

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Peptidyl-prolyl cis-trans isomerase A (Homo sapiens (Human)) | BDBM92913 (Aryl 1-indanylketone, 2) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 300 | -34.7 | n/a | n/a | n/a | n/a | n/a | 7.8 | 5 |
Max Planck Research | Assay Description PPIase activity assay were performed at 283 K in quartz cuvettes with a path length of 1 cm under vigorous stirring with a Hewlett-Packard 8453A UV-v... | Biochemistry 48: 6268-77 (2009) Article DOI: 10.1021/bi9007287 BindingDB Entry DOI: 10.7270/Q2X34W26 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase A (Homo sapiens (Human)) | BDBM92912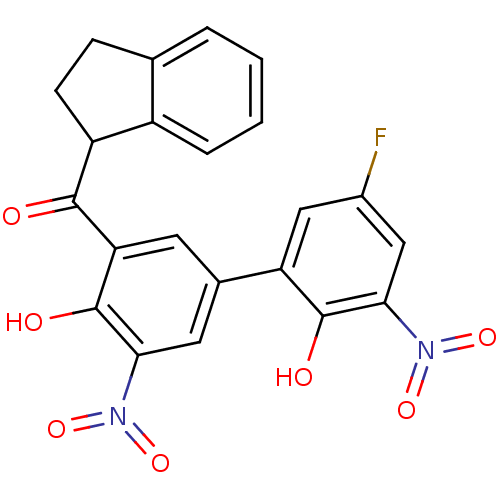 (Aryl 1-indanylketone, 1) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | 520 | -33.5 | n/a | n/a | n/a | n/a | n/a | 7.8 | 5 |
Max Planck Research | Assay Description PPIase activity assay were performed at 283 K in quartz cuvettes with a path length of 1 cm under vigorous stirring with a Hewlett-Packard 8453A UV-v... | Biochemistry 48: 6268-77 (2009) Article DOI: 10.1021/bi9007287 BindingDB Entry DOI: 10.7270/Q2X34W26 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase A (Homo sapiens (Human)) | BDBM92915 (Aryl 1-indanylketone, 4) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.20E+3 | -31.5 | n/a | n/a | n/a | n/a | n/a | 7.8 | 5 |
Max Planck Research | Assay Description PPIase activity assay were performed at 283 K in quartz cuvettes with a path length of 1 cm under vigorous stirring with a Hewlett-Packard 8453A UV-v... | Biochemistry 48: 6268-77 (2009) Article DOI: 10.1021/bi9007287 BindingDB Entry DOI: 10.7270/Q2X34W26 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase A (Homo sapiens (Human)) | BDBM92914 (Aryl 1-indanylketone, 3) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.70E+3 | -30.7 | n/a | n/a | n/a | n/a | n/a | 7.8 | 5 |
Max Planck Research | Assay Description PPIase activity assay were performed at 283 K in quartz cuvettes with a path length of 1 cm under vigorous stirring with a Hewlett-Packard 8453A UV-v... | Biochemistry 48: 6268-77 (2009) Article DOI: 10.1021/bi9007287 BindingDB Entry DOI: 10.7270/Q2X34W26 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase B (Homo sapiens (Human)) | BDBM92915 (Aryl 1-indanylketone, 4) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.10E+3 | -30.2 | n/a | n/a | n/a | n/a | n/a | 7.8 | 5 |
Max Planck Research | Assay Description PPIase activity assay were performed at 283 K in quartz cuvettes with a path length of 1 cm under vigorous stirring with a Hewlett-Packard 8453A UV-v... | Biochemistry 48: 6268-77 (2009) Article DOI: 10.1021/bi9007287 BindingDB Entry DOI: 10.7270/Q2X34W26 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase D (Homo sapiens (Human)) | BDBM92912 (Aryl 1-indanylketone, 1) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | 2.42E+3 | -29.9 | n/a | n/a | n/a | n/a | n/a | 7.8 | 5 |
Max Planck Research | Assay Description PPIase activity assay were performed at 283 K in quartz cuvettes with a path length of 1 cm under vigorous stirring with a Hewlett-Packard 8453A UV-v... | Biochemistry 48: 6268-77 (2009) Article DOI: 10.1021/bi9007287 BindingDB Entry DOI: 10.7270/Q2X34W26 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase D (Homo sapiens (Human)) | BDBM92913 (Aryl 1-indanylketone, 2) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 6.28E+3 | -27.7 | n/a | n/a | n/a | n/a | n/a | 7.8 | 5 |
Max Planck Research | Assay Description PPIase activity assay were performed at 283 K in quartz cuvettes with a path length of 1 cm under vigorous stirring with a Hewlett-Packard 8453A UV-v... | Biochemistry 48: 6268-77 (2009) Article DOI: 10.1021/bi9007287 BindingDB Entry DOI: 10.7270/Q2X34W26 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase B (Homo sapiens (Human)) | BDBM92914 (Aryl 1-indanylketone, 3) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 8.60E+3 | -27.0 | n/a | n/a | n/a | n/a | n/a | 7.8 | 5 |
Max Planck Research | Assay Description PPIase activity assay were performed at 283 K in quartz cuvettes with a path length of 1 cm under vigorous stirring with a Hewlett-Packard 8453A UV-v... | Biochemistry 48: 6268-77 (2009) Article DOI: 10.1021/bi9007287 BindingDB Entry DOI: 10.7270/Q2X34W26 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase A (Homo sapiens (Human)) | BDBM92916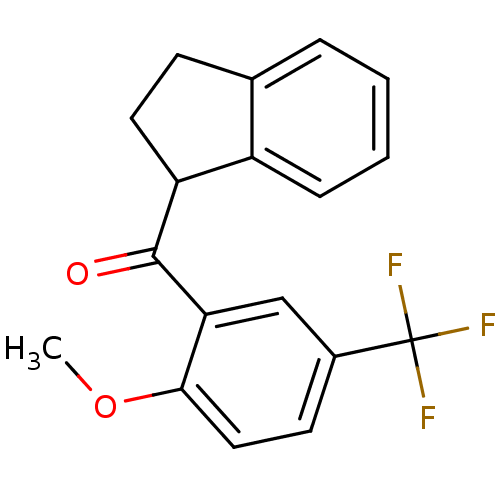 (Aryl 1-indanylketone, 5) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.00E+4 | -26.6 | n/a | n/a | n/a | n/a | n/a | 7.8 | 5 |
Max Planck Research | Assay Description PPIase activity assay were performed at 283 K in quartz cuvettes with a path length of 1 cm under vigorous stirring with a Hewlett-Packard 8453A UV-v... | Biochemistry 48: 6268-77 (2009) Article DOI: 10.1021/bi9007287 BindingDB Entry DOI: 10.7270/Q2X34W26 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase B (Homo sapiens (Human)) | BDBM92913 (Aryl 1-indanylketone, 2) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.20E+4 | -26.2 | n/a | n/a | n/a | n/a | n/a | 7.8 | 5 |
Max Planck Research | Assay Description PPIase activity assay were performed at 283 K in quartz cuvettes with a path length of 1 cm under vigorous stirring with a Hewlett-Packard 8453A UV-v... | Biochemistry 48: 6268-77 (2009) Article DOI: 10.1021/bi9007287 BindingDB Entry DOI: 10.7270/Q2X34W26 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase A (Homo sapiens (Human)) | BDBM92918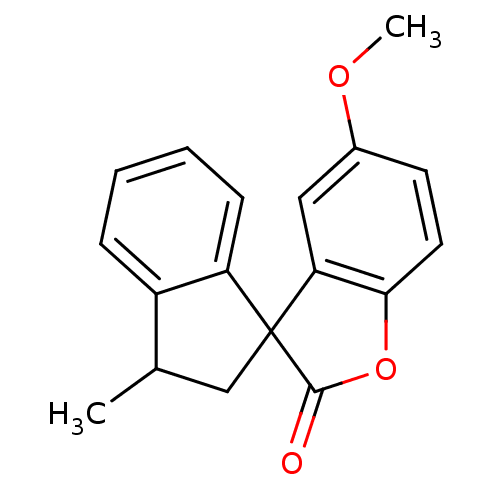 (Benzofuranone, 7) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.10E+4 | -24.9 | n/a | n/a | n/a | n/a | n/a | 7.8 | 5 |
Max Planck Research | Assay Description PPIase activity assay were performed at 283 K in quartz cuvettes with a path length of 1 cm under vigorous stirring with a Hewlett-Packard 8453A UV-v... | Biochemistry 48: 6268-77 (2009) Article DOI: 10.1021/bi9007287 BindingDB Entry DOI: 10.7270/Q2X34W26 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase-like 1 (Homo sapiens (Human)) | BDBM92912 (Aryl 1-indanylketone, 1) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | 2.90E+4 | -24.2 | n/a | n/a | n/a | n/a | n/a | 7.8 | 5 |
Max Planck Research | Assay Description PPIase activity assay were performed at 283 K in quartz cuvettes with a path length of 1 cm under vigorous stirring with a Hewlett-Packard 8453A UV-v... | Biochemistry 48: 6268-77 (2009) Article DOI: 10.1021/bi9007287 BindingDB Entry DOI: 10.7270/Q2X34W26 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase B (Homo sapiens (Human)) | BDBM92920 (Benzofuranone, 9) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 6.30E+4 | -22.4 | n/a | n/a | n/a | n/a | n/a | 7.8 | 5 |
Max Planck Research | Assay Description PPIase activity assay were performed at 283 K in quartz cuvettes with a path length of 1 cm under vigorous stirring with a Hewlett-Packard 8453A UV-v... | Biochemistry 48: 6268-77 (2009) Article DOI: 10.1021/bi9007287 BindingDB Entry DOI: 10.7270/Q2X34W26 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase B (Homo sapiens (Human)) | BDBM92912 (Aryl 1-indanylketone, 1) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | >1.00E+5 | >-21.3 | n/a | n/a | n/a | n/a | n/a | 7.8 | 5 |
Max Planck Research | Assay Description PPIase activity assay were performed at 283 K in quartz cuvettes with a path length of 1 cm under vigorous stirring with a Hewlett-Packard 8453A UV-v... | Biochemistry 48: 6268-77 (2009) Article DOI: 10.1021/bi9007287 BindingDB Entry DOI: 10.7270/Q2X34W26 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase B (Homo sapiens (Human)) | BDBM92917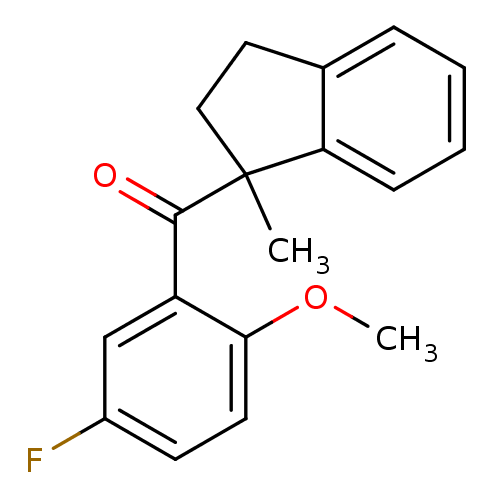 (Aryl 1-indanylketone, 6) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | >1.00E+5 | >-21.3 | n/a | n/a | n/a | n/a | n/a | 7.8 | 5 |
Max Planck Research | Assay Description PPIase activity assay were performed at 283 K in quartz cuvettes with a path length of 1 cm under vigorous stirring with a Hewlett-Packard 8453A UV-v... | Biochemistry 48: 6268-77 (2009) Article DOI: 10.1021/bi9007287 BindingDB Entry DOI: 10.7270/Q2X34W26 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase B (Homo sapiens (Human)) | BDBM92918 (Benzofuranone, 7) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >1.00E+5 | >-21.3 | n/a | n/a | n/a | n/a | n/a | 7.8 | 5 |
Max Planck Research | Assay Description PPIase activity assay were performed at 283 K in quartz cuvettes with a path length of 1 cm under vigorous stirring with a Hewlett-Packard 8453A UV-v... | Biochemistry 48: 6268-77 (2009) Article DOI: 10.1021/bi9007287 BindingDB Entry DOI: 10.7270/Q2X34W26 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase B (Homo sapiens (Human)) | BDBM92919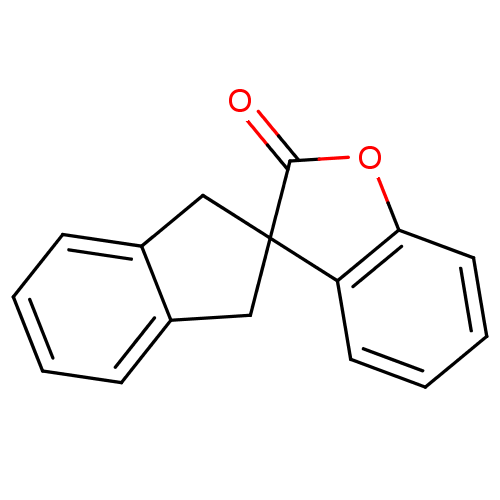 (Benzofuranone, 8) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >1.00E+5 | >-21.3 | n/a | n/a | n/a | n/a | n/a | 7.8 | 5 |
Max Planck Research | Assay Description PPIase activity assay were performed at 283 K in quartz cuvettes with a path length of 1 cm under vigorous stirring with a Hewlett-Packard 8453A UV-v... | Biochemistry 48: 6268-77 (2009) Article DOI: 10.1021/bi9007287 BindingDB Entry DOI: 10.7270/Q2X34W26 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase C (Homo sapiens (Human)) | BDBM92912 (Aryl 1-indanylketone, 1) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | >1.00E+5 | >-21.3 | n/a | n/a | n/a | n/a | n/a | 7.8 | 5 |
Max Planck Research | Assay Description PPIase activity assay were performed at 283 K in quartz cuvettes with a path length of 1 cm under vigorous stirring with a Hewlett-Packard 8453A UV-v... | Biochemistry 48: 6268-77 (2009) Article DOI: 10.1021/bi9007287 BindingDB Entry DOI: 10.7270/Q2X34W26 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase A (Homo sapiens (Human)) | BDBM92920 (Benzofuranone, 9) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >1.00E+5 | >-21.3 | n/a | n/a | n/a | n/a | n/a | 7.8 | 5 |
Max Planck Research | Assay Description PPIase activity assay were performed at 283 K in quartz cuvettes with a path length of 1 cm under vigorous stirring with a Hewlett-Packard 8453A UV-v... | Biochemistry 48: 6268-77 (2009) Article DOI: 10.1021/bi9007287 BindingDB Entry DOI: 10.7270/Q2X34W26 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase B (Homo sapiens (Human)) | BDBM92916 (Aryl 1-indanylketone, 5) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >1.00E+5 | >-21.3 | n/a | n/a | n/a | n/a | n/a | 7.8 | 5 |
Max Planck Research | Assay Description PPIase activity assay were performed at 283 K in quartz cuvettes with a path length of 1 cm under vigorous stirring with a Hewlett-Packard 8453A UV-v... | Biochemistry 48: 6268-77 (2009) Article DOI: 10.1021/bi9007287 BindingDB Entry DOI: 10.7270/Q2X34W26 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase H (Homo sapiens (Human)) | BDBM92913 (Aryl 1-indanylketone, 2) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | >1.00E+5 | >-21.3 | n/a | n/a | n/a | n/a | n/a | 7.8 | 5 |
Max Planck Research | Assay Description PPIase activity assay were performed at 283 K in quartz cuvettes with a path length of 1 cm under vigorous stirring with a Hewlett-Packard 8453A UV-v... | Biochemistry 48: 6268-77 (2009) Article DOI: 10.1021/bi9007287 BindingDB Entry DOI: 10.7270/Q2X34W26 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase A (Homo sapiens (Human)) | BDBM92917 (Aryl 1-indanylketone, 6) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | >1.00E+5 | >-21.3 | n/a | n/a | n/a | n/a | n/a | 7.8 | 5 |
Max Planck Research | Assay Description PPIase activity assay were performed at 283 K in quartz cuvettes with a path length of 1 cm under vigorous stirring with a Hewlett-Packard 8453A UV-v... | Biochemistry 48: 6268-77 (2009) Article DOI: 10.1021/bi9007287 BindingDB Entry DOI: 10.7270/Q2X34W26 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase-like 1 (Homo sapiens (Human)) | BDBM92913 (Aryl 1-indanylketone, 2) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | >1.00E+5 | >-21.3 | n/a | n/a | n/a | n/a | n/a | 7.8 | 5 |
Max Planck Research | Assay Description PPIase activity assay were performed at 283 K in quartz cuvettes with a path length of 1 cm under vigorous stirring with a Hewlett-Packard 8453A UV-v... | Biochemistry 48: 6268-77 (2009) Article DOI: 10.1021/bi9007287 BindingDB Entry DOI: 10.7270/Q2X34W26 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase C (Homo sapiens (Human)) | BDBM92913 (Aryl 1-indanylketone, 2) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | >1.00E+5 | >-21.3 | n/a | n/a | n/a | n/a | n/a | 7.8 | 5 |
Max Planck Research | Assay Description PPIase activity assay were performed at 283 K in quartz cuvettes with a path length of 1 cm under vigorous stirring with a Hewlett-Packard 8453A UV-v... | Biochemistry 48: 6268-77 (2009) Article DOI: 10.1021/bi9007287 BindingDB Entry DOI: 10.7270/Q2X34W26 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase H (Homo sapiens (Human)) | BDBM92912 (Aryl 1-indanylketone, 1) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | >1.00E+5 | >-21.3 | n/a | n/a | n/a | n/a | n/a | 7.8 | 5 |
Max Planck Research | Assay Description PPIase activity assay were performed at 283 K in quartz cuvettes with a path length of 1 cm under vigorous stirring with a Hewlett-Packard 8453A UV-v... | Biochemistry 48: 6268-77 (2009) Article DOI: 10.1021/bi9007287 BindingDB Entry DOI: 10.7270/Q2X34W26 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase A (Homo sapiens (Human)) | BDBM92919 (Benzofuranone, 8) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >1.00E+5 | >-21.3 | n/a | n/a | n/a | n/a | n/a | 7.8 | 5 |
Max Planck Research | Assay Description PPIase activity assay were performed at 283 K in quartz cuvettes with a path length of 1 cm under vigorous stirring with a Hewlett-Packard 8453A UV-v... | Biochemistry 48: 6268-77 (2009) Article DOI: 10.1021/bi9007287 BindingDB Entry DOI: 10.7270/Q2X34W26 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50032599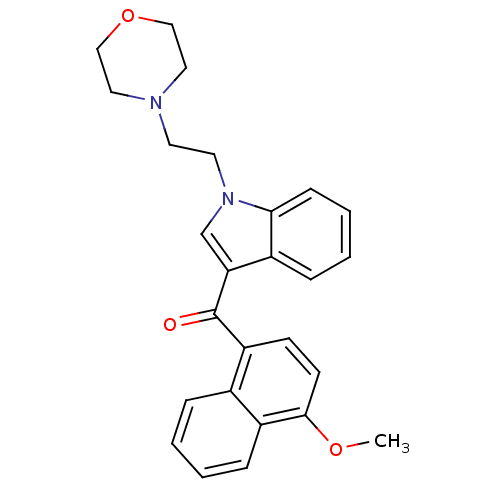 ((4-Methoxy-naphthalen-1-yl)-[1-(2-morpholin-4-yl-e...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Research Division Curated by ChEMBL | Assay Description Concentration of compound required to inhibit 50% of [3H]-WIN- 55212 binding to Cannabinoid receptor 1 in rat cerebellum membranes. | J Med Chem 38: 3094-105 (1995) BindingDB Entry DOI: 10.7270/Q2DN442Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50032565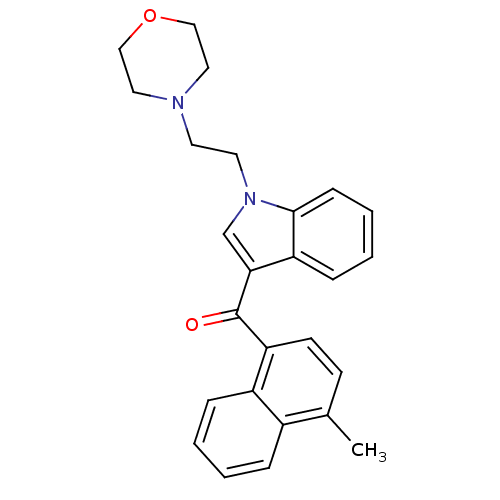 ((4-Methyl-naphthalen-1-yl)-[1-(2-morpholin-4-yl-et...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Research Division Curated by ChEMBL | Assay Description Concentration of compound required to inhibit 50% of [3H]-WIN- 55212 binding to Cannabinoid receptor 1 in rat cerebellum membranes. | J Med Chem 38: 3094-105 (1995) BindingDB Entry DOI: 10.7270/Q2DN442Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50032584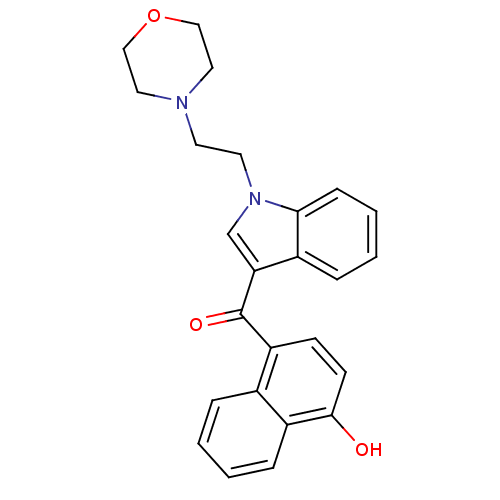 ((4-Hydroxy-naphthalen-1-yl)-[1-(2-morpholin-4-yl-e...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Research Division Curated by ChEMBL | Assay Description Concentration of compound required to inhibit 50% of [3H]-WIN- 55212 binding to Cannabinoid receptor 1 in rat cerebellum membranes. | J Med Chem 38: 3094-105 (1995) BindingDB Entry DOI: 10.7270/Q2DN442Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50032615 (CHEMBL82361 | [6-Methyl-1-(2-morpholin-4-yl-ethyl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Research Division Curated by ChEMBL | Assay Description Concentration of compound required to inhibit 50% of [3H]-WIN- 55212 binding to Cannabinoid receptor 1 in rat cerebellum membranes. | J Med Chem 38: 3094-105 (1995) BindingDB Entry DOI: 10.7270/Q2DN442Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM60994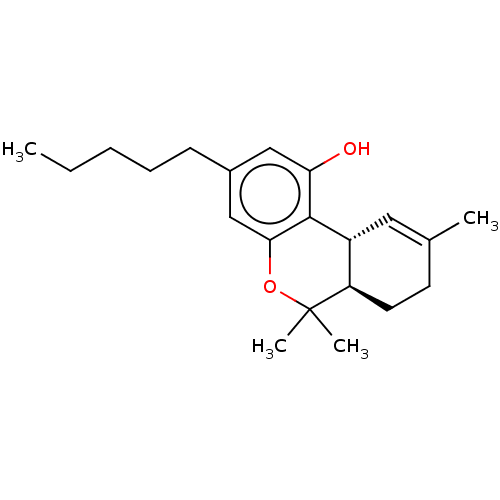 ((10R,10aR)-6,6,9-Trimethyl-3-pentyl-6a,7,8,10a-tet...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL DrugBank KEGG PC cid PC sid PDB UniChem Similars | PubMed | n/a | n/a | 5.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Research Division Curated by ChEMBL | Assay Description Concentration of compound required to inhibit 50% of [3H]-WIN- 55212 binding to Cannabinoid receptor 1 in rat cerebellum membranes. | J Med Chem 38: 3094-105 (1995) BindingDB Entry DOI: 10.7270/Q2DN442Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50032575 (CHEMBL309763 | [2-Methyl-1-(2-morpholin-4-yl-ethyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Research Division Curated by ChEMBL | Assay Description Concentration of compound required to inhibit 50% of [3H]-WIN- 55212 binding to Cannabinoid receptor 1 in rat cerebellum membranes. | J Med Chem 38: 3094-105 (1995) BindingDB Entry DOI: 10.7270/Q2DN442Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin-H2 D-isomerase (Mus musculus) | BDBM50009854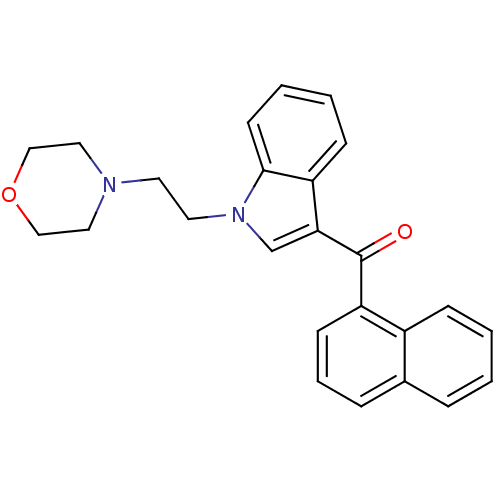 (CHEMBL13078 | [1-(2-Morpholin-4-yl-ethyl)-1H-indol...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Research Group Curated by ChEMBL | Assay Description Concentration required to inhibit 50% activity of prostaglandin synthetase was determined in vitro in mouse brain microsomes | J Med Chem 34: 1099-110 (1991) BindingDB Entry DOI: 10.7270/Q24M955S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50032583 ((4-Bromo-naphthalen-1-yl)-[2-methyl-1-(2-morpholin...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Research Division Curated by ChEMBL | Assay Description Concentration of compound required to inhibit 50% of [3H]-WIN- 55212 binding to Cannabinoid receptor 1 in rat cerebellum membranes. | J Med Chem 38: 3094-105 (1995) BindingDB Entry DOI: 10.7270/Q2DN442Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50009854 (CHEMBL13078 | [1-(2-Morpholin-4-yl-ethyl)-1H-indol...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Research Division Curated by ChEMBL | Assay Description Concentration of compound required to inhibit 50% of [3H]-WIN- 55212 binding to Cannabinoid receptor 1 in rat cerebellum membranes. | J Med Chem 38: 3094-105 (1995) BindingDB Entry DOI: 10.7270/Q2DN442Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50032621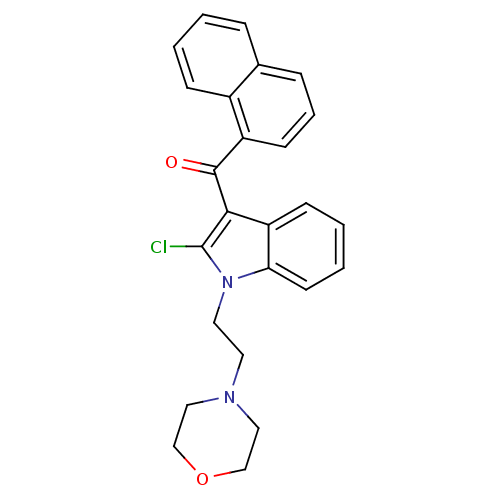 (CHEMBL78713 | [2-Chloro-1-(2-morpholin-4-yl-ethyl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Research Division Curated by ChEMBL | Assay Description Concentration of compound required to inhibit 50% of [3H]-WIN- 55212 binding to Cannabinoid receptor 1 in rat cerebellum membranes. | J Med Chem 38: 3094-105 (1995) BindingDB Entry DOI: 10.7270/Q2DN442Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50032616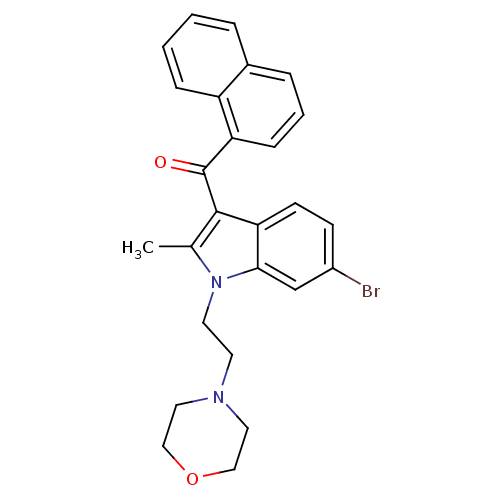 (CHEMBL78940 | [6-Bromo-2-methyl-1-(2-morpholin-4-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Research Division Curated by ChEMBL | Assay Description Concentration of compound required to inhibit 50% of [3H]-WIN- 55212 binding to Cannabinoid receptor 1 in rat cerebellum membranes. | J Med Chem 38: 3094-105 (1995) BindingDB Entry DOI: 10.7270/Q2DN442Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50032589 (CHEMBL78415 | [7-Methoxy-2-methyl-1-(2-morpholin-4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Research Division Curated by ChEMBL | Assay Description Concentration of compound required to inhibit 50% of [3H]-WIN- 55212 binding to Cannabinoid receptor 1 in rat cerebellum membranes. | J Med Chem 38: 3094-105 (1995) BindingDB Entry DOI: 10.7270/Q2DN442Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50032560 (CHEMBL78994 | [2,6-Dimethyl-1-(2-morpholin-4-yl-et...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Research Division Curated by ChEMBL | Assay Description Concentration of compound required to inhibit 50% of [3H]-WIN- 55212 binding to Cannabinoid receptor 1 in rat cerebellum membranes. | J Med Chem 38: 3094-105 (1995) BindingDB Entry DOI: 10.7270/Q2DN442Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50032561 (CHEMBL104841 | [2-Methyl-1-(1-methyl-2-morpholin-4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Research Division Curated by ChEMBL | Assay Description Concentration of compound required to inhibit 50% of [3H]-WIN- 55212 binding to Cannabinoid receptor 1 in rat cerebellum membranes. | J Med Chem 38: 3094-105 (1995) BindingDB Entry DOI: 10.7270/Q2DN442Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50032555 (4-[2-Methyl-1-(2-morpholin-4-yl-ethyl)-1H-indole-3...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Research Division Curated by ChEMBL | Assay Description Concentration of compound required to inhibit 50% of [3H]-WIN- 55212 binding to Cannabinoid receptor 1 in rat cerebellum membranes. | J Med Chem 38: 3094-105 (1995) BindingDB Entry DOI: 10.7270/Q2DN442Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin-H2 D-isomerase (Mus musculus) | BDBM50009864 (CHEMBL274833 | [2-Methyl-1-(2-morpholin-4-yl-ethyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Research Group Curated by ChEMBL | Assay Description Concentration required to inhibit 50% activity of prostaglandin synthetase was determined in vitro in mouse brain microsomes | J Med Chem 34: 1099-110 (1991) BindingDB Entry DOI: 10.7270/Q24M955S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50032567 (Benzofuran-7-yl-[1-(2-morpholin-4-yl-ethyl)-1H-ind...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Research Division Curated by ChEMBL | Assay Description Concentration of compound required to inhibit 50% of [3H]-WIN- 55212 binding to Cannabinoid receptor 1 in rat cerebellum membranes. | J Med Chem 38: 3094-105 (1995) BindingDB Entry DOI: 10.7270/Q2DN442Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin-H2 D-isomerase (Mus musculus) | BDBM50009871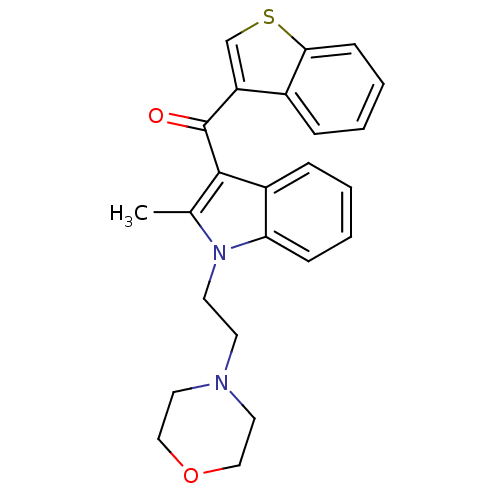 (Benzo[b]thiophen-3-yl-[2-methyl-1-(2-morpholin-4-y...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Research Group Curated by ChEMBL | Assay Description Concentration required to inhibit 50% activity of prostaglandin synthetase was determined in vitro in mouse brain microsomes | J Med Chem 34: 1099-110 (1991) BindingDB Entry DOI: 10.7270/Q24M955S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50009864 (CHEMBL274833 | [2-Methyl-1-(2-morpholin-4-yl-ethyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Research Division Curated by ChEMBL | Assay Description Concentration of compound required to inhibit 50% of [3H]-WIN- 55212 binding to Cannabinoid receptor 1 in rat cerebellum membranes. | J Med Chem 38: 3094-105 (1995) BindingDB Entry DOI: 10.7270/Q2DN442Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50032562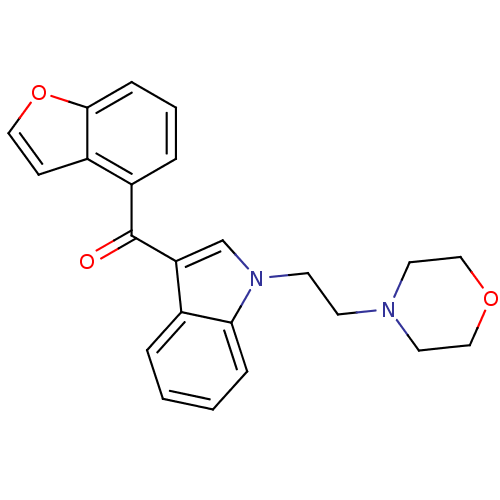 (Benzofuran-4-yl-[1-(2-morpholin-4-yl-ethyl)-1H-ind...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Research Division Curated by ChEMBL | Assay Description Concentration of compound required to inhibit 50% of [3H]-WIN- 55212 binding to Cannabinoid receptor 1 in rat cerebellum membranes. | J Med Chem 38: 3094-105 (1995) BindingDB Entry DOI: 10.7270/Q2DN442Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50032595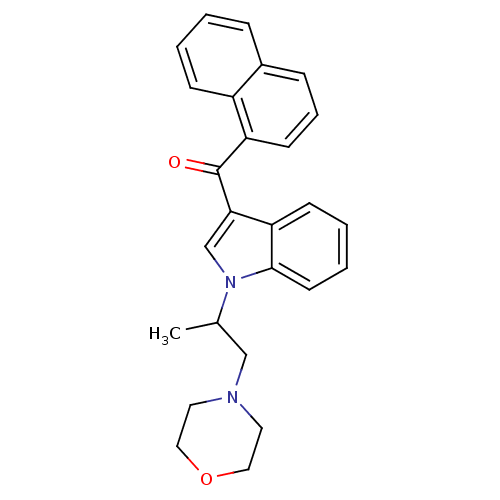 (CHEMBL107106 | [1-(1-Methyl-2-morpholin-4-yl-ethyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Research Division Curated by ChEMBL | Assay Description Concentration of compound required to inhibit 50% of [3H]-WIN- 55212 binding to Cannabinoid receptor 1 in rat cerebellum membranes. | J Med Chem 38: 3094-105 (1995) BindingDB Entry DOI: 10.7270/Q2DN442Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50032595 (CHEMBL107106 | [1-(1-Methyl-2-morpholin-4-yl-ethyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Research Division Curated by ChEMBL | Assay Description Concentration of compound required to inhibit 50% of [3H]-WIN- 55212 binding to Cannabinoid receptor 1 in rat cerebellum membranes. | J Med Chem 38: 3094-105 (1995) BindingDB Entry DOI: 10.7270/Q2DN442Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50032613 (CHEMBL78308 | [5-Fluoro-1-(2-morpholin-4-yl-ethyl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Research Division Curated by ChEMBL | Assay Description Concentration of compound required to inhibit 50% of [3H]-WIN- 55212 binding to Cannabinoid receptor 1 in rat cerebellum membranes. | J Med Chem 38: 3094-105 (1995) BindingDB Entry DOI: 10.7270/Q2DN442Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50032596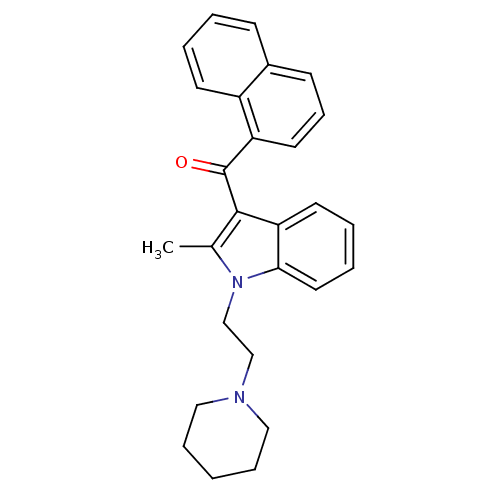 (CHEMBL311645 | [2-Methyl-1-(2-piperidin-1-yl-ethyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Research Division Curated by ChEMBL | Assay Description Concentration of compound required to inhibit 50% of [3H]-WIN- 55212 binding to Cannabinoid receptor 1 in rat cerebellum membranes. | J Med Chem 38: 3094-105 (1995) BindingDB Entry DOI: 10.7270/Q2DN442Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 118 total ) | Next | Last >> |