 Found 119 hits with Last Name = 'chowdhury' and Initial = 'sf'
Found 119 hits with Last Name = 'chowdhury' and Initial = 'sf' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50081905
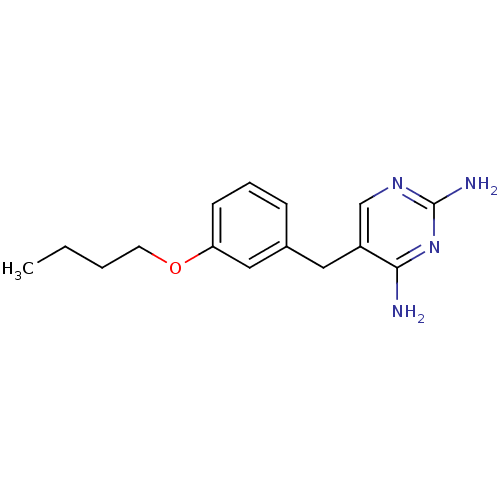
(5-(3-Butoxy-benzyl)-pyrimidine-2,4-diamine | CHEMB...)Show InChI InChI=1S/C15H20N4O/c1-2-3-7-20-13-6-4-5-11(9-13)8-12-10-18-15(17)19-14(12)16/h4-6,9-10H,2-3,7-8H2,1H3,(H4,16,17,18,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cardiff University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant Dihydrofolate reductase from humans. |
J Med Chem 42: 4300-12 (1999)
BindingDB Entry DOI: 10.7270/Q21R6PQG |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50081918
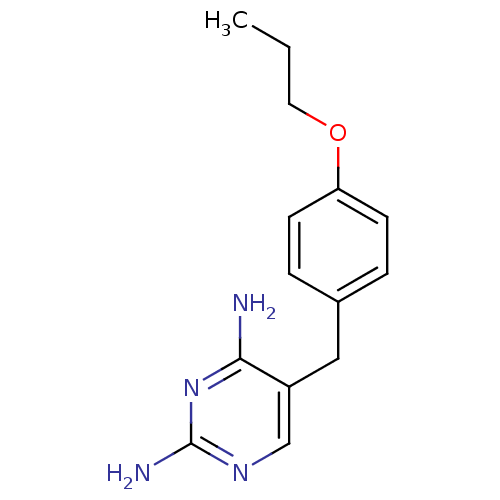
(5-(4-Propoxy-benzyl)-pyrimidine-2,4-diamine | CHEM...)Show InChI InChI=1S/C14H18N4O/c1-2-7-19-12-5-3-10(4-6-12)8-11-9-17-14(16)18-13(11)15/h3-6,9H,2,7-8H2,1H3,(H4,15,16,17,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 6.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cardiff University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant Dihydrofolate reductase from humans. |
J Med Chem 42: 4300-12 (1999)
BindingDB Entry DOI: 10.7270/Q21R6PQG |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50081916
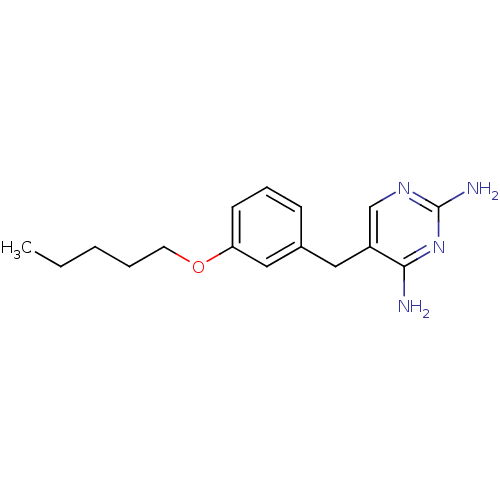
(5-(3-Pentyloxy-benzyl)-pyrimidine-2,4-diamine | CH...)Show InChI InChI=1S/C16H22N4O/c1-2-3-4-8-21-14-7-5-6-12(10-14)9-13-11-19-16(18)20-15(13)17/h5-7,10-11H,2-4,8-9H2,1H3,(H4,17,18,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 7.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cardiff University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant Dihydrofolate reductase from humans. |
J Med Chem 42: 4300-12 (1999)
BindingDB Entry DOI: 10.7270/Q21R6PQG |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50081907
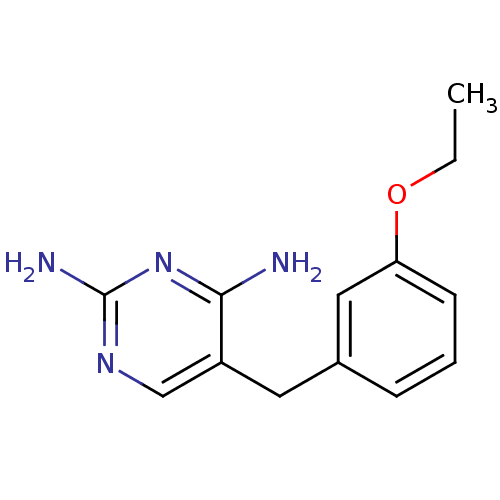
(5-(3-Ethoxy-benzyl)-pyrimidine-2,4-diamine | CHEMB...)Show InChI InChI=1S/C13H16N4O/c1-2-18-11-5-3-4-9(7-11)6-10-8-16-13(15)17-12(10)14/h3-5,7-8H,2,6H2,1H3,(H4,14,15,16,17) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 8.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cardiff University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant Dihydrofolate reductase from humans. |
J Med Chem 42: 4300-12 (1999)
BindingDB Entry DOI: 10.7270/Q21R6PQG |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50081922

(5-(3-Isopropoxy-benzyl)-pyrimidine-2,4-diamine | C...)Show InChI InChI=1S/C14H18N4O/c1-9(2)19-12-5-3-4-10(7-12)6-11-8-17-14(16)18-13(11)15/h3-5,7-9H,6H2,1-2H3,(H4,15,16,17,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 8.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cardiff University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant Dihydrofolate reductase from humans. |
J Med Chem 42: 4300-12 (1999)
BindingDB Entry DOI: 10.7270/Q21R6PQG |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50081908

(5-(3-Methoxy-benzyl)-pyrimidine-2,4-diamine | CHEM...)Show InChI InChI=1S/C12H14N4O/c1-17-10-4-2-3-8(6-10)5-9-7-15-12(14)16-11(9)13/h2-4,6-7H,5H2,1H3,(H4,13,14,15,16) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 9.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cardiff University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant Dihydrofolate reductase from humans. |
J Med Chem 42: 4300-12 (1999)
BindingDB Entry DOI: 10.7270/Q21R6PQG |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50081917
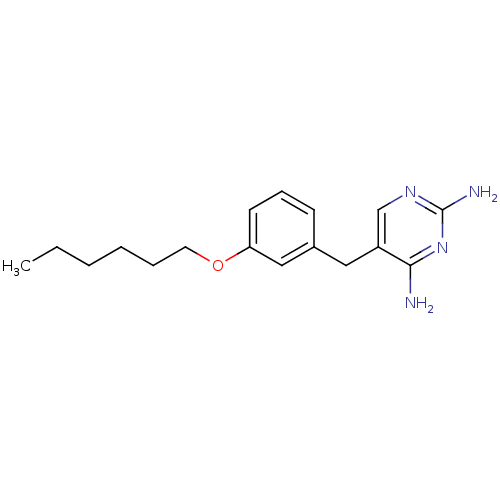
(5-(3-Hexyloxy-benzyl)-pyrimidine-2,4-diamine | CHE...)Show InChI InChI=1S/C17H24N4O/c1-2-3-4-5-9-22-15-8-6-7-13(11-15)10-14-12-20-17(19)21-16(14)18/h6-8,11-12H,2-5,9-10H2,1H3,(H4,18,19,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 9.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cardiff University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant Dihydrofolate reductase from humans. |
J Med Chem 42: 4300-12 (1999)
BindingDB Entry DOI: 10.7270/Q21R6PQG |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50081921

(5-(4-Ethoxy-benzyl)-pyrimidine-2,4-diamine | CHEMB...)Show InChI InChI=1S/C13H16N4O/c1-2-18-11-5-3-9(4-6-11)7-10-8-16-13(15)17-12(10)14/h3-6,8H,2,7H2,1H3,(H4,14,15,16,17) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 9.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cardiff University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant Dihydrofolate reductase from humans. |
J Med Chem 42: 4300-12 (1999)
BindingDB Entry DOI: 10.7270/Q21R6PQG |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM18069

(5-[(3,4,5-trimethoxyphenyl)methyl]pyrimidine-2,4-d...)Show InChI InChI=1S/C14H18N4O3/c1-19-10-5-8(6-11(20-2)12(10)21-3)4-9-7-17-14(16)18-13(9)15/h5-7H,4H2,1-3H3,(H4,15,16,17,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cardiff University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant Dihydrofolate reductase from humans. |
J Med Chem 42: 4300-12 (1999)
BindingDB Entry DOI: 10.7270/Q21R6PQG |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50081920
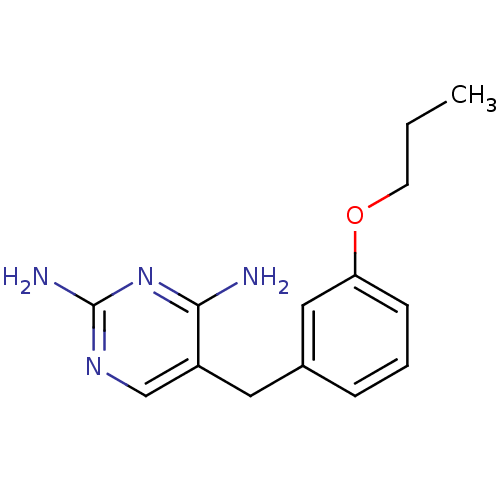
(5-(3-Propoxy-benzyl)-pyrimidine-2,4-diamine | CHEM...)Show InChI InChI=1S/C14H18N4O/c1-2-6-19-12-5-3-4-10(8-12)7-11-9-17-14(16)18-13(11)15/h3-5,8-9H,2,6-7H2,1H3,(H4,15,16,17,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 10.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cardiff University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant Dihydrofolate reductase from humans. |
J Med Chem 42: 4300-12 (1999)
BindingDB Entry DOI: 10.7270/Q21R6PQG |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50081909

(5-(3-(benzyloxy)benzyl)pyrimidine-2,4-diamine | 5-...)Show InChI InChI=1S/C18H18N4O/c19-17-15(11-21-18(20)22-17)9-14-7-4-8-16(10-14)23-12-13-5-2-1-3-6-13/h1-8,10-11H,9,12H2,(H4,19,20,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cardiff University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant Dihydrofolate reductase from humans. |
J Med Chem 42: 4300-12 (1999)
BindingDB Entry DOI: 10.7270/Q21R6PQG |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM18512

(5-(4-chlorophenyl)-6-ethylpyrimidine-2,4-diamine |...)Show InChI InChI=1S/C12H13ClN4/c1-2-9-10(11(14)17-12(15)16-9)7-3-5-8(13)6-4-7/h3-6H,2H2,1H3,(H4,14,15,16,17) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cardiff University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant Dihydrofolate reductase from humans. |
J Med Chem 42: 4300-12 (1999)
BindingDB Entry DOI: 10.7270/Q21R6PQG |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50081915

(5-[3-(Tetrahydro-pyran-2-yloxy)-benzyl]-pyrimidine...)Show InChI InChI=1S/C16H20N4O2/c17-15-12(10-19-16(18)20-15)8-11-4-3-5-13(9-11)22-14-6-1-2-7-21-14/h3-5,9-10,14H,1-2,6-8H2,(H4,17,18,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cardiff University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant Dihydrofolate reductase from humans. |
J Med Chem 42: 4300-12 (1999)
BindingDB Entry DOI: 10.7270/Q21R6PQG |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50121305
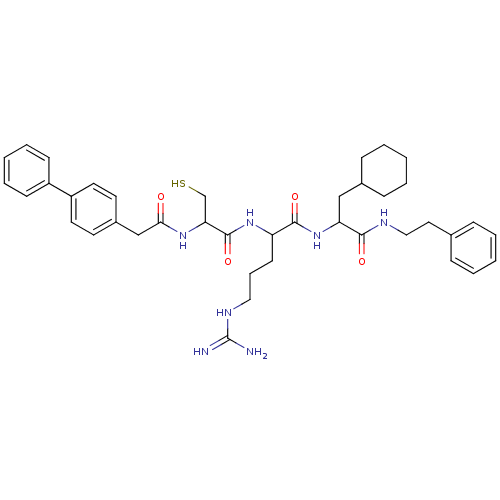
(2-[2-(2-Biphenyl-4-yl-acetylamino)-3-mercapto-prop...)Show SMILES NC(=N)NCCCC(NC(=O)C(CS)NC(=O)Cc1ccc(cc1)-c1ccccc1)C(=O)NC(CC1CCCCC1)C(=O)NCCc1ccccc1 Show InChI InChI=1S/C40H53N7O4S/c41-40(42)44-23-10-17-33(38(50)47-34(25-29-13-6-2-7-14-29)37(49)43-24-22-28-11-4-1-5-12-28)46-39(51)35(27-52)45-36(48)26-30-18-20-32(21-19-30)31-15-8-3-9-16-31/h1,3-5,8-9,11-12,15-16,18-21,29,33-35,52H,2,6-7,10,13-14,17,22-27H2,(H,43,49)(H,45,48)(H,46,51)(H,47,50)(H4,41,42,44) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Council of Canada
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Cathepsin L |
J Med Chem 45: 5321-9 (2002)
BindingDB Entry DOI: 10.7270/Q23B5ZH6 |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM21006
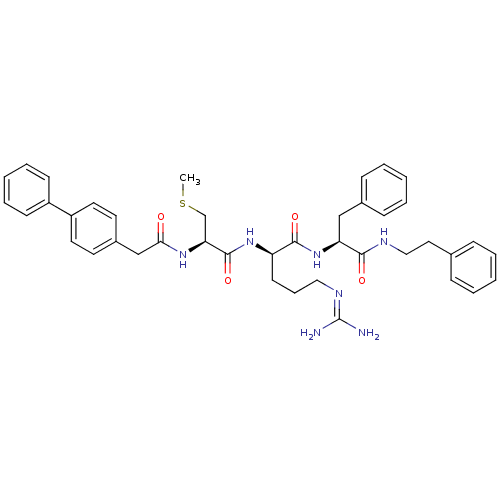
((2R)-5-[(diaminomethylidene)amino]-2-[(2R)-3-(meth...)Show SMILES [#6]-[#16]-[#6]-[#6@H](-[#7]-[#6](=O)-[#6]-c1ccc(cc1)-c1ccccc1)-[#6](=O)-[#7]-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccccc1)-[#6](=O)-[#7]-[#6]-[#6]-c1ccccc1 |r| Show InChI InChI=1S/C41H49N7O4S/c1-53-28-36(46-37(49)27-31-19-21-33(22-20-31)32-16-9-4-10-17-32)40(52)47-34(18-11-24-45-41(42)43)39(51)48-35(26-30-14-7-3-8-15-30)38(50)44-25-23-29-12-5-2-6-13-29/h2-10,12-17,19-22,34-36H,11,18,23-28H2,1H3,(H,44,50)(H,46,49)(H,47,52)(H,48,51)(H4,42,43,45)/t34-,35+,36+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 19 | -44.1 | n/a | n/a | n/a | n/a | n/a | 5.5 | 25 |
National Research Council Canada
| Assay Description
Fluorescence was monitored on a SPEX Fluorolog-2 spectrofluorometer with the excitation and emission wavelengths set at 380 and 440 nm, respectively.... |
J Med Chem 51: 1361-8 (2008)
Article DOI: 10.1021/jm701190v
BindingDB Entry DOI: 10.7270/Q21Z42QB |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50121291
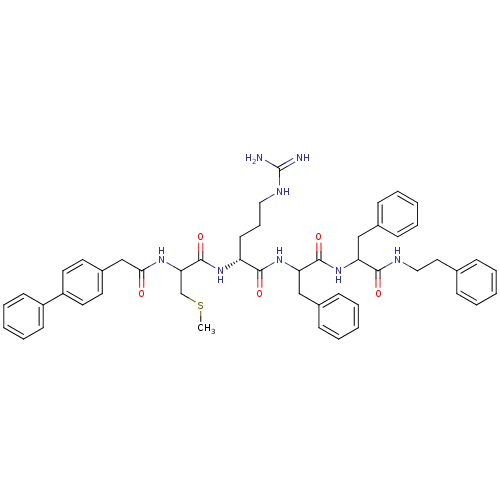
(2-[2-(2-Biphenyl-4-yl-acetylamino)-3-methylsulfany...)Show SMILES CSCC(NC(=O)Cc1ccc(cc1)-c1ccccc1)C(=O)N[C@H](CCCNC(N)=N)C(=O)NC(Cc1ccccc1)C(=O)NC(Cc1ccccc1)C(=O)NCCc1ccccc1 Show InChI InChI=1S/C50H58N8O5S/c1-64-34-44(55-45(59)33-38-24-26-40(27-25-38)39-21-12-5-13-22-39)49(63)56-41(23-14-29-54-50(51)52)47(61)58-43(32-37-19-10-4-11-20-37)48(62)57-42(31-36-17-8-3-9-18-36)46(60)53-30-28-35-15-6-2-7-16-35/h2-13,15-22,24-27,41-44H,14,23,28-34H2,1H3,(H,53,60)(H,55,59)(H,56,63)(H,57,62)(H,58,61)(H4,51,52,54)/t41-,42?,43?,44?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Council of Canada
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Cathepsin L |
J Med Chem 45: 5321-9 (2002)
BindingDB Entry DOI: 10.7270/Q23B5ZH6 |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50081906
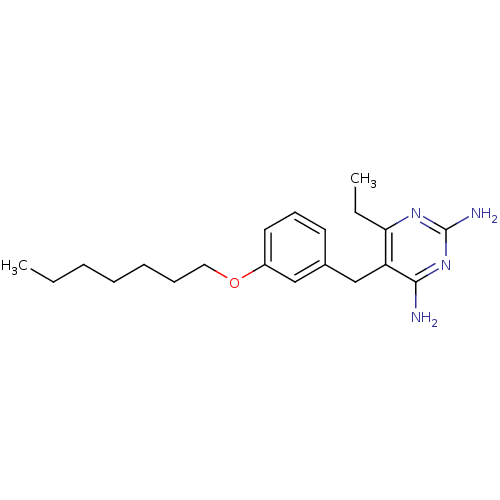
(6-Ethyl-5-(3-heptyloxy-benzyl)-pyrimidine-2,4-diam...)Show InChI InChI=1S/C20H30N4O/c1-3-5-6-7-8-12-25-16-11-9-10-15(13-16)14-17-18(4-2)23-20(22)24-19(17)21/h9-11,13H,3-8,12,14H2,1-2H3,(H4,21,22,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cardiff University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant Dihydrofolate reductase from humans. |
J Med Chem 42: 4300-12 (1999)
BindingDB Entry DOI: 10.7270/Q21R6PQG |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM20998
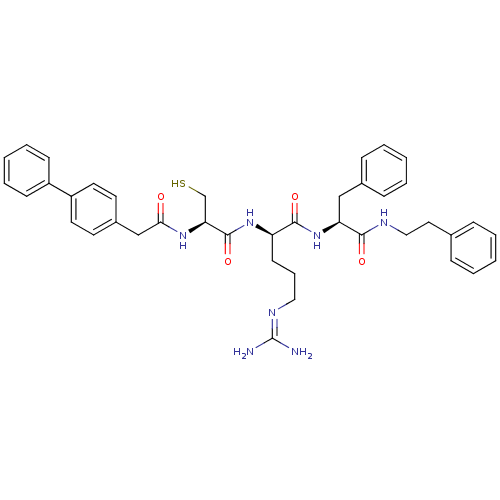
((2R)-5-[(diaminomethylidene)amino]-N-[(1S)-2-pheny...)Show SMILES [#7]\[#6](-[#7])=[#7]/[#6]-[#6]-[#6]-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#16])-[#7]-[#6](=O)-[#6]-c1ccc(cc1)-c1ccccc1)-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccccc1)-[#6](=O)-[#7]-[#6]-[#6]-c1ccccc1 |r| Show InChI InChI=1S/C40H47N7O4S/c41-40(42)44-23-10-17-33(38(50)47-34(25-29-13-6-2-7-14-29)37(49)43-24-22-28-11-4-1-5-12-28)46-39(51)35(27-52)45-36(48)26-30-18-20-32(21-19-30)31-15-8-3-9-16-31/h1-9,11-16,18-21,33-35,52H,10,17,22-27H2,(H,43,49)(H,45,48)(H,46,51)(H,47,50)(H4,41,42,44)/t33-,34+,35+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 21 | -43.8 | n/a | n/a | n/a | n/a | n/a | 5.5 | 25 |
National Research Council Canada
| Assay Description
Fluorescence was monitored on a SPEX Fluorolog-2 spectrofluorometer with the excitation and emission wavelengths set at 380 and 440 nm, respectively.... |
J Med Chem 51: 1361-8 (2008)
Article DOI: 10.1021/jm701190v
BindingDB Entry DOI: 10.7270/Q21Z42QB |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50121299
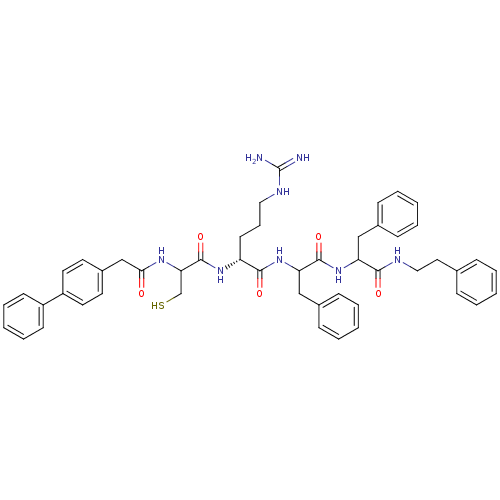
(2-[2-(2-Biphenyl-4-yl-acetylamino)-3-mercapto-prop...)Show SMILES NC(=N)NCCC[C@@H](NC(=O)C(CS)NC(=O)Cc1ccc(cc1)-c1ccccc1)C(=O)NC(Cc1ccccc1)C(=O)NC(Cc1ccccc1)C(=O)NCCc1ccccc1 Show InChI InChI=1S/C49H56N8O5S/c50-49(51)53-28-13-22-40(55-48(62)43(33-63)54-44(58)32-37-23-25-39(26-24-37)38-20-11-4-12-21-38)46(60)57-42(31-36-18-9-3-10-19-36)47(61)56-41(30-35-16-7-2-8-17-35)45(59)52-29-27-34-14-5-1-6-15-34/h1-12,14-21,23-26,40-43,63H,13,22,27-33H2,(H,52,59)(H,54,58)(H,55,62)(H,56,61)(H,57,60)(H4,50,51,53)/t40-,41?,42?,43?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Council of Canada
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Cathepsin L |
J Med Chem 45: 5321-9 (2002)
BindingDB Entry DOI: 10.7270/Q23B5ZH6 |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM20997
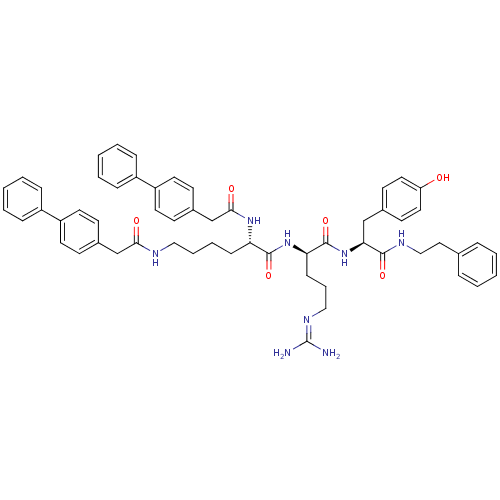
((2S)-N-[(1R)-4-[(diaminomethylidene)amino]-1-{[(1S...)Show SMILES [#7]\[#6](-[#7])=[#7]/[#6]-[#6]-[#6]-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#7]-[#6](=O)-[#6]-c1ccc(cc1)-c1ccccc1)-[#7]-[#6](=O)-[#6]-c1ccc(cc1)-c1ccccc1)-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccc(-[#8])cc1)-[#6](=O)-[#7]-[#6]-[#6]-c1ccccc1 |r| Show InChI InChI=1S/C57H64N8O6/c58-57(59)62-35-12-20-50(56(71)65-51(37-41-25-31-48(66)32-26-41)54(69)61-36-33-40-13-4-1-5-14-40)64-55(70)49(63-53(68)39-43-23-29-47(30-24-43)45-17-8-3-9-18-45)19-10-11-34-60-52(67)38-42-21-27-46(28-22-42)44-15-6-2-7-16-44/h1-9,13-18,21-32,49-51,66H,10-12,19-20,33-39H2,(H,60,67)(H,61,69)(H,63,68)(H,64,70)(H,65,71)(H4,58,59,62)/t49-,50+,51-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 23 | -43.6 | n/a | n/a | n/a | n/a | n/a | 5.5 | 25 |
National Research Council Canada
| Assay Description
Fluorescence was monitored on a SPEX Fluorolog-2 spectrofluorometer with the excitation and emission wavelengths set at 380 and 440 nm, respectively.... |
J Med Chem 51: 1361-8 (2008)
Article DOI: 10.1021/jm701190v
BindingDB Entry DOI: 10.7270/Q21Z42QB |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50081922

(5-(3-Isopropoxy-benzyl)-pyrimidine-2,4-diamine | C...)Show InChI InChI=1S/C14H18N4O/c1-9(2)19-12-5-3-4-10(7-12)6-11-8-17-14(16)18-13(11)15/h3-5,7-9H,6H2,1-2H3,(H4,15,16,17,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cardiff University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant Dihydrofolate reductase from Trypanosoma cruzi. |
J Med Chem 42: 4300-12 (1999)
BindingDB Entry DOI: 10.7270/Q21R6PQG |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50081910

(5-(3-Octyloxy-benzyl)-pyrimidine-2,4-diamine | CHE...)Show InChI InChI=1S/C19H28N4O/c1-2-3-4-5-6-7-11-24-17-10-8-9-15(13-17)12-16-14-22-19(21)23-18(16)20/h8-10,13-14H,2-7,11-12H2,1H3,(H4,20,21,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cardiff University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant Dihydrofolate reductase from humans. |
J Med Chem 42: 4300-12 (1999)
BindingDB Entry DOI: 10.7270/Q21R6PQG |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM20999
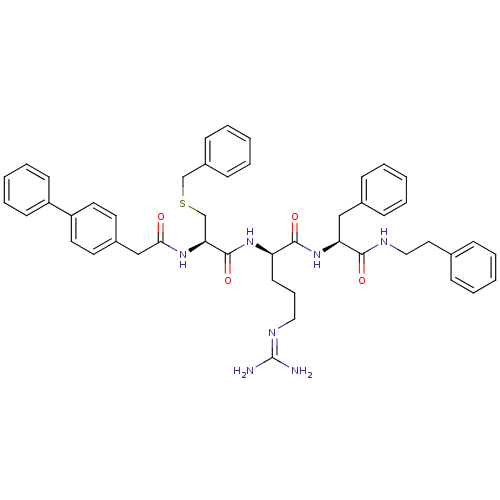
((2R)-2-[(2R)-3-(benzylsulfanyl)-2-[1-(4-phenylphen...)Show SMILES [#7]\[#6](-[#7])=[#7]/[#6]-[#6]-[#6]-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#16]-[#6]-c1ccccc1)-[#7]-[#6](=O)-[#6]-c1ccc(cc1)-c1ccccc1)-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccccc1)-[#6](=O)-[#7]-[#6]-[#6]-c1ccccc1 |r| Show InChI InChI=1S/C47H53N7O4S/c48-47(49)51-28-13-22-40(45(57)54-41(30-35-16-7-2-8-17-35)44(56)50-29-27-34-14-5-1-6-15-34)53-46(58)42(33-59-32-37-18-9-3-10-19-37)52-43(55)31-36-23-25-39(26-24-36)38-20-11-4-12-21-38/h1-12,14-21,23-26,40-42H,13,22,27-33H2,(H,50,56)(H,52,55)(H,53,58)(H,54,57)(H4,48,49,51)/t40-,41+,42+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 24 | -43.5 | n/a | n/a | n/a | n/a | n/a | 5.5 | 25 |
National Research Council Canada
| Assay Description
Fluorescence was monitored on a SPEX Fluorolog-2 spectrofluorometer with the excitation and emission wavelengths set at 380 and 440 nm, respectively.... |
J Med Chem 51: 1361-8 (2008)
Article DOI: 10.1021/jm701190v
BindingDB Entry DOI: 10.7270/Q21Z42QB |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50081911

(5-(3-Nonyloxy-benzyl)-pyrimidine-2,4-diamine | CHE...)Show InChI InChI=1S/C20H30N4O/c1-2-3-4-5-6-7-8-12-25-18-11-9-10-16(14-18)13-17-15-23-20(22)24-19(17)21/h9-11,14-15H,2-8,12-13H2,1H3,(H4,21,22,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 36 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cardiff University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant Dihydrofolate reductase from humans. |
J Med Chem 42: 4300-12 (1999)
BindingDB Entry DOI: 10.7270/Q21R6PQG |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM20993
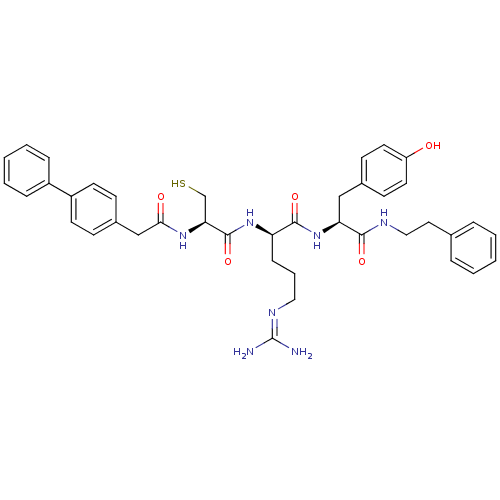
((2R)-5-[(diaminomethylidene)amino]-N-[(1S)-2-(4-hy...)Show SMILES [#7]\[#6](-[#7])=[#7]/[#6]-[#6]-[#6]-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#16])-[#7]-[#6](=O)-[#6]-c1ccc(cc1)-c1ccccc1)-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccc(-[#8])cc1)-[#6](=O)-[#7]-[#6]-[#6]-c1ccccc1 |r| Show InChI InChI=1S/C40H47N7O5S/c41-40(42)44-22-7-12-33(46-39(52)35(26-53)45-36(49)25-29-13-17-31(18-14-29)30-10-5-2-6-11-30)38(51)47-34(24-28-15-19-32(48)20-16-28)37(50)43-23-21-27-8-3-1-4-9-27/h1-6,8-11,13-20,33-35,48,53H,7,12,21-26H2,(H,43,50)(H,45,49)(H,46,52)(H,47,51)(H4,41,42,44)/t33-,34+,35+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
MMDB
PC cid
PC sid
UniChem
Similars
| MMDB
Article
PubMed
| 45 | -41.9 | n/a | n/a | n/a | n/a | n/a | 5.5 | 25 |
National Research Council Canada
| Assay Description
Fluorescence was monitored on a SPEX Fluorolog-2 spectrofluorometer with the excitation and emission wavelengths set at 380 and 440 nm, respectively.... |
J Med Chem 51: 1361-8 (2008)
Article DOI: 10.1021/jm701190v
BindingDB Entry DOI: 10.7270/Q21Z42QB |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50121290
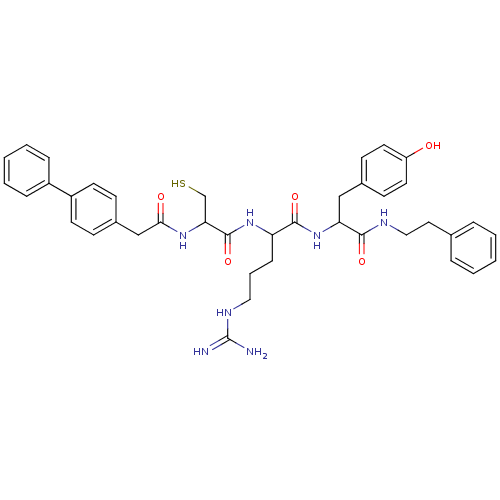
(2-[2-(2-Biphenyl-4-yl-acetylamino)-3-mercapto-prop...)Show SMILES NC(=N)NCCCC(NC(=O)C(CS)NC(=O)Cc1ccc(cc1)-c1ccccc1)C(=O)NC(Cc1ccc(O)cc1)C(=O)NCCc1ccccc1 Show InChI InChI=1S/C40H47N7O5S/c41-40(42)44-22-7-12-33(46-39(52)35(26-53)45-36(49)25-29-13-17-31(18-14-29)30-10-5-2-6-11-30)38(51)47-34(24-28-15-19-32(48)20-16-28)37(50)43-23-21-27-8-3-1-4-9-27/h1-6,8-11,13-20,33-35,48,53H,7,12,21-26H2,(H,43,50)(H,45,49)(H,46,52)(H,47,51)(H4,41,42,44) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 45 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Council of Canada
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Cathepsin L |
J Med Chem 45: 5321-9 (2002)
BindingDB Entry DOI: 10.7270/Q23B5ZH6 |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50081906

(6-Ethyl-5-(3-heptyloxy-benzyl)-pyrimidine-2,4-diam...)Show InChI InChI=1S/C20H30N4O/c1-3-5-6-7-8-12-25-16-11-9-10-15(13-16)14-17-18(4-2)23-20(22)24-19(17)21/h9-11,13H,3-8,12,14H2,1-2H3,(H4,21,22,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 48 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cardiff University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant Dihydrofolate reductase from Leishmania major. |
J Med Chem 42: 4300-12 (1999)
BindingDB Entry DOI: 10.7270/Q21R6PQG |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50081913
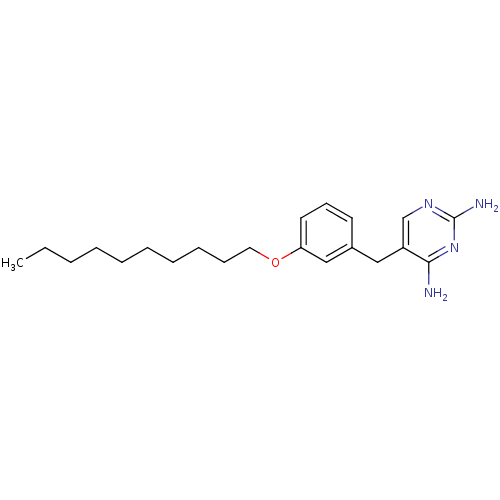
(5-(3-Decyloxy-benzyl)-pyrimidine-2,4-diamine | CHE...)Show InChI InChI=1S/C21H32N4O/c1-2-3-4-5-6-7-8-9-13-26-19-12-10-11-17(15-19)14-18-16-24-21(23)25-20(18)22/h10-12,15-16H,2-9,13-14H2,1H3,(H4,22,23,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 52 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cardiff University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant Dihydrofolate reductase from humans. |
J Med Chem 42: 4300-12 (1999)
BindingDB Entry DOI: 10.7270/Q21R6PQG |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50081909

(5-(3-(benzyloxy)benzyl)pyrimidine-2,4-diamine | 5-...)Show InChI InChI=1S/C18H18N4O/c19-17-15(11-21-18(20)22-17)9-14-7-4-8-16(10-14)23-12-13-5-2-1-3-6-13/h1-8,10-11H,9,12H2,(H4,19,20,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 65 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cardiff University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant Dihydrofolate reductase from Leishmania major. |
J Med Chem 42: 4300-12 (1999)
BindingDB Entry DOI: 10.7270/Q21R6PQG |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50121286
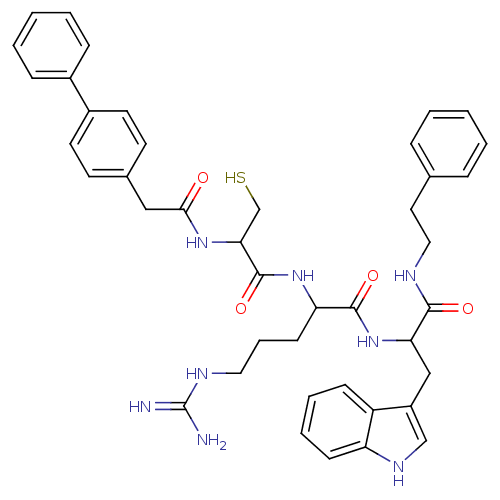
(2-[2-(2-Biphenyl-4-yl-acetylamino)-3-mercapto-prop...)Show SMILES NC(=N)NCCCC(NC(=O)C(CS)NC(=O)Cc1ccc(cc1)-c1ccccc1)C(=O)NC(Cc1c[nH]c2ccccc12)C(=O)NCCc1ccccc1 Show InChI InChI=1S/C42H48N8O4S/c43-42(44)46-22-9-16-35(49-41(54)37(27-55)48-38(51)24-29-17-19-31(20-18-29)30-12-5-2-6-13-30)40(53)50-36(25-32-26-47-34-15-8-7-14-33(32)34)39(52)45-23-21-28-10-3-1-4-11-28/h1-8,10-15,17-20,26,35-37,47,55H,9,16,21-25,27H2,(H,45,52)(H,48,51)(H,49,54)(H,50,53)(H4,43,44,46) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 67 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Council of Canada
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Cathepsin L |
J Med Chem 45: 5321-9 (2002)
BindingDB Entry DOI: 10.7270/Q23B5ZH6 |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50081912
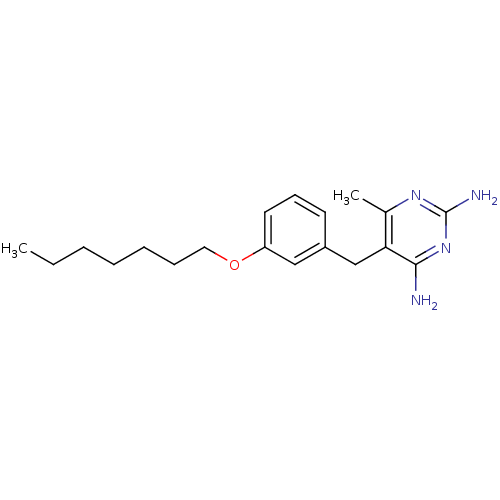
(5-(3-Heptyloxy-benzyl)-6-methyl-pyrimidine-2,4-dia...)Show InChI InChI=1S/C19H28N4O/c1-3-4-5-6-7-11-24-16-10-8-9-15(12-16)13-17-14(2)22-19(21)23-18(17)20/h8-10,12H,3-7,11,13H2,1-2H3,(H4,20,21,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cardiff University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant Dihydrofolate reductase from humans. |
J Med Chem 42: 4300-12 (1999)
BindingDB Entry DOI: 10.7270/Q21R6PQG |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50081915

(5-[3-(Tetrahydro-pyran-2-yloxy)-benzyl]-pyrimidine...)Show InChI InChI=1S/C16H20N4O2/c17-15-12(10-19-16(18)20-15)8-11-4-3-5-13(9-11)22-14-6-1-2-7-21-14/h3-5,9-10,14H,1-2,6-8H2,(H4,17,18,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 93 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cardiff University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant Dihydrofolate reductase from Leishmania major. |
J Med Chem 42: 4300-12 (1999)
BindingDB Entry DOI: 10.7270/Q21R6PQG |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50081910

(5-(3-Octyloxy-benzyl)-pyrimidine-2,4-diamine | CHE...)Show InChI InChI=1S/C19H28N4O/c1-2-3-4-5-6-7-11-24-17-10-8-9-15(13-17)12-16-14-22-19(21)23-18(16)20/h8-10,13-14H,2-7,11-12H2,1H3,(H4,20,21,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 97 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cardiff University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant Dihydrofolate reductase from Leishmania major. |
J Med Chem 42: 4300-12 (1999)
BindingDB Entry DOI: 10.7270/Q21R6PQG |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM18512

(5-(4-chlorophenyl)-6-ethylpyrimidine-2,4-diamine |...)Show InChI InChI=1S/C12H13ClN4/c1-2-9-10(11(14)17-12(15)16-9)7-3-5-8(13)6-4-7/h3-6H,2H2,1H3,(H4,14,15,16,17) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PubMed
| 98 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cardiff University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant Dihydrofolate reductase from Trypanosoma cruzi. |
J Med Chem 42: 4300-12 (1999)
BindingDB Entry DOI: 10.7270/Q21R6PQG |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM20996
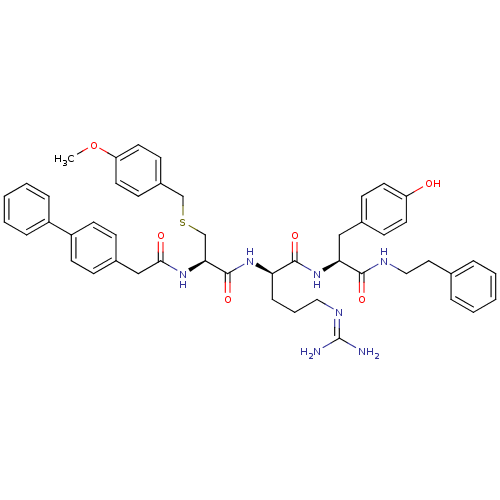
((2R)-5-[(diaminomethylidene)amino]-N-[(1S)-2-(4-hy...)Show SMILES [#6]-[#8]-c1ccc(-[#6]-[#16]-[#6]-[#6@H](-[#7]-[#6](=O)-[#6]-c2ccc(cc2)-c2ccccc2)-[#6](=O)-[#7]-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-c2ccc(-[#8])cc2)-[#6](=O)-[#7]-[#6]-[#6]-c2ccccc2)cc1 |r| Show InChI InChI=1S/C48H55N7O6S/c1-61-40-24-18-36(19-25-40)31-62-32-43(53-44(57)30-35-14-20-38(21-15-35)37-11-6-3-7-12-37)47(60)54-41(13-8-27-52-48(49)50)46(59)55-42(29-34-16-22-39(56)23-17-34)45(58)51-28-26-33-9-4-2-5-10-33/h2-7,9-12,14-25,41-43,56H,8,13,26-32H2,1H3,(H,51,58)(H,53,57)(H,54,60)(H,55,59)(H4,49,50,52)/t41-,42+,43+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 112 | -39.7 | n/a | n/a | n/a | n/a | n/a | 5.5 | 25 |
National Research Council Canada
| Assay Description
Fluorescence was monitored on a SPEX Fluorolog-2 spectrofluorometer with the excitation and emission wavelengths set at 380 and 440 nm, respectively.... |
J Med Chem 51: 1361-8 (2008)
Article DOI: 10.1021/jm701190v
BindingDB Entry DOI: 10.7270/Q21Z42QB |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM18069

(5-[(3,4,5-trimethoxyphenyl)methyl]pyrimidine-2,4-d...)Show InChI InChI=1S/C14H18N4O3/c1-19-10-5-8(6-11(20-2)12(10)21-3)4-9-7-17-14(16)18-13(9)15/h5-7H,4H2,1-3H3,(H4,15,16,17,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
PubMed
| 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cardiff University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant Dihydrofolate reductase from Leishmania major. |
J Med Chem 42: 4300-12 (1999)
BindingDB Entry DOI: 10.7270/Q21R6PQG |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM18512

(5-(4-chlorophenyl)-6-ethylpyrimidine-2,4-diamine |...)Show InChI InChI=1S/C12H13ClN4/c1-2-9-10(11(14)17-12(15)16-9)7-3-5-8(13)6-4-7/h3-6H,2H2,1H3,(H4,14,15,16,17) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PubMed
| 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cardiff University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant Dihydrofolate reductase from humans. |
J Med Chem 42: 4300-12 (1999)
BindingDB Entry DOI: 10.7270/Q21R6PQG |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50081920

(5-(3-Propoxy-benzyl)-pyrimidine-2,4-diamine | CHEM...)Show InChI InChI=1S/C14H18N4O/c1-2-6-19-12-5-3-4-10(8-12)7-11-9-17-14(16)18-13(11)15/h3-5,8-9H,2,6-7H2,1H3,(H4,15,16,17,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cardiff University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant Dihydrofolate reductase from Leishmania major. |
J Med Chem 42: 4300-12 (1999)
BindingDB Entry DOI: 10.7270/Q21R6PQG |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50081921

(5-(4-Ethoxy-benzyl)-pyrimidine-2,4-diamine | CHEMB...)Show InChI InChI=1S/C13H16N4O/c1-2-18-11-5-3-9(4-6-11)7-10-8-16-13(15)17-12(10)14/h3-6,8H,2,7H2,1H3,(H4,14,15,16,17) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cardiff University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant Dihydrofolate reductase from Leishmania major. |
J Med Chem 42: 4300-12 (1999)
BindingDB Entry DOI: 10.7270/Q21R6PQG |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50081914

(5-(3-Heptyloxy-benzyl)-pyrimidine-2,4-diamine | CH...)Show InChI InChI=1S/C18H26N4O/c1-2-3-4-5-6-10-23-16-9-7-8-14(12-16)11-15-13-21-18(20)22-17(15)19/h7-9,12-13H,2-6,10-11H2,1H3,(H4,19,20,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 131 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cardiff University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant Dihydrofolate reductase from humans. |
J Med Chem 42: 4300-12 (1999)
BindingDB Entry DOI: 10.7270/Q21R6PQG |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50081916

(5-(3-Pentyloxy-benzyl)-pyrimidine-2,4-diamine | CH...)Show InChI InChI=1S/C16H22N4O/c1-2-3-4-8-21-14-7-5-6-12(10-14)9-13-11-19-16(18)20-15(13)17/h5-7,10-11H,2-4,8-9H2,1H3,(H4,17,18,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cardiff University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant Dihydrofolate reductase from Leishmania major. |
J Med Chem 42: 4300-12 (1999)
BindingDB Entry DOI: 10.7270/Q21R6PQG |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50081905

(5-(3-Butoxy-benzyl)-pyrimidine-2,4-diamine | CHEMB...)Show InChI InChI=1S/C15H20N4O/c1-2-3-7-20-13-6-4-5-11(9-13)8-12-10-18-15(17)19-14(12)16/h4-6,9-10H,2-3,7-8H2,1H3,(H4,16,17,18,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cardiff University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant Dihydrofolate reductase from Leishmania major. |
J Med Chem 42: 4300-12 (1999)
BindingDB Entry DOI: 10.7270/Q21R6PQG |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM20995
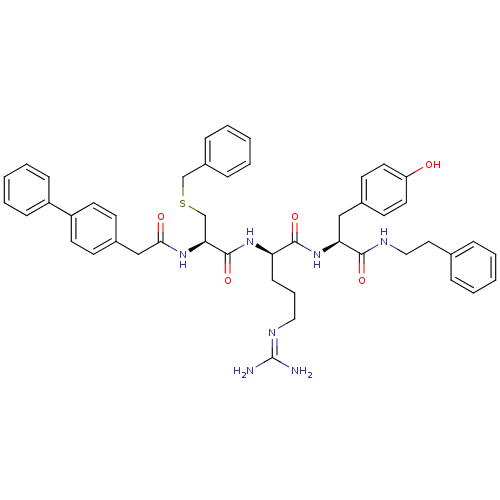
((2R)-2-[(2R)-3-(benzylsulfanyl)-2-[1-(4-phenylphen...)Show SMILES [#7]\[#6](-[#7])=[#7]/[#6]-[#6]-[#6]-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#16]-[#6]-c1ccccc1)-[#7]-[#6](=O)-[#6]-c1ccc(cc1)-c1ccccc1)-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccc(-[#8])cc1)-[#6](=O)-[#7]-[#6]-[#6]-c1ccccc1 |r| Show InChI InChI=1S/C47H53N7O5S/c48-47(49)51-27-10-17-40(45(58)54-41(29-34-20-24-39(55)25-21-34)44(57)50-28-26-33-11-4-1-5-12-33)53-46(59)42(32-60-31-36-13-6-2-7-14-36)52-43(56)30-35-18-22-38(23-19-35)37-15-8-3-9-16-37/h1-9,11-16,18-25,40-42,55H,10,17,26-32H2,(H,50,57)(H,52,56)(H,53,59)(H,54,58)(H4,48,49,51)/t40-,41+,42+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| 155 | -38.9 | n/a | n/a | n/a | n/a | n/a | 5.5 | 25 |
National Research Council Canada
| Assay Description
Fluorescence was monitored on a SPEX Fluorolog-2 spectrofluorometer with the excitation and emission wavelengths set at 380 and 440 nm, respectively.... |
J Med Chem 51: 1361-8 (2008)
Article DOI: 10.1021/jm701190v
BindingDB Entry DOI: 10.7270/Q21Z42QB |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50081907

(5-(3-Ethoxy-benzyl)-pyrimidine-2,4-diamine | CHEMB...)Show InChI InChI=1S/C13H16N4O/c1-2-18-11-5-3-4-9(7-11)6-10-8-16-13(15)17-12(10)14/h3-5,7-8H,2,6H2,1H3,(H4,14,15,16,17) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 156 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cardiff University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant Dihydrofolate reductase from Leishmania major. |
J Med Chem 42: 4300-12 (1999)
BindingDB Entry DOI: 10.7270/Q21R6PQG |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50121298
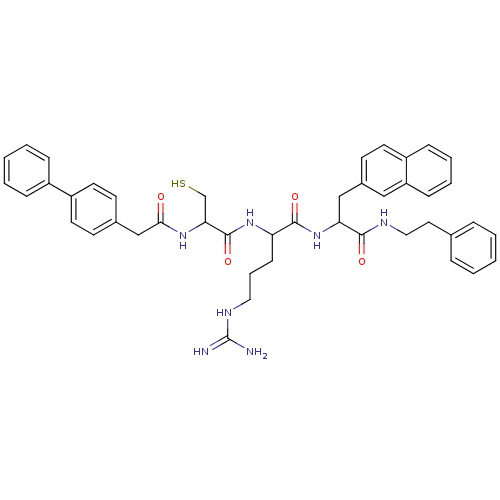
(2-[2-(2-Biphenyl-4-yl-acetylamino)-3-mercapto-prop...)Show SMILES NC(=N)NCCCC(NC(=O)C(CS)NC(=O)Cc1ccc(cc1)-c1ccccc1)C(=O)NC(Cc1ccc2ccccc2c1)C(=O)NCCc1ccccc1 Show InChI InChI=1S/C44H49N7O4S/c45-44(46)48-24-9-16-37(50-43(55)39(29-56)49-40(52)28-31-17-20-35(21-18-31)33-12-5-2-6-13-33)42(54)51-38(41(53)47-25-23-30-10-3-1-4-11-30)27-32-19-22-34-14-7-8-15-36(34)26-32/h1-8,10-15,17-22,26,37-39,56H,9,16,23-25,27-29H2,(H,47,53)(H,49,52)(H,50,55)(H,51,54)(H4,45,46,48) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Council of Canada
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Cathepsin L |
J Med Chem 45: 5321-9 (2002)
BindingDB Entry DOI: 10.7270/Q23B5ZH6 |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50081922

(5-(3-Isopropoxy-benzyl)-pyrimidine-2,4-diamine | C...)Show InChI InChI=1S/C14H18N4O/c1-9(2)19-12-5-3-4-10(7-12)6-11-8-17-14(16)18-13(11)15/h3-5,7-9H,6H2,1-2H3,(H4,15,16,17,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cardiff University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant Dihydrofolate reductase from Leishmania major. |
J Med Chem 42: 4300-12 (1999)
BindingDB Entry DOI: 10.7270/Q21R6PQG |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50121300
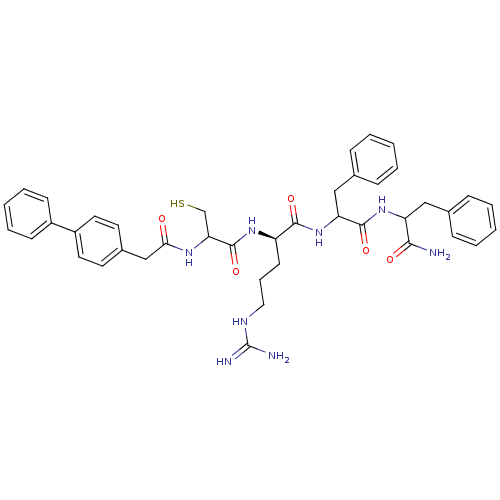
(2-[2-(2-Biphenyl-4-yl-acetylamino)-3-mercapto-prop...)Show SMILES NC(=N)NCCC[C@@H](NC(=O)C(CS)NC(=O)Cc1ccc(cc1)-c1ccccc1)C(=O)NC(Cc1ccccc1)C(=O)NC(Cc1ccccc1)C(N)=O Show InChI InChI=1S/C41H48N8O5S/c42-37(51)33(23-27-11-4-1-5-12-27)48-39(53)34(24-28-13-6-2-7-14-28)49-38(52)32(17-10-22-45-41(43)44)47-40(54)35(26-55)46-36(50)25-29-18-20-31(21-19-29)30-15-8-3-9-16-30/h1-9,11-16,18-21,32-35,55H,10,17,22-26H2,(H2,42,51)(H,46,50)(H,47,54)(H,48,53)(H,49,52)(H4,43,44,45)/t32-,33?,34?,35?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Council of Canada
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Cathepsin L |
J Med Chem 45: 5321-9 (2002)
BindingDB Entry DOI: 10.7270/Q23B5ZH6 |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50121300

(2-[2-(2-Biphenyl-4-yl-acetylamino)-3-mercapto-prop...)Show SMILES NC(=N)NCCC[C@@H](NC(=O)C(CS)NC(=O)Cc1ccc(cc1)-c1ccccc1)C(=O)NC(Cc1ccccc1)C(=O)NC(Cc1ccccc1)C(N)=O Show InChI InChI=1S/C41H48N8O5S/c42-37(51)33(23-27-11-4-1-5-12-27)48-39(53)34(24-28-13-6-2-7-14-28)49-38(52)32(17-10-22-45-41(43)44)47-40(54)35(26-55)46-36(50)25-29-18-20-31(21-19-29)30-15-8-3-9-16-30/h1-9,11-16,18-21,32-35,55H,10,17,22-26H2,(H2,42,51)(H,46,50)(H,47,54)(H,48,53)(H,49,52)(H4,43,44,45)/t32-,33?,34?,35?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Council of Canada
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Cathepsin L |
J Med Chem 45: 5321-9 (2002)
BindingDB Entry DOI: 10.7270/Q23B5ZH6 |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50081917

(5-(3-Hexyloxy-benzyl)-pyrimidine-2,4-diamine | CHE...)Show InChI InChI=1S/C17H24N4O/c1-2-3-4-5-9-22-15-8-6-7-13(11-15)10-14-12-20-17(19)21-16(14)18/h6-8,11-12H,2-5,9-10H2,1H3,(H4,18,19,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 171 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cardiff University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant Dihydrofolate reductase from Leishmania major. |
J Med Chem 42: 4300-12 (1999)
BindingDB Entry DOI: 10.7270/Q21R6PQG |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50121301
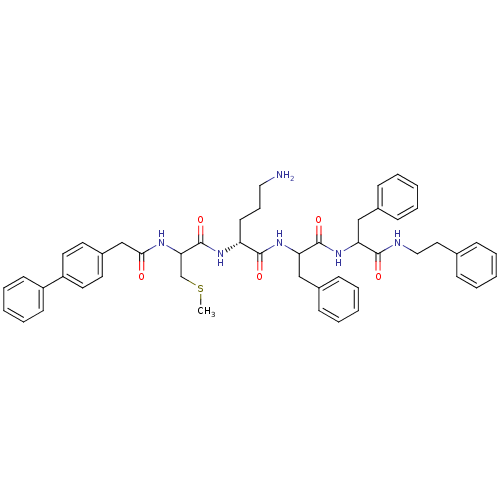
(5-Amino-2-[2-(2-biphenyl-4-yl-acetylamino)-3-methy...)Show SMILES CSCC(NC(=O)Cc1ccc(cc1)-c1ccccc1)C(=O)N[C@H](CCCN)C(=O)NC(Cc1ccccc1)C(=O)NC(Cc1ccccc1)C(=O)NCCc1ccccc1 Show InChI InChI=1S/C49H56N6O5S/c1-61-34-44(52-45(56)33-38-24-26-40(27-25-38)39-21-12-5-13-22-39)49(60)53-41(23-14-29-50)47(58)55-43(32-37-19-10-4-11-20-37)48(59)54-42(31-36-17-8-3-9-18-36)46(57)51-30-28-35-15-6-2-7-16-35/h2-13,15-22,24-27,41-44H,14,23,28-34,50H2,1H3,(H,51,57)(H,52,56)(H,53,60)(H,54,59)(H,55,58)/t41-,42?,43?,44?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Council of Canada
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Cathepsin L |
J Med Chem 45: 5321-9 (2002)
BindingDB Entry DOI: 10.7270/Q23B5ZH6 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data