 Found 161 hits with Last Name = 'furuya' and Initial = 'n'
Found 161 hits with Last Name = 'furuya' and Initial = 'n' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Procathepsin L
(Homo sapiens (Human)) | BDBM50067612
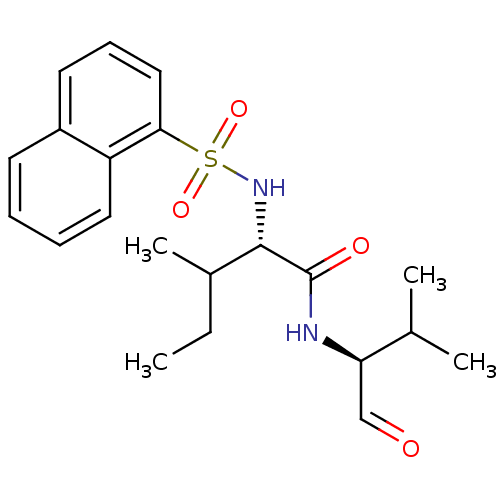
((S)-3-Methyl-2-(naphthalene-1-sulfonylamino)-penta...)Show SMILES CCC(C)[C@H](NS(=O)(=O)c1cccc2ccccc12)C(=O)N[C@H](C=O)C(C)C Show InChI InChI=1S/C21H28N2O4S/c1-5-15(4)20(21(25)22-18(13-24)14(2)3)23-28(26,27)19-12-8-10-16-9-6-7-11-17(16)19/h6-15,18,20,23H,5H2,1-4H3,(H,22,25)/t15?,18-,20+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.740 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin L. |
J Med Chem 41: 4301-8 (1998)
Article DOI: 10.1021/jm9803065
BindingDB Entry DOI: 10.7270/Q2VQ33CX |
More data for this
Ligand-Target Pair | |
BDNF/NT-3 growth factors receptor
(Homo sapiens (Human)) | BDBM50392788

(CHEMBL457614)Show SMILES CC(C)Oc1cc(Nc2nc(N[C@@H](C)c3ccc(F)cn3)ncc2Cl)[nH]n1 |r| Show InChI InChI=1S/C17H19ClFN7O/c1-9(2)27-15-6-14(25-26-15)23-16-12(18)8-21-17(24-16)22-10(3)13-5-4-11(19)7-20-13/h4-10H,1-3H3,(H3,21,22,23,24,25,26)/t10-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 0.930 | n/a | n/a | n/a | n/a | n/a | n/a |
Osaka Prefecture University
Curated by ChEMBL
| Assay Description
Inhibition of wild type human N-terminal GST-fusion tagged TRKB kinase domain (456 to 822 residues) expressed in baculovirus expression system by HTR... |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2019.126775
BindingDB Entry DOI: 10.7270/Q2Q243K6 |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50067606
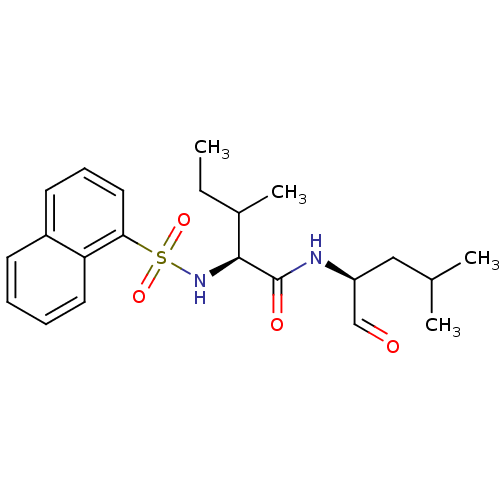
((S)-3-Methyl-2-(naphthalene-1-sulfonylamino)-penta...)Show SMILES CCC(C)[C@H](NS(=O)(=O)c1cccc2ccccc12)C(=O)N[C@@H](CC(C)C)C=O Show InChI InChI=1S/C22H30N2O4S/c1-5-16(4)21(22(26)23-18(14-25)13-15(2)3)24-29(27,28)20-12-8-10-17-9-6-7-11-19(17)20/h6-12,14-16,18,21,24H,5,13H2,1-4H3,(H,23,26)/t16?,18-,21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.950 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin L. |
J Med Chem 41: 4301-8 (1998)
Article DOI: 10.1021/jm9803065
BindingDB Entry DOI: 10.7270/Q2VQ33CX |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50067608
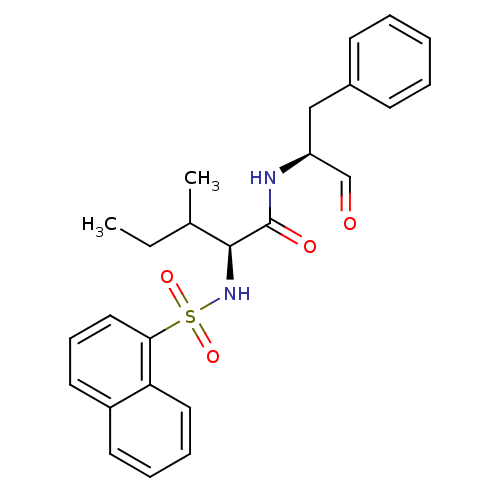
((S)-3-Methyl-2-(naphthalene-1-sulfonylamino)-penta...)Show SMILES CCC(C)[C@H](NS(=O)(=O)c1cccc2ccccc12)C(=O)N[C@@H](Cc1ccccc1)C=O Show InChI InChI=1S/C25H28N2O4S/c1-3-18(2)24(25(29)26-21(17-28)16-19-10-5-4-6-11-19)27-32(30,31)23-15-9-13-20-12-7-8-14-22(20)23/h4-15,17-18,21,24,27H,3,16H2,1-2H3,(H,26,29)/t18?,21-,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.950 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin L. |
J Med Chem 41: 4301-8 (1998)
Article DOI: 10.1021/jm9803065
BindingDB Entry DOI: 10.7270/Q2VQ33CX |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50067604

((S)-N-[(R)-1-Formyl-2-(1H-indol-3-yl)-ethyl]-3-met...)Show SMILES CC(C)[C@H](NS(=O)(=O)c1cccc2ccccc12)C(=O)N[C@H](Cc1c[nH]c2ccccc12)C=O Show InChI InChI=1S/C26H27N3O4S/c1-17(2)25(29-34(32,33)24-13-7-9-18-8-3-4-11-22(18)24)26(31)28-20(16-30)14-19-15-27-23-12-6-5-10-21(19)23/h3-13,15-17,20,25,27,29H,14H2,1-2H3,(H,28,31)/t20-,25+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.970 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin L. |
J Med Chem 41: 4301-8 (1998)
Article DOI: 10.1021/jm9803065
BindingDB Entry DOI: 10.7270/Q2VQ33CX |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50067613
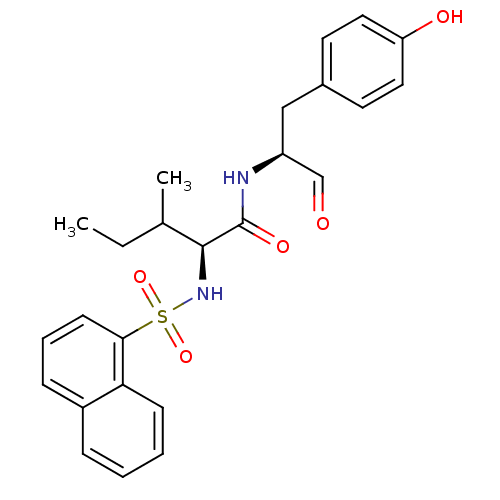
((S)-3-Methyl-2-(naphthalene-1-sulfonylamino)-penta...)Show SMILES CCC(C)[C@H](NS(=O)(=O)c1cccc2ccccc12)C(=O)N[C@@H](Cc1ccc(O)cc1)C=O Show InChI InChI=1S/C25H28N2O5S/c1-3-17(2)24(25(30)26-20(16-28)15-18-11-13-21(29)14-12-18)27-33(31,32)23-10-6-8-19-7-4-5-9-22(19)23/h4-14,16-17,20,24,27,29H,3,15H2,1-2H3,(H,26,30)/t17?,20-,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin L. |
J Med Chem 41: 4301-8 (1998)
Article DOI: 10.1021/jm9803065
BindingDB Entry DOI: 10.7270/Q2VQ33CX |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50369397
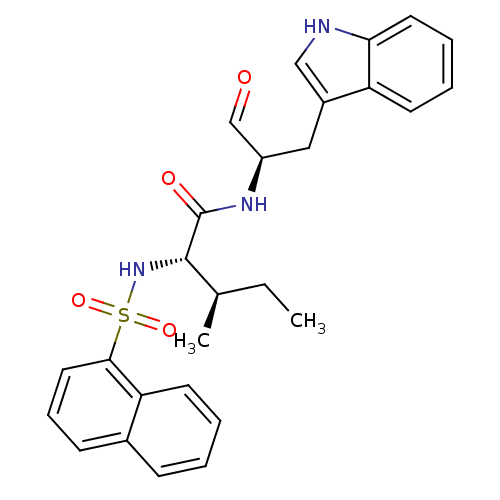
(CHEMBL1790993)Show SMILES CC[C@@H](C)[C@H](NS(=O)(=O)c1cccc2ccccc12)C(=O)N[C@H](Cc1c[nH]c2ccccc12)C=O Show InChI InChI=1S/C27H29N3O4S/c1-3-18(2)26(30-35(33,34)25-14-8-10-19-9-4-5-12-23(19)25)27(32)29-21(17-31)15-20-16-28-24-13-7-6-11-22(20)24/h4-14,16-18,21,26,28,30H,3,15H2,1-2H3,(H,29,32)/t18-,21-,26+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin L. |
J Med Chem 41: 4301-8 (1998)
Article DOI: 10.1021/jm9803065
BindingDB Entry DOI: 10.7270/Q2VQ33CX |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50369410

(CHEMBL1790989)Show SMILES CC[C@@H](C)[C@H](NC(=O)Nc1cccc2ccccc12)C(=O)N[C@H](Cc1c[nH]c2ccccc12)C=O Show InChI InChI=1S/C28H30N4O3/c1-3-18(2)26(32-28(35)31-25-14-8-10-19-9-4-5-11-22(19)25)27(34)30-21(17-33)15-20-16-29-24-13-7-6-12-23(20)24/h4-14,16-18,21,26,29H,3,15H2,1-2H3,(H,30,34)(H2,31,32,35)/t18-,21-,26+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin L. |
J Med Chem 41: 4301-8 (1998)
Article DOI: 10.1021/jm9803065
BindingDB Entry DOI: 10.7270/Q2VQ33CX |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50369405

(CHEMBL1790991)Show SMILES CC[C@@H](C)[C@H](NC(=O)Nc1ccccc1C(F)(F)F)C(=O)N[C@H](Cc1c[nH]c2ccccc12)C=O Show InChI InChI=1S/C25H27F3N4O3/c1-3-15(2)22(32-24(35)31-21-11-7-5-9-19(21)25(26,27)28)23(34)30-17(14-33)12-16-13-29-20-10-6-4-8-18(16)20/h4-11,13-15,17,22,29H,3,12H2,1-2H3,(H,30,34)(H2,31,32,35)/t15-,17-,22+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin L. |
J Med Chem 41: 4301-8 (1998)
Article DOI: 10.1021/jm9803065
BindingDB Entry DOI: 10.7270/Q2VQ33CX |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50369397

(CHEMBL1790993)Show SMILES CC[C@@H](C)[C@H](NS(=O)(=O)c1cccc2ccccc12)C(=O)N[C@H](Cc1c[nH]c2ccccc12)C=O Show InChI InChI=1S/C27H29N3O4S/c1-3-18(2)26(30-35(33,34)25-14-8-10-19-9-4-5-12-23(19)25)27(32)29-21(17-31)15-20-16-28-24-13-7-6-11-22(20)24/h4-14,16-18,21,26,28,30H,3,15H2,1-2H3,(H,29,32)/t18-,21-,26+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd.
Curated by ChEMBL
| Assay Description
Compound was measured for inhibition of collagenolytic of human Cathepsin L |
J Med Chem 41: 4301-8 (1998)
Article DOI: 10.1021/jm9803065
BindingDB Entry DOI: 10.7270/Q2VQ33CX |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50369402

(CHEMBL1790996)Show SMILES CC[C@@H](C)[C@H](NC(=O)c1ccccc1)C(=O)N[C@H](Cc1c[nH]c2ccccc12)C=O Show InChI InChI=1S/C24H27N3O3/c1-3-16(2)22(27-23(29)17-9-5-4-6-10-17)24(30)26-19(15-28)13-18-14-25-21-12-8-7-11-20(18)21/h4-12,14-16,19,22,25H,3,13H2,1-2H3,(H,26,30)(H,27,29)/t16-,19-,22+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin L. |
J Med Chem 41: 4301-8 (1998)
Article DOI: 10.1021/jm9803065
BindingDB Entry DOI: 10.7270/Q2VQ33CX |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50369403
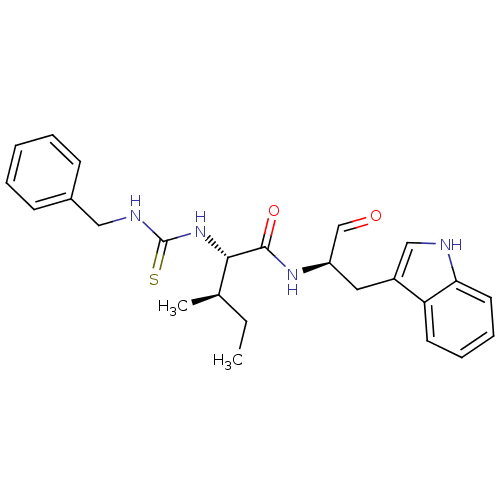
(CHEMBL1790995)Show SMILES CC[C@@H](C)[C@H](NC(=S)NCc1ccccc1)C(=O)N[C@H](Cc1c[nH]c2ccccc12)C=O Show InChI InChI=1S/C25H30N4O2S/c1-3-17(2)23(29-25(32)27-14-18-9-5-4-6-10-18)24(31)28-20(16-30)13-19-15-26-22-12-8-7-11-21(19)22/h4-12,15-17,20,23,26H,3,13-14H2,1-2H3,(H,28,31)(H2,27,29,32)/t17-,20-,23+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin L. |
J Med Chem 41: 4301-8 (1998)
Article DOI: 10.1021/jm9803065
BindingDB Entry DOI: 10.7270/Q2VQ33CX |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50369400
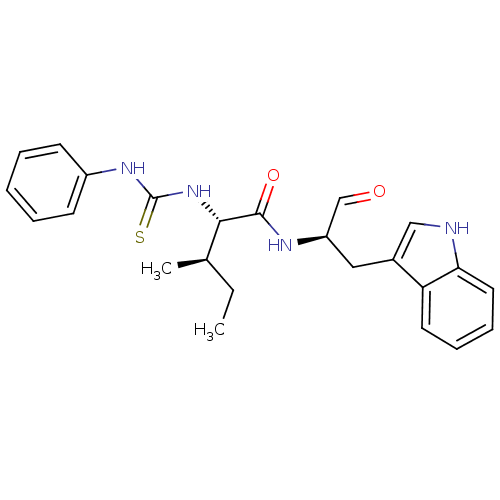
(CHEMBL1791000)Show SMILES CC[C@@H](C)[C@H](NC(=S)Nc1ccccc1)C(=O)N[C@H](Cc1c[nH]c2ccccc12)C=O Show InChI InChI=1S/C24H28N4O2S/c1-3-16(2)22(28-24(31)27-18-9-5-4-6-10-18)23(30)26-19(15-29)13-17-14-25-21-12-8-7-11-20(17)21/h4-12,14-16,19,22,25H,3,13H2,1-2H3,(H,26,30)(H2,27,28,31)/t16-,19-,22+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin L. |
J Med Chem 41: 4301-8 (1998)
Article DOI: 10.1021/jm9803065
BindingDB Entry DOI: 10.7270/Q2VQ33CX |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50369398
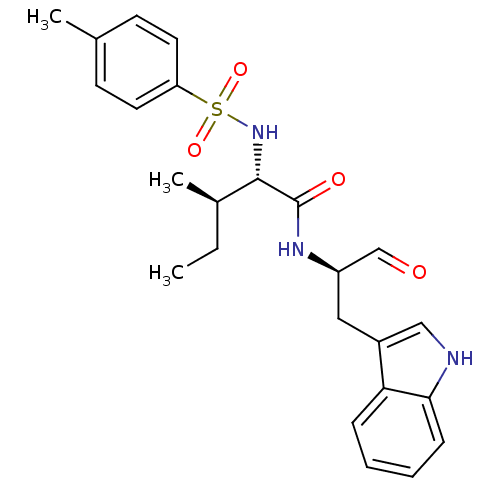
(CHEMBL1790999)Show SMILES CC[C@@H](C)[C@H](NS(=O)(=O)c1ccc(C)cc1)C(=O)N[C@H](Cc1c[nH]c2ccccc12)C=O Show InChI InChI=1S/C24H29N3O4S/c1-4-17(3)23(27-32(30,31)20-11-9-16(2)10-12-20)24(29)26-19(15-28)13-18-14-25-22-8-6-5-7-21(18)22/h5-12,14-15,17,19,23,25,27H,4,13H2,1-3H3,(H,26,29)/t17-,19-,23+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin L. |
J Med Chem 41: 4301-8 (1998)
Article DOI: 10.1021/jm9803065
BindingDB Entry DOI: 10.7270/Q2VQ33CX |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50067597
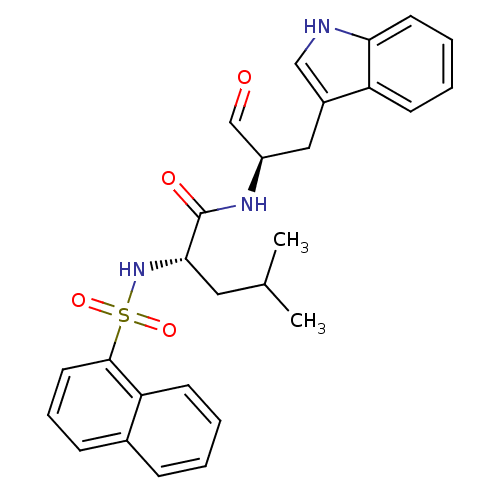
((S)-4-Methyl-2-(naphthalene-1-sulfonylamino)-penta...)Show SMILES CC(C)C[C@H](NS(=O)(=O)c1cccc2ccccc12)C(=O)N[C@H](Cc1c[nH]c2ccccc12)C=O Show InChI InChI=1S/C27H29N3O4S/c1-18(2)14-25(30-35(33,34)26-13-7-9-19-8-3-4-11-23(19)26)27(32)29-21(17-31)15-20-16-28-24-12-6-5-10-22(20)24/h3-13,16-18,21,25,28,30H,14-15H2,1-2H3,(H,29,32)/t21-,25+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin L. |
J Med Chem 41: 4301-8 (1998)
Article DOI: 10.1021/jm9803065
BindingDB Entry DOI: 10.7270/Q2VQ33CX |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50286441

((S)-2-((S)-2-Acetylamino-4-(S)-methyl-pentanoylami...)Show SMILES CC(C)C[C@H](NC(C)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCNC(N)=N)C(O)=O Show InChI InChI=1S/C20H38N6O5/c1-11(2)9-15(24-13(5)27)17(28)26-16(10-12(3)4)18(29)25-14(19(30)31)7-6-8-23-20(21)22/h11-12,14-16H,6-10H2,1-5H3,(H,24,27)(H,25,29)(H,26,28)(H,30,31)(H4,21,22,23)/t14-,15-,16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd.
Curated by ChEMBL
| Assay Description
Compound was measured for inhibition of collagenolytic of human Cathepsin L |
J Med Chem 41: 4301-8 (1998)
Article DOI: 10.1021/jm9803065
BindingDB Entry DOI: 10.7270/Q2VQ33CX |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM50392788

(CHEMBL457614)Show SMILES CC(C)Oc1cc(Nc2nc(N[C@@H](C)c3ccc(F)cn3)ncc2Cl)[nH]n1 |r| Show InChI InChI=1S/C17H19ClFN7O/c1-9(2)27-15-6-14(25-26-15)23-16-12(18)8-21-17(24-16)22-10(3)13-5-4-11(19)7-20-13/h4-10H,1-3H3,(H3,21,22,23,24,25,26)/t10-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Osaka Prefecture University
Curated by ChEMBL
| Assay Description
Inhibition of wild type human His-tagged TRKA kinase domain (441 to 796 residues) expressed in baculovirus expression system by HTRF assay |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2019.126775
BindingDB Entry DOI: 10.7270/Q2Q243K6 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM50232542

(CHEMBL4090531 | US10323022, Example 135)Show SMILES CCOc1nn(c(NC(=O)N[C@@H]2CN(CCOC)C[C@H]2c2ccc(F)c(F)c2)c1C)-c1ccccc1 |r| Show InChI InChI=1S/C26H31F2N5O3/c1-4-36-25-17(2)24(33(31-25)19-8-6-5-7-9-19)30-26(34)29-23-16-32(12-13-35-3)15-20(23)18-10-11-21(27)22(28)14-18/h5-11,14,20,23H,4,12-13,15-16H2,1-3H3,(H2,29,30,34)/t20-,23+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Osaka Prefecture University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human TrkA expressed in DHFR deficient CHO cells assessed as inhibition of human beta-nerve growth factor-induced calcium i... |
Bioorg Med Chem Lett 27: 1233-1236 (2017)
Article DOI: 10.1016/j.bmcl.2017.01.056
BindingDB Entry DOI: 10.7270/Q2TT4T51 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM50392788

(CHEMBL457614)Show SMILES CC(C)Oc1cc(Nc2nc(N[C@@H](C)c3ccc(F)cn3)ncc2Cl)[nH]n1 |r| Show InChI InChI=1S/C17H19ClFN7O/c1-9(2)27-15-6-14(25-26-15)23-16-12(18)8-21-17(24-16)22-10(3)13-5-4-11(19)7-20-13/h4-10H,1-3H3,(H3,21,22,23,24,25,26)/t10-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Osaka Prefecture University
Curated by ChEMBL
| Assay Description
Inhibition of TRKA E735A mutant (unknown origin) expressed in baculovirus infected sf9 cells assessed as kinase domain dimerization by HTRF assay |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2019.126775
BindingDB Entry DOI: 10.7270/Q2Q243K6 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Procathepsin L
(Homo sapiens (Human)) | BDBM50067594
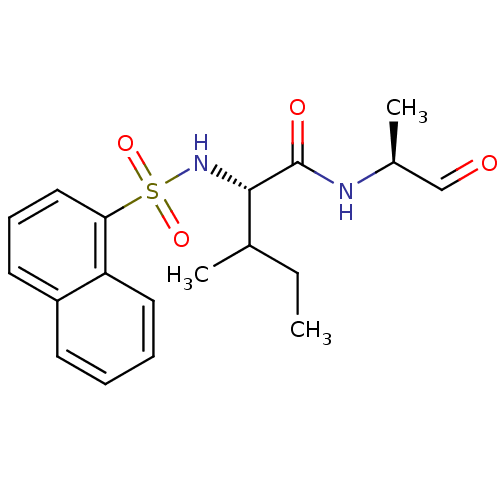
((S)-3-Methyl-2-(naphthalene-1-sulfonylamino)-penta...)Show SMILES CCC(C)[C@H](NS(=O)(=O)c1cccc2ccccc12)C(=O)N[C@@H](C)C=O Show InChI InChI=1S/C19H24N2O4S/c1-4-13(2)18(19(23)20-14(3)12-22)21-26(24,25)17-11-7-9-15-8-5-6-10-16(15)17/h5-14,18,21H,4H2,1-3H3,(H,20,23)/t13?,14-,18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin L. |
J Med Chem 41: 4301-8 (1998)
Article DOI: 10.1021/jm9803065
BindingDB Entry DOI: 10.7270/Q2VQ33CX |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM50392788

(CHEMBL457614)Show SMILES CC(C)Oc1cc(Nc2nc(N[C@@H](C)c3ccc(F)cn3)ncc2Cl)[nH]n1 |r| Show InChI InChI=1S/C17H19ClFN7O/c1-9(2)27-15-6-14(25-26-15)23-16-12(18)8-21-17(24-16)22-10(3)13-5-4-11(19)7-20-13/h4-10H,1-3H3,(H3,21,22,23,24,25,26)/t10-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Osaka Prefecture University
Curated by ChEMBL
| Assay Description
Inhibition of juxtamembrane region containing recombinant human N-terminal His6-tagged/GST-tagged TrkA (473 to 796 residues) expressed in baculovirus... |
Bioorg Med Chem Lett 27: 1233-1236 (2017)
Article DOI: 10.1016/j.bmcl.2017.01.056
BindingDB Entry DOI: 10.7270/Q2TT4T51 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Procathepsin L
(Homo sapiens (Human)) | BDBM50369406

(CHEMBL1791001)Show SMILES CC[C@@H](C)[C@H](NC(=O)OC(C)(C)C)C(=O)N[C@H](Cc1c[nH]c2ccccc12)C=O Show InChI InChI=1S/C22H31N3O4/c1-6-14(2)19(25-21(28)29-22(3,4)5)20(27)24-16(13-26)11-15-12-23-18-10-8-7-9-17(15)18/h7-10,12-14,16,19,23H,6,11H2,1-5H3,(H,24,27)(H,25,28)/t14-,16-,19+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin L. |
J Med Chem 41: 4301-8 (1998)
Article DOI: 10.1021/jm9803065
BindingDB Entry DOI: 10.7270/Q2VQ33CX |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50369408
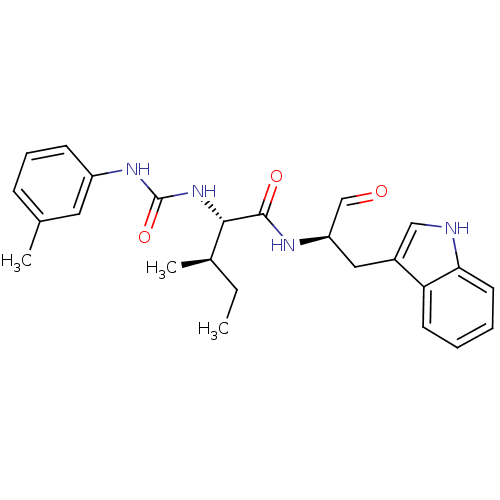
(CHEMBL1790997)Show SMILES CC[C@@H](C)[C@H](NC(=O)Nc1cccc(C)c1)C(=O)N[C@H](Cc1c[nH]c2ccccc12)C=O Show InChI InChI=1S/C25H30N4O3/c1-4-17(3)23(29-25(32)28-19-9-7-8-16(2)12-19)24(31)27-20(15-30)13-18-14-26-22-11-6-5-10-21(18)22/h5-12,14-15,17,20,23,26H,4,13H2,1-3H3,(H,27,31)(H2,28,29,32)/t17-,20-,23+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin L. |
J Med Chem 41: 4301-8 (1998)
Article DOI: 10.1021/jm9803065
BindingDB Entry DOI: 10.7270/Q2VQ33CX |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50369409
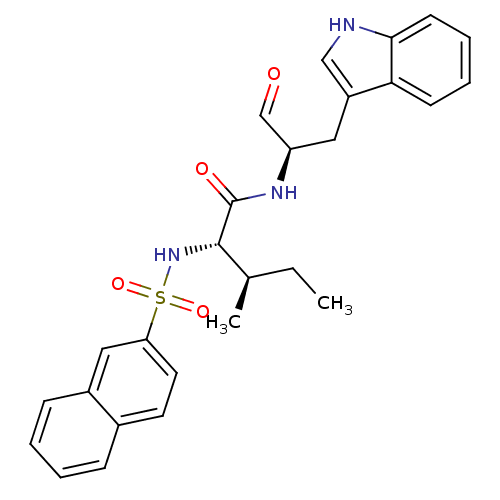
(CHEMBL1791004)Show SMILES CC[C@@H](C)[C@H](NS(=O)(=O)c1ccc2ccccc2c1)C(=O)N[C@H](Cc1c[nH]c2ccccc12)C=O Show InChI InChI=1S/C27H29N3O4S/c1-3-18(2)26(30-35(33,34)23-13-12-19-8-4-5-9-20(19)15-23)27(32)29-22(17-31)14-21-16-28-25-11-7-6-10-24(21)25/h4-13,15-18,22,26,28,30H,3,14H2,1-2H3,(H,29,32)/t18-,22-,26+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin L. |
J Med Chem 41: 4301-8 (1998)
Article DOI: 10.1021/jm9803065
BindingDB Entry DOI: 10.7270/Q2VQ33CX |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50067614
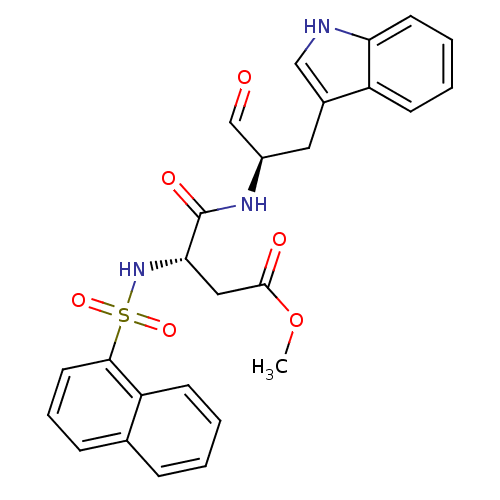
((S)-N-[(R)-1-Formyl-2-(1H-indol-3-yl)-ethyl]-3-(na...)Show SMILES COC(=O)C[C@H](NS(=O)(=O)c1cccc2ccccc12)C(=O)N[C@H](Cc1c[nH]c2ccccc12)C=O Show InChI InChI=1S/C26H25N3O6S/c1-35-25(31)14-23(29-36(33,34)24-12-6-8-17-7-2-3-10-21(17)24)26(32)28-19(16-30)13-18-15-27-22-11-5-4-9-20(18)22/h2-12,15-16,19,23,27,29H,13-14H2,1H3,(H,28,32)/t19-,23+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin L. |
J Med Chem 41: 4301-8 (1998)
Article DOI: 10.1021/jm9803065
BindingDB Entry DOI: 10.7270/Q2VQ33CX |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50067610
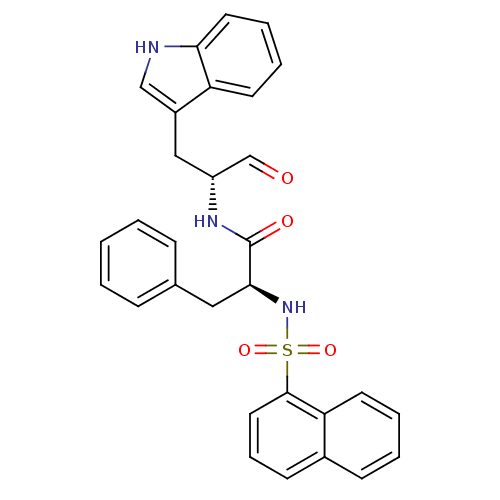
((S)-N-[(R)-1-Formyl-2-(1H-indol-3-yl)-ethyl]-2-(na...)Show SMILES O=C[C@@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](Cc1ccccc1)NS(=O)(=O)c1cccc2ccccc12 Show InChI InChI=1S/C30H27N3O4S/c34-20-24(18-23-19-31-27-15-7-6-13-25(23)27)32-30(35)28(17-21-9-2-1-3-10-21)33-38(36,37)29-16-8-12-22-11-4-5-14-26(22)29/h1-16,19-20,24,28,31,33H,17-18H2,(H,32,35)/t24-,28+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin L. |
J Med Chem 41: 4301-8 (1998)
Article DOI: 10.1021/jm9803065
BindingDB Entry DOI: 10.7270/Q2VQ33CX |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50369399
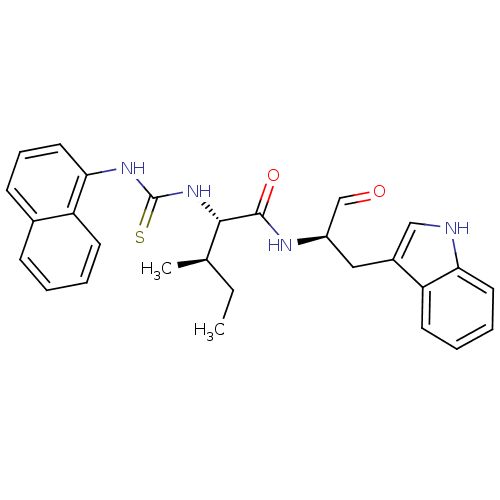
(CHEMBL1790994)Show SMILES CC[C@@H](C)[C@H](NC(=S)Nc1cccc2ccccc12)C(=O)N[C@H](Cc1c[nH]c2ccccc12)C=O Show InChI InChI=1S/C28H30N4O2S/c1-3-18(2)26(32-28(35)31-25-14-8-10-19-9-4-5-11-22(19)25)27(34)30-21(17-33)15-20-16-29-24-13-7-6-12-23(20)24/h4-14,16-18,21,26,29H,3,15H2,1-2H3,(H,30,34)(H2,31,32,35)/t18-,21-,26+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin L. |
J Med Chem 41: 4301-8 (1998)
Article DOI: 10.1021/jm9803065
BindingDB Entry DOI: 10.7270/Q2VQ33CX |
More data for this
Ligand-Target Pair | |
Acetyl-CoA carboxylase 2
(Homo sapiens (Human)) | BDBM50388655
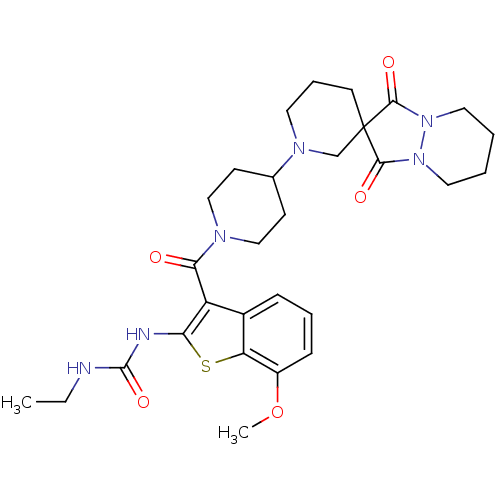
(CHEMBL2059303)Show SMILES CCNC(=O)Nc1sc2c(OC)cccc2c1C(=O)N1CCC(CC1)N1CCCC2(C1)C(=O)N1CCCCN1C2=O Show InChI InChI=1S/C29H38N6O5S/c1-3-30-28(39)31-24-22(20-8-6-9-21(40-2)23(20)41-24)25(36)32-16-10-19(11-17-32)33-13-7-12-29(18-33)26(37)34-14-4-5-15-35(34)27(29)38/h6,8-9,19H,3-5,7,10-18H2,1-2H3,(H2,30,31,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human ACC2 using acetyl-coA as substrate incubated for 60 mins prior to substrate addition measured after 20 mins by malachite green as... |
Bioorg Med Chem Lett 22: 4769-72 (2012)
Article DOI: 10.1016/j.bmcl.2012.05.062
BindingDB Entry DOI: 10.7270/Q28C9X9H |
More data for this
Ligand-Target Pair | |
Acetyl-CoA carboxylase 2
(Homo sapiens (Human)) | BDBM50388657
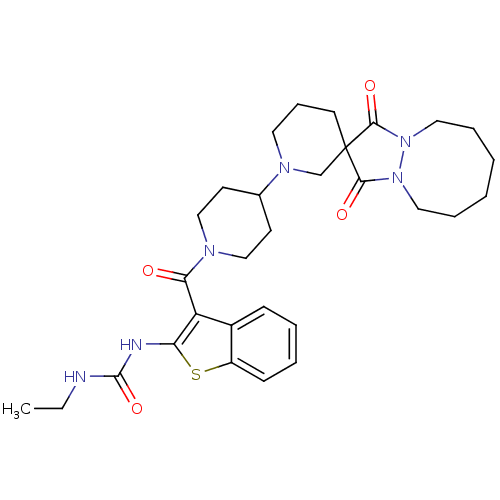
(CHEMBL2059099)Show SMILES CCNC(=O)Nc1sc2ccccc2c1C(=O)N1CCC(CC1)N1CCCC2(C1)C(=O)N1CCCCCCN1C2=O Show InChI InChI=1S/C30H40N6O4S/c1-2-31-29(40)32-25-24(22-10-5-6-11-23(22)41-25)26(37)33-18-12-21(13-19-33)34-15-9-14-30(20-34)27(38)35-16-7-3-4-8-17-36(35)28(30)39/h5-6,10-11,21H,2-4,7-9,12-20H2,1H3,(H2,31,32,40) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human ACC2 using acetyl-coA as substrate incubated for 60 mins prior to substrate addition measured after 20 mins by malachite green as... |
Bioorg Med Chem Lett 22: 4769-72 (2012)
Article DOI: 10.1016/j.bmcl.2012.05.062
BindingDB Entry DOI: 10.7270/Q28C9X9H |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50067607
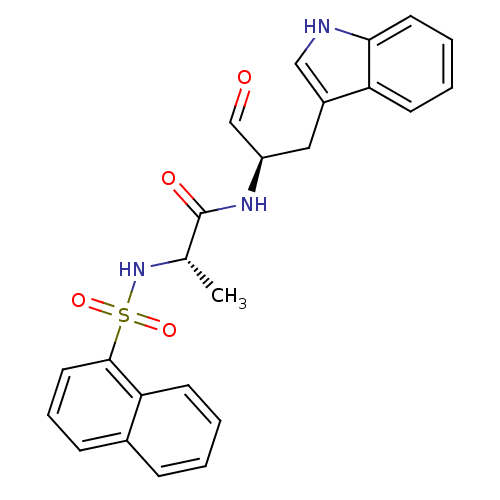
((S)-N-[(R)-1-Formyl-2-(1H-indol-3-yl)-ethyl]-2-(na...)Show SMILES C[C@H](NS(=O)(=O)c1cccc2ccccc12)C(=O)N[C@H](Cc1c[nH]c2ccccc12)C=O Show InChI InChI=1S/C24H23N3O4S/c1-16(27-32(30,31)23-12-6-8-17-7-2-3-10-21(17)23)24(29)26-19(15-28)13-18-14-25-22-11-5-4-9-20(18)22/h2-12,14-16,19,25,27H,13H2,1H3,(H,26,29)/t16-,19+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin L. |
J Med Chem 41: 4301-8 (1998)
Article DOI: 10.1021/jm9803065
BindingDB Entry DOI: 10.7270/Q2VQ33CX |
More data for this
Ligand-Target Pair | |
Acetyl-CoA carboxylase 2
(Homo sapiens (Human)) | BDBM50388656
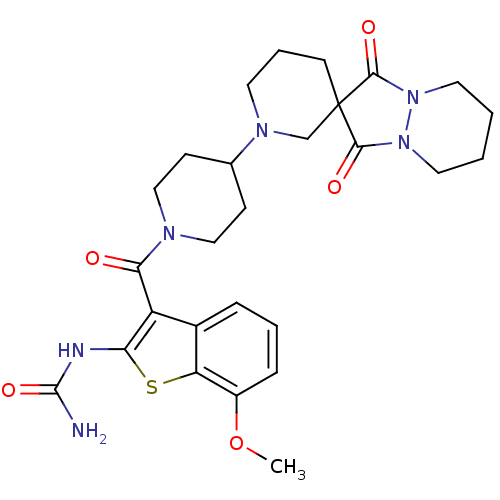
(CHEMBL2059301)Show SMILES COc1cccc2c(C(=O)N3CCC(CC3)N3CCCC4(C3)C(=O)N3CCCCN3C4=O)c(NC(N)=O)sc12 Show InChI InChI=1S/C27H34N6O5S/c1-38-19-7-4-6-18-20(22(29-26(28)37)39-21(18)19)23(34)30-14-8-17(9-15-30)31-11-5-10-27(16-31)24(35)32-12-2-3-13-33(32)25(27)36/h4,6-7,17H,2-3,5,8-16H2,1H3,(H3,28,29,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human ACC2 using acetyl-coA as substrate incubated for 60 mins prior to substrate addition measured after 20 mins by malachite green as... |
Bioorg Med Chem Lett 22: 4769-72 (2012)
Article DOI: 10.1016/j.bmcl.2012.05.062
BindingDB Entry DOI: 10.7270/Q28C9X9H |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM50232542

(CHEMBL4090531 | US10323022, Example 135)Show SMILES CCOc1nn(c(NC(=O)N[C@@H]2CN(CCOC)C[C@H]2c2ccc(F)c(F)c2)c1C)-c1ccccc1 |r| Show InChI InChI=1S/C26H31F2N5O3/c1-4-36-25-17(2)24(33(31-25)19-8-6-5-7-9-19)30-26(34)29-23-16-32(12-13-35-3)15-20(23)18-10-11-21(27)22(28)14-18/h5-11,14,20,23H,4,12-13,15-16H2,1-3H3,(H2,29,30,34)/t20-,23+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Osaka Prefecture University
Curated by ChEMBL
| Assay Description
Inhibition of juxtamembrane region containing recombinant human N-terminal His6-tagged/GST-tagged TrkA (473 to 796 residues) expressed in baculovirus... |
Bioorg Med Chem Lett 27: 1233-1236 (2017)
Article DOI: 10.1016/j.bmcl.2017.01.056
BindingDB Entry DOI: 10.7270/Q2TT4T51 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Acetyl-CoA carboxylase 2
(Homo sapiens (Human)) | BDBM50388654
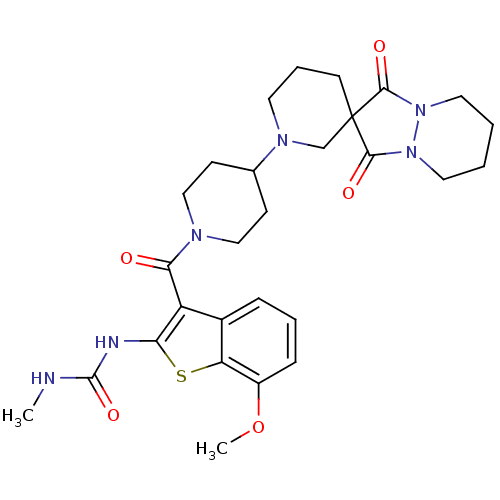
(CHEMBL2059302)Show SMILES CNC(=O)Nc1sc2c(OC)cccc2c1C(=O)N1CCC(CC1)N1CCCC2(C1)C(=O)N1CCCCN1C2=O Show InChI InChI=1S/C28H36N6O5S/c1-29-27(38)30-23-21(19-7-5-8-20(39-2)22(19)40-23)24(35)31-15-9-18(10-16-31)32-12-6-11-28(17-32)25(36)33-13-3-4-14-34(33)26(28)37/h5,7-8,18H,3-4,6,9-17H2,1-2H3,(H2,29,30,38) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human ACC2 using acetyl-coA as substrate incubated for 60 mins prior to substrate addition measured after 20 mins by malachite green as... |
Bioorg Med Chem Lett 22: 4769-72 (2012)
Article DOI: 10.1016/j.bmcl.2012.05.062
BindingDB Entry DOI: 10.7270/Q28C9X9H |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50369407

(CHEMBL1790990)Show SMILES CC[C@@H](C)[C@H](NC(=O)NCc1ccccc1)C(=O)N[C@H](Cc1c[nH]c2ccccc12)C=O Show InChI InChI=1S/C25H30N4O3/c1-3-17(2)23(29-25(32)27-14-18-9-5-4-6-10-18)24(31)28-20(16-30)13-19-15-26-22-12-8-7-11-21(19)22/h4-12,15-17,20,23,26H,3,13-14H2,1-2H3,(H,28,31)(H2,27,29,32)/t17-,20-,23+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin L. |
J Med Chem 41: 4301-8 (1998)
Article DOI: 10.1021/jm9803065
BindingDB Entry DOI: 10.7270/Q2VQ33CX |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50286441

((S)-2-((S)-2-Acetylamino-4-(S)-methyl-pentanoylami...)Show SMILES CC(C)C[C@H](NC(C)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCNC(N)=N)C(O)=O Show InChI InChI=1S/C20H38N6O5/c1-11(2)9-15(24-13(5)27)17(28)26-16(10-12(3)4)18(29)25-14(19(30)31)7-6-8-23-20(21)22/h11-12,14-16H,6-10H2,1-5H3,(H,24,27)(H,25,29)(H,26,28)(H,30,31)(H4,21,22,23)/t14-,15-,16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin L. |
J Med Chem 41: 4301-8 (1998)
Article DOI: 10.1021/jm9803065
BindingDB Entry DOI: 10.7270/Q2VQ33CX |
More data for this
Ligand-Target Pair | |
BDNF/NT-3 growth factors receptor
(Homo sapiens (Human)) | BDBM50392788

(CHEMBL457614)Show SMILES CC(C)Oc1cc(Nc2nc(N[C@@H](C)c3ccc(F)cn3)ncc2Cl)[nH]n1 |r| Show InChI InChI=1S/C17H19ClFN7O/c1-9(2)27-15-6-14(25-26-15)23-16-12(18)8-21-17(24-16)22-10(3)13-5-4-11(19)7-20-13/h4-10H,1-3H3,(H3,21,22,23,24,25,26)/t10-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 6.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Osaka Prefecture University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human TrkB expressed in DHFR deficient CHO cells assessed as inhibition of human brain-derived neurotrophic factor-induced ... |
Bioorg Med Chem Lett 27: 1233-1236 (2017)
Article DOI: 10.1016/j.bmcl.2017.01.056
BindingDB Entry DOI: 10.7270/Q2TT4T51 |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50369404

(CHEMBL1790998)Show SMILES CC[C@@H](C)[C@H](NC(=O)OCc1ccccc1)C(=O)N[C@H](Cc1c[nH]c2ccccc12)C=O Show InChI InChI=1S/C25H29N3O4/c1-3-17(2)23(28-25(31)32-16-18-9-5-4-6-10-18)24(30)27-20(15-29)13-19-14-26-22-12-8-7-11-21(19)22/h4-12,14-15,17,20,23,26H,3,13,16H2,1-2H3,(H,27,30)(H,28,31)/t17-,20-,23+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin L. |
J Med Chem 41: 4301-8 (1998)
Article DOI: 10.1021/jm9803065
BindingDB Entry DOI: 10.7270/Q2VQ33CX |
More data for this
Ligand-Target Pair | |
NT-3 growth factor receptor
(Homo sapiens (Human)) | BDBM50392788

(CHEMBL457614)Show SMILES CC(C)Oc1cc(Nc2nc(N[C@@H](C)c3ccc(F)cn3)ncc2Cl)[nH]n1 |r| Show InChI InChI=1S/C17H19ClFN7O/c1-9(2)27-15-6-14(25-26-15)23-16-12(18)8-21-17(24-16)22-10(3)13-5-4-11(19)7-20-13/h4-10H,1-3H3,(H3,21,22,23,24,25,26)/t10-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 7.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Osaka Prefecture University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human TrkC expressed in DHFR deficient CHO cells assessed as inhibition of human neurotrophin-3-induced calcium influx by F... |
Bioorg Med Chem Lett 27: 1233-1236 (2017)
Article DOI: 10.1016/j.bmcl.2017.01.056
BindingDB Entry DOI: 10.7270/Q2TT4T51 |
More data for this
Ligand-Target Pair | |
Acetyl-CoA carboxylase 2
(Homo sapiens (Human)) | BDBM50388658
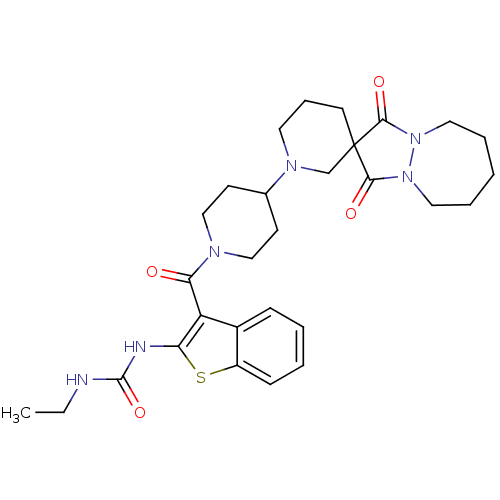
(CHEMBL2059098)Show SMILES CCNC(=O)Nc1sc2ccccc2c1C(=O)N1CCC(CC1)N1CCCC2(C1)C(=O)N1CCCCCN1C2=O Show InChI InChI=1S/C29H38N6O4S/c1-2-30-28(39)31-24-23(21-9-4-5-10-22(21)40-24)25(36)32-17-11-20(12-18-32)33-14-8-13-29(19-33)26(37)34-15-6-3-7-16-35(34)27(29)38/h4-5,9-10,20H,2-3,6-8,11-19H2,1H3,(H2,30,31,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human ACC2 using acetyl-coA as substrate incubated for 60 mins prior to substrate addition measured after 20 mins by malachite green as... |
Bioorg Med Chem Lett 22: 4769-72 (2012)
Article DOI: 10.1016/j.bmcl.2012.05.062
BindingDB Entry DOI: 10.7270/Q28C9X9H |
More data for this
Ligand-Target Pair | |
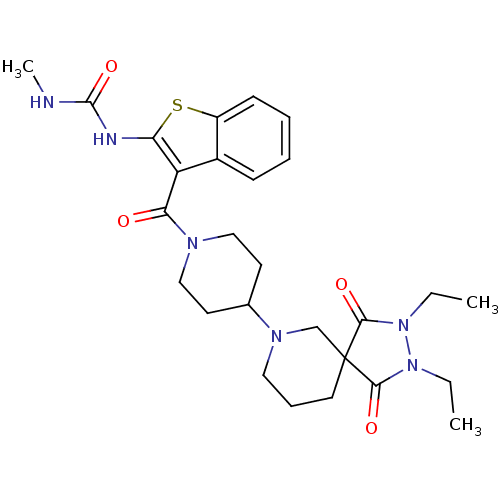
Acetyl-CoA carboxylase 2
(Homo sapiens (Human)) | BDBM50388663
(CHEMBL2059093)Show SMILES CCN1N(CC)C(=O)C2(CCCN(C2)C2CCN(CC2)C(=O)c2c(NC(=O)NC)sc3ccccc23)C1=O Show InChI InChI=1S/C27H36N6O4S/c1-4-32-24(35)27(25(36)33(32)5-2)13-8-14-31(17-27)18-11-15-30(16-12-18)23(34)21-19-9-6-7-10-20(19)38-22(21)29-26(37)28-3/h6-7,9-10,18H,4-5,8,11-17H2,1-3H3,(H2,28,29,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human ACC2 using acetyl-coA as substrate incubated for 60 mins prior to substrate addition measured after 20 mins by malachite green as... |
Bioorg Med Chem Lett 22: 4769-72 (2012)
Article DOI: 10.1016/j.bmcl.2012.05.062
BindingDB Entry DOI: 10.7270/Q28C9X9H |
More data for this
Ligand-Target Pair | |
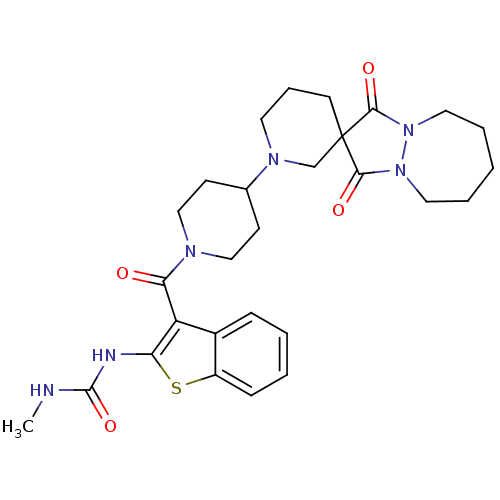
Acetyl-CoA carboxylase 2
(Homo sapiens (Human)) | BDBM50388659
(CHEMBL2059097)Show SMILES CNC(=O)Nc1sc2ccccc2c1C(=O)N1CCC(CC1)N1CCCC2(C1)C(=O)N1CCCCCN1C2=O Show InChI InChI=1S/C28H36N6O4S/c1-29-27(38)30-23-22(20-8-3-4-9-21(20)39-23)24(35)31-16-10-19(11-17-31)32-13-7-12-28(18-32)25(36)33-14-5-2-6-15-34(33)26(28)37/h3-4,8-9,19H,2,5-7,10-18H2,1H3,(H2,29,30,38) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human ACC2 using acetyl-coA as substrate incubated for 60 mins prior to substrate addition measured after 20 mins by malachite green as... |
Bioorg Med Chem Lett 22: 4769-72 (2012)
Article DOI: 10.1016/j.bmcl.2012.05.062
BindingDB Entry DOI: 10.7270/Q28C9X9H |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM50392788

(CHEMBL457614)Show SMILES CC(C)Oc1cc(Nc2nc(N[C@@H](C)c3ccc(F)cn3)ncc2Cl)[nH]n1 |r| Show InChI InChI=1S/C17H19ClFN7O/c1-9(2)27-15-6-14(25-26-15)23-16-12(18)8-21-17(24-16)22-10(3)13-5-4-11(19)7-20-13/h4-10H,1-3H3,(H3,21,22,23,24,25,26)/t10-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 8.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Osaka Prefecture University
Curated by ChEMBL
| Assay Description
Inhibition of juxtamembrane region deficient recombinant human N-terminal His6-tagged/GST-tagged TrkA (498 to 796 residues) expressed in baculovirus ... |
Bioorg Med Chem Lett 27: 1233-1236 (2017)
Article DOI: 10.1016/j.bmcl.2017.01.056
BindingDB Entry DOI: 10.7270/Q2TT4T51 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cathepsin B
(Homo sapiens (Human)) | BDBM50286441

((S)-2-((S)-2-Acetylamino-4-(S)-methyl-pentanoylami...)Show SMILES CC(C)C[C@H](NC(C)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCNC(N)=N)C(O)=O Show InChI InChI=1S/C20H38N6O5/c1-11(2)9-15(24-13(5)27)17(28)26-16(10-12(3)4)18(29)25-14(19(30)31)7-6-8-23-20(21)22/h11-12,14-16H,6-10H2,1-5H3,(H,24,27)(H,25,29)(H,26,28)(H,30,31)(H4,21,22,23)/t14-,15-,16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin B |
J Med Chem 41: 4301-8 (1998)
Article DOI: 10.1021/jm9803065
BindingDB Entry DOI: 10.7270/Q2VQ33CX |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM50392788

(CHEMBL457614)Show SMILES CC(C)Oc1cc(Nc2nc(N[C@@H](C)c3ccc(F)cn3)ncc2Cl)[nH]n1 |r| Show InChI InChI=1S/C17H19ClFN7O/c1-9(2)27-15-6-14(25-26-15)23-16-12(18)8-21-17(24-16)22-10(3)13-5-4-11(19)7-20-13/h4-10H,1-3H3,(H3,21,22,23,24,25,26)/t10-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 9.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Osaka Prefecture University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human TrkA expressed in DHFR deficient CHO cells assessed as inhibition of human beta-nerve growth factor-induced calcium i... |
Bioorg Med Chem Lett 27: 1233-1236 (2017)
Article DOI: 10.1016/j.bmcl.2017.01.056
BindingDB Entry DOI: 10.7270/Q2TT4T51 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cathepsin B
(Homo sapiens (Human)) | BDBM50157741

(CHEMBL374508 | E-64 | E64)Show SMILES [#6]-[#6](-[#6])-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H]-1-[#8]-[#6@@H]-1-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6]-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7] |r| Show InChI InChI=1S/C15H27N5O5/c1-8(2)7-9(20-13(22)10-11(25-10)14(23)24)12(21)18-5-3-4-6-19-15(16)17/h8-11H,3-7H2,1-2H3,(H,18,21)(H,20,22)(H,23,24)(H4,16,17,19)/t9-,10-,11-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin B |
J Med Chem 41: 4301-8 (1998)
Article DOI: 10.1021/jm9803065
BindingDB Entry DOI: 10.7270/Q2VQ33CX |
More data for this
Ligand-Target Pair | |
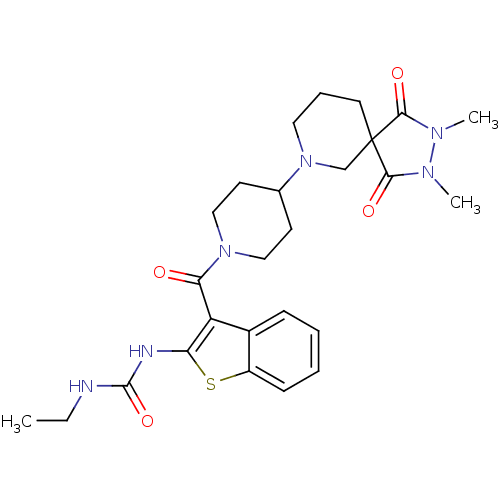
Acetyl-CoA carboxylase 2
(Homo sapiens (Human)) | BDBM50388664
(CHEMBL2059092)Show SMILES CCNC(=O)Nc1sc2ccccc2c1C(=O)N1CCC(CC1)N1CCCC2(C1)C(=O)N(C)N(C)C2=O Show InChI InChI=1S/C26H34N6O4S/c1-4-27-25(36)28-21-20(18-8-5-6-9-19(18)37-21)22(33)31-14-10-17(11-15-31)32-13-7-12-26(16-32)23(34)29(2)30(3)24(26)35/h5-6,8-9,17H,4,7,10-16H2,1-3H3,(H2,27,28,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human ACC2 using acetyl-coA as substrate incubated for 60 mins prior to substrate addition measured after 20 mins by malachite green as... |
Bioorg Med Chem Lett 22: 4769-72 (2012)
Article DOI: 10.1016/j.bmcl.2012.05.062
BindingDB Entry DOI: 10.7270/Q28C9X9H |
More data for this
Ligand-Target Pair | |
Acetyl-CoA carboxylase 1
(Homo sapiens (Human)) | BDBM50388655

(CHEMBL2059303)Show SMILES CCNC(=O)Nc1sc2c(OC)cccc2c1C(=O)N1CCC(CC1)N1CCCC2(C1)C(=O)N1CCCCN1C2=O Show InChI InChI=1S/C29H38N6O5S/c1-3-30-28(39)31-24-22(20-8-6-9-21(40-2)23(20)41-24)25(36)32-16-10-19(11-17-32)33-13-7-12-29(18-33)26(37)34-14-4-5-15-35(34)27(29)38/h6,8-9,19H,3-5,7,10-18H2,1-2H3,(H2,30,31,39) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human ACC1 using acetyl-coA as substrate incubated for 60 mins prior to substrate addition measured after 20 mins by malachite green as... |
Bioorg Med Chem Lett 22: 4769-72 (2012)
Article DOI: 10.1016/j.bmcl.2012.05.062
BindingDB Entry DOI: 10.7270/Q28C9X9H |
More data for this
Ligand-Target Pair | |
Acetyl-CoA carboxylase 1
(Homo sapiens (Human)) | BDBM50388656

(CHEMBL2059301)Show SMILES COc1cccc2c(C(=O)N3CCC(CC3)N3CCCC4(C3)C(=O)N3CCCCN3C4=O)c(NC(N)=O)sc12 Show InChI InChI=1S/C27H34N6O5S/c1-38-19-7-4-6-18-20(22(29-26(28)37)39-21(18)19)23(34)30-14-8-17(9-15-30)31-11-5-10-27(16-31)24(35)32-12-2-3-13-33(32)25(27)36/h4,6-7,17H,2-3,5,8-16H2,1H3,(H3,28,29,37) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human ACC1 using acetyl-coA as substrate incubated for 60 mins prior to substrate addition measured after 20 mins by malachite green as... |
Bioorg Med Chem Lett 22: 4769-72 (2012)
Article DOI: 10.1016/j.bmcl.2012.05.062
BindingDB Entry DOI: 10.7270/Q28C9X9H |
More data for this
Ligand-Target Pair | |
Acetyl-CoA carboxylase 1
(Homo sapiens (Human)) | BDBM50388657

(CHEMBL2059099)Show SMILES CCNC(=O)Nc1sc2ccccc2c1C(=O)N1CCC(CC1)N1CCCC2(C1)C(=O)N1CCCCCCN1C2=O Show InChI InChI=1S/C30H40N6O4S/c1-2-31-29(40)32-25-24(22-10-5-6-11-23(22)41-25)26(37)33-18-12-21(13-19-33)34-15-9-14-30(20-34)27(38)35-16-7-3-4-8-17-36(35)28(30)39/h5-6,10-11,21H,2-4,7-9,12-20H2,1H3,(H2,31,32,40) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human ACC1 using acetyl-coA as substrate incubated for 60 mins prior to substrate addition measured after 20 mins by malachite green as... |
Bioorg Med Chem Lett 22: 4769-72 (2012)
Article DOI: 10.1016/j.bmcl.2012.05.062
BindingDB Entry DOI: 10.7270/Q28C9X9H |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50369409

(CHEMBL1791004)Show SMILES CC[C@@H](C)[C@H](NS(=O)(=O)c1ccc2ccccc2c1)C(=O)N[C@H](Cc1c[nH]c2ccccc12)C=O Show InChI InChI=1S/C27H29N3O4S/c1-3-18(2)26(30-35(33,34)23-13-12-19-8-4-5-9-20(19)15-23)27(32)29-22(17-31)14-21-16-28-25-11-7-6-10-24(21)25/h4-13,15-18,22,26,28,30H,3,14H2,1-2H3,(H,29,32)/t18-,22-,26+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin B |
J Med Chem 41: 4301-8 (1998)
Article DOI: 10.1021/jm9803065
BindingDB Entry DOI: 10.7270/Q2VQ33CX |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data