 Found 2776 hits with Last Name = 'chapman' and Initial = 'k'
Found 2776 hits with Last Name = 'chapman' and Initial = 'k' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM85357
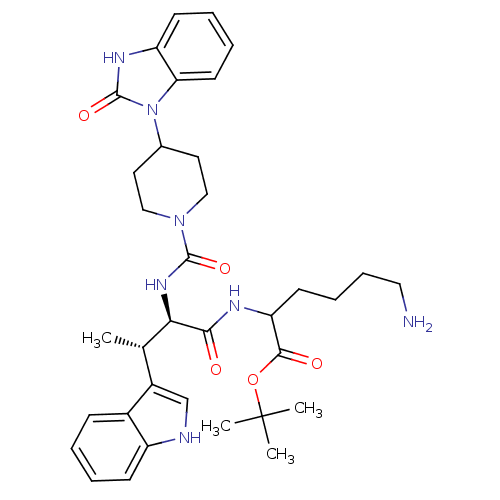
(2-[[(2R,3S)-2-[[4-[(2-Oxo-2,3-dihydro-1H-benzimida...)Show SMILES C[C@H]([C@@H](NC(=O)N1CCC(CC1)n1c2ccccc2[nH]c1=O)C(=O)NC(CCCCN)C(=O)OC(C)(C)C)c1c[nH]c2ccccc12 Show InChI InChI=1S/C35H47N7O5/c1-22(25-21-37-26-12-6-5-11-24(25)26)30(31(43)38-28(14-9-10-18-36)32(44)47-35(2,3)4)40-33(45)41-19-16-23(17-20-41)42-29-15-8-7-13-27(29)39-34(42)46/h5-8,11-13,15,21-23,28,30,37H,9-10,14,16-20,36H2,1-4H3,(H,38,43)(H,39,46)(H,40,45)/t22-,28?,30+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by PDSP Ki Database
| |
Proc Natl Acad Sci U S A 95: 10836-41 (1998)
Article DOI: 10.1073/pnas.95.18.10836
BindingDB Entry DOI: 10.7270/Q2XW4HCM |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM81767

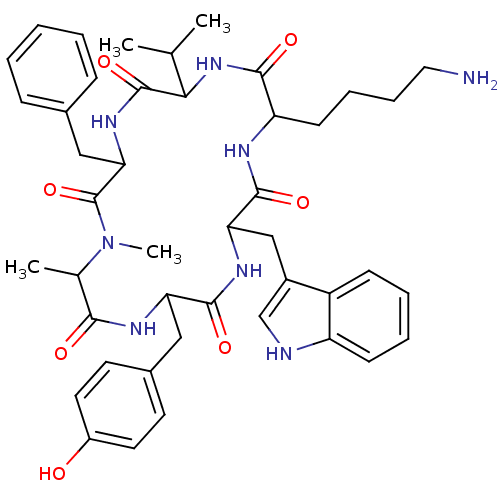
(15-28-Somatostatin-28 | CAS_38916-34-6 | CB6417646...)Show SMILES C[C@@H](O)[C@@H]1NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@@H](NC(=O)[C@H](CCCCN)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CSSC[C@H](NC(=O)[C@H](CO)NC1=O)C(O)=O)NC(=O)CNC(=O)[C@H](C)N)[C@@H](C)O |r| Show InChI InChI=1S/C76H104N18O19S2/c1-41(79)64(100)82-37-61(99)83-58-39-114-115-40-59(76(112)113)92-72(108)57(38-95)91-75(111)63(43(3)97)94-71(107)54(33-46-23-11-6-12-24-46)90-74(110)62(42(2)96)93-66(102)51(28-16-18-30-78)84-69(105)55(34-47-36-81-49-26-14-13-25-48(47)49)88-68(104)53(32-45-21-9-5-10-22-45)86-67(103)52(31-44-19-7-4-8-20-44)87-70(106)56(35-60(80)98)89-65(101)50(85-73(58)109)27-15-17-29-77/h4-14,19-26,36,41-43,50-59,62-63,81,95-97H,15-18,27-35,37-40,77-79H2,1-3H3,(H2,80,98)(H,82,100)(H,83,99)(H,84,105)(H,85,109)(H,86,103)(H,87,106)(H,88,104)(H,89,101)(H,90,110)(H,91,111)(H,92,108)(H,93,102)(H,94,107)(H,112,113)/t41-,42+,43+,50-,51-,52-,53-,54-,55-,56-,57-,58-,59-,62-,63-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by PDSP Ki Database
| |
Proc Natl Acad Sci U S A 95: 10836-41 (1998)
Article DOI: 10.1073/pnas.95.18.10836
BindingDB Entry DOI: 10.7270/Q2XW4HCM |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM81766
(CAS_3086456 | MK 678 | NSC_3086456)Show SMILES CC(C)C1NC(=O)C(CCCCN)NC(=O)C(Cc2c[nH]c3ccccc23)NC(=O)C(Cc2ccc(O)cc2)NC(=O)C(C)N(C)C(=O)C(Cc2ccccc2)NC1=O Show InChI InChI=1S/C44H56N8O7/c1-26(2)38-43(58)50-37(23-28-12-6-5-7-13-28)44(59)52(4)27(3)39(54)48-35(22-29-17-19-31(53)20-18-29)41(56)49-36(24-30-25-46-33-15-9-8-14-32(30)33)42(57)47-34(40(55)51-38)16-10-11-21-45/h5-9,12-15,17-20,25-27,34-38,46,53H,10-11,16,21-24,45H2,1-4H3,(H,47,57)(H,48,54)(H,49,56)(H,50,58)(H,51,55) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by PDSP Ki Database
| |
Proc Natl Acad Sci U S A 95: 10836-41 (1998)
Article DOI: 10.1073/pnas.95.18.10836
BindingDB Entry DOI: 10.7270/Q2XW4HCM |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50099273
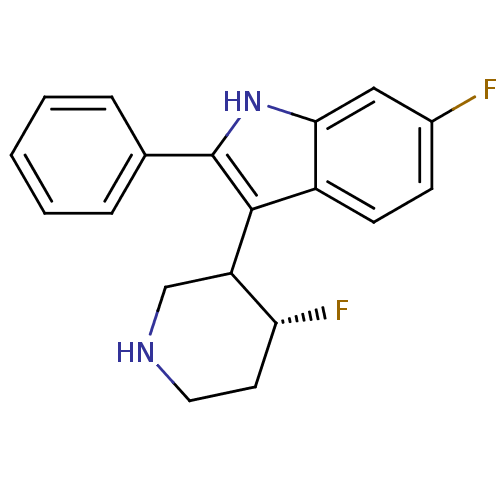
(6-Fluoro-3-(4-fluoro-piperidin-3-yl)-2-phenyl-1H-i...)Show SMILES F[C@@H]1CCNCC1c1c([nH]c2cc(F)ccc12)-c1ccccc1 Show InChI InChI=1S/C19H18F2N2/c20-13-6-7-14-17(10-13)23-19(12-4-2-1-3-5-12)18(14)15-11-22-9-8-16(15)21/h1-7,10,15-16,22-23H,8-9,11H2/t15?,16-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme
Curated by ChEMBL
| Assay Description
Binding affinity for displacement of [3H]ketanserin to human 5-hydroxytryptamine 2A receptor stably expressed in CHO cells |
J Med Chem 45: 492-503 (2002)
BindingDB Entry DOI: 10.7270/Q2KH0P27 |
More data for this
Ligand-Target Pair | |
Cholecystokinin
(GUINEA PIG) | BDBM50040671
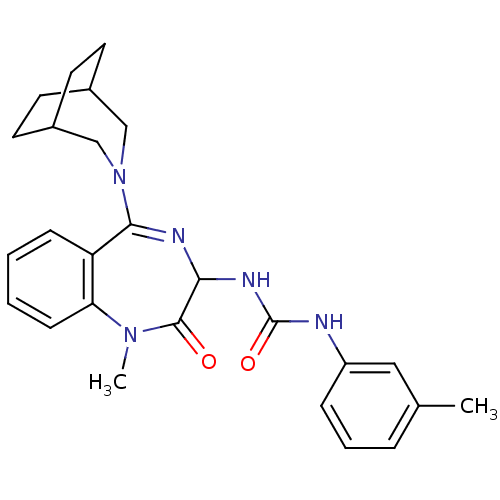
(1-[5-(3-Aza-bicyclo[3.2.2]non-3-yl)-1-methyl-2-oxo...)Show SMILES CN1c2ccccc2C(=NC(NC(=O)Nc2cccc(C)c2)C1=O)N1CC2CCC(CC2)C1 |c:9,(7.22,-6.97,;7.67,-8.45,;6.69,-9.39,;5.36,-8.63,;4.02,-9.39,;4.02,-10.93,;5.36,-11.69,;6.71,-10.96,;7.63,-11.85,;9.14,-11.6,;9.86,-10.19,;11.4,-10.21,;12.13,-11.53,;11.35,-12.86,;13.67,-11.57,;14.48,-10.26,;13.72,-8.91,;14.5,-7.6,;16.04,-7.62,;16.81,-8.95,;18.33,-8.98,;16.01,-10.29,;9.18,-8.72,;10.1,-7.49,;7.17,-13.34,;5.55,-13.5,;4.73,-14.54,;5.14,-16.05,;6.65,-16.64,;8.09,-15.8,;7.22,-14.74,;6.1,-14.2,;8.22,-14.23,)| Show InChI InChI=1S/C26H31N5O2/c1-17-6-5-7-20(14-17)27-26(33)29-23-25(32)30(2)22-9-4-3-8-21(22)24(28-23)31-15-18-10-11-19(16-31)13-12-18/h3-9,14,18-19,23H,10-13,15-16H2,1-2H3,(H2,27,29,33) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme Research Laboratories
Curated by PDSP Ki Database
| |
Mol Pharmacol 46: 943-8 (1994)
BindingDB Entry DOI: 10.7270/Q2QR4VMN |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Rattus norvegicus (rat)) | BDBM50095027
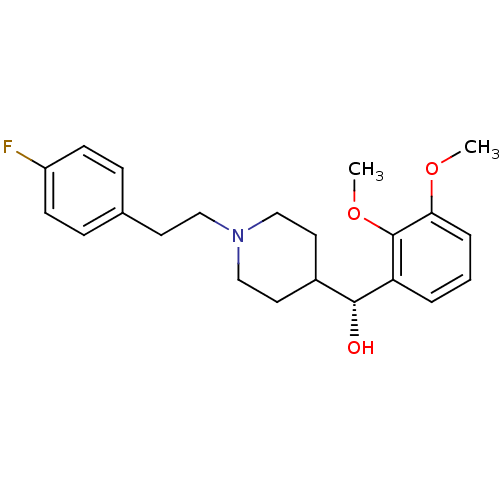
((2,3-Dimethoxy-phenyl)-{1-[2-(4-fluoro-phenyl)-eth...)Show SMILES COc1cccc([C@H](O)C2CCN(CCc3ccc(F)cc3)CC2)c1OC |r| Show InChI InChI=1S/C22H28FNO3/c1-26-20-5-3-4-19(22(20)27-2)21(25)17-11-14-24(15-12-17)13-10-16-6-8-18(23)9-7-16/h3-9,17,21,25H,10-15H2,1-2H3/t21-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme
Curated by ChEMBL
| Assay Description
Binding affinity for displacement of [3H]ketanserin to rat 5-hydroxytryptamine 2A receptor stably expressed in CHO cells |
J Med Chem 45: 492-503 (2002)
BindingDB Entry DOI: 10.7270/Q2KH0P27 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Rattus norvegicus (rat)) | BDBM50108690
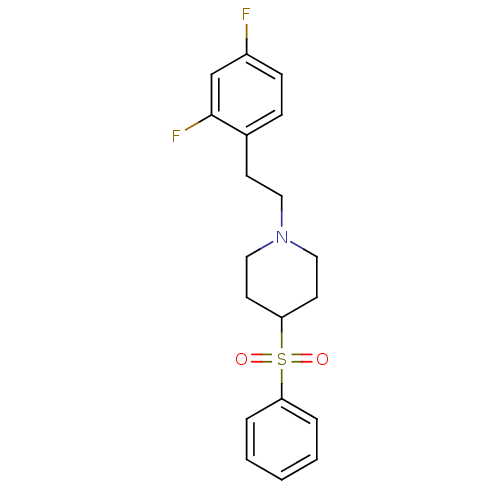
(1-(2,4-difluorophenethyl)-4-(phenylsulfonyl)piperi...)Show SMILES Fc1ccc(CCN2CCC(CC2)S(=O)(=O)c2ccccc2)c(F)c1 Show InChI InChI=1S/C19H21F2NO2S/c20-16-7-6-15(19(21)14-16)8-11-22-12-9-18(10-13-22)25(23,24)17-4-2-1-3-5-17/h1-7,14,18H,8-13H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme
Curated by ChEMBL
| Assay Description
Binding affinity for displacement of [3H]ketanserin to rat 5-hydroxytryptamine 2A receptor stably expressed in CHO cells |
J Med Chem 45: 492-503 (2002)
BindingDB Entry DOI: 10.7270/Q2KH0P27 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Rattus norvegicus (rat)) | BDBM50108689
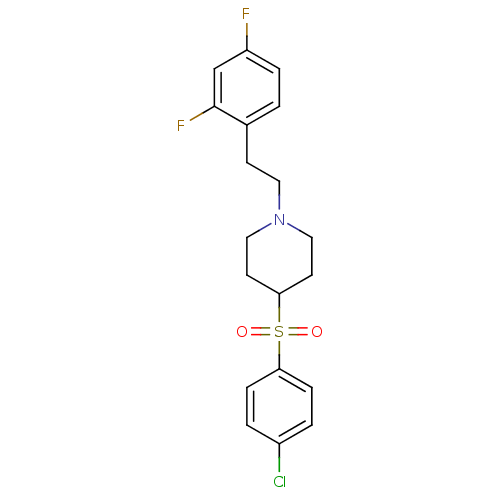
(4-(4-Chloro-benzenesulfonyl)-1-[2-(2,4-difluoro-ph...)Show SMILES Fc1ccc(CCN2CCC(CC2)S(=O)(=O)c2ccc(Cl)cc2)c(F)c1 Show InChI InChI=1S/C19H20ClF2NO2S/c20-15-2-5-17(6-3-15)26(24,25)18-8-11-23(12-9-18)10-7-14-1-4-16(21)13-19(14)22/h1-6,13,18H,7-12H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme
Curated by ChEMBL
| Assay Description
Binding affinity for displacement of [3H]ketanserin to rat 5-hydroxytryptamine 2A receptor stably expressed in CHO cells |
J Med Chem 45: 492-503 (2002)
BindingDB Entry DOI: 10.7270/Q2KH0P27 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM50059090
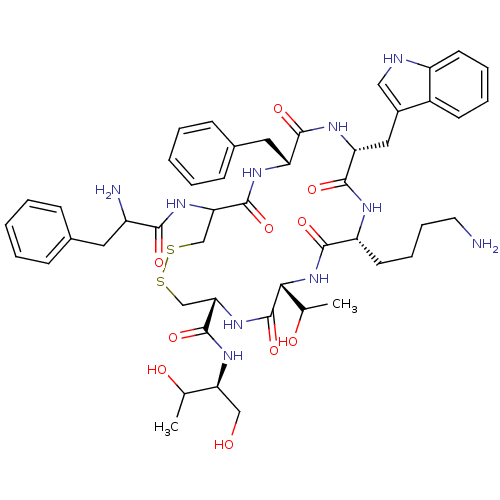
(10-(4-Amino-butyl)-19-(2-amino-3-phenyl-propionyla...)Show SMILES CC(O)[C@H](CO)NC(=O)[C@@H]1CSSCC(NC(=O)C(N)Cc2ccccc2)C(=O)N[C@H](Cc2ccccc2)C(=O)N[C@H](Cc2c[nH]c3ccccc23)C(=O)N[C@H](CCCCN)C(=O)N[C@H](C(C)O)C(=O)N1 Show InChI InChI=1S/C49H66N10O10S2/c1-28(61)39(25-60)56-48(68)41-27-71-70-26-40(57-43(63)34(51)21-30-13-5-3-6-14-30)47(67)54-37(22-31-15-7-4-8-16-31)45(65)55-38(23-32-24-52-35-18-10-9-17-33(32)35)46(66)53-36(19-11-12-20-50)44(64)59-42(29(2)62)49(69)58-41/h3-10,13-18,24,28-29,34,36-42,52,60-62H,11-12,19-23,25-27,50-51H2,1-2H3,(H,53,66)(H,54,67)(H,55,65)(H,56,68)(H,57,63)(H,58,69)(H,59,64)/t28?,29?,34?,36-,37-,38-,39+,40?,41+,42-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by PDSP Ki Database
| |
Proc Natl Acad Sci U S A 95: 10836-41 (1998)
Article DOI: 10.1073/pnas.95.18.10836
BindingDB Entry DOI: 10.7270/Q2XW4HCM |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50095027

((2,3-Dimethoxy-phenyl)-{1-[2-(4-fluoro-phenyl)-eth...)Show SMILES COc1cccc([C@H](O)C2CCN(CCc3ccc(F)cc3)CC2)c1OC |r| Show InChI InChI=1S/C22H28FNO3/c1-26-20-5-3-4-19(22(20)27-2)21(25)17-11-14-24(15-12-17)13-10-16-6-8-18(23)9-7-16/h3-9,17,21,25H,10-15H2,1-2H3/t21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme
Curated by ChEMBL
| Assay Description
Binding affinity for displacement of [3H]ketanserin to human 5-hydroxytryptamine 2A receptor stably expressed in CHO cells |
J Med Chem 45: 492-503 (2002)
BindingDB Entry DOI: 10.7270/Q2KH0P27 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50108690

(1-(2,4-difluorophenethyl)-4-(phenylsulfonyl)piperi...)Show SMILES Fc1ccc(CCN2CCC(CC2)S(=O)(=O)c2ccccc2)c(F)c1 Show InChI InChI=1S/C19H21F2NO2S/c20-16-7-6-15(19(21)14-16)8-11-22-12-9-18(10-13-22)25(23,24)17-4-2-1-3-5-17/h1-7,14,18H,8-13H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme
Curated by ChEMBL
| Assay Description
Binding affinity for displacement of [3H]ketanserin to human 5-hydroxytryptamine 2A receptor stably expressed in CHO cells |
J Med Chem 45: 492-503 (2002)
BindingDB Entry DOI: 10.7270/Q2KH0P27 |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50289127
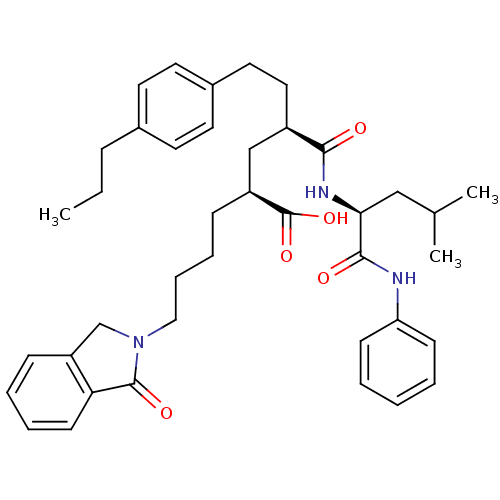
((2S,4R)-4-(3-Methyl-1-phenylcarbamoyl-butylcarbamo...)Show SMILES CCCc1ccc(CC[C@H](C[C@H](CCCCN2Cc3ccccc3C2=O)C(O)=O)C(=O)N[C@@H](CC(C)C)C(=O)Nc2ccccc2)cc1 Show InChI InChI=1S/C40H51N3O5/c1-4-12-29-18-20-30(21-19-29)22-23-31(37(44)42-36(25-28(2)3)38(45)41-34-15-6-5-7-16-34)26-32(40(47)48)13-10-11-24-43-27-33-14-8-9-17-35(33)39(43)46/h5-9,14-21,28,31-32,36H,4,10-13,22-27H2,1-3H3,(H,41,45)(H,42,44)(H,47,48)/t31-,32+,36+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of matrix metalloproteinase-3(MMP-3). |
Bioorg Med Chem Lett 6: 803-806 (1996)
Article DOI: 10.1016/0960-894X(96)00109-6
BindingDB Entry DOI: 10.7270/Q2HM58FH |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50108688
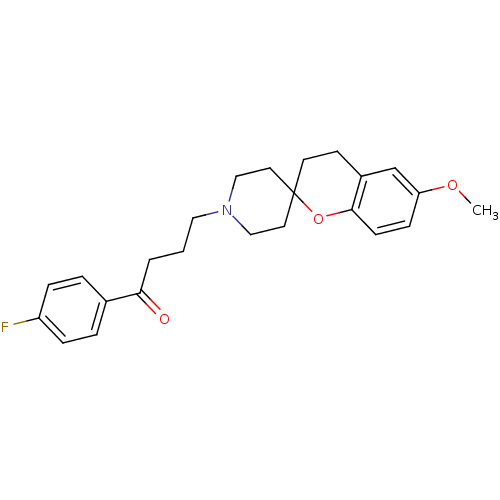
(1-(4-fluorophenyl)-4-[6-methoxyspiro[3,4-dihydro-2...)Show SMILES COc1ccc2OC3(CCN(CCCC(=O)c4ccc(F)cc4)CC3)CCc2c1 Show InChI InChI=1S/C24H28FNO3/c1-28-21-8-9-23-19(17-21)10-11-24(29-23)12-15-26(16-13-24)14-2-3-22(27)18-4-6-20(25)7-5-18/h4-9,17H,2-3,10-16H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 0.360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme
Curated by ChEMBL
| Assay Description
Binding affinity for displacement of [3H]ketanserin to human 5-hydroxytryptamine 2A receptor stably expressed in CHO cells |
J Med Chem 45: 492-503 (2002)
BindingDB Entry DOI: 10.7270/Q2KH0P27 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 1
(Homo sapiens (Human)) | BDBM81767

(15-28-Somatostatin-28 | CAS_38916-34-6 | CB6417646...)Show SMILES C[C@@H](O)[C@@H]1NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@@H](NC(=O)[C@H](CCCCN)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CSSC[C@H](NC(=O)[C@H](CO)NC1=O)C(O)=O)NC(=O)CNC(=O)[C@H](C)N)[C@@H](C)O |r| Show InChI InChI=1S/C76H104N18O19S2/c1-41(79)64(100)82-37-61(99)83-58-39-114-115-40-59(76(112)113)92-72(108)57(38-95)91-75(111)63(43(3)97)94-71(107)54(33-46-23-11-6-12-24-46)90-74(110)62(42(2)96)93-66(102)51(28-16-18-30-78)84-69(105)55(34-47-36-81-49-26-14-13-25-48(47)49)88-68(104)53(32-45-21-9-5-10-22-45)86-67(103)52(31-44-19-7-4-8-20-44)87-70(106)56(35-60(80)98)89-65(101)50(85-73(58)109)27-15-17-29-77/h4-14,19-26,36,41-43,50-59,62-63,81,95-97H,15-18,27-35,37-40,77-79H2,1-3H3,(H2,80,98)(H,82,100)(H,83,99)(H,84,105)(H,85,109)(H,86,103)(H,87,106)(H,88,104)(H,89,101)(H,90,110)(H,91,111)(H,92,108)(H,93,102)(H,94,107)(H,112,113)/t41-,42+,43+,50-,51-,52-,53-,54-,55-,56-,57-,58-,59-,62-,63-/m0/s1 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by PDSP Ki Database
| |
Proc Natl Acad Sci U S A 95: 10836-41 (1998)
Article DOI: 10.1073/pnas.95.18.10836
BindingDB Entry DOI: 10.7270/Q2XW4HCM |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Rattus norvegicus (rat)) | BDBM50108688

(1-(4-fluorophenyl)-4-[6-methoxyspiro[3,4-dihydro-2...)Show SMILES COc1ccc2OC3(CCN(CCCC(=O)c4ccc(F)cc4)CC3)CCc2c1 Show InChI InChI=1S/C24H28FNO3/c1-28-21-8-9-23-19(17-21)10-11-24(29-23)12-15-26(16-13-24)14-2-3-22(27)18-4-6-20(25)7-5-18/h4-9,17H,2-3,10-16H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme
Curated by ChEMBL
| Assay Description
Binding affinity for displacement of [3H]ketanserin to rat 5-hydroxytryptamine 2A receptor stably expressed in CHO cells |
J Med Chem 45: 492-503 (2002)
BindingDB Entry DOI: 10.7270/Q2KH0P27 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50036756
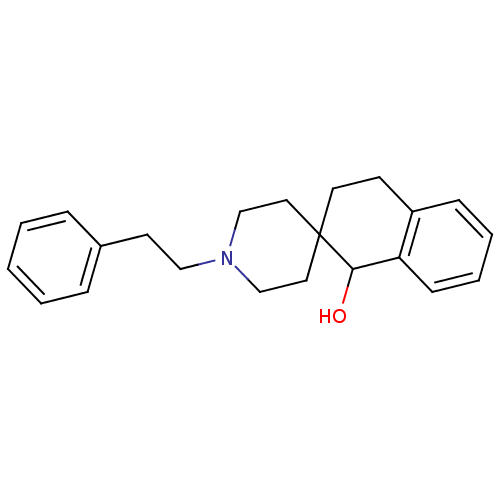
(1'-phenethylspiro[1,2,3,4-tetrahydronaphthalene-2,...)Show InChI InChI=1S/C22H27NO/c24-21-20-9-5-4-8-19(20)10-12-22(21)13-16-23(17-14-22)15-11-18-6-2-1-3-7-18/h1-9,21,24H,10-17H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme
Curated by ChEMBL
| Assay Description
Binding affinity for displacement of [3H]ketanserin to human 5-hydroxytryptamine 2A receptor stably expressed in CHO cells |
J Med Chem 45: 492-503 (2002)
BindingDB Entry DOI: 10.7270/Q2KH0P27 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50108701
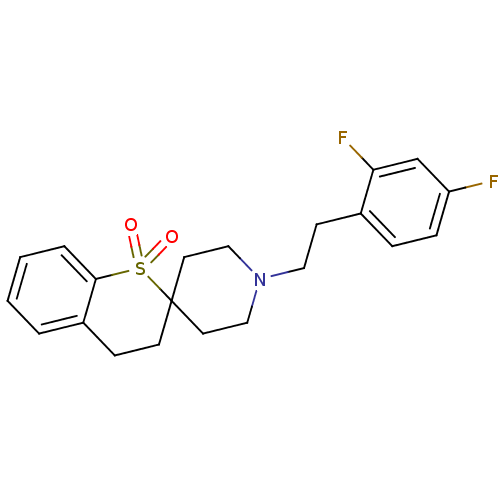
(1'-[2-(2,4-Difluorophenyl)ethyl]-3,4-dihydrospiro[...)Show SMILES Fc1ccc(CCN2CCC3(CC2)CCc2ccccc2S3(=O)=O)c(F)c1 Show InChI InChI=1S/C21H23F2NO2S/c22-18-6-5-16(19(23)15-18)8-12-24-13-10-21(11-14-24)9-7-17-3-1-2-4-20(17)27(21,25)26/h1-6,15H,7-14H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 0.410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme
Curated by ChEMBL
| Assay Description
Binding affinity for displacement of [3H]ketanserin to human 5-hydroxytryptamine 2A receptor stably expressed in CHO cells |
J Med Chem 45: 492-503 (2002)
BindingDB Entry DOI: 10.7270/Q2KH0P27 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50108699
(1-(2,4-difluorophenethyl)-4-(4-fluorophenylsulfony...)Show SMILES Fc1ccc(cc1)S(=O)(=O)C1CCN(CCc2ccc(F)cc2F)CC1 Show InChI InChI=1S/C19H20F3NO2S/c20-15-3-5-17(6-4-15)26(24,25)18-8-11-23(12-9-18)10-7-14-1-2-16(21)13-19(14)22/h1-6,13,18H,7-12H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme
Curated by ChEMBL
| Assay Description
Binding affinity to human 5HT2A |
Bioorg Med Chem Lett 16: 3201-4 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.050
BindingDB Entry DOI: 10.7270/Q20K2867 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50108699

(1-(2,4-difluorophenethyl)-4-(4-fluorophenylsulfony...)Show SMILES Fc1ccc(cc1)S(=O)(=O)C1CCN(CCc2ccc(F)cc2F)CC1 Show InChI InChI=1S/C19H20F3NO2S/c20-15-3-5-17(6-4-15)26(24,25)18-8-11-23(12-9-18)10-7-14-1-2-16(21)13-19(14)22/h1-6,13,18H,7-12H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme
Curated by ChEMBL
| Assay Description
Binding affinity for displacement of [3H]ketanserin to human 5-hydroxytryptamine 2A receptor stably expressed in CHO cells |
J Med Chem 45: 492-503 (2002)
BindingDB Entry DOI: 10.7270/Q2KH0P27 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM50064778
((5S,8R,11R,14R,17R,19aR)-11-(4-Amino-butyl)-17-ben...)Show SMILES CC(O)[C@H]1NC(=O)[C@@H](Cc2ccccc2)NC(=O)[C@H]2CCCN2C(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@@H](Cc2c[nH]c3ccccc23)NC(=O)[C@@H](CCCCN)NC1=O Show InChI InChI=1S/C44H54N8O8/c1-26(53)38-43(59)47-33(14-7-8-20-45)39(55)48-35(24-29-25-46-32-13-6-5-12-31(29)32)40(56)50-36(23-28-16-18-30(54)19-17-28)44(60)52-21-9-15-37(52)42(58)49-34(41(57)51-38)22-27-10-3-2-4-11-27/h2-6,10-13,16-19,25-26,33-38,46,53-54H,7-9,14-15,20-24,45H2,1H3,(H,47,59)(H,48,55)(H,49,58)(H,50,56)(H,51,57)/t26?,33-,34-,35-,36+,37-,38-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by PDSP Ki Database
| |
Proc Natl Acad Sci U S A 95: 10836-41 (1998)
Article DOI: 10.1073/pnas.95.18.10836
BindingDB Entry DOI: 10.7270/Q2XW4HCM |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50108696
(3-[[1-(2-(2,4-Difluorophenyl)ethyl)-4-piperidinyl]...)Show SMILES NC(=O)c1cccc(c1)S(=O)(=O)C1CCN(CCc2ccc(F)cc2F)CC1 Show InChI InChI=1S/C20H22F2N2O3S/c21-16-5-4-14(19(22)13-16)6-9-24-10-7-17(8-11-24)28(26,27)18-3-1-2-15(12-18)20(23)25/h1-5,12-13,17H,6-11H2,(H2,23,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme
Curated by ChEMBL
| Assay Description
Binding affinity for displacement of [3H]ketanserin to human 5-hydroxytryptamine 2A receptor stably expressed in CHO cells |
J Med Chem 45: 492-503 (2002)
BindingDB Entry DOI: 10.7270/Q2KH0P27 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Rattus norvegicus (rat)) | BDBM50108705
(1-(4-Fluorophenyl)-2-[4-(phenylsulfonyl)-1-piperid...)Show SMILES Fc1ccc(cc1)C(=O)CN1CCC(CC1)S(=O)(=O)c1ccccc1 Show InChI InChI=1S/C19H20FNO3S/c20-16-8-6-15(7-9-16)19(22)14-21-12-10-18(11-13-21)25(23,24)17-4-2-1-3-5-17/h1-9,18H,10-14H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme
Curated by ChEMBL
| Assay Description
Binding affinity for displacement of [3H]ketanserin to rat 5-hydroxytryptamine 2A receptor stably expressed in CHO cells |
J Med Chem 45: 492-503 (2002)
BindingDB Entry DOI: 10.7270/Q2KH0P27 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50108706
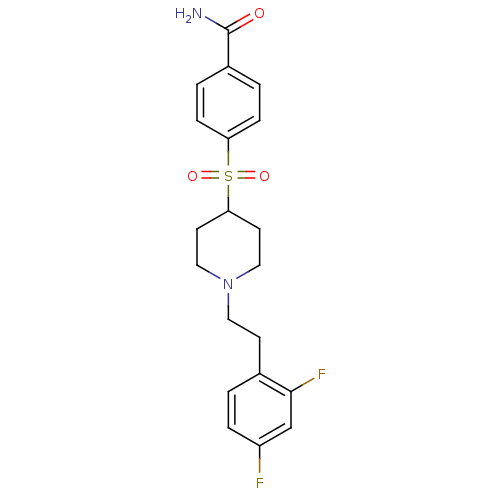
(4-[[1-(2-(2,4-Difluorophenyl)ethyl)-4-piperidinyl]...)Show SMILES NC(=O)c1ccc(cc1)S(=O)(=O)C1CCN(CCc2ccc(F)cc2F)CC1 Show InChI InChI=1S/C20H22F2N2O3S/c21-16-4-1-14(19(22)13-16)7-10-24-11-8-18(9-12-24)28(26,27)17-5-2-15(3-6-17)20(23)25/h1-6,13,18H,7-12H2,(H2,23,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme
Curated by ChEMBL
| Assay Description
Binding affinity for displacement of [3H]ketanserin to human 5-hydroxytryptamine 2A receptor stably expressed in CHO cells |
J Med Chem 45: 492-503 (2002)
BindingDB Entry DOI: 10.7270/Q2KH0P27 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50108694
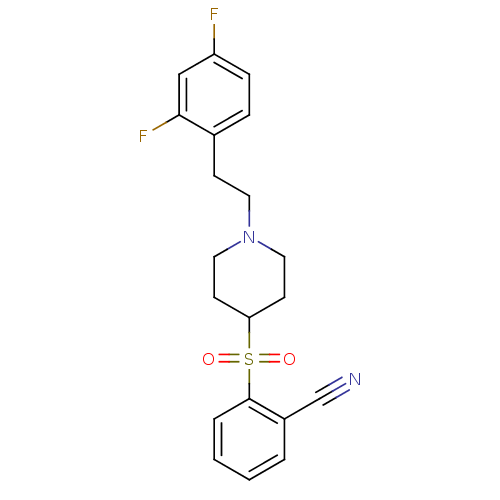
(2-[[1-(2-(2,4-Difluorophenyl)ethyl)-4-piperidinyl]...)Show SMILES Fc1ccc(CCN2CCC(CC2)S(=O)(=O)c2ccccc2C#N)c(F)c1 Show InChI InChI=1S/C20H20F2N2O2S/c21-17-6-5-15(19(22)13-17)7-10-24-11-8-18(9-12-24)27(25,26)20-4-2-1-3-16(20)14-23/h1-6,13,18H,7-12H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme
Curated by ChEMBL
| Assay Description
Binding affinity for displacement of [3H]ketanserin to human 5-hydroxytryptamine 2A receptor stably expressed in CHO cells |
J Med Chem 45: 492-503 (2002)
BindingDB Entry DOI: 10.7270/Q2KH0P27 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Rattus norvegicus (rat)) | BDBM50108707
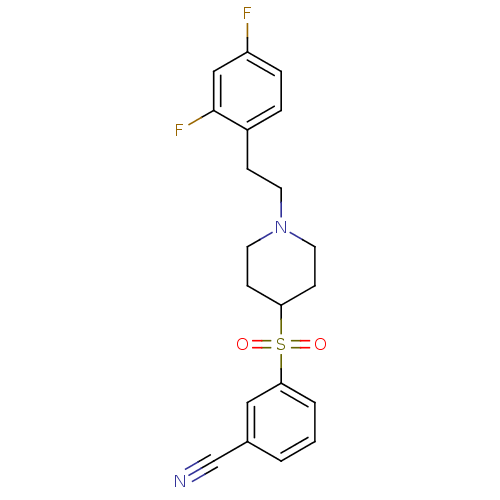
(3-[[1-(2-(2,4-Difluorophenyl)ethyl)-4-piperidinyl]...)Show SMILES Fc1ccc(CCN2CCC(CC2)S(=O)(=O)c2cccc(c2)C#N)c(F)c1 Show InChI InChI=1S/C20H20F2N2O2S/c21-17-5-4-16(20(22)13-17)6-9-24-10-7-18(8-11-24)27(25,26)19-3-1-2-15(12-19)14-23/h1-5,12-13,18H,6-11H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.540 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme
Curated by ChEMBL
| Assay Description
Binding affinity for displacement of [3H]ketanserin to rat 5-hydroxytryptamine 2A receptor stably expressed in CHO cells |
J Med Chem 45: 492-503 (2002)
BindingDB Entry DOI: 10.7270/Q2KH0P27 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Rattus norvegicus (rat)) | BDBM50036756

(1'-phenethylspiro[1,2,3,4-tetrahydronaphthalene-2,...)Show InChI InChI=1S/C22H27NO/c24-21-20-9-5-4-8-19(20)10-12-22(21)13-16-23(17-14-22)15-11-18-6-2-1-3-7-18/h1-9,21,24H,10-17H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.590 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme
Curated by ChEMBL
| Assay Description
Binding affinity for displacement of [3H]ketanserin to rat 5-hydroxytryptamine 2A receptor stably expressed in CHO cells |
J Med Chem 45: 492-503 (2002)
BindingDB Entry DOI: 10.7270/Q2KH0P27 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Rattus norvegicus (rat)) | BDBM50108701

(1'-[2-(2,4-Difluorophenyl)ethyl]-3,4-dihydrospiro[...)Show SMILES Fc1ccc(CCN2CCC3(CC2)CCc2ccccc2S3(=O)=O)c(F)c1 Show InChI InChI=1S/C21H23F2NO2S/c22-18-6-5-16(19(23)15-18)8-12-24-13-10-21(11-14-24)9-7-17-3-1-2-4-20(17)27(21,25)26/h1-6,15H,7-14H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 0.610 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme
Curated by ChEMBL
| Assay Description
Binding affinity for displacement of [3H]ketanserin to rat 5-hydroxytryptamine 2A receptor stably expressed in CHO cells |
J Med Chem 45: 492-503 (2002)
BindingDB Entry DOI: 10.7270/Q2KH0P27 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50108707

(3-[[1-(2-(2,4-Difluorophenyl)ethyl)-4-piperidinyl]...)Show SMILES Fc1ccc(CCN2CCC(CC2)S(=O)(=O)c2cccc(c2)C#N)c(F)c1 Show InChI InChI=1S/C20H20F2N2O2S/c21-17-5-4-16(20(22)13-17)6-9-24-10-7-18(8-11-24)27(25,26)19-3-1-2-15(12-19)14-23/h1-5,12-13,18H,6-11H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.660 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme
Curated by ChEMBL
| Assay Description
Binding affinity for displacement of [3H]ketanserin to human 5-hydroxytryptamine 2A receptor stably expressed in CHO cells |
J Med Chem 45: 492-503 (2002)
BindingDB Entry DOI: 10.7270/Q2KH0P27 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 3
(Homo sapiens (Human)) | BDBM81767

(15-28-Somatostatin-28 | CAS_38916-34-6 | CB6417646...)Show SMILES C[C@@H](O)[C@@H]1NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@@H](NC(=O)[C@H](CCCCN)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CSSC[C@H](NC(=O)[C@H](CO)NC1=O)C(O)=O)NC(=O)CNC(=O)[C@H](C)N)[C@@H](C)O |r| Show InChI InChI=1S/C76H104N18O19S2/c1-41(79)64(100)82-37-61(99)83-58-39-114-115-40-59(76(112)113)92-72(108)57(38-95)91-75(111)63(43(3)97)94-71(107)54(33-46-23-11-6-12-24-46)90-74(110)62(42(2)96)93-66(102)51(28-16-18-30-78)84-69(105)55(34-47-36-81-49-26-14-13-25-48(47)49)88-68(104)53(32-45-21-9-5-10-22-45)86-67(103)52(31-44-19-7-4-8-20-44)87-70(106)56(35-60(80)98)89-65(101)50(85-73(58)109)27-15-17-29-77/h4-14,19-26,36,41-43,50-59,62-63,81,95-97H,15-18,27-35,37-40,77-79H2,1-3H3,(H2,80,98)(H,82,100)(H,83,99)(H,84,105)(H,85,109)(H,86,103)(H,87,106)(H,88,104)(H,89,101)(H,90,110)(H,91,111)(H,92,108)(H,93,102)(H,94,107)(H,112,113)/t41-,42+,43+,50-,51-,52-,53-,54-,55-,56-,57-,58-,59-,62-,63-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.660 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by PDSP Ki Database
| |
Proc Natl Acad Sci U S A 95: 10836-41 (1998)
Article DOI: 10.1073/pnas.95.18.10836
BindingDB Entry DOI: 10.7270/Q2XW4HCM |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Rattus norvegicus (rat)) | BDBM50108700
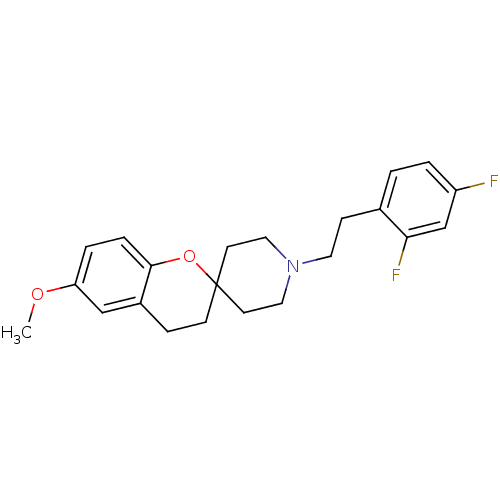
(1'-(2,4-difluorophenethyl)-6-methoxyspiro[3,4-dihy...)Show InChI InChI=1S/C22H25F2NO2/c1-26-19-4-5-21-17(14-19)6-8-22(27-21)9-12-25(13-10-22)11-7-16-2-3-18(23)15-20(16)24/h2-5,14-15H,6-13H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.690 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme
Curated by ChEMBL
| Assay Description
Binding affinity for displacement of [3H]ketanserin to rat 5-hydroxytryptamine 2A receptor stably expressed in CHO cells |
J Med Chem 45: 492-503 (2002)
BindingDB Entry DOI: 10.7270/Q2KH0P27 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Rattus norvegicus (rat)) | BDBM50108697
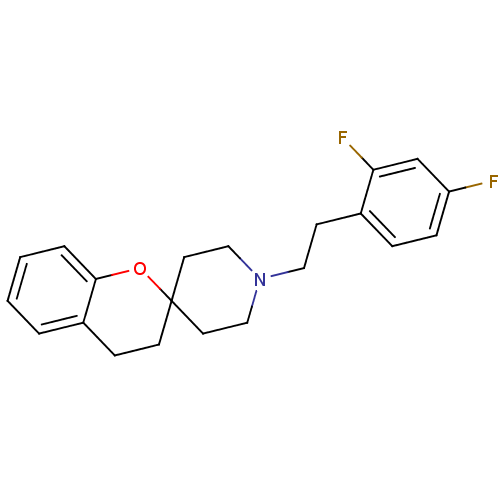
(1'-(2,4-difluorophenethyl)spiro[3,4-dihydro-2H-chr...)Show InChI InChI=1S/C21H23F2NO/c22-18-6-5-16(19(23)15-18)8-12-24-13-10-21(11-14-24)9-7-17-3-1-2-4-20(17)25-21/h1-6,15H,7-14H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.720 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme
Curated by ChEMBL
| Assay Description
Binding affinity for displacement of [3H]ketanserin to rat 5-hydroxytryptamine 2A receptor stably expressed in CHO cells |
J Med Chem 45: 492-503 (2002)
BindingDB Entry DOI: 10.7270/Q2KH0P27 |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM10355

((3S)-3-[(2S)-2-[(2S)-2-[(2S)-2-acetamido-3-(4-hydr...)Show SMILES CC(C)[C@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(C)=O)C(=O)N[C@@H](C)C(=O)N[C@@H](CC(O)=O)C=O |r| Show InChI InChI=1S/C23H32N4O8/c1-12(2)20(23(35)24-13(3)21(33)26-16(11-28)10-19(31)32)27-22(34)18(25-14(4)29)9-15-5-7-17(30)8-6-15/h5-8,11-13,16,18,20,30H,9-10H2,1-4H3,(H,24,35)(H,25,29)(H,26,33)(H,27,34)(H,31,32)/t13-,16-,18-,20-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
| 0.760 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
The compound was tested for inhibition of IL-1 beta converting enzyme in whole human blood. |
Bioorg Med Chem Lett 2: 613-618 (1992)
Article DOI: 10.1016/S0960-894X(01)81209-9
BindingDB Entry DOI: 10.7270/Q2V69JH6 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50185775
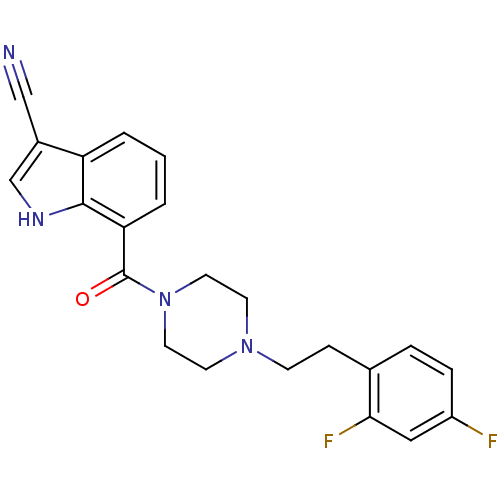
(7-(1-(2,4-difluorophenethyl)piperazine-4-carbonyl)...)Show SMILES Fc1ccc(CCN2CCN(CC2)C(=O)c2cccc3c(c[nH]c23)C#N)c(F)c1 Show InChI InChI=1S/C22H20F2N4O/c23-17-5-4-15(20(24)12-17)6-7-27-8-10-28(11-9-27)22(29)19-3-1-2-18-16(13-25)14-26-21(18)19/h1-5,12,14,26H,6-11H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.870 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme
Curated by ChEMBL
| Assay Description
Binding affinity to human 5HT2A |
Bioorg Med Chem Lett 16: 3201-4 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.050
BindingDB Entry DOI: 10.7270/Q20K2867 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Rattus norvegicus (rat)) | BDBM50108706

(4-[[1-(2-(2,4-Difluorophenyl)ethyl)-4-piperidinyl]...)Show SMILES NC(=O)c1ccc(cc1)S(=O)(=O)C1CCN(CCc2ccc(F)cc2F)CC1 Show InChI InChI=1S/C20H22F2N2O3S/c21-16-4-1-14(19(22)13-16)7-10-24-11-8-18(9-12-24)28(26,27)17-5-2-15(3-6-17)20(23)25/h1-6,13,18H,7-12H2,(H2,23,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme
Curated by ChEMBL
| Assay Description
Binding affinity for displacement of [3H]ketanserin to rat 5-hydroxytryptamine 2A receptor stably expressed in CHO cells |
J Med Chem 45: 492-503 (2002)
BindingDB Entry DOI: 10.7270/Q2KH0P27 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50108703
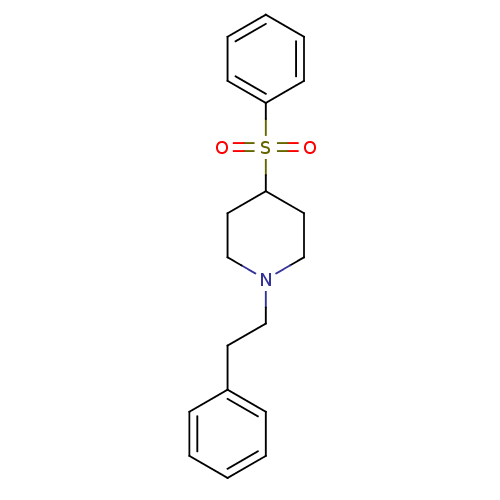
(1-(2-Phenylethyl)-4-(phenylsulfonyl)piperidine | 4...)Show InChI InChI=1S/C19H23NO2S/c21-23(22,18-9-5-2-6-10-18)19-12-15-20(16-13-19)14-11-17-7-3-1-4-8-17/h1-10,19H,11-16H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme
Curated by ChEMBL
| Assay Description
Binding affinity for displacement of [3H]ketanserin to human 5-hydroxytryptamine 2A receptor stably expressed in CHO cells |
J Med Chem 45: 492-503 (2002)
BindingDB Entry DOI: 10.7270/Q2KH0P27 |
More data for this
Ligand-Target Pair | |
Cholecystokinin
(GUINEA PIG) | BDBM82514
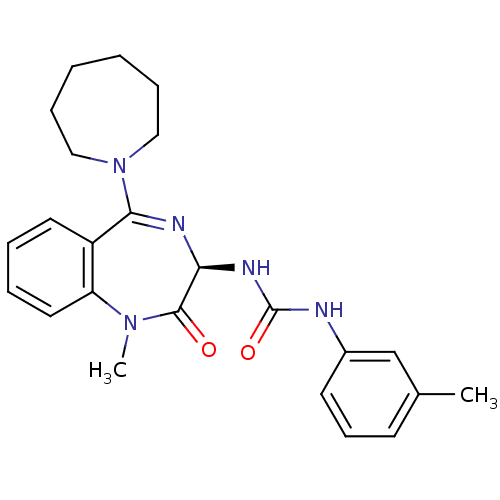
(1-[[(3R)-2,3-Dihydro-1-methyl-2-oxo-5-[(hexahydro-...)Show SMILES CN1c2ccccc2C(=N[C@@H](NC(=O)Nc2cccc(C)c2)C1=O)N1CCCCCC1 |c:9| Show InChI InChI=1S/C24H29N5O2/c1-17-10-9-11-18(16-17)25-24(31)27-21-23(30)28(2)20-13-6-5-12-19(20)22(26-21)29-14-7-3-4-8-15-29/h5-6,9-13,16,21H,3-4,7-8,14-15H2,1-2H3,(H2,25,27,31)/t21-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme Research Laboratories
Curated by PDSP Ki Database
| |
Mol Pharmacol 46: 943-8 (1994)
BindingDB Entry DOI: 10.7270/Q2QR4VMN |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50290032
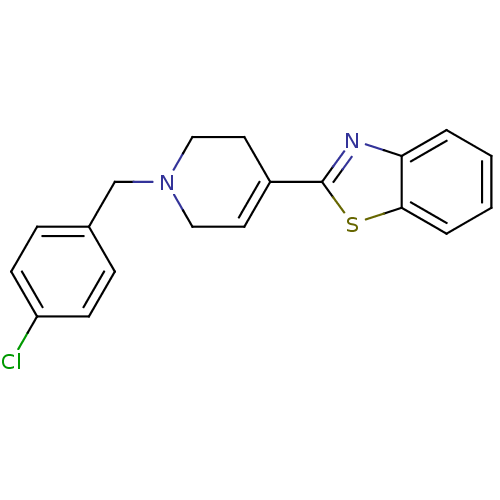
(2-[1-(4-Chloro-benzyl)-1,2,3,6-tetrahydro-pyridin-...)Show SMILES Clc1ccc(CN2CCC(=CC2)c2nc3ccccc3s2)cc1 |c:9| Show InChI InChI=1S/C19H17ClN2S/c20-16-7-5-14(6-8-16)13-22-11-9-15(10-12-22)19-21-17-3-1-2-4-18(17)23-19/h1-9H,10-13H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]-spiperone from human dopamine receptor subtype hD4 expressed in HEK293 cells |
Bioorg Med Chem Lett 7: 2211-2216 (1997)
Article DOI: 10.1016/S0960-894X(97)00402-2
BindingDB Entry DOI: 10.7270/Q2QR4X46 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50108689

(4-(4-Chloro-benzenesulfonyl)-1-[2-(2,4-difluoro-ph...)Show SMILES Fc1ccc(CCN2CCC(CC2)S(=O)(=O)c2ccc(Cl)cc2)c(F)c1 Show InChI InChI=1S/C19H20ClF2NO2S/c20-15-2-5-17(6-3-15)26(24,25)18-8-11-23(12-9-18)10-7-14-1-4-16(21)13-19(14)22/h1-6,13,18H,7-12H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme
Curated by ChEMBL
| Assay Description
Binding affinity for displacement of [3H]ketanserin to human 5-hydroxytryptamine 2A receptor stably expressed in CHO cells |
J Med Chem 45: 492-503 (2002)
BindingDB Entry DOI: 10.7270/Q2KH0P27 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Rattus norvegicus (rat)) | BDBM50108711
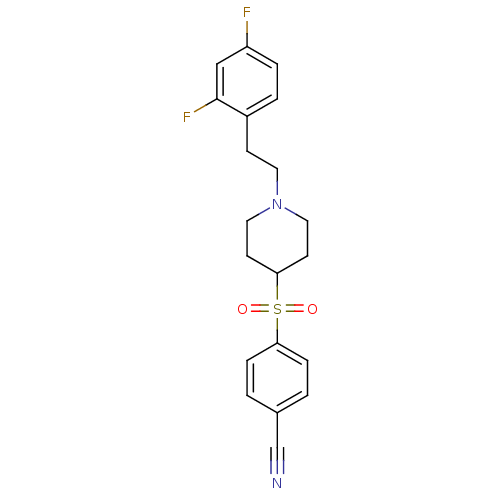
(4-[[1-(2-(2,4-Difluorophenyl)ethyl)-4-piperidinyl]...)Show SMILES Fc1ccc(CCN2CCC(CC2)S(=O)(=O)c2ccc(cc2)C#N)c(F)c1 Show InChI InChI=1S/C20H20F2N2O2S/c21-17-4-3-16(20(22)13-17)7-10-24-11-8-19(9-12-24)27(25,26)18-5-1-15(14-23)2-6-18/h1-6,13,19H,7-12H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme
Curated by ChEMBL
| Assay Description
Binding affinity for displacement of [3H]ketanserin to rat 5-hydroxytryptamine 2A receptor stably expressed in CHO cells |
J Med Chem 45: 492-503 (2002)
BindingDB Entry DOI: 10.7270/Q2KH0P27 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50185781
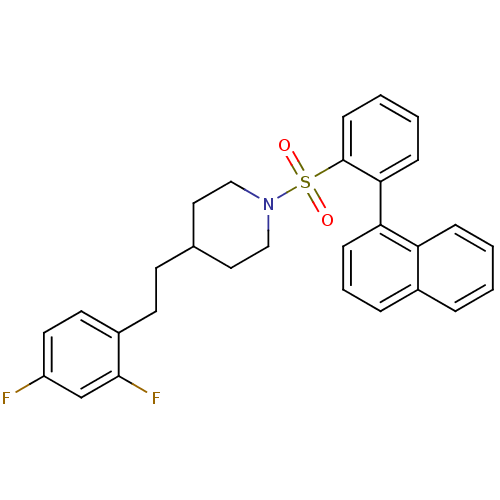
(4-(2,4-difluorophenethyl)-1-(2-(naphthalen-1-yl)ph...)Show SMILES Fc1ccc(CCC2CCN(CC2)S(=O)(=O)c2ccccc2-c2cccc3ccccc23)c(F)c1 Show InChI InChI=1S/C29H27F2NO2S/c30-24-15-14-23(28(31)20-24)13-12-21-16-18-32(19-17-21)35(33,34)29-11-4-3-9-27(29)26-10-5-7-22-6-1-2-8-25(22)26/h1-11,14-15,20-21H,12-13,16-19H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme
Curated by ChEMBL
| Assay Description
Binding affinity to human 5HT2A |
Bioorg Med Chem Lett 16: 3201-4 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.050
BindingDB Entry DOI: 10.7270/Q20K2867 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 4
(Homo sapiens (Human)) | BDBM81767

(15-28-Somatostatin-28 | CAS_38916-34-6 | CB6417646...)Show SMILES C[C@@H](O)[C@@H]1NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@@H](NC(=O)[C@H](CCCCN)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CSSC[C@H](NC(=O)[C@H](CO)NC1=O)C(O)=O)NC(=O)CNC(=O)[C@H](C)N)[C@@H](C)O |r| Show InChI InChI=1S/C76H104N18O19S2/c1-41(79)64(100)82-37-61(99)83-58-39-114-115-40-59(76(112)113)92-72(108)57(38-95)91-75(111)63(43(3)97)94-71(107)54(33-46-23-11-6-12-24-46)90-74(110)62(42(2)96)93-66(102)51(28-16-18-30-78)84-69(105)55(34-47-36-81-49-26-14-13-25-48(47)49)88-68(104)53(32-45-21-9-5-10-22-45)86-67(103)52(31-44-19-7-4-8-20-44)87-70(106)56(35-60(80)98)89-65(101)50(85-73(58)109)27-15-17-29-77/h4-14,19-26,36,41-43,50-59,62-63,81,95-97H,15-18,27-35,37-40,77-79H2,1-3H3,(H2,80,98)(H,82,100)(H,83,99)(H,84,105)(H,85,109)(H,86,103)(H,87,106)(H,88,104)(H,89,101)(H,90,110)(H,91,111)(H,92,108)(H,93,102)(H,94,107)(H,112,113)/t41-,42+,43+,50-,51-,52-,53-,54-,55-,56-,57-,58-,59-,62-,63-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.76 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by PDSP Ki Database
| |
Proc Natl Acad Sci U S A 95: 10836-41 (1998)
Article DOI: 10.1073/pnas.95.18.10836
BindingDB Entry DOI: 10.7270/Q2XW4HCM |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50108700

(1'-(2,4-difluorophenethyl)-6-methoxyspiro[3,4-dihy...)Show InChI InChI=1S/C22H25F2NO2/c1-26-19-4-5-21-17(14-19)6-8-22(27-21)9-12-25(13-10-22)11-7-16-2-3-18(23)15-20(16)24/h2-5,14-15H,6-13H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme
Curated by ChEMBL
| Assay Description
Binding affinity for displacement of [3H]ketanserin to human 5-hydroxytryptamine 2A receptor stably expressed in CHO cells |
J Med Chem 45: 492-503 (2002)
BindingDB Entry DOI: 10.7270/Q2KH0P27 |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50289128
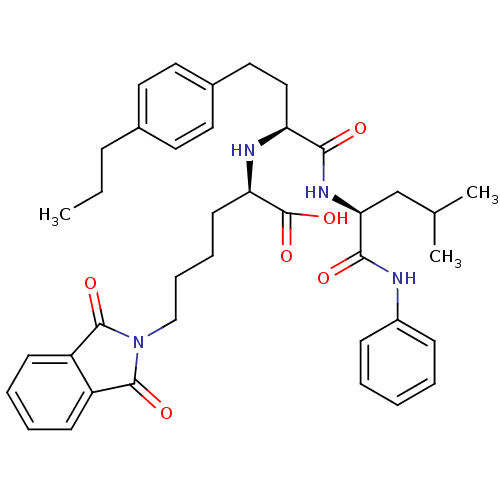
((R)-6-(1,3-Dioxo-1,3-dihydro-isoindol-2-yl)-2-[(S)...)Show SMILES CCCc1ccc(CC[C@H](N[C@H](CCCCN2C(=O)c3ccccc3C2=O)C(O)=O)C(=O)N[C@@H](CC(C)C)C(=O)Nc2ccccc2)cc1 Show InChI InChI=1S/C39H48N4O6/c1-4-12-27-18-20-28(21-19-27)22-23-32(35(44)42-34(25-26(2)3)36(45)40-29-13-6-5-7-14-29)41-33(39(48)49)17-10-11-24-43-37(46)30-15-8-9-16-31(30)38(43)47/h5-9,13-16,18-21,26,32-34,41H,4,10-12,17,22-25H2,1-3H3,(H,40,45)(H,42,44)(H,48,49)/t32-,33+,34-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of matrix metalloproteinase-3(MMP-3). |
Bioorg Med Chem Lett 6: 803-806 (1996)
Article DOI: 10.1016/0960-894X(96)00109-6
BindingDB Entry DOI: 10.7270/Q2HM58FH |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50108688

(1-(4-fluorophenyl)-4-[6-methoxyspiro[3,4-dihydro-2...)Show SMILES COc1ccc2OC3(CCN(CCCC(=O)c4ccc(F)cc4)CC3)CCc2c1 Show InChI InChI=1S/C24H28FNO3/c1-28-21-8-9-23-19(17-21)10-11-24(29-23)12-15-26(16-13-24)14-2-3-22(27)18-4-6-20(25)7-5-18/h4-9,17H,2-3,10-16H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme
Curated by ChEMBL
| Assay Description
Binding affinity for displacement of [3H]spiperone to human dopamine D2 (hD2) receptors stably expressed in CHO cells |
J Med Chem 45: 492-503 (2002)
BindingDB Entry DOI: 10.7270/Q2KH0P27 |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50057073
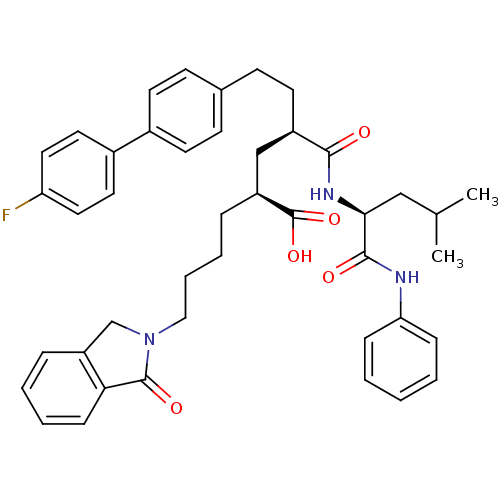
((2S,4R)-6-(4'-Fluoro-biphenyl-4-yl)-4-((S)-3-methy...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CCc1ccc(cc1)-c1ccc(F)cc1)C[C@H](CCCCN1Cc2ccccc2C1=O)C(O)=O)C(=O)Nc1ccccc1 Show InChI InChI=1S/C43H48FN3O5/c1-29(2)26-39(41(49)45-37-12-4-3-5-13-37)46-40(48)33(20-17-30-15-18-31(19-16-30)32-21-23-36(44)24-22-32)27-34(43(51)52)10-8-9-25-47-28-35-11-6-7-14-38(35)42(47)50/h3-7,11-16,18-19,21-24,29,33-34,39H,8-10,17,20,25-28H2,1-2H3,(H,45,49)(H,46,48)(H,51,52)/t33-,34+,39+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human stromelysin-1 (Matrix metalloproteinase-3) |
J Med Chem 40: 1026-40 (1997)
Article DOI: 10.1021/jm960465t
BindingDB Entry DOI: 10.7270/Q2H70DXR |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50057050
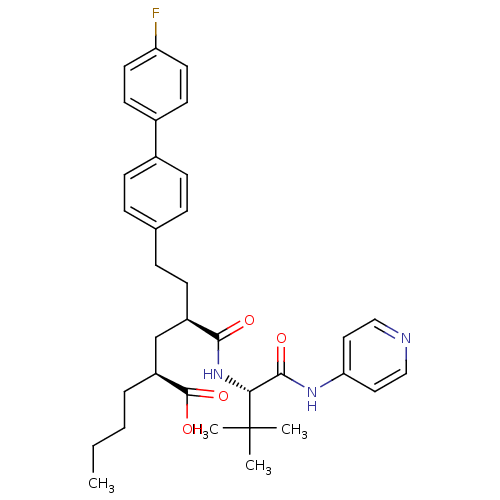
((2S,4R)-2-Butyl-4-[(S)-2,2-dimethyl-1-(pyridin-4-y...)Show SMILES CCCC[C@@H](C[C@@H](CCc1ccc(cc1)-c1ccc(F)cc1)C(=O)N[C@H](C(=O)Nc1ccncc1)C(C)(C)C)C(O)=O Show InChI InChI=1S/C34H42FN3O4/c1-5-6-7-27(33(41)42)22-26(13-10-23-8-11-24(12-9-23)25-14-16-28(35)17-15-25)31(39)38-30(34(2,3)4)32(40)37-29-18-20-36-21-19-29/h8-9,11-12,14-21,26-27,30H,5-7,10,13,22H2,1-4H3,(H,38,39)(H,41,42)(H,36,37,40)/t26-,27+,30-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human stromelysin-1 (Matrix metalloproteinase-3) |
J Med Chem 40: 1026-40 (1997)
Article DOI: 10.1021/jm960465t
BindingDB Entry DOI: 10.7270/Q2H70DXR |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50185778
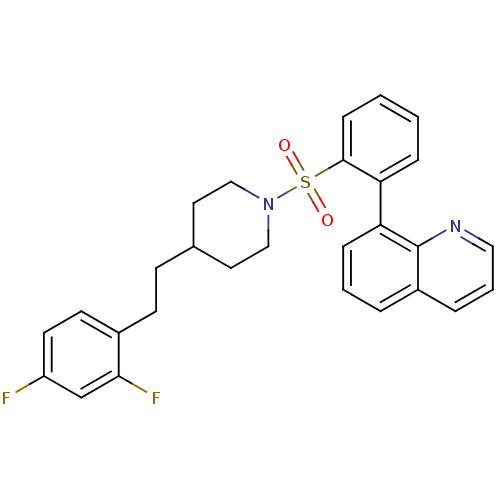
(8-(2-(4-(2,4-difluorophenethyl)piperidin-1-ylsulfo...)Show SMILES Fc1ccc(CCC2CCN(CC2)S(=O)(=O)c2ccccc2-c2cccc3cccnc23)c(F)c1 Show InChI InChI=1S/C28H26F2N2O2S/c29-23-13-12-21(26(30)19-23)11-10-20-14-17-32(18-15-20)35(33,34)27-9-2-1-7-24(27)25-8-3-5-22-6-4-16-31-28(22)25/h1-9,12-13,16,19-20H,10-11,14-15,17-18H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme
Curated by ChEMBL
| Assay Description
Binding affinity to human 5HT2A |
Bioorg Med Chem Lett 16: 3201-4 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.050
BindingDB Entry DOI: 10.7270/Q20K2867 |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50285557
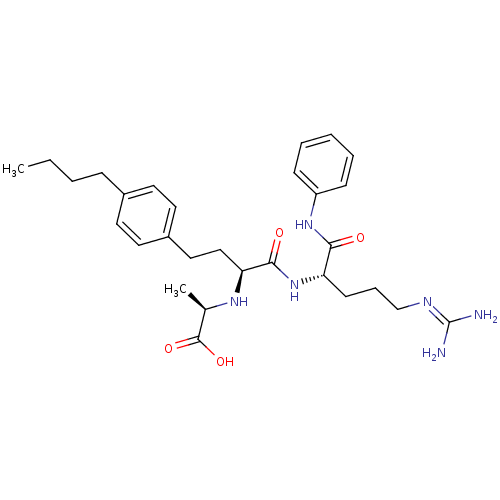
((R)-2-[(S)-3-(4-butyl-phenyl)-1-((S)-4-guanidino-1...)Show SMILES [#6]-[#6]-[#6]-[#6]-c1ccc(-[#6]-[#6]-[#6@H](-[#7]-[#6@H](-[#6])-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-c2ccccc2)cc1 Show InChI InChI=1S/C29H42N6O4/c1-3-4-9-21-13-15-22(16-14-21)17-18-25(33-20(2)28(38)39)27(37)35-24(12-8-19-32-29(30)31)26(36)34-23-10-6-5-7-11-23/h5-7,10-11,13-16,20,24-25,33H,3-4,8-9,12,17-19H2,1-2H3,(H,34,36)(H,35,37)(H,38,39)(H4,30,31,32)/t20-,24+,25+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against human gelatinase-A (MMP-2) |
Bioorg Med Chem Lett 5: 2441-2446 (1995)
Article DOI: 10.1016/0960-894X(95)00425-S
BindingDB Entry DOI: 10.7270/Q2D21XK6 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 5
(Homo sapiens (Human)) | BDBM81767

(15-28-Somatostatin-28 | CAS_38916-34-6 | CB6417646...)Show SMILES C[C@@H](O)[C@@H]1NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@@H](NC(=O)[C@H](CCCCN)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CSSC[C@H](NC(=O)[C@H](CO)NC1=O)C(O)=O)NC(=O)CNC(=O)[C@H](C)N)[C@@H](C)O |r| Show InChI InChI=1S/C76H104N18O19S2/c1-41(79)64(100)82-37-61(99)83-58-39-114-115-40-59(76(112)113)92-72(108)57(38-95)91-75(111)63(43(3)97)94-71(107)54(33-46-23-11-6-12-24-46)90-74(110)62(42(2)96)93-66(102)51(28-16-18-30-78)84-69(105)55(34-47-36-81-49-26-14-13-25-48(47)49)88-68(104)53(32-45-21-9-5-10-22-45)86-67(103)52(31-44-19-7-4-8-20-44)87-70(106)56(35-60(80)98)89-65(101)50(85-73(58)109)27-15-17-29-77/h4-14,19-26,36,41-43,50-59,62-63,81,95-97H,15-18,27-35,37-40,77-79H2,1-3H3,(H2,80,98)(H,82,100)(H,83,99)(H,84,105)(H,85,109)(H,86,103)(H,87,106)(H,88,104)(H,89,101)(H,90,110)(H,91,111)(H,92,108)(H,93,102)(H,94,107)(H,112,113)/t41-,42+,43+,50-,51-,52-,53-,54-,55-,56-,57-,58-,59-,62-,63-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by PDSP Ki Database
| |
Proc Natl Acad Sci U S A 95: 10836-41 (1998)
Article DOI: 10.1073/pnas.95.18.10836
BindingDB Entry DOI: 10.7270/Q2XW4HCM |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50108697

(1'-(2,4-difluorophenethyl)spiro[3,4-dihydro-2H-chr...)Show InChI InChI=1S/C21H23F2NO/c22-18-6-5-16(19(23)15-18)8-12-24-13-10-21(11-14-24)9-7-17-3-1-2-4-20(17)25-21/h1-6,15H,7-14H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme
Curated by ChEMBL
| Assay Description
Binding affinity for displacement of [3H]ketanserin to human 5-hydroxytryptamine 2A receptor stably expressed in CHO cells |
J Med Chem 45: 492-503 (2002)
BindingDB Entry DOI: 10.7270/Q2KH0P27 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data