

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Thyroid hormone receptor beta (Homo sapiens (Human)) | BDBM50123058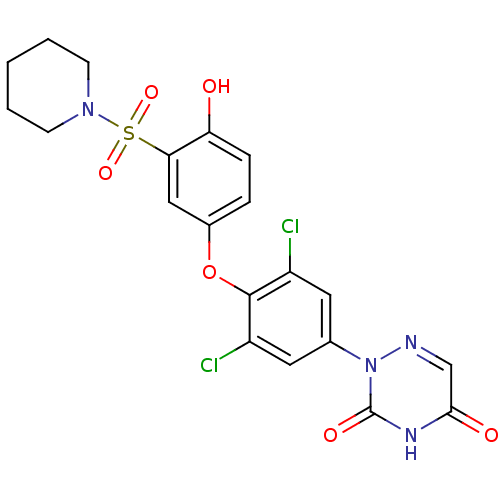 (2-(3,5-dichloro-4-(4-hydroxy-3-(piperidin-1-ylsulf...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against [125I]-T3 binding to human TRbeta1 receptor | Bioorg Med Chem Lett 13: 379-82 (2003) BindingDB Entry DOI: 10.7270/Q26T0KZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyroid hormone receptor beta (Homo sapiens (Human)) | BDBM50123046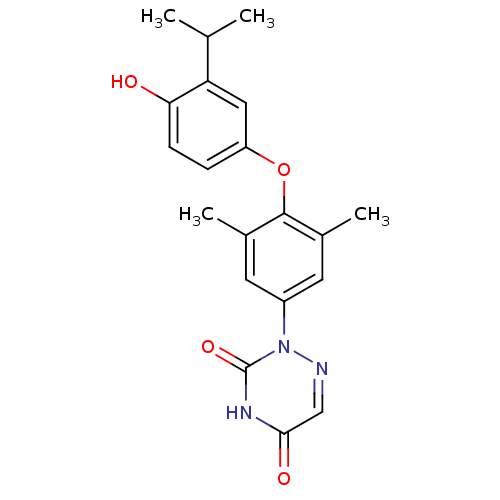 (2-[4-(4-Hydroxy-3-isopropyl-phenoxy)-3,5-dimethyl-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB PubMed | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against [125I]-T3 binding to human TRbeta1 receptor | Bioorg Med Chem Lett 13: 379-82 (2003) BindingDB Entry DOI: 10.7270/Q26T0KZB | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Thyroid hormone receptor beta (Homo sapiens (Human)) | BDBM50123044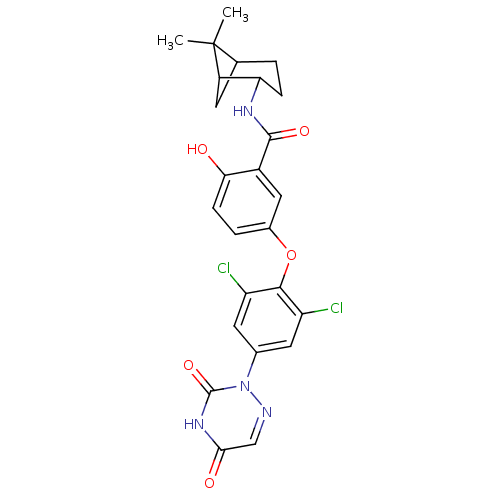 (5-(2,6-dichloro-4-(3,5-dioxo-4,5-dihydro-1,2,4-tri...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against [125I]-T3 binding to human TRbeta1 receptor | Bioorg Med Chem Lett 13: 379-82 (2003) BindingDB Entry DOI: 10.7270/Q26T0KZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyroid hormone receptor beta (Homo sapiens (Human)) | BDBM50123044 (5-(2,6-dichloro-4-(3,5-dioxo-4,5-dihydro-1,2,4-tri...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against [125I]-T3 binding to human TRbeta1 receptor | Bioorg Med Chem Lett 13: 379-82 (2003) BindingDB Entry DOI: 10.7270/Q26T0KZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyroid hormone receptor beta (Homo sapiens (Human)) | BDBM50123045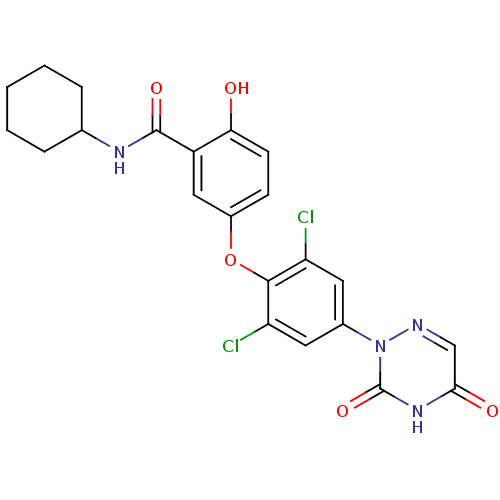 (CHEMBL413699 | N-Cyclohexyl-5-[2,6-dichloro-4-(3,5...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against [125I]-T3 binding to human TRbeta1 receptor | Bioorg Med Chem Lett 13: 379-82 (2003) BindingDB Entry DOI: 10.7270/Q26T0KZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyroid hormone receptor beta (Homo sapiens (Human)) | BDBM18860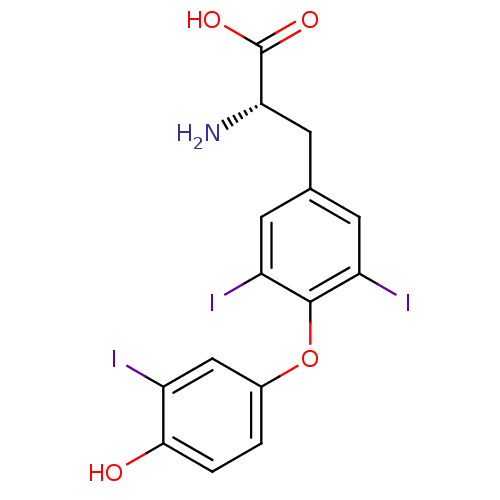 ((2R)-2-amino-3-[4-(4-hydroxy-3-iodophenoxy)-3,5-di...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB PubMed | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against [125I]-T3 binding to human TRbeta1 receptor | Bioorg Med Chem Lett 13: 379-82 (2003) BindingDB Entry DOI: 10.7270/Q26T0KZB | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50301747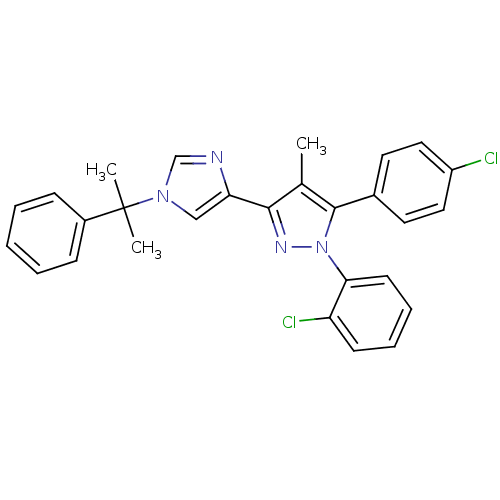 (1-(2-chlorophenyl)-5-(4-chlorophenyl)-4-methyl-3-(...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Antagonist activity at human CB1 receptor transfected in CHO-K1cells by GTPgamma[35S] binding assay | Bioorg Med Chem Lett 19: 5351-4 (2009) Article DOI: 10.1016/j.bmcl.2009.07.130 BindingDB Entry DOI: 10.7270/Q2KP827S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50301739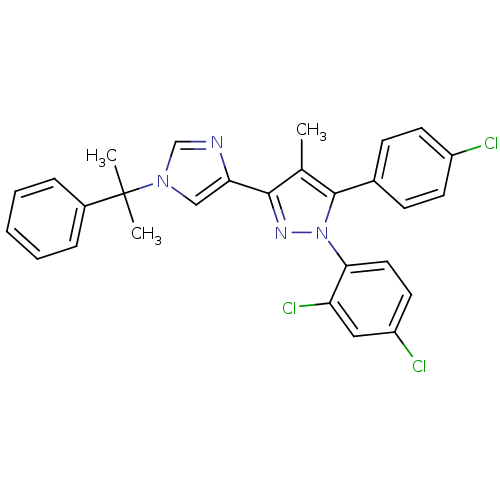 (5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Antagonist activity at human CB1 receptor transfected in CHO-K1cells by GTPgamma[35S] binding assay | Bioorg Med Chem Lett 19: 5351-4 (2009) Article DOI: 10.1016/j.bmcl.2009.07.130 BindingDB Entry DOI: 10.7270/Q2KP827S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyroid hormone receptor beta (Homo sapiens (Human)) | BDBM50123054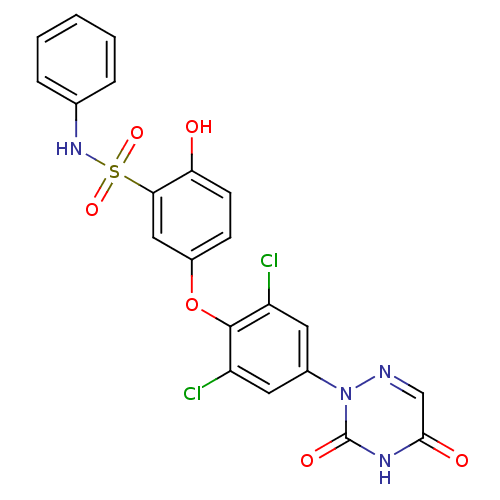 (5-(2,6-dichloro-4-(3,5-dioxo-4,5-dihydro-1,2,4-tri...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against [125I]-T3 binding to human TRbeta1 receptor | Bioorg Med Chem Lett 13: 379-82 (2003) BindingDB Entry DOI: 10.7270/Q26T0KZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyroid hormone receptor beta (Homo sapiens (Human)) | BDBM50123064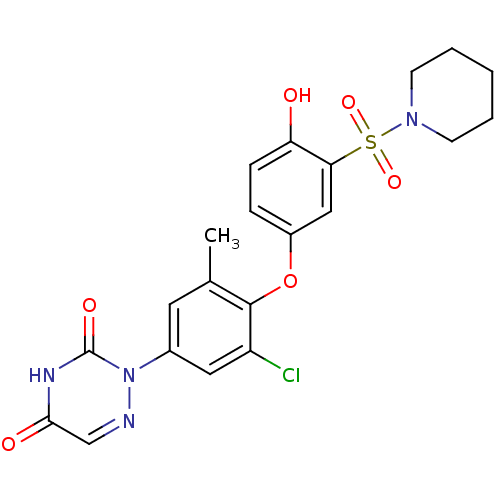 (2-(3-chloro-4-(4-hydroxy-3-(piperidin-1-ylsulfonyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against [125I]-T3 binding to human TRbeta1 receptor | Bioorg Med Chem Lett 13: 379-82 (2003) BindingDB Entry DOI: 10.7270/Q26T0KZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyroid hormone receptor beta (Homo sapiens (Human)) | BDBM50123063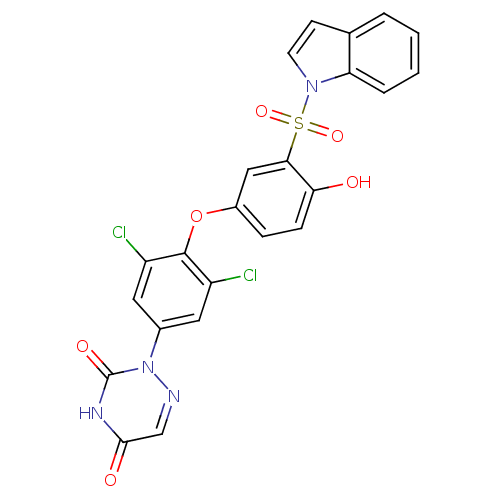 (2-{3,5-Dichloro-4-[4-hydroxy-3-(indole-1-sulfonyl)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against [125I]-T3 binding to human TRbeta1 receptor | Bioorg Med Chem Lett 13: 379-82 (2003) BindingDB Entry DOI: 10.7270/Q26T0KZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyroid hormone receptor beta (Homo sapiens (Human)) | BDBM50123052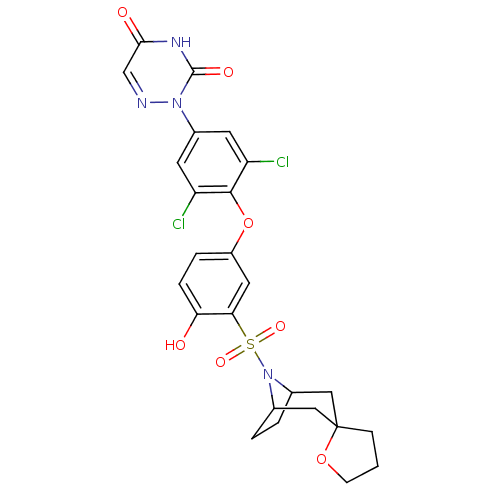 (2-[4-(3-{8-azaspiro[bicyclo[3.2.1]octane-3,2'-oxol...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against [125I]T3 binding to human TRbeta1 receptor | Bioorg Med Chem Lett 13: 379-82 (2003) BindingDB Entry DOI: 10.7270/Q26T0KZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50128931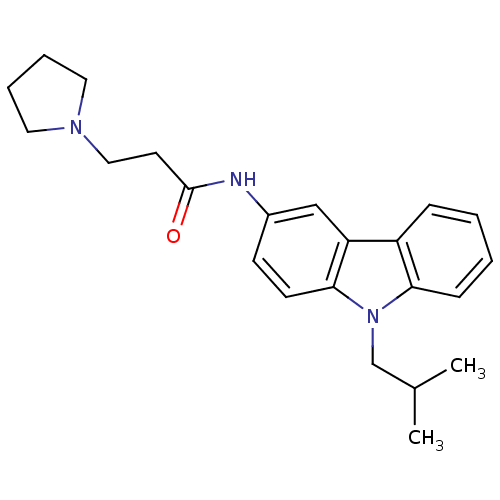 (CHEMBL61880 | N-(9-Isobutyl-9H-carbazol-3-yl)-3-py...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Binding affinity towards human neuropeptide Y receptor type 5 using 125[I]-PYY as radioligand | Bioorg Med Chem Lett 13: 1989-92 (2003) BindingDB Entry DOI: 10.7270/Q2057F9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyroid hormone receptor beta (Homo sapiens (Human)) | BDBM50123061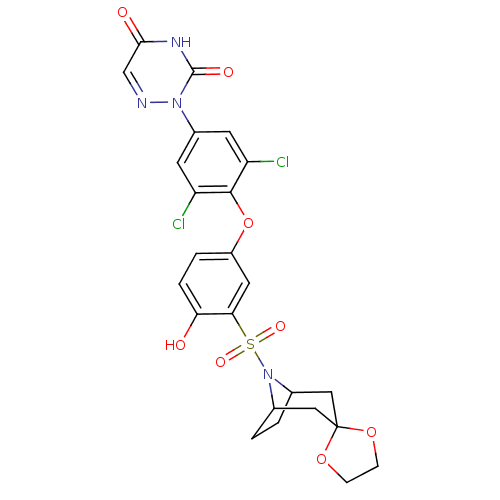 (2-[4-(3-{8-azaspiro[bicyclo[3.2.1]octane-3,2'-[1,3...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against [125I]-T3 binding to human TRbeta1 receptor | Bioorg Med Chem Lett 13: 379-82 (2003) BindingDB Entry DOI: 10.7270/Q26T0KZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Growth hormone secretagogue receptor type 1 (Homo sapiens (Human)) | BDBM50120502 (2-Amino-N-[(R)-2-(3a-benzyl-2-tert-butyl-3-oxo-2,3...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development Curated by ChEMBL | Assay Description In vitro binding affinity of the compound was determined against human type 1a growth hormone secretagogue receptor (hGHS-R1a), using [125I]-ghrelin ... | Bioorg Med Chem Lett 12: 3279-82 (2002) BindingDB Entry DOI: 10.7270/Q23F4NZZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyroid hormone receptor beta (Homo sapiens (Human)) | BDBM50123059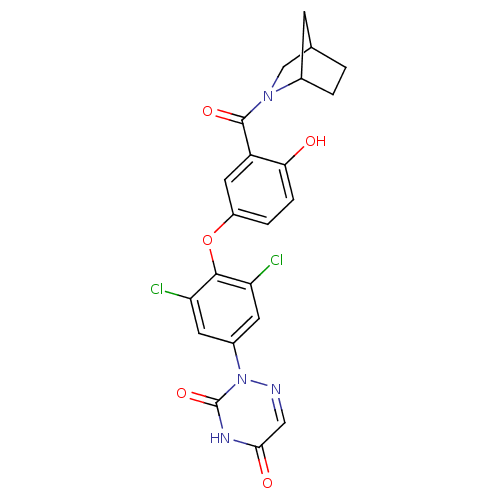 (2-{4-[3-(2-Aza-bicyclo[2.2.1]heptane-2-carbonyl)-4...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against [125I]-T3 binding to human TRbeta1 receptor | Bioorg Med Chem Lett 13: 379-82 (2003) BindingDB Entry DOI: 10.7270/Q26T0KZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyroid hormone receptor beta (Homo sapiens (Human)) | BDBM50123059 (2-{4-[3-(2-Aza-bicyclo[2.2.1]heptane-2-carbonyl)-4...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against [125I]-T3 binding to human TRbeta1 receptor | Bioorg Med Chem Lett 13: 379-82 (2003) BindingDB Entry DOI: 10.7270/Q26T0KZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyroid hormone receptor beta (Homo sapiens (Human)) | BDBM50123057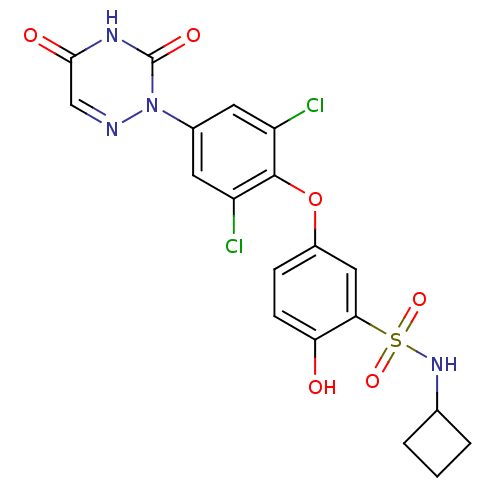 (CHEMBL124039 | N-Cyclobutyl-5-[2,6-dichloro-4-(3,5...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against [125I]-T3 binding to human TRbeta1 receptor | Bioorg Med Chem Lett 13: 379-82 (2003) BindingDB Entry DOI: 10.7270/Q26T0KZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Growth hormone secretagogue receptor type 1 (Homo sapiens (Human)) | BDBM50120504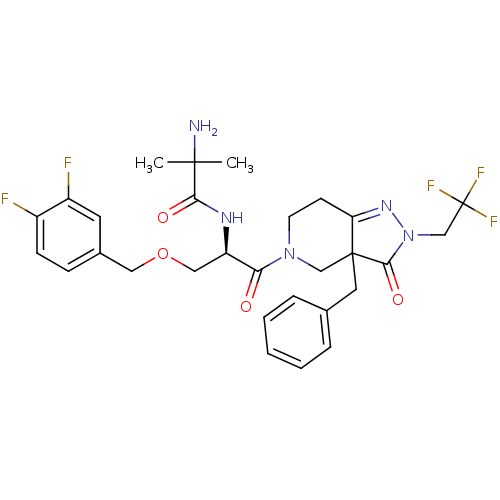 (2-Amino-N-[(R)-2-[3a-benzyl-3-oxo-2-(2,2,2-trifluo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development Curated by ChEMBL | Assay Description In vitro binding affinity of the compound was determined against human type 1a growth hormone secretagogue receptor (hGHS-R1a), using [125I]-ghrelin ... | Bioorg Med Chem Lett 12: 3279-82 (2002) BindingDB Entry DOI: 10.7270/Q23F4NZZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyroid hormone receptor beta (Homo sapiens (Human)) | BDBM50123056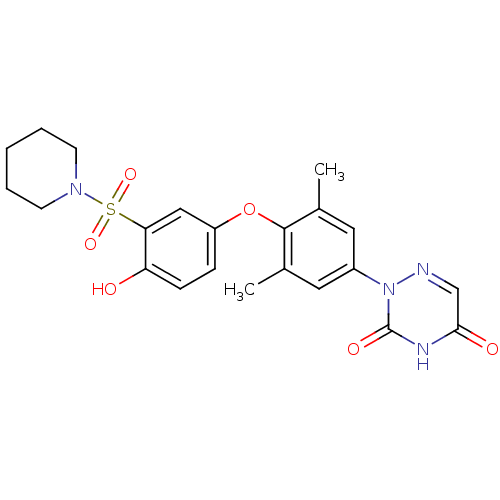 (2-(4-(4-hydroxy-3-(piperidin-1-ylsulfonyl)phenoxy)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against [125I]-T3 binding to human TRbeta1 receptor | Bioorg Med Chem Lett 13: 379-82 (2003) BindingDB Entry DOI: 10.7270/Q26T0KZB | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Thyroid hormone receptor beta (Homo sapiens (Human)) | BDBM50123062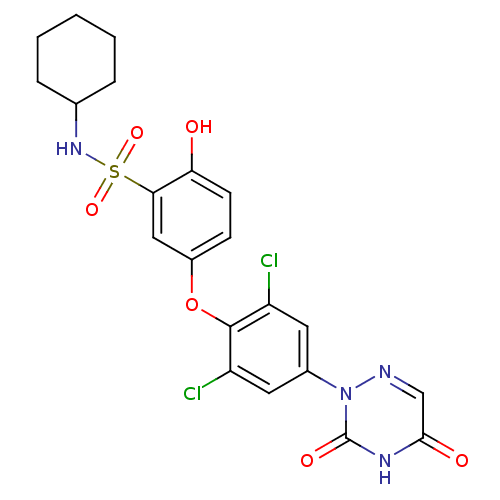 (CHEMBL340158 | N-Cyclohexyl-5-[2,6-dichloro-4-(3,5...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against [125I]-T3 binding to human TRbeta1 receptor | Bioorg Med Chem Lett 13: 379-82 (2003) BindingDB Entry DOI: 10.7270/Q26T0KZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyroid hormone receptor beta (Homo sapiens (Human)) | BDBM50123059 (2-{4-[3-(2-Aza-bicyclo[2.2.1]heptane-2-carbonyl)-4...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.590 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against [125I]-T3 binding to human TRbeta1 receptor | Bioorg Med Chem Lett 13: 379-82 (2003) BindingDB Entry DOI: 10.7270/Q26T0KZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM29061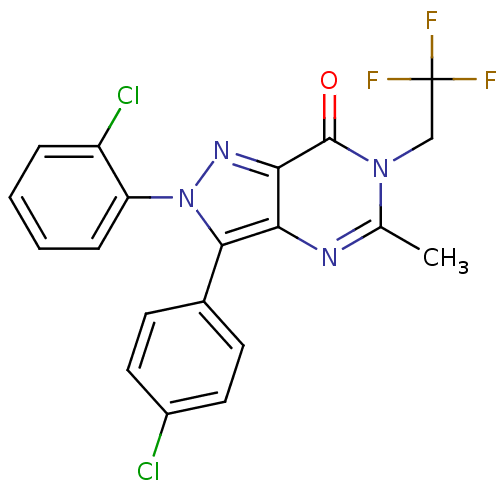 (CHEMBL201602 | pyrazolopyrimidinone-based antagoni...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.600 | -53.5 | n/a | n/a | 1 | n/a | n/a | 7.4 | 30 |
Pfizer | Assay Description IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand binding experiments. The inhibition c... | J Med Chem 52: 2652-5 (2009) Article DOI: 10.1021/jm900255t BindingDB Entry DOI: 10.7270/Q2F47MGD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyroid hormone receptor beta (Homo sapiens (Human)) | BDBM50123049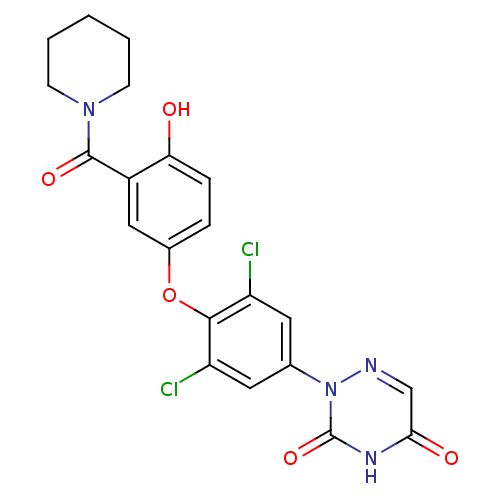 (2-(3,5-dichloro-4-(4-hydroxy-3-(piperidine-1-carbo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against [125I]-T3 binding to human TRbeta1 receptor | Bioorg Med Chem Lett 13: 379-82 (2003) BindingDB Entry DOI: 10.7270/Q26T0KZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyroid hormone receptor beta (Homo sapiens (Human)) | BDBM50123050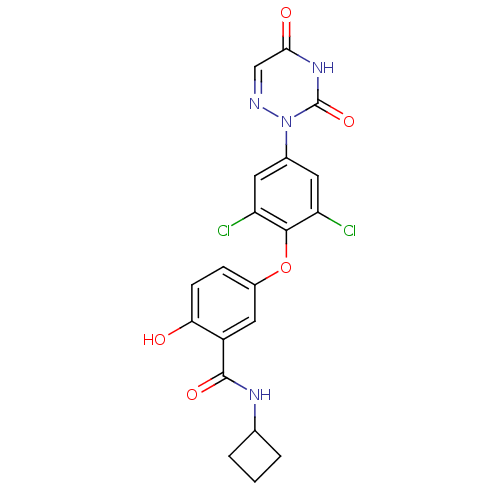 (CHEMBL124318 | N-Cyclobutyl-5-[2,6-dichloro-4-(3,5...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.640 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against [125I]-T3 binding to human TRbeta1 receptor | Bioorg Med Chem Lett 13: 379-82 (2003) BindingDB Entry DOI: 10.7270/Q26T0KZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM21278 (5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.650 | -53.3 | n/a | n/a | n/a | n/a | n/a | 7.4 | 30 |
Pfizer | Assay Description IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand binding experiments. The inhibition c... | J Med Chem 52: 234-7 (2009) Article DOI: 10.1021/jm8012932 BindingDB Entry DOI: 10.7270/Q2CN7276 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM29070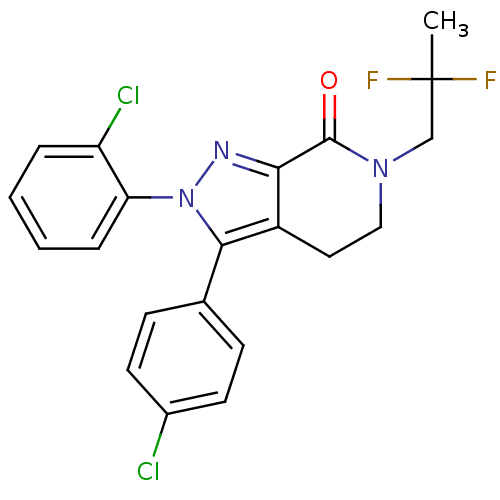 (lactam-based compound, 12i) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.700 | -53.1 | n/a | n/a | 0.800 | n/a | n/a | 7.4 | 30 |
Pfizer | Assay Description IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand binding experiments. The inhibition c... | J Med Chem 52: 2652-5 (2009) Article DOI: 10.1021/jm900255t BindingDB Entry DOI: 10.7270/Q2F47MGD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM27337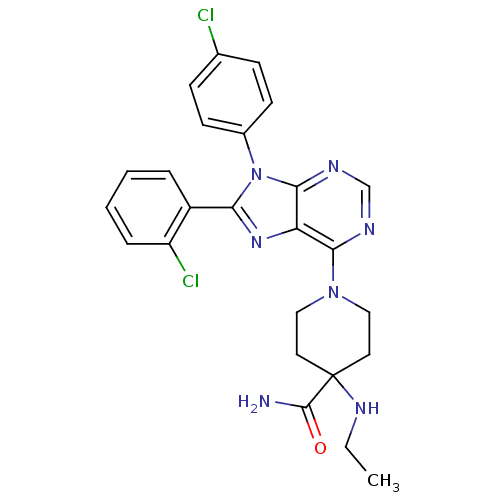 (1-[8-(2-chlorophenyl)-9-(4-chlorophenyl)-9H-purin-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.700 | -53.1 | n/a | n/a | n/a | n/a | n/a | 7.4 | 30 |
Pfizer | Assay Description IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand binding experiments. The inhibition c... | J Med Chem 52: 234-7 (2009) Article DOI: 10.1021/jm8012932 BindingDB Entry DOI: 10.7270/Q2CN7276 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyroid hormone receptor beta (Homo sapiens (Human)) | BDBM50123055 (2-(3,5-dichloro-4-(4-hydroxy-3-(morpholine-4-carbo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.720 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against [125I]-T3 binding to human TRbeta1 receptor | Bioorg Med Chem Lett 13: 379-82 (2003) BindingDB Entry DOI: 10.7270/Q26T0KZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50128927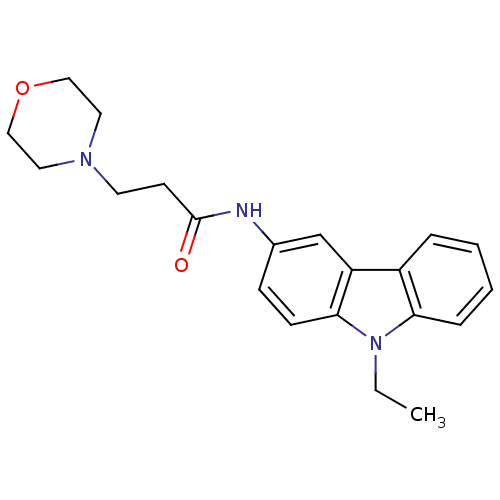 (CHEMBL59680 | N-(9-Ethyl-9H-carbazol-3-yl)-3-morph...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.840 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Binding affinity towards human neuropeptide Y receptor type 5 using 125[I]-PYY as radioligand | Bioorg Med Chem Lett 13: 1989-92 (2003) BindingDB Entry DOI: 10.7270/Q2057F9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM21278 (5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]SR141716A from rat brain CB1 receptor | Bioorg Med Chem Lett 19: 5351-4 (2009) Article DOI: 10.1016/j.bmcl.2009.07.130 BindingDB Entry DOI: 10.7270/Q2KP827S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM21278 (5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | 0.900 | -52.5 | n/a | n/a | n/a | n/a | n/a | 7.4 | 30 |
Pfizer | Assay Description IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand binding experiments. The inhibition c... | J Med Chem 52: 234-7 (2009) Article DOI: 10.1021/jm8012932 BindingDB Entry DOI: 10.7270/Q2CN7276 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM29075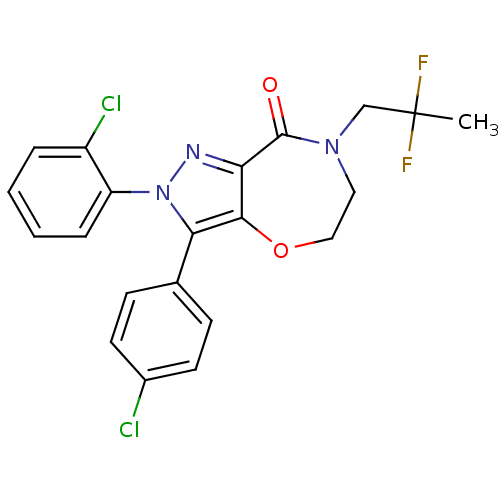 (PF-514273) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | 1 | -52.2 | n/a | n/a | 0.820 | n/a | n/a | 7.4 | 30 |
Pfizer | Assay Description IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand binding experiments. The inhibition c... | J Med Chem 52: 2652-5 (2009) Article DOI: 10.1021/jm900255t BindingDB Entry DOI: 10.7270/Q2F47MGD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50301747 (1-(2-chlorophenyl)-5-(4-chlorophenyl)-4-methyl-3-(...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]SR141716A from rat brain CB1 receptor | Bioorg Med Chem Lett 19: 5351-4 (2009) Article DOI: 10.1016/j.bmcl.2009.07.130 BindingDB Entry DOI: 10.7270/Q2KP827S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50123737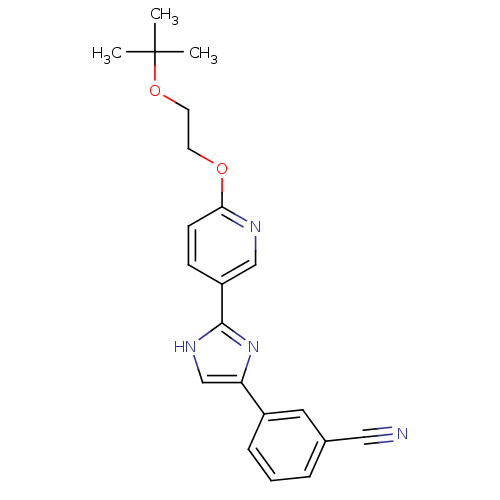 (3-{2-[6-(2-tert-Butoxy-ethoxy)-pyridin-3-yl]-3H-im...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Binding affinity of the compound to the human Neuropeptide Y receptor type 5 was determined using [125I]- [PYY] as radioligand | J Med Chem 46: 670-3 (2003) Article DOI: 10.1021/jm025584p BindingDB Entry DOI: 10.7270/Q2V987DS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM29073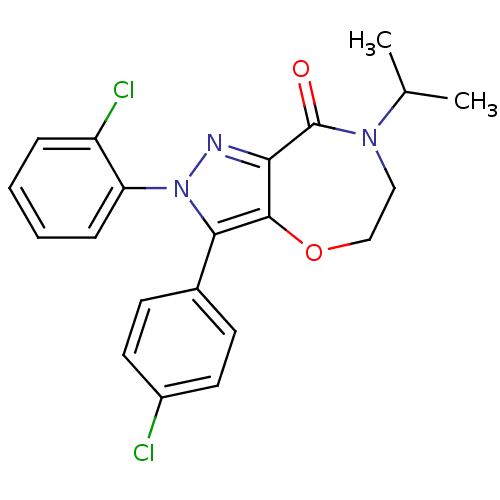 (ether-based lactam, 19b) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 1.30 | -51.6 | n/a | n/a | 4.80 | n/a | n/a | 7.4 | 30 |
Pfizer | Assay Description IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand binding experiments. The inhibition c... | J Med Chem 52: 2652-5 (2009) Article DOI: 10.1021/jm900255t BindingDB Entry DOI: 10.7270/Q2F47MGD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM29076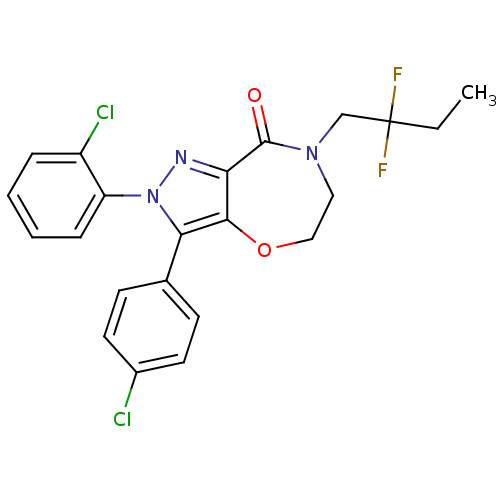 (ether-based lactam, 19e) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 1.40 | -51.4 | n/a | n/a | 0.700 | n/a | n/a | 7.4 | 30 |
Pfizer | Assay Description IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand binding experiments. The inhibition c... | J Med Chem 52: 2652-5 (2009) Article DOI: 10.1021/jm900255t BindingDB Entry DOI: 10.7270/Q2F47MGD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50128943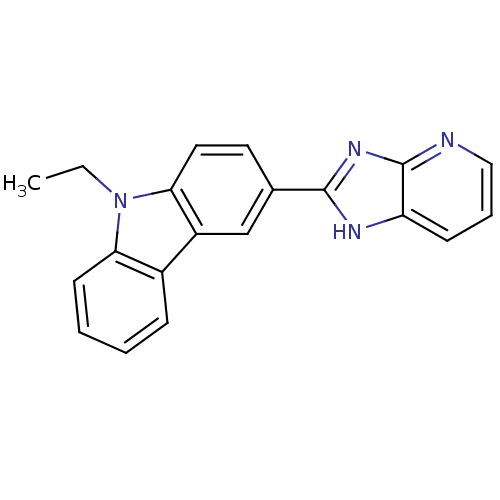 (9-Ethyl-3-(3H-imidazo[4,5-b]pyridin-2-yl)-9H-carba...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Binding affinity towards human neuropeptide Y receptor type 5 using 125[I]-PYY as radioligand | Bioorg Med Chem Lett 13: 1989-92 (2003) BindingDB Entry DOI: 10.7270/Q2057F9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM21278 (5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Antagonist activity at human CB1 receptor transfected in CHO-K1cells by GTPgamma[35S] binding assay | Bioorg Med Chem Lett 19: 5351-4 (2009) Article DOI: 10.1016/j.bmcl.2009.07.130 BindingDB Entry DOI: 10.7270/Q2KP827S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50133761 (3-[5-(3-Trifluoromethyl-phenyl)-1H-imidazol-2-yl]-...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Binding affinity towards human neuropeptide Y receptor type 5 using [125I]-PYY as radiogigand in baculovirus-infected Sf9 cells | Bioorg Med Chem Lett 13: 3593-6 (2003) BindingDB Entry DOI: 10.7270/Q2DF6QKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Rat 6B) | BDBM50123737 (3-{2-[6-(2-tert-Butoxy-ethoxy)-pyridin-3-yl]-3H-im...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Binding affinity of the compound to the rat Neuropeptide Y receptor type 5 was determined using [125I]- [Leu31,Pro34]PYY as radioligand | J Med Chem 46: 670-3 (2003) Article DOI: 10.1021/jm025584p BindingDB Entry DOI: 10.7270/Q2V987DS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM29069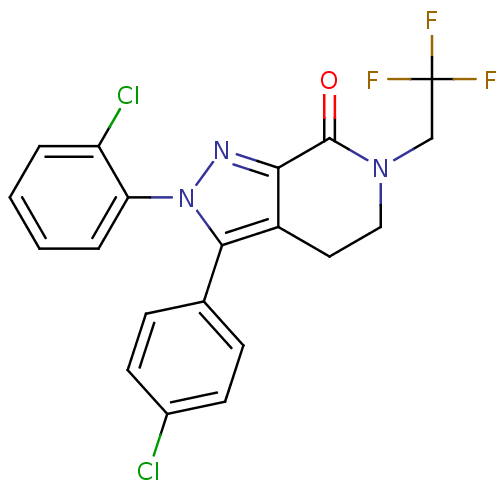 (lactam-based compound, 12h) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.70 | -50.9 | n/a | n/a | 3.10 | n/a | n/a | 7.4 | 30 |
Pfizer | Assay Description IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand binding experiments. The inhibition c... | J Med Chem 52: 2652-5 (2009) Article DOI: 10.1021/jm900255t BindingDB Entry DOI: 10.7270/Q2F47MGD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM21278 (5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | 1.80 | -50.7 | n/a | n/a | 1.60 | n/a | n/a | 7.4 | 30 |
Pfizer | Assay Description IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand binding experiments. The inhibition c... | J Med Chem 52: 2652-5 (2009) Article DOI: 10.1021/jm900255t BindingDB Entry DOI: 10.7270/Q2F47MGD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50301739 (5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]SR141716A from rat brain CB1 receptor | Bioorg Med Chem Lett 19: 5351-4 (2009) Article DOI: 10.1016/j.bmcl.2009.07.130 BindingDB Entry DOI: 10.7270/Q2KP827S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM29074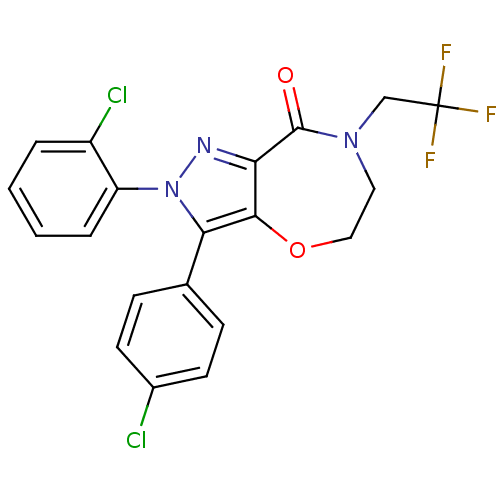 (ether-based lactam, 19c) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 1.90 | -50.6 | n/a | n/a | 1.5 | n/a | n/a | 7.4 | 30 |
Pfizer | Assay Description IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand binding experiments. The inhibition c... | J Med Chem 52: 2652-5 (2009) Article DOI: 10.1021/jm900255t BindingDB Entry DOI: 10.7270/Q2F47MGD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyroid hormone receptor beta (Homo sapiens (Human)) | BDBM50123060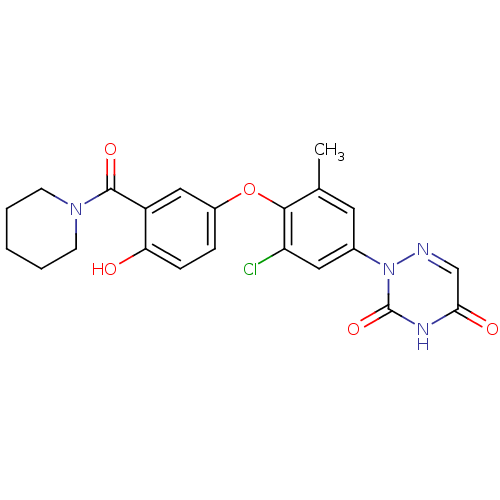 (2-{3-Chloro-4-[4-hydroxy-3-(piperidine-1-carbonyl)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against [125I]-T3 binding to human TRbeta1 receptor | Bioorg Med Chem Lett 13: 379-82 (2003) BindingDB Entry DOI: 10.7270/Q26T0KZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyroid hormone receptor beta (Homo sapiens (Human)) | BDBM50123053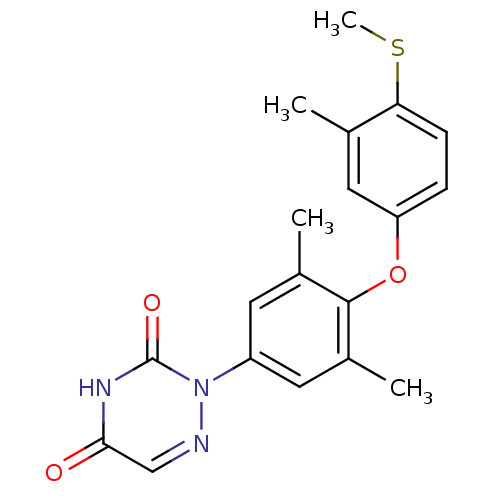 (2-[3,5-Dimethyl-4-(3-methyl-4-methylsulfanyl-pheno...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against [125I]-T3 binding to human TRbeta1 receptor | Bioorg Med Chem Lett 13: 379-82 (2003) BindingDB Entry DOI: 10.7270/Q26T0KZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM27341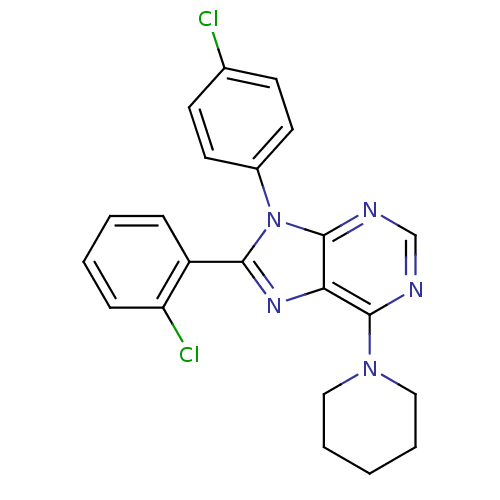 (8-(2-chlorophenyl)-9-(4-chlorophenyl)-6-(piperidin...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.10 | -50.4 | n/a | n/a | n/a | n/a | n/a | 7.4 | 30 |
Pfizer | Assay Description IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand binding experiments. The inhibition c... | J Med Chem 52: 234-7 (2009) Article DOI: 10.1021/jm8012932 BindingDB Entry DOI: 10.7270/Q2CN7276 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM27341 (8-(2-chlorophenyl)-9-(4-chlorophenyl)-6-(piperidin...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.30 | -50.1 | n/a | n/a | n/a | n/a | n/a | 7.4 | 30 |
Pfizer | Assay Description IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand binding experiments. The inhibition c... | J Med Chem 52: 234-7 (2009) Article DOI: 10.1021/jm8012932 BindingDB Entry DOI: 10.7270/Q2CN7276 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM29064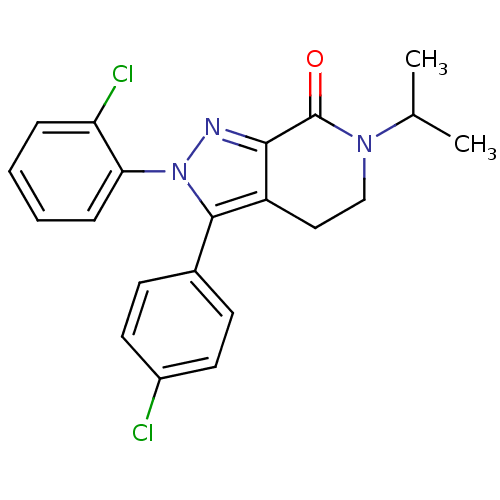 (lactam-based compound, 12c) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 2.5 | -49.9 | n/a | n/a | 5.5 | n/a | n/a | 7.4 | 30 |
Pfizer | Assay Description IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand binding experiments. The inhibition c... | J Med Chem 52: 2652-5 (2009) Article DOI: 10.1021/jm900255t BindingDB Entry DOI: 10.7270/Q2F47MGD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 826 total ) | Next | Last >> |