 Found 66 hits with Last Name = 'brenneman' and Initial = 'de'
Found 66 hits with Last Name = 'brenneman' and Initial = 'de' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50318133

((14S)-16-amino-10,14-dicyclohexyl-2-oxa-10,15,17,2...)Show SMILES NC1=Nc2cnc3Oc4cccc(CCN(C5CCCCC5)C(=O)CC[C@@H](C5CCCCC5)N1Cc2c3)c4 |r,t:1| Show InChI InChI=1S/C31H41N5O2/c32-31-34-27-20-33-29-19-24(27)21-36(31)28(23-9-3-1-4-10-23)14-15-30(37)35(25-11-5-2-6-12-25)17-16-22-8-7-13-26(18-22)38-29/h7-8,13,18-20,23,25,28H,1-6,9-12,14-17,21H2,(H2,32,34)/t28-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 |
Bioorg Med Chem Lett 20: 3158-60 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.097
BindingDB Entry DOI: 10.7270/Q2C53M1S |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50318123
((14S)-16-amino-10,14-dicyclohexyl-2-oxa-10,15,17-t...)Show SMILES NC1=Nc2ccc3Oc4cccc(CCN(C5CCCCC5)C(=O)CC[C@@H](C5CCCCC5)N1Cc2c3)c4 |r,t:1| Show InChI InChI=1S/C32H42N4O2/c33-32-34-29-15-14-28-21-25(29)22-36(32)30(24-9-3-1-4-10-24)16-17-31(37)35(26-11-5-2-6-12-26)19-18-23-8-7-13-27(20-23)38-28/h7-8,13-15,20-21,24,26,30H,1-6,9-12,16-19,22H2,(H2,33,34)/t30-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 |
Bioorg Med Chem Lett 20: 3158-60 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.097
BindingDB Entry DOI: 10.7270/Q2C53M1S |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50318131

((14S)-16-amino-10,14-dicyclohexyl-20-fluoro-2-oxa-...)Show SMILES NC1=Nc2cc(F)c3Oc4cccc(CCN(C5CCCCC5)C(=O)CC[C@@H](C5CCCCC5)N1Cc2c3)c4 |r,t:1| Show InChI InChI=1S/C32H41FN4O2/c33-27-20-28-24-19-30(27)39-26-13-7-8-22(18-26)16-17-36(25-11-5-2-6-12-25)31(38)15-14-29(23-9-3-1-4-10-23)37(21-24)32(34)35-28/h7-8,13,18-20,23,25,29H,1-6,9-12,14-17,21H2,(H2,34,35)/t29-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 |
Bioorg Med Chem Lett 20: 3158-60 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.097
BindingDB Entry DOI: 10.7270/Q2C53M1S |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50318125
((14S)-16-amino-10-cyclohexyl-14-(propan-2-yl)-2-ox...)Show SMILES CC(C)[C@@H]1CCC(=O)N(CCc2cccc(Oc3ccc4N=C(N)N1Cc4c3)c2)C1CCCCC1 |r,t:21| Show InChI InChI=1S/C29H38N4O2/c1-20(2)27-13-14-28(34)32(23-8-4-3-5-9-23)16-15-21-7-6-10-24(17-21)35-25-11-12-26-22(18-25)19-33(27)29(30)31-26/h6-7,10-12,17-18,20,23,27H,3-5,8-9,13-16,19H2,1-2H3,(H2,30,31)/t27-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 |
Bioorg Med Chem Lett 20: 3158-60 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.097
BindingDB Entry DOI: 10.7270/Q2C53M1S |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM17786
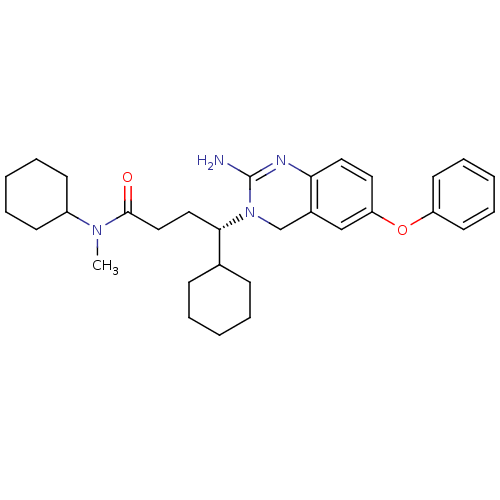
((4S)-4-(2-amino-6-phenoxy-3,4-dihydroquinazolin-3-...)Show SMILES CN(C1CCCCC1)C(=O)CC[C@@H](C1CCCCC1)N1Cc2cc(Oc3ccccc3)ccc2N=C1N |r,c:38| Show InChI InChI=1S/C31H42N4O2/c1-34(25-13-7-3-8-14-25)30(36)20-19-29(23-11-5-2-6-12-23)35-22-24-21-27(17-18-28(24)33-31(35)32)37-26-15-9-4-10-16-26/h4,9-10,15-18,21,23,25,29H,2-3,5-8,11-14,19-20,22H2,1H3,(H2,32,33)/t29-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 11 | -45.0 | n/a | n/a | n/a | n/a | n/a | 5.0 | 22 |
Johnson & Johnson Pharmaceutical
| Assay Description
BACE-1 activity was measured at pH 5 using the FS1 FRET substrate. Compounds were preincubated with recombinant BACE-1 for 20 min before adding subst... |
J Med Chem 50: 4261-4 (2007)
Article DOI: 10.1021/jm0705408
BindingDB Entry DOI: 10.7270/Q24M92T2 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50174316

(CHEMBL3809355)Show InChI InChI=1S/C21H34O2/c1-4-5-6-10-13-21(2,3)17-14-18(22)20(19(23)15-17)16-11-8-7-9-12-16/h14-16,22-23H,4-13H2,1-3H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
KannaLife Sciences
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP 55940 from human CB1 receptor after 1 hr by liquid scintillation spectrometry |
ACS Med Chem Lett 7: 424-8 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00009
BindingDB Entry DOI: 10.7270/Q2BZ680Z |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50318132

((14S)-16-amino-10,14-dicyclohexyl-20-methoxy-2-oxa...)Show SMILES COc1cc2N=C(N)N3Cc2cc1Oc1cccc(CCN(C2CCCCC2)C(=O)CC[C@H]3C2CCCCC2)c1 |r,t:5| Show InChI InChI=1S/C33H44N4O3/c1-39-30-21-28-25-20-31(30)40-27-14-8-9-23(19-27)17-18-36(26-12-6-3-7-13-26)32(38)16-15-29(24-10-4-2-5-11-24)37(22-25)33(34)35-28/h8-9,14,19-21,24,26,29H,2-7,10-13,15-18,22H2,1H3,(H2,34,35)/t29-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 |
Bioorg Med Chem Lett 20: 3158-60 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.097
BindingDB Entry DOI: 10.7270/Q2C53M1S |
More data for this
Ligand-Target Pair | |
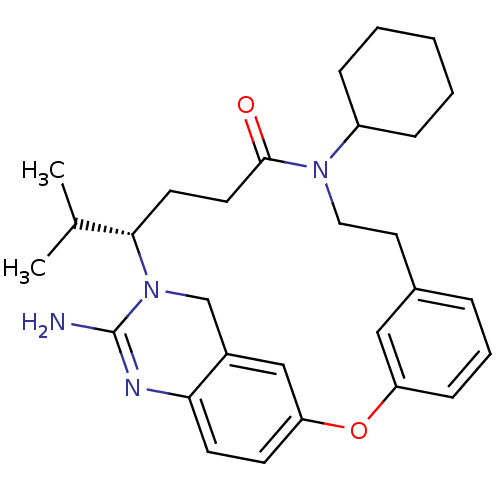
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50318124
((14S)-16-amino-14-cyclohexyl-10-(oxan-4-yl)-2-oxa-...)Show SMILES NC1=Nc2ccc3Oc4cccc(CCN(C5CCOCC5)C(=O)CC[C@@H](C5CCCCC5)N1Cc2c3)c4 |r,t:1| Show InChI InChI=1S/C31H40N4O3/c32-31-33-28-10-9-27-20-24(28)21-35(31)29(23-6-2-1-3-7-23)11-12-30(36)34(25-14-17-37-18-15-25)16-13-22-5-4-8-26(19-22)38-27/h4-5,8-10,19-20,23,25,29H,1-3,6-7,11-18,21H2,(H2,32,33)/t29-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 |
Bioorg Med Chem Lett 20: 3158-60 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.097
BindingDB Entry DOI: 10.7270/Q2C53M1S |
More data for this
Ligand-Target Pair | |
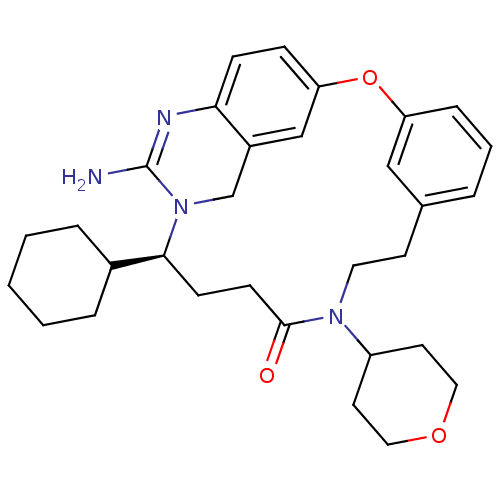
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50318129
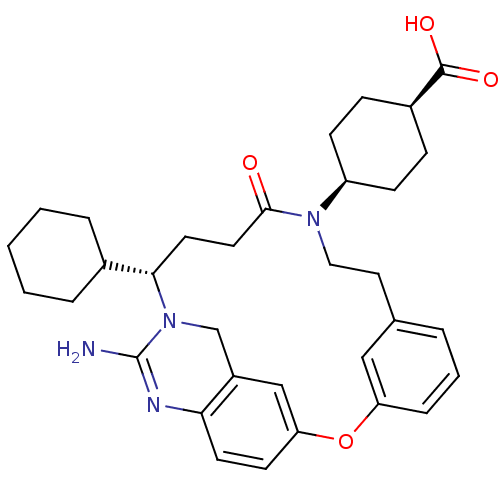
(4-[(14S)-16-amino-14-cyclohexyl-11-oxo-2-oxa-10,15...)Show SMILES NC1=Nc2ccc3Oc4cccc(CCN([C@H]5CC[C@H](CC5)C(O)=O)C(=O)CC[C@@H](C5CCCCC5)N1Cc2c3)c4 |r,wU:29.30,wD:16.15,19.22,t:1,(9.57,-14.62,;8.24,-13.86,;6.9,-14.63,;5.57,-13.86,;4.24,-14.63,;2.9,-13.86,;2.9,-12.32,;1.57,-11.53,;1.58,-10.06,;.25,-9.29,;.25,-7.75,;1.58,-6.98,;2.91,-7.74,;4.34,-6.99,;5.68,-7.8,;7.07,-7.05,;7.1,-5.51,;8.45,-4.78,;8.49,-3.25,;7.18,-2.44,;5.83,-3.17,;5.78,-4.72,;7.22,-.9,;8.58,-.16,;5.91,-.09,;8.4,-7.86,;9.75,-7.12,;8.37,-9.43,;9.68,-10.23,;9.64,-11.68,;10.91,-12.54,;10.93,-14.06,;12.26,-14.82,;13.58,-14.04,;13.57,-12.5,;12.23,-11.74,;8.23,-12.3,;6.9,-11.54,;5.57,-12.31,;4.23,-11.55,;2.92,-9.29,)| Show InChI InChI=1S/C33H42N4O4/c34-33-35-29-14-13-28-20-25(29)21-37(33)30(23-6-2-1-3-7-23)15-16-31(38)36(26-11-9-24(10-12-26)32(39)40)18-17-22-5-4-8-27(19-22)41-28/h4-5,8,13-14,19-20,23-24,26,30H,1-3,6-7,9-12,15-18,21H2,(H2,34,35)(H,39,40)/t24-,26+,30-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 |
Bioorg Med Chem Lett 20: 3158-60 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.097
BindingDB Entry DOI: 10.7270/Q2C53M1S |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50318128
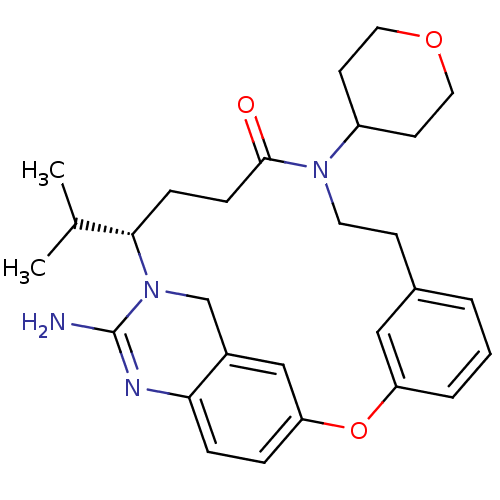
((14S)-16-amino-10-(oxan-4-yl)-14-(propan-2-yl)-2-o...)Show SMILES CC(C)[C@@H]1CCC(=O)N(CCc2cccc(Oc3ccc4N=C(N)N1Cc4c3)c2)C1CCOCC1 |r,t:21| Show InChI InChI=1S/C28H36N4O3/c1-19(2)26-8-9-27(33)31(22-11-14-34-15-12-22)13-10-20-4-3-5-23(16-20)35-24-6-7-25-21(17-24)18-32(26)28(29)30-25/h3-7,16-17,19,22,26H,8-15,18H2,1-2H3,(H2,29,30)/t26-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 |
Bioorg Med Chem Lett 20: 3158-60 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.097
BindingDB Entry DOI: 10.7270/Q2C53M1S |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM17785
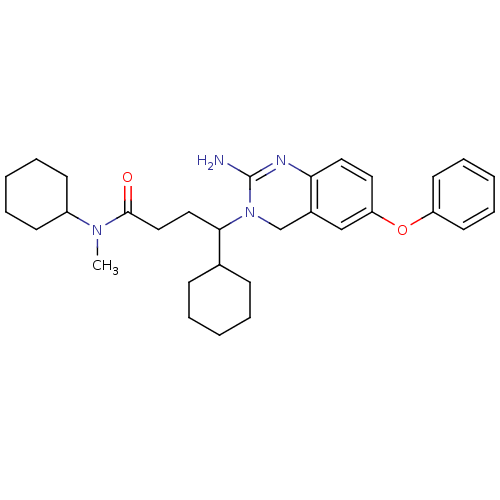
(2-aminoquinazoline, 3 | 4-(2-amino-6-phenoxy-3,4-d...)Show SMILES CN(C1CCCCC1)C(=O)CCC(C1CCCCC1)N1Cc2cc(Oc3ccccc3)ccc2N=C1N |c:38| Show InChI InChI=1S/C31H42N4O2/c1-34(25-13-7-3-8-14-25)30(36)20-19-29(23-11-5-2-6-12-23)35-22-24-21-27(17-18-28(24)33-31(35)32)37-26-15-9-4-10-16-26/h4,9-10,15-18,21,23,25,29H,2-3,5-8,11-14,19-20,22H2,1H3,(H2,32,33) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 30 | -42.5 | n/a | n/a | n/a | n/a | n/a | 5.0 | 22 |
Johnson & Johnson Pharmaceutical
| Assay Description
BACE-1 activity was measured at pH 5 using the FS1 FRET substrate. Compounds were preincubated with recombinant BACE-1 for 20 min before adding subst... |
J Med Chem 50: 4261-4 (2007)
Article DOI: 10.1021/jm0705408
BindingDB Entry DOI: 10.7270/Q24M92T2 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50318127
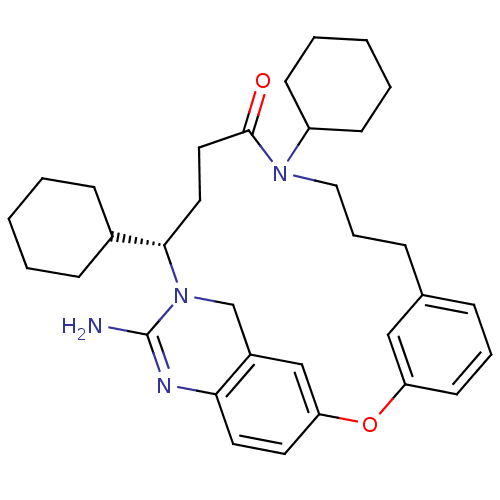
((15S)-17-amino-11,15-dicyclohexyl-2-oxa-11,16,18-t...)Show SMILES NC1=Nc2ccc3Oc4cccc(CCCN(C5CCCCC5)C(=O)CC[C@@H](C5CCCCC5)N1Cc2c3)c4 |r,t:1| Show InChI InChI=1S/C33H44N4O2/c34-33-35-30-17-16-29-22-26(30)23-37(33)31(25-11-3-1-4-12-25)18-19-32(38)36(27-13-5-2-6-14-27)20-8-10-24-9-7-15-28(21-24)39-29/h7,9,15-17,21-22,25,27,31H,1-6,8,10-14,18-20,23H2,(H2,34,35)/t31-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 |
Bioorg Med Chem Lett 20: 3158-60 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.097
BindingDB Entry DOI: 10.7270/Q2C53M1S |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50092588
(4-(1,1-Dimethyl-heptyl)-2'-isopropyl-5'-methyl-bip...)Show SMILES CCCCCCC(C)(C)c1ccc(c(O)c1)-c1cc(C)ccc1C(C)C Show InChI InChI=1S/C25H36O/c1-7-8-9-10-15-25(5,6)20-12-14-22(24(26)17-20)23-16-19(4)11-13-21(23)18(2)3/h11-14,16-18,26H,7-10,15H2,1-6H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
KannaLife Sciences
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP 55940 from human CB1 receptor after 1 hr by liquid scintillation spectrometry |
ACS Med Chem Lett 7: 424-8 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00009
BindingDB Entry DOI: 10.7270/Q2BZ680Z |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM17784
(2-aminoquinazoline, 2 | 3-[(2-amino-6-phenoxy-3,4-...)Show SMILES CN(C1CCCCC1)C(=O)c1cccc(CN2Cc3cc(Oc4ccccc4)ccc3N=C2N)c1 |c:34| Show InChI InChI=1S/C29H32N4O2/c1-32(24-11-4-2-5-12-24)28(34)22-10-8-9-21(17-22)19-33-20-23-18-26(15-16-27(23)31-29(33)30)35-25-13-6-3-7-14-25/h3,6-10,13-18,24H,2,4-5,11-12,19-20H2,1H3,(H2,30,31) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 158 | -38.4 | n/a | n/a | n/a | n/a | n/a | 5.0 | 22 |
Johnson & Johnson Pharmaceutical
| Assay Description
BACE-1 activity was measured at pH 5 using the FS1 FRET substrate. Compounds were preincubated with recombinant BACE-1 for 20 min before adding subst... |
J Med Chem 50: 4261-4 (2007)
Article DOI: 10.1021/jm0705408
BindingDB Entry DOI: 10.7270/Q24M92T2 |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50318126

((15R)-17-amino-11,15-dicyclohexyl-2-oxa-11,16,18-t...)Show SMILES NC1=Nc2ccc3Oc4cccc(CCCN(C5CCCCC5)C(=O)CC[C@H](C5CCCCC5)N1Cc2c3)c4 |r,t:1| Show InChI InChI=1S/C33H44N4O2/c34-33-35-30-17-16-29-22-26(30)23-37(33)31(25-11-3-1-4-12-25)18-19-32(38)36(27-13-5-2-6-14-27)20-8-10-24-9-7-15-28(21-24)39-29/h7,9,15-17,21-22,25,27,31H,1-6,8,10-14,18-20,23H2,(H2,34,35)/t31-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 186 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 |
Bioorg Med Chem Lett 20: 3158-60 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.097
BindingDB Entry DOI: 10.7270/Q2C53M1S |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Rattus norvegicus (rat)) | BDBM50174315
(CHEMBL3810140)Show SMILES CCCCCc1cc(O)c([C@H]2C=C(C)CC[C@@H]2C(C)=C)c(O)c1 |r,t:11| Show InChI InChI=1S/C21H30O2/c1-5-6-7-8-16-12-19(22)21(20(23)13-16)18-11-15(4)9-10-17(18)14(2)3/h11-13,17-18,22-23H,2,5-10H2,1,3-4H3/t17-,18+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 842 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
KannaLife Sciences
Curated by ChEMBL
| Assay Description
Displacement of [3H]-HU-243 from CB1 receptor in Sabra rat brain synaptosomes after 90 mins |
ACS Med Chem Lett 7: 424-8 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00009
BindingDB Entry DOI: 10.7270/Q2BZ680Z |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM17783
(2-aminoquinazoline, 1 | 3-(2-amino-6-benzoyl-3,4-d...)Show SMILES CN(C1CCCCC1)C(=O)CCN1Cc2cc(ccc2N=C1N)C(=O)c1ccccc1 |c:22| Show InChI InChI=1S/C25H30N4O2/c1-28(21-10-6-3-7-11-21)23(30)14-15-29-17-20-16-19(12-13-22(20)27-25(29)26)24(31)18-8-4-2-5-9-18/h2,4-5,8-9,12-13,16,21H,3,6-7,10-11,14-15,17H2,1H3,(H2,26,27) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| MMDB
PDB
Article
PubMed
| 900 | -34.2 | n/a | n/a | n/a | n/a | n/a | 5.0 | 22 |
Johnson & Johnson Pharmaceutical
| Assay Description
BACE-1 activity was measured at pH 5 using the FS1 FRET substrate. Compounds were preincubated with recombinant BACE-1 for 20 min before adding subst... |
J Med Chem 50: 4261-4 (2007)
Article DOI: 10.1021/jm0705408
BindingDB Entry DOI: 10.7270/Q24M92T2 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50318130
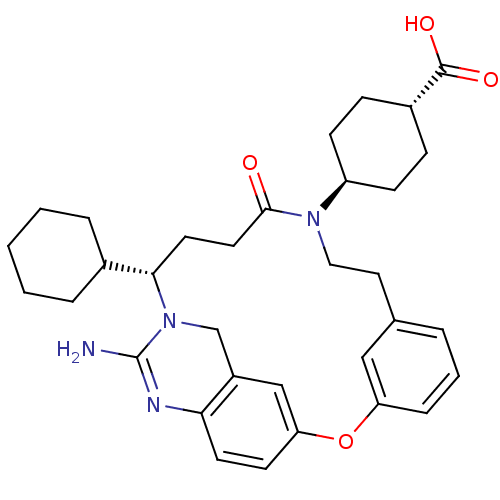
(4-[(14S)-16-amino-14-cyclohexyl-11-oxo-2-oxa-10,15...)Show SMILES NC1=Nc2ccc3Oc4cccc(CCN([C@H]5CC[C@@H](CC5)C(O)=O)C(=O)CC[C@@H](C5CCCCC5)N1Cc2c3)c4 |r,wU:29.30,19.22,wD:16.15,t:1,(5.63,-16.54,;4.29,-15.78,;2.95,-16.55,;1.62,-15.78,;.29,-16.55,;-1.05,-15.78,;-1.04,-14.24,;-2.38,-13.45,;-2.37,-11.98,;-3.7,-11.21,;-3.7,-9.67,;-2.37,-8.9,;-1.04,-9.66,;.39,-8.91,;1.74,-9.72,;3.12,-8.97,;3.15,-7.43,;4.51,-6.7,;4.54,-5.17,;3.23,-4.36,;1.88,-5.09,;1.84,-6.64,;3.27,-2.82,;4.63,-2.08,;1.96,-2.01,;4.45,-9.78,;5.8,-9.04,;4.42,-11.35,;5.73,-12.15,;5.69,-13.6,;6.96,-14.46,;6.98,-15.99,;8.31,-16.74,;9.63,-15.96,;9.62,-14.42,;8.28,-13.66,;4.29,-14.23,;2.95,-13.46,;1.62,-14.23,;.28,-13.47,;-1.03,-11.21,)| Show InChI InChI=1S/C33H42N4O4/c34-33-35-29-14-13-28-20-25(29)21-37(33)30(23-6-2-1-3-7-23)15-16-31(38)36(26-11-9-24(10-12-26)32(39)40)18-17-22-5-4-8-27(19-22)41-28/h4-5,8,13-14,19-20,23-24,26,30H,1-3,6-7,9-12,15-18,21H2,(H2,34,35)(H,39,40)/t24-,26-,30-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 |
Bioorg Med Chem Lett 20: 3158-60 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.097
BindingDB Entry DOI: 10.7270/Q2C53M1S |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Rattus norvegicus (rat)) | BDBM50318484

(2-((1R,6R)-6-Isopropenyl-3-methyl-cyclohex-2-enyl)...)Show SMILES CCCCCc1cc(O)c([C@@H]2C=C(C)CC[C@H]2C(C)=C)c(O)c1 |r,t:11| Show InChI InChI=1S/C21H30O2/c1-5-6-7-8-16-12-19(22)21(20(23)13-16)18-11-15(4)9-10-17(18)14(2)3/h11-13,17-18,22-23H,2,5-10H2,1,3-4H3/t17-,18+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
KannaLife Sciences
Curated by ChEMBL
| Assay Description
Displacement of [3H]-HU-243 from CB1 receptor in Sabra rat brain synaptosomes after 90 mins |
ACS Med Chem Lett 7: 424-8 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00009
BindingDB Entry DOI: 10.7270/Q2BZ680Z |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50060848
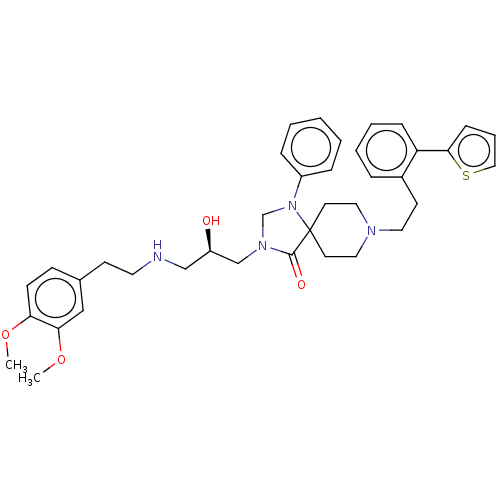
(CHEMBL3394751)Show SMILES COc1ccc(CCNC[C@@H](O)CN2CN(c3ccccc3)C3(CCN(CCc4ccccc4-c4cccs4)CC3)C2=O)cc1OC |r| Show InChI InChI=1S/C38H46N4O4S/c1-45-34-15-14-29(25-35(34)46-2)16-20-39-26-32(43)27-41-28-42(31-10-4-3-5-11-31)38(37(41)44)18-22-40(23-19-38)21-17-30-9-6-7-12-33(30)36-13-8-24-47-36/h3-15,24-25,32,39,43H,16-23,26-28H2,1-2H3/t32-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
West Chester University
Curated by ChEMBL
| Assay Description
Displacement of [125I]-Tyr14-nociceptin from human ORL1 expressed in HEK293 cells after 2 hrs by scintillation counting |
Bioorg Med Chem Lett 25: 602-6 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.015
BindingDB Entry DOI: 10.7270/Q23B61S0 |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50060837
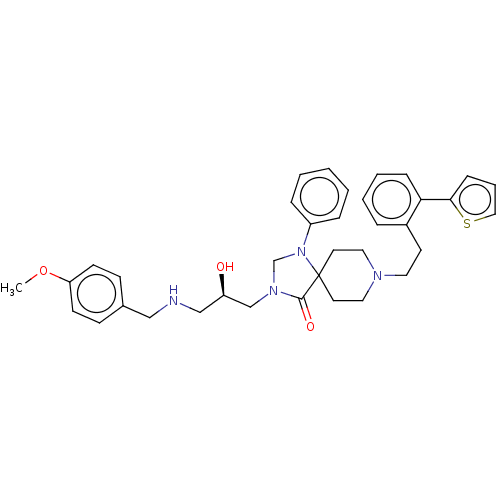
(CHEMBL3394744)Show SMILES COc1ccc(CNC[C@@H](O)CN2CN(c3ccccc3)C3(CCN(CCc4ccccc4-c4cccs4)CC3)C2=O)cc1 |r| Show InChI InChI=1S/C36H42N4O3S/c1-43-32-15-13-28(14-16-32)24-37-25-31(41)26-39-27-40(30-9-3-2-4-10-30)36(35(39)42)18-21-38(22-19-36)20-17-29-8-5-6-11-33(29)34-12-7-23-44-34/h2-16,23,31,37,41H,17-22,24-27H2,1H3/t31-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
West Chester University
Curated by ChEMBL
| Assay Description
Displacement of [125I]-Tyr14-nociceptin from human ORL1 expressed in HEK293 cells after 2 hrs by scintillation counting |
Bioorg Med Chem Lett 25: 602-6 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.015
BindingDB Entry DOI: 10.7270/Q23B61S0 |
More data for this
Ligand-Target Pair | |
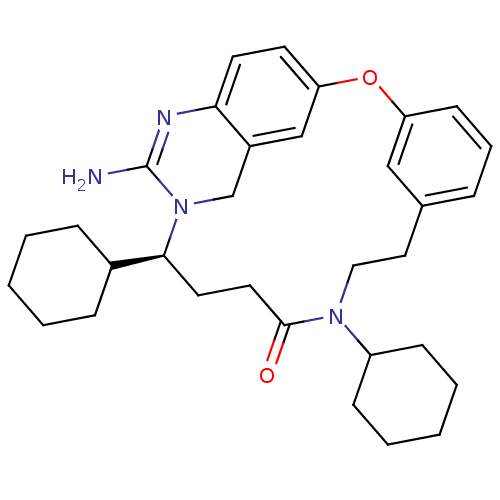
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50318133

((14S)-16-amino-10,14-dicyclohexyl-2-oxa-10,15,17,2...)Show SMILES NC1=Nc2cnc3Oc4cccc(CCN(C5CCCCC5)C(=O)CC[C@@H](C5CCCCC5)N1Cc2c3)c4 |r,t:1| Show InChI InChI=1S/C31H41N5O2/c32-31-34-27-20-33-29-19-24(27)21-36(31)28(23-9-3-1-4-10-23)14-15-30(37)35(25-11-5-2-6-12-25)17-16-22-8-7-13-26(18-22)38-29/h7-8,13,18-20,23,25,28H,1-6,9-12,14-17,21H2,(H2,32,34)/t28-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 assessed as amyloid beta (1-40) production |
Bioorg Med Chem Lett 20: 3158-60 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.097
BindingDB Entry DOI: 10.7270/Q2C53M1S |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50060843
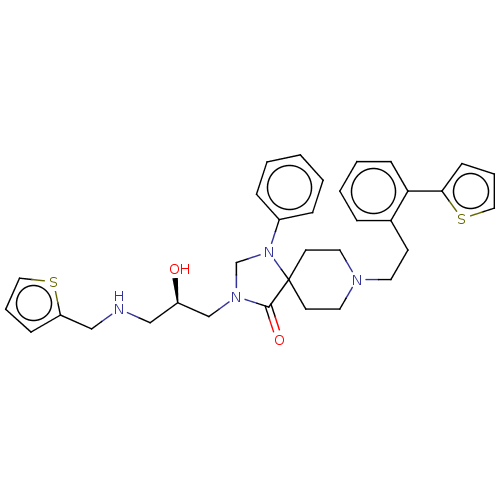
(CHEMBL3394748)Show SMILES O[C@H](CNCc1cccs1)CN1CN(c2ccccc2)C2(CCN(CCc3ccccc3-c3cccs3)CC2)C1=O |r| Show InChI InChI=1S/C33H38N4O2S2/c38-28(22-34-23-29-11-6-20-40-29)24-36-25-37(27-9-2-1-3-10-27)33(32(36)39)15-18-35(19-16-33)17-14-26-8-4-5-12-30(26)31-13-7-21-41-31/h1-13,20-21,28,34,38H,14-19,22-25H2/t28-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
West Chester University
Curated by ChEMBL
| Assay Description
Displacement of [125I]-Tyr14-nociceptin from human ORL1 expressed in HEK293 cells after 2 hrs by scintillation counting |
Bioorg Med Chem Lett 25: 602-6 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.015
BindingDB Entry DOI: 10.7270/Q23B61S0 |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50060893
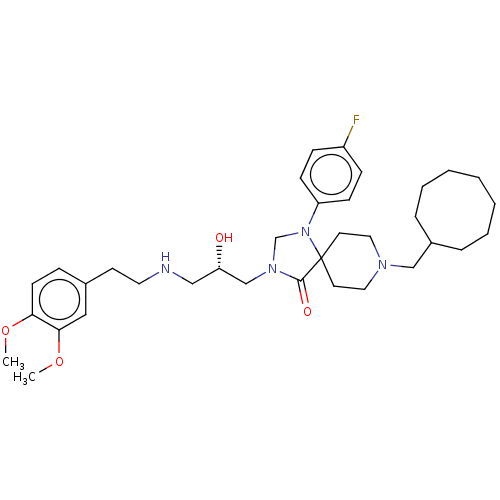
(CHEMBL3394753)Show SMILES COc1ccc(CCNC[C@H](O)CN2CN(c3ccc(F)cc3)C3(CCN(CC4CCCCCCC4)CC3)C2=O)cc1OC |r| Show InChI InChI=1S/C35H51FN4O4/c1-43-32-15-10-27(22-33(32)44-2)16-19-37-23-31(41)25-39-26-40(30-13-11-29(36)12-14-30)35(34(39)42)17-20-38(21-18-35)24-28-8-6-4-3-5-7-9-28/h10-15,22,28,31,37,41H,3-9,16-21,23-26H2,1-2H3/t31-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.80 | n/a | n/a | n/a | n/a | n/a | n/a |
West Chester University
Curated by ChEMBL
| Assay Description
Displacement of [125I]-Tyr14-nociceptin from human ORL1 expressed in HEK293 cells after 2 hrs by scintillation counting |
Bioorg Med Chem Lett 25: 602-6 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.015
BindingDB Entry DOI: 10.7270/Q23B61S0 |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50060844
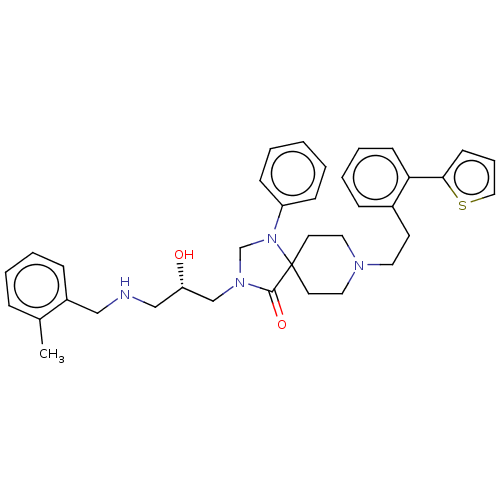
(CHEMBL3394749)Show SMILES Cc1ccccc1CNC[C@H](O)CN1CN(c2ccccc2)C2(CCN(CCc3ccccc3-c3cccs3)CC2)C1=O |r| Show InChI InChI=1S/C36H42N4O2S/c1-28-10-5-6-12-30(28)24-37-25-32(41)26-39-27-40(31-13-3-2-4-14-31)36(35(39)42)18-21-38(22-19-36)20-17-29-11-7-8-15-33(29)34-16-9-23-43-34/h2-16,23,32,37,41H,17-22,24-27H2,1H3/t32-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
West Chester University
Curated by ChEMBL
| Assay Description
Displacement of [125I]-Tyr14-nociceptin from human ORL1 expressed in HEK293 cells after 2 hrs by scintillation counting |
Bioorg Med Chem Lett 25: 602-6 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.015
BindingDB Entry DOI: 10.7270/Q23B61S0 |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50318123

((14S)-16-amino-10,14-dicyclohexyl-2-oxa-10,15,17-t...)Show SMILES NC1=Nc2ccc3Oc4cccc(CCN(C5CCCCC5)C(=O)CC[C@@H](C5CCCCC5)N1Cc2c3)c4 |r,t:1| Show InChI InChI=1S/C32H42N4O2/c33-32-34-29-15-14-28-21-25(29)22-36(32)30(24-9-3-1-4-10-24)16-17-31(37)35(26-11-5-2-6-12-26)19-18-23-8-7-13-27(20-23)38-28/h7-8,13-15,20-21,24,26,30H,1-6,9-12,16-19,22H2,(H2,33,34)/t30-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 assessed as amyloid beta (1-40) production |
Bioorg Med Chem Lett 20: 3158-60 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.097
BindingDB Entry DOI: 10.7270/Q2C53M1S |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50060903
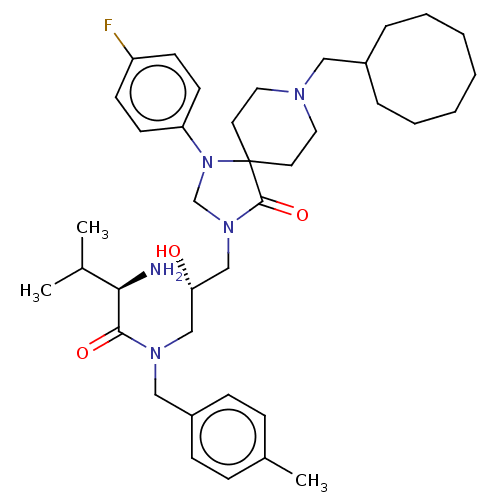
(CHEMBL3394756)Show SMILES CC(C)[C@@H](N)C(=O)N(C[C@H](O)CN1CN(c2ccc(F)cc2)C2(CCN(CC3CCCCCCC3)CC2)C1=O)Cc1ccc(C)cc1 |r| Show InChI InChI=1S/C38H56FN5O3/c1-28(2)35(40)36(46)42(24-31-13-11-29(3)12-14-31)25-34(45)26-43-27-44(33-17-15-32(39)16-18-33)38(37(43)47)19-21-41(22-20-38)23-30-9-7-5-4-6-8-10-30/h11-18,28,30,34-35,45H,4-10,19-27,40H2,1-3H3/t34-,35+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
West Chester University
Curated by ChEMBL
| Assay Description
Displacement of [125I]-Tyr14-nociceptin from human ORL1 expressed in HEK293 cells after 2 hrs by scintillation counting |
Bioorg Med Chem Lett 25: 602-6 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.015
BindingDB Entry DOI: 10.7270/Q23B61S0 |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50060846
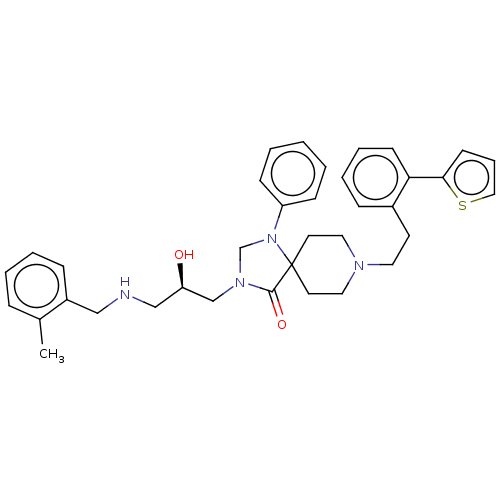
(CHEMBL3394750)Show SMILES Cc1ccccc1CNC[C@@H](O)CN1CN(c2ccccc2)C2(CCN(CCc3ccccc3-c3cccs3)CC2)C1=O |r| Show InChI InChI=1S/C36H42N4O2S/c1-28-10-5-6-12-30(28)24-37-25-32(41)26-39-27-40(31-13-3-2-4-14-31)36(35(39)42)18-21-38(22-19-36)20-17-29-11-7-8-15-33(29)34-16-9-23-43-34/h2-16,23,32,37,41H,17-22,24-27H2,1H3/t32-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
West Chester University
Curated by ChEMBL
| Assay Description
Displacement of [125I]-Tyr14-nociceptin from human ORL1 expressed in HEK293 cells after 2 hrs by scintillation counting |
Bioorg Med Chem Lett 25: 602-6 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.015
BindingDB Entry DOI: 10.7270/Q23B61S0 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50060836
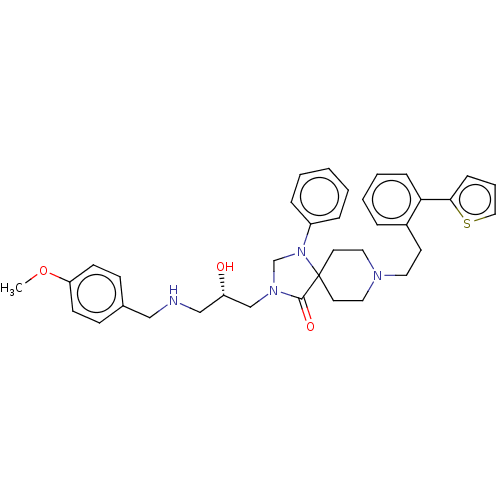
(CHEMBL3394743)Show SMILES COc1ccc(CNC[C@H](O)CN2CN(c3ccccc3)C3(CCN(CCc4ccccc4-c4cccs4)CC3)C2=O)cc1 |r| Show InChI InChI=1S/C36H42N4O3S/c1-43-32-15-13-28(14-16-32)24-37-25-31(41)26-39-27-40(30-9-3-2-4-10-30)36(35(39)42)18-21-38(22-19-36)20-17-29-8-5-6-11-33(29)34-12-7-23-44-34/h2-16,23,31,37,41H,17-22,24-27H2,1H3/t31-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
West Chester University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from human Mu opioid receptor expressed in HEK293 cells after 2 hrs by scintillation counting |
Bioorg Med Chem Lett 25: 602-6 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.015
BindingDB Entry DOI: 10.7270/Q23B61S0 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50060842
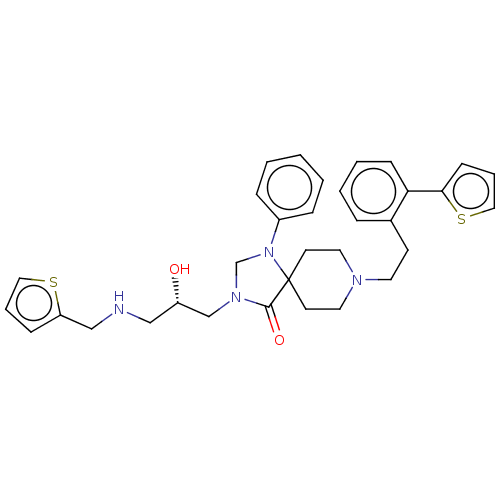
(CHEMBL3394747)Show SMILES O[C@@H](CNCc1cccs1)CN1CN(c2ccccc2)C2(CCN(CCc3ccccc3-c3cccs3)CC2)C1=O |r| Show InChI InChI=1S/C33H38N4O2S2/c38-28(22-34-23-29-11-6-20-40-29)24-36-25-37(27-9-2-1-3-10-27)33(32(36)39)15-18-35(19-16-33)17-14-26-8-4-5-12-30(26)31-13-7-21-41-31/h1-13,20-21,28,34,38H,14-19,22-25H2/t28-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
West Chester University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from human Mu opioid receptor expressed in HEK293 cells after 2 hrs by scintillation counting |
Bioorg Med Chem Lett 25: 602-6 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.015
BindingDB Entry DOI: 10.7270/Q23B61S0 |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50318131

((14S)-16-amino-10,14-dicyclohexyl-20-fluoro-2-oxa-...)Show SMILES NC1=Nc2cc(F)c3Oc4cccc(CCN(C5CCCCC5)C(=O)CC[C@@H](C5CCCCC5)N1Cc2c3)c4 |r,t:1| Show InChI InChI=1S/C32H41FN4O2/c33-27-20-28-24-19-30(27)39-26-13-7-8-22(18-26)16-17-36(25-11-5-2-6-12-25)31(38)15-14-29(23-9-3-1-4-10-23)37(21-24)32(34)35-28/h7-8,13,18-20,23,25,29H,1-6,9-12,14-17,21H2,(H2,34,35)/t29-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 assessed as amyloid beta (1-40) production |
Bioorg Med Chem Lett 20: 3158-60 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.097
BindingDB Entry DOI: 10.7270/Q2C53M1S |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50318127

((15S)-17-amino-11,15-dicyclohexyl-2-oxa-11,16,18-t...)Show SMILES NC1=Nc2ccc3Oc4cccc(CCCN(C5CCCCC5)C(=O)CC[C@@H](C5CCCCC5)N1Cc2c3)c4 |r,t:1| Show InChI InChI=1S/C33H44N4O2/c34-33-35-30-17-16-29-22-26(30)23-37(33)31(25-11-3-1-4-12-25)18-19-32(38)36(27-13-5-2-6-14-27)20-8-10-24-9-7-15-28(21-24)39-29/h7,9,15-17,21-22,25,27,31H,1-6,8,10-14,18-20,23H2,(H2,34,35)/t31-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 assessed as amyloid beta (1-40) production |
Bioorg Med Chem Lett 20: 3158-60 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.097
BindingDB Entry DOI: 10.7270/Q2C53M1S |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50060904
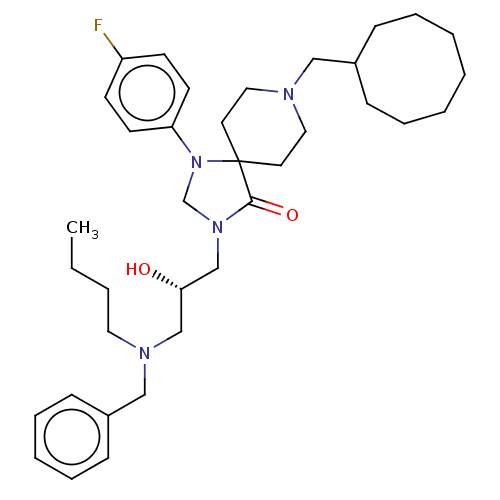
(CHEMBL3394742)Show SMILES CCCCN(C[C@H](O)CN1CN(c2ccc(F)cc2)C2(CCN(CC3CCCCCCC3)CC2)C1=O)Cc1ccccc1 |r| Show InChI InChI=1S/C36H53FN4O2/c1-2-3-22-39(26-31-14-10-7-11-15-31)27-34(42)28-40-29-41(33-18-16-32(37)17-19-33)36(35(40)43)20-23-38(24-21-36)25-30-12-8-5-4-6-9-13-30/h7,10-11,14-19,30,34,42H,2-6,8-9,12-13,20-29H2,1H3/t34-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
West Chester University
Curated by ChEMBL
| Assay Description
Displacement of [125I]-Tyr14-nociceptin from human ORL1 expressed in HEK293 cells after 2 hrs by scintillation counting |
Bioorg Med Chem Lett 25: 602-6 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.015
BindingDB Entry DOI: 10.7270/Q23B61S0 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50060838
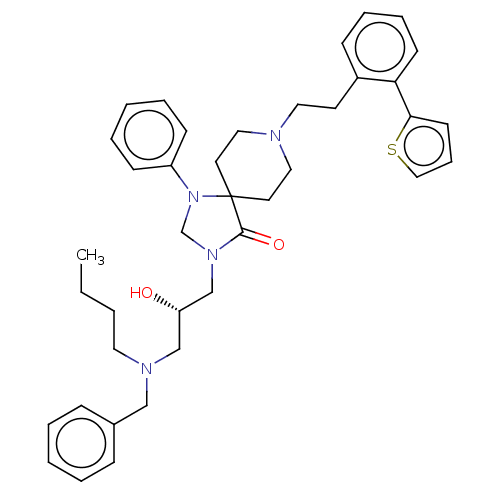
(CHEMBL3394745)Show SMILES CCCCN(C[C@H](O)CN1CN(c2ccccc2)C2(CCN(CCc3ccccc3-c3cccs3)CC2)C1=O)Cc1ccccc1 |r| Show InChI InChI=1S/C39H48N4O2S/c1-2-3-23-41(28-32-13-6-4-7-14-32)29-35(44)30-42-31-43(34-16-8-5-9-17-34)39(38(42)45)21-25-40(26-22-39)24-20-33-15-10-11-18-36(33)37-19-12-27-46-37/h4-19,27,35,44H,2-3,20-26,28-31H2,1H3/t35-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
West Chester University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from human Mu opioid receptor expressed in HEK293 cells after 2 hrs by scintillation counting |
Bioorg Med Chem Lett 25: 602-6 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.015
BindingDB Entry DOI: 10.7270/Q23B61S0 |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50318132

((14S)-16-amino-10,14-dicyclohexyl-20-methoxy-2-oxa...)Show SMILES COc1cc2N=C(N)N3Cc2cc1Oc1cccc(CCN(C2CCCCC2)C(=O)CC[C@H]3C2CCCCC2)c1 |r,t:5| Show InChI InChI=1S/C33H44N4O3/c1-39-30-21-28-25-20-31(30)40-27-14-8-9-23(19-27)17-18-36(26-12-6-3-7-13-26)32(38)16-15-29(24-10-4-2-5-11-24)37(22-25)33(34)35-28/h8-9,14,19-21,24,26,29H,2-7,10-13,15-18,22H2,1H3,(H2,34,35)/t29-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 assessed as amyloid beta (1-40) production |
Bioorg Med Chem Lett 20: 3158-60 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.097
BindingDB Entry DOI: 10.7270/Q2C53M1S |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50060897

(CHEMBL3394754)Show SMILES Cc1cccc(SC[C@H](O)CN2CN(c3ccc(F)cc3)C3(CCN(CC4CCCCCCC4)CC3)C2=O)c1 |r| Show InChI InChI=1S/C32H44FN3O2S/c1-25-8-7-11-30(20-25)39-23-29(37)22-35-24-36(28-14-12-27(33)13-15-28)32(31(35)38)16-18-34(19-17-32)21-26-9-5-3-2-4-6-10-26/h7-8,11-15,20,26,29,37H,2-6,9-10,16-19,21-24H2,1H3/t29-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
West Chester University
Curated by ChEMBL
| Assay Description
Displacement of [125I]-Tyr14-nociceptin from human ORL1 expressed in HEK293 cells after 2 hrs by scintillation counting |
Bioorg Med Chem Lett 25: 602-6 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.015
BindingDB Entry DOI: 10.7270/Q23B61S0 |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50060863

(CHEMBL3394752)Show SMILES CC(C)(C)NC[C@@H](O)CN1CN(c2ccccc2)C2(CCN(CCc3ccccc3-c3cccs3)CC2)C1=O |r| Show InChI InChI=1S/C32H42N4O2S/c1-31(2,3)33-22-27(37)23-35-24-36(26-11-5-4-6-12-26)32(30(35)38)16-19-34(20-17-32)18-15-25-10-7-8-13-28(25)29-14-9-21-39-29/h4-14,21,27,33,37H,15-20,22-24H2,1-3H3/t27-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
West Chester University
Curated by ChEMBL
| Assay Description
Displacement of [125I]-Tyr14-nociceptin from human ORL1 expressed in HEK293 cells after 2 hrs by scintillation counting |
Bioorg Med Chem Lett 25: 602-6 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.015
BindingDB Entry DOI: 10.7270/Q23B61S0 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50060841

(CHEMBL3394746)Show SMILES CCCCN(C[C@@H](O)CN1CN(c2ccccc2)C2(CCN(CCc3ccccc3-c3cccs3)CC2)C1=O)Cc1ccccc1 |r| Show InChI InChI=1S/C39H48N4O2S/c1-2-3-23-41(28-32-13-6-4-7-14-32)29-35(44)30-42-31-43(34-16-8-5-9-17-34)39(38(42)45)21-25-40(26-22-39)24-20-33-15-10-11-18-36(33)37-19-12-27-46-37/h4-19,27,35,44H,2-3,20-26,28-31H2,1H3/t35-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
West Chester University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from human Mu opioid receptor expressed in HEK293 cells after 2 hrs by scintillation counting |
Bioorg Med Chem Lett 25: 602-6 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.015
BindingDB Entry DOI: 10.7270/Q23B61S0 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50060863

(CHEMBL3394752)Show SMILES CC(C)(C)NC[C@@H](O)CN1CN(c2ccccc2)C2(CCN(CCc3ccccc3-c3cccs3)CC2)C1=O |r| Show InChI InChI=1S/C32H42N4O2S/c1-31(2,3)33-22-27(37)23-35-24-36(26-11-5-4-6-12-26)32(30(35)38)16-19-34(20-17-32)18-15-25-10-7-8-13-28(25)29-14-9-21-39-29/h4-14,21,27,33,37H,15-20,22-24H2,1-3H3/t27-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 57 | n/a | n/a | n/a | n/a | n/a | n/a |
West Chester University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from human Mu opioid receptor expressed in HEK293 cells after 2 hrs by scintillation counting |
Bioorg Med Chem Lett 25: 602-6 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.015
BindingDB Entry DOI: 10.7270/Q23B61S0 |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50060836

(CHEMBL3394743)Show SMILES COc1ccc(CNC[C@H](O)CN2CN(c3ccccc3)C3(CCN(CCc4ccccc4-c4cccs4)CC3)C2=O)cc1 |r| Show InChI InChI=1S/C36H42N4O3S/c1-43-32-15-13-28(14-16-32)24-37-25-31(41)26-39-27-40(30-9-3-2-4-10-30)36(35(39)42)18-21-38(22-19-36)20-17-29-8-5-6-11-33(29)34-12-7-23-44-34/h2-16,23,31,37,41H,17-22,24-27H2,1H3/t31-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 61 | n/a | n/a | n/a | n/a | n/a | n/a |
West Chester University
Curated by ChEMBL
| Assay Description
Displacement of [125I]-Tyr14-nociceptin from human ORL1 expressed in HEK293 cells after 2 hrs by scintillation counting |
Bioorg Med Chem Lett 25: 602-6 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.015
BindingDB Entry DOI: 10.7270/Q23B61S0 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50060844

(CHEMBL3394749)Show SMILES Cc1ccccc1CNC[C@H](O)CN1CN(c2ccccc2)C2(CCN(CCc3ccccc3-c3cccs3)CC2)C1=O |r| Show InChI InChI=1S/C36H42N4O2S/c1-28-10-5-6-12-30(28)24-37-25-32(41)26-39-27-40(31-13-3-2-4-14-31)36(35(39)42)18-21-38(22-19-36)20-17-29-11-7-8-15-33(29)34-16-9-23-43-34/h2-16,23,32,37,41H,17-22,24-27H2,1H3/t32-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 69 | n/a | n/a | n/a | n/a | n/a | n/a |
West Chester University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from human Mu opioid receptor expressed in HEK293 cells after 2 hrs by scintillation counting |
Bioorg Med Chem Lett 25: 602-6 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.015
BindingDB Entry DOI: 10.7270/Q23B61S0 |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50318124

((14S)-16-amino-14-cyclohexyl-10-(oxan-4-yl)-2-oxa-...)Show SMILES NC1=Nc2ccc3Oc4cccc(CCN(C5CCOCC5)C(=O)CC[C@@H](C5CCCCC5)N1Cc2c3)c4 |r,t:1| Show InChI InChI=1S/C31H40N4O3/c32-31-33-28-10-9-27-20-24(28)21-35(31)29(23-6-2-1-3-7-23)11-12-30(36)34(25-14-17-37-18-15-25)16-13-22-5-4-8-26(19-22)38-27/h4-5,8-10,19-20,23,25,29H,1-3,6-7,11-18,21H2,(H2,32,33)/t29-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 73 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 assessed as amyloid beta (1-40) production |
Bioorg Med Chem Lett 20: 3158-60 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.097
BindingDB Entry DOI: 10.7270/Q2C53M1S |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50060848

(CHEMBL3394751)Show SMILES COc1ccc(CCNC[C@@H](O)CN2CN(c3ccccc3)C3(CCN(CCc4ccccc4-c4cccs4)CC3)C2=O)cc1OC |r| Show InChI InChI=1S/C38H46N4O4S/c1-45-34-15-14-29(25-35(34)46-2)16-20-39-26-32(43)27-41-28-42(31-10-4-3-5-11-31)38(37(41)44)18-22-40(23-19-38)21-17-30-9-6-7-12-33(30)36-13-8-24-47-36/h3-15,24-25,32,39,43H,16-23,26-28H2,1-2H3/t32-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 79 | n/a | n/a | n/a | n/a | n/a | n/a |
West Chester University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from human Mu opioid receptor expressed in HEK293 cells after 2 hrs by scintillation counting |
Bioorg Med Chem Lett 25: 602-6 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.015
BindingDB Entry DOI: 10.7270/Q23B61S0 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50060843

(CHEMBL3394748)Show SMILES O[C@H](CNCc1cccs1)CN1CN(c2ccccc2)C2(CCN(CCc3ccccc3-c3cccs3)CC2)C1=O |r| Show InChI InChI=1S/C33H38N4O2S2/c38-28(22-34-23-29-11-6-20-40-29)24-36-25-37(27-9-2-1-3-10-27)33(32(36)39)15-18-35(19-16-33)17-14-26-8-4-5-12-30(26)31-13-7-21-41-31/h1-13,20-21,28,34,38H,14-19,22-25H2/t28-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
West Chester University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from human Mu opioid receptor expressed in HEK293 cells after 2 hrs by scintillation counting |
Bioorg Med Chem Lett 25: 602-6 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.015
BindingDB Entry DOI: 10.7270/Q23B61S0 |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50060902
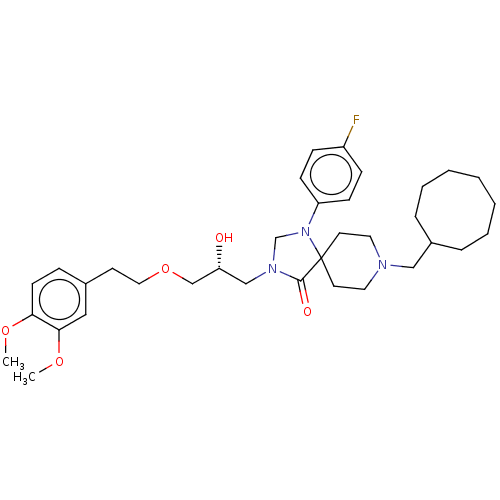
(CHEMBL3394755)Show SMILES COc1ccc(CCOC[C@H](O)CN2CN(c3ccc(F)cc3)C3(CCN(CC4CCCCCCC4)CC3)C2=O)cc1OC |r| Show InChI InChI=1S/C35H50FN3O5/c1-42-32-15-10-27(22-33(32)43-2)16-21-44-25-31(40)24-38-26-39(30-13-11-29(36)12-14-30)35(34(38)41)17-19-37(20-18-35)23-28-8-6-4-3-5-7-9-28/h10-15,22,28,31,40H,3-9,16-21,23-26H2,1-2H3/t31-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 82 | n/a | n/a | n/a | n/a | n/a | n/a |
West Chester University
Curated by ChEMBL
| Assay Description
Displacement of [125I]-Tyr14-nociceptin from human ORL1 expressed in HEK293 cells after 2 hrs by scintillation counting |
Bioorg Med Chem Lett 25: 602-6 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.015
BindingDB Entry DOI: 10.7270/Q23B61S0 |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50318125

((14S)-16-amino-10-cyclohexyl-14-(propan-2-yl)-2-ox...)Show SMILES CC(C)[C@@H]1CCC(=O)N(CCc2cccc(Oc3ccc4N=C(N)N1Cc4c3)c2)C1CCCCC1 |r,t:21| Show InChI InChI=1S/C29H38N4O2/c1-20(2)27-13-14-28(34)32(23-8-4-3-5-9-23)16-15-21-7-6-10-24(17-21)35-25-11-12-26-22(18-25)19-33(27)29(30)31-26/h6-7,10-12,17-18,20,23,27H,3-5,8-9,13-16,19H2,1-2H3,(H2,30,31)/t27-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 assessed as amyloid beta (1-40) production |
Bioorg Med Chem Lett 20: 3158-60 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.097
BindingDB Entry DOI: 10.7270/Q2C53M1S |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50060838

(CHEMBL3394745)Show SMILES CCCCN(C[C@H](O)CN1CN(c2ccccc2)C2(CCN(CCc3ccccc3-c3cccs3)CC2)C1=O)Cc1ccccc1 |r| Show InChI InChI=1S/C39H48N4O2S/c1-2-3-23-41(28-32-13-6-4-7-14-32)29-35(44)30-42-31-43(34-16-8-5-9-17-34)39(38(42)45)21-25-40(26-22-39)24-20-33-15-10-11-18-36(33)37-19-12-27-46-37/h4-19,27,35,44H,2-3,20-26,28-31H2,1H3/t35-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
West Chester University
Curated by ChEMBL
| Assay Description
Displacement of [125I]-Tyr14-nociceptin from human ORL1 expressed in HEK293 cells after 2 hrs by scintillation counting |
Bioorg Med Chem Lett 25: 602-6 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.015
BindingDB Entry DOI: 10.7270/Q23B61S0 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50060846

(CHEMBL3394750)Show SMILES Cc1ccccc1CNC[C@@H](O)CN1CN(c2ccccc2)C2(CCN(CCc3ccccc3-c3cccs3)CC2)C1=O |r| Show InChI InChI=1S/C36H42N4O2S/c1-28-10-5-6-12-30(28)24-37-25-32(41)26-39-27-40(31-13-3-2-4-14-31)36(35(39)42)18-21-38(22-19-36)20-17-29-11-7-8-15-33(29)34-16-9-23-43-34/h2-16,23,32,37,41H,17-22,24-27H2,1H3/t32-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 103 | n/a | n/a | n/a | n/a | n/a | n/a |
West Chester University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from human Mu opioid receptor expressed in HEK293 cells after 2 hrs by scintillation counting |
Bioorg Med Chem Lett 25: 602-6 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.015
BindingDB Entry DOI: 10.7270/Q23B61S0 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50060837

(CHEMBL3394744)Show SMILES COc1ccc(CNC[C@@H](O)CN2CN(c3ccccc3)C3(CCN(CCc4ccccc4-c4cccs4)CC3)C2=O)cc1 |r| Show InChI InChI=1S/C36H42N4O3S/c1-43-32-15-13-28(14-16-32)24-37-25-31(41)26-39-27-40(30-9-3-2-4-10-30)36(35(39)42)18-21-38(22-19-36)20-17-29-8-5-6-11-33(29)34-12-7-23-44-34/h2-16,23,31,37,41H,17-22,24-27H2,1H3/t31-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
West Chester University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from human Mu opioid receptor expressed in HEK293 cells after 2 hrs by scintillation counting |
Bioorg Med Chem Lett 25: 602-6 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.015
BindingDB Entry DOI: 10.7270/Q23B61S0 |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM17786

((4S)-4-(2-amino-6-phenoxy-3,4-dihydroquinazolin-3-...)Show SMILES CN(C1CCCCC1)C(=O)CC[C@@H](C1CCCCC1)N1Cc2cc(Oc3ccccc3)ccc2N=C1N |r,c:38| Show InChI InChI=1S/C31H42N4O2/c1-34(25-13-7-3-8-14-25)30(36)20-19-29(23-11-5-2-6-12-23)35-22-24-21-27(17-18-28(24)33-31(35)32)37-26-15-9-4-10-16-26/h4,9-10,15-18,21,23,25,29H,2-3,5-8,11-14,19-20,22H2,1H3,(H2,32,33)/t29-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | 4.0 | 22 |
Johnson & Johnson Pharmaceutical
| Assay Description
Cathepsin D activity was measured at pH 4 using a FRET peptide substrate. Compounds were preincubated with recombinant human liver cathepsin D for 20... |
J Med Chem 50: 4261-4 (2007)
Article DOI: 10.1021/jm0705408
BindingDB Entry DOI: 10.7270/Q24M92T2 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data