

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50252816 (2-(4-{Butyl[3-(2-hydroxyethoxy)benzyl]amino}phenyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Displacement of [N-methyl-3H]GW0438 from human biotinylated LXRbeta ligand binding domain | J Med Chem 51: 5758-65 (2008) Article DOI: 10.1021/jm800612u BindingDB Entry DOI: 10.7270/Q28052FN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50252939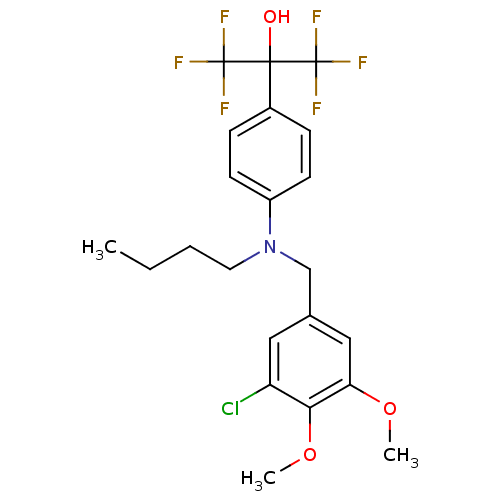 (2-[4-(Butyl{[3-chloro-4,5-bis(methyloxy)phenyl]met...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Displacement of [N-methyl-3H]GW0438 from human biotinylated LXRbeta ligand binding domain | J Med Chem 51: 5758-65 (2008) Article DOI: 10.1021/jm800612u BindingDB Entry DOI: 10.7270/Q28052FN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50252850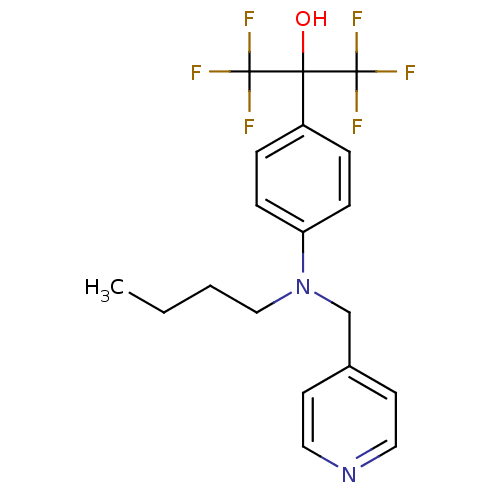 (2-{4-[Butyl(pyridin-4-ylmethyl)amino]phenyl}-1,1,1...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Displacement of [N-methyl-3H]GW0438 from human biotinylated LXRbeta ligand binding domain | J Med Chem 51: 5758-65 (2008) Article DOI: 10.1021/jm800612u BindingDB Entry DOI: 10.7270/Q28052FN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50252967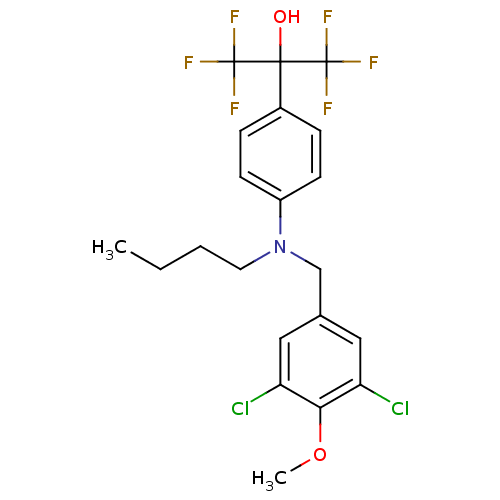 (2-{4-[Butyl(3,5-dichloro-4-methoxybenzyl)amino]phe...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Displacement of [N-methyl-3H]GW0438 from human biotinylated LXRbeta ligand binding domain | J Med Chem 51: 5758-65 (2008) Article DOI: 10.1021/jm800612u BindingDB Entry DOI: 10.7270/Q28052FN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50252817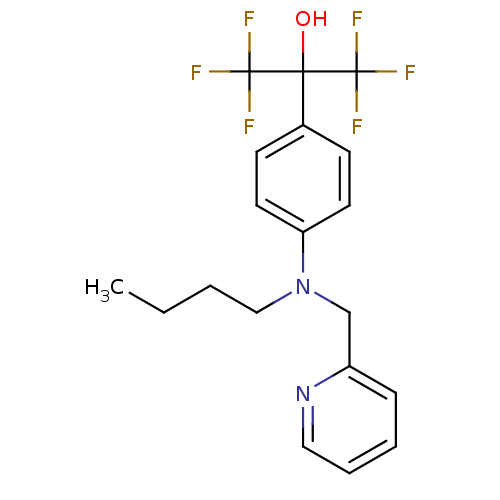 (2-{4-[Butyl(pyridin-2-ylmethyl)amino]phenyl}-1,1,1...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Displacement of [N-methyl-3H]GW0438 from human biotinylated LXRbeta ligand binding domain | J Med Chem 51: 5758-65 (2008) Article DOI: 10.1021/jm800612u BindingDB Entry DOI: 10.7270/Q28052FN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50252941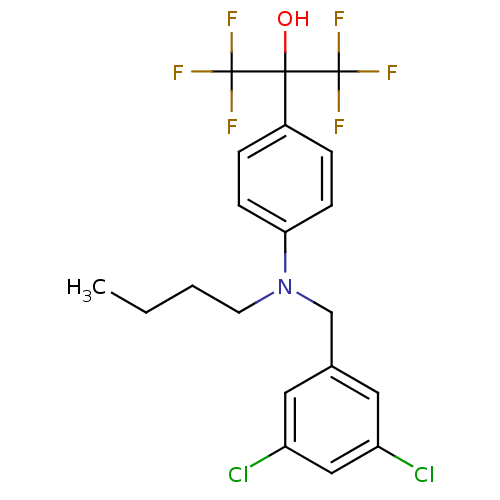 (2-{4-[Butyl(3,5-dichlorobenzyl)amino]phenyl}-1,1,1...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Displacement of [N-methyl-3H]GW0438 from human biotinylated LXRbeta ligand binding domain | J Med Chem 51: 5758-65 (2008) Article DOI: 10.1021/jm800612u BindingDB Entry DOI: 10.7270/Q28052FN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM19993 (CHEMBL62136 | N-[4-(1,1,1,3,3,3-hexafluoro-2-hydro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Displacement of [N-methyl-3H]GW0438 from human biotinylated LXRbeta ligand binding domain | J Med Chem 51: 5758-65 (2008) Article DOI: 10.1021/jm800612u BindingDB Entry DOI: 10.7270/Q28052FN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM19992 (2-[3-[3-[[2-chloro-3-(trifluoromethyl)phenyl]methy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Displacement of [N-methyl-3H]GW0438 from human biotinylated LXRbeta ligand binding domain | J Med Chem 51: 5758-65 (2008) Article DOI: 10.1021/jm800612u BindingDB Entry DOI: 10.7270/Q28052FN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50252852 (3-[(Butyl{4-[2,2,2-trifluoro-1-hydroxy-1-(trifluor...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Displacement of [N-methyl-3H]GW0438 from human biotinylated LXRbeta ligand binding domain | J Med Chem 51: 5758-65 (2008) Article DOI: 10.1021/jm800612u BindingDB Entry DOI: 10.7270/Q28052FN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50252940 (4-[(Butyl{4-[2,2,2-trifluoro-1-hydroxy-1-(trifluor...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Displacement of [N-methyl-3H]GW0438 from human biotinylated LXRbeta ligand binding domain | J Med Chem 51: 5758-65 (2008) Article DOI: 10.1021/jm800612u BindingDB Entry DOI: 10.7270/Q28052FN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50252851 (2-[(Butyl{4-[2,2,2-trifluoro-1-hydroxy-1-(trifluor...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Displacement of [N-methyl-3H]GW0438 from human biotinylated LXRbeta ligand binding domain | J Med Chem 51: 5758-65 (2008) Article DOI: 10.1021/jm800612u BindingDB Entry DOI: 10.7270/Q28052FN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50252815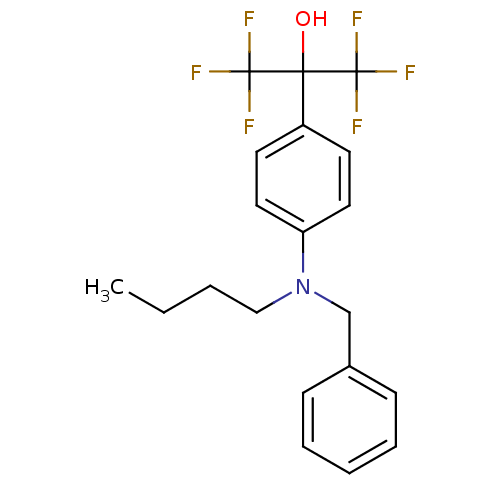 (2-{4-[Benzyl(butyl)amino]phenyl}-1,1,1,3,3,3-hexaf...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Displacement of [N-methyl-3H]GW0438 from human biotinylated LXRbeta ligand binding domain | J Med Chem 51: 5758-65 (2008) Article DOI: 10.1021/jm800612u BindingDB Entry DOI: 10.7270/Q28052FN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50252938 (4-[(Butyl{4-[2,2,2-trifluoro-1-hydroxy-1-(trifluor...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Displacement of [N-methyl-3H]GW0438 from human biotinylated LXRbeta ligand binding domain | J Med Chem 51: 5758-65 (2008) Article DOI: 10.1021/jm800612u BindingDB Entry DOI: 10.7270/Q28052FN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50252908 (4-[(Butyl{4-[2,2,2-trifluoro-1-hydroxy-1-(trifluor...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Displacement of [N-methyl-3H]GW0438 from human biotinylated LXRbeta ligand binding domain | J Med Chem 51: 5758-65 (2008) Article DOI: 10.1021/jm800612u BindingDB Entry DOI: 10.7270/Q28052FN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM19993 (CHEMBL62136 | N-[4-(1,1,1,3,3,3-hexafluoro-2-hydro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 75 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Displacement of [N-methyl-3H]GW0438 from human biotinylated LXRalpha ligand binding domain | J Med Chem 51: 5758-65 (2008) Article DOI: 10.1021/jm800612u BindingDB Entry DOI: 10.7270/Q28052FN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM50252850 (2-{4-[Butyl(pyridin-4-ylmethyl)amino]phenyl}-1,1,1...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Displacement of [N-methyl-3H]GW0438 from human biotinylated LXRalpha ligand binding domain | J Med Chem 51: 5758-65 (2008) Article DOI: 10.1021/jm800612u BindingDB Entry DOI: 10.7270/Q28052FN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50252907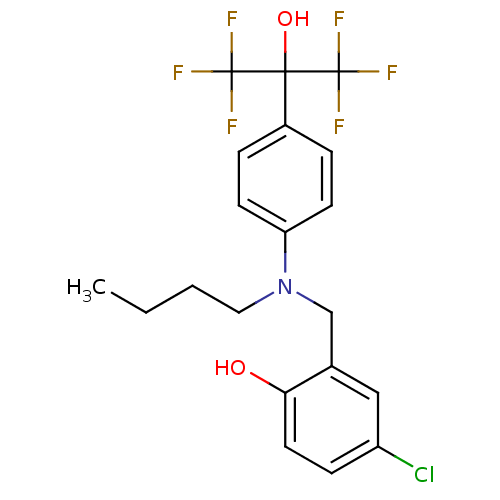 (2-[(Butyl{4-[2,2,2-trifluoro-1-hydroxy-1-(trifluor...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 115 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Displacement of [N-methyl-3H]GW0438 from human biotinylated LXRbeta ligand binding domain | J Med Chem 51: 5758-65 (2008) Article DOI: 10.1021/jm800612u BindingDB Entry DOI: 10.7270/Q28052FN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM50252816 (2-(4-{Butyl[3-(2-hydroxyethoxy)benzyl]amino}phenyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 115 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Displacement of [N-methyl-3H]GW0438 from human biotinylated LXRalpha ligand binding domain | J Med Chem 51: 5758-65 (2008) Article DOI: 10.1021/jm800612u BindingDB Entry DOI: 10.7270/Q28052FN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50252854 (2-[(Butyl{4-[2,2,2-trifluoro-1-hydroxy-1-(trifluor...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Displacement of [N-methyl-3H]GW0438 from human biotinylated LXRbeta ligand binding domain | J Med Chem 51: 5758-65 (2008) Article DOI: 10.1021/jm800612u BindingDB Entry DOI: 10.7270/Q28052FN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM50252817 (2-{4-[Butyl(pyridin-2-ylmethyl)amino]phenyl}-1,1,1...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Displacement of [N-methyl-3H]GW0438 from human biotinylated LXRalpha ligand binding domain | J Med Chem 51: 5758-65 (2008) Article DOI: 10.1021/jm800612u BindingDB Entry DOI: 10.7270/Q28052FN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM50252852 (3-[(Butyl{4-[2,2,2-trifluoro-1-hydroxy-1-(trifluor...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Displacement of [N-methyl-3H]GW0438 from human biotinylated LXRalpha ligand binding domain | J Med Chem 51: 5758-65 (2008) Article DOI: 10.1021/jm800612u BindingDB Entry DOI: 10.7270/Q28052FN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50252905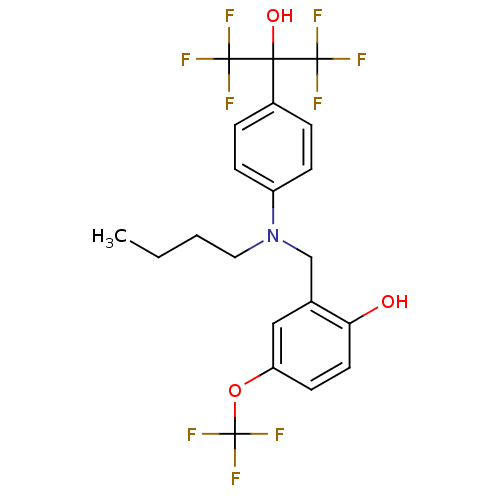 (2-[(Butyl{4-[2,2,2-trifluoro-1-hydroxy-1-(trifluor...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 165 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Displacement of [N-methyl-3H]GW0438 from human biotinylated LXRbeta ligand binding domain | J Med Chem 51: 5758-65 (2008) Article DOI: 10.1021/jm800612u BindingDB Entry DOI: 10.7270/Q28052FN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM50252939 (2-[4-(Butyl{[3-chloro-4,5-bis(methyloxy)phenyl]met...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 165 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Displacement of [N-methyl-3H]GW0438 from human biotinylated LXRalpha ligand binding domain | J Med Chem 51: 5758-65 (2008) Article DOI: 10.1021/jm800612u BindingDB Entry DOI: 10.7270/Q28052FN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM50252967 (2-{4-[Butyl(3,5-dichloro-4-methoxybenzyl)amino]phe...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Displacement of [N-methyl-3H]GW0438 from human biotinylated LXRalpha ligand binding domain | J Med Chem 51: 5758-65 (2008) Article DOI: 10.1021/jm800612u BindingDB Entry DOI: 10.7270/Q28052FN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM50252815 (2-{4-[Benzyl(butyl)amino]phenyl}-1,1,1,3,3,3-hexaf...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Displacement of [N-methyl-3H]GW0438 from human biotinylated LXRalpha ligand binding domain | J Med Chem 51: 5758-65 (2008) Article DOI: 10.1021/jm800612u BindingDB Entry DOI: 10.7270/Q28052FN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50252814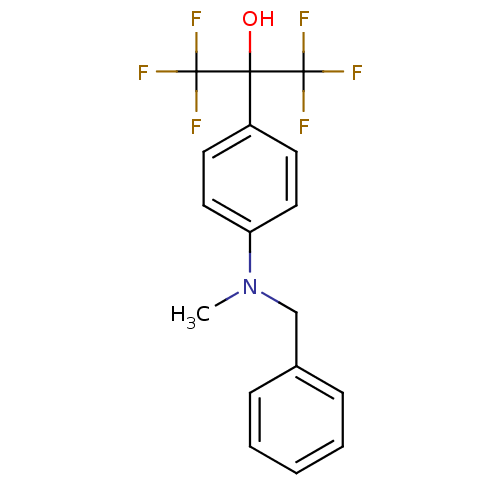 (1,1,1,3,3,3-Hexafluoro-2-{4-[methyl(phenylmethyl)a...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Displacement of [N-methyl-3H]GW0438 from human biotinylated LXRbeta ligand binding domain | J Med Chem 51: 5758-65 (2008) Article DOI: 10.1021/jm800612u BindingDB Entry DOI: 10.7270/Q28052FN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50252690 (2-(4-(dibenzylamino)phenyl)-1,1,1,3,3,3-hexafluoro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 175 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Displacement of [N-methyl-3H]GW0438 from human biotinylated LXRbeta ligand binding domain | J Med Chem 51: 5758-65 (2008) Article DOI: 10.1021/jm800612u BindingDB Entry DOI: 10.7270/Q28052FN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM50252940 (4-[(Butyl{4-[2,2,2-trifluoro-1-hydroxy-1-(trifluor...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Displacement of [N-methyl-3H]GW0438 from human biotinylated LXRalpha ligand binding domain | J Med Chem 51: 5758-65 (2008) Article DOI: 10.1021/jm800612u BindingDB Entry DOI: 10.7270/Q28052FN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM50252851 (2-[(Butyl{4-[2,2,2-trifluoro-1-hydroxy-1-(trifluor...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Displacement of [N-methyl-3H]GW0438 from human biotinylated LXRalpha ligand binding domain | J Med Chem 51: 5758-65 (2008) Article DOI: 10.1021/jm800612u BindingDB Entry DOI: 10.7270/Q28052FN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM50252905 (2-[(Butyl{4-[2,2,2-trifluoro-1-hydroxy-1-(trifluor...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Displacement of [N-methyl-3H]GW0438 from human biotinylated LXRalpha ligand binding domain | J Med Chem 51: 5758-65 (2008) Article DOI: 10.1021/jm800612u BindingDB Entry DOI: 10.7270/Q28052FN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM50252941 (2-{4-[Butyl(3,5-dichlorobenzyl)amino]phenyl}-1,1,1...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 195 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Displacement of [N-methyl-3H]GW0438 from human biotinylated LXRalpha ligand binding domain | J Med Chem 51: 5758-65 (2008) Article DOI: 10.1021/jm800612u BindingDB Entry DOI: 10.7270/Q28052FN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM50252907 (2-[(Butyl{4-[2,2,2-trifluoro-1-hydroxy-1-(trifluor...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Displacement of [N-methyl-3H]GW0438 from human biotinylated LXRalpha ligand binding domain | J Med Chem 51: 5758-65 (2008) Article DOI: 10.1021/jm800612u BindingDB Entry DOI: 10.7270/Q28052FN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM19992 (2-[3-[3-[[2-chloro-3-(trifluoromethyl)phenyl]methy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 235 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Displacement of [N-methyl-3H]GW0438 from human biotinylated LXRalpha ligand binding domain | J Med Chem 51: 5758-65 (2008) Article DOI: 10.1021/jm800612u BindingDB Entry DOI: 10.7270/Q28052FN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50252906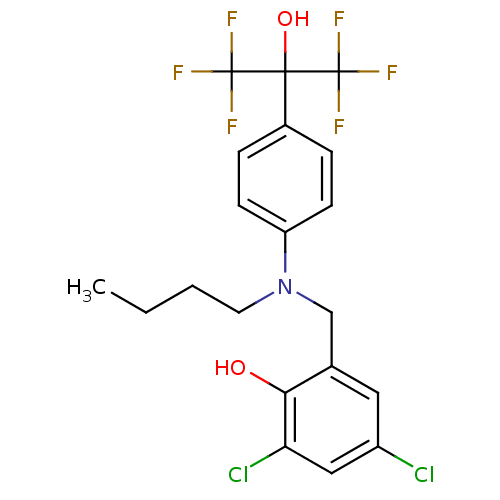 (2-[(Butyl{4-[2,2,2-trifluoro-1-hydroxy-1-(trifluor...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 290 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Displacement of [N-methyl-3H]GW0438 from human biotinylated LXRbeta ligand binding domain | J Med Chem 51: 5758-65 (2008) Article DOI: 10.1021/jm800612u BindingDB Entry DOI: 10.7270/Q28052FN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM50252814 (1,1,1,3,3,3-Hexafluoro-2-{4-[methyl(phenylmethyl)a...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 310 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Displacement of [N-methyl-3H]GW0438 from human biotinylated LXRalpha ligand binding domain | J Med Chem 51: 5758-65 (2008) Article DOI: 10.1021/jm800612u BindingDB Entry DOI: 10.7270/Q28052FN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM50252690 (2-(4-(dibenzylamino)phenyl)-1,1,1,3,3,3-hexafluoro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 315 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Displacement of [N-methyl-3H]GW0438 from human biotinylated LXRalpha ligand binding domain | J Med Chem 51: 5758-65 (2008) Article DOI: 10.1021/jm800612u BindingDB Entry DOI: 10.7270/Q28052FN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM50252908 (4-[(Butyl{4-[2,2,2-trifluoro-1-hydroxy-1-(trifluor...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 560 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Displacement of [N-methyl-3H]GW0438 from human biotinylated LXRalpha ligand binding domain | J Med Chem 51: 5758-65 (2008) Article DOI: 10.1021/jm800612u BindingDB Entry DOI: 10.7270/Q28052FN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM50252854 (2-[(Butyl{4-[2,2,2-trifluoro-1-hydroxy-1-(trifluor...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 615 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Displacement of [N-methyl-3H]GW0438 from human biotinylated LXRalpha ligand binding domain | J Med Chem 51: 5758-65 (2008) Article DOI: 10.1021/jm800612u BindingDB Entry DOI: 10.7270/Q28052FN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50252853 (4-[(Butyl{4-[2,2,2-trifluoro-1-hydroxy-1-(trifluor...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 740 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Displacement of [N-methyl-3H]GW0438 from human biotinylated LXRbeta ligand binding domain | J Med Chem 51: 5758-65 (2008) Article DOI: 10.1021/jm800612u BindingDB Entry DOI: 10.7270/Q28052FN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM50252938 (4-[(Butyl{4-[2,2,2-trifluoro-1-hydroxy-1-(trifluor...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 775 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Displacement of [N-methyl-3H]GW0438 from human biotinylated LXRalpha ligand binding domain | J Med Chem 51: 5758-65 (2008) Article DOI: 10.1021/jm800612u BindingDB Entry DOI: 10.7270/Q28052FN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM50252906 (2-[(Butyl{4-[2,2,2-trifluoro-1-hydroxy-1-(trifluor...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 890 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Displacement of [N-methyl-3H]GW0438 from human biotinylated LXRalpha ligand binding domain | J Med Chem 51: 5758-65 (2008) Article DOI: 10.1021/jm800612u BindingDB Entry DOI: 10.7270/Q28052FN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM50252853 (4-[(Butyl{4-[2,2,2-trifluoro-1-hydroxy-1-(trifluor...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 955 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Displacement of [N-methyl-3H]GW0438 from human biotinylated LXRalpha ligand binding domain | J Med Chem 51: 5758-65 (2008) Article DOI: 10.1021/jm800612u BindingDB Entry DOI: 10.7270/Q28052FN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 5 group A member 2 (Homo sapiens (Human)) | BDBM22373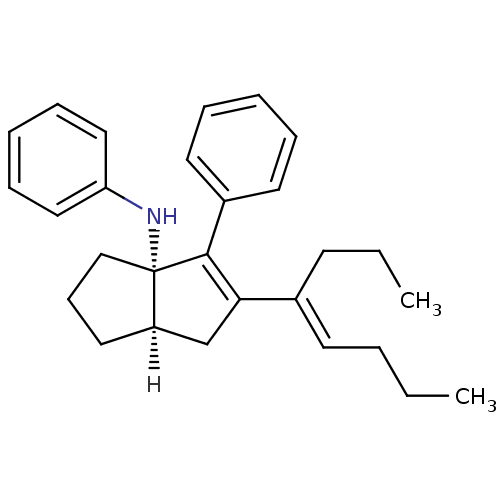 ((3aS,6aR)-5-[(4E)-oct-4-en-4-yl]-N,4-diphenyl-1,2,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | n/a | n/a | n/a | n/a | 430 | n/a | n/a | 7.5 | 22 |
University of Southampton | Assay Description The screen utilizes a ligand mediated co-factor interaction between purified bacterial expressed ligand binding domain of human LRH1 and a TIF2-deriv... | J Med Chem 49: 6652-5 (2006) Article DOI: 10.1021/jm060990k BindingDB Entry DOI: 10.7270/Q2930RGT | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Nuclear receptor subfamily 5 group A member 2 (Homo sapiens (Human)) | BDBM22374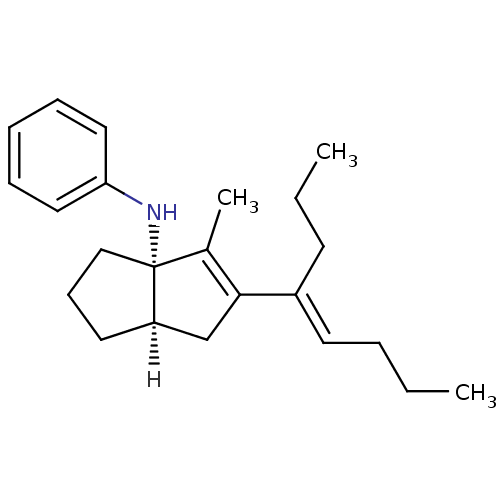 ((3aS,6aR)-4-methyl-5-[(4E)-oct-4-en-4-yl]-N-phenyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 90 | n/a | n/a | 7.5 | 22 |
University of Southampton | Assay Description The screen utilizes a ligand mediated co-factor interaction between purified bacterial expressed ligand binding domain of human LRH1 and a TIF2-deriv... | J Med Chem 49: 6652-5 (2006) Article DOI: 10.1021/jm060990k BindingDB Entry DOI: 10.7270/Q2930RGT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 5 group A member 2 (Homo sapiens (Human)) | BDBM22375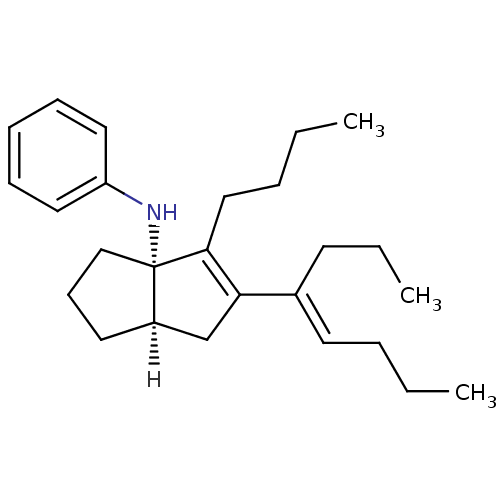 ((3aS,6aR)-4-butyl-5-[(4E)-oct-4-en-4-yl]-N-phenyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 22 | n/a | n/a | 7.5 | 22 |
University of Southampton | Assay Description The screen utilizes a ligand mediated co-factor interaction between purified bacterial expressed ligand binding domain of human LRH1 and a TIF2-deriv... | J Med Chem 49: 6652-5 (2006) Article DOI: 10.1021/jm060990k BindingDB Entry DOI: 10.7270/Q2930RGT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 5 group A member 2 (Homo sapiens (Human)) | BDBM22376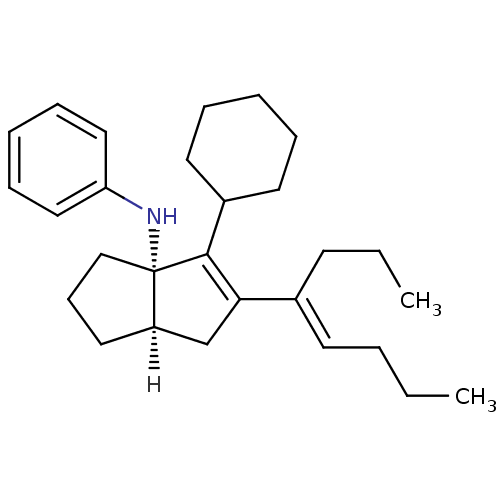 ((3aS,6aR)-4-cyclohexyl-5-[(4E)-oct-4-en-4-yl]-N-ph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 12 | n/a | n/a | 7.5 | 22 |
University of Southampton | Assay Description The screen utilizes a ligand mediated co-factor interaction between purified bacterial expressed ligand binding domain of human LRH1 and a TIF2-deriv... | J Med Chem 49: 6652-5 (2006) Article DOI: 10.1021/jm060990k BindingDB Entry DOI: 10.7270/Q2930RGT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 5 group A member 2 (Homo sapiens (Human)) | BDBM22377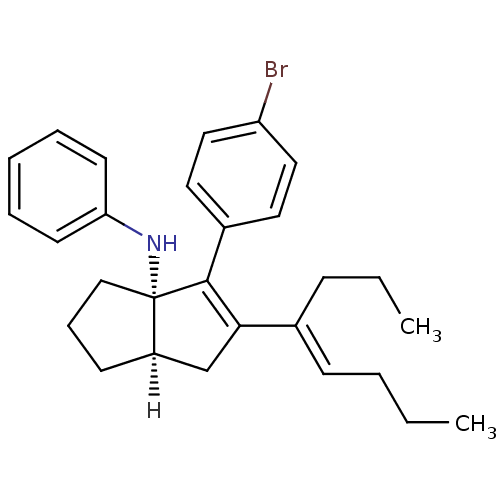 ((3aS,6aR)-4-(4-bromophenyl)-5-[(4E)-oct-4-en-4-yl]...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 160 | n/a | n/a | 7.5 | 22 |
University of Southampton | Assay Description The screen utilizes a ligand mediated co-factor interaction between purified bacterial expressed ligand binding domain of human LRH1 and a TIF2-deriv... | J Med Chem 49: 6652-5 (2006) Article DOI: 10.1021/jm060990k BindingDB Entry DOI: 10.7270/Q2930RGT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 5 group A member 2 (Homo sapiens (Human)) | BDBM22378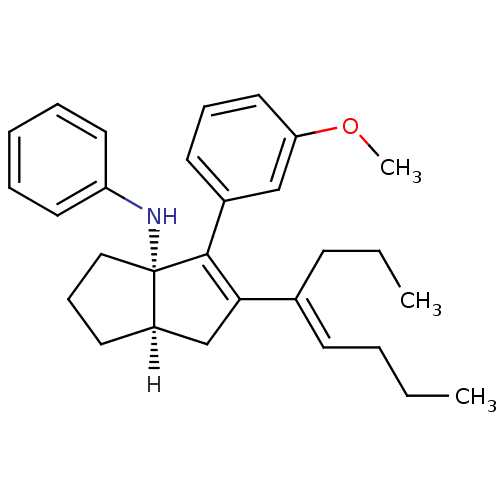 ((3aS,6aR)-4-(3-methoxyphenyl)-5-[(4E)-oct-4-en-4-y...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 100 | n/a | n/a | 7.5 | 22 |
University of Southampton | Assay Description The screen utilizes a ligand mediated co-factor interaction between purified bacterial expressed ligand binding domain of human LRH1 and a TIF2-deriv... | J Med Chem 49: 6652-5 (2006) Article DOI: 10.1021/jm060990k BindingDB Entry DOI: 10.7270/Q2930RGT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 5 group A member 2 (Homo sapiens (Human)) | BDBM22379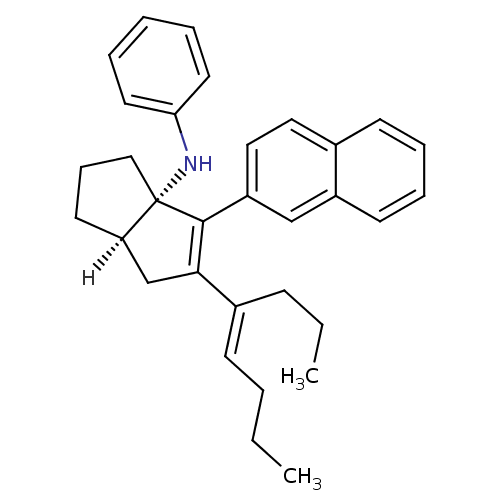 ((3aS,6aR)-4-(naphthalen-2-yl)-5-[(4E)-oct-4-en-4-y...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 60 | n/a | n/a | 7.5 | 22 |
University of Southampton | Assay Description The screen utilizes a ligand mediated co-factor interaction between purified bacterial expressed ligand binding domain of human LRH1 and a TIF2-deriv... | J Med Chem 49: 6652-5 (2006) Article DOI: 10.1021/jm060990k BindingDB Entry DOI: 10.7270/Q2930RGT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 5 group A member 2 (Homo sapiens (Human)) | BDBM22380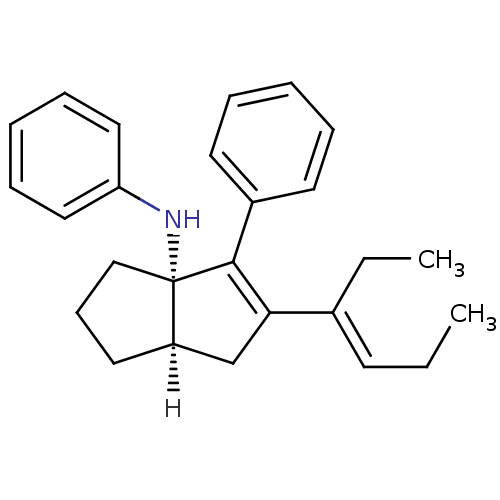 ((3aS,6aR)-5-[(3E)-hex-3-en-3-yl]-N,4-diphenyl-1,2,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.40E+3 | n/a | n/a | 7.5 | 22 |
University of Southampton | Assay Description The screen utilizes a ligand mediated co-factor interaction between purified bacterial expressed ligand binding domain of human LRH1 and a TIF2-deriv... | J Med Chem 49: 6652-5 (2006) Article DOI: 10.1021/jm060990k BindingDB Entry DOI: 10.7270/Q2930RGT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 91 total ) | Next | Last >> |