

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50191633 ((3S)-3-{(cyclopropylmethyl)[3-(5-fluoro-1H-indol-3...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Displacement of [3H]-8-OH-DPAT from human 5HT1A receptor expressed in CHO cells | J Med Chem 49: 4785-9 (2006) Article DOI: 10.1021/jm060218h BindingDB Entry DOI: 10.7270/Q261114D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50123752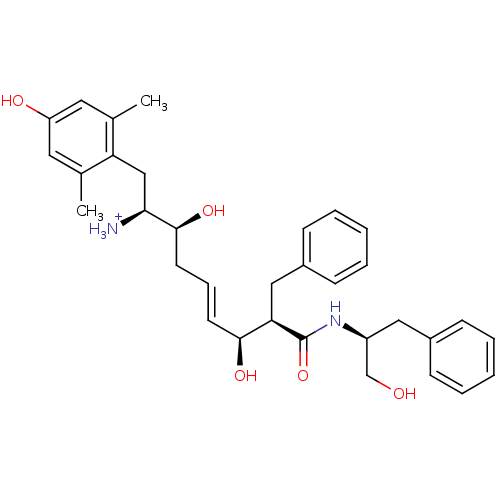 ((E)-(1S,2S,6S,7R)-2,6-Dihydroxy-1-(4-hydroxy-2,6-d...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard University Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from human Opioid receptor mu 1 expressing CHO cells | J Med Chem 46: 677-80 (2003) Article DOI: 10.1021/jm025608s BindingDB Entry DOI: 10.7270/Q2959J96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50123763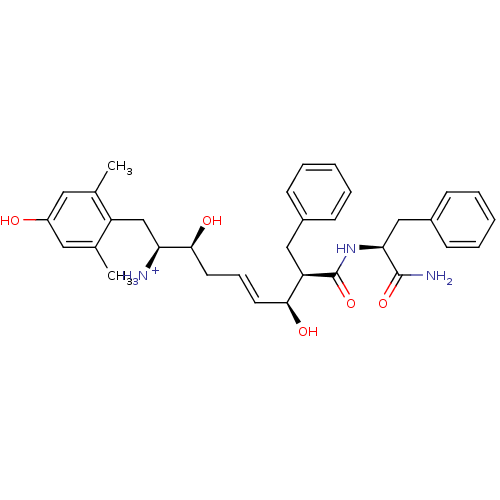 ((E)-(1S,2S,6S,7R)-7-((S)-1-Carbamoyl-2-phenyl-ethy...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard University Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from human Opioid receptor mu 1 expressing CHO cells | J Med Chem 46: 677-80 (2003) Article DOI: 10.1021/jm025608s BindingDB Entry DOI: 10.7270/Q2959J96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50123754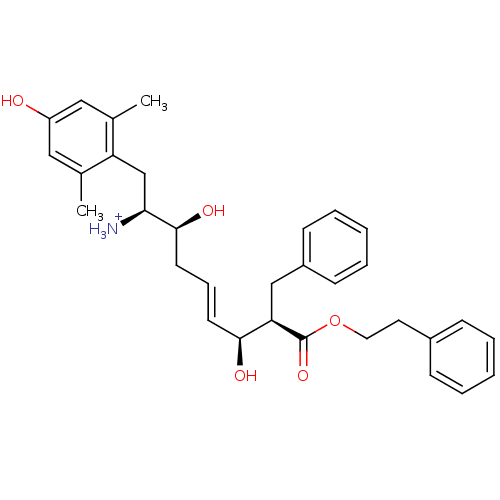 ((E)-(1S,2S,6S,7R)-2,6-Dihydroxy-1-(4-hydroxy-2,6-d...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard University Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from human Opioid receptor mu 1 expressing CHO cells | J Med Chem 46: 677-80 (2003) Article DOI: 10.1021/jm025608s BindingDB Entry DOI: 10.7270/Q2959J96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50123756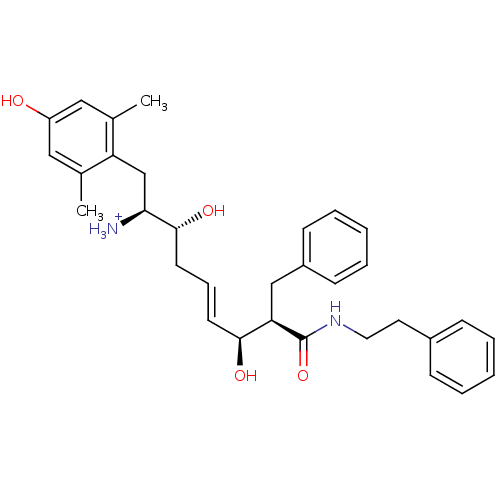 ((E)-(1S,2R,6S,7R)-2,6-Dihydroxy-1-(4-hydroxy-2,6-d...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard University Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from human Opioid receptor mu 1 expressing CHO cells | J Med Chem 46: 677-80 (2003) Article DOI: 10.1021/jm025608s BindingDB Entry DOI: 10.7270/Q2959J96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50295066 ((2S)-8-Methyl-2-{[4-(6-nitroquinolin-2-yl)piperazi...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Displacement of [3H]paroxetine from 5HT transporter in rat cortical membrane | J Med Chem 52: 4955-9 (2009) Article DOI: 10.1021/jm900374r BindingDB Entry DOI: 10.7270/Q2CZ376D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50123750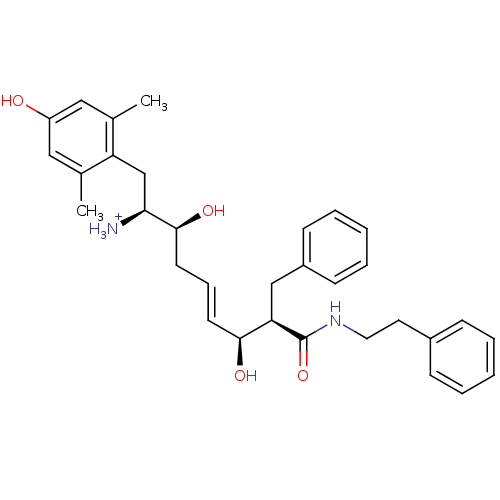 ((E)-(1S,2S,6S,7R)-2,6-Dihydroxy-1-(4-hydroxy-2,6-d...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard University Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from human Opioid receptor mu 1 expressing CHO cells | J Med Chem 46: 677-80 (2003) Article DOI: 10.1021/jm025608s BindingDB Entry DOI: 10.7270/Q2959J96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50300820 (CHEMBL565723 | N-(2-{3-[(3-Chlorophenyl)sulfonyl]-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Displacement of [3H]LSD from human cloned 5HT6 receptor expressed in HeLa cells | Bioorg Med Chem 17: 5153-63 (2009) Article DOI: 10.1016/j.bmc.2009.05.055 BindingDB Entry DOI: 10.7270/Q25T3KJS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50123751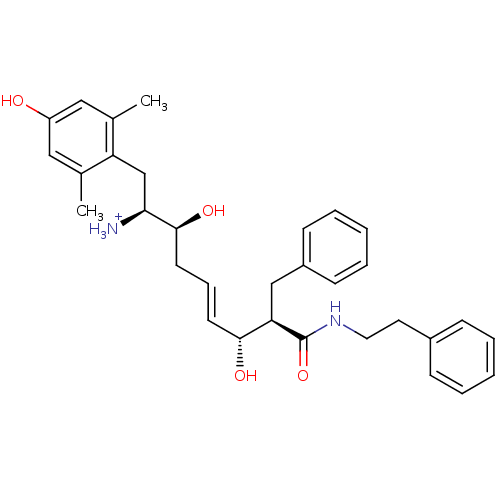 ((E)-(1S,2S,6R,7R)-2,6-Dihydroxy-1-(4-hydroxy-2,6-d...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard University Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from human Opioid receptor mu 1 expressing CHO cells | J Med Chem 46: 677-80 (2003) Article DOI: 10.1021/jm025608s BindingDB Entry DOI: 10.7270/Q2959J96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50316677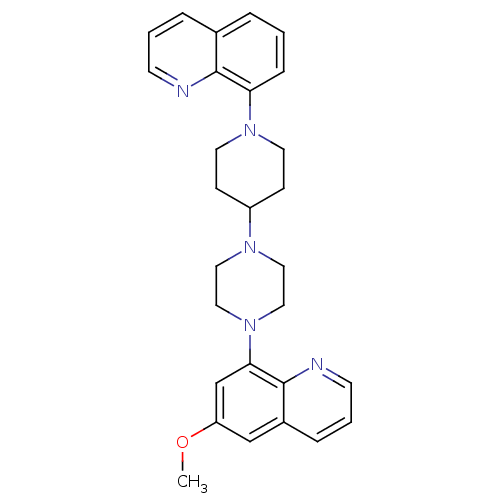 (6-Methoxy-8-{4-[1-(8-quinolinyl)-4-piperidinyl]-1-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from human 5HT1A receptor expressed in CHO cells | J Med Chem 53: 4066-84 (2010) Article DOI: 10.1021/jm1000908 BindingDB Entry DOI: 10.7270/Q28P60P7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50316673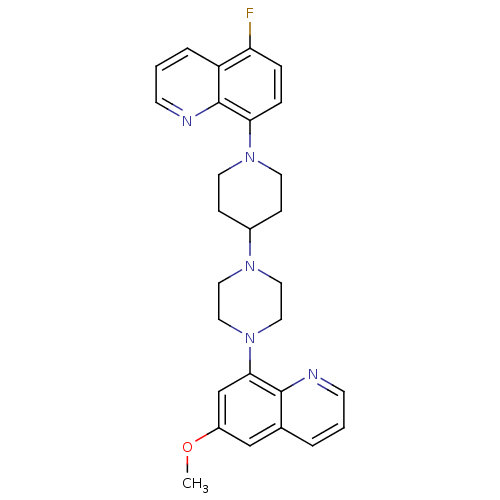 (5-Fluoro-8-{4-[4-(6-methoxyquinolin-8-yl)piperazin...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from human 5HT1A receptor expressed in CHO cells | J Med Chem 53: 4066-84 (2010) Article DOI: 10.1021/jm1000908 BindingDB Entry DOI: 10.7270/Q28P60P7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50191625 (CHEMBL208605 | rac-3-{ethyl[4-(5-fluoro-1H-indol-3...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Displacement of [3H]paroxetine from 5-HT transporter in Sprague-Dawley rat cortical membranes | J Med Chem 49: 4785-9 (2006) Article DOI: 10.1021/jm060218h BindingDB Entry DOI: 10.7270/Q261114D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50123759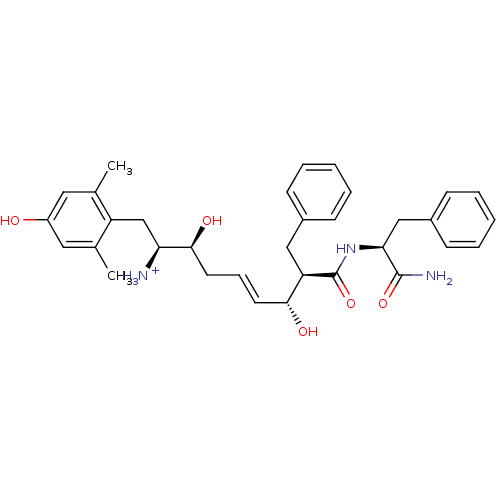 ((E)-(1S,2S,6R,7R)-7-((S)-1-Carbamoyl-2-phenyl-ethy...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard University Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from human Opioid receptor mu 1 expressing CHO cells | J Med Chem 46: 677-80 (2003) Article DOI: 10.1021/jm025608s BindingDB Entry DOI: 10.7270/Q2959J96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50123762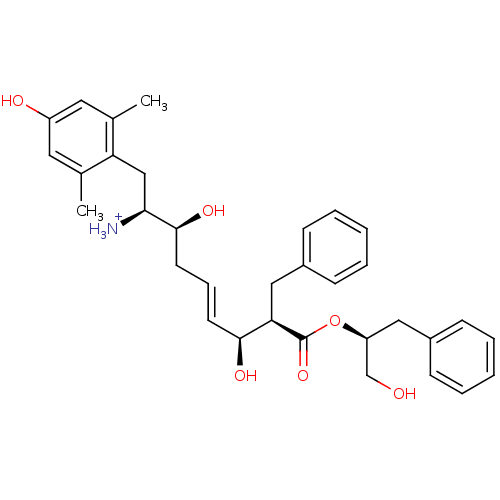 ((E)-(1S,2S,6S,7R)-2,6-Dihydroxy-1-(4-hydroxy-2,6-d...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard University Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from human Opioid receptor mu 1 expressing CHO cells | J Med Chem 46: 677-80 (2003) Article DOI: 10.1021/jm025608s BindingDB Entry DOI: 10.7270/Q2959J96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50123760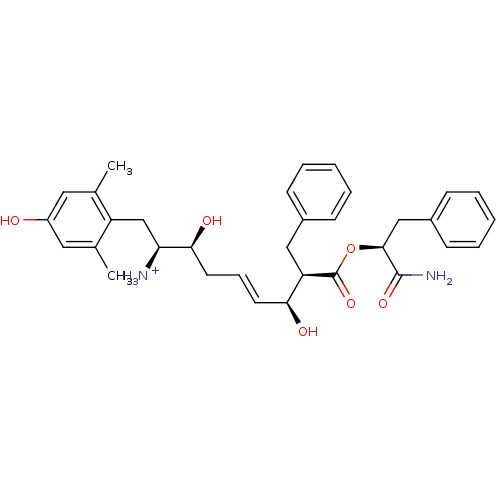 ((E)-(1S,2S,6S,7R)-7-((S)-1-Carbamoyl-2-phenyl-etho...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard University Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from human Opioid receptor mu 1 expressing CHO cells | J Med Chem 46: 677-80 (2003) Article DOI: 10.1021/jm025608s BindingDB Entry DOI: 10.7270/Q2959J96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50316680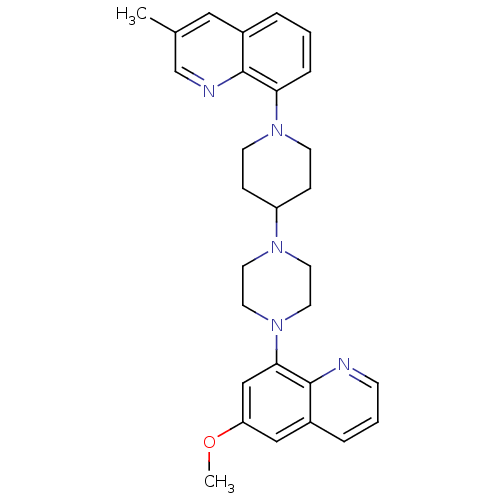 (8-{4-[4-(6-Methoxyquinolin-8-yl)piperazin-1-yl]pip...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from human 5HT1A receptor expressed in CHO cells | J Med Chem 53: 4066-84 (2010) Article DOI: 10.1021/jm1000908 BindingDB Entry DOI: 10.7270/Q28P60P7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50316684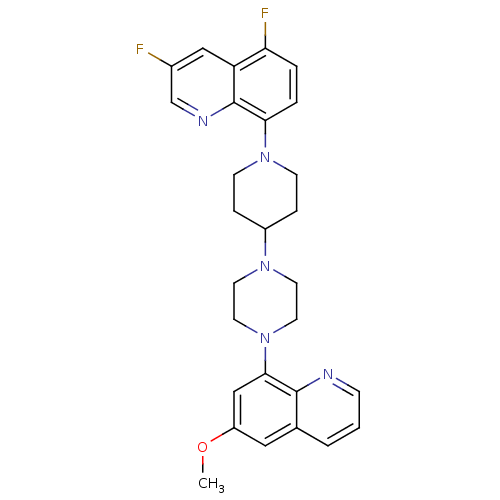 (3,5-Difluoro-8-{4-[4-(6-methoxyquinolin-8-yl)piper...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from human 5HT1A receptor expressed in CHO cells | J Med Chem 53: 4066-84 (2010) Article DOI: 10.1021/jm1000908 BindingDB Entry DOI: 10.7270/Q28P60P7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50316688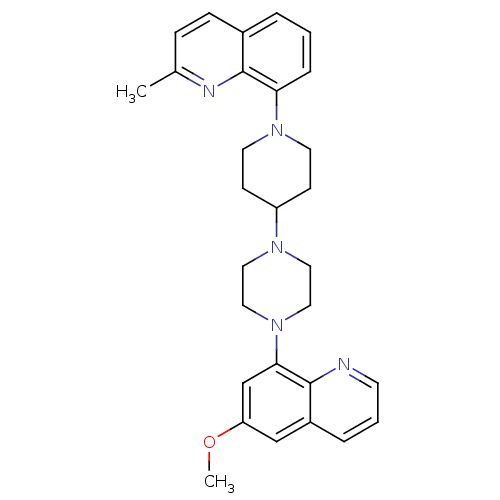 (8-{4-[4-(6-Methoxyquinolin-8-yl)piperazin-1-yl]pip...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.540 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from human 5HT1A receptor expressed in CHO cells | J Med Chem 53: 4066-84 (2010) Article DOI: 10.1021/jm1000908 BindingDB Entry DOI: 10.7270/Q28P60P7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50316690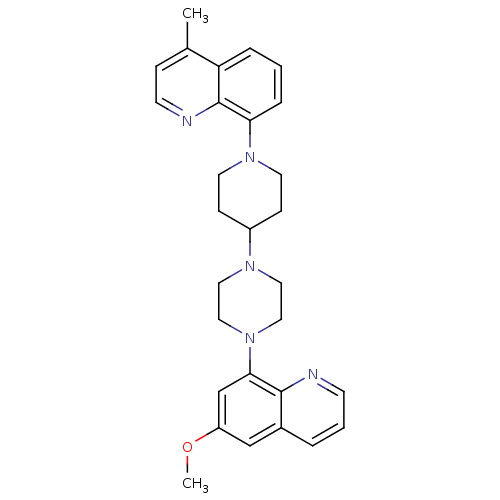 (8-{4-[4-(6-Methoxyquinolin-8-yl)piperazin-1-yl]pip...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.610 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from human 5HT1A receptor expressed in CHO cells | J Med Chem 53: 4066-84 (2010) Article DOI: 10.1021/jm1000908 BindingDB Entry DOI: 10.7270/Q28P60P7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50316682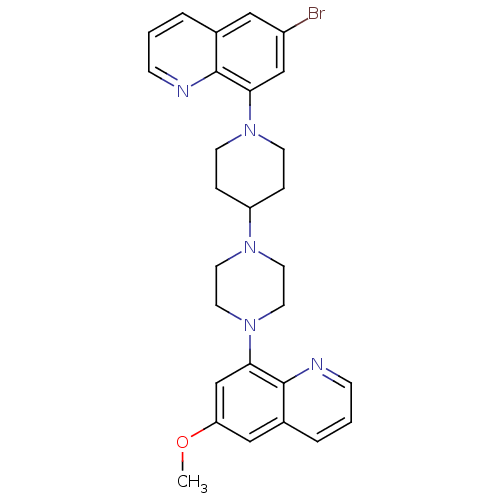 (6-Bromo-8-{4-[4-(6-methoxyquinolin-8-yl)piperazin-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.610 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from human 5HT1A receptor expressed in CHO cells | J Med Chem 53: 4066-84 (2010) Article DOI: 10.1021/jm1000908 BindingDB Entry DOI: 10.7270/Q28P60P7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50316678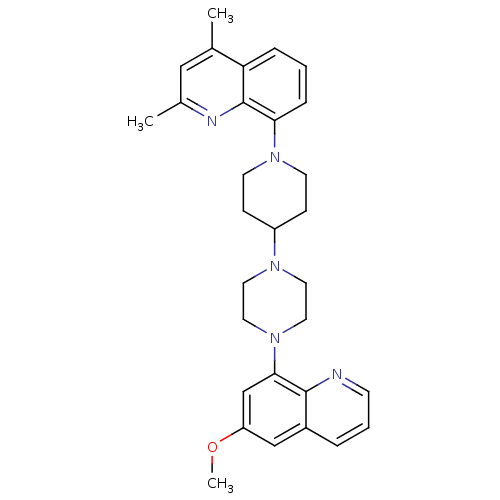 (8-{4-[4-(6-Methoxyquinolin-8-yl)piperazin-1-yl]pip...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from human 5HT1A receptor expressed in CHO cells | J Med Chem 53: 4066-84 (2010) Article DOI: 10.1021/jm1000908 BindingDB Entry DOI: 10.7270/Q28P60P7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50123757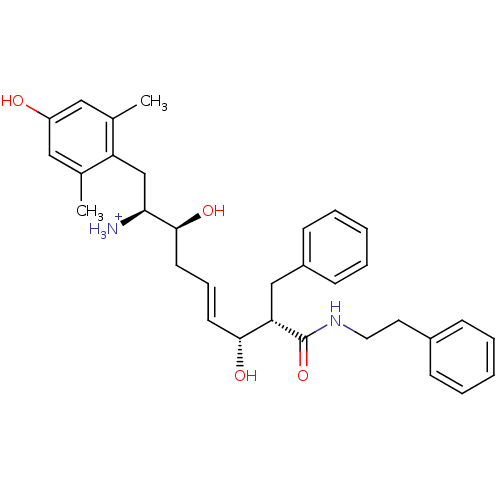 ((E)-(1S,2S,6R,7S)-2,6-Dihydroxy-1-(4-hydroxy-2,6-d...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.640 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard University Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from human Opioid receptor mu 1 expressing CHO cells | J Med Chem 46: 677-80 (2003) Article DOI: 10.1021/jm025608s BindingDB Entry DOI: 10.7270/Q2959J96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50274186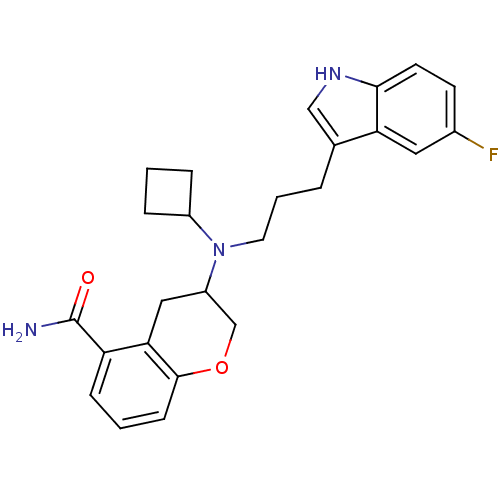 ((+)-3-{Cyclobutyl[3-(5-fluoro-1H-indol-3-yl)propyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.660 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from human cloned 5HT1A receptor expressed in CHO cells | J Med Chem 51: 6980-7004 (2008) Article DOI: 10.1021/jm8007097 BindingDB Entry DOI: 10.7270/Q2TT4RWM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50123755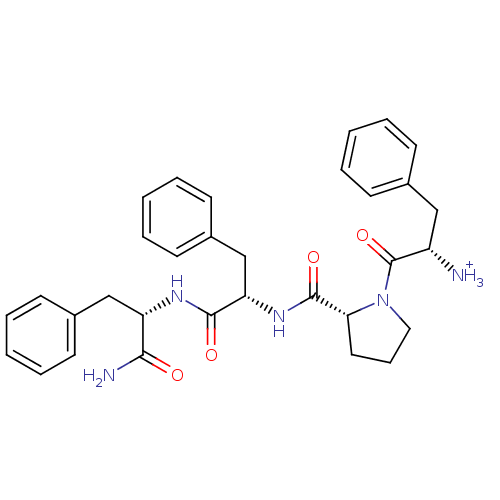 (2-{(R)-2-[1-((S)-(S)-1-Carbamoyl-2-phenyl-ethylcar...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.690 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard University Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from human Opioid receptor mu 1 expressing CHO cells | J Med Chem 46: 677-80 (2003) Article DOI: 10.1021/jm025608s BindingDB Entry DOI: 10.7270/Q2959J96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50123761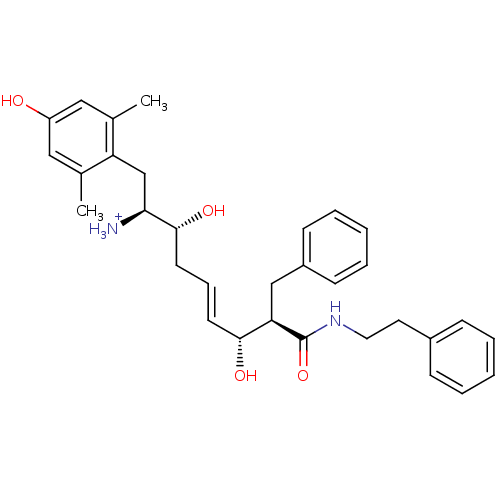 ((E)-(1S,2R,6R,7R)-2,6-Dihydroxy-1-(4-hydroxy-2,6-d...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.720 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard University Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from human Opioid receptor mu 1 expressing CHO cells | J Med Chem 46: 677-80 (2003) Article DOI: 10.1021/jm025608s BindingDB Entry DOI: 10.7270/Q2959J96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50306472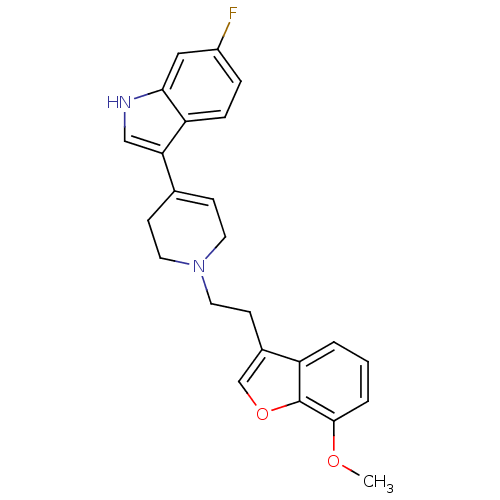 (6-fluoro-3-(1-(2-(7-methoxybenzofuran-3-yl)ethyl)-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.760 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Displacement of [3H]paroxetine from 5-HT transporter in rat frontal cortical synaptosomes | Bioorg Med Chem Lett 20: 824-7 (2010) Article DOI: 10.1016/j.bmcl.2009.12.093 BindingDB Entry DOI: 10.7270/Q2B56JVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50316679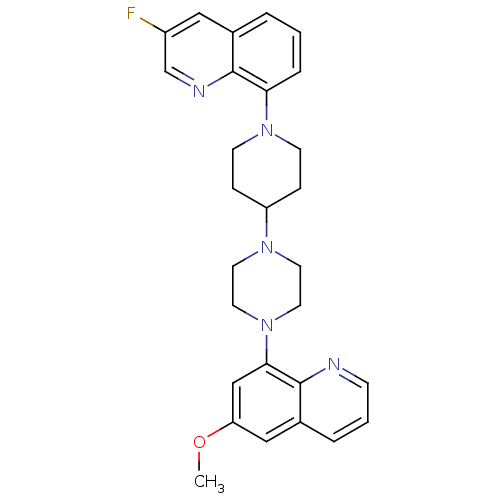 (3-Fluoro-8-{4-[4-(6-methoxyquinolin-8-yl)piperazin...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.790 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from human 5HT1A receptor expressed in CHO cells | J Med Chem 53: 4066-84 (2010) Article DOI: 10.1021/jm1000908 BindingDB Entry DOI: 10.7270/Q28P60P7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50274782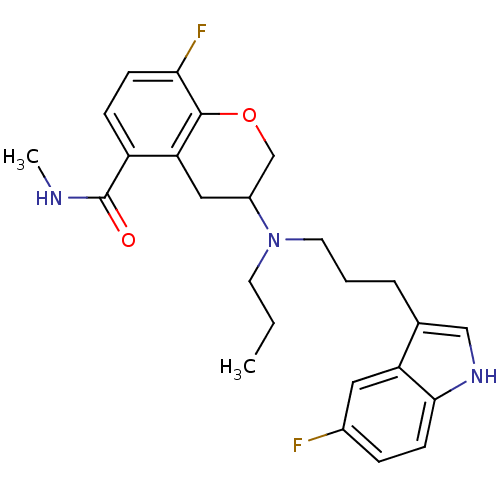 (8-Fluoro-3-{[3-(5-fluoro-1H-indol-3-yl)propyl](pro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.810 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from human cloned 5HT1A receptor expressed in CHO cells | J Med Chem 51: 6980-7004 (2008) Article DOI: 10.1021/jm8007097 BindingDB Entry DOI: 10.7270/Q2TT4RWM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50316683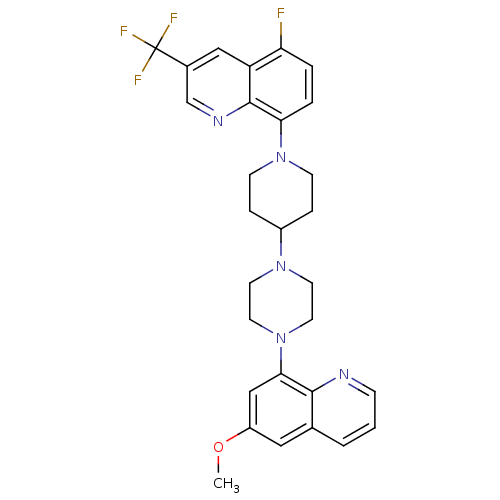 (5-Fluoro-8-{4-[4-(6-methoxyquinolin-8-yl)piperazin...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.870 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from human 5HT1A receptor expressed in CHO cells | J Med Chem 53: 4066-84 (2010) Article DOI: 10.1021/jm1000908 BindingDB Entry DOI: 10.7270/Q28P60P7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50295064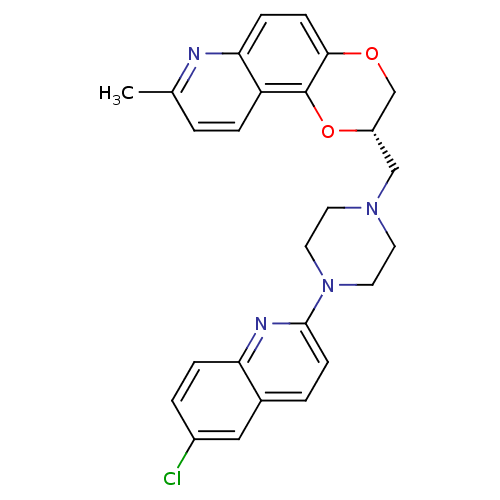 ((2S)-2-{[4-(6-Chloroquinolin-2-yl)piperazin-1-yl]m...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.880 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Displacement of [3H]paroxetine from 5HT transporter in rat cortical membrane | J Med Chem 52: 4955-9 (2009) Article DOI: 10.1021/jm900374r BindingDB Entry DOI: 10.7270/Q2CZ376D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50300845 (CHEMBL565746 | N-(2-{3-[(6-Chloroimidazo[2,1-b][1,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Displacement of [3H]LSD from human cloned 5HT6 receptor expressed in HeLa cells | Bioorg Med Chem 17: 5153-63 (2009) Article DOI: 10.1016/j.bmc.2009.05.055 BindingDB Entry DOI: 10.7270/Q25T3KJS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM86708 (CAS_146714-97-8 | CHEMBL31354 | CHEMBL514874 | CHE...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from human 5HT1A receptor expressed in CHO cells | Bioorg Med Chem 16: 6707-23 (2008) Article DOI: 10.1016/j.bmc.2008.05.075 BindingDB Entry DOI: 10.7270/Q2GT5MZ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM86708 (CAS_146714-97-8 | CHEMBL31354 | CHEMBL514874 | CHE...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Displacement of 8-OH-DPAT from human 5-hydroxytryptamine 1A receptor expressed in chinese hamster ovary cells | J Med Chem 48: 3467-70 (2005) Article DOI: 10.1021/jm049493z BindingDB Entry DOI: 10.7270/Q2S1838Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM86708 (CAS_146714-97-8 | CHEMBL31354 | CHEMBL514874 | CHE...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from human 5HT1A receptor expressed in CHO cells | J Med Chem 53: 4066-84 (2010) Article DOI: 10.1021/jm1000908 BindingDB Entry DOI: 10.7270/Q28P60P7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM86708 (CAS_146714-97-8 | CHEMBL31354 | CHEMBL514874 | CHE...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from human 5HT1A receptor expressed in CHO cells | J Med Chem 52: 4955-9 (2009) Article DOI: 10.1021/jm900374r BindingDB Entry DOI: 10.7270/Q2CZ376D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50300842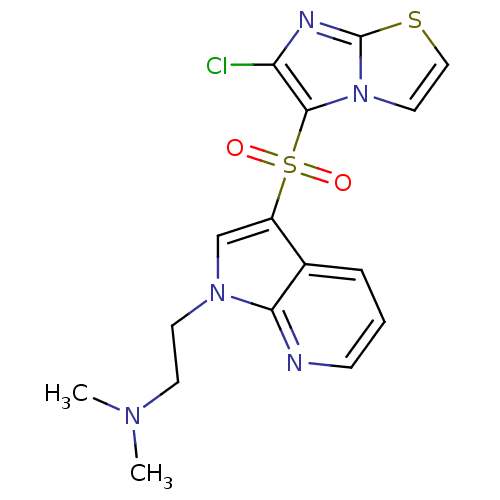 (CHEMBL565724 | N-(2-{3-[(6-Chloroimidazo[2,1-b][1,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Displacement of [3H]LSD from human cloned 5HT6 receptor expressed in HeLa cells | Bioorg Med Chem 17: 5153-63 (2009) Article DOI: 10.1016/j.bmc.2009.05.055 BindingDB Entry DOI: 10.7270/Q25T3KJS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50274784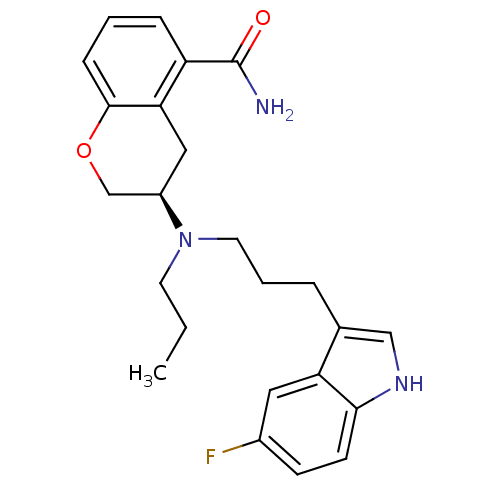 (3-{[3-(5-Fluoro-1H-indol-3-yl)propyl](propyl)amino...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.930 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from human cloned 5HT1A receptor expressed in CHO cells | J Med Chem 51: 6980-7004 (2008) Article DOI: 10.1021/jm8007097 BindingDB Entry DOI: 10.7270/Q2TT4RWM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM86708 (CAS_146714-97-8 | CHEMBL31354 | CHEMBL514874 | CHE...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.960 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Displacement of [3H]-8-OH-DPAT from human 5HT1A receptor expressed in CHO cells | J Med Chem 49: 4785-9 (2006) Article DOI: 10.1021/jm060218h BindingDB Entry DOI: 10.7270/Q261114D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM86708 (CAS_146714-97-8 | CHEMBL31354 | CHEMBL514874 | CHE...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.960 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from human cloned 5HT1A receptor expressed in CHO cells | J Med Chem 51: 6980-7004 (2008) Article DOI: 10.1021/jm8007097 BindingDB Entry DOI: 10.7270/Q2TT4RWM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50166904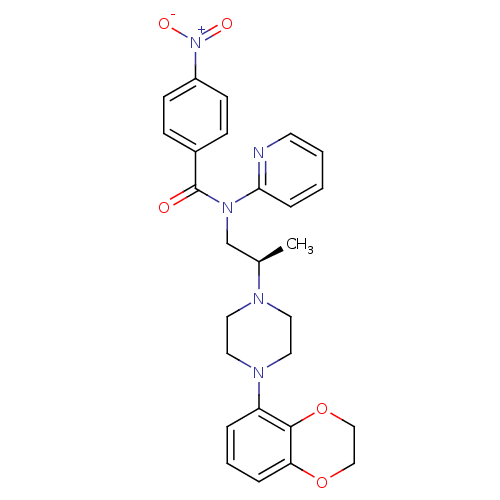 (CHEMBL193206 | N-{(R)-2-[4-(2,3-Dihydro-benzo[1,4]...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Displacement of 8-OH-DPAT from human 5-hydroxytryptamine 1A receptor expressed in chinese hamster ovary cells | J Med Chem 48: 3467-70 (2005) Article DOI: 10.1021/jm049493z BindingDB Entry DOI: 10.7270/Q2S1838Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50300838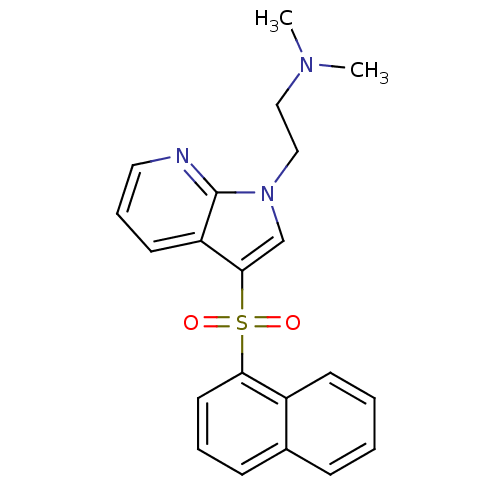 (CHEMBL565552 | N,N-Dimethyl-N-{2-[3-(1-naphthylsul...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Displacement of [3H]LSD from human cloned 5HT6 receptor expressed in HeLa cells | Bioorg Med Chem 17: 5153-63 (2009) Article DOI: 10.1016/j.bmc.2009.05.055 BindingDB Entry DOI: 10.7270/Q25T3KJS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50300843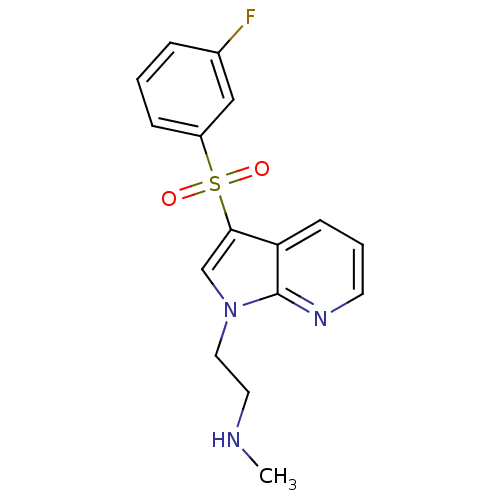 (CHEMBL565976 | N-(2-{3-[(3-Fluorophenyl)sulfonyl]-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Displacement of [3H]LSD from human cloned 5HT6 receptor expressed in HeLa cells | Bioorg Med Chem 17: 5153-63 (2009) Article DOI: 10.1016/j.bmc.2009.05.055 BindingDB Entry DOI: 10.7270/Q25T3KJS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50295055 (2-(4-{[(2S)-8-Methyl-2,3-dihydro[1,4]dioxino[2,3-f...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Displacement of [3H]paroxetine from 5HT transporter in rat cortical membrane | J Med Chem 52: 4955-9 (2009) Article DOI: 10.1021/jm900374r BindingDB Entry DOI: 10.7270/Q2CZ376D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50252278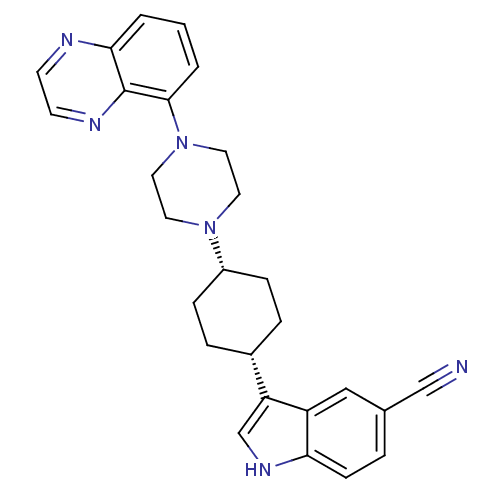 (3-[(1,4-cis)-4-(4-Quinoxalin-5-yl-piperazin-1-yl)-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Displacement of [3H]paroxetine from rat cortical 5HTT reuptake site | Bioorg Med Chem 16: 6707-23 (2008) Article DOI: 10.1016/j.bmc.2008.05.075 BindingDB Entry DOI: 10.7270/Q2GT5MZ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50274487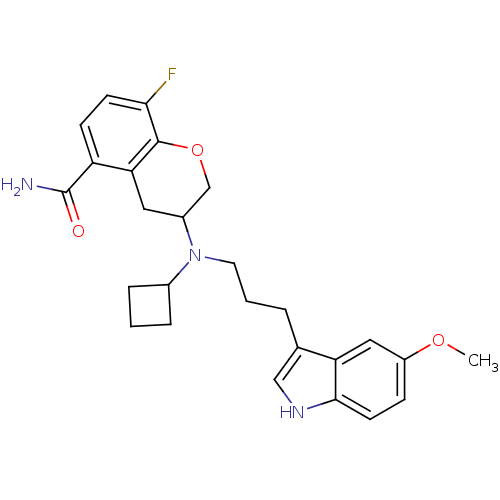 (3-{Cyclobutyl[3-(5-methoxy-1H-indol-3-yl)propyl]am...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from human cloned 5HT1A receptor expressed in CHO cells | J Med Chem 51: 6980-7004 (2008) Article DOI: 10.1021/jm8007097 BindingDB Entry DOI: 10.7270/Q2TT4RWM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50316686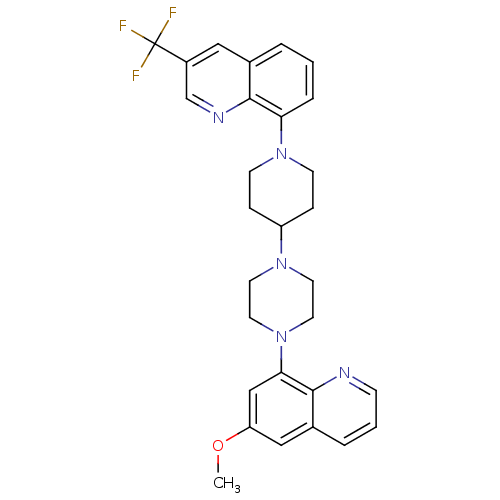 (8-{4-[4-(6-Methoxyquinolin-8-yl)piperazin-1-yl]pip...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from human 5HT1A receptor expressed in CHO cells | J Med Chem 53: 4066-84 (2010) Article DOI: 10.1021/jm1000908 BindingDB Entry DOI: 10.7270/Q28P60P7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50123753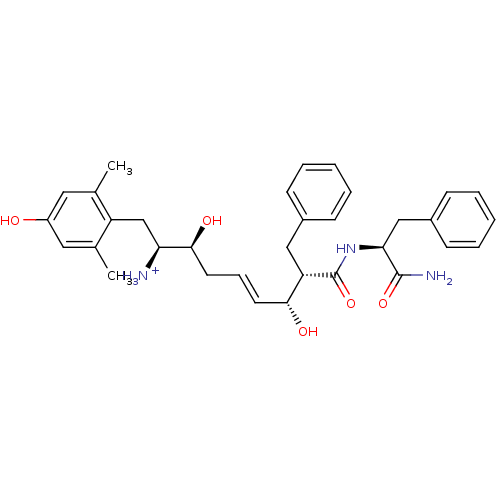 ((E)-(1S,2S,6R,7S)-7-((S)-1-Carbamoyl-2-phenyl-ethy...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard University Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from human Opioid receptor mu 1 expressing CHO cells | J Med Chem 46: 677-80 (2003) Article DOI: 10.1021/jm025608s BindingDB Entry DOI: 10.7270/Q2959J96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50191615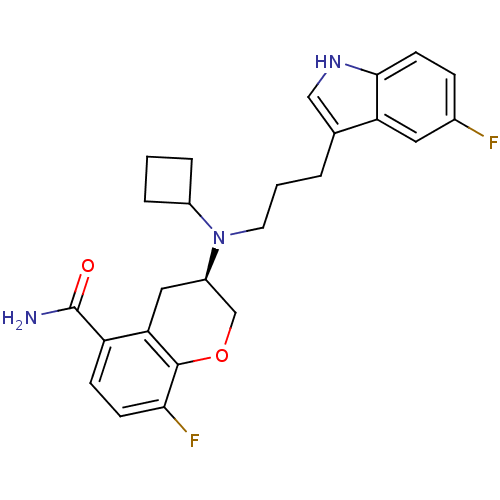 ((3R)-(-)-3-{Cyclobutyl[3-(5-fluoro-1H-indol-3-yl)p...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from human cloned 5HT1A receptor expressed in CHO cells | J Med Chem 51: 6980-7004 (2008) Article DOI: 10.1021/jm8007097 BindingDB Entry DOI: 10.7270/Q2TT4RWM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50191615 ((3R)-(-)-3-{Cyclobutyl[3-(5-fluoro-1H-indol-3-yl)p...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Displacement of [3H]-8-OH-DPAT from human 5HT1A receptor expressed in CHO cells | J Med Chem 49: 4785-9 (2006) Article DOI: 10.1021/jm060218h BindingDB Entry DOI: 10.7270/Q261114D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50123758 ((E)-(1S,2R,6S,7R)-7-((S)-1-Carbamoyl-2-phenyl-ethy...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard University Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from human Opioid receptor mu 1 expressing CHO cells | J Med Chem 46: 677-80 (2003) Article DOI: 10.1021/jm025608s BindingDB Entry DOI: 10.7270/Q2959J96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 4272 total ) | Next | Last >> |