

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sodium/hydrogen exchanger 1 (Rattus norvegicus) | BDBM50115243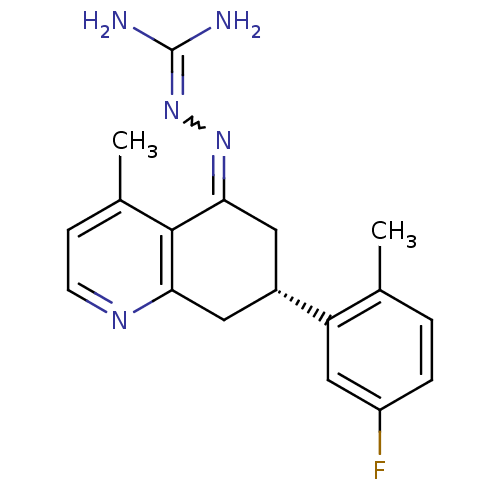 ((S)-N-[7-(5-fluoro-2-methylphenyl)-4-methyl-(7S)-5...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description In vitro inhibition of acid induced swelling in rat platelets by 50% as a measure of NHE-1 inhibition | J Med Chem 45: 3009-21 (2002) BindingDB Entry DOI: 10.7270/Q2V40VZN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/hydrogen exchanger 1 (Rattus norvegicus) | BDBM50115239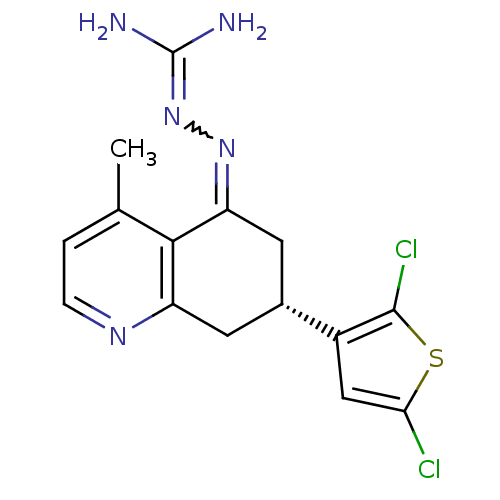 ((S)-N-[7-(2,5-dichloro-3-thienyl)-4-methyl-(7S)-5,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description In vitro inhibition of acid induced swelling in rat platelets by 50% as a measure of NHE-1 inhibition | J Med Chem 45: 3009-21 (2002) BindingDB Entry DOI: 10.7270/Q2V40VZN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/hydrogen exchanger 1 (Rattus norvegicus) | BDBM50115271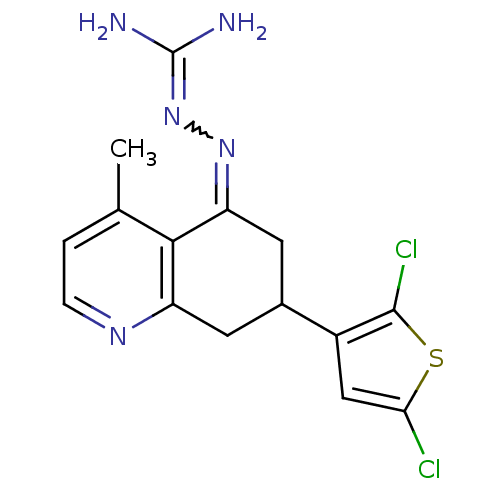 (CHEMBL556009 | N-[7-(2,5-dichloro-3-thienyl)-4-met...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description In vitro inhibition of acid induced swelling in rat platelets by 50% as a measure of NHE-1 inhibition | J Med Chem 45: 3009-21 (2002) BindingDB Entry DOI: 10.7270/Q2V40VZN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/hydrogen exchanger 1 (Rattus norvegicus) | BDBM50115246 (CHEMBL543867 | N-[7-(2-chloro-5-fluorophenyl)-4-me...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description In vitro inhibition of acid induced swelling in rat platelets by 50% as a measure of NHE-1 inhibition | J Med Chem 45: 3009-21 (2002) BindingDB Entry DOI: 10.7270/Q2V40VZN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/hydrogen exchanger 1 (Rattus norvegicus) | BDBM50115248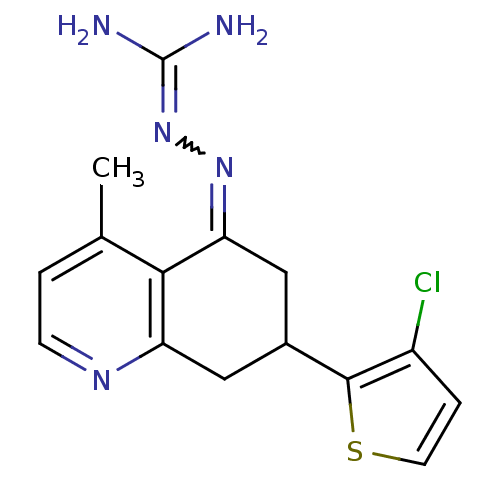 (CHEMBL552723 | N-[7-(3-chloro-2-thienyl)-4-methyl-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description In vitro inhibition of acid induced swelling in rat platelets by 50% as a measure of NHE-1 inhibition | J Med Chem 45: 3009-21 (2002) BindingDB Entry DOI: 10.7270/Q2V40VZN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/hydrogen exchanger 1 (Rattus norvegicus) | BDBM50115267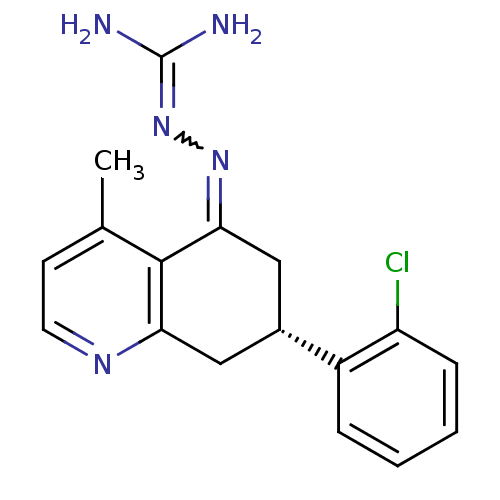 (CHEMBL102546 | N-[7-(2-chlorophenyl)-4-methyl-(7S)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description In vitro inhibition of acid induced swelling in rat platelets by 50% as a measure of NHE-1 inhibition | J Med Chem 45: 3009-21 (2002) BindingDB Entry DOI: 10.7270/Q2V40VZN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/hydrogen exchanger 1 (Rattus norvegicus) | BDBM50115268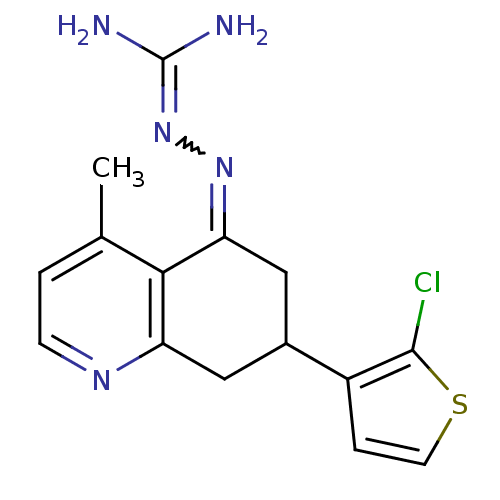 (CHEMBL554001 | N-[7-(2-chloro-3-thienyl)-4-methyl-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description In vitro inhibition of acid induced swelling in rat platelets by 50% as a measure of NHE-1 inhibition | J Med Chem 45: 3009-21 (2002) BindingDB Entry DOI: 10.7270/Q2V40VZN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/hydrogen exchanger 1 (Rattus norvegicus) | BDBM50115238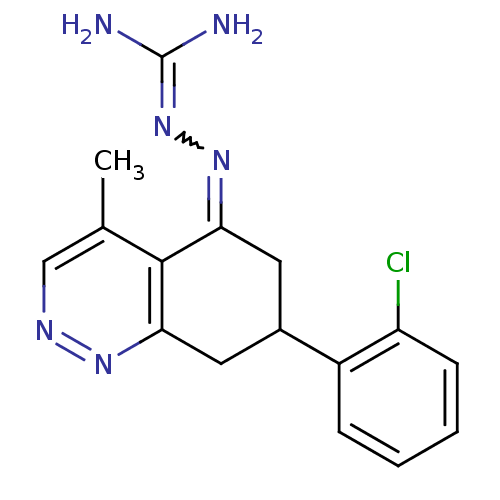 (CHEMBL552865 | N-[7-(2-chlorophenyl)-4-methyl-5,6,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description In vitro inhibition of acid induced swelling in rat platelets by 50% as a measure of NHE-1 inhibition | J Med Chem 45: 3009-21 (2002) BindingDB Entry DOI: 10.7270/Q2V40VZN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/hydrogen exchanger 1 (Rattus norvegicus) | BDBM50115245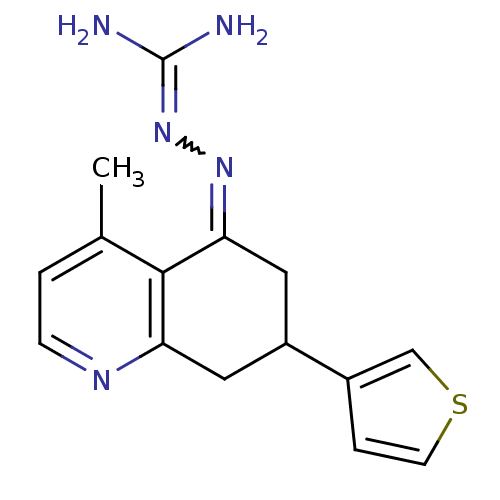 (CHEMBL554215 | N-[4-methyl-7-(3-thienyl)-5,6,7,8-t...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description In vitro inhibition of acid induced swelling in rat platelets by 50% as a measure of NHE-1 inhibition | J Med Chem 45: 3009-21 (2002) BindingDB Entry DOI: 10.7270/Q2V40VZN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Bos taurus) | BDBM50044403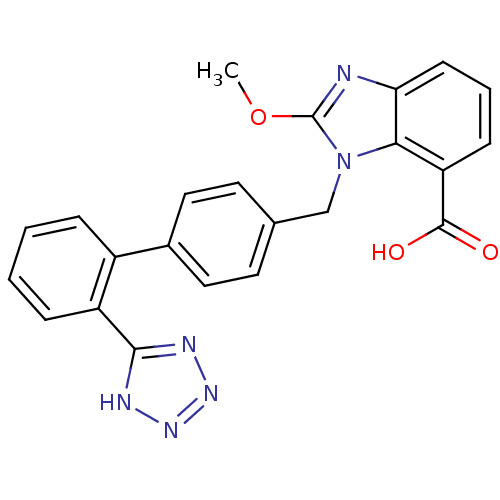 (2-Methoxy-3-[2'-(1H-tetrazol-5-yl)-biphenyl-4-ylme...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description Inhibition of specific binding of [125 I ] Angiotensin-II (0.2 nM) to bovine adrenal cortex | J Med Chem 36: 2182-95 (1993) BindingDB Entry DOI: 10.7270/Q20Z73X8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/hydrogen exchanger 1 (Rattus norvegicus) | BDBM50115236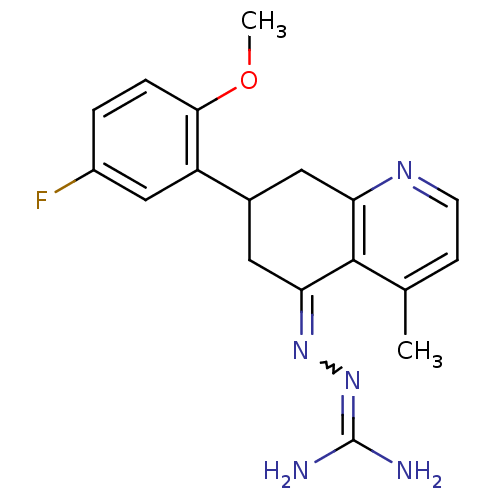 (CHEMBL542925 | N-[7-(5-fluoro-2-methoxyphenyl)-4-m...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description In vitro inhibition of acid induced swelling in rat platelets by 50% as a measure of NHE-1 inhibition | J Med Chem 45: 3009-21 (2002) BindingDB Entry DOI: 10.7270/Q2V40VZN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/hydrogen exchanger 1 (Rattus norvegicus) | BDBM50115233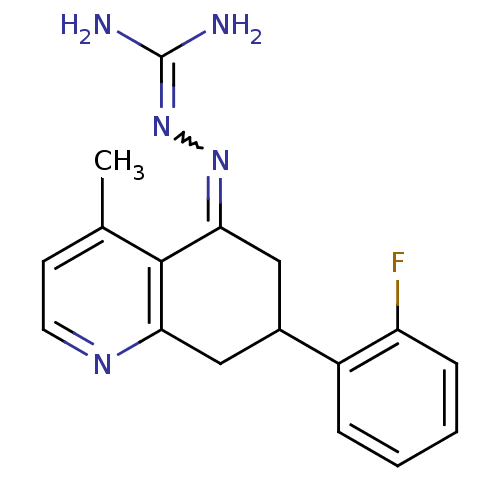 (CHEMBL541577 | N-[7-(2-fluorophenyl)-4-methyl-5,6,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description In vitro inhibition of acid induced swelling in rat platelets by 50% as a measure of NHE-1 inhibition | J Med Chem 45: 3009-21 (2002) BindingDB Entry DOI: 10.7270/Q2V40VZN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/hydrogen exchanger 1 (Rattus norvegicus) | BDBM50115270 ((R)-N-[7-(2,5-dichloro-3-thienyl)-4-methyl-(7R)-5,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description In vitro inhibition of acid induced swelling in rat platelets by 50% as a measure of NHE-1 inhibition | J Med Chem 45: 3009-21 (2002) BindingDB Entry DOI: 10.7270/Q2V40VZN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/hydrogen exchanger 1 (Rattus norvegicus) | BDBM50115264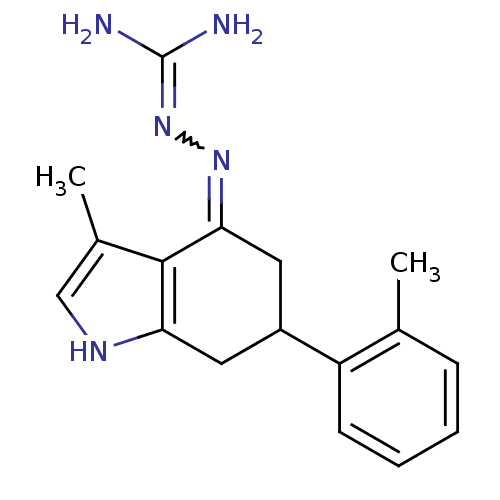 (CHEMBL552934 | N-[3-methyl-6-(2-methylphenyl)-4,5,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description In vitro inhibition of acid induced swelling in rat platelets by 50% as a measure of NHE-1 inhibition | J Med Chem 45: 3009-21 (2002) BindingDB Entry DOI: 10.7270/Q2V40VZN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/hydrogen exchanger 1 (Rattus norvegicus) | BDBM50115258 (CHEMBL555535 | N-[7-(5-fluoro-2-methylphenyl)-4-me...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description In vitro inhibition of acid induced swelling in rat platelets by 50% as a measure of NHE-1 inhibition | J Med Chem 45: 3009-21 (2002) BindingDB Entry DOI: 10.7270/Q2V40VZN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Bos taurus) | BDBM50044379 (2-Propylamino-3-[2'-(1H-tetrazol-5-yl)-biphenyl-4-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description Inhibition of specific binding of [125 I ] Angiotensin-II (0.2 nM) to bovine adrenal cortex | J Med Chem 36: 2182-95 (1993) BindingDB Entry DOI: 10.7270/Q20Z73X8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/hydrogen exchanger 1 (Rattus norvegicus) | BDBM50115257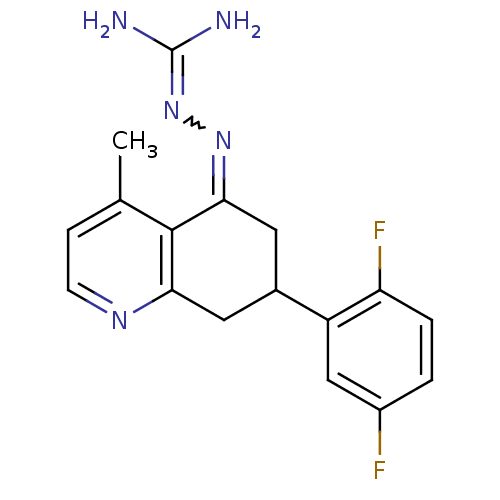 (CHEMBL542687 | N-[7-(2,5-difluorophenyl)-4-methyl-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description In vitro inhibition of acid induced swelling in rat platelets by 50% as a measure of NHE-1 inhibition | J Med Chem 45: 3009-21 (2002) BindingDB Entry DOI: 10.7270/Q2V40VZN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/hydrogen exchanger 1 (Rattus norvegicus) | BDBM50115256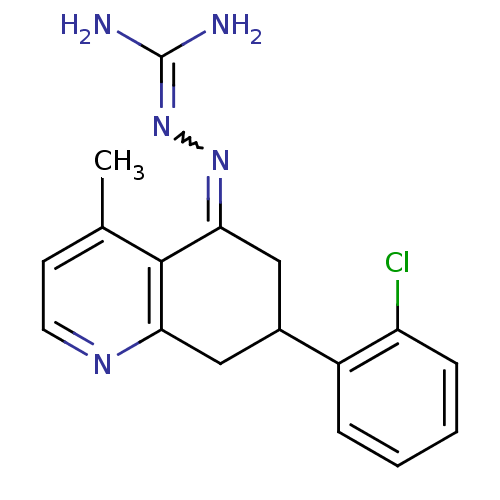 (CHEMBL543148 | N-[7-(2-chlorophenyl)-4-methyl-5,6,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description In vitro inhibition of acid induced swelling in rat platelets by 50% as a measure of NHE-1 inhibition | J Med Chem 45: 3009-21 (2002) BindingDB Entry DOI: 10.7270/Q2V40VZN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Bos taurus) | BDBM50285002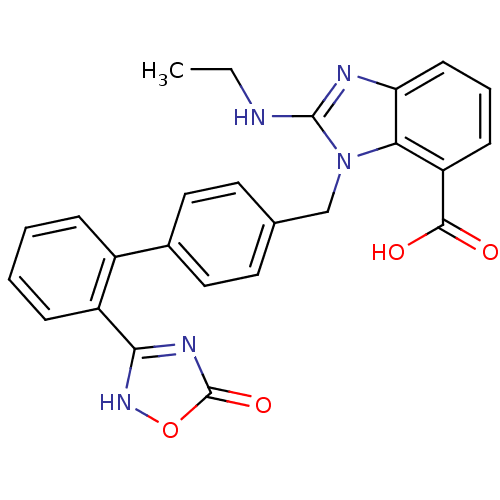 (2-Ethylamino-3-[2'-(5-oxo-4,5-dihydro-[1,2,4]oxadi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [125 I]-AII (0.2 nM) from bovine adrenal cortical membrane angiotensin II (AII) receptor at 10e-7 M | Bioorg Med Chem Lett 5: 1903-1908 (1995) Article DOI: 10.1016/0960-894X(95)00319-O BindingDB Entry DOI: 10.7270/Q26M36SM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Bos taurus) | BDBM50044410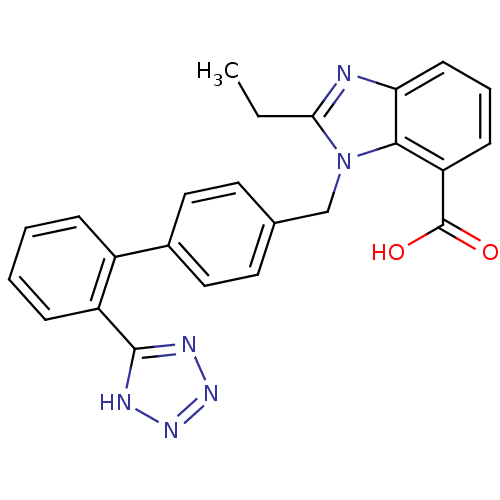 (2-Ethyl-3-[2'-(1H-tetrazol-5-yl)-biphenyl-4-ylmeth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description Inhibition of specific binding of [125 I ] Angiotensin-II (0.2 nM) to bovine adrenal cortex | J Med Chem 36: 2182-95 (1993) BindingDB Entry DOI: 10.7270/Q20Z73X8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/hydrogen exchanger 1 (Rattus norvegicus) | BDBM50115252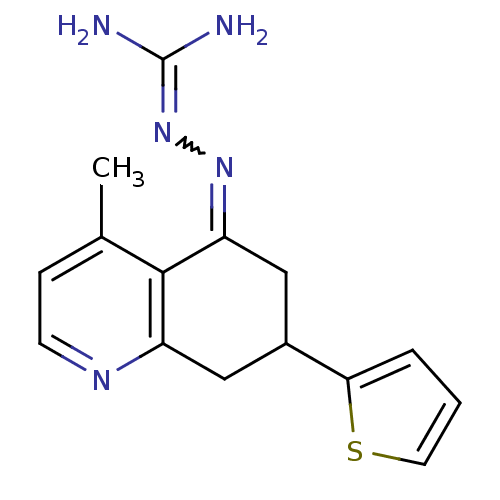 (CHEMBL552827 | N-[4-methyl-7-(2-thienyl)-5,6,7,8-t...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description In vitro inhibition of acid induced swelling in rat platelets by 50% as a measure of NHE-1 inhibition | J Med Chem 45: 3009-21 (2002) BindingDB Entry DOI: 10.7270/Q2V40VZN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/hydrogen exchanger 1 (Rattus norvegicus) | BDBM50115260 (CHEMBL545732 | N-[7-(3,5-dichloro-2-thienyl)-4-met...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 49 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description In vitro inhibition of acid induced swelling in rat platelets by 50% as a measure of NHE-1 inhibition | J Med Chem 45: 3009-21 (2002) BindingDB Entry DOI: 10.7270/Q2V40VZN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/hydrogen exchanger 1 (Rattus norvegicus) | BDBM50115234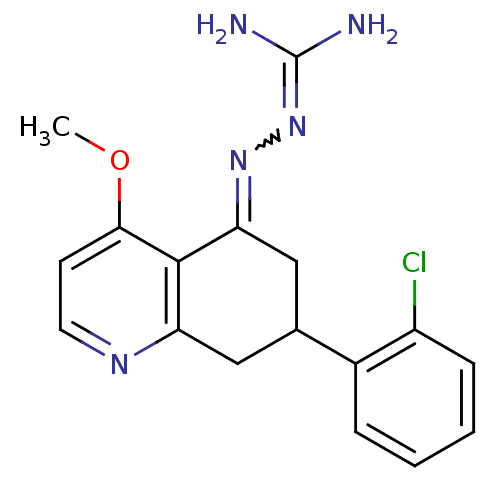 (CHEMBL105412 | N-[7-(2-chlorophenyl)-4-methoxy-5,6...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description In vitro inhibition of acid induced swelling in rat platelets by 50% as a measure of NHE-1 inhibition | J Med Chem 45: 3009-21 (2002) BindingDB Entry DOI: 10.7270/Q2V40VZN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/hydrogen exchanger 1 (Rattus norvegicus) | BDBM50115241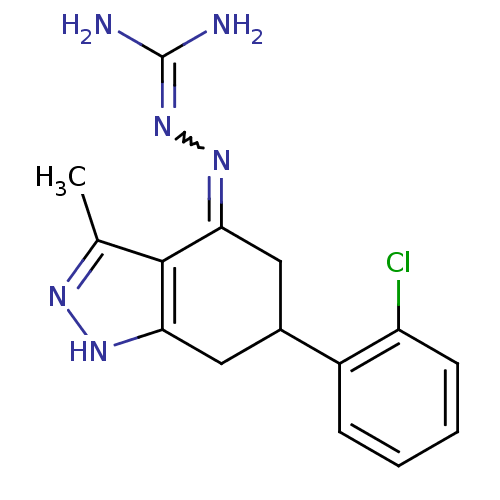 (CHEMBL541918 | N-[6-(2-chlorophenyl)-3-methyl-4,5,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description In vitro inhibition of acid induced swelling in rat platelets by 50% as a measure of NHE-1 inhibition | J Med Chem 45: 3009-21 (2002) BindingDB Entry DOI: 10.7270/Q2V40VZN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/hydrogen exchanger 1 (Rattus norvegicus) | BDBM50115259 (CHEMBL554422 | N-[7-(2-bromophenyl)-4-methyl-5,6,7...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 56 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description In vitro inhibition of acid induced swelling in rat platelets by 50% as a measure of NHE-1 inhibition | J Med Chem 45: 3009-21 (2002) BindingDB Entry DOI: 10.7270/Q2V40VZN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/hydrogen exchanger 1 (Rattus norvegicus) | BDBM50115228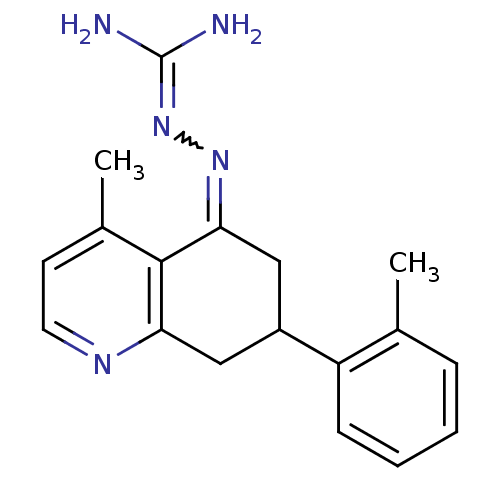 (CHEMBL552540 | N-[4-methyl-7-(2-methylphenyl)-5,6,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 57 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description In vitro inhibition of acid induced swelling in rat platelets by 50% as a measure of NHE-1 inhibition | J Med Chem 45: 3009-21 (2002) BindingDB Entry DOI: 10.7270/Q2V40VZN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/hydrogen exchanger 1 (Rattus norvegicus) | BDBM50115255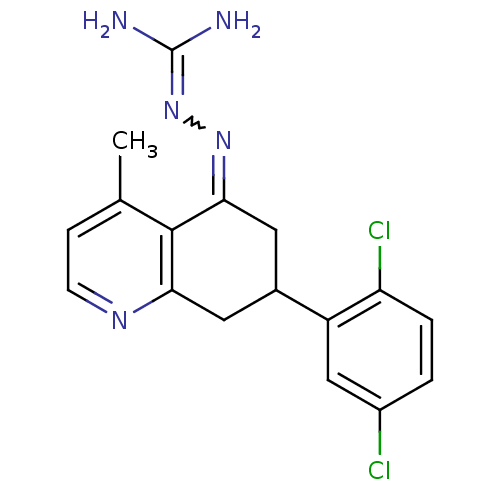 (CHEMBL540813 | N-[7-(2,5-dichlorophenyl)-4-methyl-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 58 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description In vitro inhibition of acid induced swelling in rat platelets by 50% as a measure of NHE-1 inhibition | J Med Chem 45: 3009-21 (2002) BindingDB Entry DOI: 10.7270/Q2V40VZN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/hydrogen exchanger 1 (Rattus norvegicus) | BDBM50115229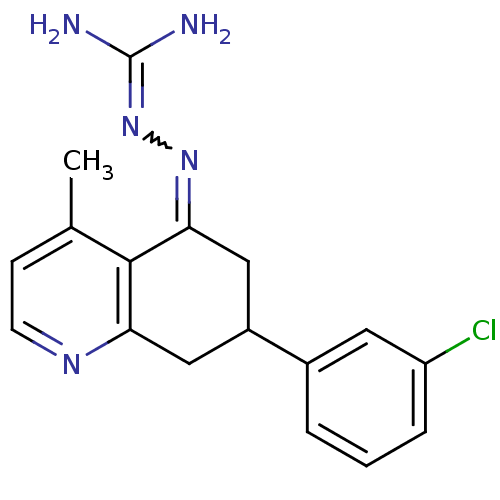 (CHEMBL553754 | N-[7-(3-chlorophenyl)-4-methyl-5,6,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 61 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description In vitro inhibition of acid induced swelling in rat platelets by 50% as a measure of NHE-1 inhibition | J Med Chem 45: 3009-21 (2002) BindingDB Entry DOI: 10.7270/Q2V40VZN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Bos taurus) | BDBM50044414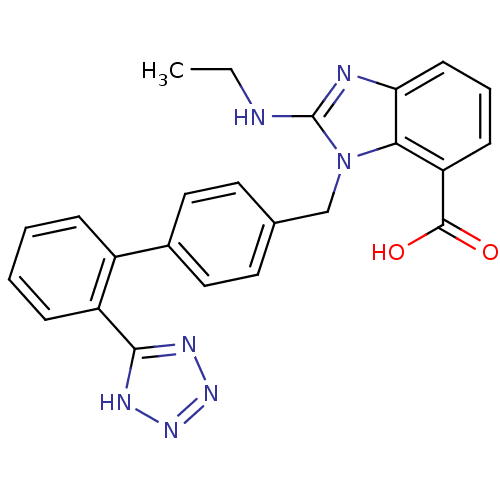 (2-Ethylamino-3-[2'-(1H-tetrazol-5-yl)-biphenyl-4-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 62 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description Inhibition of specific binding of [125 I ] Angiotensin-II (0.2 nM) to bovine adrenal cortex | J Med Chem 36: 2182-95 (1993) BindingDB Entry DOI: 10.7270/Q20Z73X8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Bos taurus) | BDBM50044396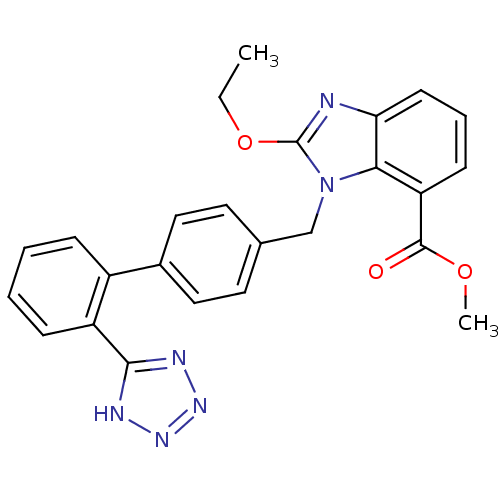 (2-Ethoxy-3-[2'-(1H-tetrazol-5-yl)-biphenyl-4-ylmet...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 66 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description Inhibition of specific binding of [125 I ] Angiotensin-II (0.2 nM) to bovine adrenal cortex | J Med Chem 36: 2182-95 (1993) BindingDB Entry DOI: 10.7270/Q20Z73X8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/hydrogen exchanger 1 (Rattus norvegicus) | BDBM50058759 (CHEMBL436559 | CHEMBL462831 | Cariporide | N-(4-Is...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 75 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description In vitro inhibition of acid induced swelling in rat platelets by 50% as a measure of NHE-1 inhibition | J Med Chem 45: 3009-21 (2002) BindingDB Entry DOI: 10.7270/Q2V40VZN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/hydrogen exchanger 1 (Rattus norvegicus) | BDBM50115235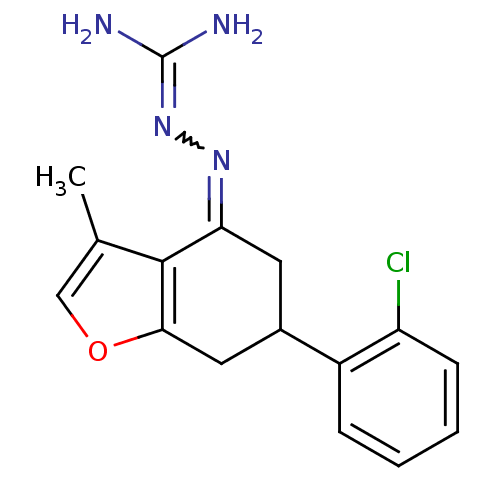 (CHEMBL541833 | N-[6-(2-chlorophenyl)-3-methyl-4,5,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 82 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description In vitro inhibition of acid induced swelling in rat platelets by 50% as a measure of NHE-1 inhibition | J Med Chem 45: 3009-21 (2002) BindingDB Entry DOI: 10.7270/Q2V40VZN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Bos taurus) | BDBM50044386 (2-Isopropyl-3-[2'-(1H-tetrazol-5-yl)-biphenyl-4-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 82 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description Inhibition of specific binding of [125 I ] Angiotensin-II (0.2 nM) to bovine adrenal cortex | J Med Chem 36: 2182-95 (1993) BindingDB Entry DOI: 10.7270/Q20Z73X8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Bos taurus) | BDBM50044389 (2-Cyclopropyl-3-[2'-(1H-tetrazol-5-yl)-biphenyl-4-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 84 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description Inhibition of specific binding of [125 I ] Angiotensin-II (0.2 nM) to bovine adrenal cortex | J Med Chem 36: 2182-95 (1993) BindingDB Entry DOI: 10.7270/Q20Z73X8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/hydrogen exchanger 1 (Rattus norvegicus) | BDBM50115269 (CHEMBL554686 | N-[7-(2,3-dichlorophenyl)-4-methyl-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 89 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description In vitro inhibition of acid induced swelling in rat platelets by 50% as a measure of NHE-1 inhibition | J Med Chem 45: 3009-21 (2002) BindingDB Entry DOI: 10.7270/Q2V40VZN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/hydrogen exchanger 1 (Rattus norvegicus) | BDBM50115261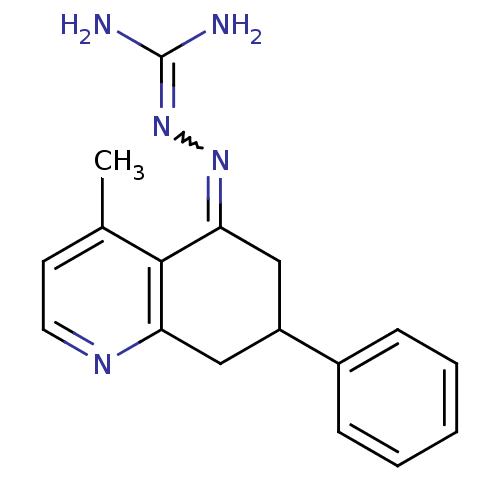 (CHEMBL543865 | N-(4-methyl-7-phenyl-5,6,7,8-tetrah...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 91 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description In vitro inhibition of acid induced swelling in rat platelets by 50% as a measure of NHE-1 inhibition | J Med Chem 45: 3009-21 (2002) BindingDB Entry DOI: 10.7270/Q2V40VZN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Bos taurus) | BDBM50044388 (2-Ethanesulfinyl-3-[2'-(1H-tetrazol-5-yl)-biphenyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >100 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description Inhibition of specific binding of [125 I ] Angiotensin-II (0.2 nM) to bovine adrenal cortex | J Med Chem 36: 2182-95 (1993) BindingDB Entry DOI: 10.7270/Q20Z73X8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/hydrogen exchanger 1 (Rattus norvegicus) | BDBM50115253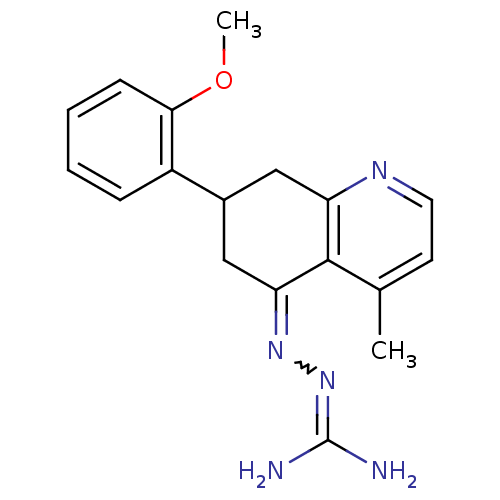 (CHEMBL555103 | N-[7-(2-methoxyphenyl)-4-methyl-5,6...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description In vitro inhibition of acid induced swelling in rat platelets by 50% as a measure of NHE-1 inhibition | J Med Chem 45: 3009-21 (2002) BindingDB Entry DOI: 10.7270/Q2V40VZN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Bos taurus) | BDBM50240609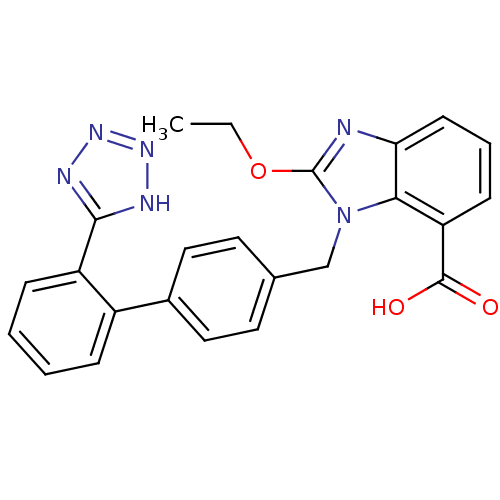 (2-Ethoxy-3-[2'-(1H-tetrazol-5-yl)-biphenyl-4-ylmet...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description Inhibition of specific binding of [125 I ] Angiotensin-II (0.2 nM) to bovine adrenal cortex | J Med Chem 36: 2182-95 (1993) BindingDB Entry DOI: 10.7270/Q20Z73X8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Bos taurus) | BDBM50240609 (2-Ethoxy-3-[2'-(1H-tetrazol-5-yl)-biphenyl-4-ylmet...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [125 I]-AII (0.2 nM) from bovine adrenal cortical membrane angiotensin II (AII) receptor at 10e-7 M | Bioorg Med Chem Lett 5: 1903-1908 (1995) Article DOI: 10.1016/0960-894X(95)00319-O BindingDB Entry DOI: 10.7270/Q26M36SM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/hydrogen exchanger 1 (Rattus norvegicus) | BDBM50115242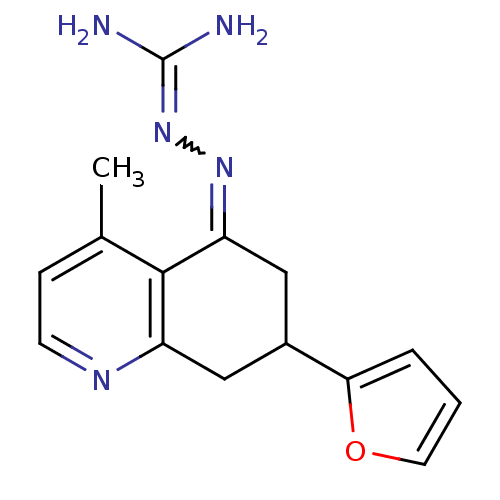 (CHEMBL554125 | N-[7-(2-furyl)-4-methyl-5,6,7,8-tet...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description In vitro inhibition of acid induced swelling in rat platelets by 50% as a measure of NHE-1 inhibition | J Med Chem 45: 3009-21 (2002) BindingDB Entry DOI: 10.7270/Q2V40VZN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Bos taurus) | BDBM50044413 (2-Propylsulfanyl-3-[2'-(1H-tetrazol-5-yl)-biphenyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description Inhibition of specific binding of [125 I ] Angiotensin-II (0.2 nM) to bovine adrenal cortex | J Med Chem 36: 2182-95 (1993) BindingDB Entry DOI: 10.7270/Q20Z73X8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Bos taurus) | BDBM50044377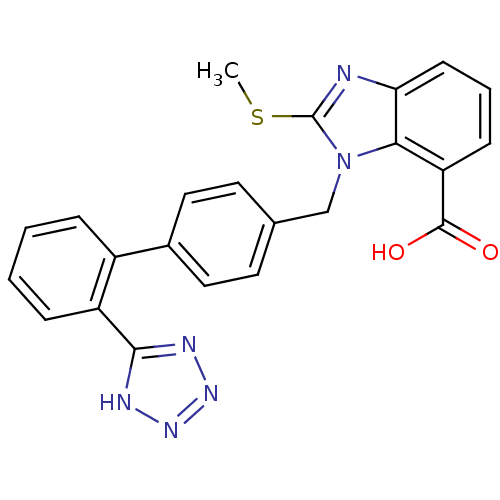 (2-Methylsulfanyl-3-[2'-(1H-tetrazol-5-yl)-biphenyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description Inhibition of specific binding of [125 I ] Angiotensin-II (0.2 nM) to bovine adrenal cortex | J Med Chem 36: 2182-95 (1993) BindingDB Entry DOI: 10.7270/Q20Z73X8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Bos taurus) | BDBM50009714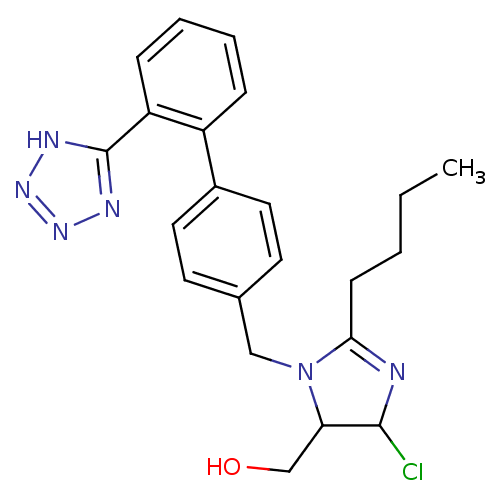 (CHEMBL191 | {2-Butyl-5-chloro-3-[2'-(2H-tetrazol-5...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [125 I]-AII (0.2 nM) from bovine adrenal cortical membrane angiotensin II (AII) receptor at 10e-7 M | Bioorg Med Chem Lett 5: 1903-1908 (1995) Article DOI: 10.1016/0960-894X(95)00319-O BindingDB Entry DOI: 10.7270/Q26M36SM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Bos taurus) | BDBM50044406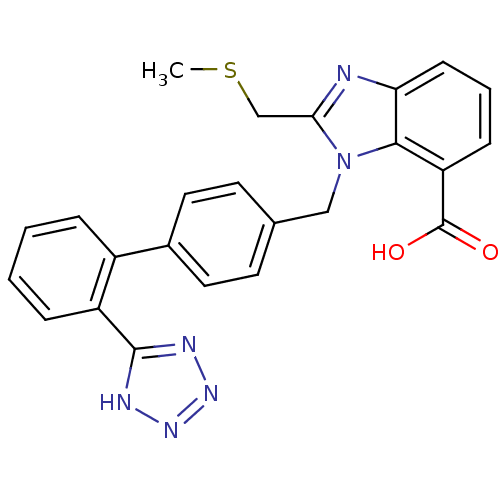 (2-Methylsulfanylmethyl-3-[2'-(1H-tetrazol-5-yl)-bi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description Inhibition of specific binding of [125 I ] Angiotensin-II (0.2 nM) to bovine adrenal cortex | J Med Chem 36: 2182-95 (1993) BindingDB Entry DOI: 10.7270/Q20Z73X8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Bos taurus) | BDBM50406795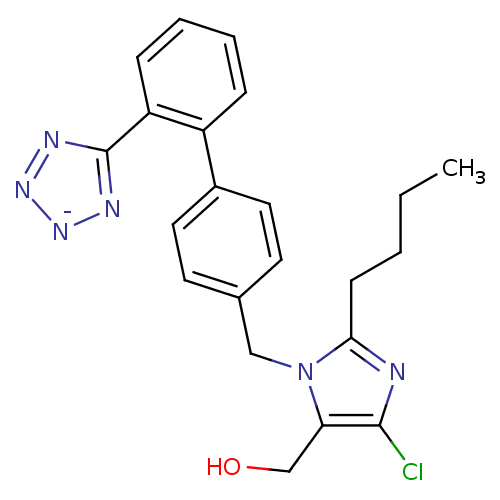 (Cozaar | LOSARTAN POTASSIUM) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description Inhibition of specific binding of [125 I ] Angiotensin-II (0.2 nM) to bovine adrenal cortex | J Med Chem 36: 2182-95 (1993) BindingDB Entry DOI: 10.7270/Q20Z73X8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Bos taurus) | BDBM50044408 (2-Methylamino-3-[2'-(1H-tetrazol-5-yl)-biphenyl-4-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description Inhibition of specific binding of [125 I ] Angiotensin-II (0.2 nM) to bovine adrenal cortex | J Med Chem 36: 2182-95 (1993) BindingDB Entry DOI: 10.7270/Q20Z73X8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Bos taurus) | BDBM50044375 (2-Propyl-3-[2'-(1H-tetrazol-5-yl)-biphenyl-4-ylmet...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description Inhibition of specific binding of [125 I ] Angiotensin-II (0.2 nM) to bovine adrenal cortex | J Med Chem 36: 2182-95 (1993) BindingDB Entry DOI: 10.7270/Q20Z73X8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Bos taurus) | BDBM50044382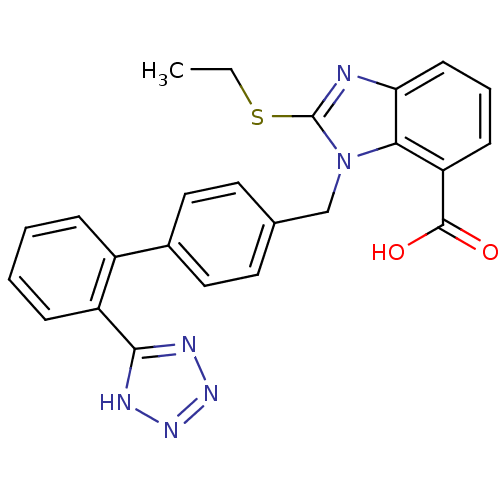 (2-Ethylsulfanyl-3-[2'-(1H-tetrazol-5-yl)-biphenyl-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description Inhibition of specific binding of [125 I ] Angiotensin-II (0.2 nM) to bovine adrenal cortex | J Med Chem 36: 2182-95 (1993) BindingDB Entry DOI: 10.7270/Q20Z73X8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/hydrogen exchanger 1 (Rattus norvegicus) | BDBM50115249 (CHEMBL545502 | N-(2,4-dimethyl-7-phenyl-5,6,7,8-te...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description In vitro inhibition of acid induced swelling in rat platelets by 50% as a measure of NHE-1 inhibition | J Med Chem 45: 3009-21 (2002) BindingDB Entry DOI: 10.7270/Q2V40VZN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 101 total ) | Next | Last >> |