 Found 1502 hits with Last Name = 'green' and Initial = 'a'
Found 1502 hits with Last Name = 'green' and Initial = 'a' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50214385
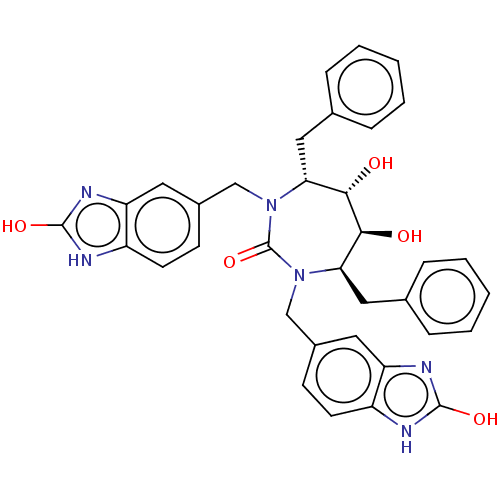
(CHEMBL316681)Show SMILES O[C@@H]1[C@@H](O)[C@@H](Cc2ccccc2)N(Cc2ccc3[nH]c(O)nc3c2)C(=O)N(Cc2ccc3[nH]c(O)nc3c2)[C@@H]1Cc1ccccc1 Show InChI InChI=1S/C35H34N6O5/c42-31-29(17-21-7-3-1-4-8-21)40(19-23-11-13-25-27(15-23)38-33(44)36-25)35(46)41(30(32(31)43)18-22-9-5-2-6-10-22)20-24-12-14-26-28(16-24)39-34(45)37-26/h1-16,29-32,42-43H,17-20H2,(H2,36,38,44)(H2,37,39,45)/t29-,30-,31+,32+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| <0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity was evaluated against HIV protease |
Bioorg Med Chem Lett 6: 2919-2924 (1996)
Article DOI: 10.1016/S0960-894X(96)00531-8
BindingDB Entry DOI: 10.7270/Q2QF8SVC |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50288430

((4R,5S,6S,7R)-4,7-Dibenzyl-5,6-dihydroxy-1,3-bis-(...)Show SMILES O[C@@H]1[C@@H](O)[C@@H](Cc2ccccc2)N(Cc2ccc3c[nH]nc3c2)C(=O)N(Cc2ccc3c[nH]nc3c2)[C@@H]1Cc1ccccc1 Show InChI InChI=1S/C35H34N6O3/c42-33-31(17-23-7-3-1-4-8-23)40(21-25-11-13-27-19-36-38-29(27)15-25)35(44)41(22-26-12-14-28-20-37-39-30(28)16-26)32(34(33)43)18-24-9-5-2-6-10-24/h1-16,19-20,31-34,42-43H,17-18,21-22H2,(H,36,38)(H,37,39)/t31-,32-,33+,34+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.0180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity was evaluated against HIV protease |
Bioorg Med Chem Lett 6: 2919-2924 (1996)
Article DOI: 10.1016/S0960-894X(96)00531-8
BindingDB Entry DOI: 10.7270/Q2QF8SVC |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM161
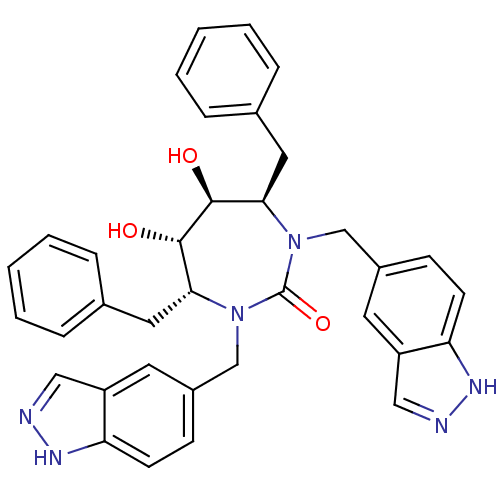
((4R,5S,6S,7R)-4,7-dibenzyl-5,6-dihydroxy-1,3-bis(1...)Show SMILES O[C@@H]1[C@@H](O)[C@@H](Cc2ccccc2)N(Cc2ccc3[nH]ncc3c2)C(=O)N(Cc2ccc3[nH]ncc3c2)[C@@H]1Cc1ccccc1 Show InChI InChI=1S/C35H34N6O3/c42-33-31(17-23-7-3-1-4-8-23)40(21-25-11-13-29-27(15-25)19-36-38-29)35(44)41(22-26-12-14-30-28(16-26)20-37-39-30)32(34(33)43)18-24-9-5-2-6-10-24/h1-16,19-20,31-34,42-43H,17-18,21-22H2,(H,36,38)(H,37,39)/t31-,32-,33+,34+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.0180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity was evaluated against HIV protease |
Bioorg Med Chem Lett 6: 2919-2924 (1996)
Article DOI: 10.1016/S0960-894X(96)00531-8
BindingDB Entry DOI: 10.7270/Q2QF8SVC |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50288424

((4R,5S,6S,7R)-1,3-Bis-(1H-benzotriazol-5-ylmethyl)...)Show SMILES O[C@@H]1[C@@H](O)[C@@H](Cc2ccccc2)N(Cc2ccc3nn[nH]c3c2)C(=O)N(Cc2ccc3nn[nH]c3c2)[C@@H]1Cc1ccccc1 Show InChI InChI=1S/C33H32N8O3/c42-31-29(17-21-7-3-1-4-8-21)40(19-23-11-13-25-27(15-23)36-38-34-25)33(44)41(20-24-12-14-26-28(16-24)37-39-35-26)30(32(31)43)18-22-9-5-2-6-10-22/h1-16,29-32,42-43H,17-20H2,(H,34,36,38)(H,35,37,39)/t29-,30-,31+,32+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity was evaluated against HIV protease |
Bioorg Med Chem Lett 6: 2919-2924 (1996)
Article DOI: 10.1016/S0960-894X(96)00531-8
BindingDB Entry DOI: 10.7270/Q2QF8SVC |
More data for this
Ligand-Target Pair | |
Sigma non-opioid intracellular receptor 1
(RAT) | BDBM50374600
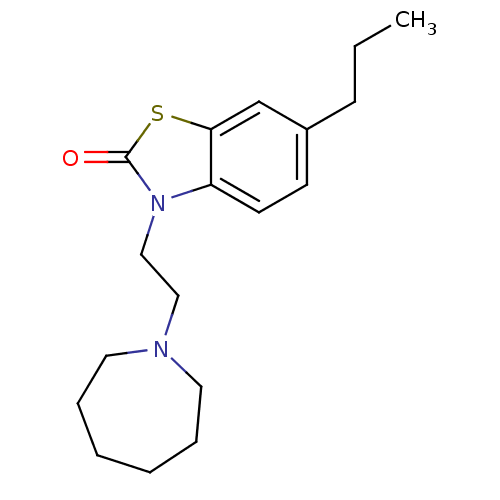
(CHEMBL272899)Show InChI InChI=1S/C18H26N2OS/c1-2-7-15-8-9-16-17(14-15)22-18(21)20(16)13-12-19-10-5-3-4-6-11-19/h8-9,14H,2-7,10-13H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Mississippi
Curated by PDSP Ki Database
| Assay Description
Displacement of [3H](+)-pentazocine from opioid sigma1 receptor in rat brain homogenate |
J Med Chem 51: 1482-6 (2008)
Article DOI: 10.1021/jm701357m
BindingDB Entry DOI: 10.7270/Q2736RTZ |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50288420

(5-[4,7-dibenzyl-5,6-dihydroxy-2-oxo-3-(2-oxo-2,3-d...)Show SMILES O[C@@H]1[C@@H](O)[C@@H](Cc2ccccc2)N(Cc2ccc3NC(=O)Cc3c2)C(=O)N(Cc2ccc3NC(=O)Cc3c2)[C@@H]1Cc1ccccc1 Show InChI InChI=1S/C37H36N4O5/c42-33-19-27-15-25(11-13-29(27)38-33)21-40-31(17-23-7-3-1-4-8-23)35(44)36(45)32(18-24-9-5-2-6-10-24)41(37(40)46)22-26-12-14-30-28(16-26)20-34(43)39-30/h1-16,31-32,35-36,44-45H,17-22H2,(H,38,42)(H,39,43)/t31-,32-,35+,36+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity was evaluated against HIV protease |
Bioorg Med Chem Lett 6: 2919-2924 (1996)
Article DOI: 10.1016/S0960-894X(96)00531-8
BindingDB Entry DOI: 10.7270/Q2QF8SVC |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50288421
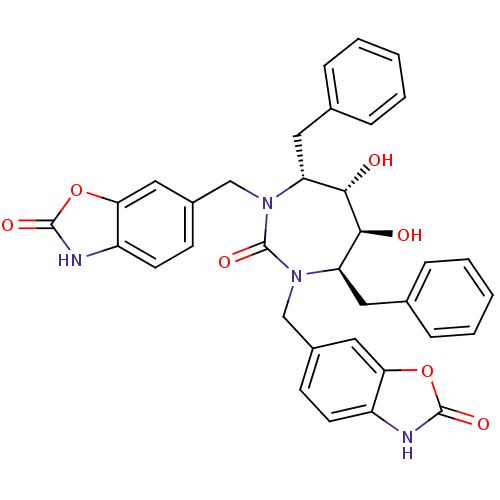
(6-[4,7-dibenzyl-5,6-dihydroxy-2-oxo-3-(2-oxo-2,3-d...)Show SMILES O[C@@H]1[C@@H](O)[C@@H](Cc2ccccc2)N(Cc2ccc3[nH]c(=O)oc3c2)C(=O)N(Cc2ccc3[nH]c(=O)oc3c2)[C@@H]1Cc1ccccc1 Show InChI InChI=1S/C35H32N4O7/c40-31-27(15-21-7-3-1-4-8-21)38(19-23-11-13-25-29(17-23)45-33(42)36-25)35(44)39(28(32(31)41)16-22-9-5-2-6-10-22)20-24-12-14-26-30(18-24)46-34(43)37-26/h1-14,17-18,27-28,31-32,40-41H,15-16,19-20H2,(H,36,42)(H,37,43)/t27-,28-,31+,32+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity was evaluated against HIV protease |
Bioorg Med Chem Lett 6: 2919-2924 (1996)
Article DOI: 10.1016/S0960-894X(96)00531-8
BindingDB Entry DOI: 10.7270/Q2QF8SVC |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50288434
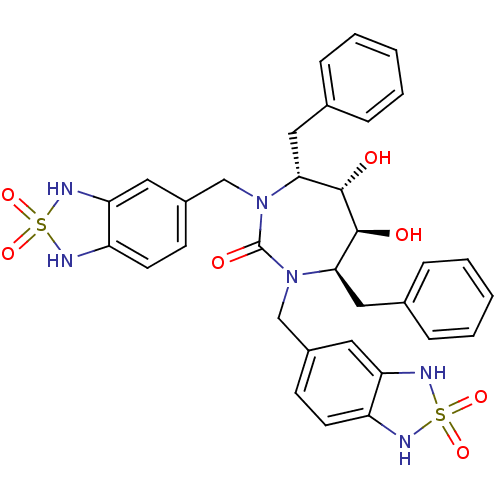
((4R,5S,6S,7R)-4,7-Dibenzyl-1,3-bis-(2,2-dioxo-2,3-...)Show SMILES O[C@@H]1[C@@H](O)[C@@H](Cc2ccccc2)N(Cc2ccc3NS(=O)(=O)Nc3c2)C(=O)N(Cc2ccc3NS(=O)(=O)Nc3c2)[C@@H]1Cc1ccccc1 Show InChI InChI=1S/C33H34N6O7S2/c40-31-29(17-21-7-3-1-4-8-21)38(19-23-11-13-25-27(15-23)36-47(43,44)34-25)33(42)39(30(32(31)41)18-22-9-5-2-6-10-22)20-24-12-14-26-28(16-24)37-48(45,46)35-26/h1-16,29-32,34-37,40-41H,17-20H2/t29-,30-,31+,32+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
| 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity was evaluated against HIV protease |
Bioorg Med Chem Lett 6: 2919-2924 (1996)
Article DOI: 10.1016/S0960-894X(96)00531-8
BindingDB Entry DOI: 10.7270/Q2QF8SVC |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50288431

((4R,5S,6S,7R)-1,3-Bis-(2-amino-benzooxazol-6-ylmet...)Show SMILES Nc1nc2ccc(CN3[C@H](Cc4ccccc4)[C@H](O)[C@@H](O)[C@@H](Cc4ccccc4)N(Cc4ccc5nc(N)oc5c4)C3=O)cc2o1 Show InChI InChI=1S/C35H34N6O5/c36-33-38-25-13-11-23(17-29(25)45-33)19-40-27(15-21-7-3-1-4-8-21)31(42)32(43)28(16-22-9-5-2-6-10-22)41(35(40)44)20-24-12-14-26-30(18-24)46-34(37)39-26/h1-14,17-18,27-28,31-32,42-43H,15-16,19-20H2,(H2,36,38)(H2,37,39)/t27-,28-,31+,32+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity was evaluated against HIV protease |
Bioorg Med Chem Lett 6: 2919-2924 (1996)
Article DOI: 10.1016/S0960-894X(96)00531-8
BindingDB Entry DOI: 10.7270/Q2QF8SVC |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50334730
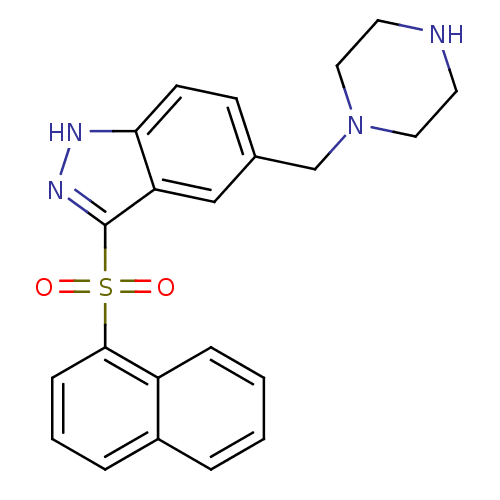
(3-(Naphthalen-1-ylsulfonyl)-5-(piperazin-1-ylmethy...)Show SMILES O=S(=O)(c1n[nH]c2ccc(CN3CCNCC3)cc12)c1cccc2ccccc12 Show InChI InChI=1S/C22H22N4O2S/c27-29(28,21-7-3-5-17-4-1-2-6-18(17)21)22-19-14-16(8-9-20(19)24-25-22)15-26-12-10-23-11-13-26/h1-9,14,23H,10-13,15H2,(H,24,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]-LSD from cloned human 5-HT6 receptor expressed in human HeLa cells |
Bioorg Med Chem 19: 650-62 (2011)
Article DOI: 10.1016/j.bmc.2010.10.033
BindingDB Entry DOI: 10.7270/Q29S1R9V |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM150
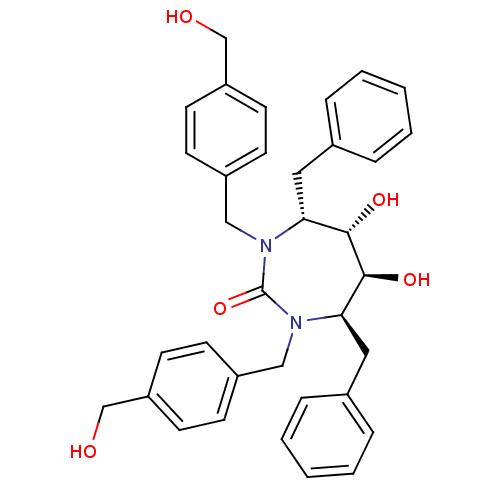
((4R,5S,6S,7R)-4,7-dibenzyl-5,6-dihydroxy-1,3-bis({...)Show SMILES OCc1ccc(CN2[C@H](Cc3ccccc3)[C@H](O)[C@@H](O)[C@@H](Cc3ccccc3)N(Cc3ccc(CO)cc3)C2=O)cc1 Show InChI InChI=1S/C35H38N2O5/c38-23-29-15-11-27(12-16-29)21-36-31(19-25-7-3-1-4-8-25)33(40)34(41)32(20-26-9-5-2-6-10-26)37(35(36)42)22-28-13-17-30(24-39)18-14-28/h1-18,31-34,38-41H,19-24H2/t31-,32-,33+,34+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
| 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity was evaluated against HIV protease |
Bioorg Med Chem Lett 6: 2919-2924 (1996)
Article DOI: 10.1016/S0960-894X(96)00531-8
BindingDB Entry DOI: 10.7270/Q2QF8SVC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50302220
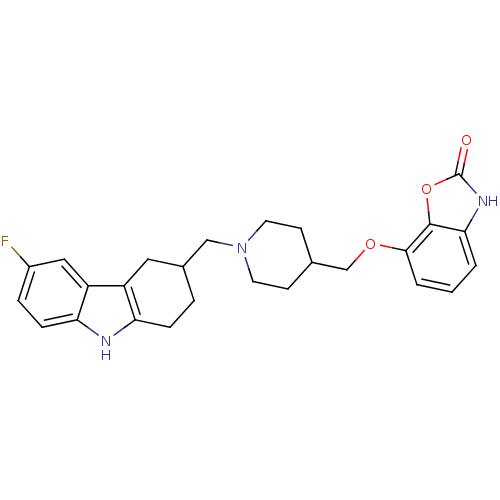
(7-((1-((6-fluoro-2,3,4,9-tetrahydro-1H-carbazol-3-...)Show SMILES Fc1ccc2[nH]c3CCC(CN4CCC(COc5cccc6[nH]c(=O)oc56)CC4)Cc3c2c1 Show InChI InChI=1S/C26H28FN3O3/c27-18-5-7-22-20(13-18)19-12-17(4-6-21(19)28-22)14-30-10-8-16(9-11-30)15-32-24-3-1-2-23-25(24)33-26(31)29-23/h1-3,5,7,13,16-17,28H,4,6,8-12,14-15H2,(H,29,31) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]citalopram from human serotonin transporter expressed in HEK293 cells by scintillation proximity assay |
Bioorg Med Chem Lett 19: 5552-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.08.050
BindingDB Entry DOI: 10.7270/Q2Z89DCC |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM335426
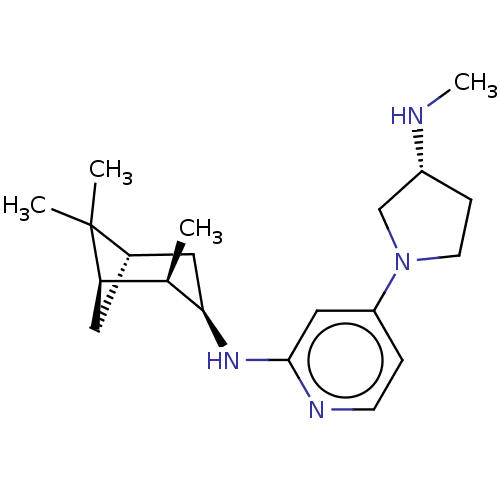
(4-[(3R)-3-(Methylamino)pyrrolidin-1-yl]-N-[(1R,2R,...)Show SMILES CN[C@@H]1CCN(C1)c1ccnc(N[C@@H]2C[C@@H]3C[C@H]([C@H]2C)C3(C)C)c1 |r| Show InChI InChI=1S/C20H32N4/c1-13-17-9-14(20(17,2)3)10-18(13)23-19-11-16(5-7-22-19)24-8-6-15(12-24)21-4/h5,7,11,13-15,17-18,21H,6,8-10,12H2,1-4H3,(H,22,23)/t13-,14+,15-,17-,18-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
JANSSEN PHARMACEUTICA N.V.
US Patent
| Assay Description
Cell pellets from SK-N-MC cells stably or transiently transfected with human H4 receptor (NCBI accession No. AF312230) were used for the binding assa... |
US Patent US9732087 (2017)
BindingDB Entry DOI: 10.7270/Q2251M9T |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50334734
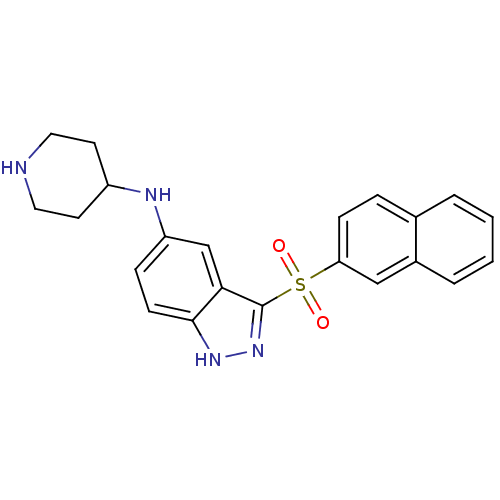
(3-(Naphthalen-2-ylsulfonyl)-N-(piperidin-4-yl)-1H-...)Show SMILES O=S(=O)(c1n[nH]c2ccc(NC3CCNCC3)cc12)c1ccc2ccccc2c1 Show InChI InChI=1S/C22H22N4O2S/c27-29(28,19-7-5-15-3-1-2-4-16(15)13-19)22-20-14-18(6-8-21(20)25-26-22)24-17-9-11-23-12-10-17/h1-8,13-14,17,23-24H,9-12H2,(H,25,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]-LSD from cloned human 5-HT6 receptor expressed in human HeLa cells |
Bioorg Med Chem 19: 650-62 (2011)
Article DOI: 10.1016/j.bmc.2010.10.033
BindingDB Entry DOI: 10.7270/Q29S1R9V |
More data for this
Ligand-Target Pair | |
Alpha-1A adrenergic receptor
(Rattus norvegicus (Rat)) | BDBM81444
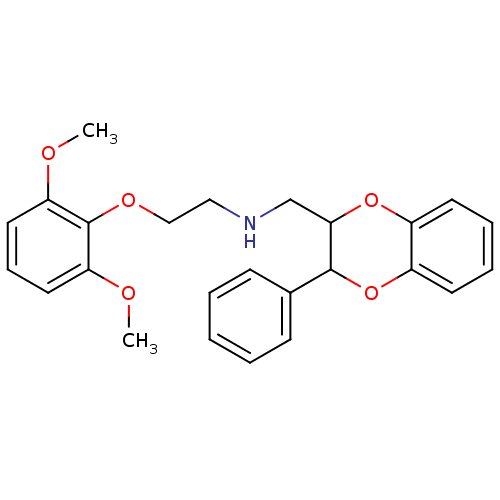
(CAS_185453 | NSC_185453 | WB 4101 | WB-4101)Show InChI InChI=1S/C25H27NO5/c1-27-21-13-8-14-22(28-2)25(21)29-16-15-26-17-23-24(18-9-4-3-5-10-18)31-20-12-7-6-11-19(20)30-23/h3-14,23-24,26H,15-17H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by PDSP Ki Database
| |
Mol Pharmacol 13: 454-73 (1977)
BindingDB Entry DOI: 10.7270/Q2TT4PFZ |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Rattus norvegicus (rat)) | BDBM81444

(CAS_185453 | NSC_185453 | WB 4101 | WB-4101)Show InChI InChI=1S/C25H27NO5/c1-27-21-13-8-14-22(28-2)25(21)29-16-15-26-17-23-24(18-9-4-3-5-10-18)31-20-12-7-6-11-19(20)30-23/h3-14,23-24,26H,15-17H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by PDSP Ki Database
| |
Life Sci 19: 69-76 (1976)
Article DOI: 10.1016/0024-3205(76)90375-1
BindingDB Entry DOI: 10.7270/Q23B5XNK |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50334731
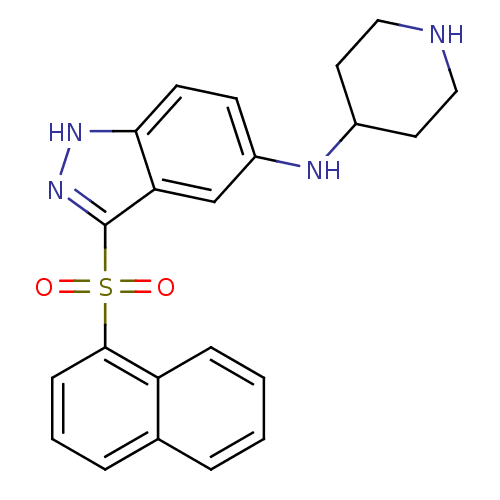
(CHEMBL1642851 | [3-(Naphthalen-1-sulfonyl)-1H-inda...)Show SMILES O=S(=O)(c1n[nH]c2ccc(NC3CCNCC3)cc12)c1cccc2ccccc12 Show InChI InChI=1S/C22H22N4O2S/c27-29(28,21-7-3-5-15-4-1-2-6-18(15)21)22-19-14-17(8-9-20(19)25-26-22)24-16-10-12-23-13-11-16/h1-9,14,16,23-24H,10-13H2,(H,25,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]-LSD from cloned human 5-HT6 receptor expressed in human HeLa cells |
Bioorg Med Chem 19: 650-62 (2011)
Article DOI: 10.1016/j.bmc.2010.10.033
BindingDB Entry DOI: 10.7270/Q29S1R9V |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50334754
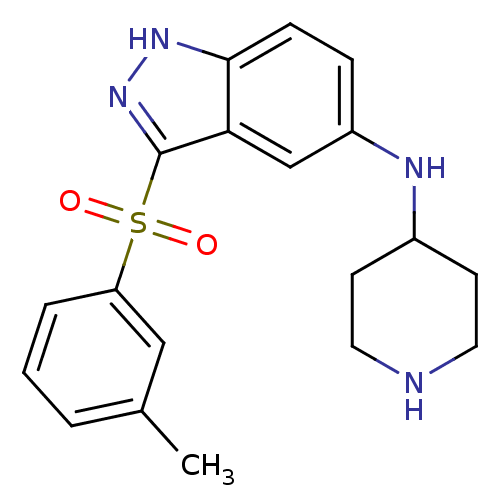
(CHEMBL1642883 | N-(Piperidin-4-yl)-3-(m-tolylsulfo...)Show SMILES Cc1cccc(c1)S(=O)(=O)c1n[nH]c2ccc(NC3CCNCC3)cc12 Show InChI InChI=1S/C19H22N4O2S/c1-13-3-2-4-16(11-13)26(24,25)19-17-12-15(5-6-18(17)22-23-19)21-14-7-9-20-10-8-14/h2-6,11-12,14,20-21H,7-10H2,1H3,(H,22,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]-LSD from cloned human 5-HT6 receptor expressed in human HeLa cells |
Bioorg Med Chem 19: 650-62 (2011)
Article DOI: 10.1016/j.bmc.2010.10.033
BindingDB Entry DOI: 10.7270/Q29S1R9V |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50288416
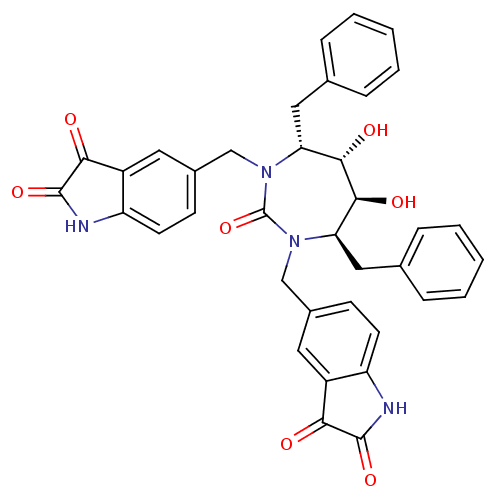
(5-[4,7-dibenzyl-3-(2,3-dioxo-2,3-dihydro-1H-5-indo...)Show SMILES O[C@@H]1[C@@H](O)[C@@H](Cc2ccccc2)N(Cc2ccc3NC(=O)C(=O)c3c2)C(=O)N(Cc2ccc3NC(=O)C(=O)c3c2)[C@@H]1Cc1ccccc1 Show InChI InChI=1S/C37H32N4O7/c42-31-25-15-23(11-13-27(25)38-35(31)46)19-40-29(17-21-7-3-1-4-8-21)33(44)34(45)30(18-22-9-5-2-6-10-22)41(37(40)48)20-24-12-14-28-26(16-24)32(43)36(47)39-28/h1-16,29-30,33-34,44-45H,17-20H2,(H,38,42,46)(H,39,43,47)/t29-,30-,33+,34+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.810 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity was evaluated against HIV protease |
Bioorg Med Chem Lett 6: 2919-2924 (1996)
Article DOI: 10.1016/S0960-894X(96)00531-8
BindingDB Entry DOI: 10.7270/Q2QF8SVC |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50288419
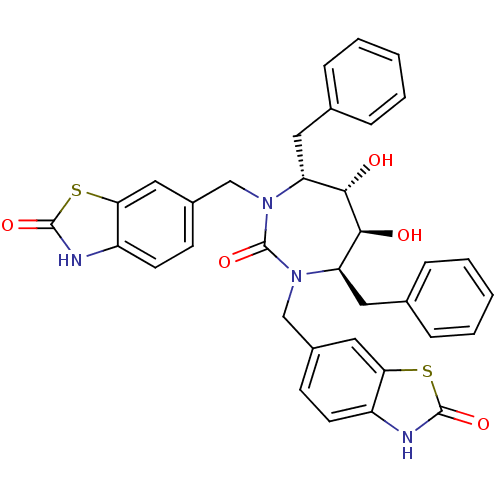
(6-[4,7-dibenzyl-5,6-dihydroxy-2-oxo-3-(2-oxo-2,3-d...)Show SMILES O[C@@H]1[C@@H](O)[C@@H](Cc2ccccc2)N(Cc2ccc3[nH]c(=O)sc3c2)C(=O)N(Cc2ccc3[nH]c(=O)sc3c2)[C@@H]1Cc1ccccc1 Show InChI InChI=1S/C35H32N4O5S2/c40-31-27(15-21-7-3-1-4-8-21)38(19-23-11-13-25-29(17-23)45-33(42)36-25)35(44)39(28(32(31)41)16-22-9-5-2-6-10-22)20-24-12-14-26-30(18-24)46-34(43)37-26/h1-14,17-18,27-28,31-32,40-41H,15-16,19-20H2,(H,36,42)(H,37,43)/t27-,28-,31+,32+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.910 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity was evaluated against HIV protease |
Bioorg Med Chem Lett 6: 2919-2924 (1996)
Article DOI: 10.1016/S0960-894X(96)00531-8
BindingDB Entry DOI: 10.7270/Q2QF8SVC |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50334733
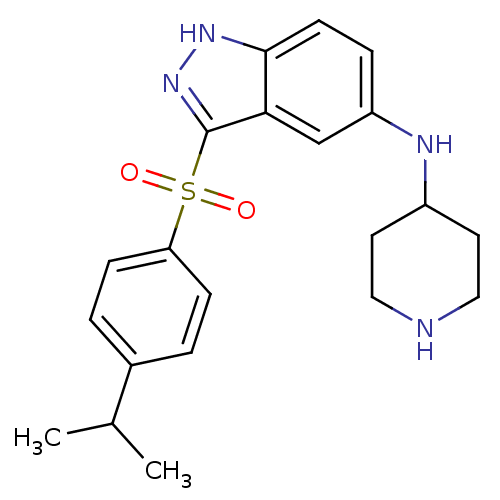
(3-(4-Isopropylphenylsulfonyl)-N-(piperidin-4-yl)-1...)Show SMILES CC(C)c1ccc(cc1)S(=O)(=O)c1n[nH]c2ccc(NC3CCNCC3)cc12 Show InChI InChI=1S/C21H26N4O2S/c1-14(2)15-3-6-18(7-4-15)28(26,27)21-19-13-17(5-8-20(19)24-25-21)23-16-9-11-22-12-10-16/h3-8,13-14,16,22-23H,9-12H2,1-2H3,(H,24,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]-LSD from cloned human 5-HT6 receptor expressed in human HeLa cells |
Bioorg Med Chem 19: 650-62 (2011)
Article DOI: 10.1016/j.bmc.2010.10.033
BindingDB Entry DOI: 10.7270/Q29S1R9V |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50061008
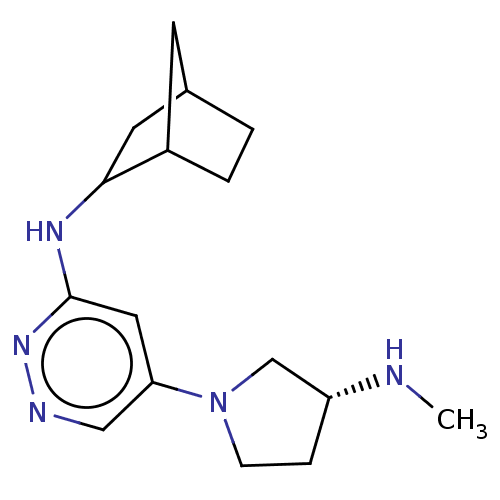
(CHEMBL3393556 | US9732087, 96)Show InChI InChI=1S/C16H25N5/c1-17-13-4-5-21(10-13)14-8-16(20-18-9-14)19-15-7-11-2-3-12(15)6-11/h8-9,11-13,15,17H,2-7,10H2,1H3,(H,19,20)/t11?,12?,13-,15?/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| US Patent
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
JANSSEN PHARMACEUTICA N.V.
US Patent
| Assay Description
Cell pellets from SK-N-MC cells stably or transiently transfected with human H4 receptor (NCBI accession No. AF312230) were used for the binding assa... |
US Patent US9732087 (2017)
BindingDB Entry DOI: 10.7270/Q2251M9T |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM335425
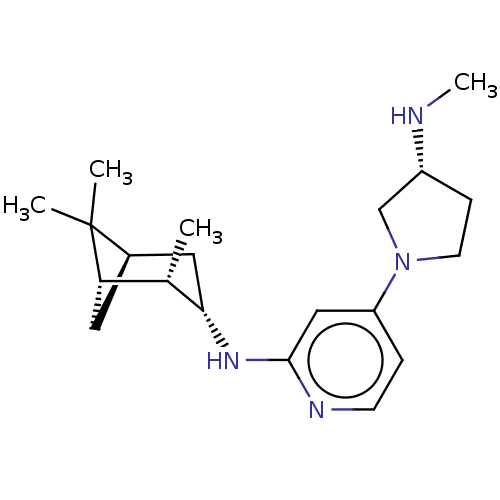
(4-[(3R)-3-(Methylamino)pyrrolidin-1-yl]-N-[(1S,2S,...)Show SMILES CN[C@@H]1CCN(C1)c1ccnc(N[C@H]2C[C@H]3C[C@@H]([C@@H]2C)C3(C)C)c1 |r| Show InChI InChI=1S/C20H32N4/c1-13-17-9-14(20(17,2)3)10-18(13)23-19-11-16(5-7-22-19)24-8-6-15(12-24)21-4/h5,7,11,13-15,17-18,21H,6,8-10,12H2,1-4H3,(H,22,23)/t13-,14+,15+,17-,18-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
JANSSEN PHARMACEUTICA N.V.
US Patent
| Assay Description
Cell pellets from SK-N-MC cells stably or transiently transfected with human H4 receptor (NCBI accession No. AF312230) were used for the binding assa... |
US Patent US9732087 (2017)
BindingDB Entry DOI: 10.7270/Q2251M9T |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50061098

(CHEMBL3393534 | US9732087, 1)Show InChI InChI=1S/C17H26N4/c1-18-14-5-7-21(11-14)15-4-6-19-17(10-15)20-16-9-12-2-3-13(16)8-12/h4,6,10,12-14,16,18H,2-3,5,7-9,11H2,1H3,(H,19,20)/t12?,13?,14-,16?/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| US Patent
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
JANSSEN PHARMACEUTICA N.V.
US Patent
| Assay Description
Cell pellets from SK-N-MC cells stably or transiently transfected with human H4 receptor (NCBI accession No. AF312230) were used for the binding assa... |
US Patent US9732087 (2017)
BindingDB Entry DOI: 10.7270/Q2251M9T |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50334732
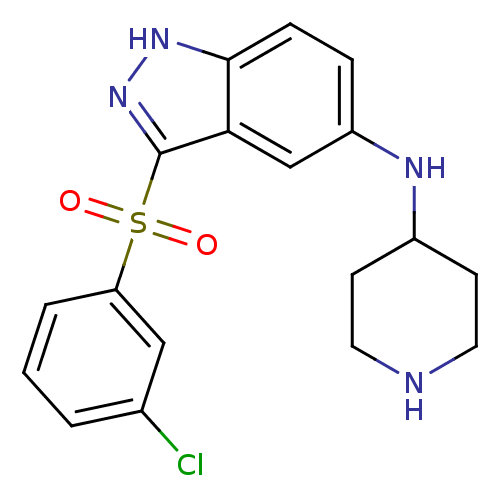
(3-(3-Chlorophenylsulfonyl)-N-(piperidin-4-yl)-1H-i...)Show SMILES Clc1cccc(c1)S(=O)(=O)c1n[nH]c2ccc(NC3CCNCC3)cc12 Show InChI InChI=1S/C18H19ClN4O2S/c19-12-2-1-3-15(10-12)26(24,25)18-16-11-14(4-5-17(16)22-23-18)21-13-6-8-20-9-7-13/h1-5,10-11,13,20-21H,6-9H2,(H,22,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]-LSD from cloned human 5-HT6 receptor expressed in human HeLa cells |
Bioorg Med Chem 19: 650-62 (2011)
Article DOI: 10.1016/j.bmc.2010.10.033
BindingDB Entry DOI: 10.7270/Q29S1R9V |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50302225
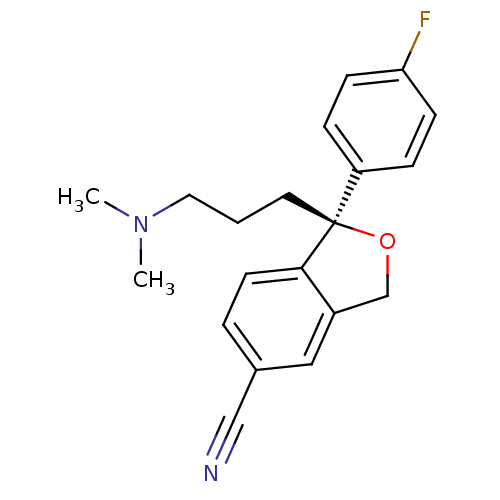
((1S)-1-[3-(dimethylamino)propyl]-1-(4-fluorophenyl...)Show SMILES CN(C)CCC[C@]1(OCc2cc(ccc12)C#N)c1ccc(F)cc1 |r| Show InChI InChI=1S/C20H21FN2O/c1-23(2)11-3-10-20(17-5-7-18(21)8-6-17)19-9-4-15(13-22)12-16(19)14-24-20/h4-9,12H,3,10-11,14H2,1-2H3/t20-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]citalopram from human serotonin transporter expressed in HEK293 cells by scintillation proximity assay |
Bioorg Med Chem Lett 19: 5552-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.08.050
BindingDB Entry DOI: 10.7270/Q2Z89DCC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50334725
(CHEMBL1642866 | N-(3-(Naphthalen-1-ylsulfonyl)-1H-...)Show SMILES O=C(Nc1ccc2[nH]nc(c2c1)S(=O)(=O)c1cccc2ccccc12)C1CCNCC1 Show InChI InChI=1S/C23H22N4O3S/c28-22(16-10-12-24-13-11-16)25-17-8-9-20-19(14-17)23(27-26-20)31(29,30)21-7-3-5-15-4-1-2-6-18(15)21/h1-9,14,16,24H,10-13H2,(H,25,28)(H,26,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]-LSD from cloned human 5-HT6 receptor expressed in human HeLa cells |
Bioorg Med Chem 19: 650-62 (2011)
Article DOI: 10.1016/j.bmc.2010.10.033
BindingDB Entry DOI: 10.7270/Q29S1R9V |
More data for this
Ligand-Target Pair | |
Sigma non-opioid intracellular receptor 1
(RAT) | BDBM50374598
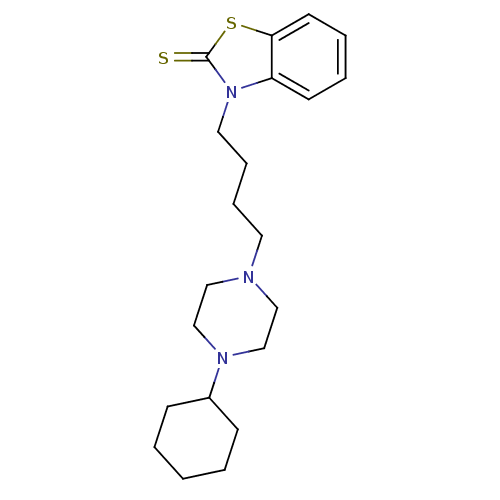
(CHEMBL272603 | US9604926, Compound CM-156 | US9724...)Show InChI InChI=1S/C21H31N3S2/c25-21-24(19-10-4-5-11-20(19)26-21)13-7-6-12-22-14-16-23(17-15-22)18-8-2-1-3-9-18/h4-5,10-11,18H,1-3,6-9,12-17H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 1.28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Mississippi
Curated by PDSP Ki Database
| Assay Description
Displacement of [3H](+)-pentazocine from opioid sigma1 receptor in rat brain homogenate |
J Med Chem 51: 1482-6 (2008)
Article DOI: 10.1021/jm701357m
BindingDB Entry DOI: 10.7270/Q2736RTZ |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50288433
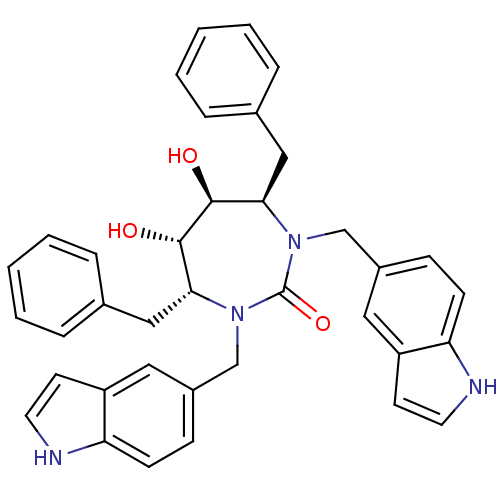
((4R,5S,6S,7R)-4,7-Dibenzyl-5,6-dihydroxy-1,3-bis-(...)Show SMILES O[C@@H]1[C@@H](O)[C@@H](Cc2ccccc2)N(Cc2ccc3[nH]ccc3c2)C(=O)N(Cc2ccc3[nH]ccc3c2)[C@@H]1Cc1ccccc1 Show InChI InChI=1S/C37H36N4O3/c42-35-33(21-25-7-3-1-4-8-25)40(23-27-11-13-31-29(19-27)15-17-38-31)37(44)41(24-28-12-14-32-30(20-28)16-18-39-32)34(36(35)43)22-26-9-5-2-6-10-26/h1-20,33-36,38-39,42-43H,21-24H2/t33-,34-,35+,36+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity was evaluated against HIV protease |
Bioorg Med Chem Lett 6: 2919-2924 (1996)
Article DOI: 10.1016/S0960-894X(96)00531-8
BindingDB Entry DOI: 10.7270/Q2QF8SVC |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50334747
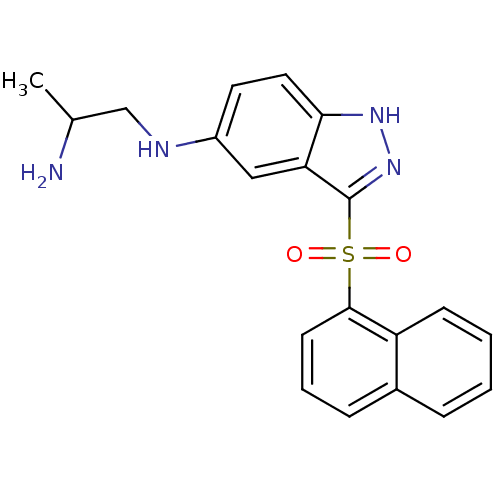
(CHEMBL1642849 | N1-(3-(Naphthalen-1-ylsulfonyl)-1H...)Show SMILES CC(N)CNc1ccc2[nH]nc(c2c1)S(=O)(=O)c1cccc2ccccc12 Show InChI InChI=1S/C20H20N4O2S/c1-13(21)12-22-15-9-10-18-17(11-15)20(24-23-18)27(25,26)19-8-4-6-14-5-2-3-7-16(14)19/h2-11,13,22H,12,21H2,1H3,(H,23,24) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]-LSD from cloned human 5-HT6 receptor expressed in human HeLa cells |
Bioorg Med Chem 19: 650-62 (2011)
Article DOI: 10.1016/j.bmc.2010.10.033
BindingDB Entry DOI: 10.7270/Q29S1R9V |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Rattus norvegicus (rat)) | BDBM30712
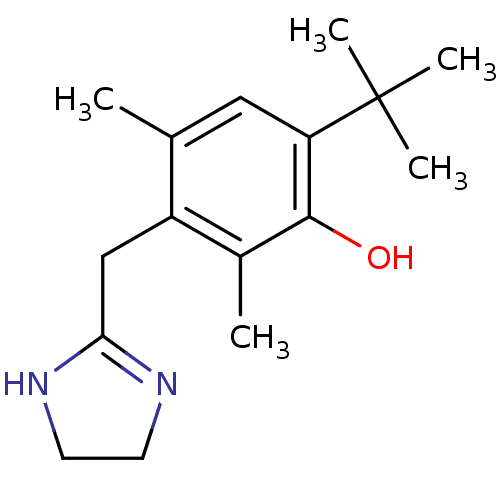
(6-tert-butyl-3-(2-imidazolin-2-ylmethyl)-2,4-dimet...)Show InChI InChI=1S/C16H24N2O/c1-10-8-13(16(3,4)5)15(19)11(2)12(10)9-14-17-6-7-18-14/h8,19H,6-7,9H2,1-5H3,(H,17,18) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
DrugBank
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by PDSP Ki Database
| |
Life Sci 19: 69-76 (1976)
Article DOI: 10.1016/0024-3205(76)90375-1
BindingDB Entry DOI: 10.7270/Q23B5XNK |
More data for this
Ligand-Target Pair | |
Sigma non-opioid intracellular receptor 1
(RAT) | BDBM50048866

(1-Cyclohexyl-4-[3-(5-methoxy-1,2,3,4-tetrahydro-na...)Show InChI InChI=1S/C24H38N2O/c1-27-24-14-6-12-22-20(8-5-13-23(22)24)9-7-15-25-16-18-26(19-17-25)21-10-3-2-4-11-21/h6,12,14,20-21H,2-5,7-11,13,15-19H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 1.48 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Mississippi
Curated by PDSP Ki Database
| Assay Description
Displacement of [3H](+)-pentazocine from opioid sigma1 receptor in rat brain homogenate |
J Med Chem 51: 1482-6 (2008)
Article DOI: 10.1021/jm701357m
BindingDB Entry DOI: 10.7270/Q2736RTZ |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
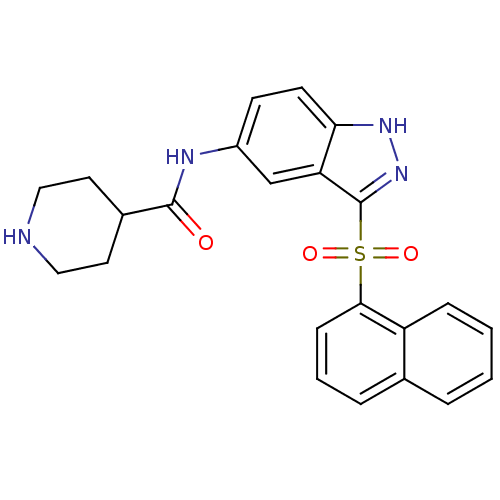
(Homo sapiens (Human)) | BDBM50334720
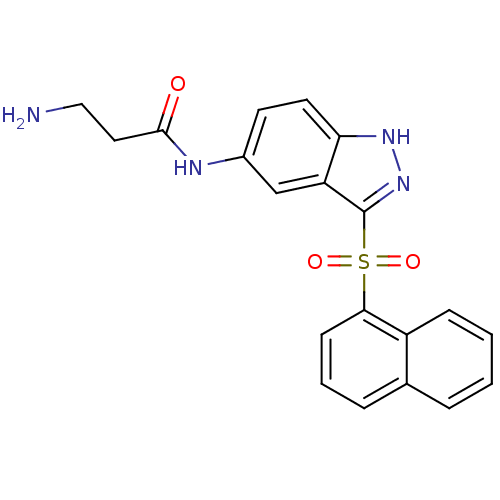
(3-Amino-N-[3-(naphthalen-1-sulfonyl)-1H-indazol-5-...)Show SMILES NCCC(=O)Nc1ccc2[nH]nc(c2c1)S(=O)(=O)c1cccc2ccccc12 Show InChI InChI=1S/C20H18N4O3S/c21-11-10-19(25)22-14-8-9-17-16(12-14)20(24-23-17)28(26,27)18-7-3-5-13-4-1-2-6-15(13)18/h1-9,12H,10-11,21H2,(H,22,25)(H,23,24) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]-LSD from cloned human 5-HT6 receptor expressed in human HeLa cells |
Bioorg Med Chem 19: 650-62 (2011)
Article DOI: 10.1016/j.bmc.2010.10.033
BindingDB Entry DOI: 10.7270/Q29S1R9V |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50334757
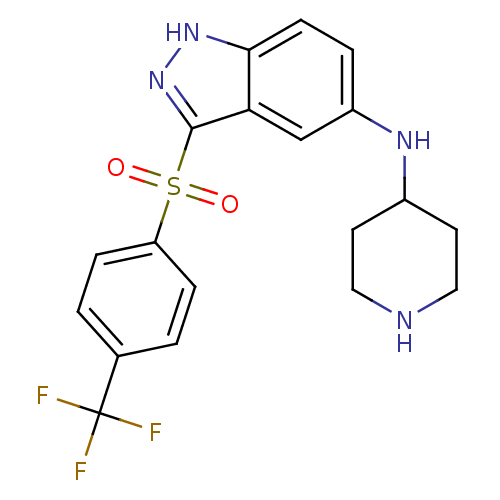
(CHEMBL1642887 | N-(Piperidin-4-yl)-3-(4-(trifluoro...)Show SMILES FC(F)(F)c1ccc(cc1)S(=O)(=O)c1n[nH]c2ccc(NC3CCNCC3)cc12 Show InChI InChI=1S/C19H19F3N4O2S/c20-19(21,22)12-1-4-15(5-2-12)29(27,28)18-16-11-14(3-6-17(16)25-26-18)24-13-7-9-23-10-8-13/h1-6,11,13,23-24H,7-10H2,(H,25,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]-LSD from cloned human 5-HT6 receptor expressed in human HeLa cells |
Bioorg Med Chem 19: 650-62 (2011)
Article DOI: 10.1016/j.bmc.2010.10.033
BindingDB Entry DOI: 10.7270/Q29S1R9V |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50299343

((2S,4R)-1-((R)-5-chloro-1-(2,4-dimethoxyphenylsulf...)Show SMILES COc1ccc(c(OC)c1)S(=O)(=O)N1C(=O)[C@@](N2C[C@H](O)C[C@H]2C(=O)N(C)C)(c2cc(Cl)ccc12)c1ccccc1OC |r| Show InChI InChI=1S/C30H32ClN3O8S/c1-32(2)28(36)24-15-19(35)17-33(24)30(21-8-6-7-9-25(21)41-4)22-14-18(31)10-12-23(22)34(29(30)37)43(38,39)27-13-11-20(40-3)16-26(27)42-5/h6-14,16,19,24,35H,15,17H2,1-5H3/t19-,24+,30+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.51 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Antagonist activity at recombinant oxytocin receptor expressed in CHO cells assessed as inhibition of vasopressin-induced calcium release after 10 mi... |
Bioorg Med Chem Lett 21: 92-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.11.061
BindingDB Entry DOI: 10.7270/Q2639R01 |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50061143
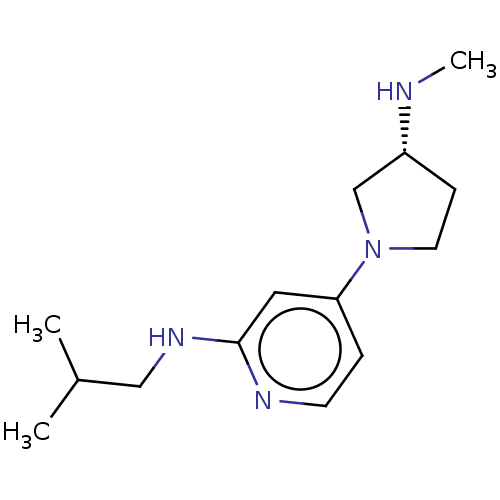
(CHEMBL3393526 | US9732087, 22)Show InChI InChI=1S/C14H24N4/c1-11(2)9-17-14-8-13(4-6-16-14)18-7-5-12(10-18)15-3/h4,6,8,11-12,15H,5,7,9-10H2,1-3H3,(H,16,17)/t12-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| US Patent
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
JANSSEN PHARMACEUTICA N.V.
US Patent
| Assay Description
Cell pellets from SK-N-MC cells stably or transiently transfected with human H4 receptor (NCBI accession No. AF312230) were used for the binding assa... |
US Patent US9732087 (2017)
BindingDB Entry DOI: 10.7270/Q2251M9T |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50334716
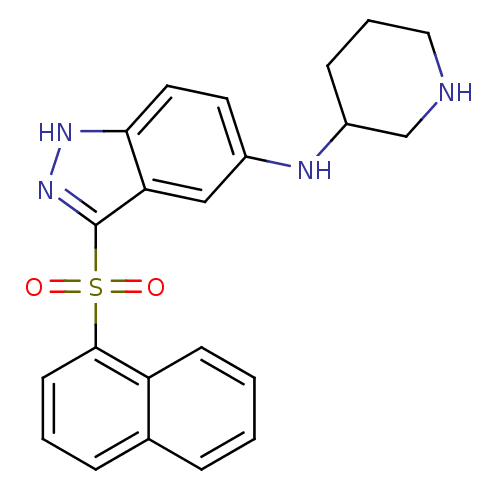
(3-(Naphthalen-1-ylsulfonyl)-N-(piperidin-5-yl)-1H-...)Show SMILES O=S(=O)(c1n[nH]c2ccc(NC3CCCNC3)cc12)c1cccc2ccccc12 Show InChI InChI=1S/C22H22N4O2S/c27-29(28,21-9-3-6-15-5-1-2-8-18(15)21)22-19-13-16(10-11-20(19)25-26-22)24-17-7-4-12-23-14-17/h1-3,5-6,8-11,13,17,23-24H,4,7,12,14H2,(H,25,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]-LSD from cloned human 5-HT6 receptor expressed in human HeLa cells |
Bioorg Med Chem 19: 650-62 (2011)
Article DOI: 10.1016/j.bmc.2010.10.033
BindingDB Entry DOI: 10.7270/Q29S1R9V |
More data for this
Ligand-Target Pair | |
Alpha-1A adrenergic receptor
(Rattus norvegicus (Rat)) | BDBM30712

(6-tert-butyl-3-(2-imidazolin-2-ylmethyl)-2,4-dimet...)Show InChI InChI=1S/C16H24N2O/c1-10-8-13(16(3,4)5)15(19)11(2)12(10)9-14-17-6-7-18-14/h8,19H,6-7,9H2,1-5H3,(H,17,18) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
DrugBank
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by PDSP Ki Database
| |
Mol Pharmacol 13: 454-73 (1977)
BindingDB Entry DOI: 10.7270/Q2TT4PFZ |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50061056
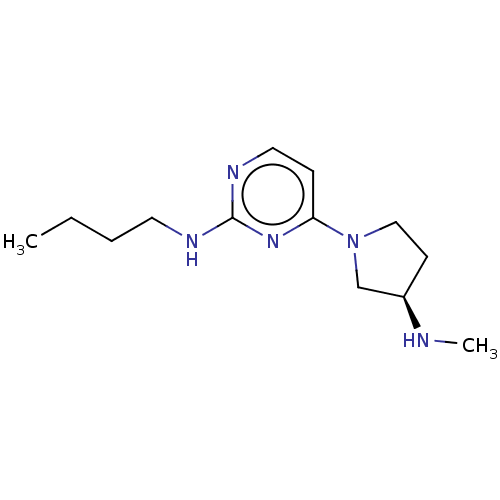
(CHEMBL3393542 | US9732087, 73)Show InChI InChI=1S/C13H23N5/c1-3-4-7-15-13-16-8-5-12(17-13)18-9-6-11(10-18)14-2/h5,8,11,14H,3-4,6-7,9-10H2,1-2H3,(H,15,16,17)/t11-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| US Patent
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
JANSSEN PHARMACEUTICA N.V.
US Patent
| Assay Description
Cell pellets from SK-N-MC cells stably or transiently transfected with human H4 receptor (NCBI accession No. AF312230) were used for the binding assa... |
US Patent US9732087 (2017)
BindingDB Entry DOI: 10.7270/Q2251M9T |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50302213
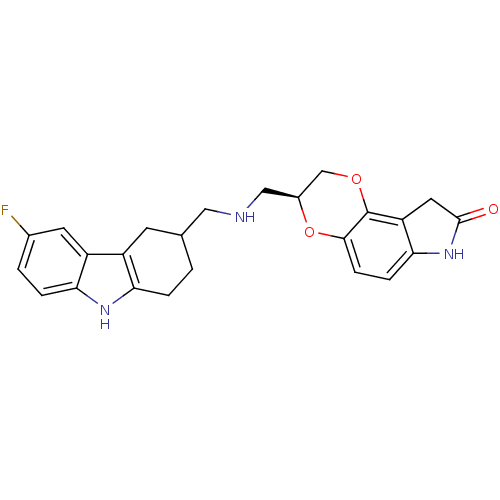
((3S)-3-(((6-fluoro-2,3,4,9-tetrahydro-1H-carbazol-...)Show SMILES Fc1ccc2[nH]c3CCC(CNC[C@H]4COc5c6CC(=O)Nc6ccc5O4)Cc3c2c1 |r| Show InChI InChI=1S/C24H24FN3O3/c25-14-2-4-20-17(8-14)16-7-13(1-3-19(16)27-20)10-26-11-15-12-30-24-18-9-23(29)28-21(18)5-6-22(24)31-15/h2,4-6,8,13,15,26-27H,1,3,7,9-12H2,(H,28,29)/t13?,15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Agonist activity at 5-HT2A receptor |
Bioorg Med Chem Lett 19: 5552-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.08.050
BindingDB Entry DOI: 10.7270/Q2Z89DCC |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50302213

((3S)-3-(((6-fluoro-2,3,4,9-tetrahydro-1H-carbazol-...)Show SMILES Fc1ccc2[nH]c3CCC(CNC[C@H]4COc5c6CC(=O)Nc6ccc5O4)Cc3c2c1 |r| Show InChI InChI=1S/C24H24FN3O3/c25-14-2-4-20-17(8-14)16-7-13(1-3-19(16)27-20)10-26-11-15-12-30-24-18-9-23(29)28-21(18)5-6-22(24)31-15/h2,4-6,8,13,15,26-27H,1,3,7,9-12H2,(H,28,29)/t13?,15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Agonist activity at 5-HT2A receptor |
Bioorg Med Chem Lett 19: 5552-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.08.050
BindingDB Entry DOI: 10.7270/Q2Z89DCC |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50334756
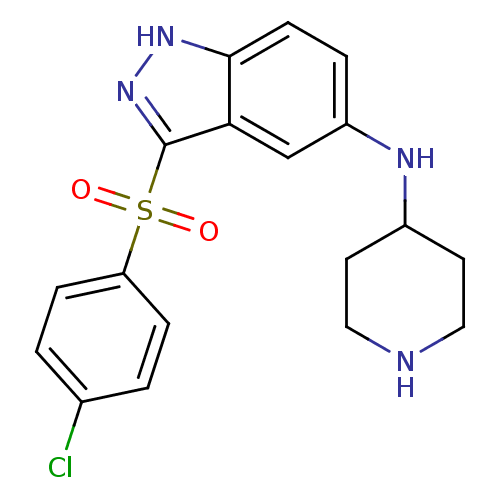
(3-(4-Chlorophenylsulfonyl)-N-(piperidin-4-yl)-1H-i...)Show SMILES Clc1ccc(cc1)S(=O)(=O)c1n[nH]c2ccc(NC3CCNCC3)cc12 Show InChI InChI=1S/C18H19ClN4O2S/c19-12-1-4-15(5-2-12)26(24,25)18-16-11-14(3-6-17(16)22-23-18)21-13-7-9-20-10-8-13/h1-6,11,13,20-21H,7-10H2,(H,22,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]-LSD from cloned human 5-HT6 receptor expressed in human HeLa cells |
Bioorg Med Chem 19: 650-62 (2011)
Article DOI: 10.1016/j.bmc.2010.10.033
BindingDB Entry DOI: 10.7270/Q29S1R9V |
More data for this
Ligand-Target Pair | |
Alpha-1A adrenergic receptor
(Rattus norvegicus (Rat)) | BDBM81453
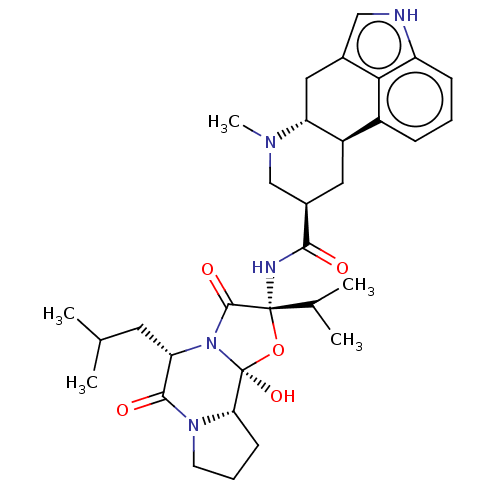
(CAS_159729 | Dihydro-alpha-ergocryptine | NSC_1597...)Show SMILES CC(C)C[C@@H]1N2C(=O)[C@](NC(=O)[C@@H]3C[C@H]4[C@@H](Cc5c[nH]c6cccc4c56)N(C)C3)(O[C@@]2(O)[C@@H]2CCCN2C1=O)C(C)C Show InChI InChI=1S/C32H43N5O5/c1-17(2)12-25-29(39)36-11-7-10-26(36)32(41)37(25)30(40)31(42-32,18(3)4)34-28(38)20-13-22-21-8-6-9-23-27(21)19(15-33-23)14-24(22)35(5)16-20/h6,8-9,15,17-18,20,22,24-26,33,41H,7,10-14,16H2,1-5H3,(H,34,38) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by PDSP Ki Database
| |
Mol Pharmacol 13: 454-73 (1977)
BindingDB Entry DOI: 10.7270/Q2TT4PFZ |
More data for this
Ligand-Target Pair | |
Alpha-1A adrenergic receptor
(Rattus norvegicus (Rat)) | BDBM50280055
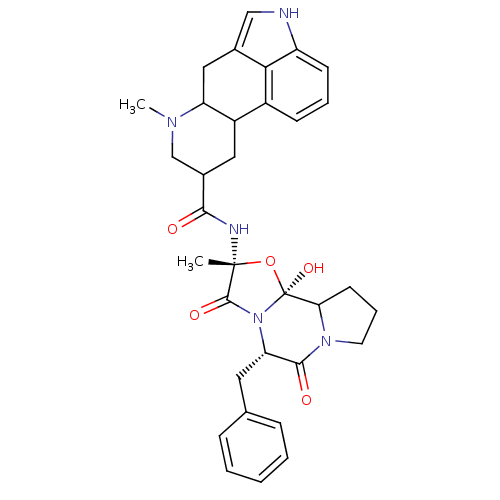
(50285557 | 7-Methyl-4,6,6a,7,8,9,10,10a-octahydro-...)Show SMILES CN1CC(CC2C1Cc1c[nH]c3cccc2c13)C(=O)N[C@]1(C)O[C@@]2(O)C3CCCN3C(=O)[C@H](Cc3ccccc3)N2C1=O Show InChI InChI=1S/C33H37N5O5/c1-32(35-29(39)21-15-23-22-10-6-11-24-28(22)20(17-34-24)16-25(23)36(2)18-21)31(41)38-26(14-19-8-4-3-5-9-19)30(40)37-13-7-12-27(37)33(38,42)43-32/h3-6,8-11,17,21,23,25-27,34,42H,7,12-16,18H2,1-2H3,(H,35,39)/t21?,23?,25?,26-,27?,32+,33-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by PDSP Ki Database
| |
Mol Pharmacol 13: 454-73 (1977)
BindingDB Entry DOI: 10.7270/Q2TT4PFZ |
More data for this
Ligand-Target Pair | |
Alpha-1A adrenergic receptor
(Rattus norvegicus (Rat)) | BDBM32638

(Dihydroergocristine | NSC409663)Show SMILES CC(C)C1(NC(=O)C2CC3C(Cc4c[nH]c5cccc3c45)N(C)C2)OC2(O)C3CCCN3C(=O)C(Cc3ccccc3)N2C1=O Show InChI InChI=1S/C35H41N5O5/c1-20(2)34(37-31(41)23-16-25-24-11-7-12-26-30(24)22(18-36-26)17-27(25)38(3)19-23)33(43)40-28(15-21-9-5-4-6-10-21)32(42)39-14-8-13-29(39)35(40,44)45-34/h4-7,9-12,18,20,23,25,27-29,36,44H,8,13-17,19H2,1-3H3,(H,37,41) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by PDSP Ki Database
| |
Mol Pharmacol 13: 454-73 (1977)
BindingDB Entry DOI: 10.7270/Q2TT4PFZ |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50334751
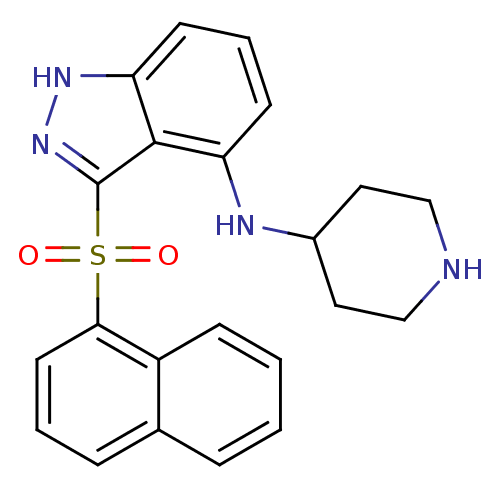
(3-(Naphthalen-1-ylsulfonyl)-N-(piperidin-4-yl)-1H-...)Show SMILES O=S(=O)(c1n[nH]c2cccc(NC3CCNCC3)c12)c1cccc2ccccc12 Show InChI InChI=1S/C22H22N4O2S/c27-29(28,20-10-3-6-15-5-1-2-7-17(15)20)22-21-18(8-4-9-19(21)25-26-22)24-16-11-13-23-14-12-16/h1-10,16,23-24H,11-14H2,(H,25,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]-LSD from cloned human 5-HT6 receptor expressed in human HeLa cells |
Bioorg Med Chem 19: 650-62 (2011)
Article DOI: 10.1016/j.bmc.2010.10.033
BindingDB Entry DOI: 10.7270/Q29S1R9V |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50334727
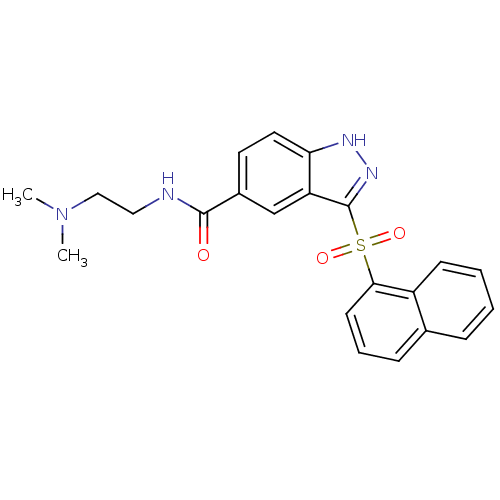
(CHEMBL1642878 | N-[2-(Dimethylamino)ethyl]-3-(1-na...)Show SMILES CN(C)CCNC(=O)c1ccc2[nH]nc(c2c1)S(=O)(=O)c1cccc2ccccc12 Show InChI InChI=1S/C22H22N4O3S/c1-26(2)13-12-23-21(27)16-10-11-19-18(14-16)22(25-24-19)30(28,29)20-9-5-7-15-6-3-4-8-17(15)20/h3-11,14H,12-13H2,1-2H3,(H,23,27)(H,24,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]-LSD from cloned human 5-HT6 receptor expressed in human HeLa cells |
Bioorg Med Chem 19: 650-62 (2011)
Article DOI: 10.1016/j.bmc.2010.10.033
BindingDB Entry DOI: 10.7270/Q29S1R9V |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50302218
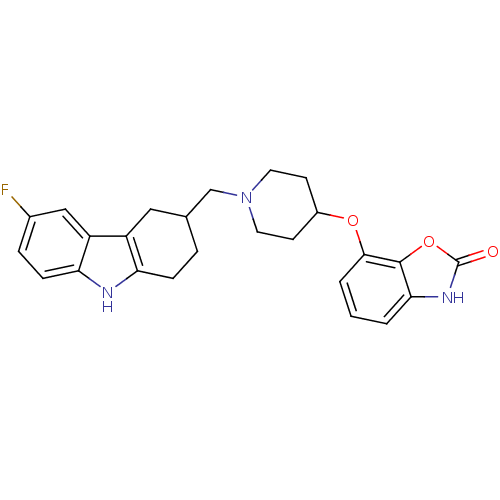
(7-(1-((6-fluoro-2,3,4,9-tetrahydro-1H-carbazol-3-y...)Show SMILES Fc1ccc2[nH]c3CCC(CN4CCC(CC4)Oc4cccc5[nH]c(=O)oc45)Cc3c2c1 Show InChI InChI=1S/C25H26FN3O3/c26-16-5-7-21-19(13-16)18-12-15(4-6-20(18)27-21)14-29-10-8-17(9-11-29)31-23-3-1-2-22-24(23)32-25(30)28-22/h1-3,5,7,13,15,17,27H,4,6,8-12,14H2,(H,28,30) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]citalopram from human serotonin transporter expressed in HEK293 cells by scintillation proximity assay |
Bioorg Med Chem Lett 19: 5552-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.08.050
BindingDB Entry DOI: 10.7270/Q2Z89DCC |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50302213

((3S)-3-(((6-fluoro-2,3,4,9-tetrahydro-1H-carbazol-...)Show SMILES Fc1ccc2[nH]c3CCC(CNC[C@H]4COc5c6CC(=O)Nc6ccc5O4)Cc3c2c1 |r| Show InChI InChI=1S/C24H24FN3O3/c25-14-2-4-20-17(8-14)16-7-13(1-3-19(16)27-20)10-26-11-15-12-30-24-18-9-23(29)28-21(18)5-6-22(24)31-15/h2,4-6,8,13,15,26-27H,1,3,7,9-12H2,(H,28,29)/t13?,15-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]spiperone from D2 receptor |
Bioorg Med Chem Lett 19: 5552-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.08.050
BindingDB Entry DOI: 10.7270/Q2Z89DCC |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50302213

((3S)-3-(((6-fluoro-2,3,4,9-tetrahydro-1H-carbazol-...)Show SMILES Fc1ccc2[nH]c3CCC(CNC[C@H]4COc5c6CC(=O)Nc6ccc5O4)Cc3c2c1 |r| Show InChI InChI=1S/C24H24FN3O3/c25-14-2-4-20-17(8-14)16-7-13(1-3-19(16)27-20)10-26-11-15-12-30-24-18-9-23(29)28-21(18)5-6-22(24)31-15/h2,4-6,8,13,15,26-27H,1,3,7,9-12H2,(H,28,29)/t13?,15-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]spiperone from D2 receptor |
Bioorg Med Chem Lett 19: 5552-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.08.050
BindingDB Entry DOI: 10.7270/Q2Z89DCC |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data