3,(H,41,42) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Amira Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of FLAP in human peripheral leukocytes assessed as inhibition of calcium ionophore A23187-induced LTB4 production after 10 mins by ELISA |
J Med Chem 54: 8013-29 (2011)
Article DOI: 10.1021/jm2008369
BindingDB Entry DOI: 10.7270/Q269740W |
More data for this
Ligand-Target Pair | |
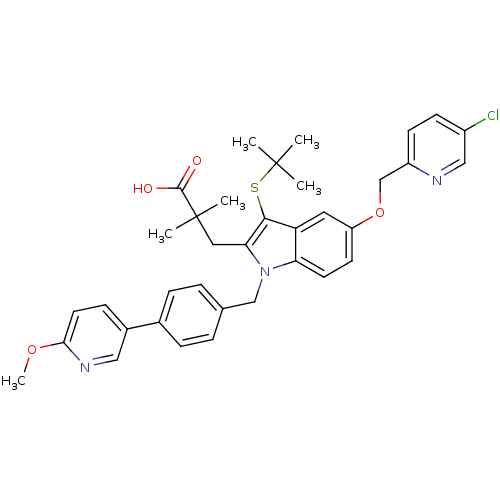
Arachidonate 5-lipoxygenase-activating protein
(Homo sapiens (Human)) | BDBM50359066
(CHEMBL1922646)Show SMILES COc1ccc(cn1)-c1ccc(Cn2c(CC(C)(C)C(O)=O)c(SC(C)(C)C)c3cc(OCc4ccc(Cl)cn4)ccc23)cc1 Show InChI InChI=1S/C36H38ClN3O4S/c1-35(2,3)45-33-29-17-28(44-22-27-13-12-26(37)20-38-27)14-15-30(29)40(31(33)18-36(4,5)34(41)42)21-23-7-9-24(10-8-23)25-11-16-32(43-6)39-19-25/h7-17,19-20H,18,21-22H2,1-6H3,(H,41,42) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Amira Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of FLAP in human peripheral leukocytes assessed as inhibition of calcium ionophore A23187-induced LTB4 production after 10 mins by ELISA |
J Med Chem 54: 8013-29 (2011)
Article DOI: 10.1021/jm2008369
BindingDB Entry DOI: 10.7270/Q269740W |
More data for this
Ligand-Target Pair | |
3-hydroxy-3-methylglutaryl-coenzyme A reductase
(Homo sapiens (Human)) | BDBM50215707
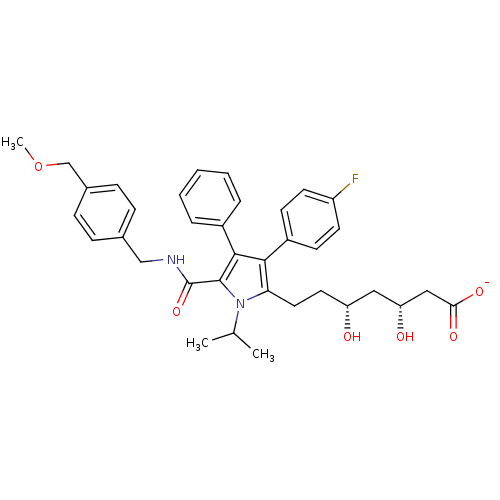
(CHEMBL250749 | sodium (3R,5R)-7-(5-((4-(methoxymet...)Show SMILES COCc1ccc(CNC(=O)c2c(c(c(CC[C@@H](O)C[C@@H](O)CC([O-])=O)n2C(C)C)-c2ccc(F)cc2)-c2ccccc2)cc1 Show InChI InChI=1S/C36H41FN2O6/c1-23(2)39-31(18-17-29(40)19-30(41)20-32(42)43)33(27-13-15-28(37)16-14-27)34(26-7-5-4-6-8-26)35(39)36(44)38-21-24-9-11-25(12-10-24)22-45-3/h4-16,23,29-30,40-41H,17-22H2,1-3H3,(H,38,44)(H,42,43)/p-1/t29-,30-/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of HMG-CoA reductase |
Bioorg Med Chem Lett 17: 4538-44 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.096
BindingDB Entry DOI: 10.7270/Q2SJ1K9Q |
More data for this
Ligand-Target Pair | |
Arachidonate 5-lipoxygenase-activating protein
(Homo sapiens (Human)) | BDBM50359095

(CHEMBL1922524)Show SMILES COc1ccc(cn1)-c1ccc(Cn2c(CC(C)(C)C(O)=O)c(SC(C)(C)C)c3cc(OCc4cnc5ccccc5n4)ccc23)cc1 Show InChI InChI=1S/C39H40N4O4S/c1-38(2,3)48-36-30-19-29(47-24-28-22-40-31-9-7-8-10-32(31)42-28)16-17-33(30)43(34(36)20-39(4,5)37(44)45)23-25-11-13-26(14-12-25)27-15-18-35(46-6)41-21-27/h7-19,21-22H,20,23-24H2,1-6H3,(H,44,45) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Amira Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of FLAP in human peripheral leukocytes assessed as inhibition of calcium ionophore A23187-induced LTB4 production after 10 mins by ELISA |
J Med Chem 54: 8013-29 (2011)
Article DOI: 10.1021/jm2008369
BindingDB Entry DOI: 10.7270/Q269740W |
More data for this
Ligand-Target Pair | |
3-hydroxy-3-methylglutaryl-coenzyme A reductase
(Homo sapiens (Human)) | BDBM50215684
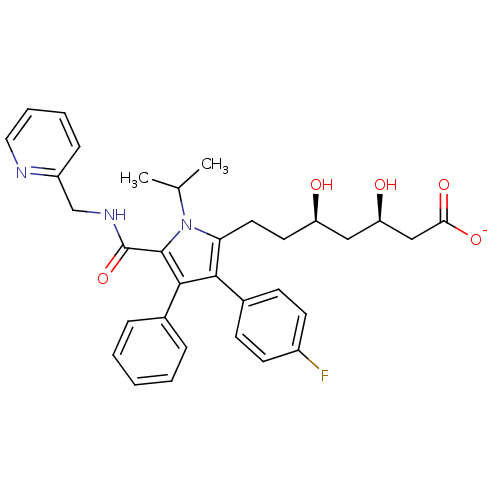
(CHEMBL398240 | sodium (3R,5R)-7-(3-(4-fluorophenyl...)Show SMILES CC(C)n1c(CC[C@@H](O)C[C@@H](O)CC([O-])=O)c(c(c1C(=O)NCc1ccccn1)-c1ccccc1)-c1ccc(F)cc1 Show InChI InChI=1S/C33H36FN3O5/c1-21(2)37-28(16-15-26(38)18-27(39)19-29(40)41)30(23-11-13-24(34)14-12-23)31(22-8-4-3-5-9-22)32(37)33(42)36-20-25-10-6-7-17-35-25/h3-14,17,21,26-27,38-39H,15-16,18-20H2,1-2H3,(H,36,42)(H,40,41)/p-1/t26-,27-/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of HMG-CoA reductase |
Bioorg Med Chem Lett 17: 4538-44 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.096
BindingDB Entry DOI: 10.7270/Q2SJ1K9Q |
More data for this
Ligand-Target Pair | |
3-hydroxy-3-methylglutaryl-coenzyme A reductase
(Homo sapiens (Human)) | BDBM50215690

(CHEMBL399313 | sodium (3R,5R)-7-(5-(benzylcarbamoy...)Show SMILES CC(C)n1c(CC[C@@H](O)C[C@@H](O)CC([O-])=O)c(c(c1C(=O)NCc1ccccc1)-c1ccccc1)-c1ccc(F)cc1 Show InChI InChI=1S/C34H37FN2O5/c1-22(2)37-29(18-17-27(38)19-28(39)20-30(40)41)31(25-13-15-26(35)16-14-25)32(24-11-7-4-8-12-24)33(37)34(42)36-21-23-9-5-3-6-10-23/h3-16,22,27-28,38-39H,17-21H2,1-2H3,(H,36,42)(H,40,41)/p-1/t27-,28-/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of HMG-CoA reductase |
Bioorg Med Chem Lett 17: 4538-44 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.096
BindingDB Entry DOI: 10.7270/Q2SJ1K9Q |
More data for this
Ligand-Target Pair | |
3-hydroxy-3-methylglutaryl-coenzyme A reductase
(Homo sapiens (Human)) | BDBM50215708
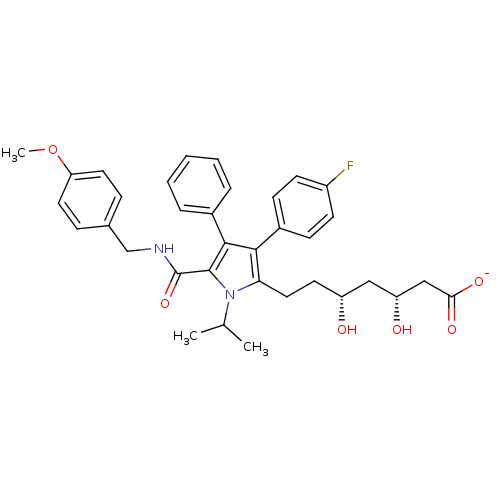
(CHEMBL249724 | sodium (3R,5R)-7-(5-((4-methoxybenz...)Show SMILES COc1ccc(CNC(=O)c2c(c(c(CC[C@@H](O)C[C@@H](O)CC([O-])=O)n2C(C)C)-c2ccc(F)cc2)-c2ccccc2)cc1 Show InChI InChI=1S/C35H39FN2O6/c1-22(2)38-30(18-15-27(39)19-28(40)20-31(41)42)32(25-11-13-26(36)14-12-25)33(24-7-5-4-6-8-24)34(38)35(43)37-21-23-9-16-29(44-3)17-10-23/h4-14,16-17,22,27-28,39-40H,15,18-21H2,1-3H3,(H,37,43)(H,41,42)/p-1/t27-,28-/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of HMG-CoA reductase |
Bioorg Med Chem Lett 17: 4538-44 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.096
BindingDB Entry DOI: 10.7270/Q2SJ1K9Q |
More data for this
Ligand-Target Pair | |
Arachidonate 5-lipoxygenase-activating protein
(Homo sapiens (Human)) | BDBM50359094
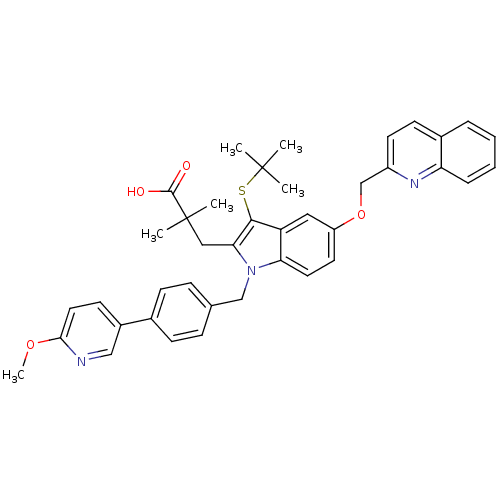
(CHEMBL1922523)Show SMILES COc1ccc(cn1)-c1ccc(Cn2c(CC(C)(C)C(O)=O)c(SC(C)(C)C)c3cc(OCc4ccc5ccccc5n4)ccc23)cc1 Show InChI InChI=1S/C40H41N3O4S/c1-39(2,3)48-37-32-21-31(47-25-30-17-15-28-9-7-8-10-33(28)42-30)18-19-34(32)43(35(37)22-40(4,5)38(44)45)24-26-11-13-27(14-12-26)29-16-20-36(46-6)41-23-29/h7-21,23H,22,24-25H2,1-6H3,(H,44,45) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Amira Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of FLAP in human peripheral leukocytes assessed as inhibition of calcium ionophore A23187-induced LTB4 production after 10 mins by ELISA |
J Med Chem 54: 8013-29 (2011)
Article DOI: 10.1021/jm2008369
BindingDB Entry DOI: 10.7270/Q269740W |
More data for this
Ligand-Target Pair | |
Arachidonate 5-lipoxygenase-activating protein
(Homo sapiens (Human)) | BDBM50359074

(CHEMBL1922654)Show SMILES COc1cccnc1-c1ccc(Cn2c(CC(C)(C)C(O)=O)c(SC(C)(C)C)c3cc(OCc4ccc(C)cn4)ccc23)cc1 Show InChI InChI=1S/C37H41N3O4S/c1-24-10-15-27(39-21-24)23-44-28-16-17-30-29(19-28)34(45-36(2,3)4)31(20-37(5,6)35(41)42)40(30)22-25-11-13-26(14-12-25)33-32(43-7)9-8-18-38-33/h8-19,21H,20,22-23H2,1-7H3,(H,41,42) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Amira Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of FLAP in human peripheral leukocytes assessed as inhibition of calcium ionophore A23187-induced LTB4 production after 10 mins by ELISA |
J Med Chem 54: 8013-29 (2011)
Article DOI: 10.1021/jm2008369
BindingDB Entry DOI: 10.7270/Q269740W |
More data for this
Ligand-Target Pair | |
Prostaglandin D2 receptor 2
(Homo sapiens (Human)) | BDBM50296979
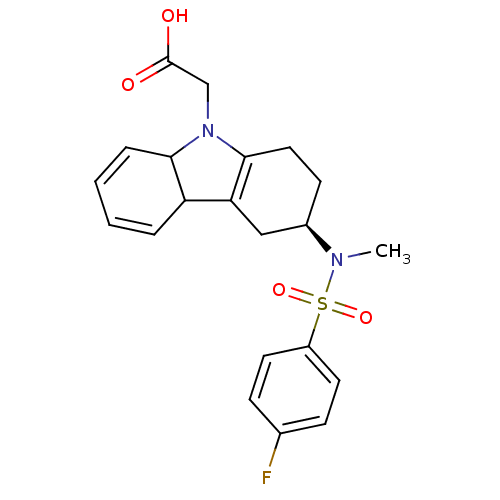
(2-((3R)-3-(4-fluoro-N-methylphenylsulfonamido)-3,4...)Show SMILES CN([C@@H]1CCC2=C(C1)C1C=CC=CC1N2CC(O)=O)S(=O)(=O)c1ccc(F)cc1 |r,c:5,10,12| Show InChI InChI=1S/C21H23FN2O4S/c1-23(29(27,28)16-9-6-14(22)7-10-16)15-8-11-20-18(12-15)17-4-2-3-5-19(17)24(20)13-21(25)26/h2-7,9-10,15,17,19H,8,11-13H2,1H3,(H,25,26)/t15-,17?,19?/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Amira Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGD2 from human prostaglandin D2 receptor |
Bioorg Med Chem Lett 19: 4647-51 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.085
BindingDB Entry DOI: 10.7270/Q25T3KHB |
More data for this
Ligand-Target Pair | |
Prostaglandin D2 receptor 2
(Homo sapiens (Human)) | BDBM50335958
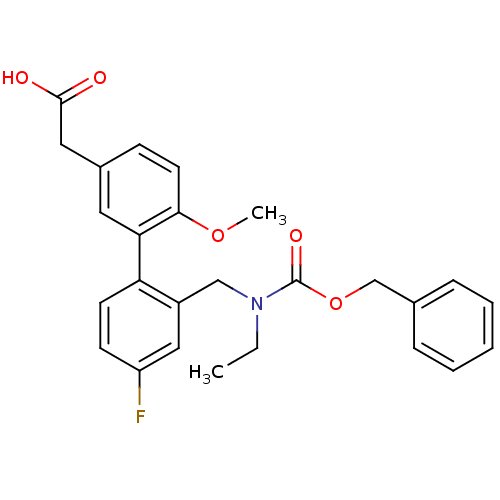
(2-(2'-(((benzyloxycarbonyl)(ethyl)amino)methyl)-4'...)Show SMILES CCN(Cc1cc(F)ccc1-c1cc(CC(O)=O)ccc1OC)C(=O)OCc1ccccc1 Show InChI InChI=1S/C26H26FNO5/c1-3-28(26(31)33-17-18-7-5-4-6-8-18)16-20-15-21(27)10-11-22(20)23-13-19(14-25(29)30)9-12-24(23)32-2/h4-13,15H,3,14,16-17H2,1-2H3,(H,29,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
USA.
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGD2 from human CRTh2 receptor expressed in 293 cells by liquid scintillation counting |
Bioorg Med Chem Lett 21: 1036-40 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.016
BindingDB Entry DOI: 10.7270/Q27P8ZNT |
More data for this
Ligand-Target Pair | |
Arachidonate 5-lipoxygenase-activating protein
(Homo sapiens (Human)) | BDBM50052018

(3-[3-tert-Butylsulfanyl-1-(4-chloro-benzyl)-5-(qui...)Show SMILES CC(C)(C)Sc1c(CC(C)(C)C([O-])=O)n(Cc2ccc(Cl)cc2)c2ccc(OCc3ccc4ccccc4n3)cc12 Show InChI InChI=1S/C34H35ClN2O3S/c1-33(2,3)41-31-27-18-26(40-21-25-15-12-23-8-6-7-9-28(23)36-25)16-17-29(27)37(20-22-10-13-24(35)14-11-22)30(31)19-34(4,5)32(38)39/h6-18H,19-21H2,1-5H3,(H,38,39)/p-1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Amira Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of FLAP in human peripheral leukocytes assessed as inhibition of calcium ionophore A23187-induced LTB4 production after 1 hr by ELISA in a... |
J Med Chem 54: 8013-29 (2011)
Article DOI: 10.1021/jm2008369
BindingDB Entry DOI: 10.7270/Q269740W |
More data for this
Ligand-Target Pair | |
Arachidonate 5-lipoxygenase-activating protein
(Homo sapiens (Human)) | BDBM50359080

(CHEMBL1922660)Show SMILES CCOc1ccc(cn1)-c1ccc(Cn2c(CC(C)(C)C(O)=O)c(SC(C)(C)C)c3cc(OCc4ccc(C)cn4)ccc23)cc1 Show InChI InChI=1S/C38H43N3O4S/c1-8-44-34-18-14-28(22-40-34)27-12-10-26(11-13-27)23-41-32-17-16-30(45-24-29-15-9-25(2)21-39-29)19-31(32)35(46-37(3,4)5)33(41)20-38(6,7)36(42)43/h9-19,21-22H,8,20,23-24H2,1-7H3,(H,42,43) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Amira Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of FLAP in human peripheral leukocytes assessed as inhibition of calcium ionophore A23187-induced LTB4 production after 5 mins by ELISA in... |
J Med Chem 54: 8013-29 (2011)
Article DOI: 10.1021/jm2008369
BindingDB Entry DOI: 10.7270/Q269740W |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50080250
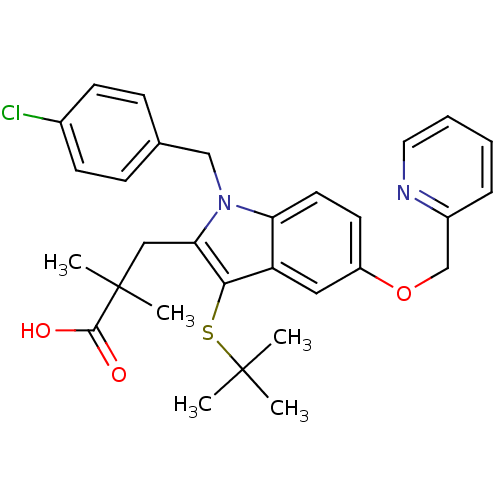
(3-(3-(tert-butylthio)-1-(4-chlorobenzyl)-5-(pyridi...)Show SMILES CC(C)(C)Sc1c(CC(C)(C)C(O)=O)n(Cc2ccc(Cl)cc2)c2ccc(OCc3ccccn3)cc12 Show InChI InChI=1S/C30H33ClN2O3S/c1-29(2,3)37-27-24-16-23(36-19-22-8-6-7-15-32-22)13-14-25(24)33(18-20-9-11-21(31)12-10-20)26(27)17-30(4,5)28(34)35/h6-16H,17-19H2,1-5H3,(H,34,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Amira Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 |
J Med Chem 52: 5803-15 (2009)
Article DOI: 10.1021/jm900945d
BindingDB Entry DOI: 10.7270/Q2G44QB1 |
More data for this
Ligand-Target Pair | |
Arachidonate 5-lipoxygenase-activating protein
(Homo sapiens (Human)) | BDBM50359080

(CHEMBL1922660)Show SMILES CCOc1ccc(cn1)-c1ccc(Cn2c(CC(C)(C)C(O)=O)c(SC(C)(C)C)c3cc(OCc4ccc(C)cn4)ccc23)cc1 Show InChI InChI=1S/C38H43N3O4S/c1-8-44-34-18-14-28(22-40-34)27-12-10-26(11-13-27)23-41-32-17-16-30(45-24-29-15-9-25(2)21-39-29)19-31(32)35(46-37(3,4)5)33(41)20-38(6,7)36(42)43/h9-19,21-22H,8,20,23-24H2,1-7H3,(H,42,43) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Amira Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of FLAP in human peripheral leukocytes assessed as inhibition of calcium ionophore A23187-induced LTB4 production after 1 hr by ELISA in a... |
J Med Chem 54: 8013-29 (2011)
Article DOI: 10.1021/jm2008369
BindingDB Entry DOI: 10.7270/Q269740W |
More data for this
Ligand-Target Pair | |
Arachidonate 5-lipoxygenase-activating protein
(Homo sapiens (Human)) | BDBM50052018

(3-[3-tert-Butylsulfanyl-1-(4-chloro-benzyl)-5-(qui...)Show SMILES CC(C)(C)Sc1c(CC(C)(C)C([O-])=O)n(Cc2ccc(Cl)cc2)c2ccc(OCc3ccc4ccccc4n3)cc12 Show InChI InChI=1S/C34H35ClN2O3S/c1-33(2,3)41-31-27-18-26(40-21-25-15-12-23-8-6-7-9-28(23)36-25)16-17-29(27)37(20-22-10-13-24(35)14-11-22)30(31)19-34(4,5)32(38)39/h6-18H,19-21H2,1-5H3,(H,38,39)/p-1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Amira Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of FLAP in human peripheral leukocytes assessed as inhibition of calcium ionophore A23187-induced LTB4 production after 5 mins by ELISA in... |
J Med Chem 54: 8013-29 (2011)
Article DOI: 10.1021/jm2008369
BindingDB Entry DOI: 10.7270/Q269740W |
More data for this
Ligand-Target Pair | |
Arachidonate 5-lipoxygenase-activating protein
(Homo sapiens (Human)) | BDBM50359059
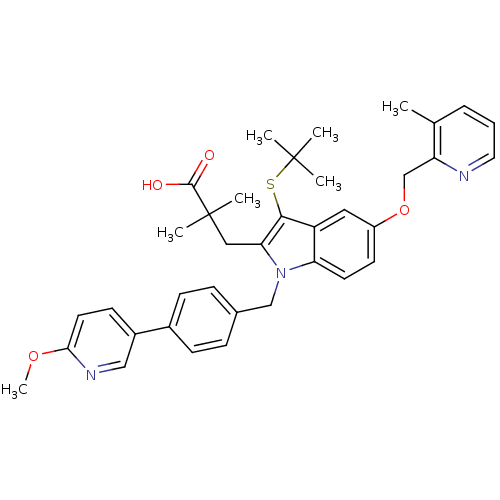
(CHEMBL1922530)Show SMILES COc1ccc(cn1)-c1ccc(Cn2c(CC(C)(C)C(O)=O)c(SC(C)(C)C)c3cc(OCc4ncccc4C)ccc23)cc1 Show InChI InChI=1S/C37H41N3O4S/c1-24-9-8-18-38-30(24)23-44-28-15-16-31-29(19-28)34(45-36(2,3)4)32(20-37(5,6)35(41)42)40(31)22-25-10-12-26(13-11-25)27-14-17-33(43-7)39-21-27/h8-19,21H,20,22-23H2,1-7H3,(H,41,42) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Amira Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of FLAP in human peripheral leukocytes assessed as inhibition of calcium ionophore A23187-induced LTB4 production after 10 mins by ELISA |
J Med Chem 54: 8013-29 (2011)
Article DOI: 10.1021/jm2008369
BindingDB Entry DOI: 10.7270/Q269740W |
More data for this
Ligand-Target Pair | |
Arachidonate 5-lipoxygenase-activating protein
(Homo sapiens (Human)) | BDBM50297385

(3-[3-tert-Butylsulfanyl-1-[4-(6-methoxy-pyridin-3-...)Show SMILES COc1ccc(cn1)-c1ccc(Cn2c(CC(C)(C)C(O)=O)c(SC(C)(C)C)c3cc(OCc4ccccn4)ccc23)cc1 Show InChI InChI=1S/C36H39N3O4S/c1-35(2,3)44-33-29-19-28(43-23-27-9-7-8-18-37-27)15-16-30(29)39(31(33)20-36(4,5)34(40)41)22-24-10-12-25(13-11-24)26-14-17-32(42-6)38-21-26/h7-19,21H,20,22-23H2,1-6H3,(H,40,41) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Amira Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of FLAP in human peripheral leukocytes assessed as inhibition of calcium ionophore A23187-induced LTB4 production after 1 hr by ELISA in a... |
J Med Chem 54: 8013-29 (2011)
Article DOI: 10.1021/jm2008369
BindingDB Entry DOI: 10.7270/Q269740W |
More data for this
Ligand-Target Pair | |
3-hydroxy-3-methylglutaryl-coenzyme A reductase
(Homo sapiens (Human)) | BDBM50215698

(CHEMBL251499 | sodium (3R,5R)-7-(5-((4-(dimethylca...)Show SMILES CC(C)n1c(CC[C@@H](O)C[C@@H](O)CC([O-])=O)c(c(c1C(=O)NCc1ccc(cc1)C(=O)N(C)C)-c1ccccc1)-c1ccc(F)cc1 Show InChI InChI=1S/C37H42FN3O6/c1-23(2)41-31(19-18-29(42)20-30(43)21-32(44)45)33(26-14-16-28(38)17-15-26)34(25-8-6-5-7-9-25)35(41)36(46)39-22-24-10-12-27(13-11-24)37(47)40(3)4/h5-17,23,29-30,42-43H,18-22H2,1-4H3,(H,39,46)(H,44,45)/p-1/t29-,30-/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of HMG-CoA reductase |
Bioorg Med Chem Lett 17: 4538-44 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.096
BindingDB Entry DOI: 10.7270/Q2SJ1K9Q |
More data for this
Ligand-Target Pair | |
Arachidonate 5-lipoxygenase-activating protein
(Homo sapiens (Human)) | BDBM50359064
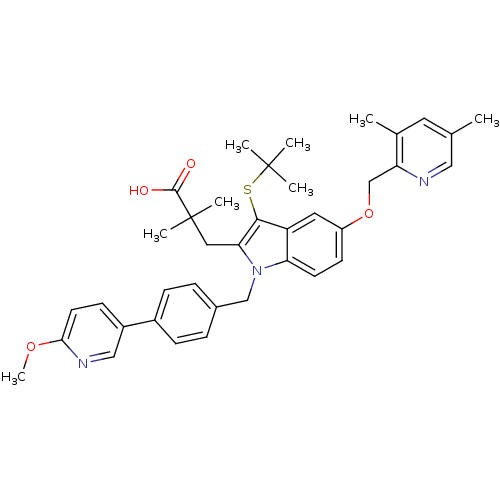
(CHEMBL1922644)Show SMILES COc1ccc(cn1)-c1ccc(Cn2c(CC(C)(C)C(O)=O)c(SC(C)(C)C)c3cc(OCc4ncc(C)cc4C)ccc23)cc1 Show InChI InChI=1S/C38H43N3O4S/c1-24-17-25(2)31(39-20-24)23-45-29-14-15-32-30(18-29)35(46-37(3,4)5)33(19-38(6,7)36(42)43)41(32)22-26-9-11-27(12-10-26)28-13-16-34(44-8)40-21-28/h9-18,20-21H,19,22-23H2,1-8H3,(H,42,43) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Amira Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of FLAP in human peripheral leukocytes assessed as inhibition of calcium ionophore A23187-induced LTB4 production after 10 mins by ELISA |
J Med Chem 54: 8013-29 (2011)
Article DOI: 10.1021/jm2008369
BindingDB Entry DOI: 10.7270/Q269740W |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data