

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| B1 bradykinin receptor (Homo sapiens (Human)) | BDBM50254456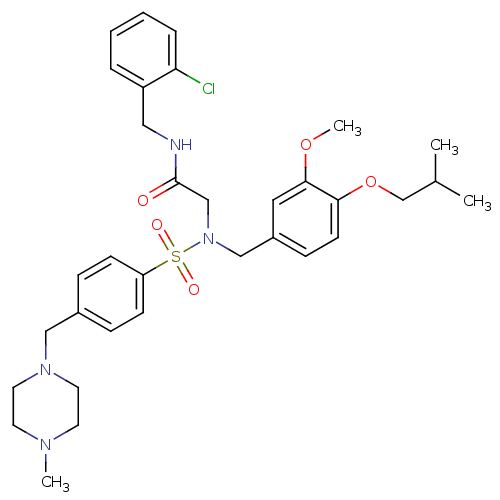 (CHEMBL447392 | N-(2-chlorobenzyl)-2-(N-(4-isobutox...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia Inc Curated by ChEMBL | Assay Description Displacement of [3H]desArg from human B1 in human WI 38 cells | Bioorg Med Chem Lett 19: 119-22 (2008) Article DOI: 10.1016/j.bmcl.2008.11.005 BindingDB Entry DOI: 10.7270/Q2KH0N6M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B1 bradykinin receptor (Homo sapiens (Human)) | BDBM50254457 (CHEMBL443207 | N-(2-chlorobenzyl)-2-(N-(3-chloro-4...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia Inc Curated by ChEMBL | Assay Description Displacement of [3H]desArg from human B1 in human WI 38 cells | Bioorg Med Chem Lett 19: 119-22 (2008) Article DOI: 10.1016/j.bmcl.2008.11.005 BindingDB Entry DOI: 10.7270/Q2KH0N6M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B1 bradykinin receptor (Homo sapiens (Human)) | BDBM50254458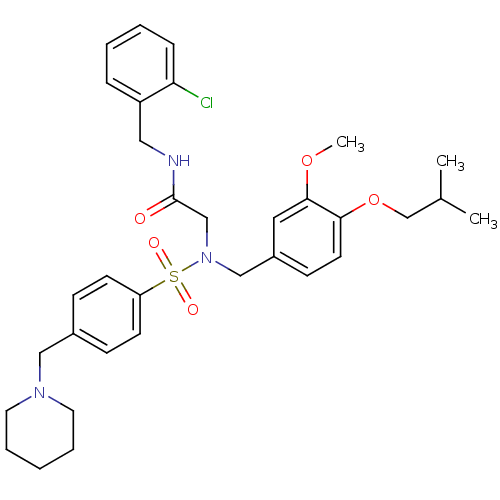 (CHEMBL452238 | N-(2-chlorobenzyl)-2-(N-(4-isobutox...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 34 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia Inc Curated by ChEMBL | Assay Description Displacement of [3H]desArg from human B1 in human WI 38 cells | Bioorg Med Chem Lett 19: 119-22 (2008) Article DOI: 10.1016/j.bmcl.2008.11.005 BindingDB Entry DOI: 10.7270/Q2KH0N6M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B1 bradykinin receptor (Homo sapiens (Human)) | BDBM50254456 (CHEMBL447392 | N-(2-chlorobenzyl)-2-(N-(4-isobutox...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia Inc Curated by ChEMBL | Assay Description Antagonist activity at human cloned B1 receptor expressed in african green monkey COS7 cells by calcium mobilization assay | Bioorg Med Chem Lett 19: 119-22 (2008) Article DOI: 10.1016/j.bmcl.2008.11.005 BindingDB Entry DOI: 10.7270/Q2KH0N6M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B1 bradykinin receptor (Homo sapiens (Human)) | BDBM50254459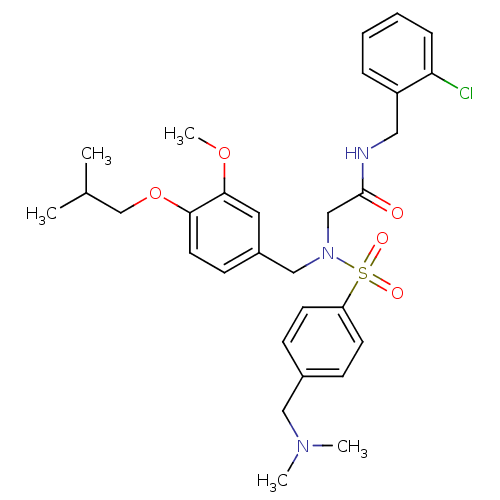 (CHEMBL512111 | N-(2-chlorobenzyl)-2-(N-(4-isobutox...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 93 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia Inc Curated by ChEMBL | Assay Description Displacement of [3H]desArg from human B1 in human WI 38 cells | Bioorg Med Chem Lett 19: 119-22 (2008) Article DOI: 10.1016/j.bmcl.2008.11.005 BindingDB Entry DOI: 10.7270/Q2KH0N6M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B1 bradykinin receptor (Homo sapiens (Human)) | BDBM50254460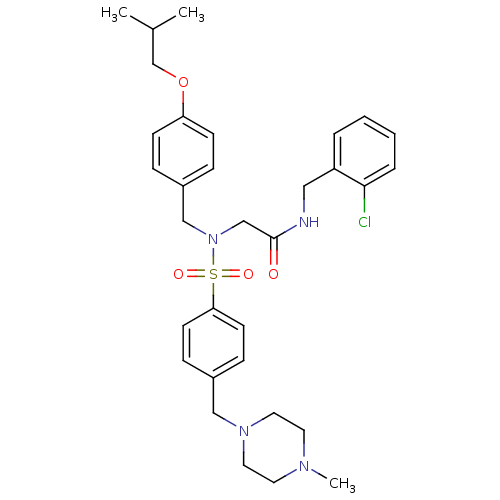 (CHEMBL499999 | N-(2-chlorobenzyl)-2-(N-(4-isobutox...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 113 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia Inc Curated by ChEMBL | Assay Description Displacement of [3H]desArg from human B1 in human WI 38 cells | Bioorg Med Chem Lett 19: 119-22 (2008) Article DOI: 10.1016/j.bmcl.2008.11.005 BindingDB Entry DOI: 10.7270/Q2KH0N6M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B1 bradykinin receptor (Homo sapiens (Human)) | BDBM50254461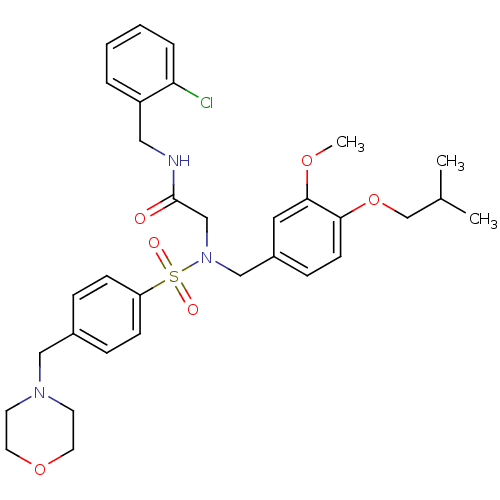 (CHEMBL507546 | N-(2-chlorobenzyl)-2-(N-(4-isobutox...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 149 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia Inc Curated by ChEMBL | Assay Description Displacement of [3H]desArg from human B1 in human WI 38 cells | Bioorg Med Chem Lett 19: 119-22 (2008) Article DOI: 10.1016/j.bmcl.2008.11.005 BindingDB Entry DOI: 10.7270/Q2KH0N6M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B1 bradykinin receptor (Homo sapiens (Human)) | BDBM50254457 (CHEMBL443207 | N-(2-chlorobenzyl)-2-(N-(3-chloro-4...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 188 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia Inc Curated by ChEMBL | Assay Description Antagonist activity at human cloned B1 receptor expressed in african green monkey COS7 cells by calcium mobilization assay | Bioorg Med Chem Lett 19: 119-22 (2008) Article DOI: 10.1016/j.bmcl.2008.11.005 BindingDB Entry DOI: 10.7270/Q2KH0N6M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B1 bradykinin receptor (Homo sapiens (Human)) | BDBM50254462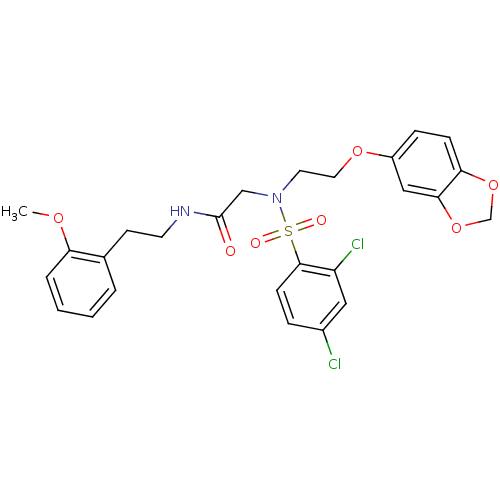 (CHEMBL468492 | N-(2-methoxyphenethyl)-2-(N-(2-(ben...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia Inc Curated by ChEMBL | Assay Description Displacement of [3H]desArg from human B1 in human WI 38 cells | Bioorg Med Chem Lett 19: 119-22 (2008) Article DOI: 10.1016/j.bmcl.2008.11.005 BindingDB Entry DOI: 10.7270/Q2KH0N6M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B1 bradykinin receptor (Homo sapiens (Human)) | BDBM50254458 (CHEMBL452238 | N-(2-chlorobenzyl)-2-(N-(4-isobutox...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 267 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia Inc Curated by ChEMBL | Assay Description Antagonist activity at human cloned B1 receptor expressed in african green monkey COS7 cells by calcium mobilization assay | Bioorg Med Chem Lett 19: 119-22 (2008) Article DOI: 10.1016/j.bmcl.2008.11.005 BindingDB Entry DOI: 10.7270/Q2KH0N6M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B1 bradykinin receptor (Homo sapiens (Human)) | BDBM50254463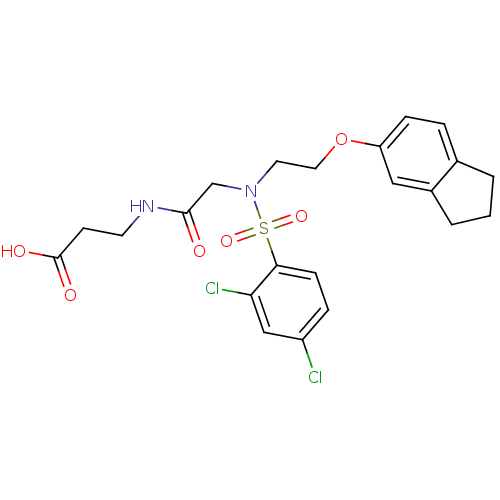 (3-(2-(2,4-dichloro-N-(2-(2,3-dihydro-1H-inden-5-yl...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia Inc Curated by ChEMBL | Assay Description Displacement of [3H]desArg from human B1 in human WI 38 cells | Bioorg Med Chem Lett 19: 119-22 (2008) Article DOI: 10.1016/j.bmcl.2008.11.005 BindingDB Entry DOI: 10.7270/Q2KH0N6M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B1 bradykinin receptor (Homo sapiens (Human)) | BDBM50254461 (CHEMBL507546 | N-(2-chlorobenzyl)-2-(N-(4-isobutox...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 463 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia Inc Curated by ChEMBL | Assay Description Antagonist activity at human cloned B1 receptor expressed in african green monkey COS7 cells by calcium mobilization assay | Bioorg Med Chem Lett 19: 119-22 (2008) Article DOI: 10.1016/j.bmcl.2008.11.005 BindingDB Entry DOI: 10.7270/Q2KH0N6M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B1 bradykinin receptor (Homo sapiens (Human)) | BDBM50254459 (CHEMBL512111 | N-(2-chlorobenzyl)-2-(N-(4-isobutox...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 497 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia Inc Curated by ChEMBL | Assay Description Antagonist activity at human cloned B1 receptor expressed in african green monkey COS7 cells by calcium mobilization assay | Bioorg Med Chem Lett 19: 119-22 (2008) Article DOI: 10.1016/j.bmcl.2008.11.005 BindingDB Entry DOI: 10.7270/Q2KH0N6M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B1 bradykinin receptor (Homo sapiens (Human)) | BDBM50254508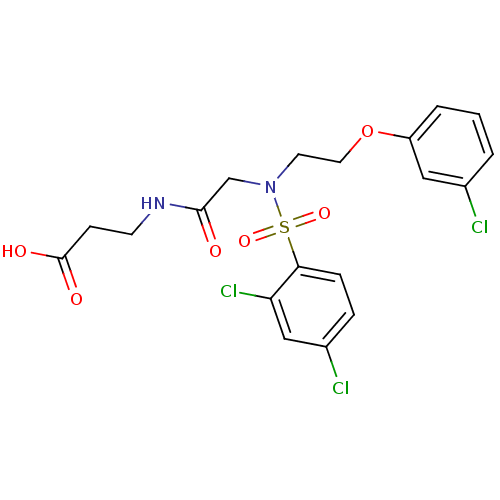 (3-(2-(2,4-dichloro-N-(2-(3-chlorophenoxy)ethyl)phe...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.09E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia Inc Curated by ChEMBL | Assay Description Displacement of [3H]desArg from human B1 in human WI 38 cells | Bioorg Med Chem Lett 19: 119-22 (2008) Article DOI: 10.1016/j.bmcl.2008.11.005 BindingDB Entry DOI: 10.7270/Q2KH0N6M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B1 bradykinin receptor (Homo sapiens (Human)) | BDBM50254632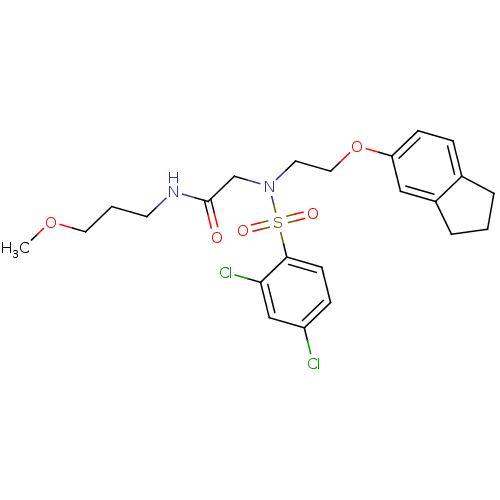 (2-(2,4-dichloro-N-(2-(2,3-dihydro-1H-inden-5-yloxy...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.21E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia Inc Curated by ChEMBL | Assay Description Displacement of [3H]desArg from human B1 in human WI 38 cells | Bioorg Med Chem Lett 19: 119-22 (2008) Article DOI: 10.1016/j.bmcl.2008.11.005 BindingDB Entry DOI: 10.7270/Q2KH0N6M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B1 bradykinin receptor (Homo sapiens (Human)) | BDBM50254633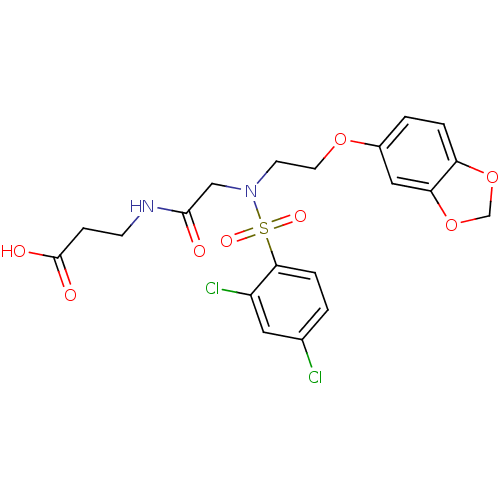 (3-(2-(N-(2-(benzo[d][1,3]dioxol-5-yloxy)ethyl)-2,4...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.57E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia Inc Curated by ChEMBL | Assay Description Displacement of [3H]desArg from human B1 in human WI 38 cells | Bioorg Med Chem Lett 19: 119-22 (2008) Article DOI: 10.1016/j.bmcl.2008.11.005 BindingDB Entry DOI: 10.7270/Q2KH0N6M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B1 bradykinin receptor (Homo sapiens (Human)) | BDBM50254634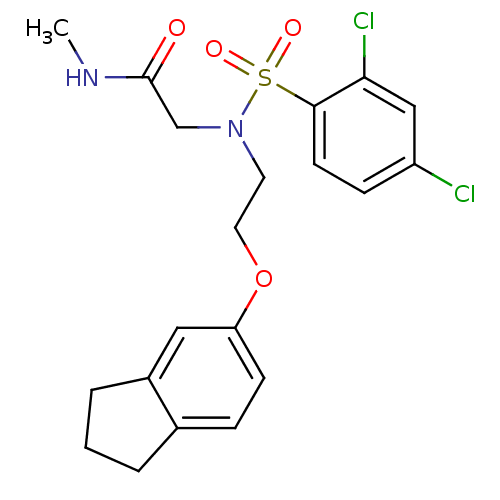 (2-(2,4-dichloro-N-(2-(2,3-dihydro-1H-inden-5-yloxy...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia Inc Curated by ChEMBL | Assay Description Displacement of [3H]desArg from human B1 in human WI 38 cells | Bioorg Med Chem Lett 19: 119-22 (2008) Article DOI: 10.1016/j.bmcl.2008.11.005 BindingDB Entry DOI: 10.7270/Q2KH0N6M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B1 bradykinin receptor (Homo sapiens (Human)) | BDBM50254635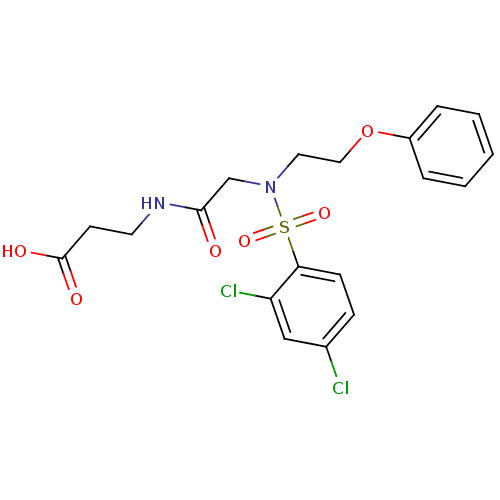 (3-(2-(2,4-dichloro-N-(2-phenoxyethyl)phenylsulfona...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia Inc Curated by ChEMBL | Assay Description Displacement of [3H]desArg from human B1 in human WI 38 cells | Bioorg Med Chem Lett 19: 119-22 (2008) Article DOI: 10.1016/j.bmcl.2008.11.005 BindingDB Entry DOI: 10.7270/Q2KH0N6M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50072775 (2-((1R,2R,5R)-5-hydroxy-2-(3-hydroxypropyl)cyclohe...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 0.770 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Displacement of [3H]CP-55940 from human CB1 receptor expressed in HEK293 cells | J Med Chem 50: 3851-6 (2007) Article DOI: 10.1021/jm070317a BindingDB Entry DOI: 10.7270/Q2K64JWR | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cyclin-dependent kinase 4 (Homo sapiens (Human)) | BDBM146964 (US8957074, 3) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Novartis AG US Patent | Assay Description An assay for monitoring CDK4/cyclin D1-catalyzed phosphorylation of pRb at the Ser780 site was performed using TR-FRET in a 384-well format, and was ... | US Patent US8957074 (2015) BindingDB Entry DOI: 10.7270/Q2K0730S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50072775 (2-((1R,2R,5R)-5-hydroxy-2-(3-hydroxypropyl)cyclohe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Displacement of [3H]CP-55940 human CB2 receptor expressed in CHOK1 cells | J Med Chem 50: 3851-6 (2007) Article DOI: 10.1021/jm070317a BindingDB Entry DOI: 10.7270/Q2K64JWR | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cyclin-dependent kinase 4 (Oryctolagus cuniculus (Rabbit)) | BDBM50420304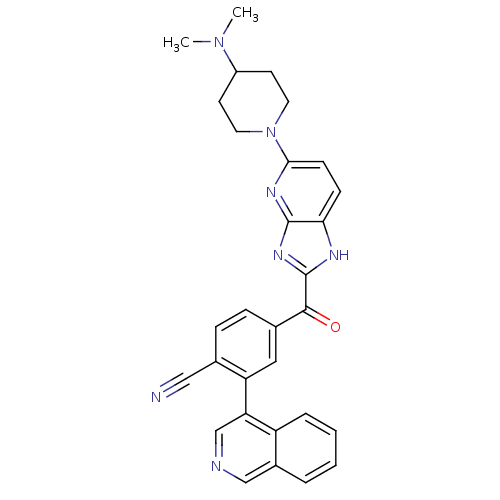 (CHEMBL2089065 | US8598217, 165) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Astex Therapeutics Ltd.; Novartis AG US Patent | Assay Description A 384-well microtiter Lance TR-FRET (time-resolved-fluorescence energy transfer) endpoint assay was used for CDK4/cyclin D1 kinase activity measureme... | US Patent US8598217 (2013) BindingDB Entry DOI: 10.7270/Q2BG2MN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 4 (Homo sapiens (Human)) | BDBM146997 (US8957074, 36) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Novartis AG US Patent | Assay Description An assay for monitoring CDK4/cyclin D1-catalyzed phosphorylation of pRb at the Ser780 site was performed using TR-FRET in a 384-well format, and was ... | US Patent US8957074 (2015) BindingDB Entry DOI: 10.7270/Q2K0730S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 4 (Homo sapiens (Human)) | BDBM147014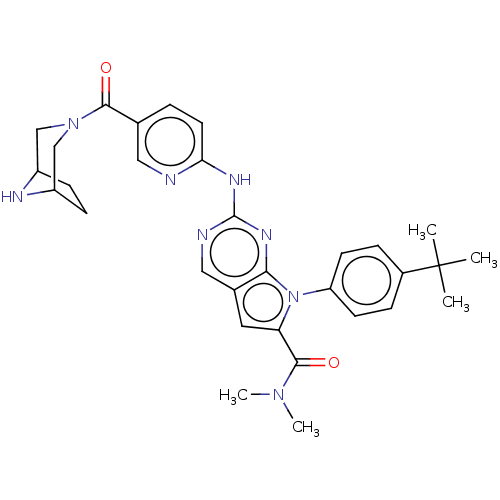 (US8957074, 53) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Novartis AG US Patent | Assay Description An assay for monitoring CDK4/cyclin D1-catalyzed phosphorylation of pRb at the Ser780 site was performed using TR-FRET in a 384-well format, and was ... | US Patent US8957074 (2015) BindingDB Entry DOI: 10.7270/Q2K0730S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 4 (Homo sapiens (Human)) | BDBM147083 (US8957074, 123) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Novartis AG US Patent | Assay Description An assay for monitoring CDK4/cyclin D1-catalyzed phosphorylation of pRb at the Ser780 site was performed using TR-FRET in a 384-well format, and was ... | US Patent US8957074 (2015) BindingDB Entry DOI: 10.7270/Q2K0730S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 4 (Homo sapiens (Human)) | BDBM147027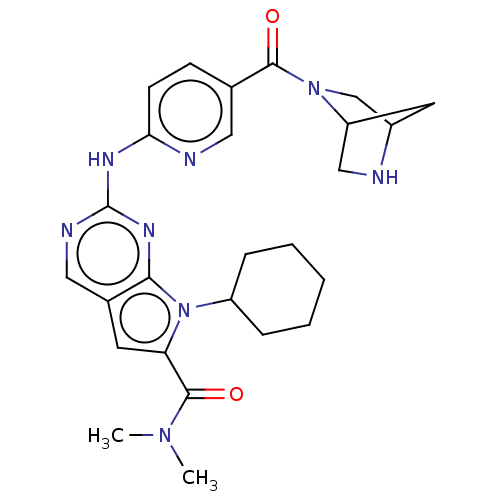 (US8957074, 67) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Novartis AG US Patent | Assay Description An assay for monitoring CDK4/cyclin D1-catalyzed phosphorylation of pRb at the Ser780 site was performed using TR-FRET in a 384-well format, and was ... | US Patent US8957074 (2015) BindingDB Entry DOI: 10.7270/Q2K0730S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 4 (Homo sapiens (Human)) | BDBM147041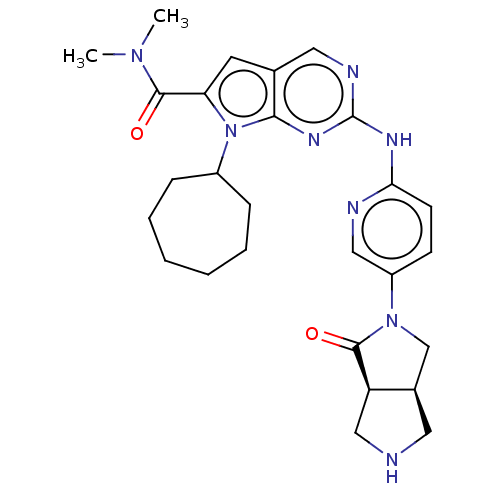 (US8957074, 83) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Novartis AG US Patent | Assay Description An assay for monitoring CDK4/cyclin D1-catalyzed phosphorylation of pRb at the Ser780 site was performed using TR-FRET in a 384-well format, and was ... | US Patent US8957074 (2015) BindingDB Entry DOI: 10.7270/Q2K0730S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 4 (Homo sapiens (Human)) | BDBM147049 (US8957074, 89) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Novartis AG US Patent | Assay Description An assay for monitoring CDK4/cyclin D1-catalyzed phosphorylation of pRb at the Ser780 site was performed using TR-FRET in a 384-well format, and was ... | US Patent US8957074 (2015) BindingDB Entry DOI: 10.7270/Q2K0730S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 4 (Homo sapiens (Human)) | BDBM147015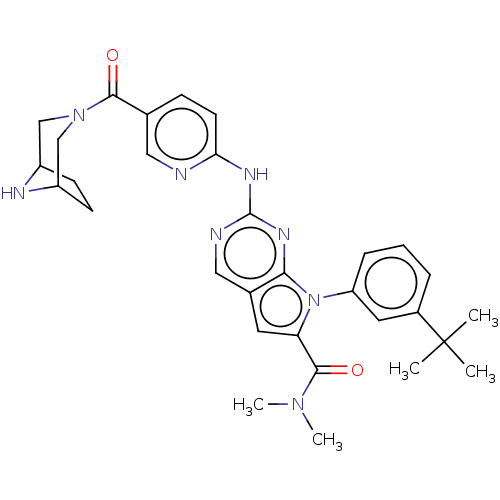 (US8957074, 54) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Novartis AG US Patent | Assay Description An assay for monitoring CDK4/cyclin D1-catalyzed phosphorylation of pRb at the Ser780 site was performed using TR-FRET in a 384-well format, and was ... | US Patent US8957074 (2015) BindingDB Entry DOI: 10.7270/Q2K0730S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 4 (Homo sapiens (Human)) | BDBM146965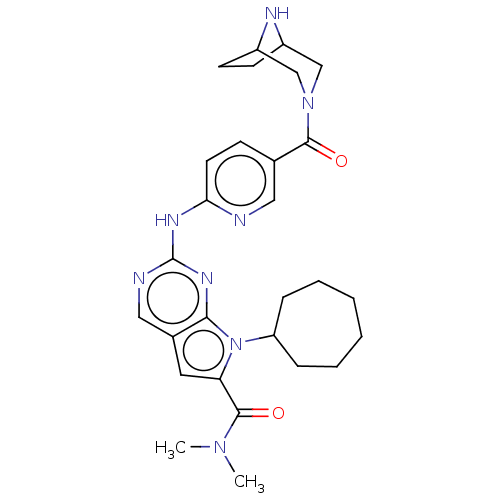 (US8957074, 4) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Novartis AG US Patent | Assay Description An assay for monitoring CDK4/cyclin D1-catalyzed phosphorylation of pRb at the Ser780 site was performed using TR-FRET in a 384-well format, and was ... | US Patent US8957074 (2015) BindingDB Entry DOI: 10.7270/Q2K0730S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 4 (Homo sapiens (Human)) | BDBM146970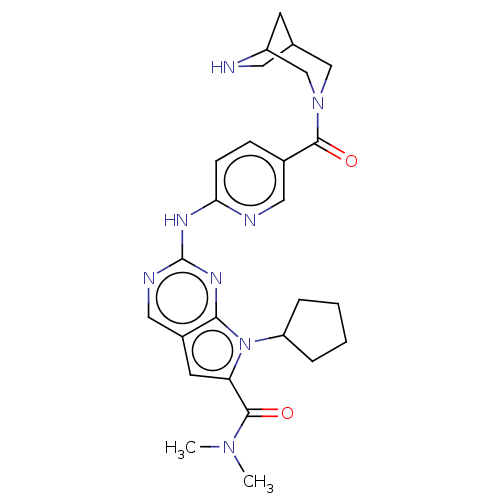 (US8957074, 9) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <4 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Novartis AG US Patent | Assay Description An assay for monitoring CDK4/cyclin D1-catalyzed phosphorylation of pRb at the Ser780 site was performed using TR-FRET in a 384-well format, and was ... | US Patent US8957074 (2015) BindingDB Entry DOI: 10.7270/Q2K0730S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 4 (Homo sapiens (Human)) | BDBM147091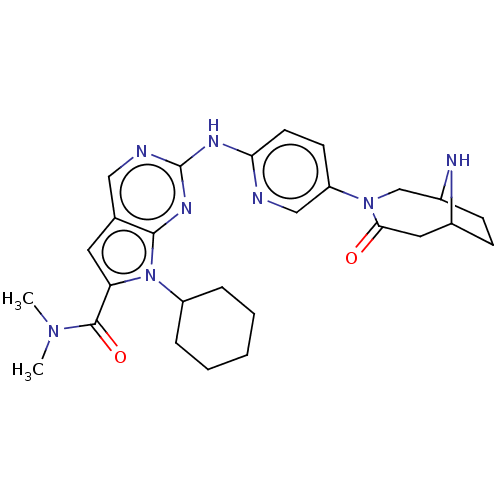 (US8957074, 131) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Novartis AG US Patent | Assay Description An assay for monitoring CDK4/cyclin D1-catalyzed phosphorylation of pRb at the Ser780 site was performed using TR-FRET in a 384-well format, and was ... | US Patent US8957074 (2015) BindingDB Entry DOI: 10.7270/Q2K0730S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 4 (Oryctolagus cuniculus (Rabbit)) | BDBM107751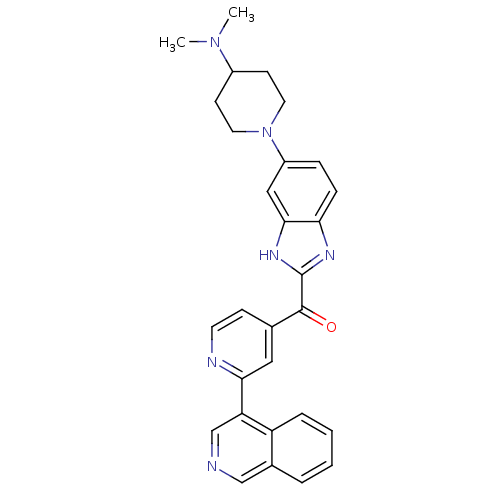 (CHEMBL2089063 | US8598217, 89) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Astex Therapeutics Ltd.; Novartis AG US Patent | Assay Description A 384-well microtiter Lance TR-FRET (time-resolved-fluorescence energy transfer) endpoint assay was used for CDK4/cyclin D1 kinase activity measureme... | US Patent US8598217 (2013) BindingDB Entry DOI: 10.7270/Q2BG2MN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 4 (Oryctolagus cuniculus (Rabbit)) | BDBM107755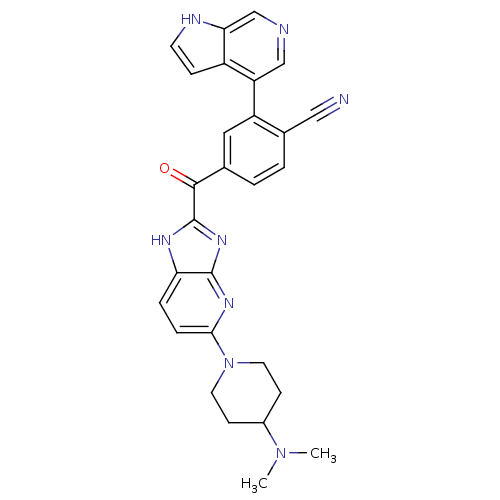 (US8598217, 140) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Astex Therapeutics Ltd.; Novartis AG US Patent | Assay Description A 384-well microtiter Lance TR-FRET (time-resolved-fluorescence energy transfer) endpoint assay was used for CDK4/cyclin D1 kinase activity measureme... | US Patent US8598217 (2013) BindingDB Entry DOI: 10.7270/Q2BG2MN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 4 (Homo sapiens (Human)) | BDBM147042 (US8957074, 82) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Novartis AG US Patent | Assay Description An assay for monitoring CDK4/cyclin D1-catalyzed phosphorylation of pRb at the Ser780 site was performed using TR-FRET in a 384-well format, and was ... | US Patent US8957074 (2015) BindingDB Entry DOI: 10.7270/Q2K0730S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 4 (Oryctolagus cuniculus (Rabbit)) | BDBM107761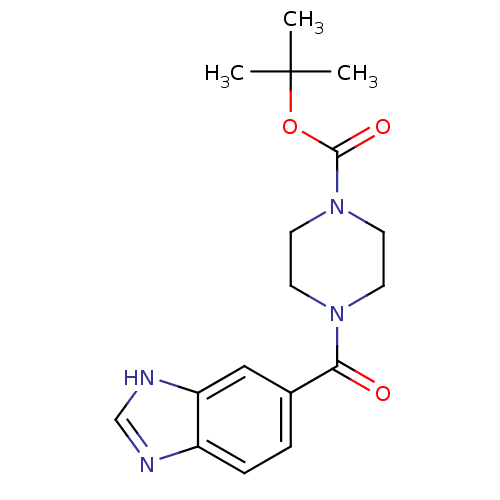 (US8598217, 174) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Astex Therapeutics Ltd.; Novartis AG US Patent | Assay Description A 384-well microtiter Lance TR-FRET (time-resolved-fluorescence energy transfer) endpoint assay was used for CDK4/cyclin D1 kinase activity measureme... | US Patent US8598217 (2013) BindingDB Entry DOI: 10.7270/Q2BG2MN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 4 (Homo sapiens (Human)) | BDBM146963 (US8957074, 2) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Novartis AG US Patent | Assay Description An assay for monitoring CDK4/cyclin D1-catalyzed phosphorylation of pRb at the Ser780 site was performed using TR-FRET in a 384-well format, and was ... | US Patent US8957074 (2015) BindingDB Entry DOI: 10.7270/Q2K0730S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 4 (Homo sapiens (Human)) | BDBM147093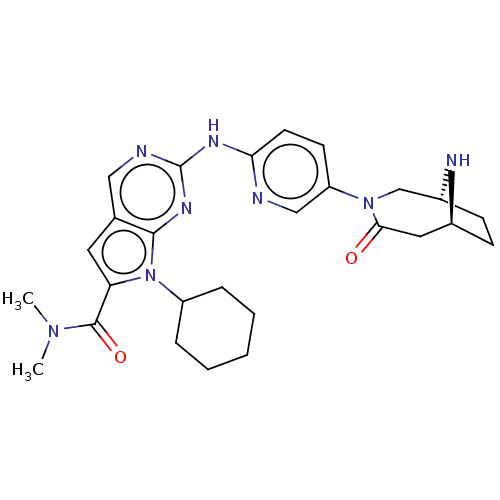 (US8957074, 133) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <5 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Novartis AG US Patent | Assay Description An assay for monitoring CDK4/cyclin D1-catalyzed phosphorylation of pRb at the Ser780 site was performed using TR-FRET in a 384-well format, and was ... | US Patent US8957074 (2015) BindingDB Entry DOI: 10.7270/Q2K0730S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 4 (Homo sapiens (Human)) | BDBM147087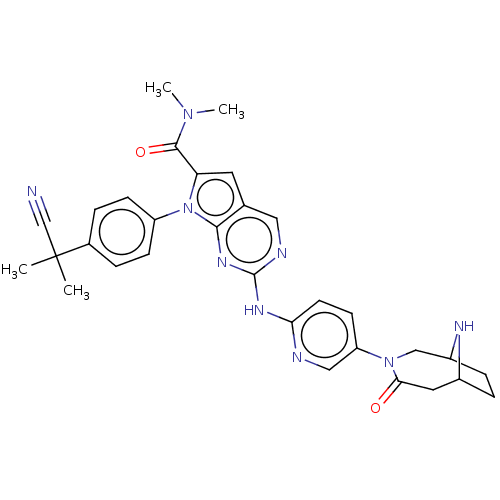 (US8957074, 127) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Novartis AG US Patent | Assay Description An assay for monitoring CDK4/cyclin D1-catalyzed phosphorylation of pRb at the Ser780 site was performed using TR-FRET in a 384-well format, and was ... | US Patent US8957074 (2015) BindingDB Entry DOI: 10.7270/Q2K0730S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 4 (Homo sapiens (Human)) | BDBM147053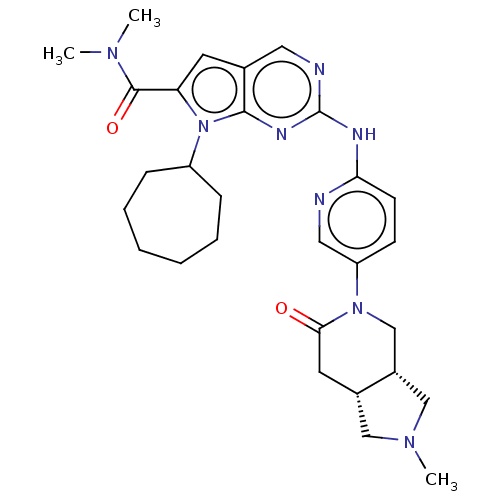 (US8957074, 93) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <5 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Novartis AG US Patent | Assay Description An assay for monitoring CDK4/cyclin D1-catalyzed phosphorylation of pRb at the Ser780 site was performed using TR-FRET in a 384-well format, and was ... | US Patent US8957074 (2015) BindingDB Entry DOI: 10.7270/Q2K0730S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 4 (Homo sapiens (Human)) | BDBM147050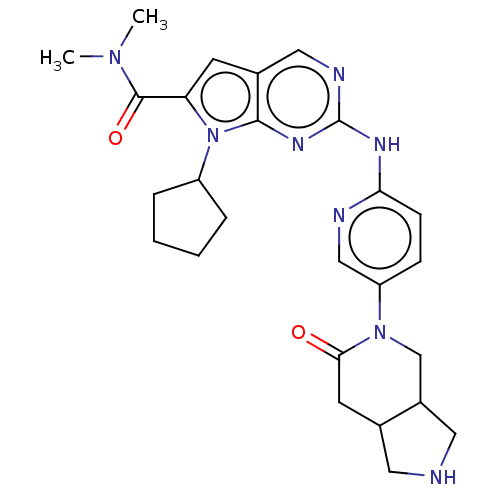 (US8957074, 90) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Novartis AG US Patent | Assay Description An assay for monitoring CDK4/cyclin D1-catalyzed phosphorylation of pRb at the Ser780 site was performed using TR-FRET in a 384-well format, and was ... | US Patent US8957074 (2015) BindingDB Entry DOI: 10.7270/Q2K0730S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 4 (Homo sapiens (Human)) | BDBM146971 (US8957074, 10) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Novartis AG US Patent | Assay Description An assay for monitoring CDK4/cyclin D1-catalyzed phosphorylation of pRb at the Ser780 site was performed using TR-FRET in a 384-well format, and was ... | US Patent US8957074 (2015) BindingDB Entry DOI: 10.7270/Q2K0730S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 4 (Homo sapiens (Human)) | BDBM47154 (US8957074, 81) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <5 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Novartis AG US Patent | Assay Description An assay for monitoring CDK4/cyclin D1-catalyzed phosphorylation of pRb at the Ser780 site was performed using TR-FRET in a 384-well format, and was ... | US Patent US8957074 (2015) BindingDB Entry DOI: 10.7270/Q2K0730S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 4 (Homo sapiens (Human)) | BDBM147044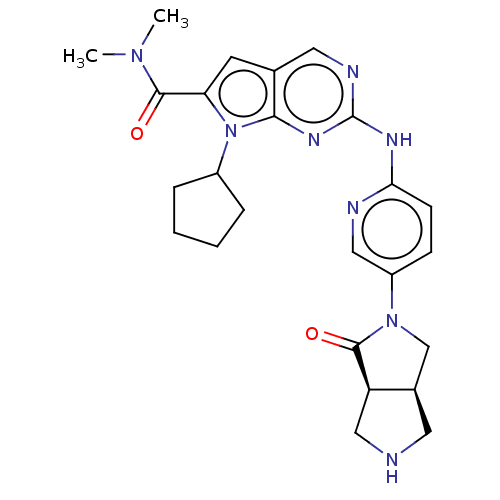 (US8957074, 84 | US8957074, 86) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <5 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Novartis AG US Patent | Assay Description An assay for monitoring CDK4/cyclin D1-catalyzed phosphorylation of pRb at the Ser780 site was performed using TR-FRET in a 384-well format, and was ... | US Patent US8957074 (2015) BindingDB Entry DOI: 10.7270/Q2K0730S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 4 (Homo sapiens (Human)) | BDBM147047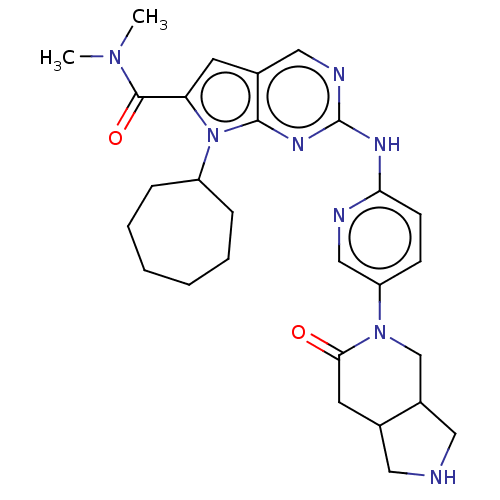 (US8957074, 87) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <5 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Novartis AG US Patent | Assay Description An assay for monitoring CDK4/cyclin D1-catalyzed phosphorylation of pRb at the Ser780 site was performed using TR-FRET in a 384-well format, and was ... | US Patent US8957074 (2015) BindingDB Entry DOI: 10.7270/Q2K0730S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 4 (Homo sapiens (Human)) | BDBM147048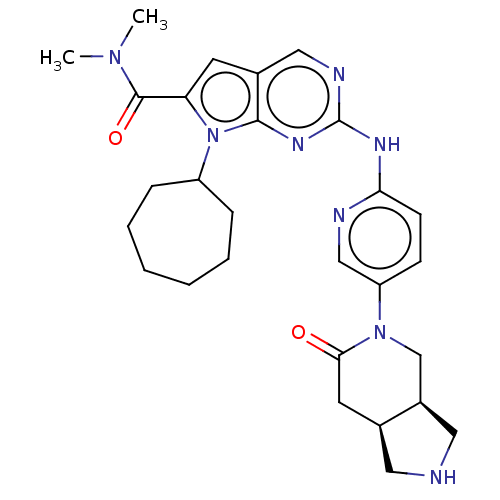 (US8957074, 88) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Novartis AG US Patent | Assay Description An assay for monitoring CDK4/cyclin D1-catalyzed phosphorylation of pRb at the Ser780 site was performed using TR-FRET in a 384-well format, and was ... | US Patent US8957074 (2015) BindingDB Entry DOI: 10.7270/Q2K0730S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 4 (Homo sapiens (Human)) | BDBM147031 (US8957074, 71) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Novartis AG US Patent | Assay Description An assay for monitoring CDK4/cyclin D1-catalyzed phosphorylation of pRb at the Ser780 site was performed using TR-FRET in a 384-well format, and was ... | US Patent US8957074 (2015) BindingDB Entry DOI: 10.7270/Q2K0730S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 4 (Homo sapiens (Human)) | BDBM147026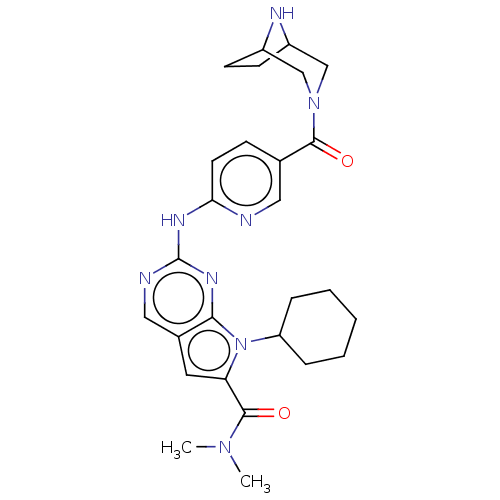 (US8957074, 66) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Novartis AG US Patent | Assay Description An assay for monitoring CDK4/cyclin D1-catalyzed phosphorylation of pRb at the Ser780 site was performed using TR-FRET in a 384-well format, and was ... | US Patent US8957074 (2015) BindingDB Entry DOI: 10.7270/Q2K0730S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 4 (Homo sapiens (Human)) | BDBM146998 (US8957074, 37) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Novartis AG US Patent | Assay Description An assay for monitoring CDK4/cyclin D1-catalyzed phosphorylation of pRb at the Ser780 site was performed using TR-FRET in a 384-well format, and was ... | US Patent US8957074 (2015) BindingDB Entry DOI: 10.7270/Q2K0730S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 4 (Homo sapiens (Human)) | BDBM147044 (US8957074, 84 | US8957074, 86) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Novartis AG US Patent | Assay Description An assay for monitoring CDK4/cyclin D1-catalyzed phosphorylation of pRb at the Ser780 site was performed using TR-FRET in a 384-well format, and was ... | US Patent US8957074 (2015) BindingDB Entry DOI: 10.7270/Q2K0730S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 573 total ) | Next | Last >> |