

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Coagulation factor X (Homo sapiens (Human)) | BDBM12776 (2-(5-carbamimidoyl-1H-indol-3-yl)-3-phenyl-N-[4-(2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.630 | -52.0 | n/a | n/a | n/a | n/a | n/a | 7.0 | 22 |
Bristol-Myers Squibb Company | Assay Description Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter... | Bioorg Med Chem Lett 14: 5269-73 (2004) Article DOI: 10.1016/j.bmcl.2004.08.037 BindingDB Entry DOI: 10.7270/Q2PR7T6P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12778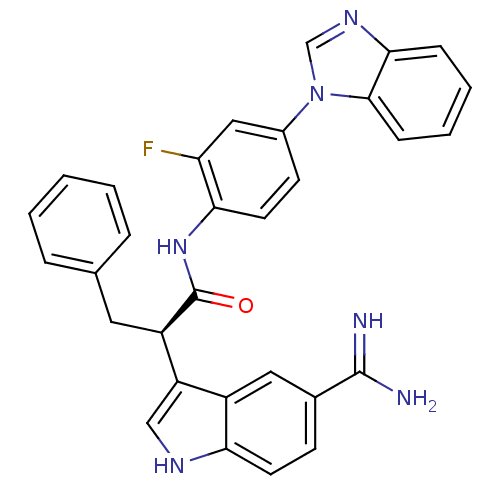 ((2R)-N-[4-(1H-1,3-benzodiazol-1-yl)-2-fluorophenyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.30 | -50.2 | n/a | n/a | n/a | n/a | n/a | 7.0 | 22 |
Bristol-Myers Squibb Company | Assay Description Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter... | Bioorg Med Chem Lett 14: 5269-73 (2004) Article DOI: 10.1016/j.bmcl.2004.08.037 BindingDB Entry DOI: 10.7270/Q2PR7T6P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12786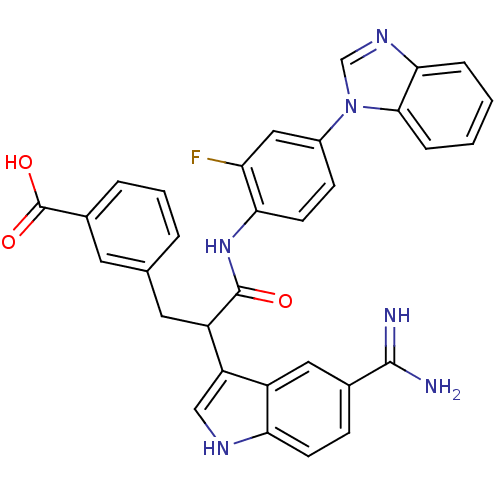 (3-(2-{[4-(1H-1,3-benzodiazol-1-yl)-2-fluorophenyl]...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.80 | -49.4 | n/a | n/a | n/a | n/a | n/a | 7.0 | 22 |
Bristol-Myers Squibb Company | Assay Description Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter... | Bioorg Med Chem Lett 14: 5269-73 (2004) Article DOI: 10.1016/j.bmcl.2004.08.037 BindingDB Entry DOI: 10.7270/Q2PR7T6P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12781 (5-amidinoindole 15 | N-[4-(1H-1,3-benzodiazol-1-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.20 | -48.9 | n/a | n/a | n/a | n/a | n/a | 7.0 | 22 |
Bristol-Myers Squibb Company | Assay Description Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter... | Bioorg Med Chem Lett 14: 5269-73 (2004) Article DOI: 10.1016/j.bmcl.2004.08.037 BindingDB Entry DOI: 10.7270/Q2PR7T6P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12777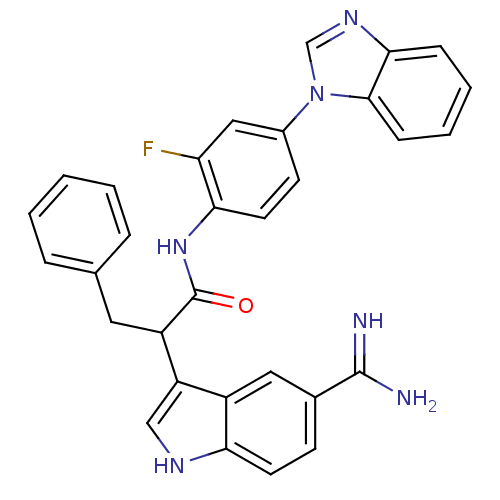 (5-amidinoindole 13a | N-[4-(1H-1,3-benzodiazol-1-y...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.5 | -48.6 | n/a | n/a | n/a | n/a | n/a | 7.0 | 22 |
Bristol-Myers Squibb Company | Assay Description Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter... | Bioorg Med Chem Lett 14: 5269-73 (2004) Article DOI: 10.1016/j.bmcl.2004.08.037 BindingDB Entry DOI: 10.7270/Q2PR7T6P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12782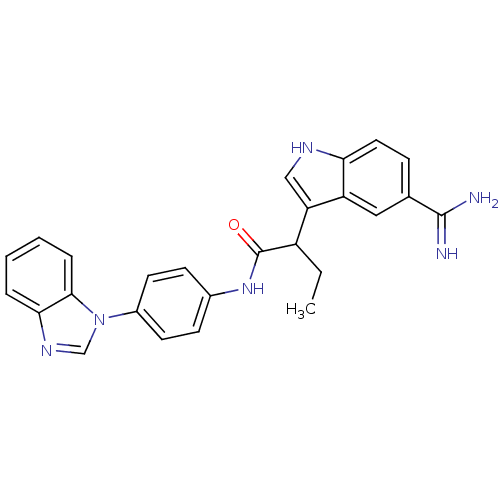 (5-amidinoindole 16 | N-[4-(1H-1,3-benzodiazol-1-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.80 | -48.3 | n/a | n/a | n/a | n/a | n/a | 7.0 | 22 |
Bristol-Myers Squibb Company | Assay Description Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter... | Bioorg Med Chem Lett 14: 5269-73 (2004) Article DOI: 10.1016/j.bmcl.2004.08.037 BindingDB Entry DOI: 10.7270/Q2PR7T6P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12780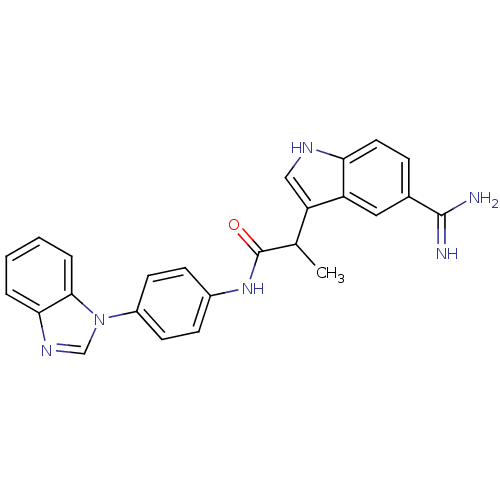 (5-amidinoindole 14 | N-[4-(1H-1,3-benzodiazol-1-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3 | -48.2 | n/a | n/a | n/a | n/a | n/a | 7.0 | 22 |
Bristol-Myers Squibb Company | Assay Description Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter... | Bioorg Med Chem Lett 14: 5269-73 (2004) Article DOI: 10.1016/j.bmcl.2004.08.037 BindingDB Entry DOI: 10.7270/Q2PR7T6P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12775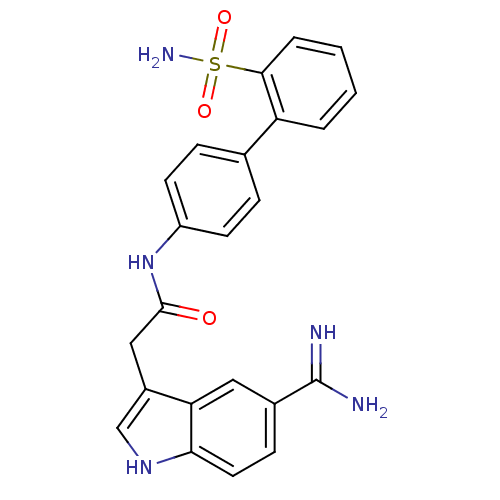 (2-(5-carbamimidoyl-1H-indol-3-yl)-N-[4-(2-sulfamoy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3.20 | -48.0 | n/a | n/a | n/a | n/a | n/a | 7.0 | 22 |
Bristol-Myers Squibb Company | Assay Description Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter... | Bioorg Med Chem Lett 14: 5269-73 (2004) Article DOI: 10.1016/j.bmcl.2004.08.037 BindingDB Entry DOI: 10.7270/Q2PR7T6P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12787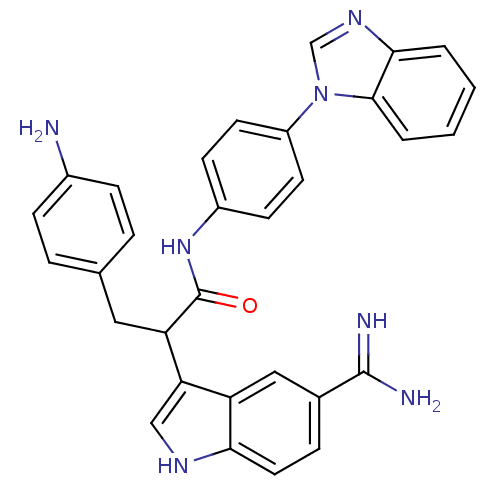 (3-(4-aminophenyl)-N-[4-(1H-1,3-benzodiazol-1-yl)ph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 4 | -47.5 | n/a | n/a | n/a | n/a | n/a | 7.0 | 22 |
Bristol-Myers Squibb Company | Assay Description Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter... | Bioorg Med Chem Lett 14: 5269-73 (2004) Article DOI: 10.1016/j.bmcl.2004.08.037 BindingDB Entry DOI: 10.7270/Q2PR7T6P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12791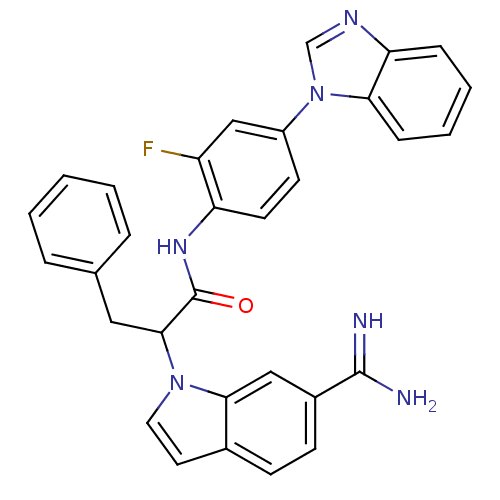 (6-amidinoindole 25 | N-[4-(1H-1,3-benzodiazol-1-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 5.60 | -46.6 | n/a | n/a | n/a | n/a | n/a | 7.0 | 22 |
Bristol-Myers Squibb Company | Assay Description Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter... | Bioorg Med Chem Lett 14: 5269-73 (2004) Article DOI: 10.1016/j.bmcl.2004.08.037 BindingDB Entry DOI: 10.7270/Q2PR7T6P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12784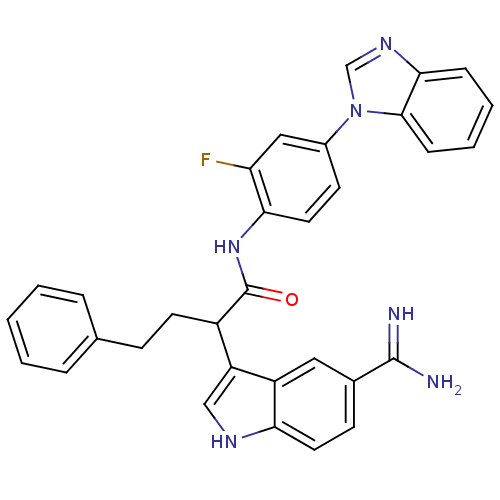 (5-amidinoindole 18 | N-[4-(1H-1,3-benzodiazol-1-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 6.10 | -46.4 | n/a | n/a | n/a | n/a | n/a | 7.0 | 22 |
Bristol-Myers Squibb Company | Assay Description Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter... | Bioorg Med Chem Lett 14: 5269-73 (2004) Article DOI: 10.1016/j.bmcl.2004.08.037 BindingDB Entry DOI: 10.7270/Q2PR7T6P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12788 (5-amidinoindole 22 | N-[4-(1H-1,3-benzodiazol-1-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 7 | -46.1 | n/a | n/a | n/a | n/a | n/a | 7.0 | 22 |
Bristol-Myers Squibb Company | Assay Description Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter... | Bioorg Med Chem Lett 14: 5269-73 (2004) Article DOI: 10.1016/j.bmcl.2004.08.037 BindingDB Entry DOI: 10.7270/Q2PR7T6P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12783 (5-amidinoindole 17 | N-[4-(1H-1,3-benzodiazol-1-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 14 | -44.4 | n/a | n/a | n/a | n/a | n/a | 7.0 | 22 |
Bristol-Myers Squibb Company | Assay Description Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter... | Bioorg Med Chem Lett 14: 5269-73 (2004) Article DOI: 10.1016/j.bmcl.2004.08.037 BindingDB Entry DOI: 10.7270/Q2PR7T6P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12789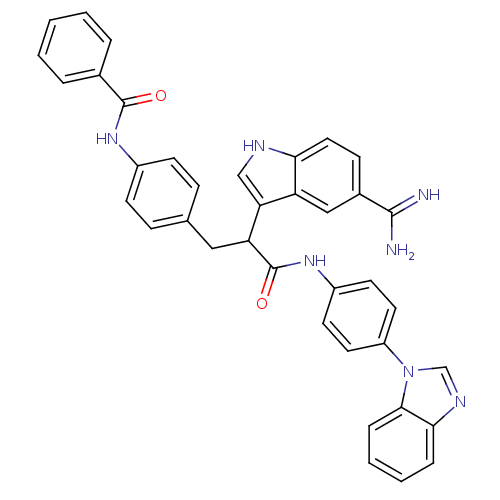 (5-amidinoindole 23 | N-[4-(2-{[4-(1H-1,3-benzodiaz...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 18 | -43.8 | n/a | n/a | n/a | n/a | n/a | 7.0 | 22 |
Bristol-Myers Squibb Company | Assay Description Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter... | Bioorg Med Chem Lett 14: 5269-73 (2004) Article DOI: 10.1016/j.bmcl.2004.08.037 BindingDB Entry DOI: 10.7270/Q2PR7T6P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12790 (3-(4-benzenesulfonamidophenyl)-N-[4-(1H-1,3-benzod...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 19 | -43.6 | n/a | n/a | n/a | n/a | n/a | 7.0 | 22 |
Bristol-Myers Squibb Company | Assay Description Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter... | Bioorg Med Chem Lett 14: 5269-73 (2004) Article DOI: 10.1016/j.bmcl.2004.08.037 BindingDB Entry DOI: 10.7270/Q2PR7T6P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor IX (Homo sapiens (Human)) | BDBM12778 ((2R)-N-[4-(1H-1,3-benzodiazol-1-yl)-2-fluorophenyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter... | Bioorg Med Chem Lett 14: 5269-73 (2004) Article DOI: 10.1016/j.bmcl.2004.08.037 BindingDB Entry DOI: 10.7270/Q2PR7T6P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12779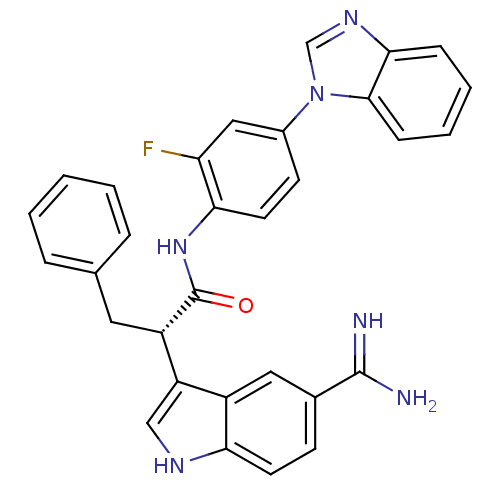 ((2S)-N-[4-(1H-1,3-benzodiazol-1-yl)-2-fluorophenyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 26 | -42.9 | n/a | n/a | n/a | n/a | n/a | 7.0 | 22 |
Bristol-Myers Squibb Company | Assay Description Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter... | Bioorg Med Chem Lett 14: 5269-73 (2004) Article DOI: 10.1016/j.bmcl.2004.08.037 BindingDB Entry DOI: 10.7270/Q2PR7T6P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12785 (5-amidinoindole 19 | N-[4-(1H-1,3-benzodiazol-1-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 28 | -42.7 | n/a | n/a | n/a | n/a | n/a | 7.0 | 22 |
Bristol-Myers Squibb Company | Assay Description Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter... | Bioorg Med Chem Lett 14: 5269-73 (2004) Article DOI: 10.1016/j.bmcl.2004.08.037 BindingDB Entry DOI: 10.7270/Q2PR7T6P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12794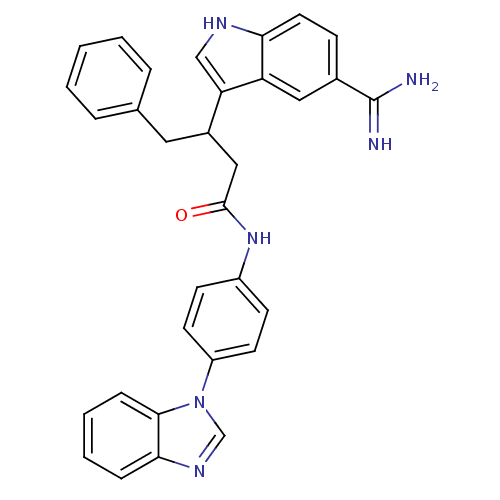 (5-amidinoindole 28 | N-[4-(1H-1,3-benzodiazol-1-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 36 | -42.1 | n/a | n/a | n/a | n/a | n/a | 7.0 | 22 |
Bristol-Myers Squibb Company | Assay Description Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter... | Bioorg Med Chem Lett 14: 5269-73 (2004) Article DOI: 10.1016/j.bmcl.2004.08.037 BindingDB Entry DOI: 10.7270/Q2PR7T6P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12793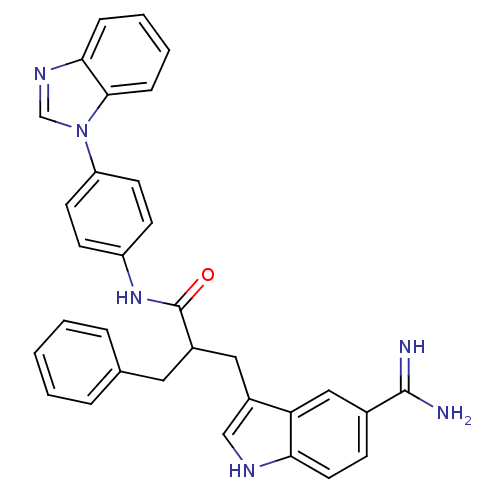 (5-amidinoindole 27 | N-[4-(1H-1,3-benzodiazol-1-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 45 | -41.5 | n/a | n/a | n/a | n/a | n/a | 7.0 | 22 |
Bristol-Myers Squibb Company | Assay Description Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter... | Bioorg Med Chem Lett 14: 5269-73 (2004) Article DOI: 10.1016/j.bmcl.2004.08.037 BindingDB Entry DOI: 10.7270/Q2PR7T6P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor IX (Homo sapiens (Human)) | BDBM12786 (3-(2-{[4-(1H-1,3-benzodiazol-1-yl)-2-fluorophenyl]...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 59 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter... | Bioorg Med Chem Lett 14: 5269-73 (2004) Article DOI: 10.1016/j.bmcl.2004.08.037 BindingDB Entry DOI: 10.7270/Q2PR7T6P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor IX (Homo sapiens (Human)) | BDBM12777 (5-amidinoindole 13a | N-[4-(1H-1,3-benzodiazol-1-y...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 63 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter... | Bioorg Med Chem Lett 14: 5269-73 (2004) Article DOI: 10.1016/j.bmcl.2004.08.037 BindingDB Entry DOI: 10.7270/Q2PR7T6P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12795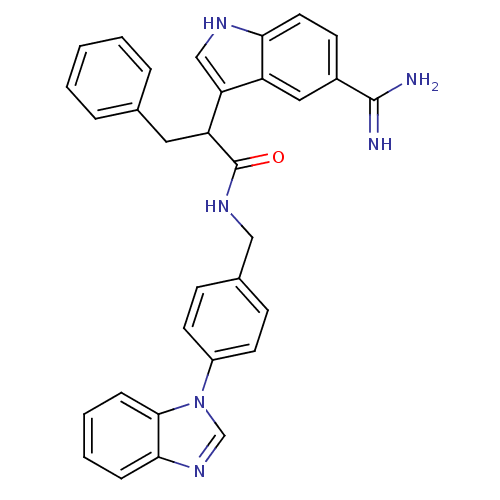 (5-amidinoindole 29 | N-{[4-(1H-1,3-benzodiazol-1-y...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 72 | -40.4 | n/a | n/a | n/a | n/a | n/a | 7.0 | 22 |
Bristol-Myers Squibb Company | Assay Description Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter... | Bioorg Med Chem Lett 14: 5269-73 (2004) Article DOI: 10.1016/j.bmcl.2004.08.037 BindingDB Entry DOI: 10.7270/Q2PR7T6P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor IX (Homo sapiens (Human)) | BDBM12787 (3-(4-aminophenyl)-N-[4-(1H-1,3-benzodiazol-1-yl)ph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter... | Bioorg Med Chem Lett 14: 5269-73 (2004) Article DOI: 10.1016/j.bmcl.2004.08.037 BindingDB Entry DOI: 10.7270/Q2PR7T6P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12798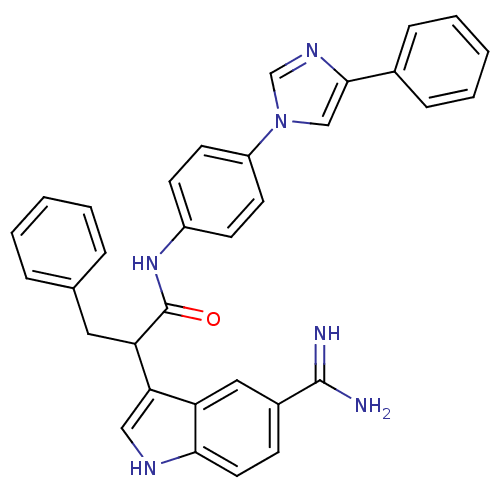 (2-(5-carbamimidoyl-1H-indol-3-yl)-3-phenyl-N-[4-(4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 79 | -40.1 | n/a | n/a | n/a | n/a | n/a | 7.0 | 22 |
Bristol-Myers Squibb Company | Assay Description Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter... | Bioorg Med Chem Lett 14: 5269-73 (2004) Article DOI: 10.1016/j.bmcl.2004.08.037 BindingDB Entry DOI: 10.7270/Q2PR7T6P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor IX (Homo sapiens (Human)) | BDBM12785 (5-amidinoindole 19 | N-[4-(1H-1,3-benzodiazol-1-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 98 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter... | Bioorg Med Chem Lett 14: 5269-73 (2004) Article DOI: 10.1016/j.bmcl.2004.08.037 BindingDB Entry DOI: 10.7270/Q2PR7T6P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor IX (Homo sapiens (Human)) | BDBM12793 (5-amidinoindole 27 | N-[4-(1H-1,3-benzodiazol-1-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter... | Bioorg Med Chem Lett 14: 5269-73 (2004) Article DOI: 10.1016/j.bmcl.2004.08.037 BindingDB Entry DOI: 10.7270/Q2PR7T6P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor IX (Homo sapiens (Human)) | BDBM12790 (3-(4-benzenesulfonamidophenyl)-N-[4-(1H-1,3-benzod...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter... | Bioorg Med Chem Lett 14: 5269-73 (2004) Article DOI: 10.1016/j.bmcl.2004.08.037 BindingDB Entry DOI: 10.7270/Q2PR7T6P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12792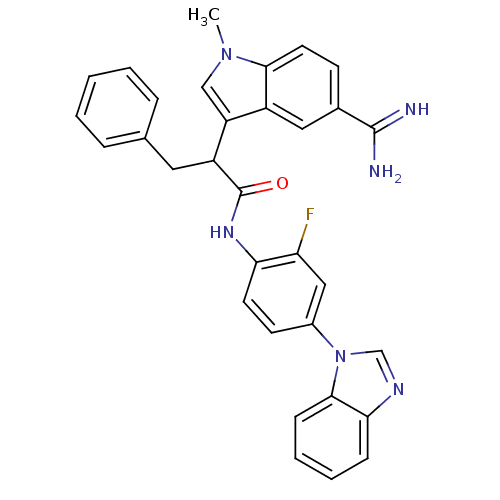 (5-amidinoindole 26 | N-[4-(1H-1,3-benzodiazol-1-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 160 | -38.4 | n/a | n/a | n/a | n/a | n/a | 7.0 | 22 |
Bristol-Myers Squibb Company | Assay Description Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter... | Bioorg Med Chem Lett 14: 5269-73 (2004) Article DOI: 10.1016/j.bmcl.2004.08.037 BindingDB Entry DOI: 10.7270/Q2PR7T6P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor IX (Homo sapiens (Human)) | BDBM12782 (5-amidinoindole 16 | N-[4-(1H-1,3-benzodiazol-1-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter... | Bioorg Med Chem Lett 14: 5269-73 (2004) Article DOI: 10.1016/j.bmcl.2004.08.037 BindingDB Entry DOI: 10.7270/Q2PR7T6P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor IX (Homo sapiens (Human)) | BDBM12784 (5-amidinoindole 18 | N-[4-(1H-1,3-benzodiazol-1-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter... | Bioorg Med Chem Lett 14: 5269-73 (2004) Article DOI: 10.1016/j.bmcl.2004.08.037 BindingDB Entry DOI: 10.7270/Q2PR7T6P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor IX (Homo sapiens (Human)) | BDBM12794 (5-amidinoindole 28 | N-[4-(1H-1,3-benzodiazol-1-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter... | Bioorg Med Chem Lett 14: 5269-73 (2004) Article DOI: 10.1016/j.bmcl.2004.08.037 BindingDB Entry DOI: 10.7270/Q2PR7T6P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor IX (Homo sapiens (Human)) | BDBM12789 (5-amidinoindole 23 | N-[4-(2-{[4-(1H-1,3-benzodiaz...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter... | Bioorg Med Chem Lett 14: 5269-73 (2004) Article DOI: 10.1016/j.bmcl.2004.08.037 BindingDB Entry DOI: 10.7270/Q2PR7T6P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor IX (Homo sapiens (Human)) | BDBM12798 (2-(5-carbamimidoyl-1H-indol-3-yl)-3-phenyl-N-[4-(4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter... | Bioorg Med Chem Lett 14: 5269-73 (2004) Article DOI: 10.1016/j.bmcl.2004.08.037 BindingDB Entry DOI: 10.7270/Q2PR7T6P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor IX (Homo sapiens (Human)) | BDBM12791 (6-amidinoindole 25 | N-[4-(1H-1,3-benzodiazol-1-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter... | Bioorg Med Chem Lett 14: 5269-73 (2004) Article DOI: 10.1016/j.bmcl.2004.08.037 BindingDB Entry DOI: 10.7270/Q2PR7T6P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor IX (Homo sapiens (Human)) | BDBM12795 (5-amidinoindole 29 | N-{[4-(1H-1,3-benzodiazol-1-y...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter... | Bioorg Med Chem Lett 14: 5269-73 (2004) Article DOI: 10.1016/j.bmcl.2004.08.037 BindingDB Entry DOI: 10.7270/Q2PR7T6P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor IX (Homo sapiens (Human)) | BDBM12792 (5-amidinoindole 26 | N-[4-(1H-1,3-benzodiazol-1-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter... | Bioorg Med Chem Lett 14: 5269-73 (2004) Article DOI: 10.1016/j.bmcl.2004.08.037 BindingDB Entry DOI: 10.7270/Q2PR7T6P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor IX (Homo sapiens (Human)) | BDBM12780 (5-amidinoindole 14 | N-[4-(1H-1,3-benzodiazol-1-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter... | Bioorg Med Chem Lett 14: 5269-73 (2004) Article DOI: 10.1016/j.bmcl.2004.08.037 BindingDB Entry DOI: 10.7270/Q2PR7T6P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Homo sapiens (Human)) | BDBM50209971 (1-((1R,2S)-2-(((S)-3-(4-fluorobenzyl)piperidin-1-y...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity to dopamine transporter | Bioorg Med Chem Lett 17: 2992-7 (2007) Article DOI: 10.1016/j.bmcl.2007.03.065 BindingDB Entry DOI: 10.7270/Q2V987SQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor IX (Homo sapiens (Human)) | BDBM12799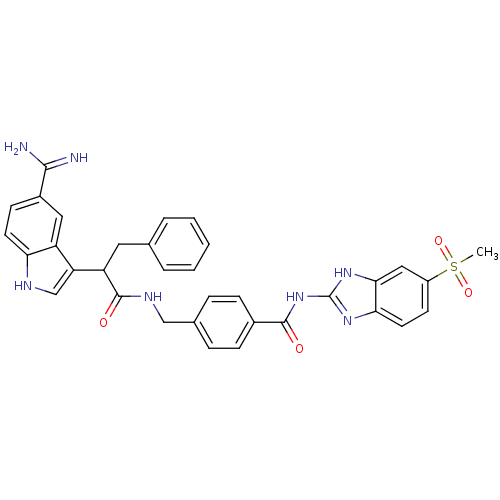 (4-{[2-(5-carbamimidoyl-1H-indol-3-yl)-3-phenylprop...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 660 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter... | Bioorg Med Chem Lett 14: 5269-73 (2004) Article DOI: 10.1016/j.bmcl.2004.08.037 BindingDB Entry DOI: 10.7270/Q2PR7T6P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor IX (Homo sapiens (Human)) | BDBM12776 (2-(5-carbamimidoyl-1H-indol-3-yl)-3-phenyl-N-[4-(2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 660 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter... | Bioorg Med Chem Lett 14: 5269-73 (2004) Article DOI: 10.1016/j.bmcl.2004.08.037 BindingDB Entry DOI: 10.7270/Q2PR7T6P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor IX (Homo sapiens (Human)) | BDBM12781 (5-amidinoindole 15 | N-[4-(1H-1,3-benzodiazol-1-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 750 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter... | Bioorg Med Chem Lett 14: 5269-73 (2004) Article DOI: 10.1016/j.bmcl.2004.08.037 BindingDB Entry DOI: 10.7270/Q2PR7T6P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12796 (5-amidinoindole 30 | N-(1H-1,3-benzodiazol-2-yl)-4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 810 | -34.4 | n/a | n/a | n/a | n/a | n/a | 7.0 | 22 |
Bristol-Myers Squibb Company | Assay Description Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter... | Bioorg Med Chem Lett 14: 5269-73 (2004) Article DOI: 10.1016/j.bmcl.2004.08.037 BindingDB Entry DOI: 10.7270/Q2PR7T6P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor IX (Homo sapiens (Human)) | BDBM12779 ((2S)-N-[4-(1H-1,3-benzodiazol-1-yl)-2-fluorophenyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 820 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter... | Bioorg Med Chem Lett 14: 5269-73 (2004) Article DOI: 10.1016/j.bmcl.2004.08.037 BindingDB Entry DOI: 10.7270/Q2PR7T6P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor IX (Homo sapiens (Human)) | BDBM12796 (5-amidinoindole 30 | N-(1H-1,3-benzodiazol-2-yl)-4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 930 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter... | Bioorg Med Chem Lett 14: 5269-73 (2004) Article DOI: 10.1016/j.bmcl.2004.08.037 BindingDB Entry DOI: 10.7270/Q2PR7T6P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent noradrenaline transporter (Homo sapiens (Human)) | BDBM50209971 (1-((1R,2S)-2-(((S)-3-(4-fluorobenzyl)piperidin-1-y...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 950 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity to norepinephrine transporter | Bioorg Med Chem Lett 17: 2992-7 (2007) Article DOI: 10.1016/j.bmcl.2007.03.065 BindingDB Entry DOI: 10.7270/Q2V987SQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12799 (4-{[2-(5-carbamimidoyl-1H-indol-3-yl)-3-phenylprop...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.10E+3 | -33.7 | n/a | n/a | n/a | n/a | n/a | 7.0 | 22 |
Bristol-Myers Squibb Company | Assay Description Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter... | Bioorg Med Chem Lett 14: 5269-73 (2004) Article DOI: 10.1016/j.bmcl.2004.08.037 BindingDB Entry DOI: 10.7270/Q2PR7T6P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor IX (Homo sapiens (Human)) | BDBM12788 (5-amidinoindole 22 | N-[4-(1H-1,3-benzodiazol-1-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter... | Bioorg Med Chem Lett 14: 5269-73 (2004) Article DOI: 10.1016/j.bmcl.2004.08.037 BindingDB Entry DOI: 10.7270/Q2PR7T6P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor IX (Homo sapiens (Human)) | BDBM12797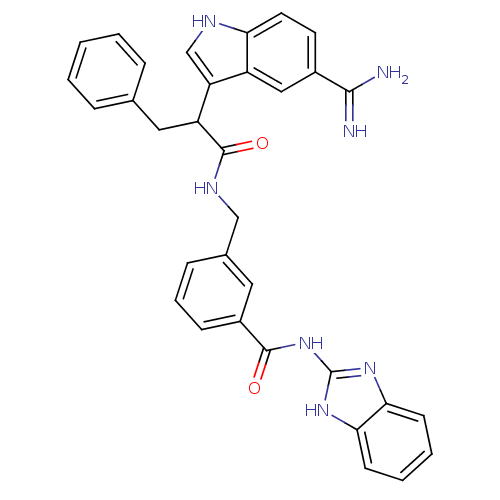 (5-amidinoindole 31 | N-(1H-1,3-benzodiazol-2-yl)-3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter... | Bioorg Med Chem Lett 14: 5269-73 (2004) Article DOI: 10.1016/j.bmcl.2004.08.037 BindingDB Entry DOI: 10.7270/Q2PR7T6P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12797 (5-amidinoindole 31 | N-(1H-1,3-benzodiazol-2-yl)-3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.60E+3 | -31.6 | n/a | n/a | n/a | n/a | n/a | 7.0 | 22 |
Bristol-Myers Squibb Company | Assay Description Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter... | Bioorg Med Chem Lett 14: 5269-73 (2004) Article DOI: 10.1016/j.bmcl.2004.08.037 BindingDB Entry DOI: 10.7270/Q2PR7T6P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 376 total ) | Next | Last >> |