 Found 6612 hits with Last Name = 'go' and Initial = 'n'
Found 6612 hits with Last Name = 'go' and Initial = 'n' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50113782

((10R,13S,17S)-17-hydroxy-13-methyl-10-(4-methylben...)Show SMILES CC#C[C@]1(O)CCC2C3CCC4=CC(=O)CC[C@]4(Cc4ccc(C)cc4)C3=CC[C@]12C |c:29,t:11| Show InChI InChI=1S/C29H34O2/c1-4-14-29(31)17-13-25-24-10-9-22-18-23(30)11-16-28(22,26(24)12-15-27(25,29)3)19-21-7-5-20(2)6-8-21/h5-8,12,18,24-25,31H,9-11,13,15-17,19H2,1-3H3/t24?,25?,27-,28+,29-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trinity College
Curated by ChEMBL
| Assay Description
Displacement of [3H]DEX from human glucocorticoid receptor |
J Med Chem 53: 3065-74 (2010)
Article DOI: 10.1021/jm901452y
BindingDB Entry DOI: 10.7270/Q2VQ33NH |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50113783
((2R,4aS,10aR)-4a-benzyl-2-(chloroethynyl)-1,2,3,4,...)Show SMILES Oc1ccc2c(CC[C@@H]3C[C@](O)(CC[C@@]23Cc2ccccc2)C#CCl)c1 Show InChI InChI=1S/C23H23ClO2/c24-13-12-22(26)10-11-23(15-17-4-2-1-3-5-17)19(16-22)7-6-18-14-20(25)8-9-21(18)23/h1-5,8-9,14,19,25-26H,6-7,10-11,15-16H2/t19-,22+,23+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trinity College
Curated by ChEMBL
| Assay Description
Displacement of [3H]DEX from human glucocorticoid receptor |
J Med Chem 53: 3065-74 (2010)
Article DOI: 10.1021/jm901452y
BindingDB Entry DOI: 10.7270/Q2VQ33NH |
More data for this
Ligand-Target Pair | |
D(1B) dopamine receptor
(RAT) | BDBM82247

(8-Chloro-3-methyl-5-phenyl-2,3,4,5-tetrahydro-1H-b...)Show InChI InChI=1S/C17H18ClNO/c1-19-8-7-13-9-16(18)17(20)10-14(13)15(11-19)12-5-3-2-4-6-12/h2-6,9-10,15,20H,7-8,11H2,1H3/t15-/m1/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University
Curated by PDSP Ki Database
| |
Proc Natl Acad Sci U S A 88: 7491-5 (1991)
Article DOI: 10.1073/pnas.88.17.7491
BindingDB Entry DOI: 10.7270/Q2DB80B9 |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(Homo sapiens (Human)) | BDBM82247

(8-Chloro-3-methyl-5-phenyl-2,3,4,5-tetrahydro-1H-b...)Show InChI InChI=1S/C17H18ClNO/c1-19-8-7-13-9-16(18)17(20)10-14(13)15(11-19)12-5-3-2-4-6-12/h2-6,9-10,15,20H,7-8,11H2,1H3/t15-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University
Curated by PDSP Ki Database
| |
Proc Natl Acad Sci U S A 88: 7491-5 (1991)
Article DOI: 10.1073/pnas.88.17.7491
BindingDB Entry DOI: 10.7270/Q2DB80B9 |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50113780
((2R,4aS,10aR)-4a-benzyl-2-(prop-1-ynyl)-1,2,3,4,4a...)Show SMILES CC#C[C@@]1(O)CC[C@]2(Cc3ccccc3)[C@H](CCc3cc(O)ccc23)C1 Show InChI InChI=1S/C24H26O2/c1-2-12-23(26)13-14-24(16-18-6-4-3-5-7-18)20(17-23)9-8-19-15-21(25)10-11-22(19)24/h3-7,10-11,15,20,25-26H,8-9,13-14,16-17H2,1H3/t20-,23-,24+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trinity College
Curated by ChEMBL
| Assay Description
Displacement of [3H]DEX from human glucocorticoid receptor |
J Med Chem 53: 3065-74 (2010)
Article DOI: 10.1021/jm901452y
BindingDB Entry DOI: 10.7270/Q2VQ33NH |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM18627

((10S,11S,14S,15S,17R)-17-[4-(dimethylamino)phenyl]...)Show SMILES [H][C@@]12CC[C@@](O)(C#CC)[C@@]1(C)C[C@@H](C1=C3CCC(=O)C=C3CC[C@@]21[H])c1ccc(cc1)N(C)C |r,c:14,20| Show InChI InChI=1S/C29H35NO2/c1-5-15-29(32)16-14-26-24-12-8-20-17-22(31)11-13-23(20)27(24)25(18-28(26,29)2)19-6-9-21(10-7-19)30(3)4/h6-7,9-10,17,24-26,32H,8,11-14,16,18H2,1-4H3/t24-,25+,26-,28-,29-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trinity College
Curated by ChEMBL
| Assay Description
Displacement of [3H]DEX from human glucocorticoid receptor |
J Med Chem 53: 3065-74 (2010)
Article DOI: 10.1021/jm901452y
BindingDB Entry DOI: 10.7270/Q2VQ33NH |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Metabotropic glutamate receptor 5
(Rattus norvegicus (Rat)) | BDBM50181745
(3-[(2-methyl-4-thiazolyl)ethynyl]benzonitrile | CH...)Show InChI InChI=1S/C13H8N2S/c1-10-15-13(9-16-10)6-5-11-3-2-4-12(7-11)8-14/h2-4,7,9H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Displacement of [3H]MPEP from mGluR5 in rat brain membranes |
J Med Chem 49: 1080-100 (2006)
Article DOI: 10.1021/jm050570f
BindingDB Entry DOI: 10.7270/Q27S7NC5 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Cavia porcellus) | BDBM21147

((3S)-3-[(2S)-2-[(2S)-2-{2-[(2S)-2-[(2S)-2-[(3S)-3-...)Show SMILES CSCC[C@H](NC(=O)[C@H](Cc1ccc(OS(O)(=O)=O)cc1)NC(=O)[C@@H](N)CC(O)=O)C(=O)NCC(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CCSC)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C49H62N10O16S3/c1-76-18-16-34(55-47(69)37(58-44(66)32(50)23-41(61)62)21-28-12-14-30(15-13-28)75-78(72,73)74)45(67)53-26-40(60)54-38(22-29-25-52-33-11-7-6-10-31(29)33)48(70)56-35(17-19-77-2)46(68)59-39(24-42(63)64)49(71)57-36(43(51)65)20-27-8-4-3-5-9-27/h3-15,25,32,34-39,52H,16-24,26,50H2,1-2H3,(H2,51,65)(H,53,67)(H,54,60)(H,55,69)(H,56,70)(H,57,71)(H,58,66)(H,59,68)(H,61,62)(H,63,64)(H,72,73,74)/t32-,34-,35-,36-,37-,38-,39-/m0/s1 | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Inhibition of [3H]pCCK-8 binding to Cholecystokinin type A receptor of Guinea pig pancreatic membranes |
J Med Chem 40: 647-58 (1997)
Article DOI: 10.1021/jm9603072
BindingDB Entry DOI: 10.7270/Q2PG1SDX |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50185473
(4-(3-(4-chlorophenyl)-3-hydroxy-8-aza-bicyclo[3.2....)Show SMILES OC1(CC2CCC(C1)N2CCCC(=O)c1ccc(F)cc1)c1ccc(Cl)cc1 |TLB:9:8:1.2.7:4.5,0:1:8:4.5| Show InChI InChI=1S/C23H25ClFNO2/c24-18-7-5-17(6-8-18)23(28)14-20-11-12-21(15-23)26(20)13-1-2-22(27)16-3-9-19(25)10-4-16/h3-10,20-21,28H,1-2,11-15H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Florida A&M University
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-methylspiperone from human dopamine D2 receptor by liquid scintillation counting |
Bioorg Med Chem Lett 24: 4294-7 (2014)
Article DOI: 10.1016/j.bmcl.2014.07.018
BindingDB Entry DOI: 10.7270/Q2222WF8 |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 5
(Homo sapiens (Human)) | BDBM50181745

(3-[(2-methyl-4-thiazolyl)ethynyl]benzonitrile | CH...)Show InChI InChI=1S/C13H8N2S/c1-10-15-13(9-16-10)6-5-11-3-2-4-12(7-11)8-14/h2-4,7,9H,1H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Displacement of [3H]MPEP from cloned human mGluR5 transfected in HEK293-T cells |
J Med Chem 49: 1080-100 (2006)
Article DOI: 10.1021/jm050570f
BindingDB Entry DOI: 10.7270/Q27S7NC5 |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM14774

(3-(cyclopropylmethoxy)-N-(3,5-dichloropyridin-4-yl...)Show SMILES FC(F)Oc1ccc(cc1OCC1CC1)C(=O)Nc1c(Cl)cncc1Cl Show InChI InChI=1S/C17H14Cl2F2N2O3/c18-11-6-22-7-12(19)15(11)23-16(24)10-3-4-13(26-17(20)21)14(5-10)25-8-9-1-2-9/h3-7,9,17H,1-2,8H2,(H,22,23,24) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Amsterdam
Curated by ChEMBL
| Assay Description
Inhibition of full length recombinant human PDE4B1 expressed in Sf21 insect cells using cAMP as substrate by scintillation proximity assay |
J Med Chem 61: 3870-3888 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01670
BindingDB Entry DOI: 10.7270/Q2P84FF9 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
D(4) dopamine receptor
(RAT) | BDBM85093
(CAS_3853 | CHEMBL267014 | CHEMBL555670 | L 745,870...)Show InChI InChI=1S/C18H19ClN4/c19-15-3-5-16(6-4-15)23-10-8-22(9-11-23)13-14-12-21-18-17(14)2-1-7-20-18/h1-7,12H,8-11,13H2,(H,20,21) | Reactome pathway
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Florida A&M University
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-methylspiperone from rat dopamine D4 receptor by PDSP assay |
Bioorg Med Chem 22: 3105-14 (2014)
Article DOI: 10.1016/j.bmc.2014.04.026
BindingDB Entry DOI: 10.7270/Q25140RW |
More data for this
Ligand-Target Pair | |
Genome polyprotein [1027-1206,R1052K]
(Hepatitis C virus) | BDBM92407
(BI201335)Show SMILES COc1ccc2c(O[C@@H]3CC(N(C3)C(=O)[C@@H](NC(=O)OC3CCCC3)C(C)(C)C)C(=O)N[C@@]3(CC3C=C)C(O)=O)cc(nc2c1Br)-c1csc(NC(=O)C(C)C)n1 |r| Show InChI InChI=1S/C40H49BrN6O9S/c1-8-21-17-40(21,36(51)52)46-34(49)27-15-23(18-47(27)35(50)32(39(4,5)6)44-38(53)56-22-11-9-10-12-22)55-29-16-25(26-19-57-37(43-26)45-33(48)20(2)3)42-31-24(29)13-14-28(54-7)30(31)41/h8,13-14,16,19-23,27,32H,1,9-12,15,17-18H2,2-7H3,(H,44,53)(H,46,49)(H,51,52)(H,43,45,48)/t21?,23-,27?,32-,40-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.530 | n/a | n/a | n/a | n/a | 0.000440 | 8.50E+5 | 8.0 | n/a |
Boehringer Ingelheim (Canada) Ltd.
| Assay Description
Kinetic analysis of NS3 inhibitor binding experiment were performed using the KinTek stopped flow instrument (SF-2005; excitation, 325 nm; and emissi... |
J Biol Chem 286: 11434-43 (2011)
Article DOI: 10.1074/jbc.M110.211417
BindingDB Entry DOI: 10.7270/Q2WM1C0T |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(RAT) | BDBM21147

((3S)-3-[(2S)-2-[(2S)-2-{2-[(2S)-2-[(2S)-2-[(3S)-3-...)Show SMILES CSCC[C@H](NC(=O)[C@H](Cc1ccc(OS(O)(=O)=O)cc1)NC(=O)[C@@H](N)CC(O)=O)C(=O)NCC(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CCSC)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C49H62N10O16S3/c1-76-18-16-34(55-47(69)37(58-44(66)32(50)23-41(61)62)21-28-12-14-30(15-13-28)75-78(72,73)74)45(67)53-26-40(60)54-38(22-29-25-52-33-11-7-6-10-31(29)33)48(70)56-35(17-19-77-2)46(68)59-39(24-42(63)64)49(71)57-36(43(51)65)20-27-8-4-3-5-9-27/h3-15,25,32,34-39,52H,16-24,26,50H2,1-2H3,(H2,51,65)(H,53,67)(H,54,60)(H,55,69)(H,56,70)(H,57,71)(H,58,66)(H,59,68)(H,61,62)(H,63,64)(H,72,73,74)/t32-,34-,35-,36-,37-,38-,39-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Affinity to inhibit [3H]pCCK-8 specific binding on rat brain Cholecystokinin type B receptor expressed in CHO cells |
J Med Chem 40: 647-58 (1997)
Article DOI: 10.1021/jm9603072
BindingDB Entry DOI: 10.7270/Q2PG1SDX |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2b
(Homo sapiens (Human)) | BDBM50336996
(CHEMBL1672631 | N-[6-(2-Fluorophenyl)-5-(3-fluorop...)Show InChI InChI=1S/C19H14F2N4O/c20-14-4-2-1-3-12(14)18-17(13-7-8-22-9-15(13)21)23-10-16(24-18)25-19(26)11-5-6-11/h1-4,7-11H,5-6H2,(H,24,25,26) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]DPCPX from human adenosine A2B receptor expressed in HEK293 cells after 60 mins by filtration binding assay |
ACS Med Chem Lett 2: 213-218 (2011)
Article DOI: 10.1021/ml100249e
BindingDB Entry DOI: 10.7270/Q2PG1S10 |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM50427452
(CHEMBL2326941)Show SMILES COc1ccc(cc1OCCCCOc1ccc(cc1)-c1nnn[nH]1)C1=NN(C2CCCCCC2)C(=O)[C@@H]2CC=CC[C@H]12 |r,c:43,t:28| Show InChI InChI=1S/C33H40N6O4/c1-41-29-19-16-24(31-27-12-6-7-13-28(27)33(40)39(36-31)25-10-4-2-3-5-11-25)22-30(29)43-21-9-8-20-42-26-17-14-23(15-18-26)32-34-37-38-35-32/h6-7,14-19,22,25,27-28H,2-5,8-13,20-21H2,1H3,(H,34,35,37,38)/t27-,28+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Amsterdam
Curated by ChEMBL
| Assay Description
Inhibition of full length recombinant human PDE4B1 expressed in Sf21 insect cells using cAMP as substrate by scintillation proximity assay |
J Med Chem 61: 3870-3888 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01670
BindingDB Entry DOI: 10.7270/Q2P84FF9 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM21147

((3S)-3-[(2S)-2-[(2S)-2-{2-[(2S)-2-[(2S)-2-[(3S)-3-...)Show SMILES CSCC[C@H](NC(=O)[C@H](Cc1ccc(OS(O)(=O)=O)cc1)NC(=O)[C@@H](N)CC(O)=O)C(=O)NCC(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CCSC)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C49H62N10O16S3/c1-76-18-16-34(55-47(69)37(58-44(66)32(50)23-41(61)62)21-28-12-14-30(15-13-28)75-78(72,73)74)45(67)53-26-40(60)54-38(22-29-25-52-33-11-7-6-10-31(29)33)48(70)56-35(17-19-77-2)46(68)59-39(24-42(63)64)49(71)57-36(43(51)65)20-27-8-4-3-5-9-27/h3-15,25,32,34-39,52H,16-24,26,50H2,1-2H3,(H2,51,65)(H,53,67)(H,54,60)(H,55,69)(H,56,70)(H,57,71)(H,58,66)(H,59,68)(H,61,62)(H,63,64)(H,72,73,74)/t32-,34-,35-,36-,37-,38-,39-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.640 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Inhibition of [3H]pCCK-8 binding to Guinea pig cortex membrane Cholecystokinin type B receptor |
J Med Chem 40: 647-58 (1997)
Article DOI: 10.1021/jm9603072
BindingDB Entry DOI: 10.7270/Q2PG1SDX |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(Homo sapiens (Human)) | BDBM81490
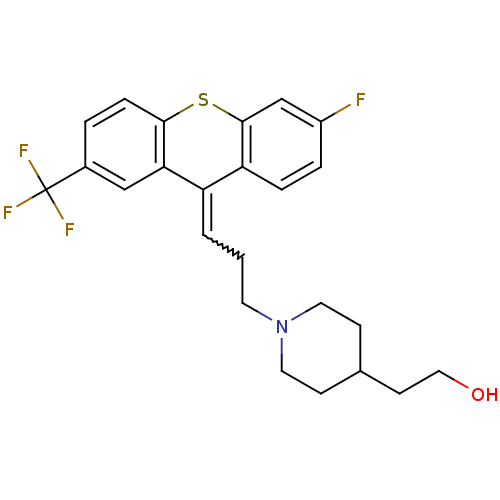
(CAS_60197-32-2 | CIS PIFLUTIXOL | NSC_68714 | Pifl...)Show SMILES OCCC1CCN(CCC=C2c3ccc(F)cc3Sc3ccc(cc23)C(F)(F)F)CC1 |w:9.8| Show InChI InChI=1S/C24H25F4NOS/c25-18-4-5-20-19(2-1-10-29-11-7-16(8-12-29)9-13-30)21-14-17(24(26,27)28)3-6-22(21)31-23(20)15-18/h2-6,14-16,30H,1,7-13H2 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University
Curated by PDSP Ki Database
| |
Proc Natl Acad Sci U S A 88: 7491-5 (1991)
Article DOI: 10.1073/pnas.88.17.7491
BindingDB Entry DOI: 10.7270/Q2DB80B9 |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(RAT) | BDBM50406692
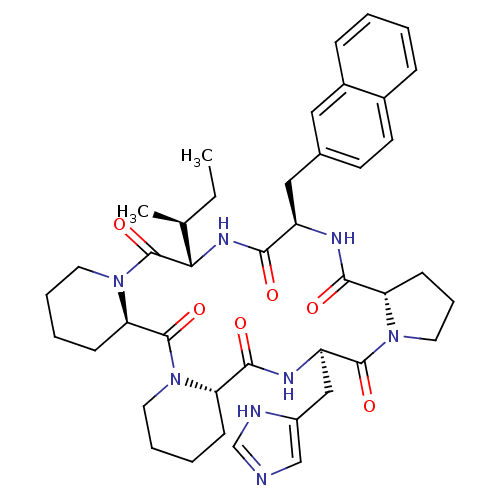
(CHEMBL2112249)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@@H](Cc2ccc3ccccc3c2)NC(=O)[C@@H]2CCCN2C(=O)[C@@H](Cc2cnc[nH]2)NC(=O)[C@@H]2CCCCN2C(=O)[C@H]2CCCCN2C1=O Show InChI InChI=1S/C42H54N8O6/c1-3-26(2)36-42(56)50-19-9-7-14-35(50)41(55)49-18-8-6-13-33(49)39(53)46-32(23-30-24-43-25-44-30)40(54)48-20-10-15-34(48)38(52)45-31(37(51)47-36)22-27-16-17-28-11-4-5-12-29(28)21-27/h4-5,11-12,16-17,21,24-26,31-36H,3,6-10,13-15,18-20,22-23H2,1-2H3,(H,43,44)(H,45,52)(H,46,53)(H,47,51)/t26-,31+,32+,33-,34-,35+,36-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Compound was evaluated for OT receptor affinity by displacement of [3H]OT from binding sites in uterine tissue taken from pregnant rats |
J Med Chem 35: 3905-18 (1992)
BindingDB Entry DOI: 10.7270/Q2K64JP2 |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50055521
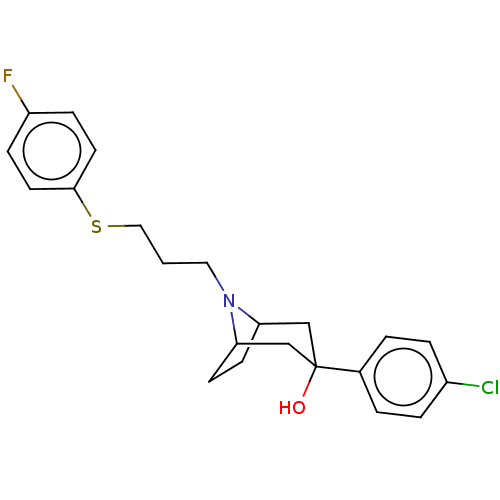
(CHEMBL3321790)Show SMILES OC1(CC2CCC(C1)N2CCCSc1ccc(F)cc1)c1ccc(Cl)cc1 |TLB:9:8:4.5:2.1.7,20:1:8:4.5,0:1:8:4.5| Show InChI InChI=1S/C22H25ClFNOS/c23-17-4-2-16(3-5-17)22(26)14-19-8-9-20(15-22)25(19)12-1-13-27-21-10-6-18(24)7-11-21/h2-7,10-11,19-20,26H,1,8-9,12-15H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Florida A&M University
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-methylspiperone from human dopamine D3 receptor by liquid scintillation counting |
Bioorg Med Chem Lett 24: 4294-7 (2014)
Article DOI: 10.1016/j.bmcl.2014.07.018
BindingDB Entry DOI: 10.7270/Q2222WF8 |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50185473

(4-(3-(4-chlorophenyl)-3-hydroxy-8-aza-bicyclo[3.2....)Show SMILES OC1(CC2CCC(C1)N2CCCC(=O)c1ccc(F)cc1)c1ccc(Cl)cc1 |TLB:9:8:1.2.7:4.5,0:1:8:4.5| Show InChI InChI=1S/C23H25ClFNO2/c24-18-7-5-17(6-8-18)23(28)14-20-11-12-21(15-23)26(20)13-1-2-22(27)16-3-9-19(25)10-4-16/h3-10,20-21,28H,1-2,11-15H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.710 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Florida A&M University
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-methylspiperone from human dopamine D3 receptor by liquid scintillation counting |
Bioorg Med Chem Lett 24: 4294-7 (2014)
Article DOI: 10.1016/j.bmcl.2014.07.018
BindingDB Entry DOI: 10.7270/Q2222WF8 |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 5
(Rattus norvegicus (Rat)) | BDBM50181753
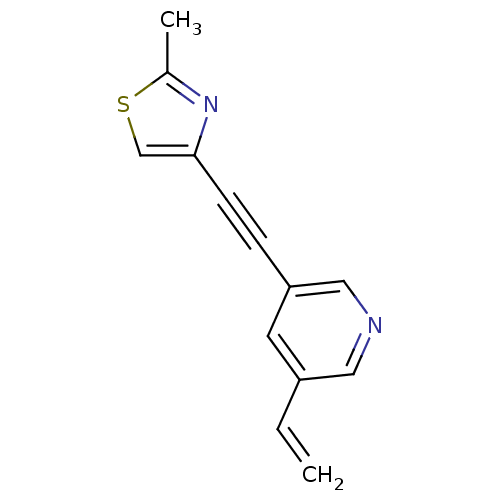
(3-[(2-methyl-4-thiazolyl)ethynyl]-5-vinylpyridine ...)Show InChI InChI=1S/C13H10N2S/c1-3-11-6-12(8-14-7-11)4-5-13-9-16-10(2)15-13/h3,6-9H,1H2,2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.710 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Displacement of [3H]MPEP from mGluR5 in rat brain membranes |
J Med Chem 49: 1080-100 (2006)
Article DOI: 10.1021/jm050570f
BindingDB Entry DOI: 10.7270/Q27S7NC5 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50056453
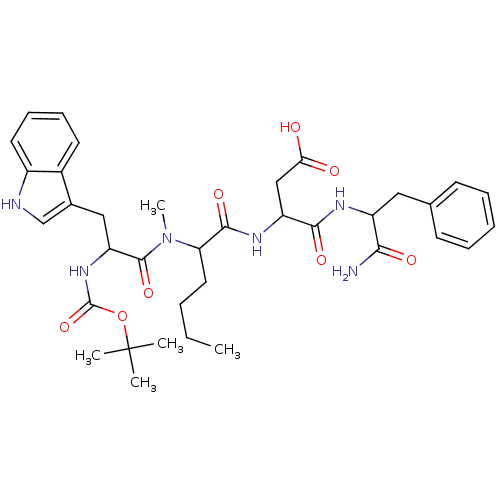
(3-(2-{[2-tert-Butoxycarbonylamino-3-(1H-indol-3-yl...)Show SMILES CCCCC(N(C)C(=O)C(Cc1c[nH]c2ccccc12)NC(=O)OC(C)(C)C)C(=O)NC(CC(O)=O)C(=O)NC(Cc1ccccc1)C(N)=O Show InChI InChI=1S/C36H48N6O8/c1-6-7-17-29(33(47)40-27(20-30(43)44)32(46)39-26(31(37)45)18-22-13-9-8-10-14-22)42(5)34(48)28(41-35(49)50-36(2,3)4)19-23-21-38-25-16-12-11-15-24(23)25/h8-16,21,26-29,38H,6-7,17-20H2,1-5H3,(H2,37,45)(H,39,46)(H,40,47)(H,41,49)(H,43,44) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Inhibition of [3H]pCCK-8 binding to Guinea pig cortex membrane Cholecystokinin type B receptor |
J Med Chem 40: 647-58 (1997)
Article DOI: 10.1021/jm9603072
BindingDB Entry DOI: 10.7270/Q2PG1SDX |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(RAT) | BDBM50384954
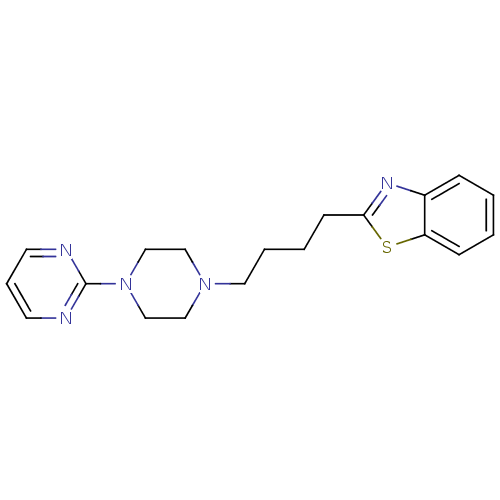
(CHEMBL2037521)Show InChI InChI=1S/C19H23N5S/c1-2-7-17-16(6-1)22-18(25-17)8-3-4-11-23-12-14-24(15-13-23)19-20-9-5-10-21-19/h1-2,5-7,9-10H,3-4,8,11-15H2 | Reactome pathway
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.840 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Florida A&M University
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-methylspiperone from rat dopamine D4 receptor by PDSP assay |
Bioorg Med Chem 22: 3105-14 (2014)
Article DOI: 10.1016/j.bmc.2014.04.026
BindingDB Entry DOI: 10.7270/Q25140RW |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM21398

(4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...)Show SMILES OC1(CCN(CCCC(=O)c2ccc(F)cc2)CC1)c1ccc(Cl)cc1 Show InChI InChI=1S/C21H23ClFNO2/c22-18-7-5-17(6-8-18)21(26)11-14-24(15-12-21)13-1-2-20(25)16-3-9-19(23)10-4-16/h3-10,26H,1-2,11-15H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 0.890 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Florida A&M University
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-methylspiperone from human dopamine D2 receptor by liquid scintillation counting |
Bioorg Med Chem Lett 24: 4294-7 (2014)
Article DOI: 10.1016/j.bmcl.2014.07.018
BindingDB Entry DOI: 10.7270/Q2222WF8 |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(Hepatitis C Virus) | BDBM92407

(BI201335)Show SMILES COc1ccc2c(O[C@@H]3CC(N(C3)C(=O)[C@@H](NC(=O)OC3CCCC3)C(C)(C)C)C(=O)N[C@@]3(CC3C=C)C(O)=O)cc(nc2c1Br)-c1csc(NC(=O)C(C)C)n1 |r| Show InChI InChI=1S/C40H49BrN6O9S/c1-8-21-17-40(21,36(51)52)46-34(49)27-15-23(18-47(27)35(50)32(39(4,5)6)44-38(53)56-22-11-9-10-12-22)55-29-16-25(26-19-57-37(43-26)45-33(48)20(2)3)42-31-24(29)13-14-28(54-7)30(31)41/h8,13-14,16,19-23,27,32H,1,9-12,15,17-18H2,2-7H3,(H,44,53)(H,46,49)(H,51,52)(H,43,45,48)/t21?,23-,27?,32-,40-/m1/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.970 | n/a | n/a | n/a | n/a | 0.000530 | 5.40E+5 | 8.0 | n/a |
Boehringer Ingelheim (Canada) Ltd.
| Assay Description
Kinetic analysis of NS3 inhibitor binding experiment were performed using the KinTek stopped flow instrument (SF-2005; excitation, 325 nm; and emissi... |
J Biol Chem 286: 11434-43 (2011)
Article DOI: 10.1074/jbc.M110.211417
BindingDB Entry DOI: 10.7270/Q2WM1C0T |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] A
(Homo sapiens (Human)) | BDBM50557368
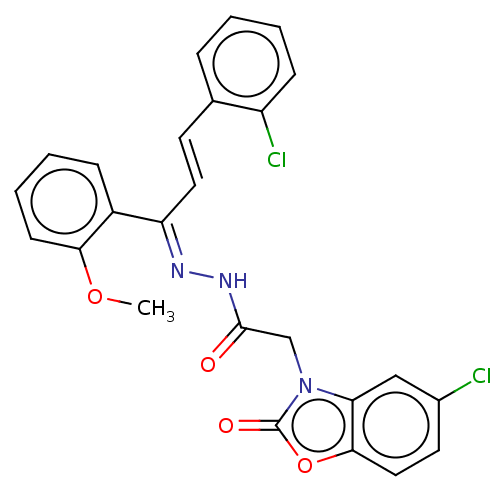
(CHEMBL4762228)Show SMILES COc1ccccc1\C(\C=C\c1ccccc1Cl)=N\NC(=O)Cn1c2cc(Cl)ccc2oc1=O | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human MAO-A expressed in baculovirus infected BTI insect cells using p-tyramine as substrate incubated for 30 mins by Ample... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01504
BindingDB Entry DOI: 10.7270/Q2G44V06 |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] A
(Homo sapiens (Human)) | BDBM50557363
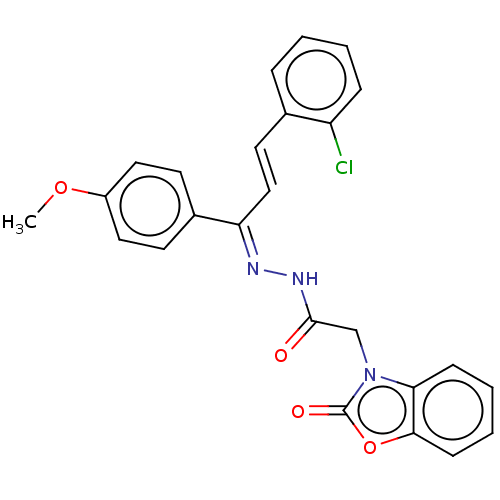
(CHEMBL4757953)Show SMILES COc1ccc(cc1)\C(\C=C\c1ccccc1Cl)=N\NC(=O)Cn1c2ccccc2oc1=O | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human MAO-A expressed in baculovirus infected BTI insect cells using p-tyramine as substrate incubated for 30 mins by Ample... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01504
BindingDB Entry DOI: 10.7270/Q2G44V06 |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] A
(Homo sapiens (Human)) | BDBM50557361
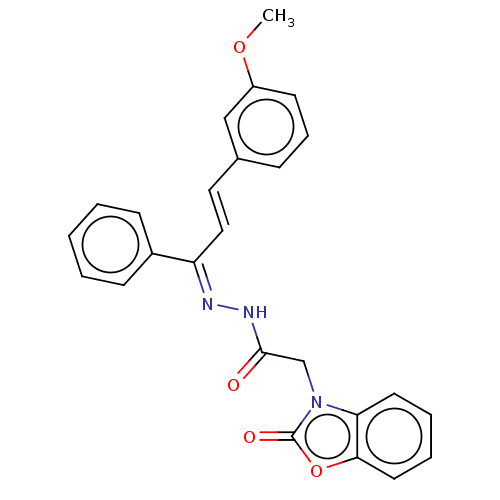
(CHEMBL4761363)Show SMILES COc1cccc(\C=C\C(=N/NC(=O)Cn2c3ccccc3oc2=O)\c2ccccc2)c1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human MAO-A expressed in baculovirus infected BTI insect cells using p-tyramine as substrate incubated for 30 mins by Ample... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01504
BindingDB Entry DOI: 10.7270/Q2G44V06 |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] A
(Homo sapiens (Human)) | BDBM50557366
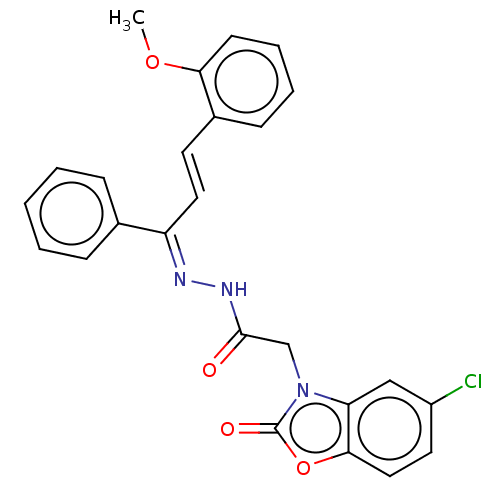
(CHEMBL4787184)Show SMILES COc1ccccc1\C=C\C(=N/NC(=O)Cn1c2cc(Cl)ccc2oc1=O)\c1ccccc1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human MAO-A expressed in baculovirus infected BTI insect cells using p-tyramine as substrate incubated for 30 mins by Ample... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01504
BindingDB Entry DOI: 10.7270/Q2G44V06 |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(Homo sapiens (Human)) | BDBM50008735

((+)-3-(tert-butyl)-(3S,4aS,13bS)-2,3,4,4a,8,9,13b,...)Show SMILES CC(C)(C)[C@]1(O)CCN2C[C@H]3c4ccccc4CCc4cccc([C@@H]2C1)c34 Show InChI InChI=1S/C25H31NO/c1-24(2,3)25(27)13-14-26-16-21-19-9-5-4-7-17(19)11-12-18-8-6-10-20(23(18)21)22(26)15-25/h4-10,21-22,27H,11-16H2,1-3H3/t21-,22-,25-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University
Curated by PDSP Ki Database
| |
Proc Natl Acad Sci U S A 88: 7491-5 (1991)
Article DOI: 10.1073/pnas.88.17.7491
BindingDB Entry DOI: 10.7270/Q2DB80B9 |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] A
(Homo sapiens (Human)) | BDBM50557339
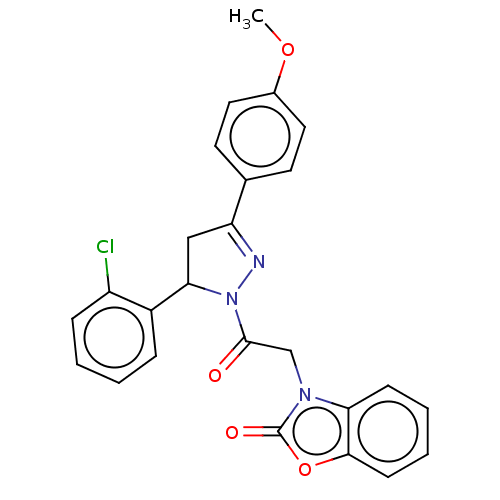
(CHEMBL4748517)Show SMILES COc1ccc(cc1)C1=NN(C(C1)c1ccccc1Cl)C(=O)Cn1c2ccccc2oc1=O |t:9| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human MAO-A expressed in baculovirus infected BTI insect cells using p-tyramine as substrate incubated for 30 mins by Ample... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01504
BindingDB Entry DOI: 10.7270/Q2G44V06 |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] A
(Homo sapiens (Human)) | BDBM50557360
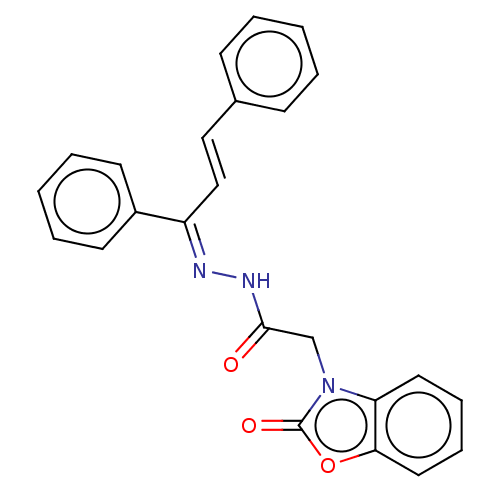
(CHEMBL4787516)Show SMILES O=C(Cn1c2ccccc2oc1=O)N\N=C(/C=C/c1ccccc1)\c1ccccc1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human MAO-A expressed in baculovirus infected BTI insect cells using p-tyramine as substrate incubated for 30 mins by Ample... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01504
BindingDB Entry DOI: 10.7270/Q2G44V06 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2b
(Homo sapiens (Human)) | BDBM50336991

(CHEMBL1672623 | N-[6-(2-Fluorophenyl)-5-pyridin-4-...)Show InChI InChI=1S/C19H15FN4O/c20-15-4-2-1-3-14(15)18-17(12-7-9-21-10-8-12)22-11-16(23-18)24-19(25)13-5-6-13/h1-4,7-11,13H,5-6H2,(H,23,24,25) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]DPCPX from human adenosine A2B receptor expressed in HEK293 cells after 60 mins by filtration binding assay |
ACS Med Chem Lett 2: 213-218 (2011)
Article DOI: 10.1021/ml100249e
BindingDB Entry DOI: 10.7270/Q2PG1S10 |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 5
(Homo sapiens (Human)) | BDBM50181753

(3-[(2-methyl-4-thiazolyl)ethynyl]-5-vinylpyridine ...)Show InChI InChI=1S/C13H10N2S/c1-3-11-6-12(8-14-7-11)4-5-13-9-16-10(2)15-13/h3,6-9H,1H2,2H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.00 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Displacement of [3H]MPEP from cloned human mGluR5 transfected in HEK293-T cells |
J Med Chem 49: 1080-100 (2006)
Article DOI: 10.1021/jm050570f
BindingDB Entry DOI: 10.7270/Q27S7NC5 |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM18207

((1R,2S,10S,11S,13R,14R,15S,17S)-1-fluoro-14,17-dih...)Show SMILES [H][C@@]12C[C@@H](C)[C@](O)(C(=O)CO)[C@@]1(C)C[C@H](O)[C@@]1(F)[C@@]2([H])CCC2=CC(=O)C=C[C@]12C |c:28,t:24| Show InChI InChI=1S/C22H29FO5/c1-12-8-16-15-5-4-13-9-14(25)6-7-19(13,2)21(15,23)17(26)10-20(16,3)22(12,28)18(27)11-24/h6-7,9,12,15-17,24,26,28H,4-5,8,10-11H2,1-3H3/t12-,15+,16+,17+,19+,20+,21+,22+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trinity College
Curated by ChEMBL
| Assay Description
Displacement of [3H]DEX from human glucocorticoid receptor |
J Med Chem 53: 3065-74 (2010)
Article DOI: 10.1021/jm901452y
BindingDB Entry DOI: 10.7270/Q2VQ33NH |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
D(4) dopamine receptor
(RAT) | BDBM50017969
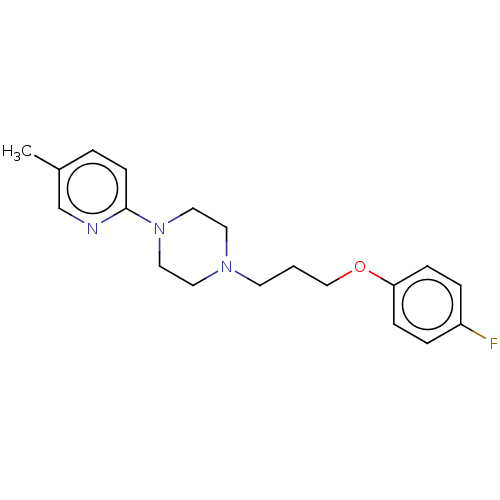
(CHEMBL3289646)Show InChI InChI=1S/C19H24FN3O/c1-16-3-8-19(21-15-16)23-12-10-22(11-13-23)9-2-14-24-18-6-4-17(20)5-7-18/h3-8,15H,2,9-14H2,1H3 | Reactome pathway
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Florida A&M University
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-methylspiperone from rat dopamine D4 receptor by PDSP assay |
Bioorg Med Chem 22: 3105-14 (2014)
Article DOI: 10.1016/j.bmc.2014.04.026
BindingDB Entry DOI: 10.7270/Q25140RW |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50063477

((3S,10R,13S)-17-Imidazol-1-yl-10,13-dimethyl-2,3,4...)Show SMILES C[C@]12CCC3C(CC=C4C[C@@H](O)CC[C@]34C)C1CC=C2n1ccnc1 |c:21,t:7| Show InChI InChI=1S/C22H30N2O/c1-21-9-7-16(25)13-15(21)3-4-17-18-5-6-20(24-12-11-23-14-24)22(18,2)10-8-19(17)21/h3,6,11-12,14,16-19,25H,4-5,7-10,13H2,1-2H3/t16-,17?,18?,19?,21-,22-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by ChEMBL
| Assay Description
Binding affinity for Cytochrome P450 17A1 (17-alpha-hydroxypregnenolone Km=560 nM) |
J Med Chem 41: 902-12 (1998)
Article DOI: 10.1021/jm970568r
BindingDB Entry DOI: 10.7270/Q2GH9H2N |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50336989

(CHEMBL1672619 | N-[6-(5-Methyl-2-furyl)-5-pyridin-...)Show InChI InChI=1S/C18H16N4O2/c1-11-2-5-14(24-11)17-16(12-6-8-19-9-7-12)20-10-15(21-17)22-18(23)13-3-4-13/h2,5-10,13H,3-4H2,1H3,(H,21,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]ZM241385 from human adenosine A2A receptor expressed in HeLa cells after 60 mins by scintillation proximity assay |
ACS Med Chem Lett 2: 213-218 (2011)
Article DOI: 10.1021/ml100249e
BindingDB Entry DOI: 10.7270/Q2PG1S10 |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50055523

(CHEMBL3321792)Show SMILES OC1(CC2CCC(C1)N2CCCSc1ccccc1F)c1ccc(Cl)cc1 |TLB:20:1:8:4.5,0:1:8:4.5,9:8:4.5:2.1.7| Show InChI InChI=1S/C22H25ClFNOS/c23-17-8-6-16(7-9-17)22(26)14-18-10-11-19(15-22)25(18)12-3-13-27-21-5-2-1-4-20(21)24/h1-2,4-9,18-19,26H,3,10-15H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Florida A&M University
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-methylspiperone from human dopamine D3 receptor by liquid scintillation counting |
Bioorg Med Chem Lett 24: 4294-7 (2014)
Article DOI: 10.1016/j.bmcl.2014.07.018
BindingDB Entry DOI: 10.7270/Q2222WF8 |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM81894
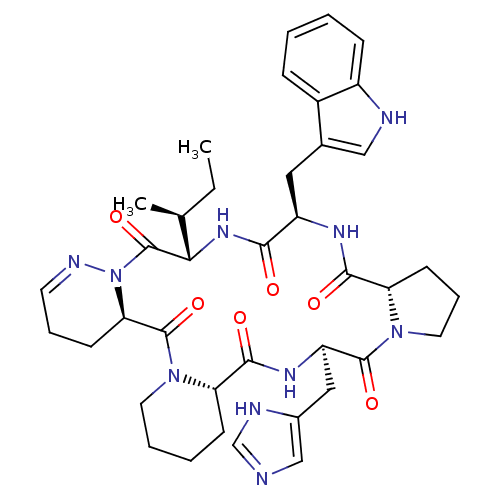
(Cyclo[L-Pro-D-Trp-L-Ile-1,6-didehydro-D-Pyz-L-Pip-...)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@@H](Cc2c[nH]c3ccccc23)NC(=O)[C@@H]2CCCN2C(=O)[C@@H](Cc2cnc[nH]2)NC(=O)[C@@H]2CCCCN2C(=O)[C@H]2CCC=NN2C1=O |c:55| Show InChI InChI=1S/C39H50N10O6/c1-3-23(2)33-39(55)49-32(13-8-15-43-49)38(54)48-16-7-6-12-30(48)36(52)45-29(19-25-21-40-22-42-25)37(53)47-17-9-14-31(47)35(51)44-28(34(50)46-33)18-24-20-41-27-11-5-4-10-26(24)27/h4-5,10-11,15,20-23,28-33,41H,3,6-9,12-14,16-19H2,1-2H3,(H,40,42)(H,44,51)(H,45,52)(H,46,50)/t23-,28+,29+,30-,31-,32+,33-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Compound was evaluated for OT receptor affinity by displacement of [3H]OT from binding sites in uterine tissue taken from near-term pregnant rhesus m... |
J Med Chem 35: 3905-18 (1992)
BindingDB Entry DOI: 10.7270/Q2K64JP2 |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM81894

(Cyclo[L-Pro-D-Trp-L-Ile-1,6-didehydro-D-Pyz-L-Pip-...)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@@H](Cc2c[nH]c3ccccc23)NC(=O)[C@@H]2CCCN2C(=O)[C@@H](Cc2cnc[nH]2)NC(=O)[C@@H]2CCCCN2C(=O)[C@H]2CCC=NN2C1=O |c:55| Show InChI InChI=1S/C39H50N10O6/c1-3-23(2)33-39(55)49-32(13-8-15-43-49)38(54)48-16-7-6-12-30(48)36(52)45-29(19-25-21-40-22-42-25)37(53)47-17-9-14-31(47)35(51)44-28(34(50)46-33)18-24-20-41-27-11-5-4-10-26(24)27/h4-5,10-11,15,20-23,28-33,41H,3,6-9,12-14,16-19H2,1-2H3,(H,40,42)(H,44,51)(H,45,52)(H,46,50)/t23-,28+,29+,30-,31-,32+,33-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Compound was evaluated for OT receptor affinity by displacement of [3H]OT from binding sites in uterine tissue taken from nonlabor pregnant women |
J Med Chem 35: 3905-18 (1992)
BindingDB Entry DOI: 10.7270/Q2K64JP2 |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(RAT) | BDBM81894

(Cyclo[L-Pro-D-Trp-L-Ile-1,6-didehydro-D-Pyz-L-Pip-...)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@@H](Cc2c[nH]c3ccccc23)NC(=O)[C@@H]2CCCN2C(=O)[C@@H](Cc2cnc[nH]2)NC(=O)[C@@H]2CCCCN2C(=O)[C@H]2CCC=NN2C1=O |c:55| Show InChI InChI=1S/C39H50N10O6/c1-3-23(2)33-39(55)49-32(13-8-15-43-49)38(54)48-16-7-6-12-30(48)36(52)45-29(19-25-21-40-22-42-25)37(53)47-17-9-14-31(47)35(51)44-28(34(50)46-33)18-24-20-41-27-11-5-4-10-26(24)27/h4-5,10-11,15,20-23,28-33,41H,3,6-9,12-14,16-19H2,1-2H3,(H,40,42)(H,44,51)(H,45,52)(H,46,50)/t23-,28+,29+,30-,31-,32+,33-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Ability to inhibit AVP stimulation of adenylate cyclase activity in the rat kidney medulla (AVP-V2) receptor |
J Med Chem 35: 3905-18 (1992)
BindingDB Entry DOI: 10.7270/Q2K64JP2 |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50063476

((3S,10R,13S)-10,13-Dimethyl-17-[1,2,3]triazol-1-yl...)Show SMILES C[C@]12CCC3C(CC=C4C[C@@H](O)CC[C@]34C)C1CC=C2n1ccnn1 |c:21,t:7| Show InChI InChI=1S/C21H29N3O/c1-20-9-7-15(25)13-14(20)3-4-16-17-5-6-19(24-12-11-22-23-24)21(17,2)10-8-18(16)20/h3,6,11-12,15-18,25H,4-5,7-10,13H2,1-2H3/t15-,16?,17?,18?,20-,21-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by ChEMBL
| Assay Description
Binding affinity for Cytochrome P450 17A1 (17-alpha-hydroxypregnenolone Km=560 nM) |
J Med Chem 41: 902-12 (1998)
Article DOI: 10.1021/jm970568r
BindingDB Entry DOI: 10.7270/Q2GH9H2N |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase 14
(Homo sapiens (Human)) | BDBM50422425
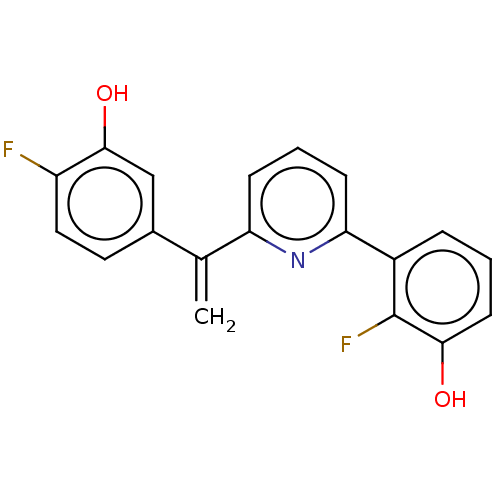
(CHEMBL4175585)Show InChI InChI=1S/C42H69N5O5/c1-50-37-23-22-35-32-38(42(49)52-40(35)33-37)41(48)47-31-19-30-45-28-17-8-7-15-26-43-24-13-5-3-4-6-14-25-44-27-16-9-10-18-29-46-34-36-20-11-12-21-39(36)51-2/h11-12,20-23,32-33,35,40,43-46H,3-10,13-19,24-31,34H2,1-2H3,(H,47,48) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University Marburg
Curated by ChEMBL
| Assay Description
Binding affinity to recombinant 17beta-HSD14 (unknown origin) expressed in Escherichia coli BL21 pLysS strain using E2 as substrate in presence of NA... |
Eur J Med Chem 155: 61-76 (2018)
Article DOI: 10.1016/j.ejmech.2018.05.029
BindingDB Entry DOI: 10.7270/Q23F4S76 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
D(4) dopamine receptor
(RAT) | BDBM50362866
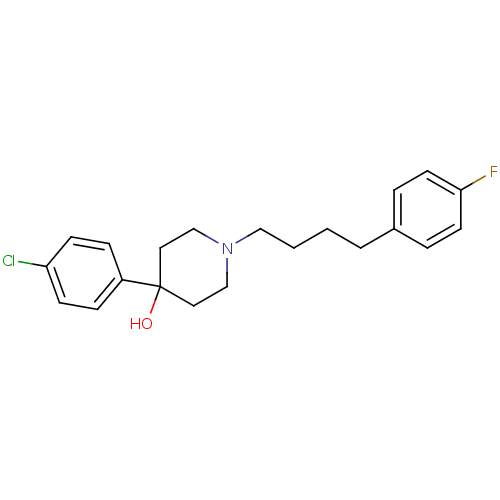
(CHEMBL1940402)Show InChI InChI=1S/C21H25ClFNO/c22-19-8-6-18(7-9-19)21(25)12-15-24(16-13-21)14-2-1-3-17-4-10-20(23)11-5-17/h4-11,25H,1-3,12-16H2 | Reactome pathway
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Florida A&M University
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-methylspiperone from rat dopamine D4 receptor by liquid scintillation counting |
Bioorg Med Chem Lett 24: 4294-7 (2014)
Article DOI: 10.1016/j.bmcl.2014.07.018
BindingDB Entry DOI: 10.7270/Q2222WF8 |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(RAT) | BDBM50362865

(CHEMBL1940420)Show InChI InChI=1S/C18H22FN3O/c19-16-5-7-17(8-6-16)23-15-3-10-21-11-13-22(14-12-21)18-4-1-2-9-20-18/h1-2,4-9H,3,10-15H2 | Reactome pathway
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Florida A&M University
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-methylspiperone from rat dopamine D4 receptor by PDSP assay |
Bioorg Med Chem 22: 3105-14 (2014)
Article DOI: 10.1016/j.bmc.2014.04.026
BindingDB Entry DOI: 10.7270/Q25140RW |
More data for this
Ligand-Target Pair | |
Genome polyprotein [1027-1206,R1052K]
(Hepatitis C virus) | BDBM92408

(NS3 protease inhibitor, analog 1)Show SMILES COc1ccc2c(O[C@@H]3CC(N(C3)C(=O)[C@@H](NC(=O)OC3CCCC3)C(C)(C)C)C(=O)N[C@@]3(CC3C=C)C(O)=O)cc(nc2c1)-c1csc(NC(=O)C(C)C)n1 |r| Show InChI InChI=1S/C40H50N6O9S/c1-8-22-18-40(22,36(50)51)45-34(48)30-16-25(19-46(30)35(49)32(39(4,5)6)43-38(52)55-23-11-9-10-12-23)54-31-17-28(41-27-15-24(53-7)13-14-26(27)31)29-20-56-37(42-29)44-33(47)21(2)3/h8,13-15,17,20-23,25,30,32H,1,9-12,16,18-19H2,2-7H3,(H,43,52)(H,45,48)(H,50,51)(H,42,44,47)/t22?,25-,30?,32-,40-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.89 | n/a | n/a | n/a | n/a | 0.00220 | 1.10E+6 | 8.0 | n/a |
Boehringer Ingelheim (Canada) Ltd.
| Assay Description
Kinetic analysis of NS3 inhibitor binding experiment were performed using the KinTek stopped flow instrument (SF-2005; excitation, 325 nm; and emissi... |
J Biol Chem 286: 11434-43 (2011)
Article DOI: 10.1074/jbc.M110.211417
BindingDB Entry DOI: 10.7270/Q2WM1C0T |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50063475
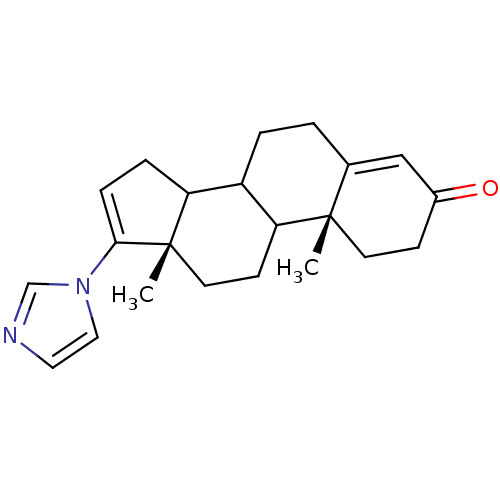
((10R,13S)-17-Imidazol-1-yl-10,13-dimethyl-1,2,6,7,...)Show SMILES C[C@]12CCC3C(CCC4=CC(=O)CC[C@]34C)C1CC=C2n1ccnc1 |c:21,t:8| Show InChI InChI=1S/C22H28N2O/c1-21-9-7-16(25)13-15(21)3-4-17-18-5-6-20(24-12-11-23-14-24)22(18,2)10-8-19(17)21/h6,11-14,17-19H,3-5,7-10H2,1-2H3/t17?,18?,19?,21-,22-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by ChEMBL
| Assay Description
Binding affinity for Cytochrome P450 17A1 (17-alpha-hydroxypregnenolone Km=560 nM) |
J Med Chem 41: 902-12 (1998)
Article DOI: 10.1021/jm970568r
BindingDB Entry DOI: 10.7270/Q2GH9H2N |
More data for this
Ligand-Target Pair | |
D(1B) dopamine receptor
(RAT) | BDBM81490

(CAS_60197-32-2 | CIS PIFLUTIXOL | NSC_68714 | Pifl...)Show SMILES OCCC1CCN(CCC=C2c3ccc(F)cc3Sc3ccc(cc23)C(F)(F)F)CC1 |w:9.8| Show InChI InChI=1S/C24H25F4NOS/c25-18-4-5-20-19(2-1-10-29-11-7-16(8-12-29)9-13-30)21-14-17(24(26,27)28)3-6-22(21)31-23(20)15-18/h2-6,14-16,30H,1,7-13H2 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University
Curated by PDSP Ki Database
| |
Proc Natl Acad Sci U S A 88: 7491-5 (1991)
Article DOI: 10.1073/pnas.88.17.7491
BindingDB Entry DOI: 10.7270/Q2DB80B9 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data