

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Chymase (Homo sapiens (Human)) | BDBM50208224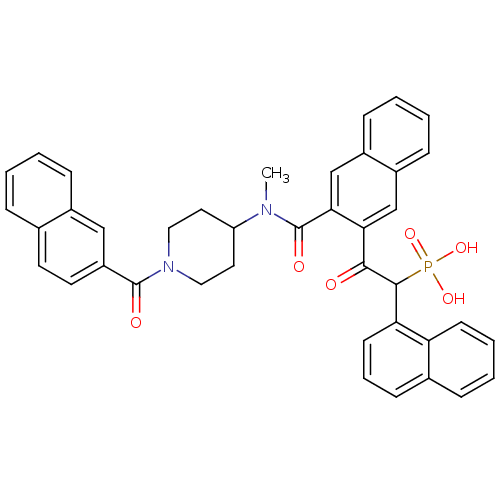 (2-(3-((1-(2-naphthoyl)piperidin-4-yl)(methyl)carba...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of human skin chymase | J Med Chem 50: 1727-30 (2007) Article DOI: 10.1021/jm0700619 BindingDB Entry DOI: 10.7270/Q26W99RF | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM50208222 ((E)-2-(3-chloro-5-fluorostyrylamino)-1-(5-chlorobe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of human skin chymase | J Med Chem 50: 1727-30 (2007) Article DOI: 10.1021/jm0700619 BindingDB Entry DOI: 10.7270/Q26W99RF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM50208228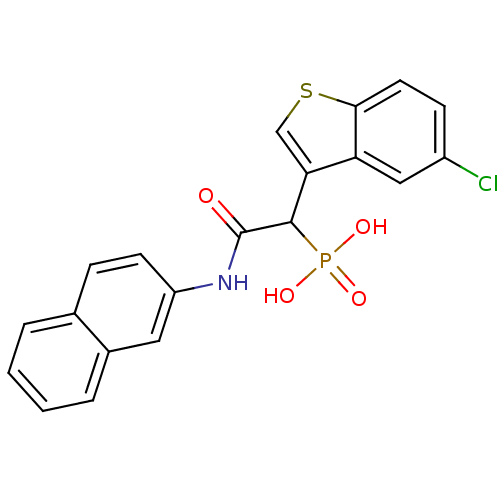 (1-(5-chlorobenzo[b]thiophen-3-yl)-2-(naphthalen-2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 36 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of human skin chymase | J Med Chem 50: 1727-30 (2007) Article DOI: 10.1021/jm0700619 BindingDB Entry DOI: 10.7270/Q26W99RF | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cathepsin G (Homo sapiens (Human)) | BDBM50208224 (2-(3-((1-(2-naphthoyl)piperidin-4-yl)(methyl)carba...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 38 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of human neutrophil Cat G | J Med Chem 50: 1727-30 (2007) Article DOI: 10.1021/jm0700619 BindingDB Entry DOI: 10.7270/Q26W99RF | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cathepsin G (Homo sapiens (Human)) | BDBM50208228 (1-(5-chlorobenzo[b]thiophen-3-yl)-2-(naphthalen-2-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 9.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of human neutrophil Cat G | J Med Chem 50: 1727-30 (2007) Article DOI: 10.1021/jm0700619 BindingDB Entry DOI: 10.7270/Q26W99RF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM50208225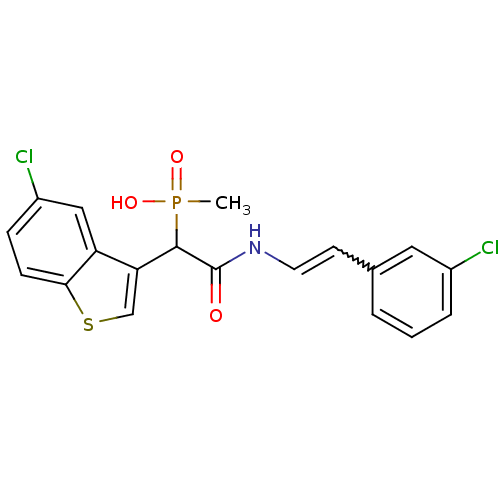 ((E)-2-(3-chlorostyrylamino)-1-(5-chlorobenzo[b]thi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of human skin chymase | J Med Chem 50: 1727-30 (2007) Article DOI: 10.1021/jm0700619 BindingDB Entry DOI: 10.7270/Q26W99RF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM50208224 (2-(3-((1-(2-naphthoyl)piperidin-4-yl)(methyl)carba...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of human skin chymase | J Med Chem 50: 1727-30 (2007) Article DOI: 10.1021/jm0700619 BindingDB Entry DOI: 10.7270/Q26W99RF | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM50208236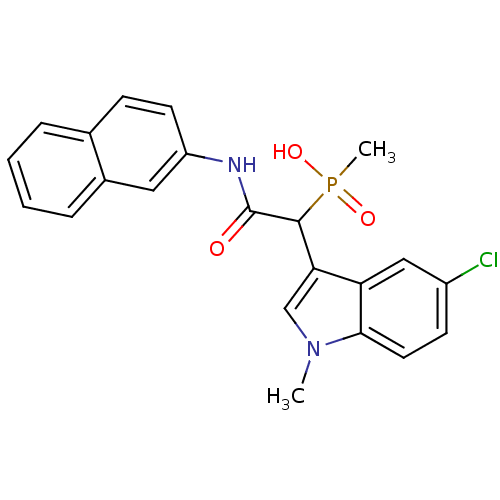 (1-(5-chloro-1-methyl-1H-indol-3-yl)-2-(naphthalen-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of human skin chymase | J Med Chem 50: 1727-30 (2007) Article DOI: 10.1021/jm0700619 BindingDB Entry DOI: 10.7270/Q26W99RF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM50208243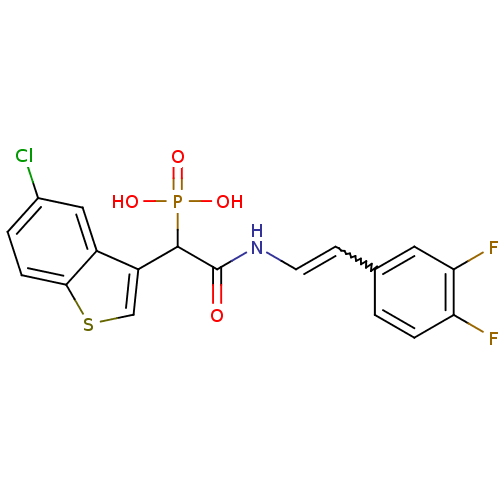 ((E)-2-(3,4-difluorostyrylamino)-1-(5-chlorobenzo[b...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of human skin chymase | J Med Chem 50: 1727-30 (2007) Article DOI: 10.1021/jm0700619 BindingDB Entry DOI: 10.7270/Q26W99RF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM50208232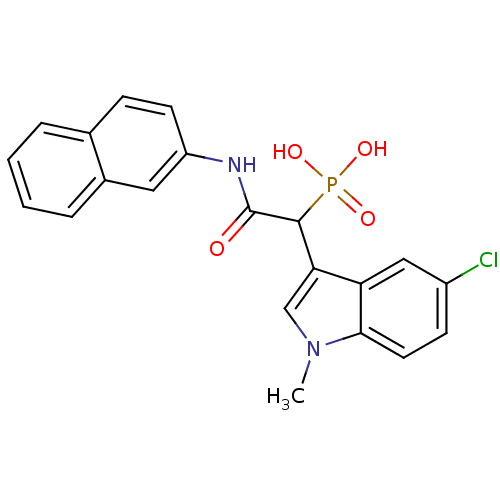 (1-(5-chloro-1-methyl-1H-indol-3-yl)-2-(naphthalen-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of human skin chymase | J Med Chem 50: 1727-30 (2007) Article DOI: 10.1021/jm0700619 BindingDB Entry DOI: 10.7270/Q26W99RF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM50208244 ((E)-2-(4-fluorostyrylamino)-1-(5-chloro-1-methyl-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of human skin chymase | J Med Chem 50: 1727-30 (2007) Article DOI: 10.1021/jm0700619 BindingDB Entry DOI: 10.7270/Q26W99RF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM50208222 ((E)-2-(3-chloro-5-fluorostyrylamino)-1-(5-chlorobe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of human skin chymase | J Med Chem 50: 1727-30 (2007) Article DOI: 10.1021/jm0700619 BindingDB Entry DOI: 10.7270/Q26W99RF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM50208229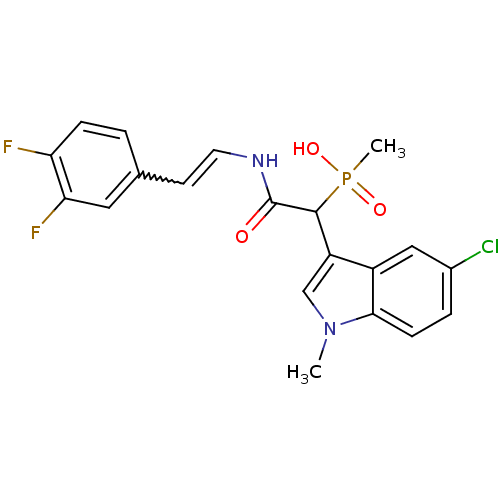 ((E)-2-(3,4-difluorostyrylamino)-1-(5-chloro-1-meth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of human skin chymase | J Med Chem 50: 1727-30 (2007) Article DOI: 10.1021/jm0700619 BindingDB Entry DOI: 10.7270/Q26W99RF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM50208228 (1-(5-chlorobenzo[b]thiophen-3-yl)-2-(naphthalen-2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of human skin chymase | J Med Chem 50: 1727-30 (2007) Article DOI: 10.1021/jm0700619 BindingDB Entry DOI: 10.7270/Q26W99RF | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM50208241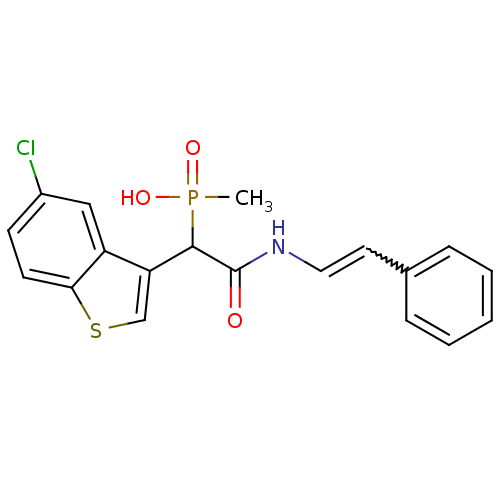 ((E)-1-(5-chlorobenzo[b]thiophen-3-yl)-2-oxo-2-(sty...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of human skin chymase | J Med Chem 50: 1727-30 (2007) Article DOI: 10.1021/jm0700619 BindingDB Entry DOI: 10.7270/Q26W99RF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM50208242 ((E)-2-(3,4-difluorostyrylamino)-1-(5-chlorobenzo[b...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 58 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of human skin chymase | J Med Chem 50: 1727-30 (2007) Article DOI: 10.1021/jm0700619 BindingDB Entry DOI: 10.7270/Q26W99RF | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM50208226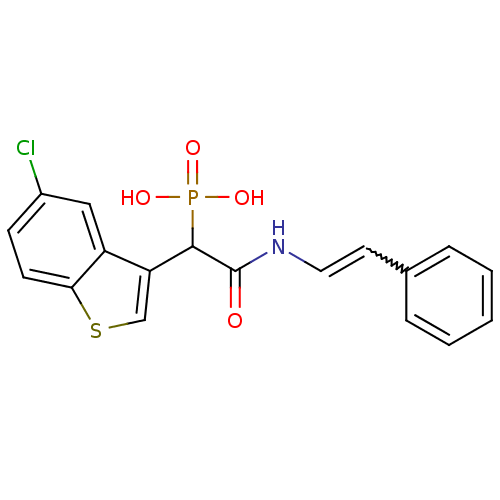 ((E)-1-(5-chlorobenzo[b]thiophen-3-yl)-2-oxo-2-(sty...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of human skin chymase | J Med Chem 50: 1727-30 (2007) Article DOI: 10.1021/jm0700619 BindingDB Entry DOI: 10.7270/Q26W99RF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM50208239 ((E)-2-(4-fluorostyrylamino)-1-(5-chlorobenzo[b]thi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 66 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of human skin chymase | J Med Chem 50: 1727-30 (2007) Article DOI: 10.1021/jm0700619 BindingDB Entry DOI: 10.7270/Q26W99RF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM50208221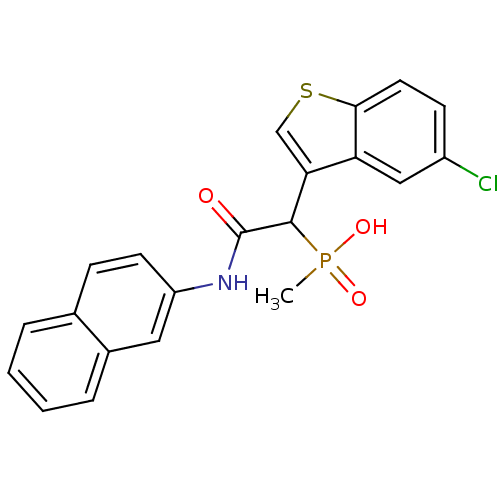 (1-(5-chlorobenzo[b]thiophen-3-yl)-2-(naphthalen-2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of human skin chymase | J Med Chem 50: 1727-30 (2007) Article DOI: 10.1021/jm0700619 BindingDB Entry DOI: 10.7270/Q26W99RF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM50208227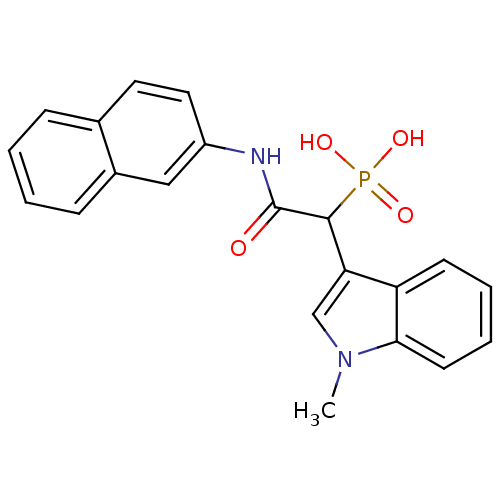 (1-(1-methyl-1H-indol-3-yl)-2-(naphthalen-2-ylamino...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of human skin chymase | J Med Chem 50: 1727-30 (2007) Article DOI: 10.1021/jm0700619 BindingDB Entry DOI: 10.7270/Q26W99RF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM50208234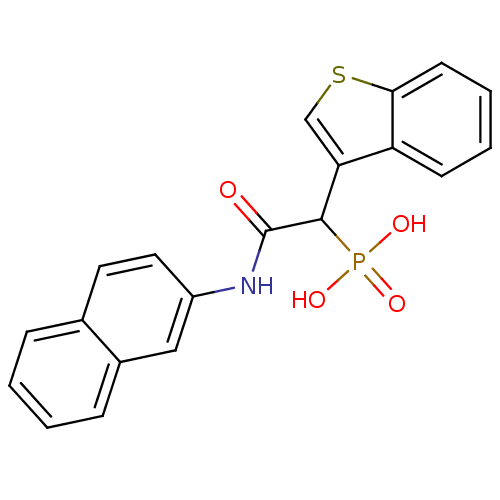 (1-(benzo[b]thiophen-3-yl)-2-(naphthalen-2-ylamino)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of human skin chymase | J Med Chem 50: 1727-30 (2007) Article DOI: 10.1021/jm0700619 BindingDB Entry DOI: 10.7270/Q26W99RF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM50208223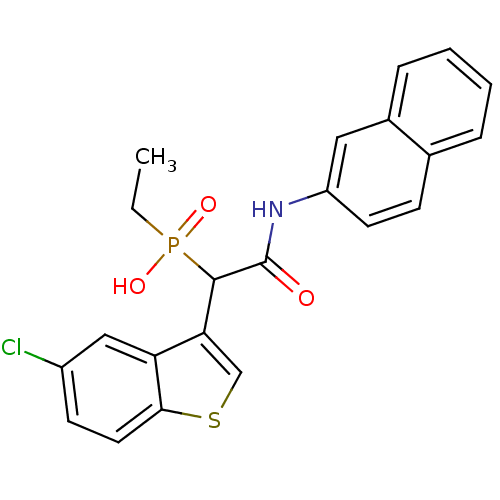 (1-(5-chlorobenzo[b]thiophen-3-yl)-2-(naphthalen-2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of human skin chymase | J Med Chem 50: 1727-30 (2007) Article DOI: 10.1021/jm0700619 BindingDB Entry DOI: 10.7270/Q26W99RF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM50208235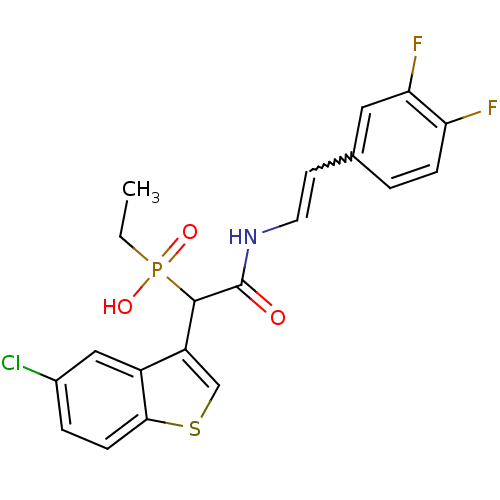 ((E)-2-(3,4-difluorostyrylamino)-1-(5-chlorobenzo[b...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 165 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of human skin chymase | J Med Chem 50: 1727-30 (2007) Article DOI: 10.1021/jm0700619 BindingDB Entry DOI: 10.7270/Q26W99RF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM50208237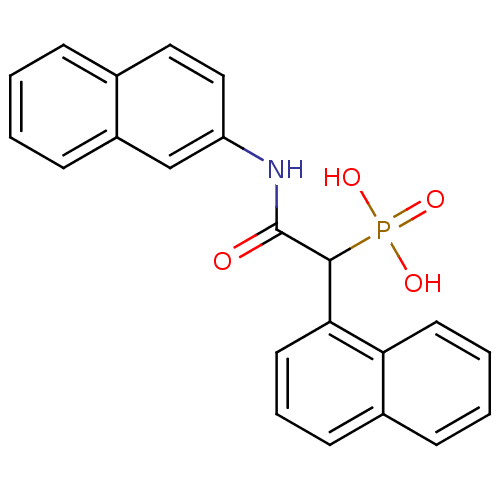 (1-(naphthalen-1-yl)-2-(naphthalen-2-ylamino)-2-oxo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of human skin chymase | J Med Chem 50: 1727-30 (2007) Article DOI: 10.1021/jm0700619 BindingDB Entry DOI: 10.7270/Q26W99RF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM50208238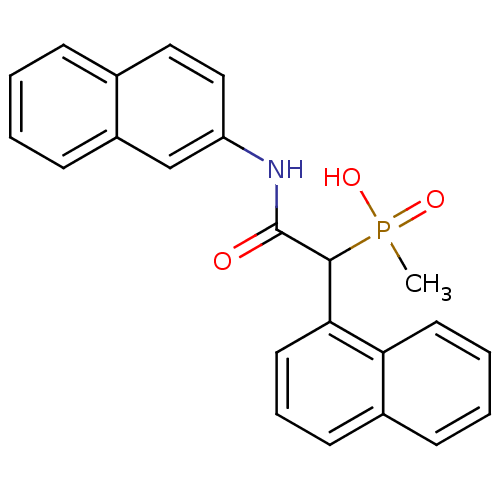 (CHEMBL223624 | methyl(1-(naphthalen-1-yl)-2-(napht...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of human skin chymase | J Med Chem 50: 1727-30 (2007) Article DOI: 10.1021/jm0700619 BindingDB Entry DOI: 10.7270/Q26W99RF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM50208220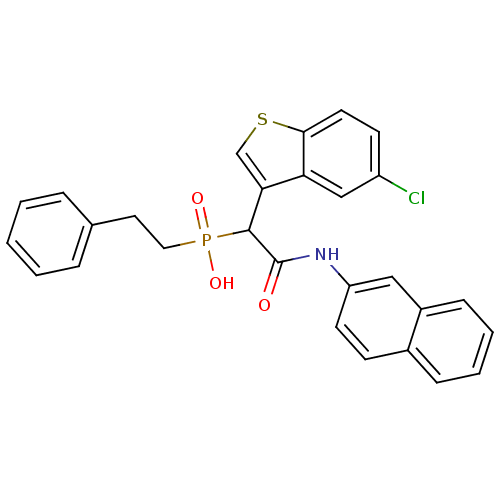 (1-(5-chlorobenzo[b]thiophen-3-yl)-2-(naphthalen-2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of human skin chymase | J Med Chem 50: 1727-30 (2007) Article DOI: 10.1021/jm0700619 BindingDB Entry DOI: 10.7270/Q26W99RF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM50208219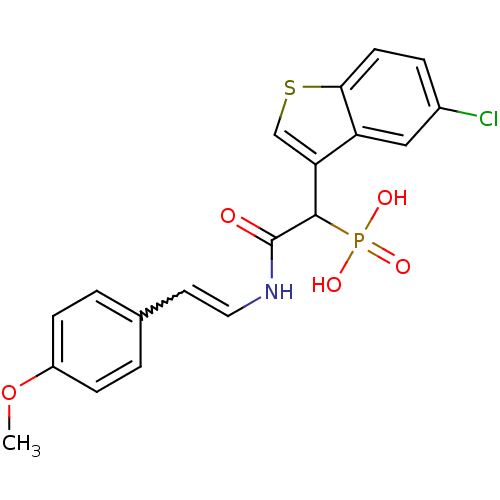 ((E)-2-(4-methoxystyrylamino)-1-(5-chlorobenzo[b]th...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 280 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of human skin chymase | J Med Chem 50: 1727-30 (2007) Article DOI: 10.1021/jm0700619 BindingDB Entry DOI: 10.7270/Q26W99RF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 1 (Homo sapiens (Human)) | BDBM50031392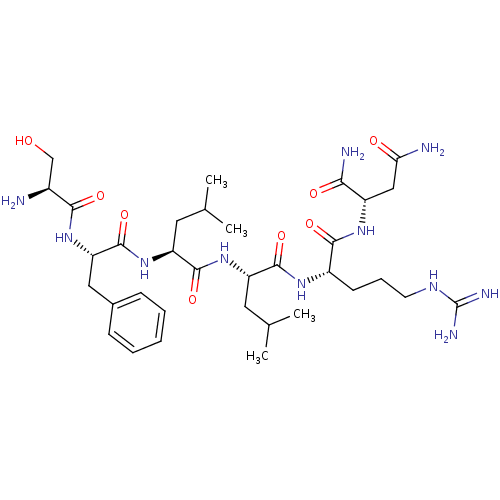 ((S)-2-[(S)-2-((S)-2-{(S)-2-[(S)-2-((S)-2-Amino-3-h...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 550 | n/a | n/a | n/a | n/a | n/a | n/a |
The R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Activation of human platelet aggregation (gel-filtered platelets) induced by alpha thrombin | Bioorg Med Chem Lett 9: 1423-8 (1999) BindingDB Entry DOI: 10.7270/Q2R78DFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM50208231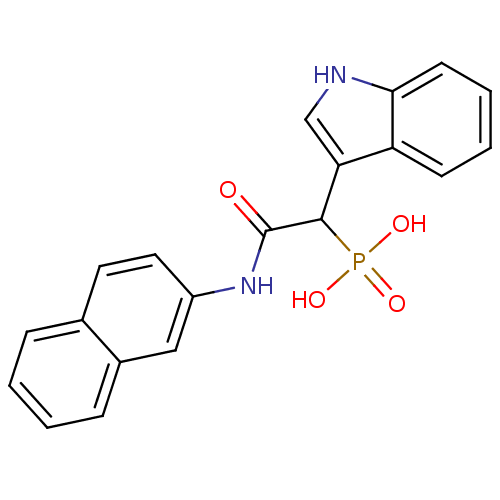 (1-(1H-indol-3-yl)-2-(naphthalen-2-ylamino)-2-oxoet...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 920 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of human skin chymase | J Med Chem 50: 1727-30 (2007) Article DOI: 10.1021/jm0700619 BindingDB Entry DOI: 10.7270/Q26W99RF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 1 (Homo sapiens (Human)) | BDBM50077725 (5-[(E)-3-(4-Fluoro-phenyl)-acryloylamino]-1H-[1,2,...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of [3H]-S-(p-F-Phe)-Har-L-Har-KY-NH2 binding to Thrombin receptor 1 (PAR-1) on membranes from CHRF cells | Bioorg Med Chem Lett 9: 1423-8 (1999) BindingDB Entry DOI: 10.7270/Q2R78DFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 1 (Homo sapiens (Human)) | BDBM50077730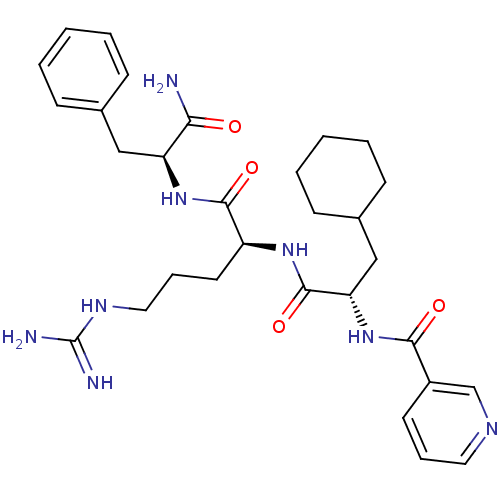 (CHEMBL284498 | N-{(S)-1-[(S)-1-((S)-1-Carbamoyl-2-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Activation of human platelet aggregation (gel-filtered platelets) induced by alpha thrombin | Bioorg Med Chem Lett 9: 1423-8 (1999) BindingDB Entry DOI: 10.7270/Q2R78DFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 1 (Homo sapiens (Human)) | BDBM50077723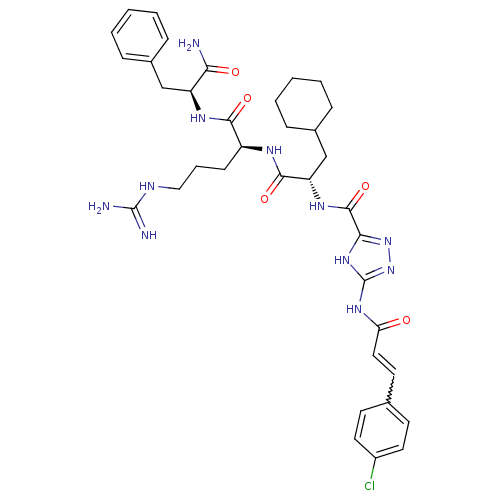 (5-[(E)-3-(4-Chloro-phenyl)-acryloylamino]-1H-[1,2,...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of [3H]-S-(p-F-Phe)-Har-L-Har-KY-NH2 binding to Thrombin receptor 1 (PAR-1) on membranes from CHRF cells | Bioorg Med Chem Lett 9: 1423-8 (1999) BindingDB Entry DOI: 10.7270/Q2R78DFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 1 (Homo sapiens (Human)) | BDBM50077741 (5-[(E)-3-(2-Chloro-phenyl)-acryloylamino]-1H-[1,2,...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of [3H]-S-(p-F-Phe)-Har-L-Har-KY-NH2 binding to thrombin receptor (PAR-1) on membranes from CHRF cells | Bioorg Med Chem Lett 9: 1423-8 (1999) BindingDB Entry DOI: 10.7270/Q2R78DFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 1 (Homo sapiens (Human)) | BDBM50077751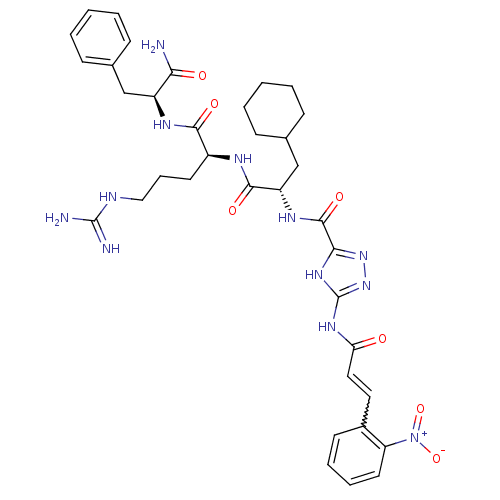 (5-[(E)-3-(2-Nitro-phenyl)-acryloylamino]-1H-[1,2,4...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of [3H]-S-(p-F-Phe)-Har-L-Har-KY-NH2 binding to thrombin receptor (PAR-1) on membranes from CHRF cells | Bioorg Med Chem Lett 9: 1423-8 (1999) BindingDB Entry DOI: 10.7270/Q2R78DFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 1 (Homo sapiens (Human)) | BDBM50077747 (4-Oxo-4H-chromene-2-carboxylic acid {(S)-1-[(S)-1-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of [3H]-S-(p-F-Phe)-Har-L-Har-KY-NH2 binding to thrombin receptor (PAR-1) on membranes from CHRF cells | Bioorg Med Chem Lett 9: 1423-8 (1999) BindingDB Entry DOI: 10.7270/Q2R78DFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 1 (Homo sapiens (Human)) | BDBM50077726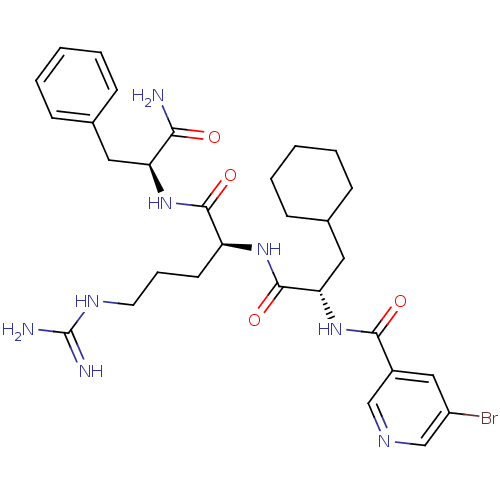 (5-Bromo-N-{(S)-1-[(S)-1-((S)-1-carbamoyl-2-phenyl-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Activation of human platelet aggregation (gel-filtered platelets) induced by alpha thrombin | Bioorg Med Chem Lett 9: 1423-8 (1999) BindingDB Entry DOI: 10.7270/Q2R78DFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 1 (Homo sapiens (Human)) | BDBM50077748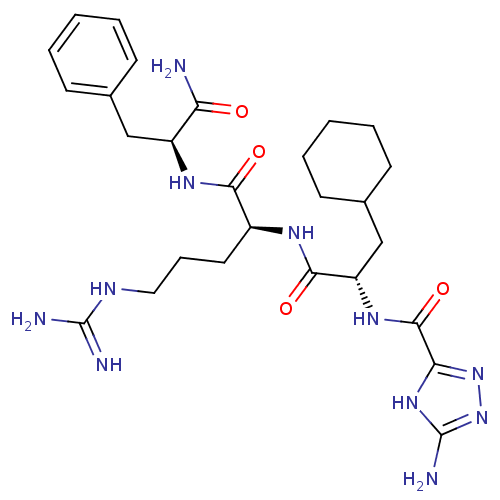 (5-Amino-1H-[1,2,4]triazole-3-carboxylic acid {(S)-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of [3H]-S-(p-F-Phe)-Har-L-Har-KY-NH2 binding to thrombin receptor (PAR-1) on membranes from CHRF cells | Bioorg Med Chem Lett 9: 1423-8 (1999) BindingDB Entry DOI: 10.7270/Q2R78DFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 1 (Homo sapiens (Human)) | BDBM50077744 (5-Amino-1H-[1,2,4]triazole-3-carboxylic acid {(S)-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of [3H]-S-(p-F-Phe)-Har-L-Har-KY-NH2 binding to thrombin receptor (PAR-1) on membranes from CHRF cells | Bioorg Med Chem Lett 9: 1423-8 (1999) BindingDB Entry DOI: 10.7270/Q2R78DFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 1 (Homo sapiens (Human)) | BDBM50077764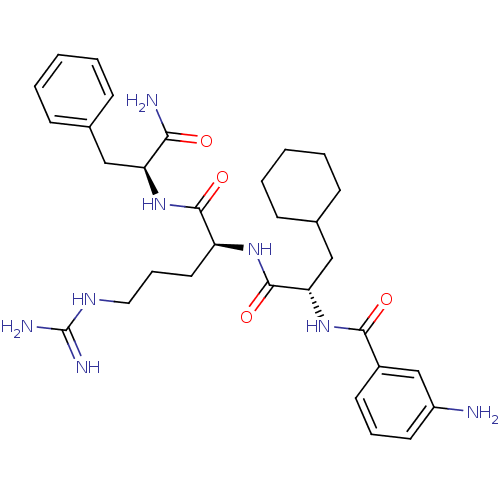 (3-Amino-N-{(S)-1-[(S)-1-((S)-1-carbamoyl-2-phenyl-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Activation of human platelet aggregation (gel-filtered platelets) induced by alpha thrombin | Bioorg Med Chem Lett 9: 1423-8 (1999) BindingDB Entry DOI: 10.7270/Q2R78DFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 1 (Homo sapiens (Human)) | BDBM50077746 (5-Amino-1H-[1,2,4]triazole-3-carboxylic acid {(S)-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of [3H]-S-(p-F-Phe)-Har-L-Har-KY-NH2 binding to thrombin receptor (PAR-1) on membranes from CHRF cells | Bioorg Med Chem Lett 9: 1423-8 (1999) BindingDB Entry DOI: 10.7270/Q2R78DFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 1 (Homo sapiens (Human)) | BDBM50077750 (6-Amino-N-{(S)-1-[(S)-1-((S)-1-carbamoyl-2-phenyl-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of [3H]-S-(p-F-Phe)-Har-L-Har-KY-NH2 binding to thrombin receptor (PAR-1) on membranes from CHRF cells | Bioorg Med Chem Lett 9: 1423-8 (1999) BindingDB Entry DOI: 10.7270/Q2R78DFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM50208240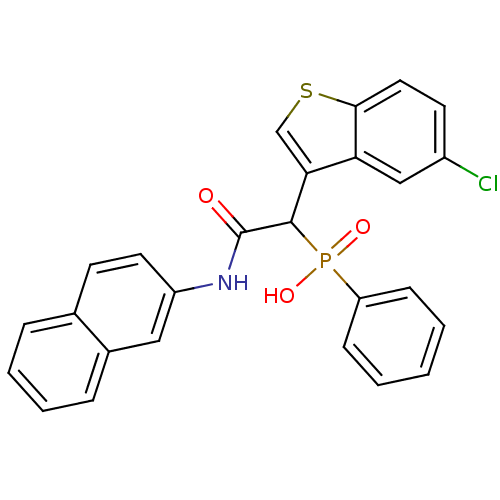 (1-(5-chlorobenzo[b]thiophen-3-yl)-2-(naphthalen-2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of human skin chymase | J Med Chem 50: 1727-30 (2007) Article DOI: 10.1021/jm0700619 BindingDB Entry DOI: 10.7270/Q26W99RF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 1 (Homo sapiens (Human)) | BDBM50077749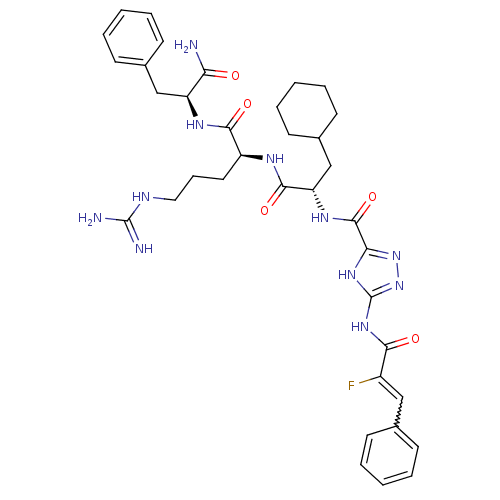 (5-((Z)-2-Fluoro-3-phenyl-acryloylamino)-1H-[1,2,4]...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of [3H]-S-(p-F-Phe)-Har-L-Har-KY-NH2 binding to thrombin receptor (PAR-1) on membranes from CHRF cells | Bioorg Med Chem Lett 9: 1423-8 (1999) BindingDB Entry DOI: 10.7270/Q2R78DFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 1 (Homo sapiens (Human)) | BDBM50077742 (2-Amino-N-{(S)-1-[(S)-1-((S)-1-carbamoyl-2-phenyl-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of [3H]-S-(p-F-Phe)-Har-L-Har-KY-NH2 binding to thrombin receptor (PAR-1) on membranes from CHRF cells | Bioorg Med Chem Lett 9: 1423-8 (1999) BindingDB Entry DOI: 10.7270/Q2R78DFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 1 (Homo sapiens (Human)) | BDBM50077733 (Benzo[b]thiophene-2-carboxylic acid {(S)-1-[(S)-1-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of [3H]-S-(p-F-Phe)-Har-L-Har-KY-NH2 binding to Thrombin receptor 1 (PAR-1) on membranes from CHRF cells | Bioorg Med Chem Lett 9: 1423-8 (1999) BindingDB Entry DOI: 10.7270/Q2R78DFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 1 (Homo sapiens (Human)) | BDBM50077736 (CHEMBL33945 | N-{(S)-1-[(S)-1-((S)-1-Carbamoyl-2-p...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of [3H]-S-(p-F-Phe)-Har-L-Har-KY-NH2 binding to Thrombin receptor 1 (PAR-1) on membranes from CHRF cells | Bioorg Med Chem Lett 9: 1423-8 (1999) BindingDB Entry DOI: 10.7270/Q2R78DFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 1 (Homo sapiens (Human)) | BDBM50077728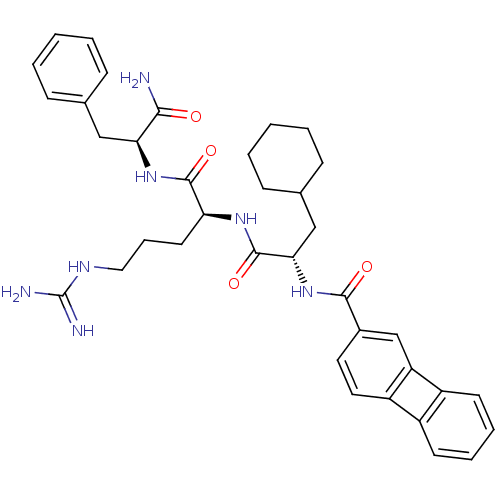 (Biphenylene-2-carboxylic acid {(S)-1-[(S)-1-((S)-1...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Activation of human platelet aggregation (gel-filtered platelets) induced by alpha thrombin | Bioorg Med Chem Lett 9: 1423-8 (1999) BindingDB Entry DOI: 10.7270/Q2R78DFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM50208233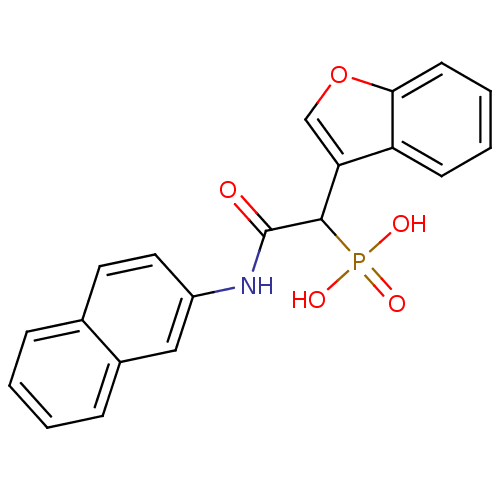 (1-(benzofuran-3-yl)-2-(naphthalen-2-ylamino)-2-oxo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of human skin chymase | J Med Chem 50: 1727-30 (2007) Article DOI: 10.1021/jm0700619 BindingDB Entry DOI: 10.7270/Q26W99RF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 1 (Homo sapiens (Human)) | BDBM50077753 (CHEMBL287270 | N-{(S)-1-[(S)-1-((S)-1-Carbamoyl-2-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of [3H]-S-(p-F-Phe)-Har-L-Har-KY-NH2 binding to Thrombin receptor 1 (PAR-1) on membranes from CHRF cells | Bioorg Med Chem Lett 9: 1423-8 (1999) BindingDB Entry DOI: 10.7270/Q2R78DFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 1 (Homo sapiens (Human)) | BDBM50077737 (4-Amino-N-{(S)-1-[(S)-1-((S)-1-carbamoyl-2-phenyl-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of [3H]-S-(p-F-Phe)-Har-L-Har-KY-NH2 binding to thrombin receptor (PAR-1) on membranes from CHRF cells | Bioorg Med Chem Lett 9: 1423-8 (1999) BindingDB Entry DOI: 10.7270/Q2R78DFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 128 total ) | Next | Last >> |