 Found 407 hits with Last Name = 'bylund' and Initial = 'j'
Found 407 hits with Last Name = 'bylund' and Initial = 'j' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Substance-P receptor
(Homo sapiens (Human)) | BDBM50188489
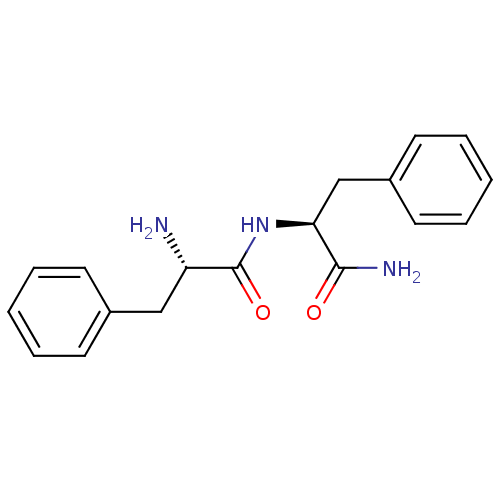
((S)-2-Amino-N-((S)-1-carbamoyl-2-phenyl-ethyl)-3-p...)Show SMILES N[C@@H](Cc1ccccc1)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C18H21N3O2/c19-15(11-13-7-3-1-4-8-13)18(23)21-16(17(20)22)12-14-9-5-2-6-10-14/h1-10,15-16H,11-12,19H2,(H2,20,22)(H,21,23)/t15-,16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
Binding affinity to NK1 receptor (unknown origin) |
ACS Med Chem Lett 5: 1272-7 (2014)
Article DOI: 10.1021/ml5002954
BindingDB Entry DOI: 10.7270/Q2Q52R7V |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50188489

((S)-2-Amino-N-((S)-1-carbamoyl-2-phenyl-ethyl)-3-p...)Show SMILES N[C@@H](Cc1ccccc1)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C18H21N3O2/c19-15(11-13-7-3-1-4-8-13)18(23)21-16(17(20)22)12-14-9-5-2-6-10-14/h1-10,15-16H,11-12,19H2,(H2,20,22)(H,21,23)/t15-,16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
Displacement of [3H]-SP1-7 from NK1 receptor (unknown origin) by scintillation counting analysis |
ACS Med Chem Lett 5: 1272-7 (2014)
Article DOI: 10.1021/ml5002954
BindingDB Entry DOI: 10.7270/Q2Q52R7V |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50436553
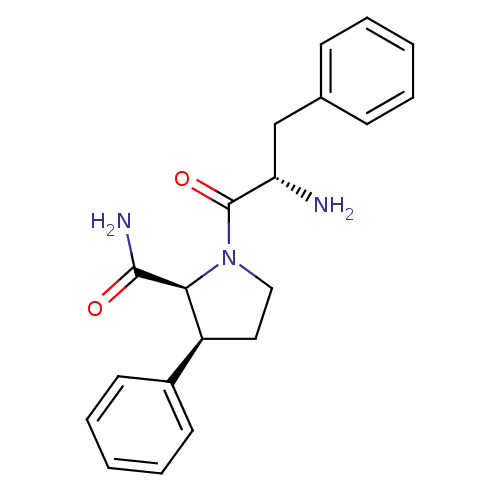
(CHEMBL2396664)Show SMILES N[C@@H](Cc1ccccc1)C(=O)N1CC[C@H]([C@H]1C(N)=O)c1ccccc1 |r| Show InChI InChI=1S/C20H23N3O2/c21-17(13-14-7-3-1-4-8-14)20(25)23-12-11-16(18(23)19(22)24)15-9-5-2-6-10-15/h1-10,16-18H,11-13,21H2,(H2,22,24)/t16-,17-,18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
Displacement of [3H]-SP1-7 from NK1 receptor (unknown origin) by scintillation counting analysis |
ACS Med Chem Lett 5: 1272-7 (2014)
Article DOI: 10.1021/ml5002954
BindingDB Entry DOI: 10.7270/Q2Q52R7V |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50436553

(CHEMBL2396664)Show SMILES N[C@@H](Cc1ccccc1)C(=O)N1CC[C@H]([C@H]1C(N)=O)c1ccccc1 |r| Show InChI InChI=1S/C20H23N3O2/c21-17(13-14-7-3-1-4-8-14)20(25)23-12-11-16(18(23)19(22)24)15-9-5-2-6-10-15/h1-10,16-18H,11-13,21H2,(H2,22,24)/t16-,17-,18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
Binding affinity to NK1 receptor (unknown origin) |
ACS Med Chem Lett 5: 1272-7 (2014)
Article DOI: 10.1021/ml5002954
BindingDB Entry DOI: 10.7270/Q2Q52R7V |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50041558
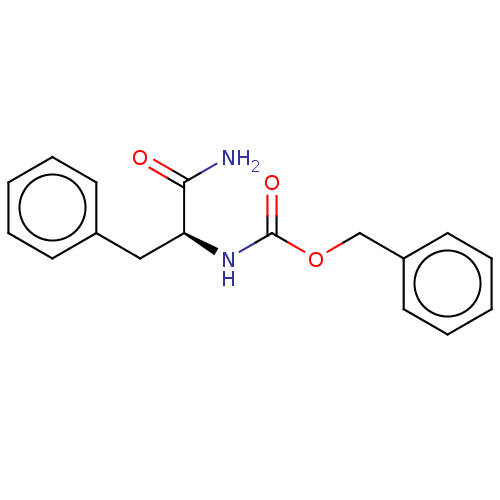
(CHEMBL2042018)Show InChI InChI=1S/C17H18N2O3/c18-16(20)15(11-13-7-3-1-4-8-13)19-17(21)22-12-14-9-5-2-6-10-14/h1-10,15H,11-12H2,(H2,18,20)(H,19,21)/t15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
Binding affinity to NK1 receptor (unknown origin) |
ACS Med Chem Lett 5: 1272-7 (2014)
Article DOI: 10.1021/ml5002954
BindingDB Entry DOI: 10.7270/Q2Q52R7V |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50041558

(CHEMBL2042018)Show InChI InChI=1S/C17H18N2O3/c18-16(20)15(11-13-7-3-1-4-8-13)19-17(21)22-12-14-9-5-2-6-10-14/h1-10,15H,11-12H2,(H2,18,20)(H,19,21)/t15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
Displacement of [3H]-SP1-7 from NK1 receptor (unknown origin) by scintillation counting analysis |
ACS Med Chem Lett 5: 1272-7 (2014)
Article DOI: 10.1021/ml5002954
BindingDB Entry DOI: 10.7270/Q2Q52R7V |
More data for this
Ligand-Target Pair | |
Presenilin-1
(Homo sapiens (Human)) | BDBM50543132

(CHEMBL4646562)Show SMILES [H][C@]12CC[C@]([H])(CN(C1)c1cc(C)ns1)[C@H]2Nc1nc2c(OCC(F)(F)F)ccc(n2n1)C(F)(F)F |r,TLB:9:7:15:3.2| Show InChI InChI=1S/C20H20F6N6OS/c1-10-6-15(34-30-10)31-7-11-2-3-12(8-31)16(11)27-18-28-17-13(33-9-19(21,22)23)4-5-14(20(24,25)26)32(17)29-18/h4-6,11-12,16H,2-3,7-9H2,1H3,(H,27,29)/t11-,12+,16- | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche AG
Curated by ChEMBL
| Assay Description
Modulation of gamma secretase in human H4 cells over expressing human APP695 harboring K595N/M596L Swedish double mutant assessed as reduction in Abe... |
J Med Chem 63: 8534-8553 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00909
BindingDB Entry DOI: 10.7270/Q2HT2SXX |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50391055
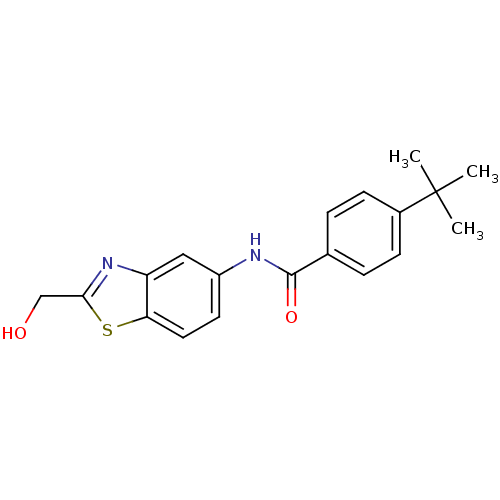
(CHEMBL2088399)Show InChI InChI=1S/C19H20N2O2S/c1-19(2,3)13-6-4-12(5-7-13)18(23)20-14-8-9-16-15(10-14)21-17(11-22)24-16/h4-10,22H,11H2,1-3H3,(H,20,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Antagonist activity at human recombinant TRPV1 receptor assessed as inhibition of capsaicin-induced calcium uptake by FLIPR assay |
Bioorg Med Chem Lett 22: 6205-11 (2012)
Article DOI: 10.1016/j.bmcl.2012.08.018
BindingDB Entry DOI: 10.7270/Q29C6ZGB |
More data for this
Ligand-Target Pair | |
Presenilin-1
(Homo sapiens (Human)) | BDBM50543124

(CHEMBL4635478)Show SMILES [H][C@]12CC[C@]([H])(CN(C1)c1cc(C)ns1)[C@H]2Nc1nc2c(OCC(F)(F)F)cccn2n1 |r,TLB:9:7:15:3.2| Show InChI InChI=1S/C19H21F3N6OS/c1-11-7-15(30-26-11)27-8-12-4-5-13(9-27)16(12)23-18-24-17-14(29-10-19(20,21)22)3-2-6-28(17)25-18/h2-3,6-7,12-13,16H,4-5,8-10H2,1H3,(H,23,25)/t12-,13+,16- | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche AG
Curated by ChEMBL
| Assay Description
Modulation of gamma secretase in human H4 cells over expressing human APP695 harboring K595N/M596L Swedish double mutant assessed as reduction in Abe... |
J Med Chem 63: 8534-8553 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00909
BindingDB Entry DOI: 10.7270/Q2HT2SXX |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50041561
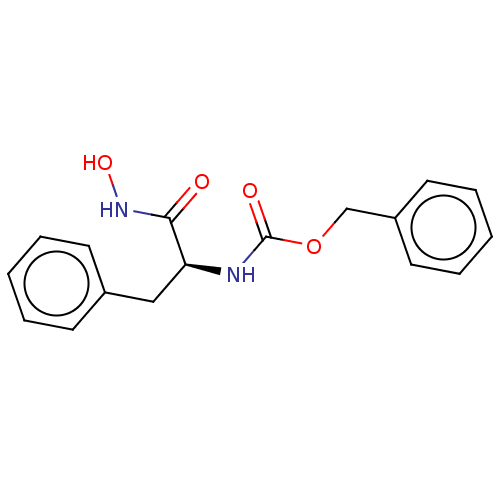
(CHEMBL3358422)Show SMILES ONC(=O)[C@H](Cc1ccccc1)NC(=O)OCc1ccccc1 |r| Show InChI InChI=1S/C17H18N2O4/c20-16(19-22)15(11-13-7-3-1-4-8-13)18-17(21)23-12-14-9-5-2-6-10-14/h1-10,15,22H,11-12H2,(H,18,21)(H,19,20)/t15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
Displacement of [3H]-SP1-7 from NK1 receptor (unknown origin) by scintillation counting analysis |
ACS Med Chem Lett 5: 1272-7 (2014)
Article DOI: 10.1021/ml5002954
BindingDB Entry DOI: 10.7270/Q2Q52R7V |
More data for this
Ligand-Target Pair | |
Presenilin-1
(Homo sapiens (Human)) | BDBM50543117

(CHEMBL4649825)Show SMILES Cc1nsc(n1)N1CC2CCC(C1)C2Nc1nc2c(cccn2n1)-c1ccc(F)c(F)c1F |@@:13,TLB:4:6:13:9.10| Show InChI InChI=1S/C22H20F3N7S/c1-11-26-22(33-30-11)31-9-12-4-5-13(10-31)19(12)27-21-28-20-15(3-2-8-32(20)29-21)14-6-7-16(23)18(25)17(14)24/h2-3,6-8,12-13,19H,4-5,9-10H2,1H3,(H,27,29) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche AG
Curated by ChEMBL
| Assay Description
Modulation of gamma secretase in human H4 cells over expressing human APP695 harboring K595N/M596L Swedish double mutant assessed as reduction in Abe... |
J Med Chem 63: 8534-8553 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00909
BindingDB Entry DOI: 10.7270/Q2HT2SXX |
More data for this
Ligand-Target Pair | |
Presenilin-1
(Homo sapiens (Human)) | BDBM50543125

(CHEMBL4637839)Show SMILES [H][C@]12CC[C@]([H])(CN(C1)c1cc(C)ns1)[C@H]2Nc1nc2c(O[C@@H](C)C(F)(F)F)cccn2n1 |r,TLB:9:7:15:3.2| Show InChI InChI=1S/C20H23F3N6OS/c1-11-8-16(31-27-11)28-9-13-5-6-14(10-28)17(13)24-19-25-18-15(4-3-7-29(18)26-19)30-12(2)20(21,22)23/h3-4,7-8,12-14,17H,5-6,9-10H2,1-2H3,(H,24,26)/t12-,13-,14+,17-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche AG
Curated by ChEMBL
| Assay Description
Modulation of gamma secretase in human H4 cells over expressing human APP695 harboring K595N/M596L Swedish double mutant assessed as reduction in Abe... |
J Med Chem 63: 8534-8553 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00909
BindingDB Entry DOI: 10.7270/Q2HT2SXX |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50041558

(CHEMBL2042018)Show InChI InChI=1S/C17H18N2O3/c18-16(20)15(11-13-7-3-1-4-8-13)19-17(21)22-12-14-9-5-2-6-10-14/h1-10,15H,11-12H2,(H2,18,20)(H,19,21)/t15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
Displacement of [3H]-SP1-7 from NK1 receptor (unknown origin) by scintillation counting analysis |
ACS Med Chem Lett 5: 1272-7 (2014)
Article DOI: 10.1021/ml5002954
BindingDB Entry DOI: 10.7270/Q2Q52R7V |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50041562
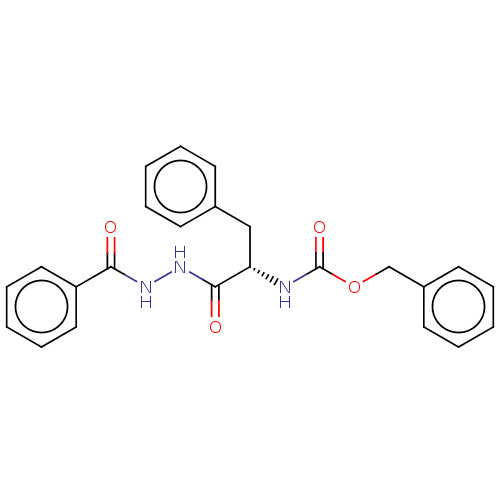
(CHEMBL3358423)Show SMILES O=C(N[C@@H](Cc1ccccc1)C(=O)NNC(=O)c1ccccc1)OCc1ccccc1 |r| Show InChI InChI=1S/C24H23N3O4/c28-22(20-14-8-3-9-15-20)26-27-23(29)21(16-18-10-4-1-5-11-18)25-24(30)31-17-19-12-6-2-7-13-19/h1-15,21H,16-17H2,(H,25,30)(H,26,28)(H,27,29)/t21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
Displacement of [3H]-SP1-7 from NK1 receptor (unknown origin) by scintillation counting analysis |
ACS Med Chem Lett 5: 1272-7 (2014)
Article DOI: 10.1021/ml5002954
BindingDB Entry DOI: 10.7270/Q2Q52R7V |
More data for this
Ligand-Target Pair | |
Presenilin-1
(Homo sapiens (Human)) | BDBM50543131

(CHEMBL4645326)Show SMILES [H][C@]12CC[C@]([H])(CN(C1)c1cc(C)no1)[C@H]2Nc1nc2c(OCC(F)(F)C(F)(F)F)cccn2n1 |r,TLB:9:7:15:3.2| Show InChI InChI=1S/C20H21F5N6O2/c1-11-7-15(33-29-11)30-8-12-4-5-13(9-30)16(12)26-18-27-17-14(3-2-6-31(17)28-18)32-10-19(21,22)20(23,24)25/h2-3,6-7,12-13,16H,4-5,8-10H2,1H3,(H,26,28)/t12-,13+,16- | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche AG
Curated by ChEMBL
| Assay Description
Modulation of gamma secretase in human H4 cells over expressing human APP695 harboring K595N/M596L Swedish double mutant assessed as reduction in Abe... |
J Med Chem 63: 8534-8553 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00909
BindingDB Entry DOI: 10.7270/Q2HT2SXX |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50041563
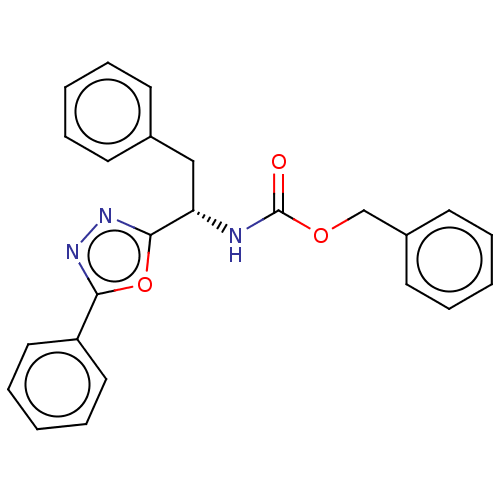
(CHEMBL3358424)Show SMILES O=C(N[C@@H](Cc1ccccc1)c1nnc(o1)-c1ccccc1)OCc1ccccc1 |r| Show InChI InChI=1S/C24H21N3O3/c28-24(29-17-19-12-6-2-7-13-19)25-21(16-18-10-4-1-5-11-18)23-27-26-22(30-23)20-14-8-3-9-15-20/h1-15,21H,16-17H2,(H,25,28)/t21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
Displacement of [3H]-SP1-7 from NK1 receptor (unknown origin) by scintillation counting analysis |
ACS Med Chem Lett 5: 1272-7 (2014)
Article DOI: 10.1021/ml5002954
BindingDB Entry DOI: 10.7270/Q2Q52R7V |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM50418800

(CHEMBL1797395)Show SMILES CCc1onc(c1NC(=O)OCc1c(F)cccc1Cl)-c1c(Cl)cccc1Cl |(12.19,-25.21,;12.82,-26.62,;11.92,-27.87,;10.38,-27.87,;9.9,-29.34,;11.15,-30.25,;12.4,-29.33,;13.86,-29.8,;15.18,-29.01,;15.16,-27.47,;16.53,-29.75,;17.85,-28.96,;19.2,-29.71,;19.21,-31.24,;17.89,-32.03,;20.56,-31.99,;21.88,-31.2,;21.85,-29.65,;20.5,-28.91,;20.47,-27.37,;11.16,-31.79,;9.83,-32.56,;8.49,-31.79,;9.83,-34.1,;11.16,-34.87,;12.5,-34.1,;12.49,-32.55,;13.82,-31.78,)| Show InChI InChI=1S/C19H14Cl3FN2O3/c1-2-15-17(18(25-28-15)16-12(21)6-3-7-13(16)22)24-19(26)27-9-10-11(20)5-4-8-14(10)23/h3-8H,2,9H2,1H3,(H,24,26) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.33 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of Nav1.7 by cell based whole-cell voltage clamp electrophysiology assay |
Bioorg Med Chem Lett 21: 3871-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.041
BindingDB Entry DOI: 10.7270/Q21837RK |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
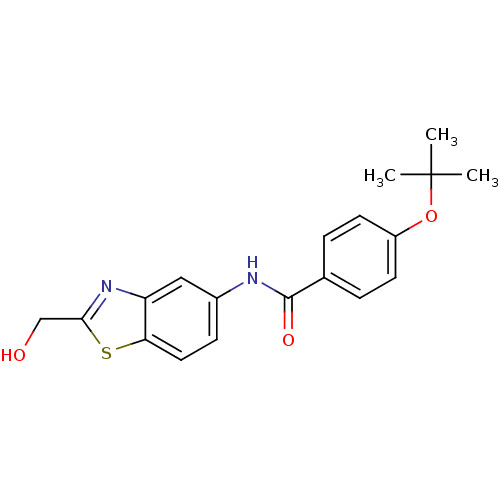
(Homo sapiens (Human)) | BDBM50391031
(CHEMBL2088393)Show InChI InChI=1S/C19H20N2O3S/c1-19(2,3)24-14-7-4-12(5-8-14)18(23)20-13-6-9-16-15(10-13)21-17(11-22)25-16/h4-10,22H,11H2,1-3H3,(H,20,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.80 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Antagonist activity at human recombinant TRPV1 receptor assessed as inhibition of capsaicin-induced calcium uptake by FLIPR assay |
Bioorg Med Chem Lett 22: 6205-11 (2012)
Article DOI: 10.1016/j.bmcl.2012.08.018
BindingDB Entry DOI: 10.7270/Q29C6ZGB |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM50418784
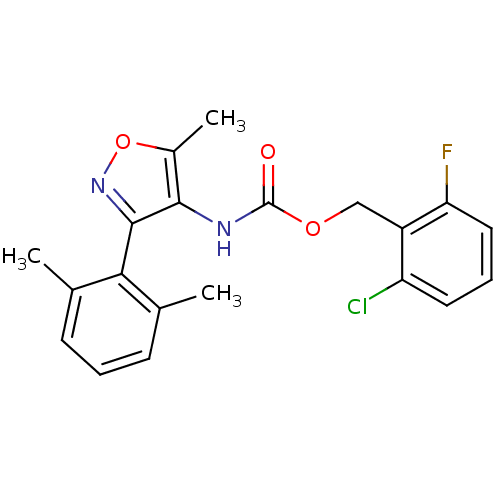
(CHEMBL1797399)Show SMILES Cc1onc(c1NC(=O)OCc1c(F)cccc1Cl)-c1c(C)cccc1C |(33.16,-37.18,;32.26,-38.43,;30.72,-38.43,;30.24,-39.9,;31.49,-40.81,;32.74,-39.89,;34.2,-40.36,;35.52,-39.57,;35.49,-38.03,;36.87,-40.31,;38.19,-39.52,;39.54,-40.27,;39.55,-41.81,;38.23,-42.6,;40.9,-42.55,;42.22,-41.76,;42.19,-40.21,;40.84,-39.47,;40.81,-37.93,;31.5,-42.35,;30.17,-43.12,;28.83,-42.35,;30.17,-44.66,;31.5,-45.44,;32.84,-44.66,;32.83,-43.11,;34.16,-42.34,)| Show InChI InChI=1S/C20H18ClFN2O3/c1-11-6-4-7-12(2)17(11)19-18(13(3)27-24-19)23-20(25)26-10-14-15(21)8-5-9-16(14)22/h4-9H,10H2,1-3H3,(H,23,25) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10.5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of Nav1.7 by cell based whole-cell voltage clamp electrophysiology assay |
Bioorg Med Chem Lett 21: 3871-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.041
BindingDB Entry DOI: 10.7270/Q21837RK |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50391029
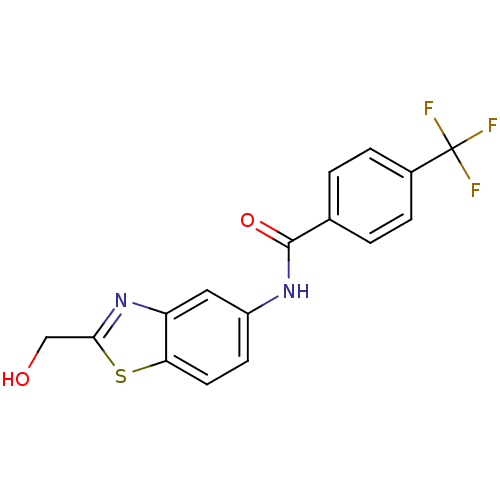
(CHEMBL2088391)Show InChI InChI=1S/C16H11F3N2O2S/c17-16(18,19)10-3-1-9(2-4-10)15(23)20-11-5-6-13-12(7-11)21-14(8-22)24-13/h1-7,22H,8H2,(H,20,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Antagonist activity at human recombinant TRPV1 receptor assessed as inhibition of capsaicin-induced calcium uptake by FLIPR assay |
Bioorg Med Chem Lett 22: 6205-11 (2012)
Article DOI: 10.1016/j.bmcl.2012.08.018
BindingDB Entry DOI: 10.7270/Q29C6ZGB |
More data for this
Ligand-Target Pair | |
Presenilin-1
(Homo sapiens (Human)) | BDBM50543122

(CHEMBL4638946)Show SMILES [H][C@]12CC[C@]([H])(CN(C1)c1nc(C)ns1)[C@H]2Nc1nc2c(OCCCC(F)(F)F)cccn2n1 |r,TLB:9:7:15:3.2| Show InChI InChI=1S/C20H24F3N7OS/c1-12-24-19(32-28-12)29-10-13-5-6-14(11-29)16(13)25-18-26-17-15(4-2-8-30(17)27-18)31-9-3-7-20(21,22)23/h2,4,8,13-14,16H,3,5-7,9-11H2,1H3,(H,25,27)/t13-,14+,16- | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche AG
Curated by ChEMBL
| Assay Description
Modulation of gamma secretase in human H4 cells over expressing human APP695 harboring K595N/M596L Swedish double mutant assessed as reduction in Abe... |
J Med Chem 63: 8534-8553 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00909
BindingDB Entry DOI: 10.7270/Q2HT2SXX |
More data for this
Ligand-Target Pair | |
Presenilin-1
(Homo sapiens (Human)) | BDBM50543121
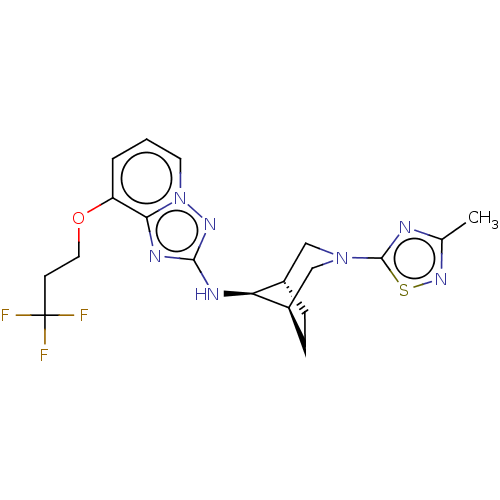
(CHEMBL4638642)Show SMILES [H][C@]12CC[C@]([H])(CN(C1)c1nc(C)ns1)[C@H]2Nc1nc2c(OCCC(F)(F)F)cccn2n1 |r,TLB:9:7:15:3.2| Show InChI InChI=1S/C19H22F3N7OS/c1-11-23-18(31-27-11)28-9-12-4-5-13(10-28)15(12)24-17-25-16-14(3-2-7-29(16)26-17)30-8-6-19(20,21)22/h2-3,7,12-13,15H,4-6,8-10H2,1H3,(H,24,26)/t12-,13+,15- | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche AG
Curated by ChEMBL
| Assay Description
Modulation of gamma secretase in human H4 cells over expressing human APP695 harboring K595N/M596L Swedish double mutant assessed as reduction in Abe... |
J Med Chem 63: 8534-8553 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00909
BindingDB Entry DOI: 10.7270/Q2HT2SXX |
More data for this
Ligand-Target Pair | |
Presenilin-1
(Homo sapiens (Human)) | BDBM50543123

(CHEMBL4647782)Show SMILES [H][C@]12CC[C@]([H])(CN(C1)c1nc(C)ns1)[C@H]2Nc1nc2c(O[C@@H](C)C(F)(F)F)cccn2n1 |r,TLB:9:7:15:3.2| Show InChI InChI=1S/C19H22F3N7OS/c1-10(19(20,21)22)30-14-4-3-7-29-16(14)25-17(26-29)24-15-12-5-6-13(15)9-28(8-12)18-23-11(2)27-31-18/h3-4,7,10,12-13,15H,5-6,8-9H2,1-2H3,(H,24,26)/t10-,12-,13+,15-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche AG
Curated by ChEMBL
| Assay Description
Modulation of gamma secretase in human H4 cells over expressing human APP695 harboring K595N/M596L Swedish double mutant assessed as reduction in Abe... |
J Med Chem 63: 8534-8553 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00909
BindingDB Entry DOI: 10.7270/Q2HT2SXX |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50041559
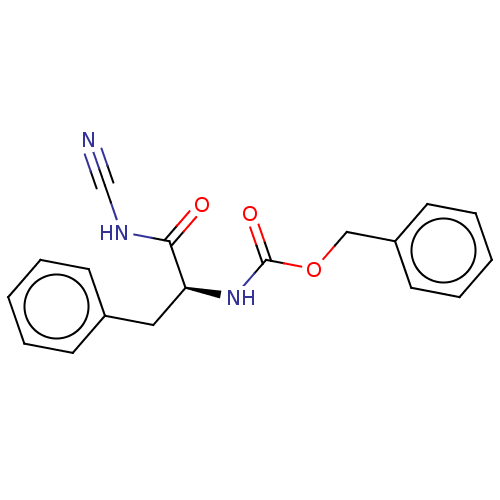
(CHEMBL3358420)Show SMILES O=C(N[C@@H](Cc1ccccc1)C(=O)NC#N)OCc1ccccc1 |r| Show InChI InChI=1S/C18H17N3O3/c19-13-20-17(22)16(11-14-7-3-1-4-8-14)21-18(23)24-12-15-9-5-2-6-10-15/h1-10,16H,11-12H2,(H,20,22)(H,21,23)/t16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
Displacement of [3H]-SP1-7 from NK1 receptor (unknown origin) by scintillation counting analysis |
ACS Med Chem Lett 5: 1272-7 (2014)
Article DOI: 10.1021/ml5002954
BindingDB Entry DOI: 10.7270/Q2Q52R7V |
More data for this
Ligand-Target Pair | |
Presenilin-1
(Homo sapiens (Human)) | BDBM50543130

(CHEMBL4639905)Show SMILES [H][C@]12CC[C@]([H])(CN(C1)c1nc(C)no1)[C@H]2Nc1nc2c(OCC(F)(F)C(F)(F)F)cccn2n1 |r,TLB:9:7:15:3.2| Show InChI InChI=1S/C19H20F5N7O2/c1-10-25-17(33-29-10)30-7-11-4-5-12(8-30)14(11)26-16-27-15-13(3-2-6-31(15)28-16)32-9-18(20,21)19(22,23)24/h2-3,6,11-12,14H,4-5,7-9H2,1H3,(H,26,28)/t11-,12+,14- | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche AG
Curated by ChEMBL
| Assay Description
Modulation of gamma secretase in human H4 cells over expressing human APP695 harboring K595N/M596L Swedish double mutant assessed as reduction in Abe... |
J Med Chem 63: 8534-8553 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00909
BindingDB Entry DOI: 10.7270/Q2HT2SXX |
More data for this
Ligand-Target Pair | |
Presenilin-1
(Homo sapiens (Human)) | BDBM50543120

(CHEMBL4639184)Show SMILES [H][C@]12CC[C@]([H])(CN(C1)c1nc(C)ns1)[C@H]2Nc1nc2c(OCC(F)(F)F)cccn2n1 |r,TLB:9:7:15:3.2| Show InChI InChI=1S/C18H20F3N7OS/c1-10-22-17(30-26-10)27-7-11-4-5-12(8-27)14(11)23-16-24-15-13(29-9-18(19,20)21)3-2-6-28(15)25-16/h2-3,6,11-12,14H,4-5,7-9H2,1H3,(H,23,25)/t11-,12+,14- | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche AG
Curated by ChEMBL
| Assay Description
Modulation of gamma secretase in human H4 cells over expressing human APP695 harboring K595N/M596L Swedish double mutant assessed as reduction in Abe... |
J Med Chem 63: 8534-8553 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00909
BindingDB Entry DOI: 10.7270/Q2HT2SXX |
More data for this
Ligand-Target Pair | |
Presenilin-1
(Homo sapiens (Human)) | BDBM50543135
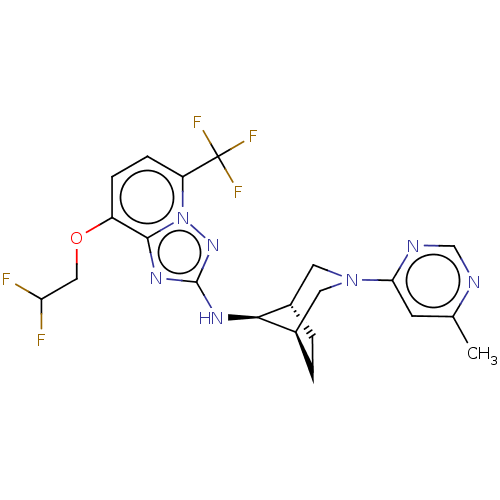
(CHEMBL4642178)Show SMILES [H][C@]12CC[C@]([H])(CN(C1)c1cc(C)ncn1)[C@H]2Nc1nc2c(OCC(F)F)ccc(n2n1)C(F)(F)F |r,TLB:9:7:16:3.2| Show InChI InChI=1S/C21H22F5N7O/c1-11-6-17(28-10-27-11)32-7-12-2-3-13(8-32)18(12)29-20-30-19-14(34-9-16(22)23)4-5-15(21(24,25)26)33(19)31-20/h4-6,10,12-13,16,18H,2-3,7-9H2,1H3,(H,29,31)/t12-,13+,18- | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche AG
Curated by ChEMBL
| Assay Description
Modulation of gamma secretase in human H4 cells over expressing human APP695 harboring K595N/M596L Swedish double mutant assessed as reduction in Abe... |
J Med Chem 63: 8534-8553 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00909
BindingDB Entry DOI: 10.7270/Q2HT2SXX |
More data for this
Ligand-Target Pair | |
Prostaglandin E synthase 2
(Homo sapiens (Human)) | BDBM181966
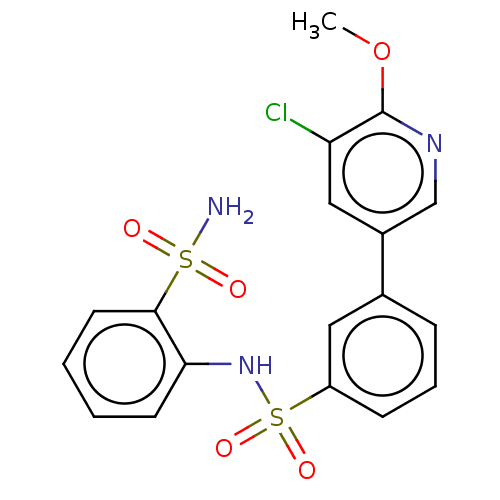
(US9145380, 169)Show SMILES COc1ncc(cc1Cl)-c1cccc(c1)S(=O)(=O)Nc1ccccc1S(N)(=O)=O Show InChI InChI=1S/C18H16ClN3O5S2/c1-27-18-15(19)10-13(11-21-18)12-5-4-6-14(9-12)29(25,26)22-16-7-2-3-8-17(16)28(20,23)24/h2-11,22H,1H3,(H2,20,23,24) | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | 6.8 | n/a |
Astrazeneca AB
US Patent
| Assay Description
A solution of test compound was added to a diluted microsome preparation containing human mPGES-1 and pre-incubated for 15 minutes in potassium phosp... |
US Patent US9145380 (2015)
BindingDB Entry DOI: 10.7270/Q2XG9PXM |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM50418789
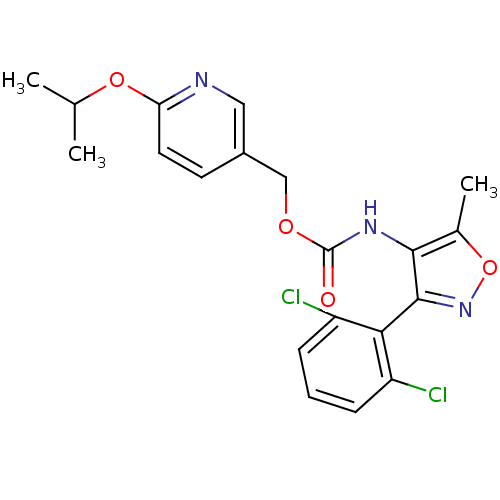
(CHEMBL1797391)Show SMILES CC(C)Oc1ccc(COC(=O)Nc2c(C)onc2-c2c(Cl)cccc2Cl)cn1 |(7.49,-20.61,;6.15,-19.87,;6.12,-18.33,;4.83,-20.66,;3.48,-19.92,;3.45,-18.37,;2.1,-17.63,;.79,-18.43,;-.55,-17.68,;-1.87,-18.47,;-3.22,-17.73,;-3.25,-16.19,;-4.54,-18.52,;-6.01,-18.05,;-6.49,-16.59,;-5.58,-15.34,;-8.03,-16.59,;-8.5,-18.06,;-7.25,-18.97,;-7.24,-20.51,;-8.57,-21.28,;-9.91,-20.51,;-8.58,-22.82,;-7.24,-23.6,;-5.9,-22.82,;-5.91,-21.27,;-4.58,-20.5,;.81,-19.97,;2.16,-20.71,)| Show InChI InChI=1S/C20H19Cl2N3O4/c1-11(2)28-16-8-7-13(9-23-16)10-27-20(26)24-18-12(3)29-25-19(18)17-14(21)5-4-6-15(17)22/h4-9,11H,10H2,1-3H3,(H,24,26) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 22.9 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of Nav1.7 by cell based whole-cell voltage clamp electrophysiology assay |
Bioorg Med Chem Lett 21: 3871-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.041
BindingDB Entry DOI: 10.7270/Q21837RK |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM50418791
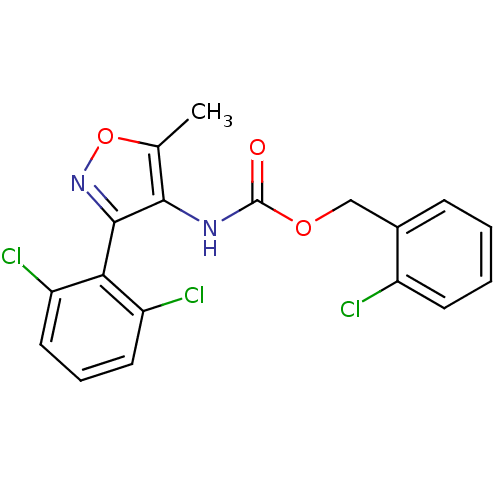
(CHEMBL1797388)Show SMILES Cc1onc(c1NC(=O)OCc1ccccc1Cl)-c1c(Cl)cccc1Cl |(15.73,5.12,;14.83,3.87,;13.29,3.87,;12.82,2.4,;14.07,1.49,;15.31,2.41,;16.78,1.94,;18.1,2.73,;18.07,4.27,;19.45,1.99,;20.77,2.78,;22.11,2.04,;22.13,.5,;23.48,-.25,;24.8,.54,;24.77,2.09,;23.42,2.83,;23.38,4.37,;14.07,-.05,;12.75,-.82,;11.41,-.05,;12.74,-2.36,;14.08,-3.13,;15.41,-2.36,;15.41,-.81,;16.74,-.03,)| Show InChI InChI=1S/C18H13Cl3N2O3/c1-10-16(17(23-26-10)15-13(20)7-4-8-14(15)21)22-18(24)25-9-11-5-2-3-6-12(11)19/h2-8H,9H2,1H3,(H,22,24) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 24.0 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of Nav1.7 by cell based whole-cell voltage clamp electrophysiology assay |
Bioorg Med Chem Lett 21: 3871-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.041
BindingDB Entry DOI: 10.7270/Q21837RK |
More data for this
Ligand-Target Pair | |
Presenilin-1
(Homo sapiens (Human)) | BDBM50543128

(CHEMBL4638937)Show SMILES [H][C@]12CC[C@]([H])(CN(C1)c1ccnc(c1)C(F)(F)F)[C@H]2Nc1nc2c(OCC(F)(F)F)cccn2n1 |r,TLB:9:7:19:3.2| Show InChI InChI=1S/C21H20F6N6O/c22-20(23,24)11-34-15-2-1-7-33-18(15)30-19(31-33)29-17-12-3-4-13(17)10-32(9-12)14-5-6-28-16(8-14)21(25,26)27/h1-2,5-8,12-13,17H,3-4,9-11H2,(H,29,31)/t12-,13+,17- | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche AG
Curated by ChEMBL
| Assay Description
Modulation of gamma secretase in human H4 cells over expressing human APP695 harboring K595N/M596L Swedish double mutant assessed as reduction in Abe... |
J Med Chem 63: 8534-8553 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00909
BindingDB Entry DOI: 10.7270/Q2HT2SXX |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50391025
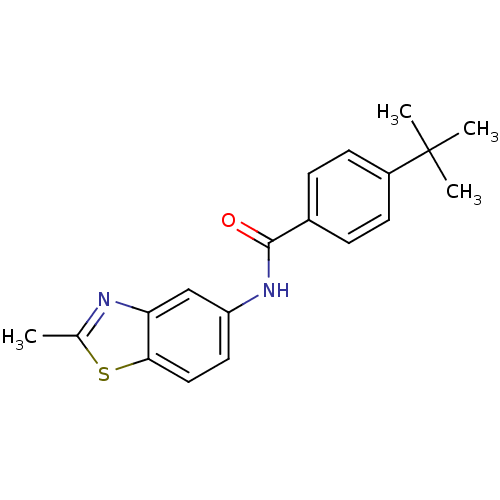
(CHEMBL2088398)Show InChI InChI=1S/C19H20N2OS/c1-12-20-16-11-15(9-10-17(16)23-12)21-18(22)13-5-7-14(8-6-13)19(2,3)4/h5-11H,1-4H3,(H,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Antagonist activity at human recombinant TRPV1 receptor assessed as inhibition of capsaicin-induced calcium uptake by FLIPR assay |
Bioorg Med Chem Lett 22: 6205-11 (2012)
Article DOI: 10.1016/j.bmcl.2012.08.018
BindingDB Entry DOI: 10.7270/Q29C6ZGB |
More data for this
Ligand-Target Pair | |
Presenilin-1
(Homo sapiens (Human)) | BDBM50543134

(CHEMBL4645689)Show SMILES [H][C@]12CC[C@]([H])(CN(C1)c1nnc(C)o1)[C@H]2Nc1nc2c(OC(C)C(F)(F)F)ccc(n2n1)C(F)(F)F |r,TLB:9:7:15:3.2| Show InChI InChI=1S/C20H21F6N7O2/c1-9(19(21,22)23)34-13-5-6-14(20(24,25)26)33-16(13)28-17(31-33)27-15-11-3-4-12(15)8-32(7-11)18-30-29-10(2)35-18/h5-6,9,11-12,15H,3-4,7-8H2,1-2H3,(H,27,31)/t9?,11-,12+,15- | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche AG
Curated by ChEMBL
| Assay Description
Modulation of gamma secretase in human H4 cells over expressing human APP695 harboring K595N/M596L Swedish double mutant assessed as reduction in Abe... |
J Med Chem 63: 8534-8553 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00909
BindingDB Entry DOI: 10.7270/Q2HT2SXX |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50391027
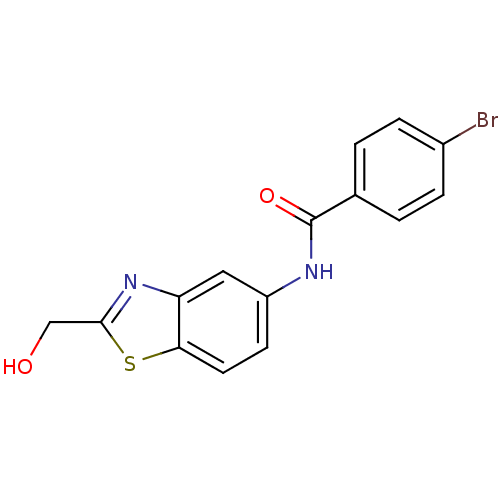
(CHEMBL2088401)Show InChI InChI=1S/C15H11BrN2O2S/c16-10-3-1-9(2-4-10)15(20)17-11-5-6-13-12(7-11)18-14(8-19)21-13/h1-7,19H,8H2,(H,17,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Antagonist activity at human recombinant TRPV1 receptor assessed as inhibition of capsaicin-induced calcium uptake by FLIPR assay |
Bioorg Med Chem Lett 22: 6205-11 (2012)
Article DOI: 10.1016/j.bmcl.2012.08.018
BindingDB Entry DOI: 10.7270/Q29C6ZGB |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM50418774

(CHEMBL1797387)Show SMILES Cc1onc(c1NC(=O)OCc1c(F)cccc1Cl)-c1c(Cl)cccc1Cl |(-4.41,5.18,;-5.31,3.94,;-6.85,3.93,;-7.32,2.47,;-6.08,1.56,;-4.83,2.47,;-3.36,2,;-2.04,2.8,;-2.07,4.34,;-.7,2.05,;.62,2.84,;1.97,2.1,;1.99,.56,;.66,-.23,;3.33,-.19,;4.65,.61,;4.62,2.15,;3.27,2.89,;3.24,4.43,;-6.07,.02,;-7.4,-.75,;-8.73,.02,;-7.4,-2.3,;-6.07,-3.07,;-4.73,-2.3,;-4.74,-.75,;-3.4,.03,)| Show InChI InChI=1S/C18H12Cl3FN2O3/c1-9-16(17(24-27-9)15-12(20)5-2-6-13(15)21)23-18(25)26-8-10-11(19)4-3-7-14(10)22/h2-7H,8H2,1H3,(H,23,25) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 28.2 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of Nav1.7 by cell based whole-cell voltage clamp electrophysiology assay |
Bioorg Med Chem Lett 21: 3871-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.041
BindingDB Entry DOI: 10.7270/Q21837RK |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
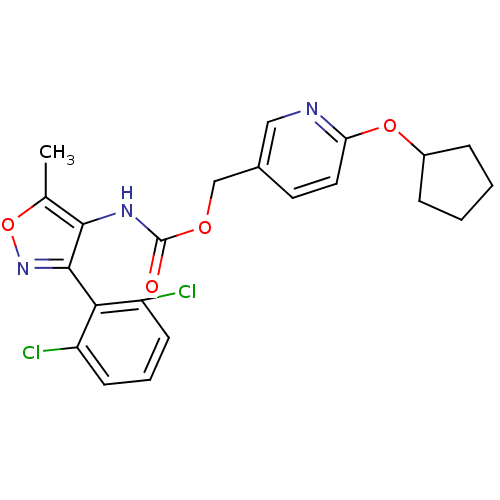
(Homo sapiens (Human)) | BDBM50418788
(CHEMBL1797392)Show SMILES Cc1onc(c1NC(=O)OCc1ccc(OC2CCCC2)nc1)-c1c(Cl)cccc1Cl |(15.59,-15.68,;14.69,-16.93,;13.15,-16.94,;12.67,-18.4,;13.92,-19.31,;15.17,-18.39,;16.63,-18.86,;17.95,-18.07,;17.92,-16.53,;19.3,-18.82,;20.62,-18.02,;21.97,-18.77,;23.27,-17.97,;24.62,-18.71,;24.65,-20.26,;26,-21.01,;27.32,-20.21,;28.78,-20.7,;29.69,-19.46,;28.8,-18.2,;27.33,-18.67,;23.33,-21.05,;21.98,-20.31,;13.93,-20.85,;12.6,-21.62,;11.26,-20.85,;12.6,-23.17,;13.93,-23.94,;15.27,-23.17,;15.26,-21.62,;16.59,-20.84,)| Show InChI InChI=1S/C22H21Cl2N3O4/c1-13-20(21(27-31-13)19-16(23)7-4-8-17(19)24)26-22(28)29-12-14-9-10-18(25-11-14)30-15-5-2-3-6-15/h4,7-11,15H,2-3,5-6,12H2,1H3,(H,26,28) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 28.8 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of Nav1.7 by cell based whole-cell voltage clamp electrophysiology assay |
Bioorg Med Chem Lett 21: 3871-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.041
BindingDB Entry DOI: 10.7270/Q21837RK |
More data for this
Ligand-Target Pair | |
Presenilin-1
(Homo sapiens (Human)) | BDBM50543136
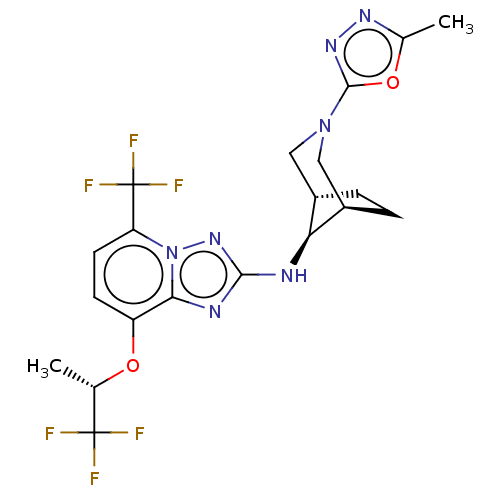
(CHEMBL4638913)Show SMILES [H][C@]12CC[C@]([H])(CN(C1)c1nnc(C)o1)[C@H]2Nc1nc2c(O[C@@H](C)C(F)(F)F)ccc(n2n1)C(F)(F)F |r,TLB:9:7:15:3.2| Show InChI InChI=1S/C20H21F6N7O2/c1-9(19(21,22)23)34-13-5-6-14(20(24,25)26)33-16(13)28-17(31-33)27-15-11-3-4-12(15)8-32(7-11)18-30-29-10(2)35-18/h5-6,9,11-12,15H,3-4,7-8H2,1-2H3,(H,27,31)/t9-,11-,12+,15-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche AG
Curated by ChEMBL
| Assay Description
Modulation of gamma secretase in human H4 cells over expressing human APP695 harboring K595N/M596L Swedish double mutant assessed as reduction in Abe... |
J Med Chem 63: 8534-8553 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00909
BindingDB Entry DOI: 10.7270/Q2HT2SXX |
More data for this
Ligand-Target Pair | |
Prostaglandin E synthase 2
(Homo sapiens (Human)) | BDBM182037
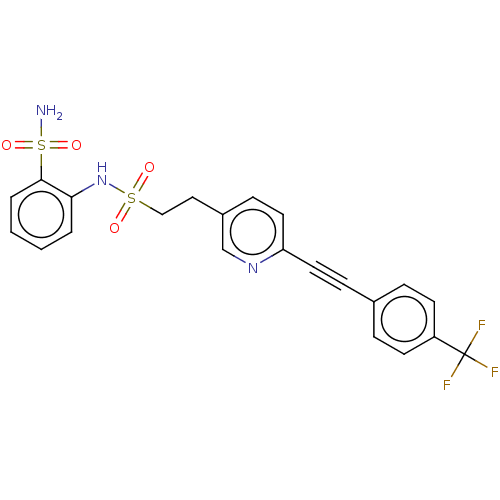
(US9145380, 240)Show SMILES NS(=O)(=O)c1ccccc1NS(=O)(=O)CCc1ccc(nc1)C#Cc1ccc(cc1)C(F)(F)F Show InChI InChI=1S/C22H18F3N3O4S2/c23-22(24,25)18-9-5-16(6-10-18)7-11-19-12-8-17(15-27-19)13-14-33(29,30)28-20-3-1-2-4-21(20)34(26,31)32/h1-6,8-10,12,15,28H,13-14H2,(H2,26,31,32) | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 31 | n/a | n/a | n/a | n/a | 6.8 | n/a |
Astrazeneca AB
US Patent
| Assay Description
A solution of test compound was added to a diluted microsome preparation containing human mPGES-1 and pre-incubated for 15 minutes in potassium phosp... |
US Patent US9145380 (2015)
BindingDB Entry DOI: 10.7270/Q2XG9PXM |
More data for this
Ligand-Target Pair | |
Presenilin-1
(Homo sapiens (Human)) | BDBM50543133

(CHEMBL4649550)Show SMILES [H][C@]12CC[C@]([H])(CN(C1)c1nc(C)no1)[C@H]2Nc1nc2c(OCC(F)(F)F)ccc(n2n1)C(F)(F)F |r,TLB:9:7:15:3.2| Show InChI InChI=1S/C19H19F6N7O2/c1-9-26-17(34-30-9)31-6-10-2-3-11(7-31)14(10)27-16-28-15-12(33-8-18(20,21)22)4-5-13(19(23,24)25)32(15)29-16/h4-5,10-11,14H,2-3,6-8H2,1H3,(H,27,29)/t10-,11+,14- | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche AG
Curated by ChEMBL
| Assay Description
Modulation of gamma secretase in human H4 cells over expressing human APP695 harboring K595N/M596L Swedish double mutant assessed as reduction in Abe... |
J Med Chem 63: 8534-8553 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00909
BindingDB Entry DOI: 10.7270/Q2HT2SXX |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50391032
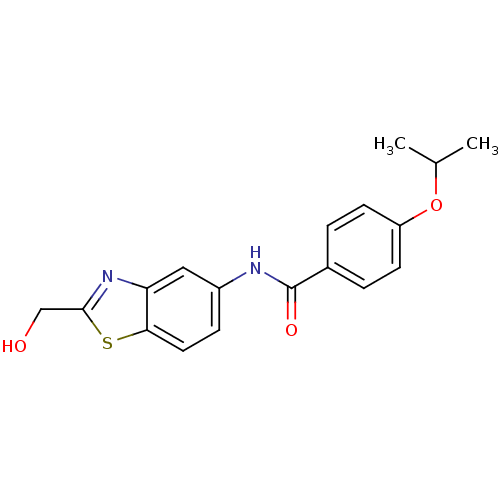
(CHEMBL2088394)Show InChI InChI=1S/C18H18N2O3S/c1-11(2)23-14-6-3-12(4-7-14)18(22)19-13-5-8-16-15(9-13)20-17(10-21)24-16/h3-9,11,21H,10H2,1-2H3,(H,19,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Antagonist activity at human recombinant TRPV1 receptor assessed as inhibition of capsaicin-induced calcium uptake by FLIPR assay |
Bioorg Med Chem Lett 22: 6205-11 (2012)
Article DOI: 10.1016/j.bmcl.2012.08.018
BindingDB Entry DOI: 10.7270/Q29C6ZGB |
More data for this
Ligand-Target Pair | |
Prostaglandin E synthase 2
(Homo sapiens (Human)) | BDBM181846
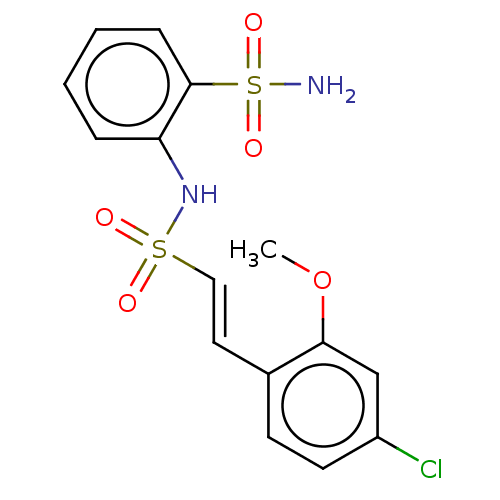
(US9145380, 49)Show SMILES COc1cc(Cl)ccc1\C=C\S(=O)(=O)Nc1ccccc1S(N)(=O)=O Show InChI InChI=1S/C15H15ClN2O5S2/c1-23-14-10-12(16)7-6-11(14)8-9-24(19,20)18-13-4-2-3-5-15(13)25(17,21)22/h2-10,18H,1H3,(H2,17,21,22)/b9-8+ | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 36 | n/a | n/a | n/a | n/a | 6.8 | n/a |
Astrazeneca AB
US Patent
| Assay Description
A solution of test compound was added to a diluted microsome preparation containing human mPGES-1 and pre-incubated for 15 minutes in potassium phosp... |
US Patent US9145380 (2015)
BindingDB Entry DOI: 10.7270/Q2XG9PXM |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM50418782
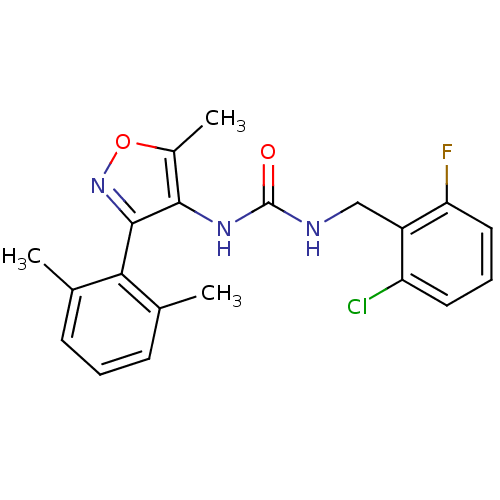
(CHEMBL1797401)Show SMILES Cc1onc(c1NC(=O)NCc1c(F)cccc1Cl)-c1c(C)cccc1C |(12.41,5.36,;11.51,4.11,;9.97,4.1,;9.5,2.64,;10.75,1.73,;11.99,2.64,;13.46,2.17,;14.78,2.97,;14.75,4.51,;16.13,2.22,;17.45,3.02,;18.79,2.27,;18.81,.73,;17.49,-.06,;20.15,-.02,;21.48,.78,;21.44,2.33,;20.1,3.06,;20.06,4.6,;10.75,.19,;9.42,-.58,;8.09,.19,;9.42,-2.13,;10.76,-2.9,;12.09,-2.13,;12.09,-.58,;13.42,.2,)| Show InChI InChI=1S/C20H19ClFN3O2/c1-11-6-4-7-12(2)17(11)19-18(13(3)27-25-19)24-20(26)23-10-14-15(21)8-5-9-16(14)22/h4-9H,10H2,1-3H3,(H2,23,24,26) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 38.0 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of Nav1.7 by cell based whole-cell voltage clamp electrophysiology assay |
Bioorg Med Chem Lett 21: 3871-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.041
BindingDB Entry DOI: 10.7270/Q21837RK |
More data for this
Ligand-Target Pair | |
Prostaglandin E synthase 2
(Homo sapiens (Human)) | BDBM181802

(US9145380, 5)Show SMILES NS(=O)(=O)c1ccc(F)cc1NS(=O)(=O)\C=C\c1ccc(cc1)-c1cc2ccccc2o1 Show InChI InChI=1S/C22H17FN2O5S2/c23-18-9-10-22(32(24,28)29)19(14-18)25-31(26,27)12-11-15-5-7-16(8-6-15)21-13-17-3-1-2-4-20(17)30-21/h1-14,25H,(H2,24,28,29)/b12-11+ | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | 6.8 | n/a |
Astrazeneca AB
US Patent
| Assay Description
A solution of test compound was added to a diluted microsome preparation containing human mPGES-1 and pre-incubated for 15 minutes in potassium phosp... |
US Patent US9145380 (2015)
BindingDB Entry DOI: 10.7270/Q2XG9PXM |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50391040

(CHEMBL2088406)Show SMILES OCc1nc2cc(NC(=O)c3ccc(OCC(F)(F)F)nc3)ccc2s1 Show InChI InChI=1S/C16H12F3N3O3S/c17-16(18,19)8-25-13-4-1-9(6-20-13)15(24)21-10-2-3-12-11(5-10)22-14(7-23)26-12/h1-6,23H,7-8H2,(H,21,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Antagonist activity at human recombinant TRPV1 receptor assessed as inhibition of capsaicin-induced calcium uptake by FLIPR assay |
Bioorg Med Chem Lett 22: 6205-11 (2012)
Article DOI: 10.1016/j.bmcl.2012.08.018
BindingDB Entry DOI: 10.7270/Q29C6ZGB |
More data for this
Ligand-Target Pair | |
Prostaglandin E synthase 2
(Homo sapiens (Human)) | BDBM182009
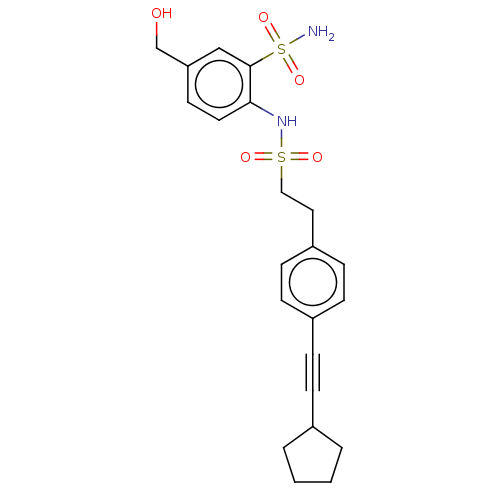
(US9145380, 212)Show SMILES NS(=O)(=O)c1cc(CO)ccc1NS(=O)(=O)CCc1ccc(cc1)C#CC1CCCC1 Show InChI InChI=1S/C22H26N2O5S2/c23-31(28,29)22-15-20(16-25)11-12-21(22)24-30(26,27)14-13-19-9-7-18(8-10-19)6-5-17-3-1-2-4-17/h7-12,15,17,24-25H,1-4,13-14,16H2,(H2,23,28,29) | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 41 | n/a | n/a | n/a | n/a | 6.8 | n/a |
Astrazeneca AB
US Patent
| Assay Description
A solution of test compound was added to a diluted microsome preparation containing human mPGES-1 and pre-incubated for 15 minutes in potassium phosp... |
US Patent US9145380 (2015)
BindingDB Entry DOI: 10.7270/Q2XG9PXM |
More data for this
Ligand-Target Pair | |
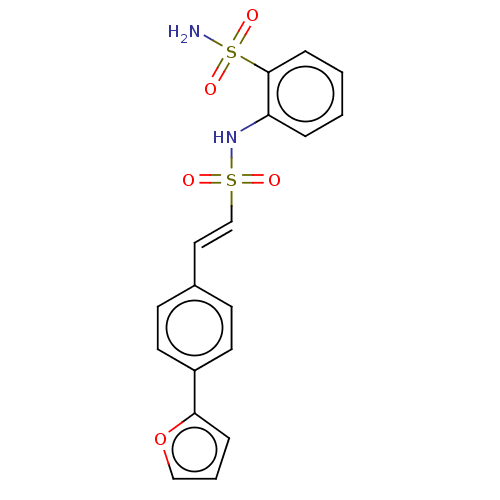
Prostaglandin E synthase 2
(Homo sapiens (Human)) | BDBM181815
(US9145380, 18)Show SMILES NS(=O)(=O)c1ccccc1NS(=O)(=O)\C=C\c1ccc(cc1)-c1ccco1 Show InChI InChI=1S/C18H16N2O5S2/c19-27(23,24)18-6-2-1-4-16(18)20-26(21,22)13-11-14-7-9-15(10-8-14)17-5-3-12-25-17/h1-13,20H,(H2,19,23,24)/b13-11+ | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 44 | n/a | n/a | n/a | n/a | 6.8 | n/a |
Astrazeneca AB
US Patent
| Assay Description
A solution of test compound was added to a diluted microsome preparation containing human mPGES-1 and pre-incubated for 15 minutes in potassium phosp... |
US Patent US9145380 (2015)
BindingDB Entry DOI: 10.7270/Q2XG9PXM |
More data for this
Ligand-Target Pair | |
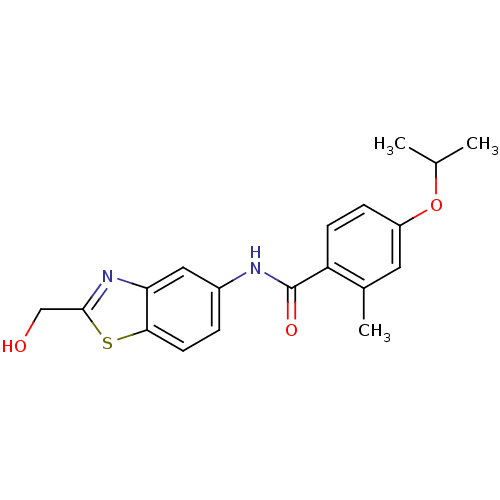
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50391052
(CHEMBL2088419)Show InChI InChI=1S/C19H20N2O3S/c1-11(2)24-14-5-6-15(12(3)8-14)19(23)20-13-4-7-17-16(9-13)21-18(10-22)25-17/h4-9,11,22H,10H2,1-3H3,(H,20,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Antagonist activity at human recombinant TRPV1 receptor assessed as inhibition of capsaicin-induced calcium uptake by FLIPR assay |
Bioorg Med Chem Lett 22: 6205-11 (2012)
Article DOI: 10.1016/j.bmcl.2012.08.018
BindingDB Entry DOI: 10.7270/Q29C6ZGB |
More data for this
Ligand-Target Pair | |
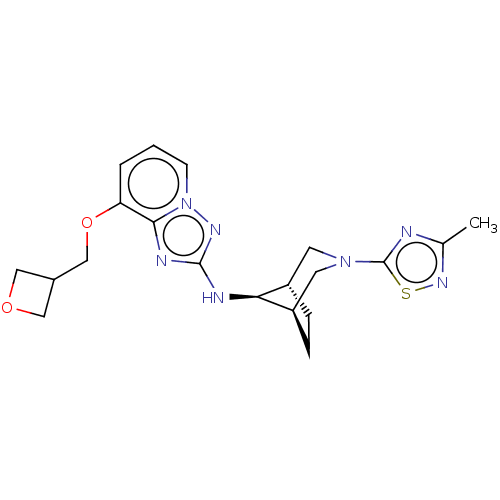
Presenilin-1
(Homo sapiens (Human)) | BDBM50543126
(CHEMBL4638537)Show SMILES [H][C@]12CC[C@]([H])(CN(C1)c1nc(C)ns1)[C@H]2Nc1nc2c(OCC3COC3)cccn2n1 |r,TLB:9:7:15:3.2| Show InChI InChI=1S/C20H25N7O2S/c1-12-21-20(30-25-12)26-7-14-4-5-15(8-26)17(14)22-19-23-18-16(3-2-6-27(18)24-19)29-11-13-9-28-10-13/h2-3,6,13-15,17H,4-5,7-11H2,1H3,(H,22,24)/t14-,15+,17- | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 49 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche AG
Curated by ChEMBL
| Assay Description
Modulation of gamma secretase in human H4 cells over expressing human APP695 harboring K595N/M596L Swedish double mutant assessed as reduction in Abe... |
J Med Chem 63: 8534-8553 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00909
BindingDB Entry DOI: 10.7270/Q2HT2SXX |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50391037
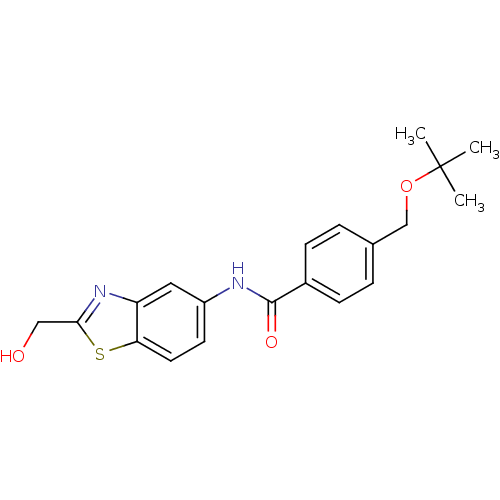
(CHEMBL2088403)Show SMILES CC(C)(C)OCc1ccc(cc1)C(=O)Nc1ccc2sc(CO)nc2c1 Show InChI InChI=1S/C20H22N2O3S/c1-20(2,3)25-12-13-4-6-14(7-5-13)19(24)21-15-8-9-17-16(10-15)22-18(11-23)26-17/h4-10,23H,11-12H2,1-3H3,(H,21,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 52 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Antagonist activity at human recombinant TRPV1 receptor assessed as inhibition of capsaicin-induced calcium uptake by FLIPR assay |
Bioorg Med Chem Lett 22: 6205-11 (2012)
Article DOI: 10.1016/j.bmcl.2012.08.018
BindingDB Entry DOI: 10.7270/Q29C6ZGB |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50391053

(CHEMBL2088420)Show InChI InChI=1S/C16H13BrN2O2S/c1-9-6-10(17)2-4-12(9)16(21)18-11-3-5-14-13(7-11)19-15(8-20)22-14/h2-7,20H,8H2,1H3,(H,18,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 52 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Antagonist activity at human recombinant TRPV1 receptor assessed as inhibition of capsaicin-induced calcium uptake by FLIPR assay |
Bioorg Med Chem Lett 22: 6205-11 (2012)
Article DOI: 10.1016/j.bmcl.2012.08.018
BindingDB Entry DOI: 10.7270/Q29C6ZGB |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data