 Found 37 hits with Last Name = 'hollenberg' and Initial = 'nk'
Found 37 hits with Last Name = 'hollenberg' and Initial = 'nk' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Renin
(Homo sapiens (Human)) | BDBM50021989
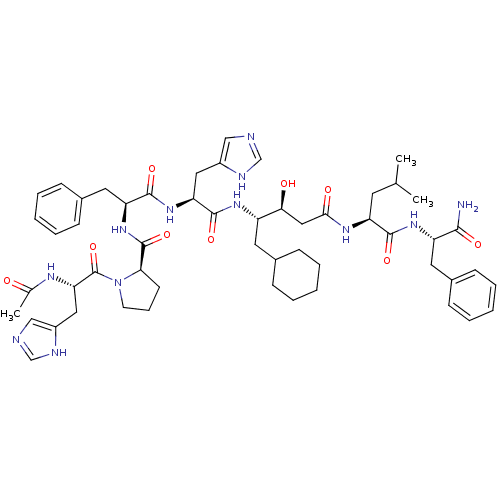
(Ac-His-Pro-Phe-His-ACHPA-Leu-Phe-NH2 | CHEMBL38686...)Show SMILES CC(C)C[C@H](NC(=O)C[C@H](O)[C@H](CC1CCCCC1)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H]1CCCN1C(=O)[C@H](Cc1cnc[nH]1)NC(C)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C54H74N12O9/c1-33(2)22-42(50(71)63-41(49(55)70)24-36-16-9-5-10-17-36)61-48(69)28-47(68)40(23-35-14-7-4-8-15-35)62-52(73)44(26-38-29-56-31-58-38)64-51(72)43(25-37-18-11-6-12-19-37)65-53(74)46-20-13-21-66(46)54(75)45(60-34(3)67)27-39-30-57-32-59-39/h5-6,9-12,16-19,29-33,35,40-47,68H,4,7-8,13-15,20-28H2,1-3H3,(H2,55,70)(H,56,58)(H,57,59)(H,60,67)(H,61,69)(H,62,73)(H,63,71)(H,64,72)(H,65,74)/t40-,41-,42-,43-,44-,45-,46+,47-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Massachusetts General Hospital
Curated by ChEMBL
| Assay Description
Compound was tested for its inhibitory activity against human plasma renin |
J Med Chem 31: 1679-86 (1988)
BindingDB Entry DOI: 10.7270/Q2NC61TR |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50022002
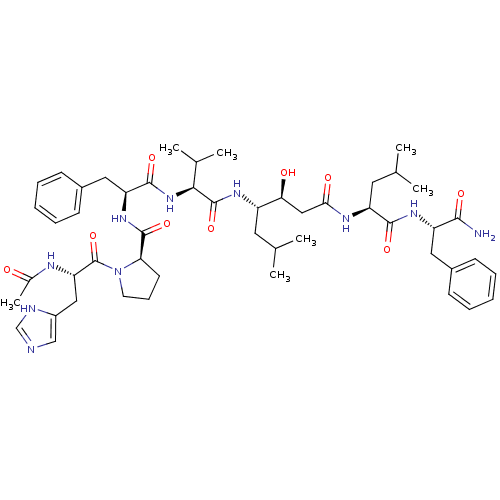
(1-[2-Acetylamino-3-(3H-imidazol-4-yl)-propionyl]-p...)Show SMILES CC(C)C[C@H](NC(=O)[C@@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H]1CCCN1C(=O)[C@H](Cc1cnc[nH]1)NC(C)=O)C(C)C)[C@@H](O)CC(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C50H72N10O9/c1-29(2)21-36(42(62)26-43(63)55-38(22-30(3)4)46(65)57-37(45(51)64)23-33-15-10-8-11-16-33)56-49(68)44(31(5)6)59-47(66)39(24-34-17-12-9-13-18-34)58-48(67)41-19-14-20-60(41)50(69)40(54-32(7)61)25-35-27-52-28-53-35/h8-13,15-18,27-31,36-42,44,62H,14,19-26H2,1-7H3,(H2,51,64)(H,52,53)(H,54,61)(H,55,63)(H,56,68)(H,57,65)(H,58,67)(H,59,66)/t36-,37-,38-,39-,40-,41+,42-,44-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Massachusetts General Hospital
Curated by ChEMBL
| Assay Description
Compound was tested for its inhibitory activity against human plasma renin |
J Med Chem 31: 1679-86 (1988)
BindingDB Entry DOI: 10.7270/Q2NC61TR |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50022005

(Ac-His-Pro-Phe-His-Statine-Leu-Phe-NH2 | CHEMBL266...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H]1CCCN1C(=O)[C@H](Cc1cnc[nH]1)NC(C)=O)[C@@H](O)CC(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C51H70N12O9/c1-30(2)19-37(44(65)25-45(66)58-39(20-31(3)4)47(68)60-38(46(52)67)21-33-13-8-6-9-14-33)59-49(70)41(23-35-26-53-28-55-35)61-48(69)40(22-34-15-10-7-11-16-34)62-50(71)43-17-12-18-63(43)51(72)42(57-32(5)64)24-36-27-54-29-56-36/h6-11,13-16,26-31,37-44,65H,12,17-25H2,1-5H3,(H2,52,67)(H,53,55)(H,54,56)(H,57,64)(H,58,66)(H,59,70)(H,60,68)(H,61,69)(H,62,71)/t37-,38-,39-,40-,41-,42-,43+,44-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Massachusetts General Hospital
Curated by ChEMBL
| Assay Description
Compound was tested for its inhibitory activity against human plasma renin |
J Med Chem 31: 1679-86 (1988)
BindingDB Entry DOI: 10.7270/Q2NC61TR |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50022007
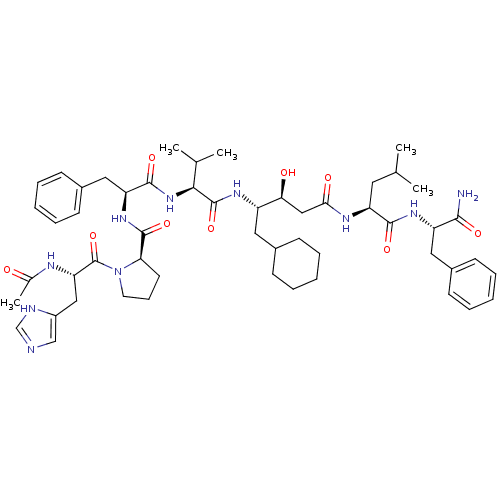
(1-[2-Acetylamino-3-(3H-imidazol-4-yl)-propionyl]-p...)Show SMILES CC(C)C[C@H](NC(=O)C[C@H](O)[C@H](CC1CCCCC1)NC(=O)[C@@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H]1CCCN1C(=O)[C@H](Cc1cnc[nH]1)NC(C)=O)C(C)C)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C53H76N10O9/c1-32(2)24-41(49(68)60-40(48(54)67)26-36-18-11-7-12-19-36)58-46(66)29-45(65)39(25-35-16-9-6-10-17-35)59-52(71)47(33(3)4)62-50(69)42(27-37-20-13-8-14-21-37)61-51(70)44-22-15-23-63(44)53(72)43(57-34(5)64)28-38-30-55-31-56-38/h7-8,11-14,18-21,30-33,35,39-45,47,65H,6,9-10,15-17,22-29H2,1-5H3,(H2,54,67)(H,55,56)(H,57,64)(H,58,66)(H,59,71)(H,60,68)(H,61,70)(H,62,69)/t39-,40-,41-,42-,43-,44+,45-,47-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Massachusetts General Hospital
Curated by ChEMBL
| Assay Description
Compound was tested for its inhibitory activity against human plasma renin |
J Med Chem 31: 1679-86 (1988)
BindingDB Entry DOI: 10.7270/Q2NC61TR |
More data for this
Ligand-Target Pair | |
Renin
(Rattus norvegicus) | BDBM50022002

(1-[2-Acetylamino-3-(3H-imidazol-4-yl)-propionyl]-p...)Show SMILES CC(C)C[C@H](NC(=O)[C@@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H]1CCCN1C(=O)[C@H](Cc1cnc[nH]1)NC(C)=O)C(C)C)[C@@H](O)CC(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C50H72N10O9/c1-29(2)21-36(42(62)26-43(63)55-38(22-30(3)4)46(65)57-37(45(51)64)23-33-15-10-8-11-16-33)56-49(68)44(31(5)6)59-47(66)39(24-34-17-12-9-13-18-34)58-48(67)41-19-14-20-60(41)50(69)40(54-32(7)61)25-35-27-52-28-53-35/h8-13,15-18,27-31,36-42,44,62H,14,19-26H2,1-7H3,(H2,51,64)(H,52,53)(H,54,61)(H,55,63)(H,56,68)(H,57,65)(H,58,67)(H,59,66)/t36-,37-,38-,39-,40-,41+,42-,44-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Massachusetts General Hospital
Curated by ChEMBL
| Assay Description
Inhibitory activity against rat plasma renin in vitro. |
J Med Chem 31: 1679-86 (1988)
BindingDB Entry DOI: 10.7270/Q2NC61TR |
More data for this
Ligand-Target Pair | |
Renin
(Rattus norvegicus) | BDBM50022007

(1-[2-Acetylamino-3-(3H-imidazol-4-yl)-propionyl]-p...)Show SMILES CC(C)C[C@H](NC(=O)C[C@H](O)[C@H](CC1CCCCC1)NC(=O)[C@@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H]1CCCN1C(=O)[C@H](Cc1cnc[nH]1)NC(C)=O)C(C)C)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C53H76N10O9/c1-32(2)24-41(49(68)60-40(48(54)67)26-36-18-11-7-12-19-36)58-46(66)29-45(65)39(25-35-16-9-6-10-17-35)59-52(71)47(33(3)4)62-50(69)42(27-37-20-13-8-14-21-37)61-51(70)44-22-15-23-63(44)53(72)43(57-34(5)64)28-38-30-55-31-56-38/h7-8,11-14,18-21,30-33,35,39-45,47,65H,6,9-10,15-17,22-29H2,1-5H3,(H2,54,67)(H,55,56)(H,57,64)(H,58,66)(H,59,71)(H,60,68)(H,61,70)(H,62,69)/t39-,40-,41-,42-,43-,44+,45-,47-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
Massachusetts General Hospital
Curated by ChEMBL
| Assay Description
Inhibitory activity against rat plasma renin in vitro. |
J Med Chem 31: 1679-86 (1988)
BindingDB Entry DOI: 10.7270/Q2NC61TR |
More data for this
Ligand-Target Pair | |
Renin
(Rattus norvegicus) | BDBM50021993

(1-[2-Acetylamino-3-(4-methoxy-phenyl)-propionyl]-p...)Show SMILES COc1ccc(C[C@H](NC(C)=O)C(=O)N2CCC[C@@H]2C(=O)N[C@@H](Cc2ccccc2)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CC(C)C)[C@@H](O)CC(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](Cc2ccccc2)C(N)=O)cc1 Show InChI InChI=1S/C54H76N8O10/c1-32(2)26-40(46(64)31-47(65)57-42(27-33(3)4)50(67)59-41(49(55)66)28-36-16-11-9-12-17-36)58-53(70)48(34(5)6)61-51(68)43(29-37-18-13-10-14-19-37)60-52(69)45-20-15-25-62(45)54(71)44(56-35(7)63)30-38-21-23-39(72-8)24-22-38/h9-14,16-19,21-24,32-34,40-46,48,64H,15,20,25-31H2,1-8H3,(H2,55,66)(H,56,63)(H,57,65)(H,58,70)(H,59,67)(H,60,69)(H,61,68)/t40-,41-,42-,43-,44-,45+,46-,48-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
Massachusetts General Hospital
Curated by ChEMBL
| Assay Description
Inhibitory activity against rat plasma renin in vitro. |
J Med Chem 31: 1679-86 (1988)
BindingDB Entry DOI: 10.7270/Q2NC61TR |
More data for this
Ligand-Target Pair | |
Renin
(Rattus norvegicus) | BDBM50022021
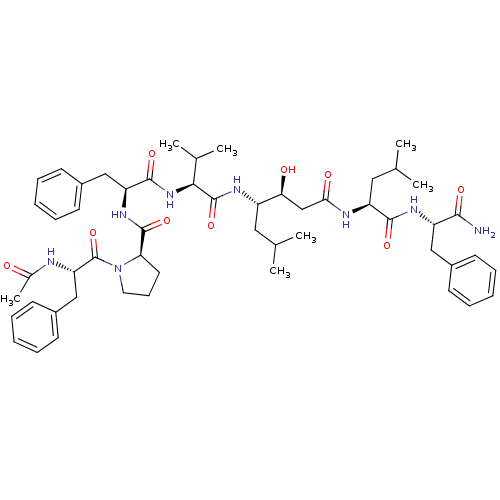
(1-(2-Acetylamino-3-phenyl-propionyl)-pyrrolidine-2...)Show SMILES CC(C)C[C@H](NC(=O)[C@@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H]1CCCN1C(=O)[C@H](Cc1ccccc1)NC(C)=O)C(C)C)[C@@H](O)CC(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C53H74N8O9/c1-32(2)26-39(45(63)31-46(64)56-41(27-33(3)4)49(66)58-40(48(54)65)28-36-18-11-8-12-19-36)57-52(69)47(34(5)6)60-50(67)42(29-37-20-13-9-14-21-37)59-51(68)44-24-17-25-61(44)53(70)43(55-35(7)62)30-38-22-15-10-16-23-38/h8-16,18-23,32-34,39-45,47,63H,17,24-31H2,1-7H3,(H2,54,65)(H,55,62)(H,56,64)(H,57,69)(H,58,66)(H,59,68)(H,60,67)/t39-,40-,41-,42-,43-,44+,45-,47-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
Massachusetts General Hospital
Curated by ChEMBL
| Assay Description
Inhibitory activity against rat plasma renin in vitro. |
J Med Chem 31: 1679-86 (1988)
BindingDB Entry DOI: 10.7270/Q2NC61TR |
More data for this
Ligand-Target Pair | |
Renin
(Rattus norvegicus) | BDBM50022015

(1-(2-Acetylamino-3-naphthalen-1-yl-propionyl)-pyrr...)Show SMILES CC(C)C[C@H](NC(=O)[C@@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H]1CCCN1C(=O)[C@H](Cc1cccc2ccccc12)NC(C)=O)C(C)C)[C@@H](O)CC(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C57H76N8O9/c1-34(2)28-43(49(67)33-50(68)60-45(29-35(3)4)53(70)62-44(52(58)69)30-38-18-10-8-11-19-38)61-56(73)51(36(5)6)64-54(71)46(31-39-20-12-9-13-21-39)63-55(72)48-26-17-27-65(48)57(74)47(59-37(7)66)32-41-24-16-23-40-22-14-15-25-42(40)41/h8-16,18-25,34-36,43-49,51,67H,17,26-33H2,1-7H3,(H2,58,69)(H,59,66)(H,60,68)(H,61,73)(H,62,70)(H,63,72)(H,64,71)/t43-,44-,45-,46-,47-,48+,49-,51-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Massachusetts General Hospital
Curated by ChEMBL
| Assay Description
Inhibitory activity against rat plasma renin in vitro. |
J Med Chem 31: 1679-86 (1988)
BindingDB Entry DOI: 10.7270/Q2NC61TR |
More data for this
Ligand-Target Pair | |
Renin
(Rattus norvegicus) | BDBM50021989

(Ac-His-Pro-Phe-His-ACHPA-Leu-Phe-NH2 | CHEMBL38686...)Show SMILES CC(C)C[C@H](NC(=O)C[C@H](O)[C@H](CC1CCCCC1)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H]1CCCN1C(=O)[C@H](Cc1cnc[nH]1)NC(C)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C54H74N12O9/c1-33(2)22-42(50(71)63-41(49(55)70)24-36-16-9-5-10-17-36)61-48(69)28-47(68)40(23-35-14-7-4-8-15-35)62-52(73)44(26-38-29-56-31-58-38)64-51(72)43(25-37-18-11-6-12-19-37)65-53(74)46-20-13-21-66(46)54(75)45(60-34(3)67)27-39-30-57-32-59-39/h5-6,9-12,16-19,29-33,35,40-47,68H,4,7-8,13-15,20-28H2,1-3H3,(H2,55,70)(H,56,58)(H,57,59)(H,60,67)(H,61,69)(H,62,73)(H,63,71)(H,64,72)(H,65,74)/t40-,41-,42-,43-,44-,45-,46+,47-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Massachusetts General Hospital
Curated by ChEMBL
| Assay Description
Inhibitory activity against rat plasma renin in vitro. |
J Med Chem 31: 1679-86 (1988)
BindingDB Entry DOI: 10.7270/Q2NC61TR |
More data for this
Ligand-Target Pair | |
Renin
(Rattus norvegicus) | BDBM50367761

(CHEMBL1790252)Show SMILES CC[C@@H](C)[C@H](NC(=O)C[C@H](O)[C@H](CC1CCCCC1)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H]1CCCN1C(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H]1CCCN1)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C57H79N13O9/c1-3-35(2)50(56(78)65-43(51(58)73)26-37-17-9-5-10-18-37)69-49(72)30-48(71)42(25-36-15-7-4-8-16-36)64-54(76)45(28-39-31-59-33-62-39)66-53(75)44(27-38-19-11-6-12-20-38)67-55(77)47-22-14-24-70(47)57(79)46(29-40-32-60-34-63-40)68-52(74)41-21-13-23-61-41/h5-6,9-12,17-20,31-36,41-48,50,61,71H,3-4,7-8,13-16,21-30H2,1-2H3,(H2,58,73)(H,59,62)(H,60,63)(H,64,76)(H,65,78)(H,66,75)(H,67,77)(H,68,74)(H,69,72)/t35-,41-,42+,43+,44+,45+,46+,47-,48+,50+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 65 | n/a | n/a | n/a | n/a | n/a | n/a |
Massachusetts General Hospital
Curated by ChEMBL
| Assay Description
Inhibitory activity against rat plasma renin in vitro. |
J Med Chem 31: 1679-86 (1988)
BindingDB Entry DOI: 10.7270/Q2NC61TR |
More data for this
Ligand-Target Pair | |
Renin
(Rattus norvegicus) | BDBM50021994
(1-[2-Acetylamino-3-(3H-imidazol-4-yl)-propionyl]-p...)Show SMILES CC(C)C[C@H](NC(=O)C[C@H](O)[C@H](Cc1ccccc1)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H]1CCCN1C(=O)[C@H](Cc1cnc[nH]1)NC(C)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C54H68N12O9/c1-33(2)22-42(50(71)63-41(49(55)70)24-36-16-9-5-10-17-36)61-48(69)28-47(68)40(23-35-14-7-4-8-15-35)62-52(73)44(26-38-29-56-31-58-38)64-51(72)43(25-37-18-11-6-12-19-37)65-53(74)46-20-13-21-66(46)54(75)45(60-34(3)67)27-39-30-57-32-59-39/h4-12,14-19,29-33,40-47,68H,13,20-28H2,1-3H3,(H2,55,70)(H,56,58)(H,57,59)(H,60,67)(H,61,69)(H,62,73)(H,63,71)(H,64,72)(H,65,74)/t40-,41-,42-,43-,44-,45-,46+,47-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Massachusetts General Hospital
Curated by ChEMBL
| Assay Description
Inhibitory activity against rat plasma renin in vitro. |
J Med Chem 31: 1679-86 (1988)
BindingDB Entry DOI: 10.7270/Q2NC61TR |
More data for this
Ligand-Target Pair | |
Renin
(Rattus norvegicus) | BDBM50022006

(Ac-His-Pro-Phe-His-Statine-Leu-CHA-NH2 | CHEMBL217...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H]1CCCN1C(=O)[C@H](Cc1cnc[nH]1)NC(C)=O)[C@@H](O)CC(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C1CCCCC1)C(N)=O Show InChI InChI=1S/C50H74N12O9/c1-29(2)19-36(42(64)24-43(65)57-37(20-30(3)4)48(69)61-44(45(51)66)33-15-10-7-11-16-33)58-47(68)39(22-34-25-52-27-54-34)59-46(67)38(21-32-13-8-6-9-14-32)60-49(70)41-17-12-18-62(41)50(71)40(56-31(5)63)23-35-26-53-28-55-35/h6,8-9,13-14,25-30,33,36-42,44,64H,7,10-12,15-24H2,1-5H3,(H2,51,66)(H,52,54)(H,53,55)(H,56,63)(H,57,65)(H,58,68)(H,59,67)(H,60,70)(H,61,69)/t36-,37-,38-,39-,40-,41+,42-,44-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Massachusetts General Hospital
Curated by ChEMBL
| Assay Description
Inhibitory activity against rat plasma renin in vitro. |
J Med Chem 31: 1679-86 (1988)
BindingDB Entry DOI: 10.7270/Q2NC61TR |
More data for this
Ligand-Target Pair | |
Renin
(Rattus norvegicus) | BDBM50022005

(Ac-His-Pro-Phe-His-Statine-Leu-Phe-NH2 | CHEMBL266...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H]1CCCN1C(=O)[C@H](Cc1cnc[nH]1)NC(C)=O)[C@@H](O)CC(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C51H70N12O9/c1-30(2)19-37(44(65)25-45(66)58-39(20-31(3)4)47(68)60-38(46(52)67)21-33-13-8-6-9-14-33)59-49(70)41(23-35-26-53-28-55-35)61-48(69)40(22-34-15-10-7-11-16-34)62-50(71)43-17-12-18-63(43)51(72)42(57-32(5)64)24-36-27-54-29-56-36/h6-11,13-16,26-31,37-44,65H,12,17-25H2,1-5H3,(H2,52,67)(H,53,55)(H,54,56)(H,57,64)(H,58,66)(H,59,70)(H,60,68)(H,61,69)(H,62,71)/t37-,38-,39-,40-,41-,42-,43+,44-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
Massachusetts General Hospital
Curated by ChEMBL
| Assay Description
Inhibitory activity against rat plasma renin in vitro. |
J Med Chem 31: 1679-86 (1988)
BindingDB Entry DOI: 10.7270/Q2NC61TR |
More data for this
Ligand-Target Pair | |
Renin
(Rattus norvegicus) | BDBM50022001
(Ac-His-Pro-Napa-His-Statine-Leu-Phe-NH2 | CHEMBL27...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](Cc1cccc2ccccc12)NC(=O)[C@H]1CCCN1C(=O)[C@H](Cc1cnc[nH]1)NC(C)=O)[C@@H](O)CC(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C55H72N12O9/c1-32(2)21-41(48(69)27-49(70)62-43(22-33(3)4)51(72)64-42(50(56)71)23-35-13-7-6-8-14-35)63-53(74)45(25-38-28-57-30-59-38)65-52(73)44(24-37-17-11-16-36-15-9-10-18-40(36)37)66-54(75)47-19-12-20-67(47)55(76)46(61-34(5)68)26-39-29-58-31-60-39/h6-11,13-18,28-33,41-48,69H,12,19-27H2,1-5H3,(H2,56,71)(H,57,59)(H,58,60)(H,61,68)(H,62,70)(H,63,74)(H,64,72)(H,65,73)(H,66,75)/t41-,42-,43-,44-,45-,46-,47+,48-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
Massachusetts General Hospital
Curated by ChEMBL
| Assay Description
Inhibitory activity against rat plasma renin in vitro. |
J Med Chem 31: 1679-86 (1988)
BindingDB Entry DOI: 10.7270/Q2NC61TR |
More data for this
Ligand-Target Pair | |
Renin
(Rattus norvegicus) | BDBM50022008

(Ac-His-Pro-Trp-His-Statine-Leu-Phe-NH2 | CHEMBL384...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H]1CCCN1C(=O)[C@H](Cc1cnc[nH]1)NC(C)=O)[C@@H](O)CC(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C53H71N13O9/c1-30(2)18-39(46(68)24-47(69)61-41(19-31(3)4)49(71)63-40(48(54)70)20-33-12-7-6-8-13-33)62-51(73)43(22-35-26-55-28-58-35)64-50(72)42(21-34-25-57-38-15-10-9-14-37(34)38)65-52(74)45-16-11-17-66(45)53(75)44(60-32(5)67)23-36-27-56-29-59-36/h6-10,12-15,25-31,39-46,57,68H,11,16-24H2,1-5H3,(H2,54,70)(H,55,58)(H,56,59)(H,60,67)(H,61,69)(H,62,73)(H,63,71)(H,64,72)(H,65,74)/t39-,40-,41-,42-,43-,44-,45+,46-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
Massachusetts General Hospital
Curated by ChEMBL
| Assay Description
Inhibitory activity against rat plasma renin in vitro. |
J Med Chem 31: 1679-86 (1988)
BindingDB Entry DOI: 10.7270/Q2NC61TR |
More data for this
Ligand-Target Pair | |
Renin
(Rattus norvegicus) | BDBM50022017
(CHEMBL414537 | Pro-His-Pro-Phe-His-Statine-Leu-Phe...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H]1CCCN1C(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H]1CCCN1)[C@@H](O)CC(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C54H75N13O9/c1-32(2)21-39(46(68)27-47(69)61-41(22-33(3)4)50(72)63-40(48(55)70)23-34-13-7-5-8-14-34)62-52(74)43(25-36-28-56-30-59-36)64-51(73)42(24-35-15-9-6-10-16-35)65-53(75)45-18-12-20-67(45)54(76)44(26-37-29-57-31-60-37)66-49(71)38-17-11-19-58-38/h5-10,13-16,28-33,38-46,58,68H,11-12,17-27H2,1-4H3,(H2,55,70)(H,56,59)(H,57,60)(H,61,69)(H,62,74)(H,63,72)(H,64,73)(H,65,75)(H,66,71)/t38-,39+,40+,41+,42+,43+,44+,45-,46+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
Massachusetts General Hospital
Curated by ChEMBL
| Assay Description
Inhibitory activity against rat plasma renin in vitro. |
J Med Chem 31: 1679-86 (1988)
BindingDB Entry DOI: 10.7270/Q2NC61TR |
More data for this
Ligand-Target Pair | |
Renin
(Rattus norvegicus) | BDBM50021996

(Ac-His-Pro-Phe-His-Statine-Leu-Val-NH2 | CHEMBL295...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H]1CCCN1C(=O)[C@H](Cc1cnc[nH]1)NC(C)=O)[C@@H](O)CC(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C(C)C)C(N)=O Show InChI InChI=1S/C47H70N12O9/c1-26(2)16-33(39(61)21-40(62)54-34(17-27(3)4)45(66)58-41(28(5)6)42(48)63)55-44(65)36(19-31-22-49-24-51-31)56-43(64)35(18-30-12-9-8-10-13-30)57-46(67)38-14-11-15-59(38)47(68)37(53-29(7)60)20-32-23-50-25-52-32/h8-10,12-13,22-28,33-39,41,61H,11,14-21H2,1-7H3,(H2,48,63)(H,49,51)(H,50,52)(H,53,60)(H,54,62)(H,55,65)(H,56,64)(H,57,67)(H,58,66)/t33-,34-,35-,36-,37-,38+,39-,41-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 270 | n/a | n/a | n/a | n/a | n/a | n/a |
Massachusetts General Hospital
Curated by ChEMBL
| Assay Description
Inhibitory activity against rat plasma renin in vitro. |
J Med Chem 31: 1679-86 (1988)
BindingDB Entry DOI: 10.7270/Q2NC61TR |
More data for this
Ligand-Target Pair | |
Renin
(Rattus norvegicus) | BDBM50022014
(1-(2-Acetylamino-2-cyclohexyl-acetyl)-pyrrolidine-...)Show SMILES CC(C)C[C@H](NC(=O)[C@@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H]1CCCN1C(=O)[C@@H](NC(C)=O)C1CCCCC1)C(C)C)[C@@H](O)CC(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C52H78N8O9/c1-31(2)26-38(43(62)30-44(63)55-40(27-32(3)4)48(65)57-39(47(53)64)28-35-18-11-8-12-19-35)56-51(68)45(33(5)6)59-49(66)41(29-36-20-13-9-14-21-36)58-50(67)42-24-17-25-60(42)52(69)46(54-34(7)61)37-22-15-10-16-23-37/h8-9,11-14,18-21,31-33,37-43,45-46,62H,10,15-17,22-30H2,1-7H3,(H2,53,64)(H,54,61)(H,55,63)(H,56,68)(H,57,65)(H,58,67)(H,59,66)/t38-,39-,40-,41-,42+,43-,45-,46-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 280 | n/a | n/a | n/a | n/a | n/a | n/a |
Massachusetts General Hospital
Curated by ChEMBL
| Assay Description
Inhibitory activity against rat plasma renin in vitro. |
J Med Chem 31: 1679-86 (1988)
BindingDB Entry DOI: 10.7270/Q2NC61TR |
More data for this
Ligand-Target Pair | |
Renin
(Rattus norvegicus) | BDBM50021997

(1-[2-Acetylamino-3-(3H-imidazol-4-yl)-propionyl]-p...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H]1CCCN1C(=O)[C@H](Cc1cnc[nH]1)NC(C)=O)[C@@H](O)CC(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C51H74N10O9/c1-30(2)21-37(44(63)27-45(64)56-39(22-31(3)4)47(66)58-38(46(52)65)24-34-15-10-8-11-16-34)57-48(67)40(23-32(5)6)59-49(68)41(25-35-17-12-9-13-18-35)60-50(69)43-19-14-20-61(43)51(70)42(55-33(7)62)26-36-28-53-29-54-36/h8-13,15-18,28-32,37-44,63H,14,19-27H2,1-7H3,(H2,52,65)(H,53,54)(H,55,62)(H,56,64)(H,57,67)(H,58,66)(H,59,68)(H,60,69)/t37-,38-,39-,40-,41-,42-,43+,44-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 310 | n/a | n/a | n/a | n/a | n/a | n/a |
Massachusetts General Hospital
Curated by ChEMBL
| Assay Description
Inhibitory activity against rat plasma renin in vitro. |
J Med Chem 31: 1679-86 (1988)
BindingDB Entry DOI: 10.7270/Q2NC61TR |
More data for this
Ligand-Target Pair | |
Renin
(Rattus norvegicus) | BDBM50367760

(CHEMBL2367544)Show SMILES CC[C@H](C)[C@H](NC(=O)C[C@H](O)[C@H](Cc1ccccc1)NC(=O)[C@H](Cc1c[nH]cn1)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H]1CCCN1C(=O)[C@H](Cc1c[nH]cn1)NC(=O)[C@H]1CCCN1)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C57H73N13O9/c1-3-35(2)50(56(78)65-43(51(58)73)26-37-17-9-5-10-18-37)69-49(72)30-48(71)42(25-36-15-7-4-8-16-36)64-54(76)45(28-39-31-59-33-62-39)66-53(75)44(27-38-19-11-6-12-20-38)67-55(77)47-22-14-24-70(47)57(79)46(29-40-32-60-34-63-40)68-52(74)41-21-13-23-61-41/h4-12,15-20,31-35,41-48,50,61,71H,3,13-14,21-30H2,1-2H3,(H2,58,73)(H,59,62)(H,60,63)(H,64,76)(H,65,78)(H,66,75)(H,67,77)(H,68,74)(H,69,72)/t35-,41+,42-,43-,44-,45-,46-,47+,48-,50-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 370 | n/a | n/a | n/a | n/a | n/a | n/a |
Massachusetts General Hospital
Curated by ChEMBL
| Assay Description
Inhibitory activity against rat plasma renin in vitro. |
J Med Chem 31: 1679-86 (1988)
BindingDB Entry DOI: 10.7270/Q2NC61TR |
More data for this
Ligand-Target Pair | |
Renin
(Rattus norvegicus) | BDBM50022019
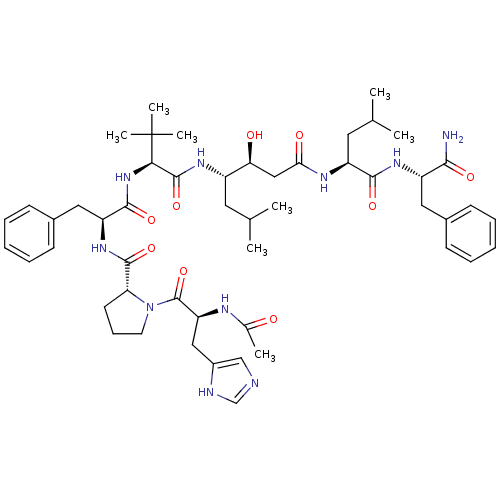
(1-[2-Acetylamino-3-(3H-imidazol-4-yl)-propionyl]-p...)Show SMILES CC(C)C[C@H](NC(=O)[C@@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H]1CCCN1C(=O)[C@H](Cc1cnc[nH]1)NC(C)=O)C(C)(C)C)[C@@H](O)CC(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C51H74N10O9/c1-30(2)22-36(42(63)27-43(64)56-38(23-31(3)4)46(66)58-37(45(52)65)24-33-16-11-9-12-17-33)57-49(69)44(51(6,7)8)60-47(67)39(25-34-18-13-10-14-19-34)59-48(68)41-20-15-21-61(41)50(70)40(55-32(5)62)26-35-28-53-29-54-35/h9-14,16-19,28-31,36-42,44,63H,15,20-27H2,1-8H3,(H2,52,65)(H,53,54)(H,55,62)(H,56,64)(H,57,69)(H,58,66)(H,59,68)(H,60,67)/t36-,37-,38-,39-,40-,41+,42-,44+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
Massachusetts General Hospital
Curated by ChEMBL
| Assay Description
Inhibitory activity against rat plasma renin in vitro. |
J Med Chem 31: 1679-86 (1988)
BindingDB Entry DOI: 10.7270/Q2NC61TR |
More data for this
Ligand-Target Pair | |
Renin
(Rattus norvegicus) | BDBM50022011
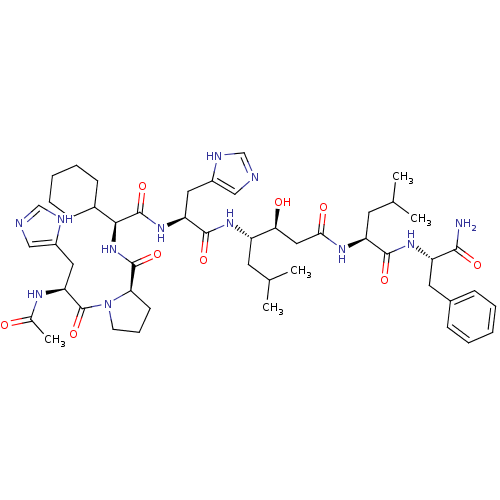
(Ac-His-Pro-CHA-His-Statine-Leu-Phe-NH2 | CHEMBL269...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@@H](NC(=O)[C@H]1CCCN1C(=O)[C@H](Cc1cnc[nH]1)NC(C)=O)C1CCCCC1)[C@@H](O)CC(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C50H74N12O9/c1-29(2)19-36(42(64)24-43(65)57-38(20-30(3)4)46(67)59-37(45(51)66)21-32-13-8-6-9-14-32)58-47(68)39(22-34-25-52-27-54-34)60-49(70)44(33-15-10-7-11-16-33)61-48(69)41-17-12-18-62(41)50(71)40(56-31(5)63)23-35-26-53-28-55-35/h6,8-9,13-14,25-30,33,36-42,44,64H,7,10-12,15-24H2,1-5H3,(H2,51,66)(H,52,54)(H,53,55)(H,56,63)(H,57,65)(H,58,68)(H,59,67)(H,60,70)(H,61,69)/t36-,37-,38-,39-,40-,41+,42-,44-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 650 | n/a | n/a | n/a | n/a | n/a | n/a |
Massachusetts General Hospital
Curated by ChEMBL
| Assay Description
Inhibitory activity against rat plasma renin in vitro. |
J Med Chem 31: 1679-86 (1988)
BindingDB Entry DOI: 10.7270/Q2NC61TR |
More data for this
Ligand-Target Pair | |
Renin
(Rattus norvegicus) | BDBM50022013
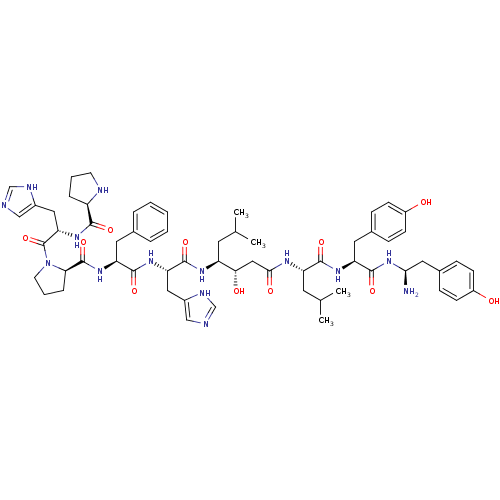
(CHEMBL384354 | Pro-His-Pro-Phe-His-Statine-Leu-Tyr...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H]1CCCN1C(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H]1CCCN1)[C@@H](O)CC(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@H](N)Cc1ccc(O)cc1 Show InChI InChI=1S/C62H84N14O11/c1-36(2)24-46(53(79)31-55(80)69-47(25-37(3)4)57(82)71-49(27-39-14-18-43(77)19-15-39)60(85)75-54(63)28-40-16-20-44(78)21-17-40)70-59(84)50(29-41-32-64-34-67-41)72-58(83)48(26-38-10-6-5-7-11-38)73-61(86)52-13-9-23-76(52)62(87)51(30-42-33-65-35-68-42)74-56(81)45-12-8-22-66-45/h5-7,10-11,14-21,32-37,45-54,66,77-79H,8-9,12-13,22-31,63H2,1-4H3,(H,64,67)(H,65,68)(H,69,80)(H,70,84)(H,71,82)(H,72,83)(H,73,86)(H,74,81)(H,75,85)/t45-,46+,47+,48+,49+,50+,51+,52-,53+,54+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 775 | n/a | n/a | n/a | n/a | n/a | n/a |
Massachusetts General Hospital
Curated by ChEMBL
| Assay Description
Inhibitory activity against rat plasma renin in vitro. |
J Med Chem 31: 1679-86 (1988)
BindingDB Entry DOI: 10.7270/Q2NC61TR |
More data for this
Ligand-Target Pair | |
Renin
(Rattus norvegicus) | BDBM50367763
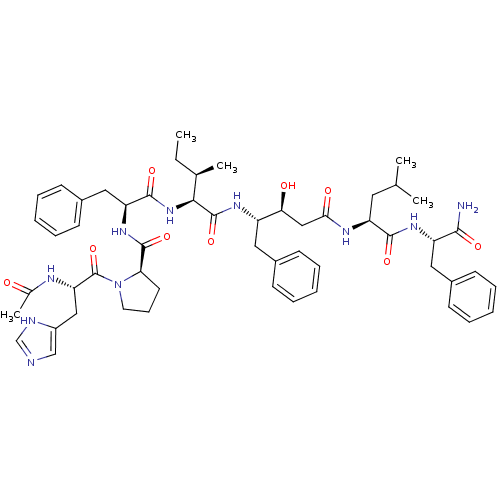
(CHEMBL1790270)Show SMILES CC[C@@H](C)[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H]1CCCN1C(=O)[C@H](Cc1cnc[nH]1)NC(C)=O)C(=O)N[C@@H](Cc1ccccc1)[C@@H](O)CC(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C54H72N10O9/c1-6-34(4)48(63-51(70)43(28-38-21-14-9-15-22-38)62-52(71)45-23-16-24-64(45)54(73)44(58-35(5)65)29-39-31-56-32-57-39)53(72)60-40(26-36-17-10-7-11-18-36)46(66)30-47(67)59-42(25-33(2)3)50(69)61-41(49(55)68)27-37-19-12-8-13-20-37/h7-15,17-22,31-34,40-46,48,66H,6,16,23-30H2,1-5H3,(H2,55,68)(H,56,57)(H,58,65)(H,59,67)(H,60,72)(H,61,69)(H,62,71)(H,63,70)/t34-,40+,41+,42+,43+,44+,45-,46+,48+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.27E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Massachusetts General Hospital
Curated by ChEMBL
| Assay Description
Inhibitory activity against rat plasma renin in vitro. |
J Med Chem 31: 1679-86 (1988)
BindingDB Entry DOI: 10.7270/Q2NC61TR |
More data for this
Ligand-Target Pair | |
Renin
(Rattus norvegicus) | BDBM50022009
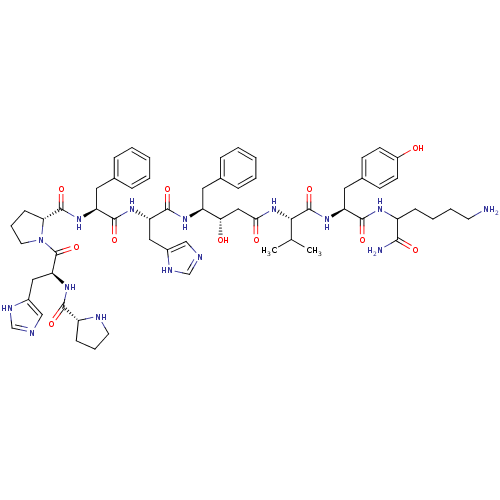
(CHEMBL263849 | Pro-His-Pro-Phe-His-AHPPA-Val-Tyr-L...)Show SMILES CC(C)[C@H](NC(=O)C[C@H](O)[C@H](Cc1ccccc1)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H]1CCCN1C(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H]1CCCN1)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)NC(CCCCN)C(N)=O Show InChI InChI=1S/C62H83N15O11/c1-37(2)54(61(87)74-48(29-40-20-22-43(78)23-21-40)57(83)70-44(55(64)81)17-9-10-24-63)76-53(80)32-52(79)46(27-38-13-5-3-6-14-38)71-59(85)49(30-41-33-65-35-68-41)72-58(84)47(28-39-15-7-4-8-16-39)73-60(86)51-19-12-26-77(51)62(88)50(31-42-34-66-36-69-42)75-56(82)45-18-11-25-67-45/h3-8,13-16,20-23,33-37,44-52,54,67,78-79H,9-12,17-19,24-32,63H2,1-2H3,(H2,64,81)(H,65,68)(H,66,69)(H,70,83)(H,71,85)(H,72,84)(H,73,86)(H,74,87)(H,75,82)(H,76,80)/t44?,45-,46+,47+,48+,49+,50+,51-,52+,54+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Massachusetts General Hospital
Curated by ChEMBL
| Assay Description
Inhibitory activity against rat plasma renin in vitro. |
J Med Chem 31: 1679-86 (1988)
BindingDB Entry DOI: 10.7270/Q2NC61TR |
More data for this
Ligand-Target Pair | |
Renin
(Rattus norvegicus) | BDBM50021998
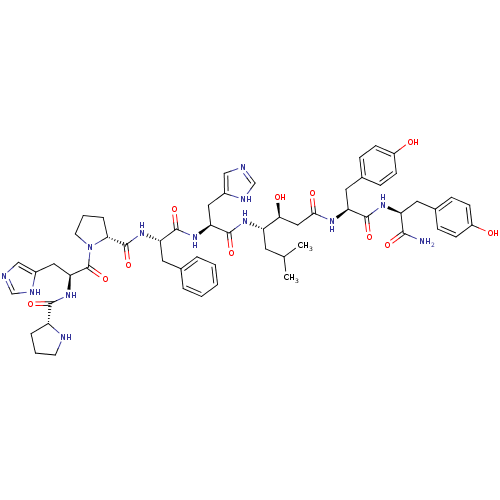
(CHEMBL437044 | Pro-His-Pro-Phe-His-Statine-Tyr-Tyr...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H]1CCCN1C(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H]1CCCN1)[C@@H](O)CC(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](Cc1ccc(O)cc1)C(N)=O Show InChI InChI=1S/C57H73N13O11/c1-33(2)22-42(49(73)28-50(74)64-44(25-36-14-18-40(72)19-15-36)53(77)66-43(51(58)75)23-35-12-16-39(71)17-13-35)65-55(79)46(26-37-29-59-31-62-37)67-54(78)45(24-34-8-4-3-5-9-34)68-56(80)48-11-7-21-70(48)57(81)47(27-38-30-60-32-63-38)69-52(76)41-10-6-20-61-41/h3-5,8-9,12-19,29-33,41-49,61,71-73H,6-7,10-11,20-28H2,1-2H3,(H2,58,75)(H,59,62)(H,60,63)(H,64,74)(H,65,79)(H,66,77)(H,67,78)(H,68,80)(H,69,76)/t41-,42+,43+,44+,45+,46+,47+,48-,49+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Massachusetts General Hospital
Curated by ChEMBL
| Assay Description
Inhibitory activity against rat plasma renin in vitro. |
J Med Chem 31: 1679-86 (1988)
BindingDB Entry DOI: 10.7270/Q2NC61TR |
More data for this
Ligand-Target Pair | |
Renin
(Rattus norvegicus) | BDBM50022012
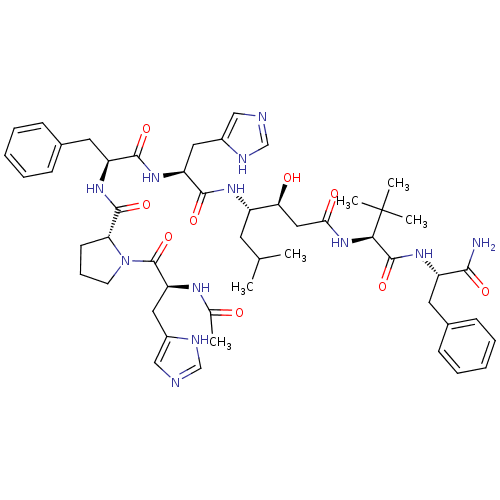
(Ac-His-Pro-Phe-His-Statine-tLeu-Phe-NH2 | CHEMBL40...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H]1CCCN1C(=O)[C@H](Cc1cnc[nH]1)NC(C)=O)[C@@H](O)CC(=O)N[C@H](C(=O)N[C@@H](Cc1ccccc1)C(N)=O)C(C)(C)C Show InChI InChI=1S/C51H70N12O9/c1-30(2)20-36(42(65)25-43(66)62-44(51(4,5)6)49(71)59-37(45(52)67)21-32-14-9-7-10-15-32)58-47(69)39(23-34-26-53-28-55-34)60-46(68)38(22-33-16-11-8-12-17-33)61-48(70)41-18-13-19-63(41)50(72)40(57-31(3)64)24-35-27-54-29-56-35/h7-12,14-17,26-30,36-42,44,65H,13,18-25H2,1-6H3,(H2,52,67)(H,53,55)(H,54,56)(H,57,64)(H,58,69)(H,59,71)(H,60,68)(H,61,70)(H,62,66)/t36-,37-,38-,39-,40-,41+,42-,44+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Massachusetts General Hospital
Curated by ChEMBL
| Assay Description
Inhibitory activity against rat plasma renin in vitro. |
J Med Chem 31: 1679-86 (1988)
BindingDB Entry DOI: 10.7270/Q2NC61TR |
More data for this
Ligand-Target Pair | |
Renin
(Rattus norvegicus) | BDBM50022016
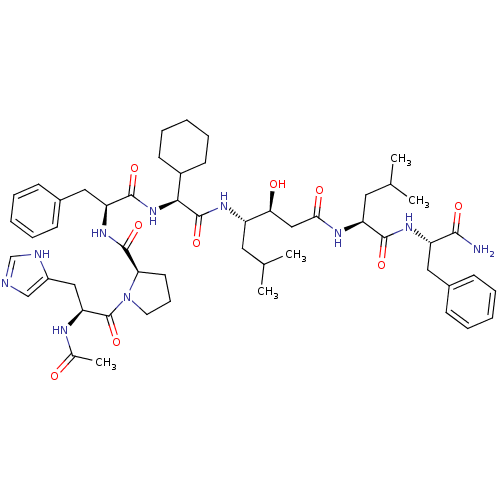
(1-[2-Acetylamino-3-(3H-imidazol-4-yl)-propionyl]-p...)Show SMILES CC(C)C[C@H](NC(=O)[C@@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H]1CCCN1C(=O)[C@H](Cc1cnc[nH]1)NC(C)=O)C1CCCCC1)[C@@H](O)CC(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C53H76N10O9/c1-32(2)24-39(45(65)29-46(66)58-41(25-33(3)4)49(68)60-40(48(54)67)26-35-16-9-6-10-17-35)59-52(71)47(37-20-13-8-14-21-37)62-50(69)42(27-36-18-11-7-12-19-36)61-51(70)44-22-15-23-63(44)53(72)43(57-34(5)64)28-38-30-55-31-56-38/h6-7,9-12,16-19,30-33,37,39-45,47,65H,8,13-15,20-29H2,1-5H3,(H2,54,67)(H,55,56)(H,57,64)(H,58,66)(H,59,71)(H,60,68)(H,61,70)(H,62,69)/t39-,40-,41-,42-,43-,44+,45-,47-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Massachusetts General Hospital
Curated by ChEMBL
| Assay Description
Inhibitory activity against rat plasma renin in vitro. |
J Med Chem 31: 1679-86 (1988)
BindingDB Entry DOI: 10.7270/Q2NC61TR |
More data for this
Ligand-Target Pair | |
Renin
(Rattus norvegicus) | BDBM50022010

(Ac-His-Pro-Phe-His-Statine-CHA-Phe-NH2 | CHEMBL443...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H]1CCCN1C(=O)[C@H](Cc1cnc[nH]1)NC(C)=O)[C@@H](O)CC(=O)N[C@@H](C1CCCCC1)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C53H72N12O9/c1-32(2)22-39(45(67)27-46(68)64-47(36-18-11-6-12-19-36)52(73)61-40(48(54)69)23-34-14-7-4-8-15-34)60-50(71)42(25-37-28-55-30-57-37)62-49(70)41(24-35-16-9-5-10-17-35)63-51(72)44-20-13-21-65(44)53(74)43(59-33(3)66)26-38-29-56-31-58-38/h4-5,7-10,14-17,28-32,36,39-45,47,67H,6,11-13,18-27H2,1-3H3,(H2,54,69)(H,55,57)(H,56,58)(H,59,66)(H,60,71)(H,61,73)(H,62,70)(H,63,72)(H,64,68)/t39-,40-,41-,42-,43-,44+,45-,47-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Massachusetts General Hospital
Curated by ChEMBL
| Assay Description
Inhibitory activity against rat plasma renin in vitro. |
J Med Chem 31: 1679-86 (1988)
BindingDB Entry DOI: 10.7270/Q2NC61TR |
More data for this
Ligand-Target Pair | |
Renin
(Rattus norvegicus) | BDBM50022004
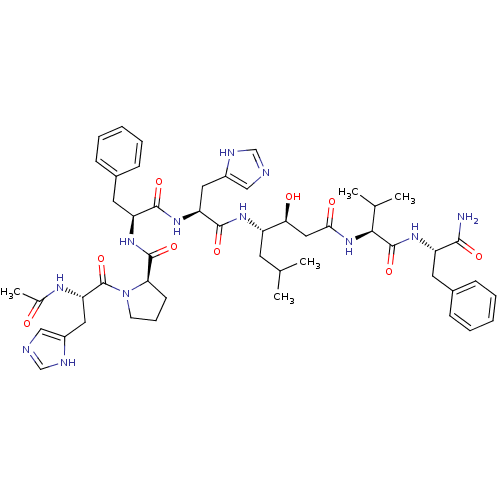
(Ac-His-Pro-Phe-His-Statine-Val-Phe-NH2 | CHEMBL276...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H]1CCCN1C(=O)[C@H](Cc1cnc[nH]1)NC(C)=O)[C@@H](O)CC(=O)N[C@@H](C(C)C)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C50H68N12O9/c1-29(2)19-36(42(64)24-43(65)61-44(30(3)4)49(70)58-37(45(51)66)20-32-13-8-6-9-14-32)57-47(68)39(22-34-25-52-27-54-34)59-46(67)38(21-33-15-10-7-11-16-33)60-48(69)41-17-12-18-62(41)50(71)40(56-31(5)63)23-35-26-53-28-55-35/h6-11,13-16,25-30,36-42,44,64H,12,17-24H2,1-5H3,(H2,51,66)(H,52,54)(H,53,55)(H,56,63)(H,57,68)(H,58,70)(H,59,67)(H,60,69)(H,61,65)/t36-,37-,38-,39-,40-,41+,42-,44-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Massachusetts General Hospital
Curated by ChEMBL
| Assay Description
Inhibitory activity against rat plasma renin in vitro. |
J Med Chem 31: 1679-86 (1988)
BindingDB Entry DOI: 10.7270/Q2NC61TR |
More data for this
Ligand-Target Pair | |
Renin
(Rattus norvegicus) | BDBM50022018

(CHEMBL2372761 | Pro-His-Pro-Phe-His-Statine-Tyr-Ty...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1c[nH]cn1)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H]1CCCN1C(=O)[C@H](Cc1c[nH]cn1)NC(=O)[C@@H]1CCCN1)[C@@H](O)CC(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CO)C(=O)N[C@@H](CCCCN)C(N)=O Show InChI InChI=1S/C66H90N16O14/c1-38(2)26-48(56(86)32-57(87)74-49(28-40-15-19-44(84)20-16-40)60(90)77-50(29-41-17-21-45(85)22-18-41)62(92)81-54(35-83)64(94)75-46(58(68)88)12-6-7-23-67)76-63(93)52(30-42-33-69-36-72-42)78-61(91)51(27-39-10-4-3-5-11-39)79-65(95)55-14-9-25-82(55)66(96)53(31-43-34-70-37-73-43)80-59(89)47-13-8-24-71-47/h3-5,10-11,15-22,33-34,36-38,46-56,71,83-86H,6-9,12-14,23-32,35,67H2,1-2H3,(H2,68,88)(H,69,72)(H,70,73)(H,74,87)(H,75,94)(H,76,93)(H,77,90)(H,78,91)(H,79,95)(H,80,89)(H,81,92)/t46-,47-,48-,49-,50-,51-,52-,53-,54-,55-,56-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 7.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Massachusetts General Hospital
Curated by ChEMBL
| Assay Description
Inhibitory activity against rat plasma renin in vitro. |
J Med Chem 31: 1679-86 (1988)
BindingDB Entry DOI: 10.7270/Q2NC61TR |
More data for this
Ligand-Target Pair | |
Renin
(Rattus norvegicus) | BDBM50022000
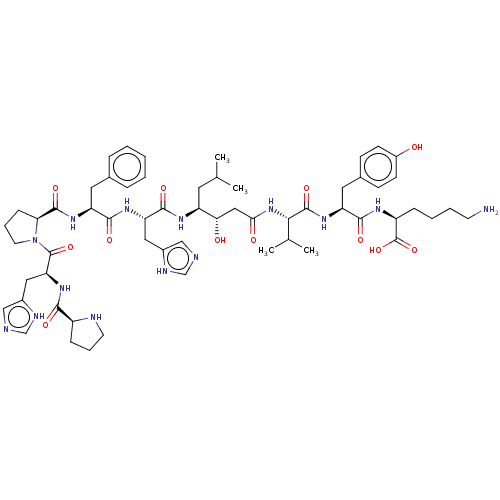
(CHEMBL2371853 | Pro-His-Pro-Phe-His-Statine-Val-Ty...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1c[nH]cn1)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H]1CCCN1C(=O)[C@H](Cc1c[nH]cn1)NC(=O)[C@@H]1CCCN1)[C@@H](O)CC(=O)N[C@@H](C(C)C)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CCCCN)C(O)=O Show InChI InChI=1S/C59H84N14O12/c1-34(2)24-43(49(75)29-50(76)72-51(35(3)4)57(82)70-45(26-37-17-19-40(74)20-18-37)53(78)66-42(59(84)85)14-8-9-21-60)67-55(80)46(27-38-30-61-32-64-38)68-54(79)44(25-36-12-6-5-7-13-36)69-56(81)48-16-11-23-73(48)58(83)47(28-39-31-62-33-65-39)71-52(77)41-15-10-22-63-41/h5-7,12-13,17-20,30-35,41-49,51,63,74-75H,8-11,14-16,21-29,60H2,1-4H3,(H,61,64)(H,62,65)(H,66,78)(H,67,80)(H,68,79)(H,69,81)(H,70,82)(H,71,77)(H,72,76)(H,84,85)/t41-,42-,43-,44-,45-,46-,47-,48-,49-,51-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 8.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Massachusetts General Hospital
Curated by ChEMBL
| Assay Description
Inhibitory activity against rat plasma renin in vitro. |
J Med Chem 31: 1679-86 (1988)
BindingDB Entry DOI: 10.7270/Q2NC61TR |
More data for this
Ligand-Target Pair | |
Renin
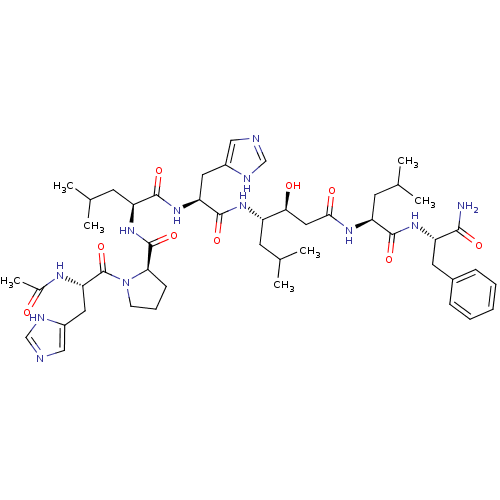
(Rattus norvegicus) | BDBM50022020
(Ac-His-Pro-Leu-His-Statine-Leu-Phe-NH2 | CHEMBL428...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](CC(C)C)NC(=O)[C@H]1CCCN1C(=O)[C@H](Cc1cnc[nH]1)NC(C)=O)[C@@H](O)CC(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C48H72N12O9/c1-27(2)16-34(41(62)22-42(63)55-36(17-28(3)4)44(65)57-35(43(49)64)19-31-12-9-8-10-13-31)56-46(67)38(20-32-23-50-25-52-32)58-45(66)37(18-29(5)6)59-47(68)40-14-11-15-60(40)48(69)39(54-30(7)61)21-33-24-51-26-53-33/h8-10,12-13,23-29,34-41,62H,11,14-22H2,1-7H3,(H2,49,64)(H,50,52)(H,51,53)(H,54,61)(H,55,63)(H,56,67)(H,57,65)(H,58,66)(H,59,68)/t34-,35-,36-,37-,38-,39-,40+,41-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 9.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Massachusetts General Hospital
Curated by ChEMBL
| Assay Description
Inhibitory activity against rat plasma renin in vitro. |
J Med Chem 31: 1679-86 (1988)
BindingDB Entry DOI: 10.7270/Q2NC61TR |
More data for this
Ligand-Target Pair | |
Renin
(Rattus norvegicus) | BDBM50367764
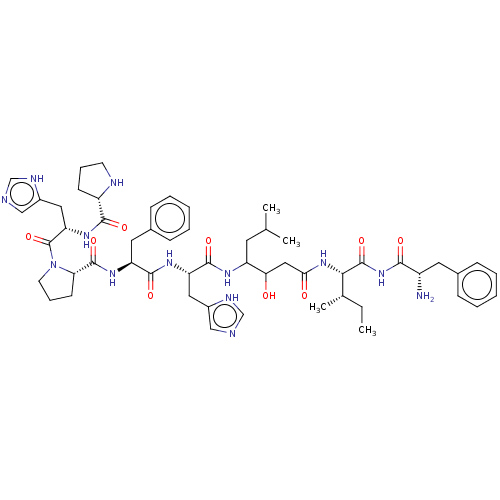
(CHEMBL264339)Show SMILES CC[C@H](C)[C@H](NC(=O)CC(O)C(CC(C)C)NC(=O)[C@H](Cc1c[nH]cn1)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H]1CCCN1C(=O)[C@H](Cc1c[nH]cn1)NC(=O)[C@@H]1CCCN1)C(=O)NC(=O)[C@@H](N)Cc1ccccc1 Show InChI InChI=1S/C54H75N13O9/c1-5-33(4)47(53(75)66-48(70)38(55)23-34-14-8-6-9-15-34)65-46(69)27-45(68)40(22-32(2)3)61-51(73)42(25-36-28-56-30-59-36)62-50(72)41(24-35-16-10-7-11-17-35)63-52(74)44-19-13-21-67(44)54(76)43(26-37-29-57-31-60-37)64-49(71)39-18-12-20-58-39/h6-11,14-17,28-33,38-45,47,58,68H,5,12-13,18-27,55H2,1-4H3,(H,56,59)(H,57,60)(H,61,73)(H,62,72)(H,63,74)(H,64,71)(H,65,69)(H,66,70,75)/t33-,38-,39-,40?,41-,42-,43-,44-,45?,47-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Massachusetts General Hospital
Curated by ChEMBL
| Assay Description
Inhibitory activity against rat plasma renin in vitro. |
J Med Chem 31: 1679-86 (1988)
BindingDB Entry DOI: 10.7270/Q2NC61TR |
More data for this
Ligand-Target Pair | |
Renin
(Rattus norvegicus) | BDBM50021990
(CHEMBL427798 | Pro-His-Pro-Phe-His-Statine-Tyr-Tyr...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H]1CCCN1C(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H]1CCCN1)[C@@H](O)CC(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)NC(CCCCN)C(N)=O Show InChI InChI=1S/C63H85N15O12/c1-37(2)26-47(54(81)32-55(82)71-48(28-39-15-19-43(79)20-16-39)58(85)74-49(29-40-17-21-44(80)22-18-40)59(86)72-45(56(65)83)12-6-7-23-64)73-61(88)51(30-41-33-66-35-69-41)75-60(87)50(27-38-10-4-3-5-11-38)76-62(89)53-14-9-25-78(53)63(90)52(31-42-34-67-36-70-42)77-57(84)46-13-8-24-68-46/h3-5,10-11,15-22,33-37,45-54,68,79-81H,6-9,12-14,23-32,64H2,1-2H3,(H2,65,83)(H,66,69)(H,67,70)(H,71,82)(H,72,86)(H,73,88)(H,74,85)(H,75,87)(H,76,89)(H,77,84)/t45?,46-,47+,48+,49+,50+,51+,52+,53-,54+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Massachusetts General Hospital
Curated by ChEMBL
| Assay Description
Inhibitory activity against rat plasma renin in vitro. |
J Med Chem 31: 1679-86 (1988)
BindingDB Entry DOI: 10.7270/Q2NC61TR |
More data for this
Ligand-Target Pair | |
Renin
(Rattus norvegicus) | BDBM50367762
(CHEMBL1790272)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H]1CCCN1C(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H]1CCCN1)[C@@H](O)CC(=O)N[C@@H]([C@H](C)O)C(=O)N[C@@H]([C@H](C)O)C(N)=O Show InChI InChI=1S/C47H69N13O11/c1-25(2)16-32(37(63)20-38(64)58-40(27(4)62)46(70)59-39(26(3)61)41(48)65)54-44(68)34(18-29-21-49-23-52-29)55-43(67)33(17-28-10-6-5-7-11-28)56-45(69)36-13-9-15-60(36)47(71)35(19-30-22-50-24-53-30)57-42(66)31-12-8-14-51-31/h5-7,10-11,21-27,31-37,39-40,51,61-63H,8-9,12-20H2,1-4H3,(H2,48,65)(H,49,52)(H,50,53)(H,54,68)(H,55,67)(H,56,69)(H,57,66)(H,58,64)(H,59,70)/t26-,27-,31+,32-,33-,34-,35-,36+,37-,39-,40-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.05E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Massachusetts General Hospital
Curated by ChEMBL
| Assay Description
Inhibitory activity against rat plasma renin in vitro. |
J Med Chem 31: 1679-86 (1988)
BindingDB Entry DOI: 10.7270/Q2NC61TR |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data




































