

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sodium channel protein type 9 subunit alpha (Homo sapiens (Human)) | BDBM50325767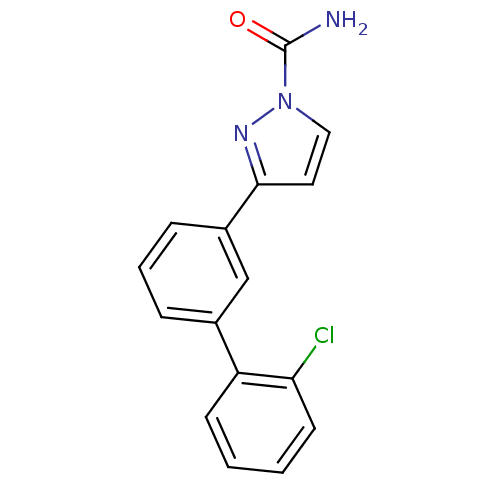 (3-(2'-chlorobiphenyl-3-yl)-1H-pyrazole-1-carboxami...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human Nav 1.7 channel by electrophysiology | Bioorg Med Chem Lett 20: 5480-3 (2010) Article DOI: 10.1016/j.bmcl.2010.07.080 BindingDB Entry DOI: 10.7270/Q2PV6KJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 9 subunit alpha (Homo sapiens (Human)) | BDBM50325766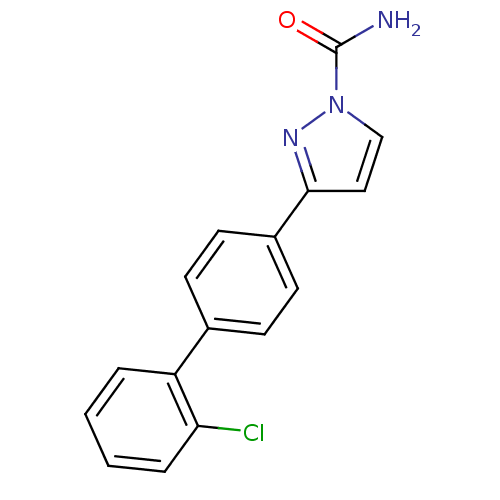 (3-(2'-chlorobiphenyl-4-yl)-1H-pyrazole-1-carboxami...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.111 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human Nav 1.7 channel by electrophysiology | Bioorg Med Chem Lett 20: 5480-3 (2010) Article DOI: 10.1016/j.bmcl.2010.07.080 BindingDB Entry DOI: 10.7270/Q2PV6KJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 9 subunit alpha (Homo sapiens (Human)) | BDBM50325765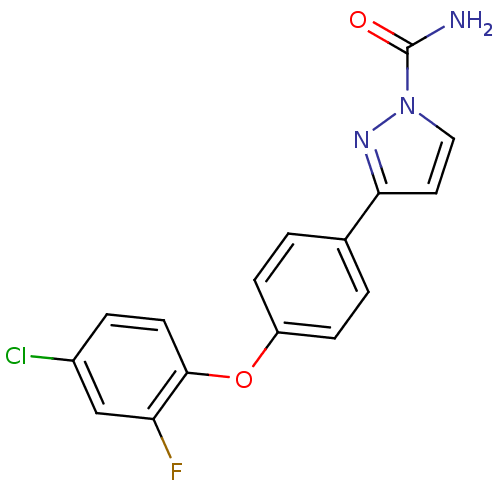 (3-(4-(4-chloro-2-fluorophenoxy)phenyl)-1H-pyrazole...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human Nav 1.7 channel by electrophysiology | Bioorg Med Chem Lett 20: 5480-3 (2010) Article DOI: 10.1016/j.bmcl.2010.07.080 BindingDB Entry DOI: 10.7270/Q2PV6KJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 9 subunit alpha (Homo sapiens (Human)) | BDBM50325764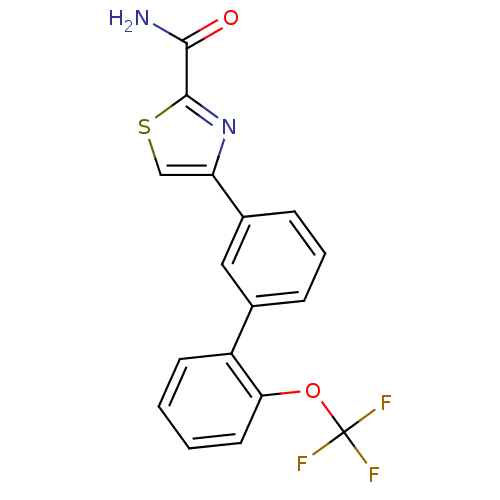 (4-(2'-(trifluoromethoxy)biphenyl-3-yl)thiazole-2-c...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human Nav 1.7 channel by electrophysiology | Bioorg Med Chem Lett 20: 5480-3 (2010) Article DOI: 10.1016/j.bmcl.2010.07.080 BindingDB Entry DOI: 10.7270/Q2PV6KJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 9 subunit alpha (Homo sapiens (Human)) | BDBM50325819 ((Z)-5-((2'-(trifluoromethoxy)biphenyl-3-yl)methyle...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity to human Nav1.7 | Bioorg Med Chem Lett 20: 5536-40 (2010) Article DOI: 10.1016/j.bmcl.2010.07.064 BindingDB Entry DOI: 10.7270/Q2FB535G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 9 subunit alpha (Homo sapiens (Human)) | BDBM50325764 (4-(2'-(trifluoromethoxy)biphenyl-3-yl)thiazole-2-c...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human Nav1.7 by VIPR assay | Bioorg Med Chem Lett 20: 7479-82 (2010) Article DOI: 10.1016/j.bmcl.2010.10.017 BindingDB Entry DOI: 10.7270/Q2MW2HDH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 9 subunit alpha (Homo sapiens (Human)) | BDBM50325762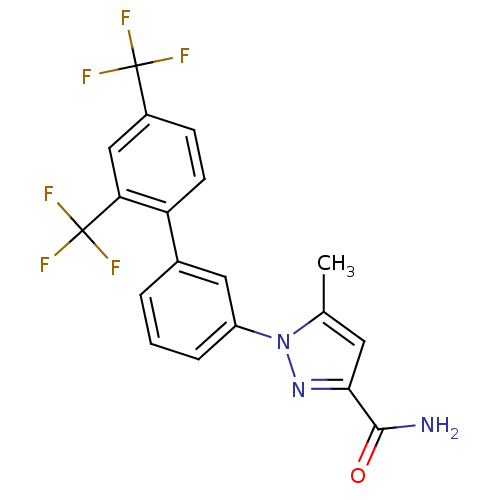 (1-(2',4'-bis(trifluoromethyl)biphenyl-3-yl)-5-meth...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human Nav1.7 by VIPR assay | Bioorg Med Chem Lett 20: 7479-82 (2010) Article DOI: 10.1016/j.bmcl.2010.10.017 BindingDB Entry DOI: 10.7270/Q2MW2HDH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 9 subunit alpha (Homo sapiens (Human)) | BDBM50141073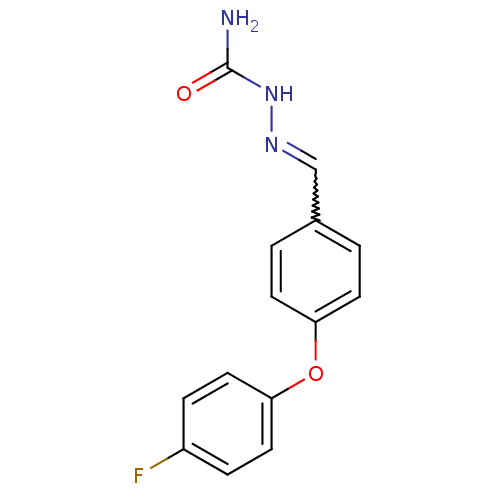 ((E)-2-(4-(4-fluorophenoxy)benzylidene)hydrazinecar...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity to human Nav1.7 | Bioorg Med Chem Lett 20: 5536-40 (2010) Article DOI: 10.1016/j.bmcl.2010.07.064 BindingDB Entry DOI: 10.7270/Q2FB535G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (RABBIT) | BDBM50035445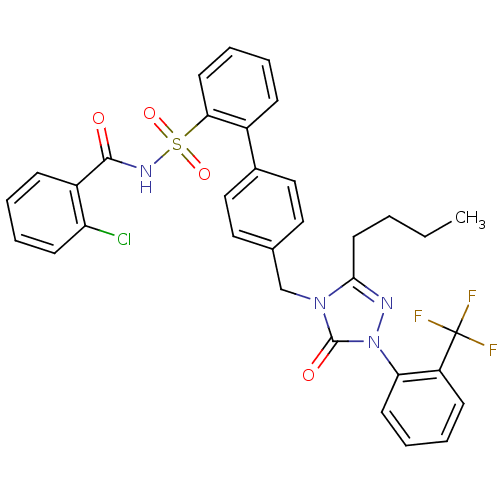 (4'-[3-Butyl-5-oxo-1-(2-trifluoromethyl-phenyl)-1,5...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity against angiotensin II rabbit aorta AT1 receptor using radioligand [125I]-Sar Ile-AII | J Med Chem 37: 2808-24 (1994) BindingDB Entry DOI: 10.7270/Q2M907QM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (RABBIT) | BDBM50039862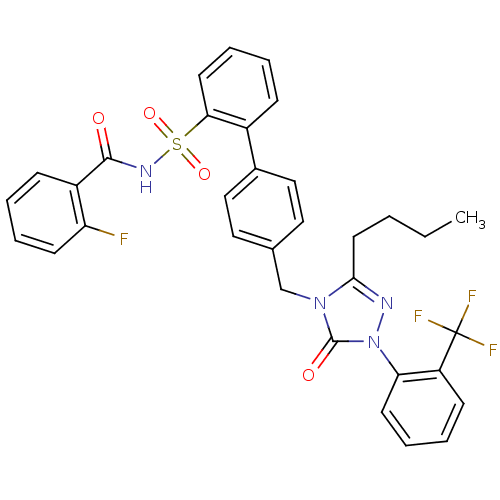 (4'-[3-Butyl-5-oxo-1-(2-trifluoromethyl-phenyl)-1,5...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity against angiotensin II rabbit aorta AT1 receptor using radioligand [125I]-Sar Ile-AII | J Med Chem 37: 2808-24 (1994) BindingDB Entry DOI: 10.7270/Q2M907QM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (RABBIT) | BDBM50039880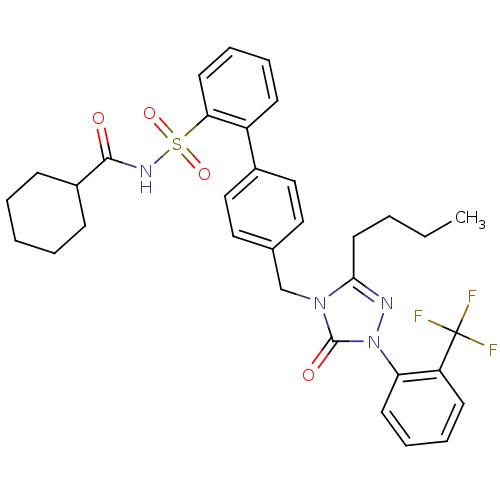 (4'-[3-Butyl-5-oxo-1-(2-trifluoromethyl-phenyl)-1,5...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity against angiotensin II rabbit aorta AT1 receptor using radioligand [125I]-Sar Ile-AII | J Med Chem 37: 2808-24 (1994) BindingDB Entry DOI: 10.7270/Q2M907QM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (RABBIT) | BDBM50039929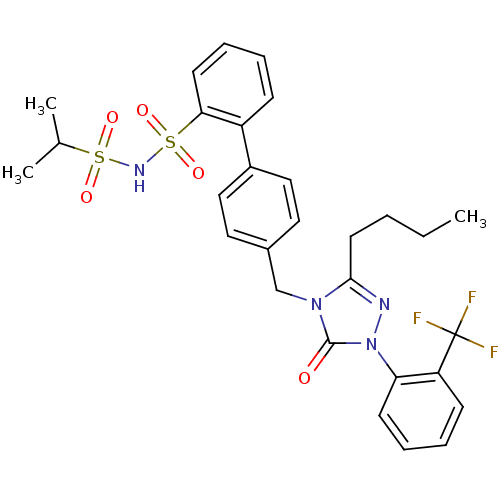 (2-{4-[3-butyl-5-oxo-1-(2-trifluoromethylphenyl)-4,...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity against angiotensin II rabbit aorta AT1 receptor using radioligand [125I]-Sar Ile-AII | J Med Chem 37: 2808-24 (1994) BindingDB Entry DOI: 10.7270/Q2M907QM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (RABBIT) | BDBM50039887 (4'-[3-Butyl-5-oxo-1-(2-trifluoromethyl-phenyl)-1,5...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity against angiotensin II rabbit aorta AT1 receptor using radioligand [125I]-Sar Ile-AII | J Med Chem 37: 2808-24 (1994) BindingDB Entry DOI: 10.7270/Q2M907QM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (RABBIT) | BDBM50039882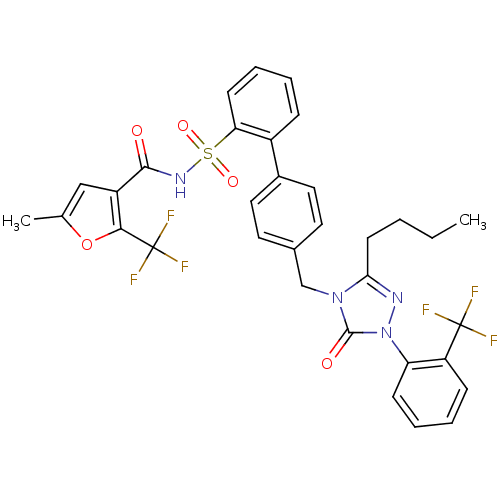 (4'-[3-Butyl-5-oxo-1-(2-trifluoromethyl-phenyl)-1,5...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity against angiotensin II rabbit aorta AT1 receptor using radioligand [125I]-Sar Ile-AII | J Med Chem 37: 2808-24 (1994) BindingDB Entry DOI: 10.7270/Q2M907QM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (RABBIT) | BDBM50039930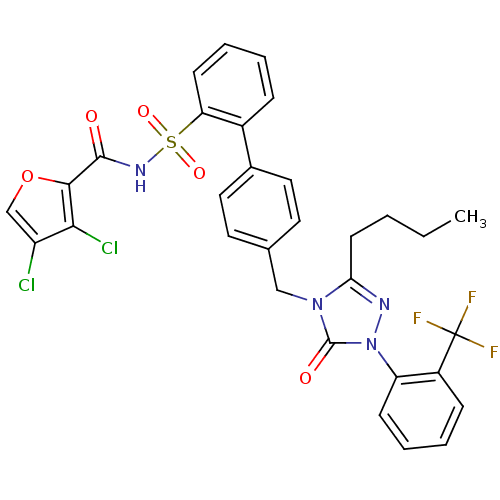 (4'-[3-Butyl-5-oxo-1-(2-trifluoromethyl-phenyl)-1,5...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity against angiotensin II rabbit aorta AT1 receptor using radioligand [125I]-Sar Ile-AII | J Med Chem 37: 2808-24 (1994) BindingDB Entry DOI: 10.7270/Q2M907QM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (RABBIT) | BDBM50038189 (4'-(2-Ethyl-5,7-dimethyl-imidazo[4,5-b]pyridin-3-y...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro binding affinity of the compound for angiotensin II AT1 receptor in rabbit aorta | J Med Chem 37: 4068-72 (1995) BindingDB Entry DOI: 10.7270/Q2DV1HZ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (RABBIT) | BDBM50039895 (2-{4-[3-butyl-5-oxo-1-(2-trifluoromethylphenyl)-4,...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity against angiotensin II rabbit aorta AT1 receptor using radioligand [125I]-Sar Ile-AII | J Med Chem 37: 2808-24 (1994) BindingDB Entry DOI: 10.7270/Q2M907QM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (RABBIT) | BDBM50282431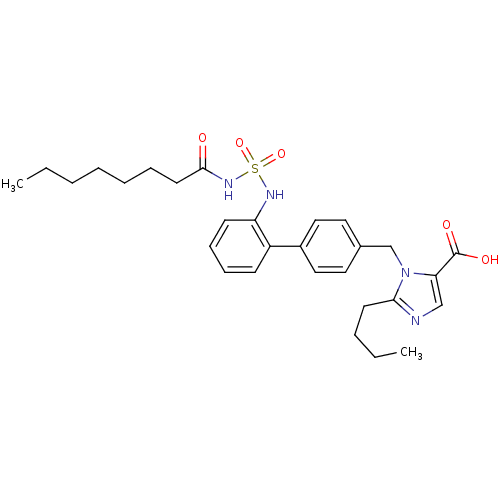 (Acyl sulfonamide derivative | CHEMBL350121) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity against Angiotensin II receptor, type 1 to displace 125I-Sar,Ile8-AII in rabbit aorta | Bioorg Med Chem Lett 4: 69-74 (1994) Article DOI: 10.1016/S0960-894X(01)81124-0 BindingDB Entry DOI: 10.7270/Q2NS0TVK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1/Type-2 angiotensin II receptor (Homo sapiens (Human)) | BDBM50229575 (CHEMBL2369937) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Exploratory Chemistry Merck Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description Binding affinity towards Angiotensin receptor from rabbit aorta | J Med Chem 34: 2919-22 (1991) BindingDB Entry DOI: 10.7270/Q26Q20H6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (RABBIT) | BDBM50039921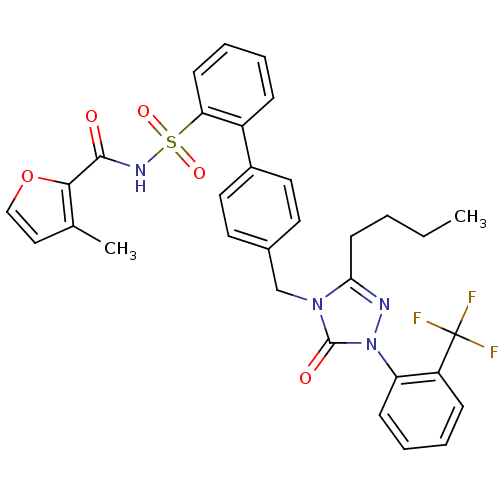 (4'-[3-Butyl-5-oxo-1-(2-trifluoromethyl-phenyl)-1,5...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity against angiotensin II rabbit aorta AT1 receptor using radioligand [125I]-Sar Ile-AII | J Med Chem 37: 2808-24 (1994) BindingDB Entry DOI: 10.7270/Q2M907QM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (RABBIT) | BDBM50038190 (4'-(2-Ethyl-5,7-dimethyl-imidazo[4,5-b]pyridin-3-y...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro binding affinity of the compound for angiotensin II AT1 receptor in rabbit aorta | J Med Chem 37: 4068-72 (1995) BindingDB Entry DOI: 10.7270/Q2DV1HZ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (RABBIT) | BDBM50039946 (4'-[3-Butyl-5-oxo-1-(2-trifluoromethyl-phenyl)-1,5...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity against angiotensin II rabbit aorta AT1 receptor using radioligand [125I]-Sar Ile-AII | J Med Chem 37: 2808-24 (1994) BindingDB Entry DOI: 10.7270/Q2M907QM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (RABBIT) | BDBM50039899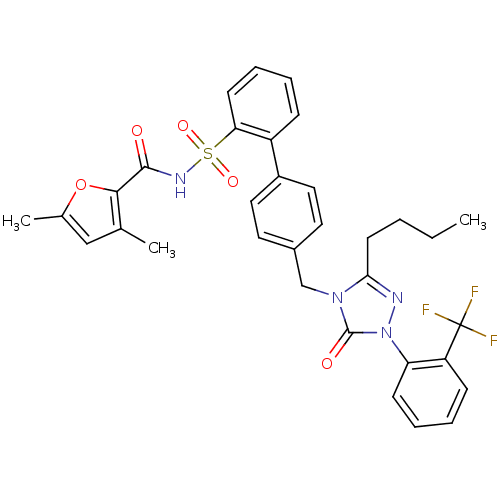 (4'-[3-Butyl-5-oxo-1-(2-trifluoromethyl-phenyl)-1,5...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity against angiotensin II rabbit aorta AT1 receptor using radioligand [125I]-Sar Ile-AII | J Med Chem 37: 2808-24 (1994) BindingDB Entry DOI: 10.7270/Q2M907QM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (RABBIT) | BDBM50039922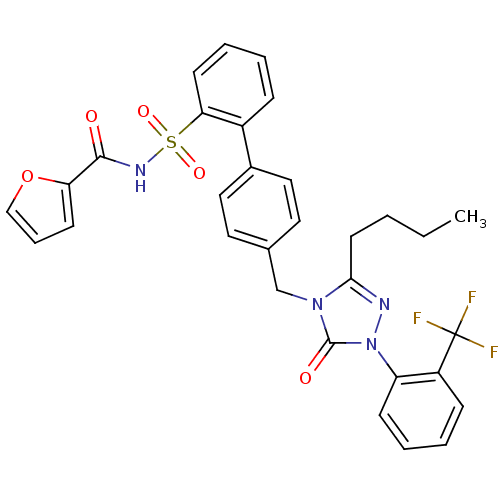 (4'-[3-Butyl-5-oxo-1-(2-trifluoromethyl-phenyl)-1,5...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity against angiotensin II rabbit aorta AT1 receptor using radioligand [125I]-Sar Ile-AII | J Med Chem 37: 2808-24 (1994) BindingDB Entry DOI: 10.7270/Q2M907QM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (RABBIT) | BDBM50039885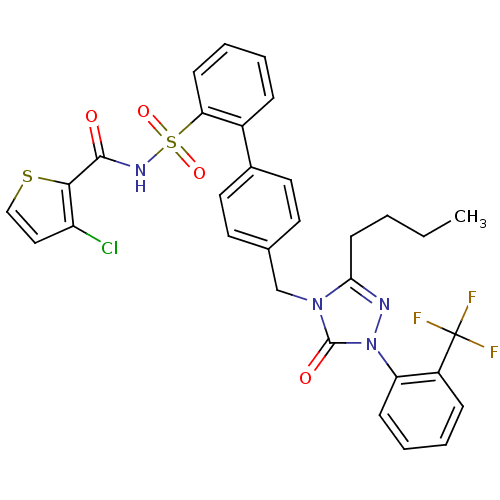 (4'-[3-Butyl-5-oxo-1-(2-trifluoromethyl-phenyl)-1,5...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity against angiotensin II rabbit aorta AT1 receptor using radioligand [125I]-Sar Ile-AII | J Med Chem 37: 2808-24 (1994) BindingDB Entry DOI: 10.7270/Q2M907QM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (RABBIT) | BDBM50039926 (4'-[3-Butyl-5-oxo-1-(2-trifluoromethyl-phenyl)-1,5...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity against angiotensin II rabbit aorta AT1 receptor using radioligand [125I]-Sar Ile-AII | J Med Chem 37: 2808-24 (1994) BindingDB Entry DOI: 10.7270/Q2M907QM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (RABBIT) | BDBM50038192 (4'-(2-Ethyl-5,7-dimethyl-imidazo[4,5-b]pyridin-3-y...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro binding affinity of the compound for angiotensin II AT1 receptor in rabbit aorta | J Med Chem 37: 4068-72 (1995) BindingDB Entry DOI: 10.7270/Q2DV1HZ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1/Type-2 angiotensin II receptor (Homo sapiens (Human)) | BDBM50229912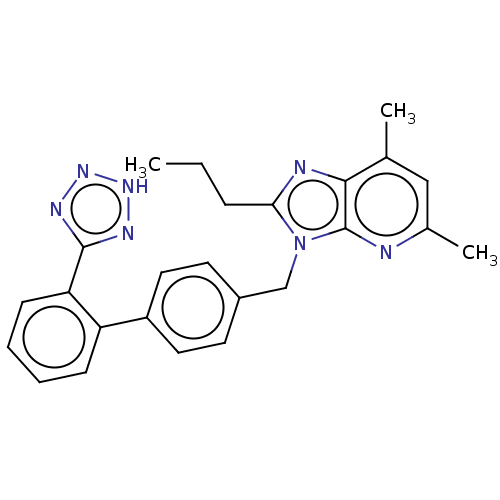 (CHEMBL315104) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Exploratory Chemistry Merck Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description Binding affinity towards Angiotensin receptor from rabbit aorta | J Med Chem 34: 2919-22 (1991) BindingDB Entry DOI: 10.7270/Q26Q20H6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (RABBIT) | BDBM50039904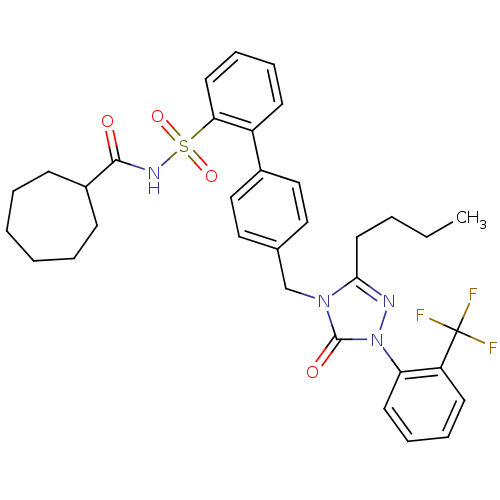 (4'-[3-Butyl-5-oxo-1-(2-trifluoromethyl-phenyl)-1,5...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity against angiotensin II rabbit aorta AT1 receptor using radioligand [125I]-Sar Ile-AII | J Med Chem 37: 2808-24 (1994) BindingDB Entry DOI: 10.7270/Q2M907QM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1/Type-2 angiotensin II receptor (Homo sapiens (Human)) | BDBM50009718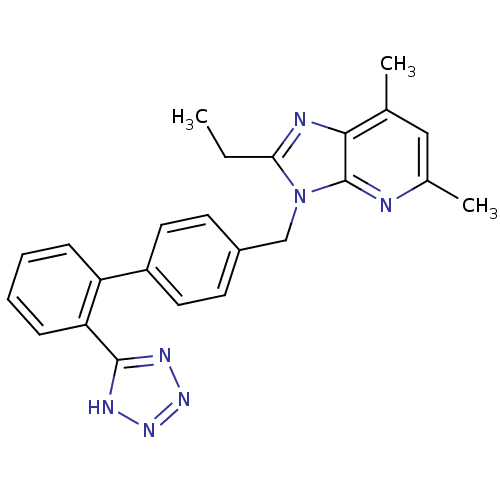 (2-Ethyl-5,7-dimethyl-3-[2'-(1H-tetrazol-5-yl)-biph...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Exploratory Chemistry Merck Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description Binding affinity towards Angiotensin receptor from rabbit aorta | J Med Chem 34: 2919-22 (1991) BindingDB Entry DOI: 10.7270/Q26Q20H6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (RABBIT) | BDBM50009718 (2-Ethyl-5,7-dimethyl-3-[2'-(1H-tetrazol-5-yl)-biph...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro binding affinity of the compound for angiotensin II AT1 receptor in rabbit aorta | J Med Chem 37: 4068-72 (1995) BindingDB Entry DOI: 10.7270/Q2DV1HZ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (RABBIT) | BDBM50039938 (4'-[3-Butyl-5-oxo-1-(2-trifluoromethyl-phenyl)-1,5...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity against angiotensin II rabbit aorta AT1 receptor using radioligand [125I]-Sar Ile-AII | J Med Chem 37: 2808-24 (1994) BindingDB Entry DOI: 10.7270/Q2M907QM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor B (RAT) | BDBM50030727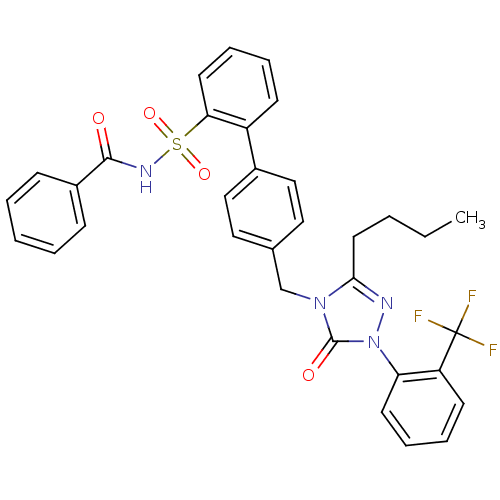 (4''-[3-Butyl-5-oxo-1-(2-trifluoromethyl-phenyl)-1,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro binding affinity of the compound towards Angiotensin II receptor, type 1 to displace 125I[Sar,Ile] from rat brain tissue preparation | Bioorg Med Chem Lett 4: 115-120 (1994) Article DOI: 10.1016/S0960-894X(01)81132-X BindingDB Entry DOI: 10.7270/Q2057FT4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (RABBIT) | BDBM50039924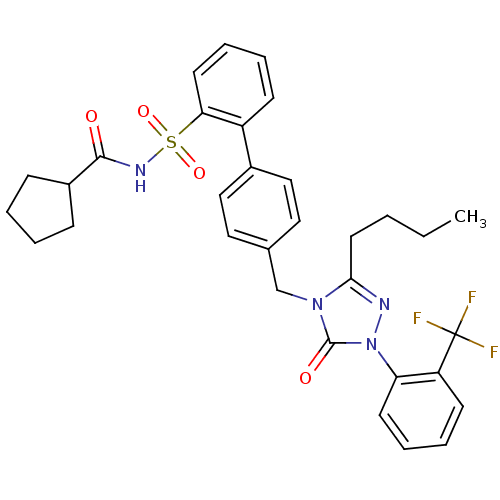 (4'-[3-Butyl-5-oxo-1-(2-trifluoromethyl-phenyl)-1,5...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity against angiotensin II rabbit aorta AT1 receptor using radioligand [125I]-Sar Ile-AII | J Med Chem 37: 2808-24 (1994) BindingDB Entry DOI: 10.7270/Q2M907QM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor B (RAT) | BDBM50030727 (4''-[3-Butyl-5-oxo-1-(2-trifluoromethyl-phenyl)-1,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro binding affinity of the compound towards Angiotensin II receptor, type 1 to displace 125I[Sar,Ile] from rat adrenal tissue preparation | Bioorg Med Chem Lett 4: 115-120 (1994) Article DOI: 10.1016/S0960-894X(01)81132-X BindingDB Entry DOI: 10.7270/Q2057FT4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (RABBIT) | BDBM50039894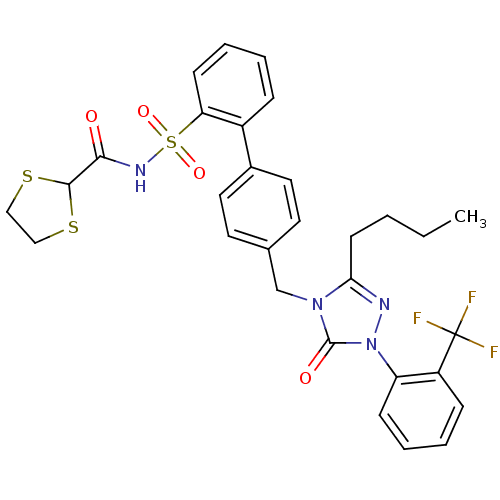 (4'-[3-Butyl-5-oxo-1-(2-trifluoromethyl-phenyl)-1,5...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity against angiotensin II rabbit aorta AT1 receptor using radioligand [125I]-Sar Ile-AII | J Med Chem 37: 2808-24 (1994) BindingDB Entry DOI: 10.7270/Q2M907QM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (RABBIT) | BDBM50039858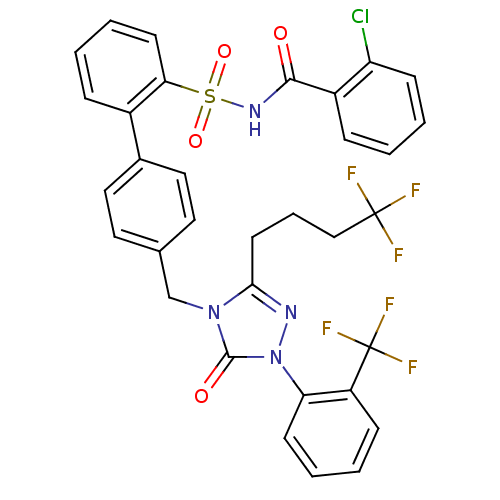 (4'-[5-Oxo-3-(4,4,4-trifluoro-butyl)-1-(2-trifluoro...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.360 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity against angiotensin II rabbit aorta AT1 receptor using radioligand [125I]-Sar Ile-AII | J Med Chem 37: 2808-24 (1994) BindingDB Entry DOI: 10.7270/Q2M907QM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (RABBIT) | BDBM50039853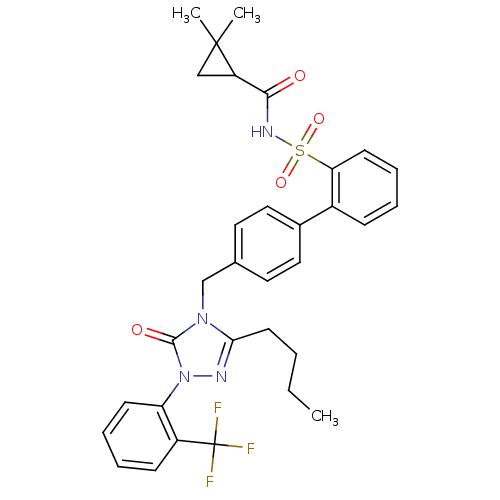 (4'-[3-Butyl-5-oxo-1-(2-trifluoromethyl-phenyl)-1,5...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.360 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity against angiotensin II rabbit aorta AT1 receptor using radioligand [125I]-Sar Ile-AII | J Med Chem 37: 2808-24 (1994) BindingDB Entry DOI: 10.7270/Q2M907QM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (RABBIT) | BDBM50039870 (4'-[3-Butyl-5-oxo-1-(2-trifluoromethyl-phenyl)-1,5...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.370 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity against angiotensin II rabbit aorta AT1 receptor using radioligand [125I]-Sar Ile-AII | J Med Chem 37: 2808-24 (1994) BindingDB Entry DOI: 10.7270/Q2M907QM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (RABBIT) | BDBM50039865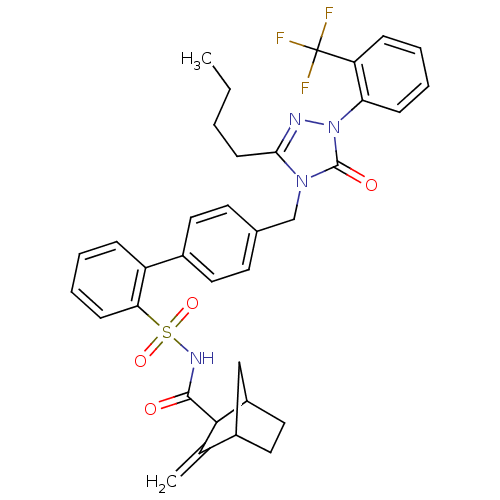 (4'-[3-Butyl-5-oxo-1-(2-trifluoromethyl-phenyl)-1,5...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.390 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity against angiotensin II rabbit aorta AT1 receptor using radioligand [125I]-Sar Ile-AII | J Med Chem 37: 2808-24 (1994) BindingDB Entry DOI: 10.7270/Q2M907QM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (RABBIT) | BDBM50039923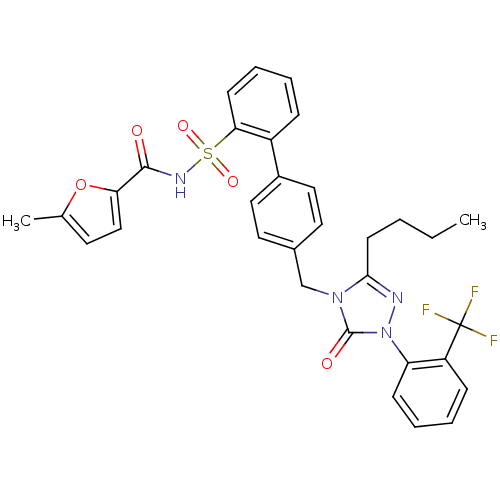 (4'-[3-Butyl-5-oxo-1-(2-trifluoromethyl-phenyl)-1,5...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity against angiotensin II rabbit aorta AT1 receptor using radioligand [125I]-Sar Ile-AII | J Med Chem 37: 2808-24 (1994) BindingDB Entry DOI: 10.7270/Q2M907QM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (RABBIT) | BDBM50039863 (4'-[3-Butyl-5-oxo-1-(2-trifluoromethyl-phenyl)-1,5...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity against angiotensin II rabbit aorta AT1 receptor using radioligand [125I]-Sar Ile-AII | J Med Chem 37: 2808-24 (1994) BindingDB Entry DOI: 10.7270/Q2M907QM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (RABBIT) | BDBM50039913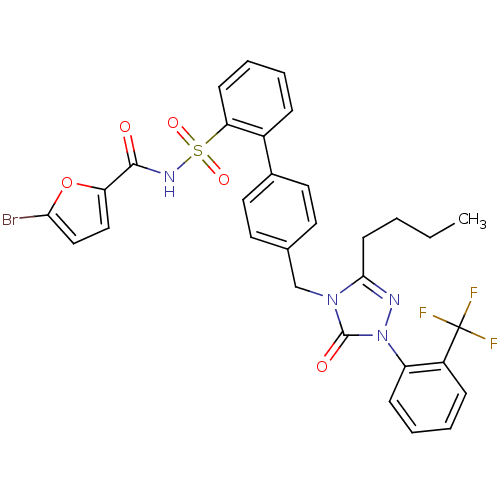 (4'-[3-Butyl-5-oxo-1-(2-trifluoromethyl-phenyl)-1,5...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity against angiotensin II rabbit aorta AT1 receptor using radioligand [125I]-Sar Ile-AII | J Med Chem 37: 2808-24 (1994) BindingDB Entry DOI: 10.7270/Q2M907QM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (RABBIT) | BDBM50039903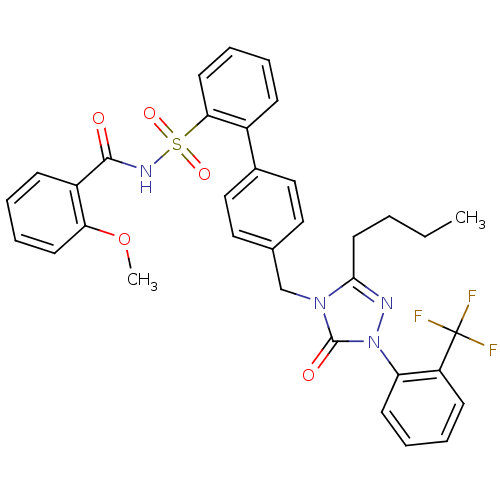 (4'-[3-Butyl-5-oxo-1-(2-trifluoromethyl-phenyl)-1,5...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.410 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity against angiotensin II rabbit aorta AT1 receptor using radioligand [125I]-Sar Ile-AII | J Med Chem 37: 2808-24 (1994) BindingDB Entry DOI: 10.7270/Q2M907QM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (RABBIT) | BDBM50039864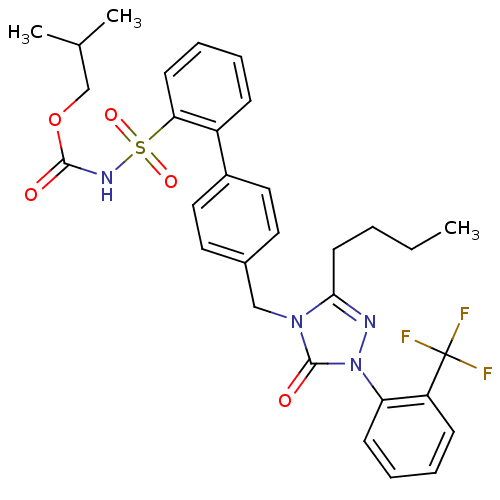 (2-{4-[3-butyl-5-oxo-1-(2-trifluoromethylphenyl)-4,...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.420 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity against angiotensin II rabbit aorta AT1 receptor using radioligand [125I]-Sar Ile-AII | J Med Chem 37: 2808-24 (1994) BindingDB Entry DOI: 10.7270/Q2M907QM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (RABBIT) | BDBM50030727 (4''-[3-Butyl-5-oxo-1-(2-trifluoromethyl-phenyl)-1,...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of 125I[Sar,Ile] from rabbit aorta membrane Angiotensin II receptor type 1 | Bioorg Med Chem Lett 4: 115-120 (1994) Article DOI: 10.1016/S0960-894X(01)81132-X BindingDB Entry DOI: 10.7270/Q2057FT4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (RABBIT) | BDBM50039877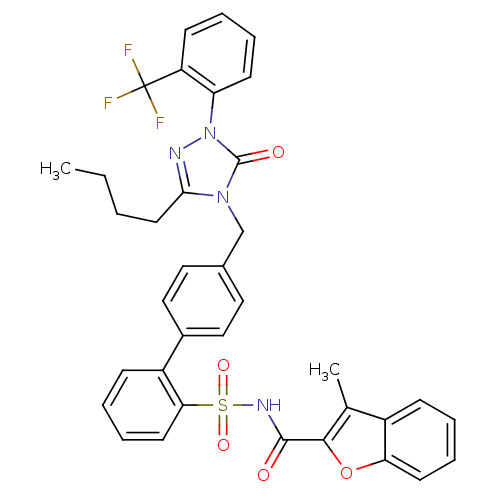 (4'-[3-Butyl-5-oxo-1-(2-trifluoromethyl-phenyl)-1,5...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity against angiotensin II rabbit aorta AT1 receptor using radioligand [125I]-Sar Ile-AII | J Med Chem 37: 2808-24 (1994) BindingDB Entry DOI: 10.7270/Q2M907QM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (RABBIT) | BDBM50030727 (4''-[3-Butyl-5-oxo-1-(2-trifluoromethyl-phenyl)-1,...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity against angiotensin II rabbit aorta AT1 receptor using radioligand [125I]-Sar Ile-AII | J Med Chem 37: 2808-24 (1994) BindingDB Entry DOI: 10.7270/Q2M907QM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (RABBIT) | BDBM50035451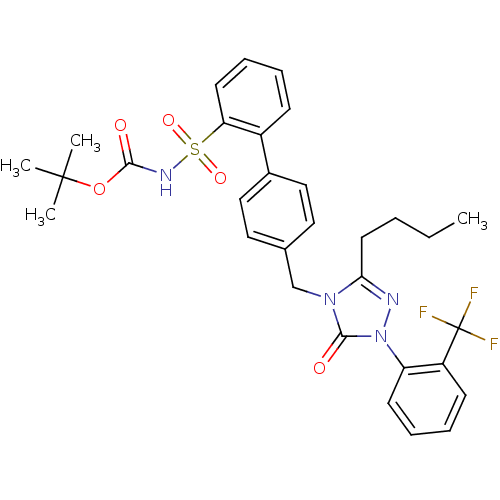 (2-{4-[3-butyl-5-oxo-1-(2-trifluoromethylphenyl)-4,...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity against angiotensin II rabbit aorta AT1 receptor using radioligand [125I]-Sar Ile-AII | J Med Chem 37: 2808-24 (1994) BindingDB Entry DOI: 10.7270/Q2M907QM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (RABBIT) | BDBM50042742 (CHEMBL340367 | [4-(2-Ethyl-5,7-dimethyl-imidazo[4,...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.470 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [1251][Sar1,IIe8]AII from rabbit aorta Angiotensin II receptor, type 1 | J Med Chem 36: 3738-42 (1994) BindingDB Entry DOI: 10.7270/Q2J1027W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 805 total ) | Next | Last >> |