

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Coagulation factor X (Homo sapiens (Human)) | BDBM12569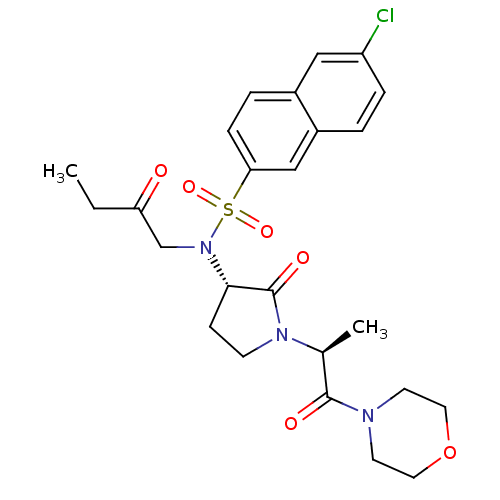 (GTC000006A | N-(6-chloronaphthalen-2-yl)-N'-[(3S)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1 | -51.4 | n/a | n/a | n/a | n/a | n/a | 7.8 | 25 |
GlaxoSmithKline | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrate. The hydrolysis rates of chromogenic substrate... | Bioorg Med Chem Lett 16: 3784-8 (2006) Article DOI: 10.1016/j.bmcl.2006.04.053 BindingDB Entry DOI: 10.7270/Q2416V9V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12557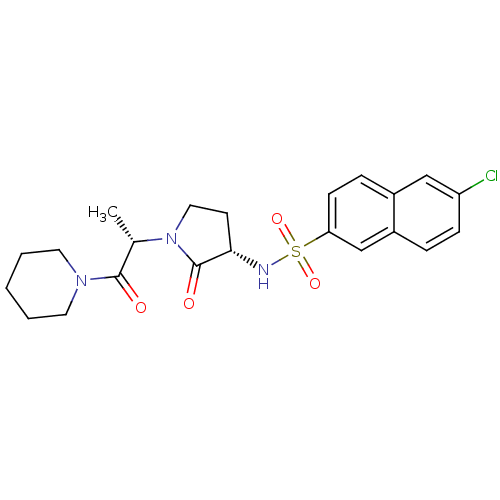 (6-chloro-N-[(3S)-2-oxo-1-[(2S)-1-oxo-1-(piperidin-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1 | -51.4 | n/a | n/a | n/a | n/a | n/a | 7.8 | 25 |
GlaxoSmithKline | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrate. The hydrolysis rates of chromogenic substrate... | Bioorg Med Chem Lett 16: 3784-8 (2006) Article DOI: 10.1016/j.bmcl.2006.04.053 BindingDB Entry DOI: 10.7270/Q2416V9V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12567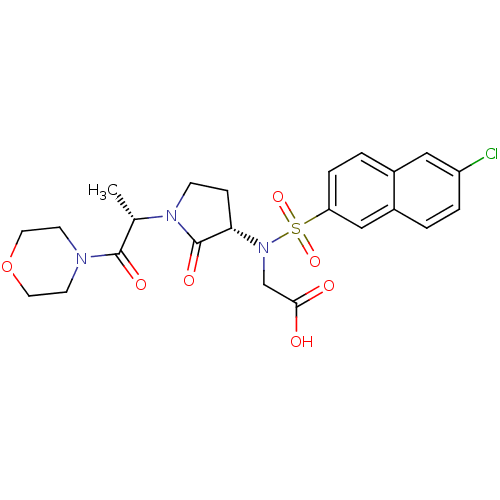 (2-[(6-chloronaphthalene-2-)[(3S)-1-[(2S)-1-(morpho...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2 | -49.7 | n/a | n/a | n/a | n/a | n/a | 7.8 | 25 |
GlaxoSmithKline | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrate. The hydrolysis rates of chromogenic substrate... | Bioorg Med Chem Lett 16: 3784-8 (2006) Article DOI: 10.1016/j.bmcl.2006.04.053 BindingDB Entry DOI: 10.7270/Q2416V9V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12558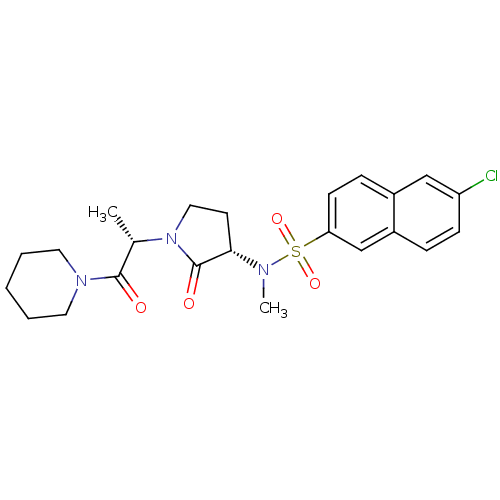 (6-chloro-N-methyl-N-[(3S)-2-oxo-1-[(2S)-1-oxo-1-(p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2 | -49.7 | n/a | n/a | n/a | n/a | n/a | 7.8 | 25 |
GlaxoSmithKline | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrate. The hydrolysis rates of chromogenic substrate... | Bioorg Med Chem Lett 16: 3784-8 (2006) Article DOI: 10.1016/j.bmcl.2006.04.053 BindingDB Entry DOI: 10.7270/Q2416V9V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12561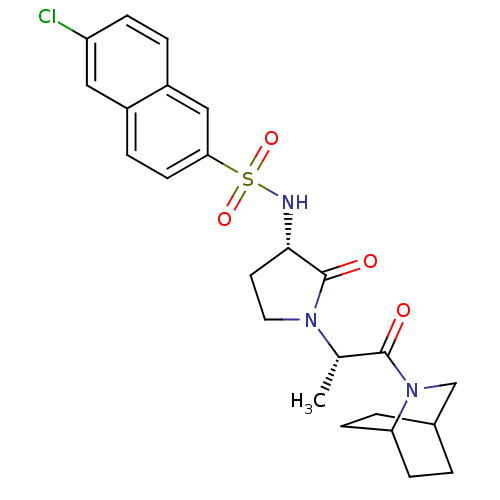 (N-[(3S)-1-[(2S)-1-{2-azabicyclo[2.2.2]octan-2-yl}-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3 | -48.6 | n/a | n/a | n/a | n/a | n/a | 7.8 | 25 |
GlaxoSmithKline | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrate. The hydrolysis rates of chromogenic substrate... | Bioorg Med Chem Lett 16: 3784-8 (2006) Article DOI: 10.1016/j.bmcl.2006.04.053 BindingDB Entry DOI: 10.7270/Q2416V9V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12568 (2-[(6-chloronaphthalene-2-)[(3S)-1-[(2S)-1-(morpho...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3 | -48.6 | n/a | n/a | n/a | n/a | n/a | 7.8 | 25 |
GlaxoSmithKline | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrate. The hydrolysis rates of chromogenic substrate... | Bioorg Med Chem Lett 16: 3784-8 (2006) Article DOI: 10.1016/j.bmcl.2006.04.053 BindingDB Entry DOI: 10.7270/Q2416V9V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12538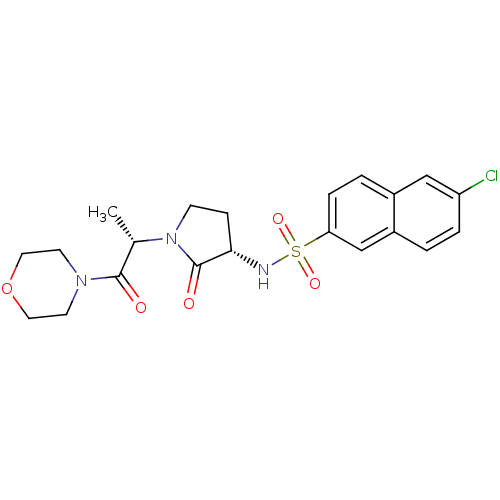 (6-chloro-N-[(3S)-1-[(2S)-1-(morpholin-4-yl)-1-oxop...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 6 | -46.9 | n/a | n/a | n/a | n/a | n/a | 7.8 | 25 |
GlaxoSmithKline | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrate. The hydrolysis rates of chromogenic substrate... | Bioorg Med Chem Lett 16: 3784-8 (2006) Article DOI: 10.1016/j.bmcl.2006.04.053 BindingDB Entry DOI: 10.7270/Q2416V9V | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12566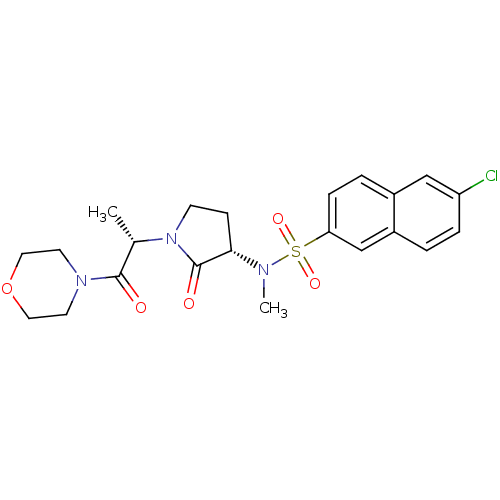 (6-chloro-N-methyl-N-[(3S)-1-[(2S)-1-(morpholin-4-y...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 10 | -45.7 | n/a | n/a | n/a | n/a | n/a | 7.8 | 25 |
GlaxoSmithKline | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrate. The hydrolysis rates of chromogenic substrate... | Bioorg Med Chem Lett 16: 3784-8 (2006) Article DOI: 10.1016/j.bmcl.2006.04.053 BindingDB Entry DOI: 10.7270/Q2416V9V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12562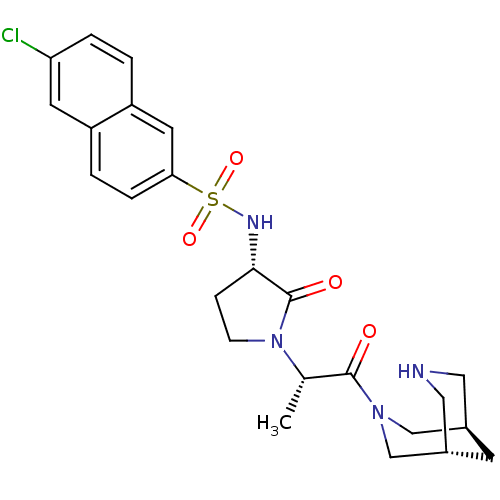 (6-chloro-N-[(3S)-1-[(2S)-1-[(1R,5S)-3,7-diazabicyc...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 13 | -45.0 | n/a | n/a | n/a | n/a | n/a | 7.8 | 25 |
GlaxoSmithKline | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrate. The hydrolysis rates of chromogenic substrate... | Bioorg Med Chem Lett 16: 3784-8 (2006) Article DOI: 10.1016/j.bmcl.2006.04.053 BindingDB Entry DOI: 10.7270/Q2416V9V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12574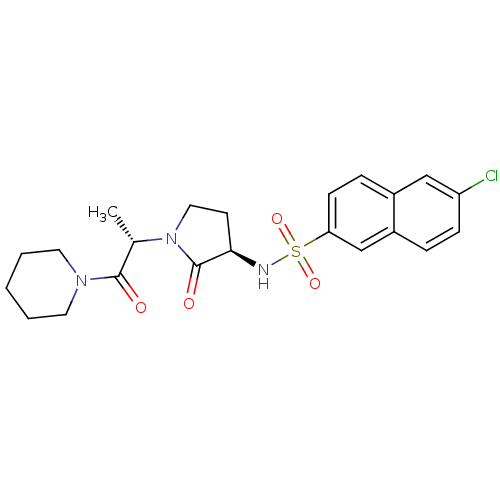 (6-chloro-N-[(3R)-2-oxo-1-[(2S)-1-oxo-1-(piperidin-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 16 | -44.5 | n/a | n/a | n/a | n/a | n/a | 7.8 | 25 |
GlaxoSmithKline | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrate. The hydrolysis rates of chromogenic substrate... | Bioorg Med Chem Lett 16: 3784-8 (2006) Article DOI: 10.1016/j.bmcl.2006.04.053 BindingDB Entry DOI: 10.7270/Q2416V9V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12573 (6-chloro-N-[(3R)-2-oxo-1-[(2R)-1-oxo-1-(piperidin-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 40 | -42.2 | n/a | n/a | n/a | n/a | n/a | 7.8 | 25 |
GlaxoSmithKline | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrate. The hydrolysis rates of chromogenic substrate... | Bioorg Med Chem Lett 16: 3784-8 (2006) Article DOI: 10.1016/j.bmcl.2006.04.053 BindingDB Entry DOI: 10.7270/Q2416V9V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12560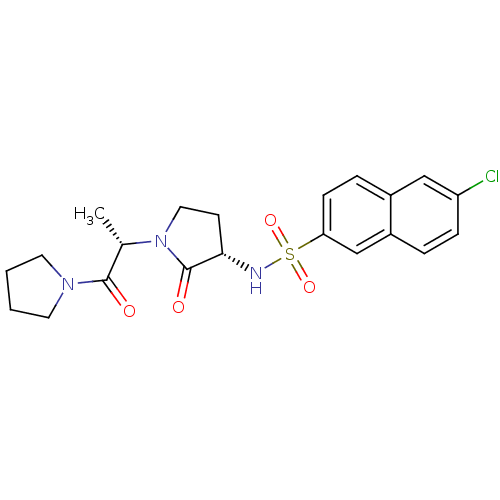 (6-chloro-N-[(3S)-2-oxo-1-[(2S)-1-oxo-1-(pyrrolidin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 50 | -41.7 | n/a | n/a | n/a | n/a | n/a | 7.8 | 25 |
GlaxoSmithKline | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrate. The hydrolysis rates of chromogenic substrate... | Bioorg Med Chem Lett 16: 3784-8 (2006) Article DOI: 10.1016/j.bmcl.2006.04.053 BindingDB Entry DOI: 10.7270/Q2416V9V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12571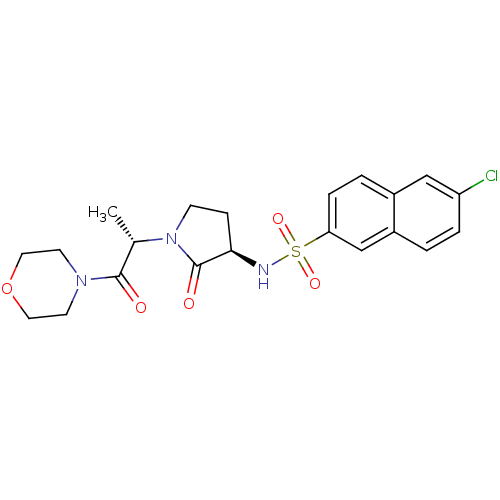 (6-chloro-N-[(3R)-1-[(2S)-1-(morpholin-4-yl)-1-oxop...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 53 | -41.5 | n/a | n/a | n/a | n/a | n/a | 7.8 | 25 |
GlaxoSmithKline | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrate. The hydrolysis rates of chromogenic substrate... | Bioorg Med Chem Lett 16: 3784-8 (2006) Article DOI: 10.1016/j.bmcl.2006.04.053 BindingDB Entry DOI: 10.7270/Q2416V9V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12554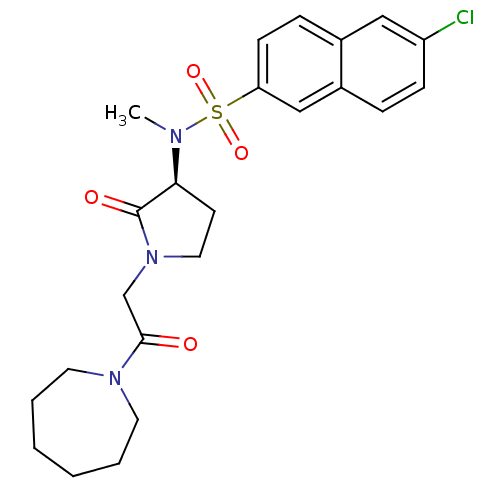 (N-[(3S)-1-[2-(azepan-1-yl)-2-oxoethyl]-2-oxopyrrol...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 60 | -41.2 | n/a | n/a | n/a | n/a | n/a | 7.8 | 25 |
GlaxoSmithKline | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrate. The hydrolysis rates of chromogenic substrate... | Bioorg Med Chem Lett 16: 3784-8 (2006) Article DOI: 10.1016/j.bmcl.2006.04.053 BindingDB Entry DOI: 10.7270/Q2416V9V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12556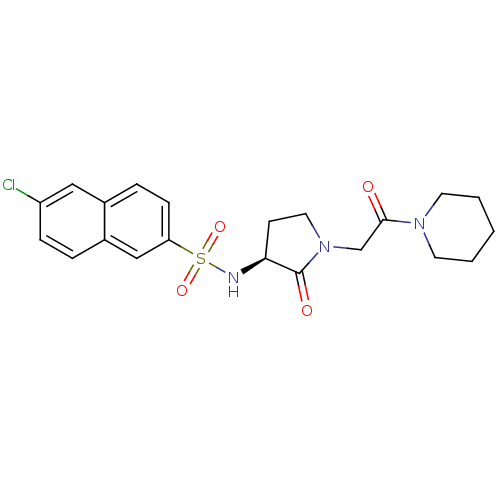 (6-chloro-N-[(3S)-2-oxo-1-[2-oxo-2-(piperidin-1-yl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 63 | -41.1 | n/a | n/a | n/a | n/a | n/a | 7.8 | 25 |
GlaxoSmithKline | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrate. The hydrolysis rates of chromogenic substrate... | Bioorg Med Chem Lett 16: 3784-8 (2006) Article DOI: 10.1016/j.bmcl.2006.04.053 BindingDB Entry DOI: 10.7270/Q2416V9V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12555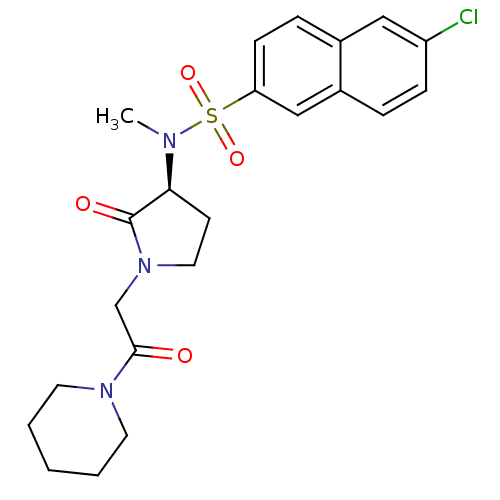 (6-chloro-N-methyl-N-[(3S)-2-oxo-1-[2-oxo-2-(piperi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 72 | -40.8 | n/a | n/a | n/a | n/a | n/a | 7.8 | 25 |
GlaxoSmithKline | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrate. The hydrolysis rates of chromogenic substrate... | Bioorg Med Chem Lett 16: 3784-8 (2006) Article DOI: 10.1016/j.bmcl.2006.04.053 BindingDB Entry DOI: 10.7270/Q2416V9V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12570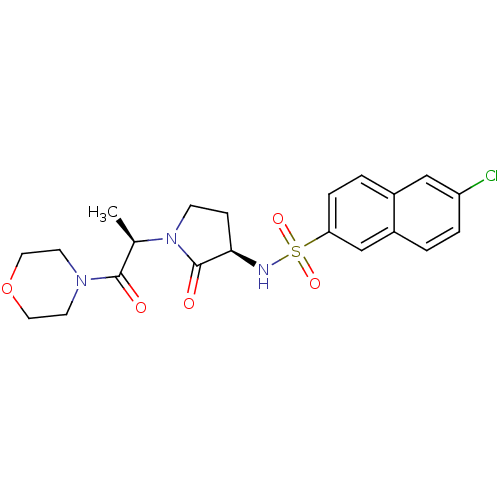 (6-chloro-N-[(3R)-1-[(2R)-1-(morpholin-4-yl)-1-oxop...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 82 | -40.4 | n/a | n/a | n/a | n/a | n/a | 7.8 | 25 |
GlaxoSmithKline | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrate. The hydrolysis rates of chromogenic substrate... | Bioorg Med Chem Lett 16: 3784-8 (2006) Article DOI: 10.1016/j.bmcl.2006.04.053 BindingDB Entry DOI: 10.7270/Q2416V9V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12575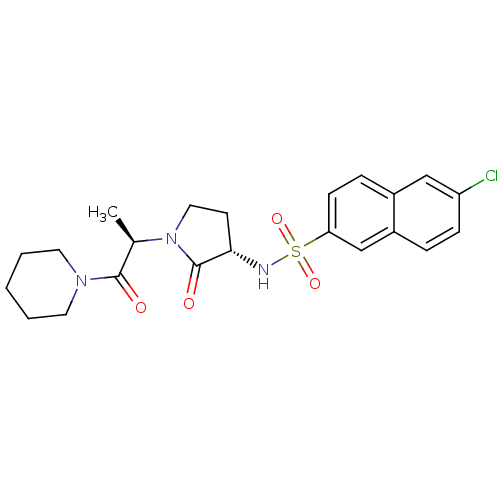 (6-chloro-N-[(3S)-2-oxo-1-[(2R)-1-oxo-1-(piperidin-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 82 | -40.4 | n/a | n/a | n/a | n/a | n/a | 7.8 | 25 |
GlaxoSmithKline | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrate. The hydrolysis rates of chromogenic substrate... | Bioorg Med Chem Lett 16: 3784-8 (2006) Article DOI: 10.1016/j.bmcl.2006.04.053 BindingDB Entry DOI: 10.7270/Q2416V9V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12572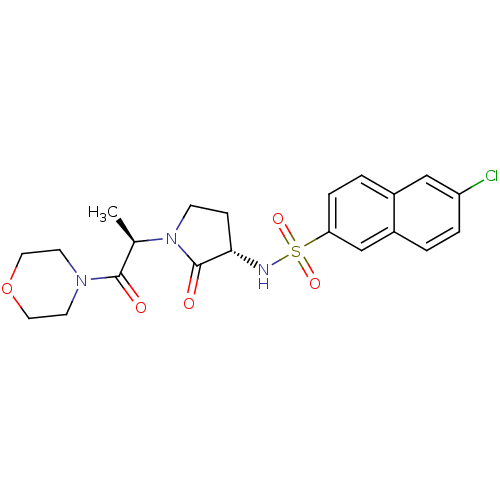 (6-chloro-N-[(3S)-1-[(2R)-1-(morpholin-4-yl)-1-oxop...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 160 | -38.8 | n/a | n/a | n/a | n/a | n/a | 7.8 | 25 |
GlaxoSmithKline | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrate. The hydrolysis rates of chromogenic substrate... | Bioorg Med Chem Lett 16: 3784-8 (2006) Article DOI: 10.1016/j.bmcl.2006.04.053 BindingDB Entry DOI: 10.7270/Q2416V9V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12563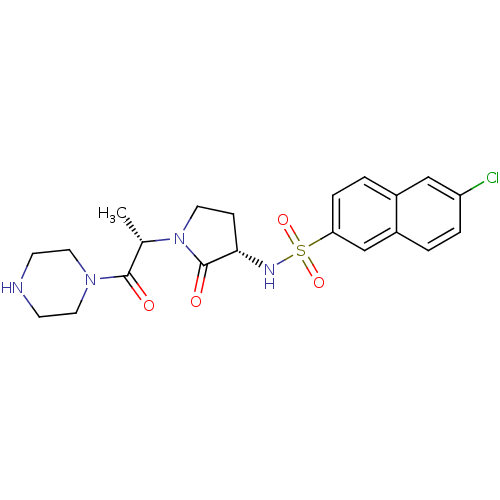 (6-chloro-N-[(3S)-2-oxo-1-[(2S)-1-oxo-1-(piperazin-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 320 | -37.1 | n/a | n/a | n/a | n/a | n/a | 7.8 | 25 |
GlaxoSmithKline | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrate. The hydrolysis rates of chromogenic substrate... | Bioorg Med Chem Lett 16: 3784-8 (2006) Article DOI: 10.1016/j.bmcl.2006.04.053 BindingDB Entry DOI: 10.7270/Q2416V9V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12564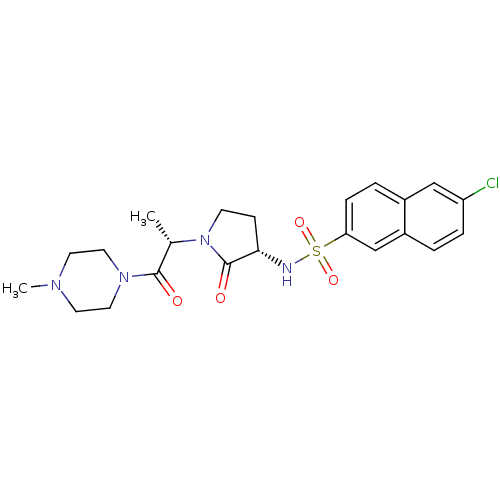 (6-chloro-N-[(3S)-1-[(2S)-1-(4-methylpiperazin-1-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.06E+3 | -32.5 | n/a | n/a | n/a | n/a | n/a | 7.8 | 25 |
GlaxoSmithKline | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrate. The hydrolysis rates of chromogenic substrate... | Bioorg Med Chem Lett 16: 3784-8 (2006) Article DOI: 10.1016/j.bmcl.2006.04.053 BindingDB Entry DOI: 10.7270/Q2416V9V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12559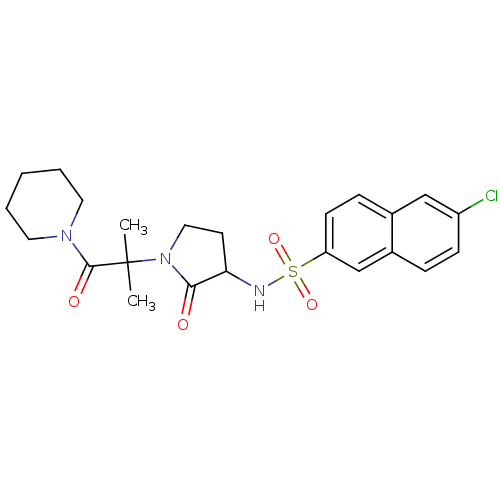 (6-chloro-N-{1-[2-methyl-1-oxo-1-(piperidin-1-yl)pr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.06E+4 | -28.4 | n/a | n/a | n/a | n/a | n/a | 7.8 | 25 |
GlaxoSmithKline | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrate. The hydrolysis rates of chromogenic substrate... | Bioorg Med Chem Lett 16: 3784-8 (2006) Article DOI: 10.1016/j.bmcl.2006.04.053 BindingDB Entry DOI: 10.7270/Q2416V9V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50276291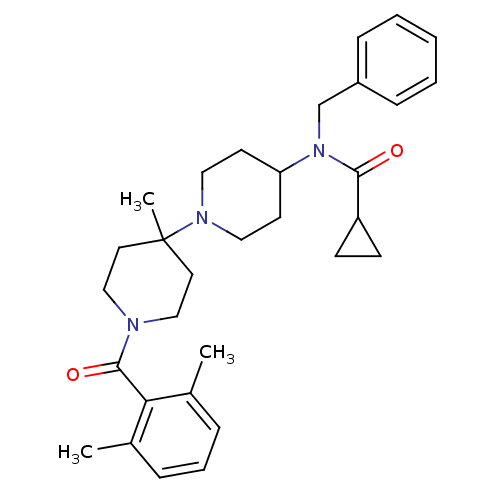 (CHEMBL472464 | N-benzyl-N-(1'-(2,6-dimethylbenzoyl...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Antagonist activity at human recombinant CCR5 expressed in human HeLa-P4 cells assessed as inhibition of human HeLa-P4 cells binding to HIV1 gp160 ex... | Bioorg Med Chem Lett 19: 1084-8 (2009) Article DOI: 10.1016/j.bmcl.2009.01.012 BindingDB Entry DOI: 10.7270/Q2W095SG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50276259 (CHEMBL481068 | exo-N-benzyl-N-((R)-1-(8-(2,6-dimet...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Antagonist activity at human recombinant CCR5 expressed in human HeLa-P4 cells assessed as inhibition of human HeLa-P4 cells binding to HIV1 gp160 ex... | Bioorg Med Chem Lett 19: 1084-8 (2009) Article DOI: 10.1016/j.bmcl.2009.01.012 BindingDB Entry DOI: 10.7270/Q2W095SG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50276260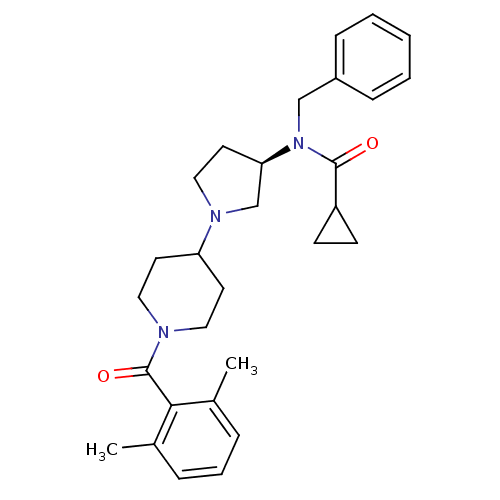 ((R)-N-benzyl-N-(1-(1-(2,6-dimethylbenzoyl)piperidi...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Antagonist activity at human recombinant CCR5 expressed in human HeLa-P4 cells assessed as inhibition of human HeLa-P4 cells binding to HIV1 gp160 ex... | Bioorg Med Chem Lett 19: 1084-8 (2009) Article DOI: 10.1016/j.bmcl.2009.01.012 BindingDB Entry DOI: 10.7270/Q2W095SG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 10 subunit alpha (Homo sapiens (Human)) | BDBM50507856 (CHEMBL4483603) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human Nav1.8 expressed in HEK cells by patch-clamp electrophysiology method | Bioorg Med Chem 27: 230-239 (2019) Article DOI: 10.1016/j.bmc.2018.12.002 BindingDB Entry DOI: 10.7270/Q2ZK5KZN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50276290 (CHEMBL472260 | N-benzyl-N-(1'-(2,6-dimethylbenzoyl...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Antagonist activity at human recombinant CCR5 expressed in human HeLa-P4 cells assessed as inhibition of human HeLa-P4 cells binding to HIV1 gp160 ex... | Bioorg Med Chem Lett 19: 1084-8 (2009) Article DOI: 10.1016/j.bmcl.2009.01.012 BindingDB Entry DOI: 10.7270/Q2W095SG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50276260 ((R)-N-benzyl-N-(1-(1-(2,6-dimethylbenzoyl)piperidi...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [125]Mip1beta form CCR5 receptor (unknown origin) expressed in MIP34.10 cells | Bioorg Med Chem Lett 19: 1084-8 (2009) Article DOI: 10.1016/j.bmcl.2009.01.012 BindingDB Entry DOI: 10.7270/Q2W095SG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 10 subunit alpha (Homo sapiens (Human)) | BDBM50507839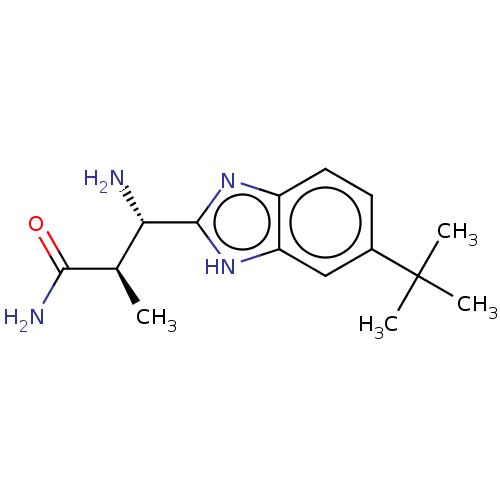 (PF-6305591 | Pf-06305591) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human Nav1.8 expressed in HEK cells by patch-clamp electrophysiology method | Bioorg Med Chem 27: 230-239 (2019) Article DOI: 10.1016/j.bmc.2018.12.002 BindingDB Entry DOI: 10.7270/Q2ZK5KZN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 10 subunit alpha (Homo sapiens (Human)) | BDBM50507845 (CHEMBL4455868) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human Nav1.8 expressed in HEK cells by patch-clamp electrophysiology method | Bioorg Med Chem 27: 230-239 (2019) Article DOI: 10.1016/j.bmc.2018.12.002 BindingDB Entry DOI: 10.7270/Q2ZK5KZN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 10 subunit alpha (Homo sapiens (Human)) | BDBM50507851 (CHEMBL4474590) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human Nav1.8 expressed in HEK cells by patch-clamp electrophysiology method | Bioorg Med Chem 27: 230-239 (2019) Article DOI: 10.1016/j.bmc.2018.12.002 BindingDB Entry DOI: 10.7270/Q2ZK5KZN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50276623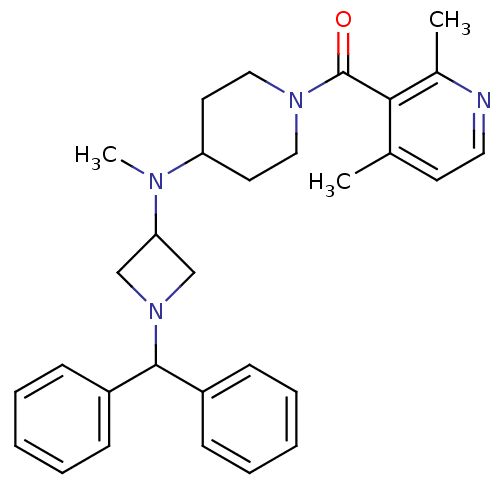 ((4-((1-benzhydrylazetidin-3-yl)(methyl)amino)piper...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Antagonist activity at human recombinant CCR5 expressed in human HeLa-P4 cells assessed as inhibition of human HeLa-P4 cells binding to HIV1 gp160 ex... | Bioorg Med Chem Lett 19: 1084-8 (2009) Article DOI: 10.1016/j.bmcl.2009.01.012 BindingDB Entry DOI: 10.7270/Q2W095SG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 10 subunit alpha (Homo sapiens (Human)) | BDBM50507854 (CHEMBL4562194) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human Nav1.8 expressed in HEK cells by patch-clamp electrophysiology method | Bioorg Med Chem 27: 230-239 (2019) Article DOI: 10.1016/j.bmc.2018.12.002 BindingDB Entry DOI: 10.7270/Q2ZK5KZN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50276672 ((4-(3-benzhydryl-3,8-diazabicyclo[3.2.1]octan-8-yl...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Antagonist activity at human recombinant CCR5 expressed in human HeLa-P4 cells assessed as inhibition of human HeLa-P4 cells binding to HIV1 gp160 ex... | Bioorg Med Chem Lett 19: 1084-8 (2009) Article DOI: 10.1016/j.bmcl.2009.01.012 BindingDB Entry DOI: 10.7270/Q2W095SG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 10 subunit alpha (Homo sapiens (Human)) | BDBM50507848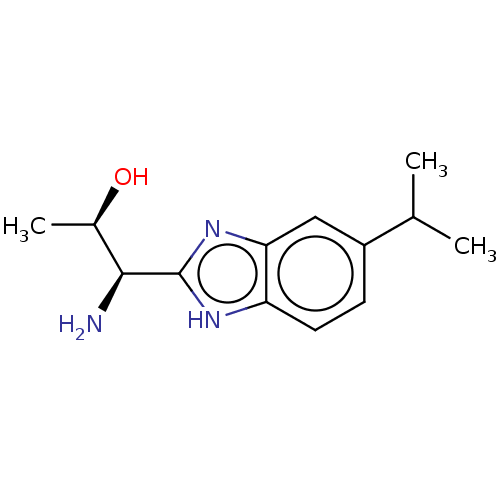 (CHEMBL4457824) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human Nav1.8 expressed in HEK cells by patch-clamp electrophysiology method | Bioorg Med Chem 27: 230-239 (2019) Article DOI: 10.1016/j.bmc.2018.12.002 BindingDB Entry DOI: 10.7270/Q2ZK5KZN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 10 subunit alpha (Homo sapiens (Human)) | BDBM50507855 (CHEMBL4564889) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human Nav1.8 expressed in HEK cells by patch-clamp electrophysiology method | Bioorg Med Chem 27: 230-239 (2019) Article DOI: 10.1016/j.bmc.2018.12.002 BindingDB Entry DOI: 10.7270/Q2ZK5KZN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 10 subunit alpha (Homo sapiens (Human)) | BDBM50507840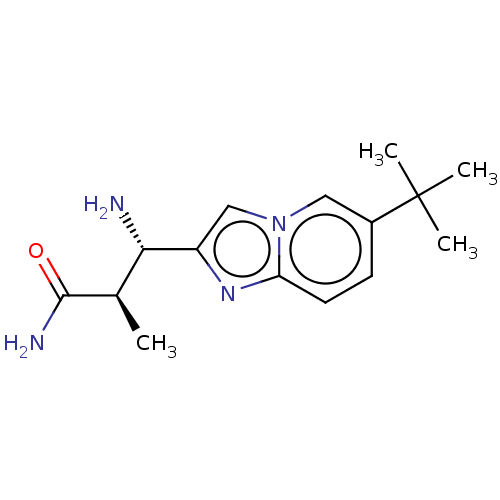 (CHEMBL4452291) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human Nav1.8 expressed in HEK cells by patch-clamp electrophysiology method | Bioorg Med Chem 27: 230-239 (2019) Article DOI: 10.1016/j.bmc.2018.12.002 BindingDB Entry DOI: 10.7270/Q2ZK5KZN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50276793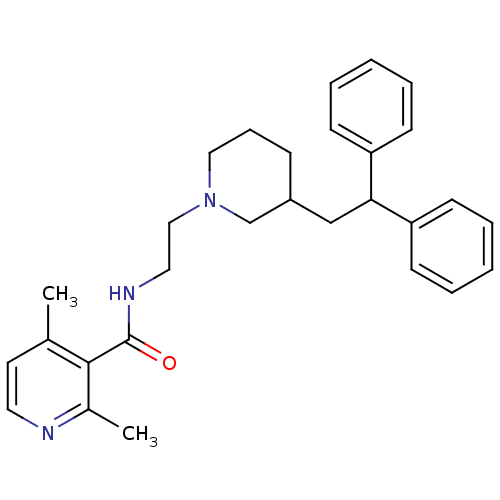 (CHEMBL459986 | rac-N-(2-(3-(2,2-diphenylethyl)pipe...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 56 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Antagonist activity at human recombinant CCR5 expressed in human HeLa-P4 cells assessed as inhibition of human HeLa-P4 cells binding to HIV1 gp160 ex... | Bioorg Med Chem Lett 19: 1084-8 (2009) Article DOI: 10.1016/j.bmcl.2009.01.012 BindingDB Entry DOI: 10.7270/Q2W095SG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 10 subunit alpha (Homo sapiens (Human)) | BDBM50507861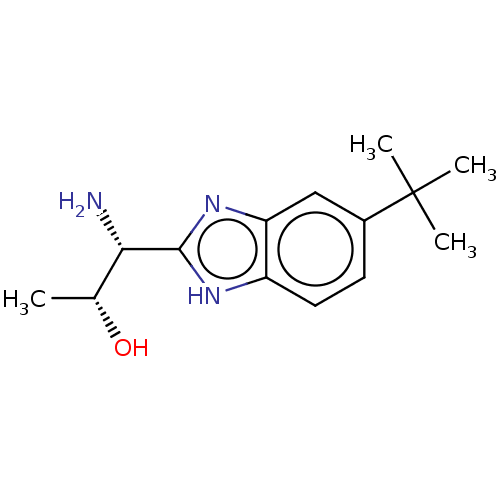 (CHEMBL4571436) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 59 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human Nav1.8 expressed in HEK cells by patch-clamp electrophysiology method | Bioorg Med Chem 27: 230-239 (2019) Article DOI: 10.1016/j.bmc.2018.12.002 BindingDB Entry DOI: 10.7270/Q2ZK5KZN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 10 subunit alpha (Homo sapiens (Human)) | BDBM50507837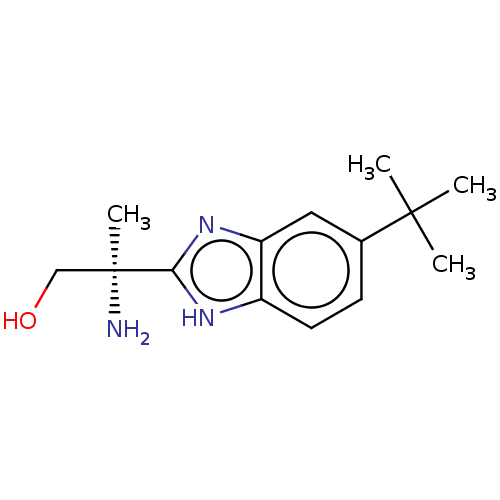 (CHEMBL4546247) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 65 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human Nav1.8 expressed in HEK cells by patch-clamp electrophysiology method | Bioorg Med Chem 27: 230-239 (2019) Article DOI: 10.1016/j.bmc.2018.12.002 BindingDB Entry DOI: 10.7270/Q2ZK5KZN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 10 subunit alpha (Homo sapiens (Human)) | BDBM50507862 (CHEMBL4521566) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 95 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human Nav1.8 expressed in HEK cells by patch-clamp electrophysiology method | Bioorg Med Chem 27: 230-239 (2019) Article DOI: 10.1016/j.bmc.2018.12.002 BindingDB Entry DOI: 10.7270/Q2ZK5KZN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50276792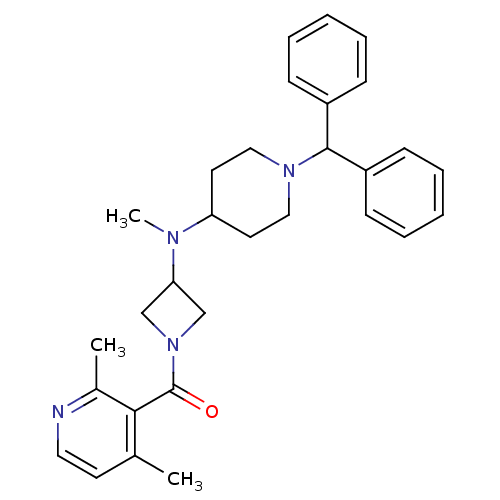 ((3-((1-benzhydrylpiperidin-4-yl)(methyl)amino)azet...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 102 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Antagonist activity at human recombinant CCR5 expressed in human HeLa-P4 cells assessed as inhibition of human HeLa-P4 cells binding to HIV1 gp160 ex... | Bioorg Med Chem Lett 19: 1084-8 (2009) Article DOI: 10.1016/j.bmcl.2009.01.012 BindingDB Entry DOI: 10.7270/Q2W095SG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 10 subunit alpha (Homo sapiens (Human)) | BDBM50507850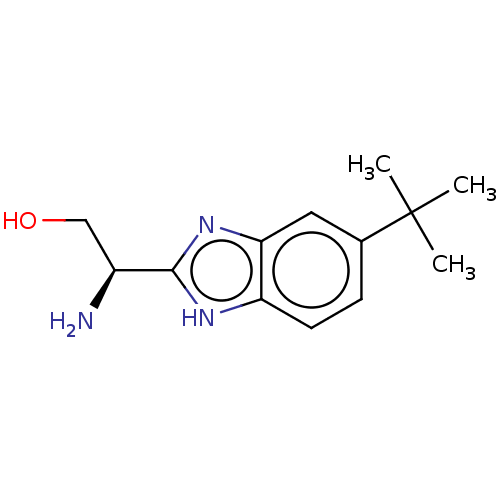 (CHEMBL4468605) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 106 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human Nav1.8 expressed in HEK cells by patch-clamp electrophysiology method | Bioorg Med Chem 27: 230-239 (2019) Article DOI: 10.1016/j.bmc.2018.12.002 BindingDB Entry DOI: 10.7270/Q2ZK5KZN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 10 subunit alpha (Homo sapiens (Human)) | BDBM50507863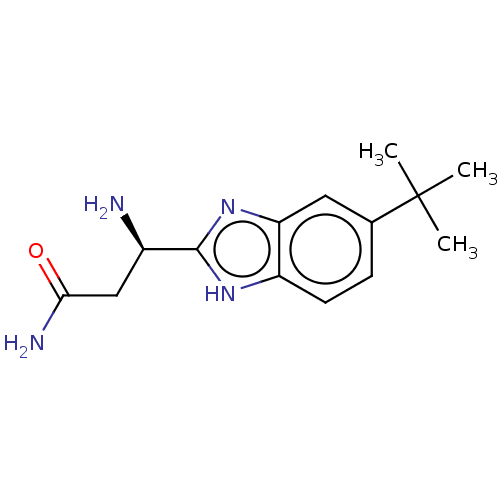 (CHEMBL4590816) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human Nav1.8 expressed in HEK cells by patch-clamp electrophysiology method | Bioorg Med Chem 27: 230-239 (2019) Article DOI: 10.1016/j.bmc.2018.12.002 BindingDB Entry DOI: 10.7270/Q2ZK5KZN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50276217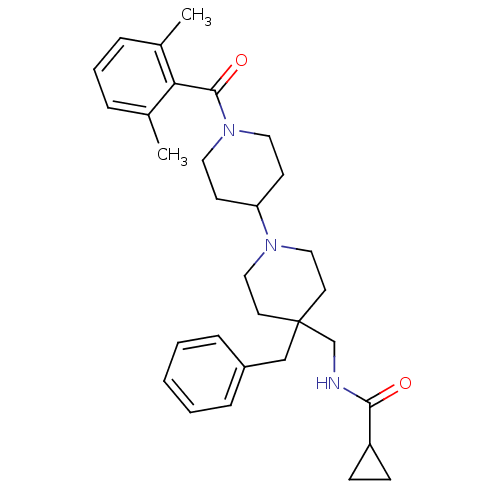 (CHEMBL471403 | N-((4-benzyl-1'-(2,6-dimethylbenzoy...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 145 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Antagonist activity at human recombinant CCR5 expressed in human HeLa-P4 cells assessed as inhibition of human HeLa-P4 cells binding to HIV1 gp160 ex... | Bioorg Med Chem Lett 19: 1084-8 (2009) Article DOI: 10.1016/j.bmcl.2009.01.012 BindingDB Entry DOI: 10.7270/Q2W095SG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 10 subunit alpha (Homo sapiens (Human)) | BDBM50507849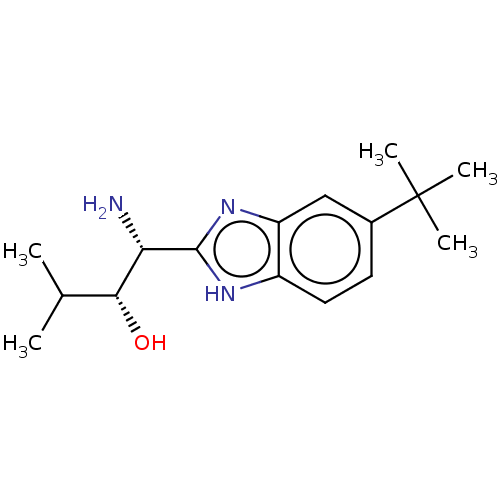 (CHEMBL4583673) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 227 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human Nav1.8 expressed in HEK cells by patch-clamp electrophysiology method | Bioorg Med Chem 27: 230-239 (2019) Article DOI: 10.1016/j.bmc.2018.12.002 BindingDB Entry DOI: 10.7270/Q2ZK5KZN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50276671 ((4-(8-benzhydryl-3,8-diazabicyclo[3.2.1]octan-3-yl...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 313 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Antagonist activity at human recombinant CCR5 expressed in human HeLa-P4 cells assessed as inhibition of human HeLa-P4 cells binding to HIV1 gp160 ex... | Bioorg Med Chem Lett 19: 1084-8 (2009) Article DOI: 10.1016/j.bmcl.2009.01.012 BindingDB Entry DOI: 10.7270/Q2W095SG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 10 subunit alpha (Homo sapiens (Human)) | BDBM50507846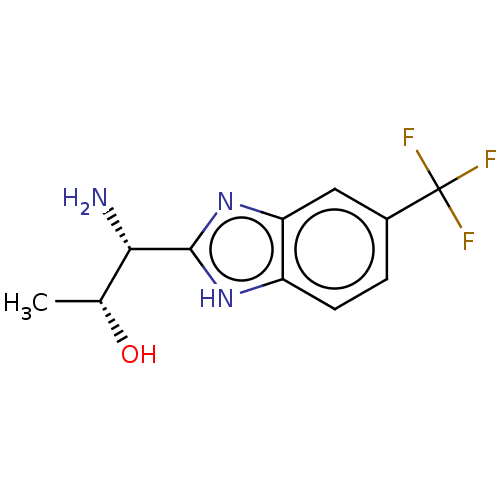 (CHEMBL4447338) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 534 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human Nav1.8 expressed in HEK cells by patch-clamp electrophysiology method | Bioorg Med Chem 27: 230-239 (2019) Article DOI: 10.1016/j.bmc.2018.12.002 BindingDB Entry DOI: 10.7270/Q2ZK5KZN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 10 subunit alpha (Homo sapiens (Human)) | BDBM50507844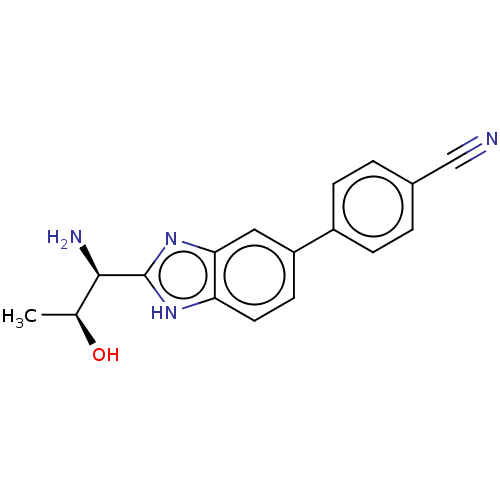 (CHEMBL4453894) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 579 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human Nav1.8 expressed in HEK cells by patch-clamp electrophysiology method | Bioorg Med Chem 27: 230-239 (2019) Article DOI: 10.1016/j.bmc.2018.12.002 BindingDB Entry DOI: 10.7270/Q2ZK5KZN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 10 subunit alpha (Homo sapiens (Human)) | BDBM50507836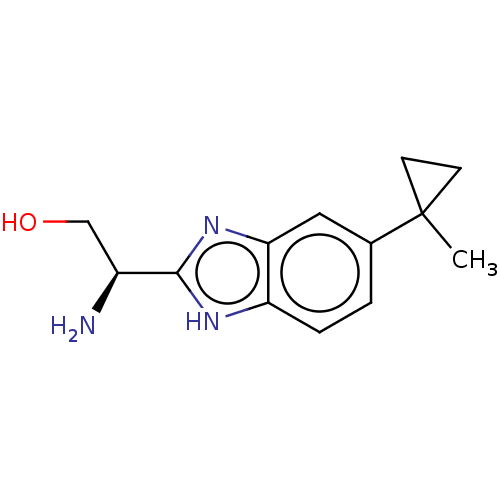 (CHEMBL4443309) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 593 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human Nav1.8 expressed in HEK cells by patch-clamp electrophysiology method | Bioorg Med Chem 27: 230-239 (2019) Article DOI: 10.1016/j.bmc.2018.12.002 BindingDB Entry DOI: 10.7270/Q2ZK5KZN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 89 total ) | Next | Last >> |