 Found 1236 hits with Last Name = 'jenkins' and Initial = 'k'
Found 1236 hits with Last Name = 'jenkins' and Initial = 'k' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Promotilin
(RABBIT) | BDBM85389
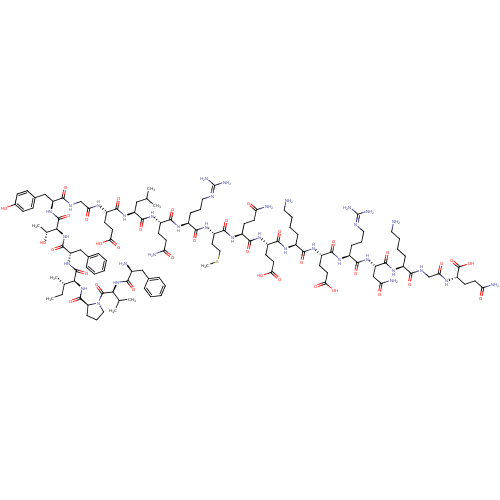
(CAS_52906-92-0 | Motilin)Show SMILES [#6]-[#6]-[#6@H](-[#6])-[#6@H](-[#7]-[#6](=O)-[#6@@H]-1-[#6]-[#6]-[#6]-[#7]-1-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@@H](-[#7])-[#6]-c1ccccc1)-[#6](-[#6])-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccccc1)-[#6](=O)-[#7]-[#6@@H](-[#6@@H](-[#6])-[#8])-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccc(-[#8])cc1)-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#6])-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#16]-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](-[#7])=O)-[#6](-[#8])=O |r| Show InChI InChI=1S/C120H188N34O35S/c1-9-64(6)97(152-114(184)86-31-22-53-154(86)117(187)96(63(4)5)151-99(169)70(123)56-66-23-12-10-13-24-66)115(185)150-84(57-67-25-14-11-15-26-67)113(183)153-98(65(7)155)116(186)149-83(58-68-32-34-69(156)35-33-68)101(171)135-60-91(161)136-75(39-45-93(163)164)105(175)147-82(55-62(2)3)111(181)145-77(37-43-88(125)158)106(176)140-73(29-20-51-132-119(128)129)103(173)146-80(48-54-190-8)110(180)142-76(36-42-87(124)157)107(177)144-79(41-47-95(167)168)108(178)139-72(28-17-19-50-122)102(172)143-78(40-46-94(165)166)109(179)141-74(30-21-52-133-120(130)131)104(174)148-85(59-90(127)160)112(182)138-71(27-16-18-49-121)100(170)134-61-92(162)137-81(118(188)189)38-44-89(126)159/h10-15,23-26,32-35,62-65,70-86,96-98,155-156H,9,16-22,27-31,36-61,121-123H2,1-8H3,(H2,124,157)(H2,125,158)(H2,126,159)(H2,127,160)(H,134,170)(H,135,171)(H,136,161)(H,137,162)(H,138,182)(H,139,178)(H,140,176)(H,141,179)(H,142,180)(H,143,172)(H,144,177)(H,145,181)(H,146,173)(H,147,175)(H,148,174)(H,149,186)(H,150,185)(H,151,169)(H,152,184)(H,153,183)(H,163,164)(H,165,166)(H,167,168)(H,188,189)(H4,128,129,132)(H4,130,131,133)/t64-,65+,70-,71-,72-,73-,74-,75-,76-,77-,78-,79-,80-,81-,82-,83-,84-,85-,86-,96-,97-,98-/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.98 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Melbourne
Curated by PDSP Ki Database
| |
Clin Exp Pharmacol Physiol 26: 242-5 (1999)
Article DOI: 10.1046/j.1440-1681.1999.03022.x
BindingDB Entry DOI: 10.7270/Q2VX0F2Z |
More data for this
Ligand-Target Pair | |
Promotilin
(RABBIT) | BDBM85390
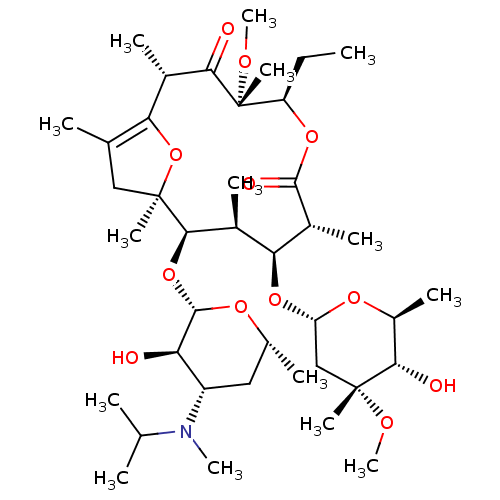
(GM 611 | Mitemcinal)Show SMILES CC[C@H]1OC(=O)[C@H](C)[C@@H](O[C@H]2C[C@@](C)(OC)[C@@H](O)[C@H](C)O2)[C@H](C)[C@@H](O[C@@H]2O[C@H](C)C[C@@H]([C@H]2O)N(C)C(C)C)[C@@]2(C)CC(C)=C(O2)[C@H](C)C(=O)[C@]1(C)OC |c:44| Show InChI InChI=1S/C40H69NO12/c1-16-28-40(12,47-15)33(43)23(6)31-21(4)18-39(11,53-31)35(52-37-30(42)27(17-22(5)48-37)41(13)20(2)3)24(7)32(25(8)36(45)50-28)51-29-19-38(10,46-14)34(44)26(9)49-29/h20,22-30,32,34-35,37,42,44H,16-19H2,1-15H3/t22-,23+,24+,25-,26+,27+,28-,29+,30-,32+,34+,35-,37+,38-,39-,40-/m1/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 31.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Melbourne
Curated by PDSP Ki Database
| |
Clin Exp Pharmacol Physiol 26: 242-5 (1999)
Article DOI: 10.1046/j.1440-1681.1999.03022.x
BindingDB Entry DOI: 10.7270/Q2VX0F2Z |
More data for this
Ligand-Target Pair | |
Promotilin
(RABBIT) | BDBM50004656

((2-Carbamoyloxy-ethyl)-trimethyl-ammonium | (2-Car...)Show InChI InChI=1S/C6H14N2O2/c1-8(2,3)4-5-10-6(7)9/h4-5H2,1-3H3,(H-,7,9)/p+1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 158 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Melbourne
Curated by PDSP Ki Database
| |
Clin Exp Pharmacol Physiol 26: 242-5 (1999)
Article DOI: 10.1046/j.1440-1681.1999.03022.x
BindingDB Entry DOI: 10.7270/Q2VX0F2Z |
More data for this
Ligand-Target Pair | |
Promotilin
(RABBIT) | BDBM50127141
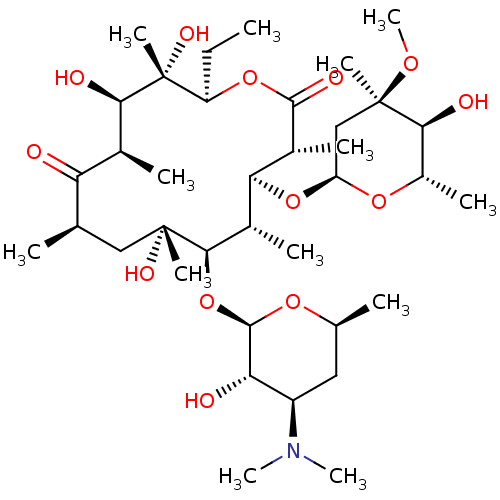
((3R,4S,5S,6R,7R,9R,11R,12R,13S,14R)-6-{[(2R,3S,4R,...)Show SMILES CC[C@H]1OC(=O)[C@H](C)[C@@H](O[C@H]2C[C@@](C)(OC)[C@@H](O)[C@H](C)O2)[C@H](C)[C@@H](O[C@H]2O[C@@H](C)C[C@H]([C@@H]2O)N(C)C)[C@](C)(O)C[C@@H](C)C(=O)[C@H](C)[C@@H](O)[C@]1(C)O |r| Show InChI InChI=1S/C37H67NO13/c1-14-25-37(10,45)30(41)20(4)27(39)18(2)16-35(8,44)32(51-34-28(40)24(38(11)12)15-19(3)47-34)21(5)29(22(6)33(43)49-25)50-26-17-36(9,46-13)31(42)23(7)48-26/h18-26,28-32,34,40-42,44-45H,14-17H2,1-13H3/t18-,19+,20+,21+,22-,23+,24-,25-,26+,28+,29+,30-,31+,32-,34-,35-,36-,37-/m1/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Melbourne
Curated by PDSP Ki Database
| |
Clin Exp Pharmacol Physiol 26: 242-5 (1999)
Article DOI: 10.1046/j.1440-1681.1999.03022.x
BindingDB Entry DOI: 10.7270/Q2VX0F2Z |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50377170
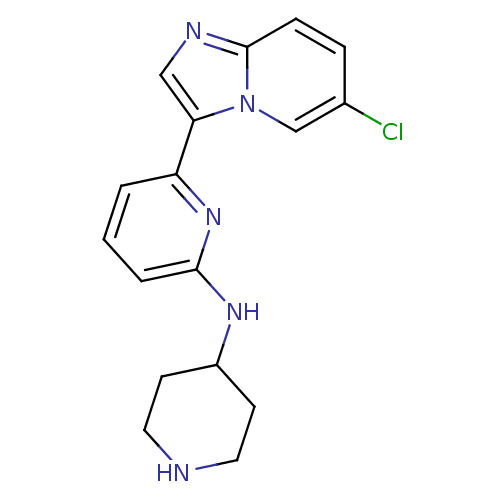
(CHEMBL256570 | US11254667, Compound I-2 | US115422...)Show InChI InChI=1S/C17H18ClN5/c18-12-4-5-17-20-10-15(23(17)11-12)14-2-1-3-16(22-14)21-13-6-8-19-9-7-13/h1-5,10-11,13,19H,6-9H2,(H,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
UCB
Curated by ChEMBL
| Assay Description
Inhibition of IRAK4 |
Bioorg Med Chem Lett 18: 3656-60 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.042
BindingDB Entry DOI: 10.7270/Q2JH3N2N |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50377180
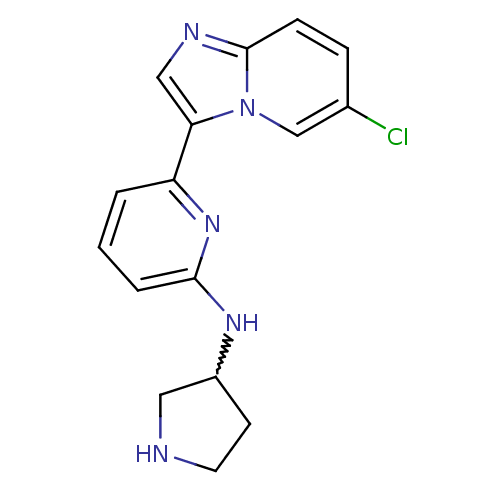
(CHEMBL401633)Show SMILES Clc1ccc2ncc(-c3cccc(NC4CCNC4)n3)n2c1 |w:14.13| Show InChI InChI=1S/C16H16ClN5/c17-11-4-5-16-19-9-14(22(16)10-11)13-2-1-3-15(21-13)20-12-6-7-18-8-12/h1-5,9-10,12,18H,6-8H2,(H,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
UCB
Curated by ChEMBL
| Assay Description
Inhibition of IRAK4 |
Bioorg Med Chem Lett 18: 3656-60 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.042
BindingDB Entry DOI: 10.7270/Q2JH3N2N |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50377165
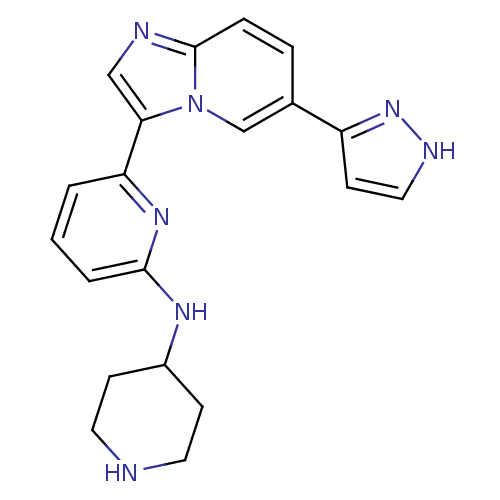
(CHEMBL255867)Show SMILES C1CC(CCN1)Nc1cccc(n1)-c1cnc2ccc(cn12)-c1cc[nH]n1 Show InChI InChI=1S/C20H21N7/c1-2-17(25-19(3-1)24-15-6-9-21-10-7-15)18-12-22-20-5-4-14(13-27(18)20)16-8-11-23-26-16/h1-5,8,11-13,15,21H,6-7,9-10H2,(H,23,26)(H,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
UCB
Curated by ChEMBL
| Assay Description
Inhibition of IRAK4 |
Bioorg Med Chem Lett 18: 3656-60 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.042
BindingDB Entry DOI: 10.7270/Q2JH3N2N |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50377175
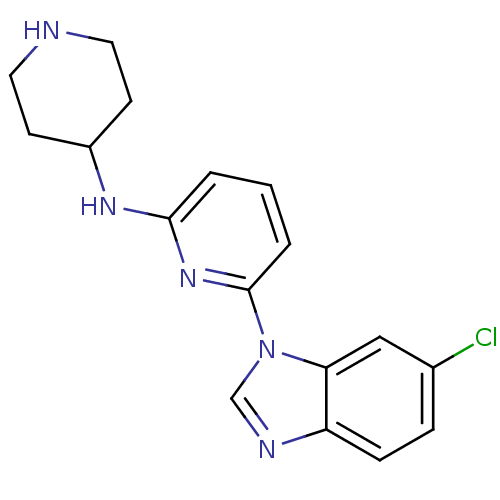
(CHEMBL436653)Show InChI InChI=1S/C17H18ClN5/c18-12-4-5-14-15(10-12)23(11-20-14)17-3-1-2-16(22-17)21-13-6-8-19-9-7-13/h1-5,10-11,13,19H,6-9H2,(H,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
UCB
Curated by ChEMBL
| Assay Description
Inhibition of IRAK4 |
Bioorg Med Chem Lett 18: 3656-60 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.042
BindingDB Entry DOI: 10.7270/Q2JH3N2N |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 10
(Homo sapiens (Human)) | BDBM50271563
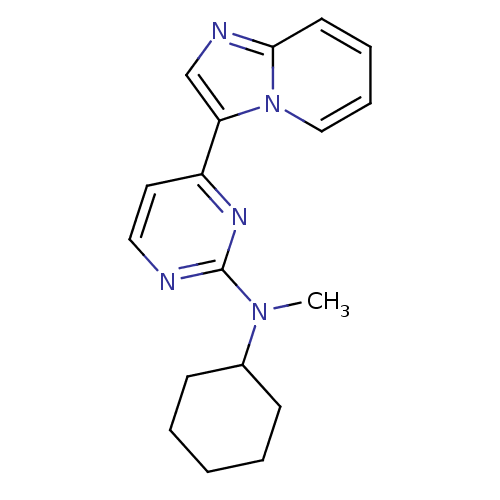
(CHEMBL482708 | N-cyclohexyl-4-(H-imidazo[1,2-a]pyr...)Show InChI InChI=1S/C18H21N5/c1-22(14-7-3-2-4-8-14)18-19-11-10-15(21-18)16-13-20-17-9-5-6-12-23(16)17/h5-6,9-14H,2-4,7-8H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
UCB
Curated by ChEMBL
| Assay Description
Inhibition of JNK3 (unknown origin) |
Bioorg Med Chem Lett 18: 3291-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.039
BindingDB Entry DOI: 10.7270/Q2NZ87FX |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Peptidase 1
(Dermatophagoides pteronyssinus (European house dus...) | BDBM50031688
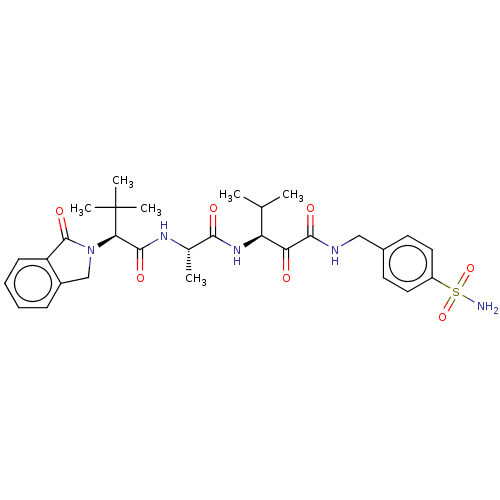
(CHEMBL3360299)Show SMILES CC(C)[C@H](NC(=O)[C@H](C)NC(=O)[C@@H](N1Cc2ccccc2C1=O)C(C)(C)C)C(=O)C(=O)NCc1ccc(cc1)S(N)(=O)=O |r| Show InChI InChI=1S/C30H39N5O7S/c1-17(2)23(24(36)27(38)32-15-19-11-13-21(14-12-19)43(31,41)42)34-26(37)18(3)33-28(39)25(30(4,5)6)35-16-20-9-7-8-10-22(20)29(35)40/h7-14,17-18,23,25H,15-16H2,1-6H3,(H,32,38)(H,33,39)(H,34,37)(H2,31,41,42)/t18-,23-,25+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Domainex Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of house dust mite Derp-1 after 20 mins |
J Med Chem 57: 9447-62 (2014)
Article DOI: 10.1021/jm501102h
BindingDB Entry DOI: 10.7270/Q2R212ZW |
More data for this
Ligand-Target Pair | |
Peptidase 1
(Dermatophagoides pteronyssinus (European house dus...) | BDBM50031689

(CHEMBL3360298)Show SMILES CC(C)[C@H](NC(=O)[C@H](C)NC(=O)[C@@H](NC(=O)c1ccnc2ccccc12)C(C)(C)C)C(=O)C(=O)NCC(=O)N1CCN(CC1)C(C)C |r| Show InChI InChI=1S/C34H49N7O6/c1-20(2)27(28(43)32(46)36-19-26(42)41-17-15-40(16-18-41)21(3)4)38-30(44)22(5)37-33(47)29(34(6,7)8)39-31(45)24-13-14-35-25-12-10-9-11-23(24)25/h9-14,20-22,27,29H,15-19H2,1-8H3,(H,36,46)(H,37,47)(H,38,44)(H,39,45)/t22-,27-,29+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Domainex Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of house dust mite Derp-1 after 20 mins |
J Med Chem 57: 9447-62 (2014)
Article DOI: 10.1021/jm501102h
BindingDB Entry DOI: 10.7270/Q2R212ZW |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50377168

(CHEMBL255873)Show InChI InChI=1S/C18H18N6/c19-10-13-4-5-18-21-11-16(24(18)12-13)15-2-1-3-17(23-15)22-14-6-8-20-9-7-14/h1-5,11-12,14,20H,6-9H2,(H,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
UCB
Curated by ChEMBL
| Assay Description
Inhibition of IRAK4 |
Bioorg Med Chem Lett 18: 3656-60 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.042
BindingDB Entry DOI: 10.7270/Q2JH3N2N |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50264777
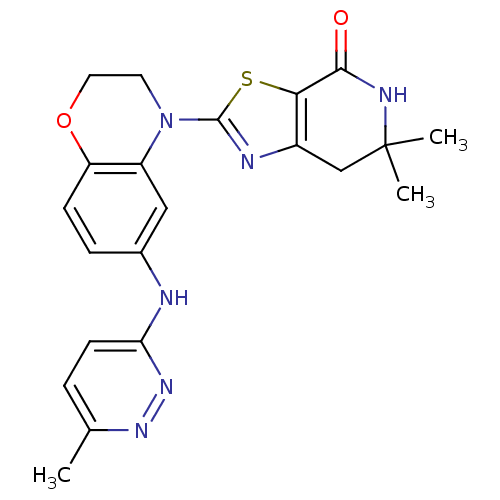
(6,6-dimethyl-2-(6-(6-methylpyridazin-3-ylamino)-2H...)Show SMILES Cc1ccc(Nc2ccc3OCCN(c4nc5CC(C)(C)NC(=O)c5s4)c3c2)nn1 Show InChI InChI=1S/C21H22N6O2S/c1-12-4-7-17(26-25-12)22-13-5-6-16-15(10-13)27(8-9-29-16)20-23-14-11-21(2,3)24-19(28)18(14)30-20/h4-7,10H,8-9,11H2,1-3H3,(H,22,26)(H,24,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
UCB
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) |
Bioorg Med Chem Lett 18: 5299-302 (2008)
Article DOI: 10.1016/j.bmcl.2008.08.042
BindingDB Entry DOI: 10.7270/Q2WS8T3X |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50265362
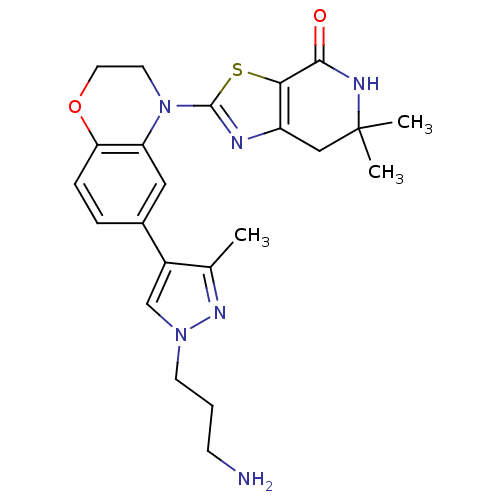
(2-(6-(1-(3-aminopropyl)-3-methyl-1H-pyrazol-4-yl)-...)Show SMILES Cc1nn(CCCN)cc1-c1ccc2OCCN(c3nc4CC(C)(C)NC(=O)c4s3)c2c1 Show InChI InChI=1S/C23H28N6O2S/c1-14-16(13-28(27-14)8-4-7-24)15-5-6-19-18(11-15)29(9-10-31-19)22-25-17-12-23(2,3)26-21(30)20(17)32-22/h5-6,11,13H,4,7-10,12,24H2,1-3H3,(H,26,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
UCB
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) |
Bioorg Med Chem Lett 18: 5299-302 (2008)
Article DOI: 10.1016/j.bmcl.2008.08.042
BindingDB Entry DOI: 10.7270/Q2WS8T3X |
More data for this
Ligand-Target Pair | |
Peptidase 1
(Dermatophagoides pteronyssinus (European house dus...) | BDBM50031684

(CHEMBL3360302)Show SMILES CC(C)[C@H](NC(=O)[C@H](C)NC(=O)[C@@H](NC(=O)c1cccc2ccccc12)C(C)(C)C)C(=O)C(=O)NCc1ccc(cc1)S(N)(=O)=O |r| Show InChI InChI=1S/C33H41N5O7S/c1-19(2)26(27(39)31(42)35-18-21-14-16-23(17-15-21)46(34,44)45)37-29(40)20(3)36-32(43)28(33(4,5)6)38-30(41)25-13-9-11-22-10-7-8-12-24(22)25/h7-17,19-20,26,28H,18H2,1-6H3,(H,35,42)(H,36,43)(H,37,40)(H,38,41)(H2,34,44,45)/t20-,26-,28+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Domainex Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of house dust mite Derp-1 after 20 mins |
J Med Chem 57: 9447-62 (2014)
Article DOI: 10.1021/jm501102h
BindingDB Entry DOI: 10.7270/Q2R212ZW |
More data for this
Ligand-Target Pair | |
Peptidase 1
(Dermatophagoides pteronyssinus (European house dus...) | BDBM50031685

(CHEMBL3360301)Show SMILES CC(C)[C@H](NC(=O)[C@H](C)NC(=O)[C@@H](N1Cc2ccccc2C1=O)C(C)(C)C)C(=O)C(=O)NCc1ccc(CN(C)C)cc1 |r| Show InChI InChI=1S/C33H45N5O5/c1-20(2)26(27(39)30(41)34-17-22-13-15-23(16-14-22)18-37(7)8)36-29(40)21(3)35-31(42)28(33(4,5)6)38-19-24-11-9-10-12-25(24)32(38)43/h9-16,20-21,26,28H,17-19H2,1-8H3,(H,34,41)(H,35,42)(H,36,40)/t21-,26-,28+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Domainex Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of house dust mite Derp-1 after 20 mins |
J Med Chem 57: 9447-62 (2014)
Article DOI: 10.1021/jm501102h
BindingDB Entry DOI: 10.7270/Q2R212ZW |
More data for this
Ligand-Target Pair | |
Peptidase 1
(Dermatophagoides pteronyssinus (European house dus...) | BDBM50031687
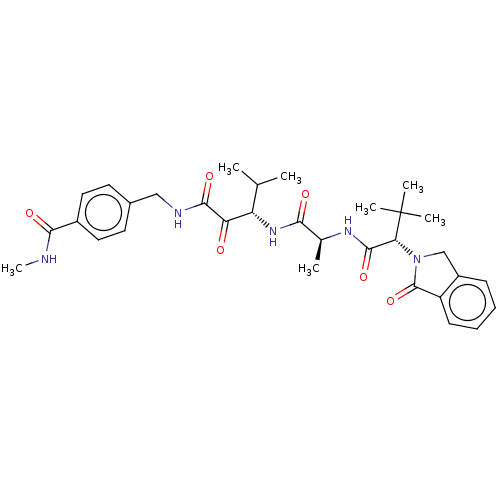
(CHEMBL3360300)Show SMILES CNC(=O)c1ccc(CNC(=O)C(=O)[C@@H](NC(=O)[C@H](C)NC(=O)[C@@H](N2Cc3ccccc3C2=O)C(C)(C)C)C(C)C)cc1 |r| Show InChI InChI=1S/C32H41N5O6/c1-18(2)24(25(38)29(41)34-16-20-12-14-21(15-13-20)28(40)33-7)36-27(39)19(3)35-30(42)26(32(4,5)6)37-17-22-10-8-9-11-23(22)31(37)43/h8-15,18-19,24,26H,16-17H2,1-7H3,(H,33,40)(H,34,41)(H,35,42)(H,36,39)/t19-,24-,26+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Domainex Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of house dust mite Derp-1 after 20 mins |
J Med Chem 57: 9447-62 (2014)
Article DOI: 10.1021/jm501102h
BindingDB Entry DOI: 10.7270/Q2R212ZW |
More data for this
Ligand-Target Pair | |
Peptidase 1
(Dermatophagoides pteronyssinus (European house dus...) | BDBM50031695
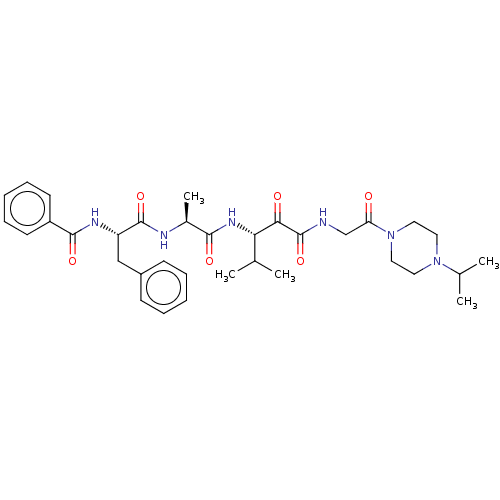
(CHEMBL3360292)Show SMILES CC(C)[C@H](NC(=O)[C@H](C)NC(=O)[C@H](Cc1ccccc1)NC(=O)c1ccccc1)C(=O)C(=O)NCC(=O)N1CCN(CC1)C(C)C |r| Show InChI InChI=1S/C34H46N6O6/c1-22(2)29(30(42)34(46)35-21-28(41)40-18-16-39(17-19-40)23(3)4)38-31(43)24(5)36-33(45)27(20-25-12-8-6-9-13-25)37-32(44)26-14-10-7-11-15-26/h6-15,22-24,27,29H,16-21H2,1-5H3,(H,35,46)(H,36,45)(H,37,44)(H,38,43)/t24-,27-,29-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Domainex Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of house dust mite Derp-1 after 20 mins |
J Med Chem 57: 9447-62 (2014)
Article DOI: 10.1021/jm501102h
BindingDB Entry DOI: 10.7270/Q2R212ZW |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50265317
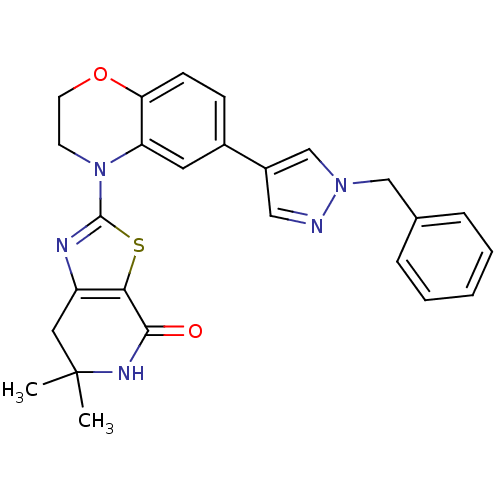
(2-(6-(1-benzyl-1H-pyrazol-4-yl)-2H-benzo[b][1,4]ox...)Show SMILES CC1(C)Cc2nc(sc2C(=O)N1)N1CCOc2ccc(cc12)-c1cnn(Cc2ccccc2)c1 Show InChI InChI=1S/C26H25N5O2S/c1-26(2)13-20-23(24(32)29-26)34-25(28-20)31-10-11-33-22-9-8-18(12-21(22)31)19-14-27-30(16-19)15-17-6-4-3-5-7-17/h3-9,12,14,16H,10-11,13,15H2,1-2H3,(H,29,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
UCB
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) |
Bioorg Med Chem Lett 18: 5299-302 (2008)
Article DOI: 10.1016/j.bmcl.2008.08.042
BindingDB Entry DOI: 10.7270/Q2WS8T3X |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50265318
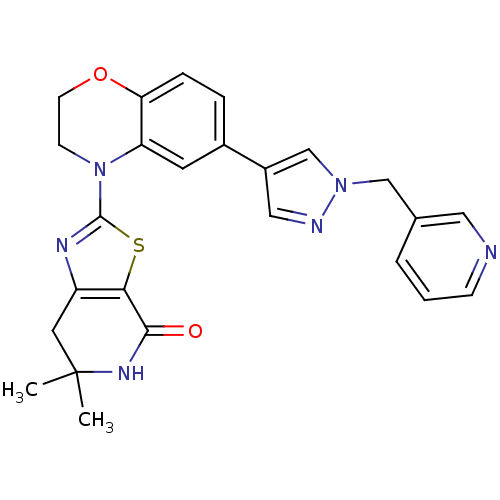
(6,6-dimethyl-2-(6-(1-(pyridin-3-ylmethyl)-1H-pyraz...)Show SMILES CC1(C)Cc2nc(sc2C(=O)N1)N1CCOc2ccc(cc12)-c1cnn(Cc2cccnc2)c1 Show InChI InChI=1S/C25H24N6O2S/c1-25(2)11-19-22(23(32)29-25)34-24(28-19)31-8-9-33-21-6-5-17(10-20(21)31)18-13-27-30(15-18)14-16-4-3-7-26-12-16/h3-7,10,12-13,15H,8-9,11,14H2,1-2H3,(H,29,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
UCB
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) |
Bioorg Med Chem Lett 18: 5299-302 (2008)
Article DOI: 10.1016/j.bmcl.2008.08.042
BindingDB Entry DOI: 10.7270/Q2WS8T3X |
More data for this
Ligand-Target Pair | |
Prolyl hydroxylase EGLN2
(Homo sapiens (Human)) | BDBM385676
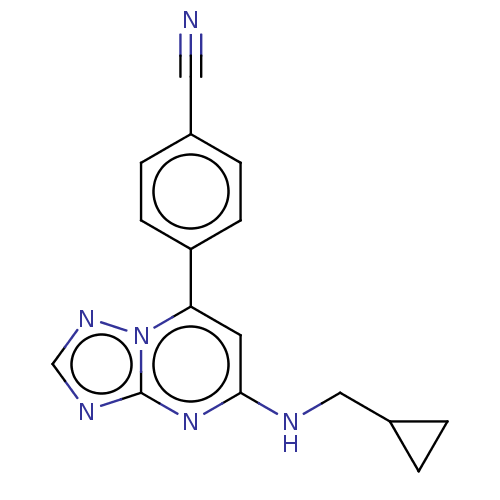
(4-{5- [(cyclopropylmethyl) amino]-[1,2,4] triazolo...)Show InChI InChI=1S/C16H14N6/c17-8-11-3-5-13(6-4-11)14-7-15(18-9-12-1-2-12)21-16-19-10-20-22(14)16/h3-7,10,12H,1-2,9H2,(H,18,19,20,21) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi di Firenze
| Assay Description
The IC50 values for the PHD1 enzyme (residues 1-407) were determined by mixing increasing amounts of a compound of the invention with a fixed amount ... |
Bioorg Med Chem 16: 7424-8 (2008)
BindingDB Entry DOI: 10.7270/Q2QJ7KMD |
More data for this
Ligand-Target Pair | |
Prolyl hydroxylase EGLN2
(Homo sapiens (Human)) | BDBM385701
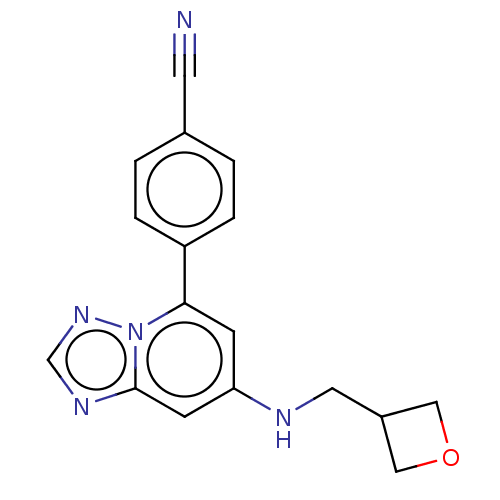
(4-{7-[(oxetan-3- ylmethyl)amino]- [1,2,4]triazolo[...)Show InChI InChI=1S/C17H15N5O/c18-7-12-1-3-14(4-2-12)16-5-15(19-8-13-9-23-10-13)6-17-20-11-21-22(16)17/h1-6,11,13,19H,8-10H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi di Firenze
| Assay Description
The IC50 values for the PHD1 enzyme (residues 1-407) were determined by mixing increasing amounts of a compound of the invention with a fixed amount ... |
Bioorg Med Chem 16: 7424-8 (2008)
BindingDB Entry DOI: 10.7270/Q2QJ7KMD |
More data for this
Ligand-Target Pair | |
Prolyl hydroxylase EGLN2
(Homo sapiens (Human)) | BDBM385711
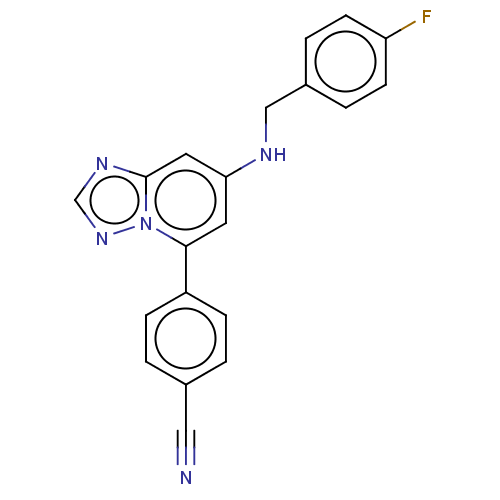
(4-(7-{[(4- fluorophenyl) methyl]amino}- [1,2,4]tri...)Show InChI InChI=1S/C20H14FN5/c21-17-7-3-15(4-8-17)12-23-18-9-19(26-20(10-18)24-13-25-26)16-5-1-14(11-22)2-6-16/h1-10,13,23H,12H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi di Firenze
| Assay Description
The IC50 values for the PHD1 enzyme (residues 1-407) were determined by mixing increasing amounts of a compound of the invention with a fixed amount ... |
Bioorg Med Chem 16: 7424-8 (2008)
BindingDB Entry DOI: 10.7270/Q2QJ7KMD |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50377163

(CHEMBL403358)Show InChI InChI=1S/C18H20N6O/c19-18(25)12-4-5-17-21-10-15(24(17)11-12)14-2-1-3-16(23-14)22-13-6-8-20-9-7-13/h1-5,10-11,13,20H,6-9H2,(H2,19,25)(H,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
UCB
Curated by ChEMBL
| Assay Description
Inhibition of IRAK4 |
Bioorg Med Chem Lett 18: 3656-60 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.042
BindingDB Entry DOI: 10.7270/Q2JH3N2N |
More data for this
Ligand-Target Pair | |
Peptidase 1
(Dermatophagoides pteronyssinus (European house dus...) | BDBM50031690

(CHEMBL3360297)Show SMILES CC(C)[C@H](NC(=O)[C@H](C)NC(=O)[C@@H](NC(=O)c1ccccc1)C12CC3CC(CC(C3)C1)C2)C(=O)C(=O)NCC(=O)N1CCN(C)CC1 |r,TLB:12:22:25:29.27.28,THB:27:26:23:29.28.30,27:28:25.26.31:23,30:28:25:31.22.23,30:22:25:29.27.28| Show InChI InChI=1S/C35H50N6O6/c1-21(2)28(29(43)33(46)36-20-27(42)41-12-10-40(4)11-13-41)38-31(44)22(3)37-34(47)30(39-32(45)26-8-6-5-7-9-26)35-17-23-14-24(18-35)16-25(15-23)19-35/h5-9,21-25,28,30H,10-20H2,1-4H3,(H,36,46)(H,37,47)(H,38,44)(H,39,45)/t22-,23?,24?,25?,28-,30+,35?/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Domainex Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of house dust mite Derp-1 after 20 mins |
J Med Chem 57: 9447-62 (2014)
Article DOI: 10.1021/jm501102h
BindingDB Entry DOI: 10.7270/Q2R212ZW |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50377169

(CHEMBL402629)Show InChI InChI=1S/C18H21N5O/c1-24-14-5-6-18-20-11-16(23(18)12-14)15-3-2-4-17(22-15)21-13-7-9-19-10-8-13/h2-6,11-13,19H,7-10H2,1H3,(H,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
UCB
Curated by ChEMBL
| Assay Description
Inhibition of IRAK4 |
Bioorg Med Chem Lett 18: 3656-60 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.042
BindingDB Entry DOI: 10.7270/Q2JH3N2N |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50264873

(6,6-dimethyl-2-(6-(6-phenylpyridazin-3-ylamino)-2H...)Show SMILES CC1(C)Cc2nc(sc2C(=O)N1)N1CCOc2ccc(Nc3ccc(nn3)-c3ccccc3)cc12 Show InChI InChI=1S/C26H24N6O2S/c1-26(2)15-19-23(24(33)29-26)35-25(28-19)32-12-13-34-21-10-8-17(14-20(21)32)27-22-11-9-18(30-31-22)16-6-4-3-5-7-16/h3-11,14H,12-13,15H2,1-2H3,(H,27,31)(H,29,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
UCB
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) |
Bioorg Med Chem Lett 18: 5299-302 (2008)
Article DOI: 10.1016/j.bmcl.2008.08.042
BindingDB Entry DOI: 10.7270/Q2WS8T3X |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50377181
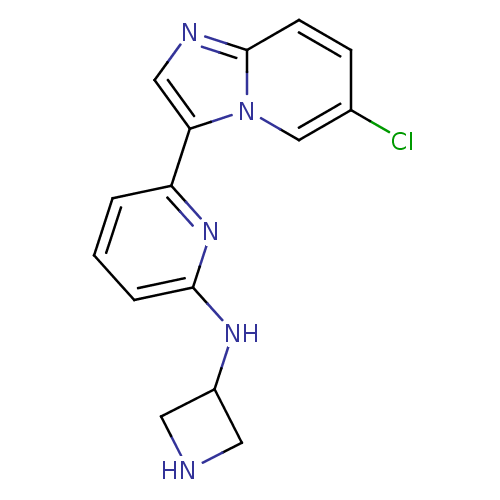
(CHEMBL256963 | US11254667, Compound I-15 | US11542...)Show InChI InChI=1S/C15H14ClN5/c16-10-4-5-15-18-8-13(21(15)9-10)12-2-1-3-14(20-12)19-11-6-17-7-11/h1-5,8-9,11,17H,6-7H2,(H,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
UCB
Curated by ChEMBL
| Assay Description
Inhibition of IRAK4 |
Bioorg Med Chem Lett 18: 3656-60 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.042
BindingDB Entry DOI: 10.7270/Q2JH3N2N |
More data for this
Ligand-Target Pair | |
Peptidase 1
(Dermatophagoides pteronyssinus (European house dus...) | BDBM50031693
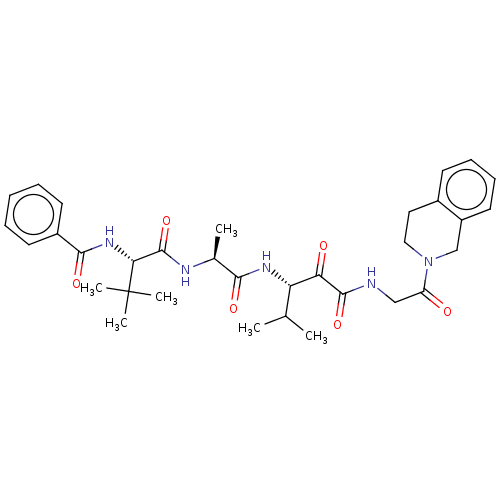
(CHEMBL3360294)Show SMILES CC(C)[C@H](NC(=O)[C@H](C)NC(=O)[C@@H](NC(=O)c1ccccc1)C(C)(C)C)C(=O)C(=O)NCC(=O)N1CCc2ccccc2C1 |r| Show InChI InChI=1S/C33H43N5O6/c1-20(2)26(27(40)31(43)34-18-25(39)38-17-16-22-12-10-11-15-24(22)19-38)36-29(41)21(3)35-32(44)28(33(4,5)6)37-30(42)23-13-8-7-9-14-23/h7-15,20-21,26,28H,16-19H2,1-6H3,(H,34,43)(H,35,44)(H,36,41)(H,37,42)/t21-,26-,28+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Domainex Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of house dust mite Derp-1 after 20 mins |
J Med Chem 57: 9447-62 (2014)
Article DOI: 10.1021/jm501102h
BindingDB Entry DOI: 10.7270/Q2R212ZW |
More data for this
Ligand-Target Pair | |
Peptidase 1
(Dermatophagoides pteronyssinus (European house dus...) | BDBM50031713

(CHEMBL3360282)Show SMILES CC(C)[C@H](NC(=O)[C@H](C)NC(=O)[C@@H](NC(=O)c1ccccc1)C(C)(C)C)C(=O)C(=O)NCc1ccc(cc1)S(N)(=O)=O |r| Show InChI InChI=1S/C29H39N5O7S/c1-17(2)22(23(35)27(38)31-16-19-12-14-21(15-13-19)42(30,40)41)33-25(36)18(3)32-28(39)24(29(4,5)6)34-26(37)20-10-8-7-9-11-20/h7-15,17-18,22,24H,16H2,1-6H3,(H,31,38)(H,32,39)(H,33,36)(H,34,37)(H2,30,40,41)/t18-,22-,24+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Domainex Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of house dust mite Derp-1 after 20 mins |
J Med Chem 57: 9447-62 (2014)
Article DOI: 10.1021/jm501102h
BindingDB Entry DOI: 10.7270/Q2R212ZW |
More data for this
Ligand-Target Pair | |
Peptidase 1
(Dermatophagoides pteronyssinus (European house dus...) | BDBM50031632
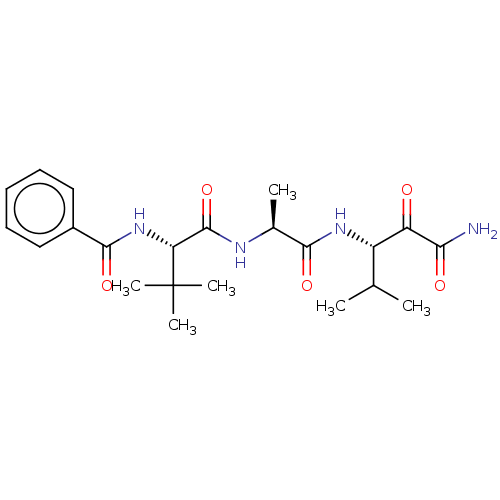
(CHEMBL3359790)Show SMILES CC(C)[C@H](NC(=O)[C@H](C)NC(=O)[C@@H](NC(=O)c1ccccc1)C(C)(C)C)C(=O)C(N)=O |r| Show InChI InChI=1S/C22H32N4O5/c1-12(2)15(16(27)18(23)28)25-19(29)13(3)24-21(31)17(22(4,5)6)26-20(30)14-10-8-7-9-11-14/h7-13,15,17H,1-6H3,(H2,23,28)(H,24,31)(H,25,29)(H,26,30)/t13-,15-,17+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Domainex Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of house dust mite Derp-1 after 20 mins |
J Med Chem 57: 9447-62 (2014)
Article DOI: 10.1021/jm501102h
BindingDB Entry DOI: 10.7270/Q2R212ZW |
More data for this
Ligand-Target Pair | |
Peptidase 1
(Dermatophagoides pteronyssinus (European house dus...) | BDBM50031635

(CHEMBL3359786)Show SMILES [O-]C=O.CC(C)[C@H](NC(=O)[C@H](C)NC(=O)[C@@H](NC(=O)C1CC[N+](C)(C)CC1)C(C)(C)C)C(=O)C(=O)NCc1ccccc1 |r| Show InChI InChI=1S/C30H47N5O5/c1-19(2)23(24(36)28(39)31-18-21-12-10-9-11-13-21)33-26(37)20(3)32-29(40)25(30(4,5)6)34-27(38)22-14-16-35(7,8)17-15-22/h9-13,19-20,22-23,25H,14-18H2,1-8H3,(H3-,31,32,33,34,37,38,39,40)/p+1/t20-,23-,25+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Domainex Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of house dust mite Derp-1 after 20 mins |
J Med Chem 57: 9447-62 (2014)
Article DOI: 10.1021/jm501102h
BindingDB Entry DOI: 10.7270/Q2R212ZW |
More data for this
Ligand-Target Pair | |
Peptidase 1
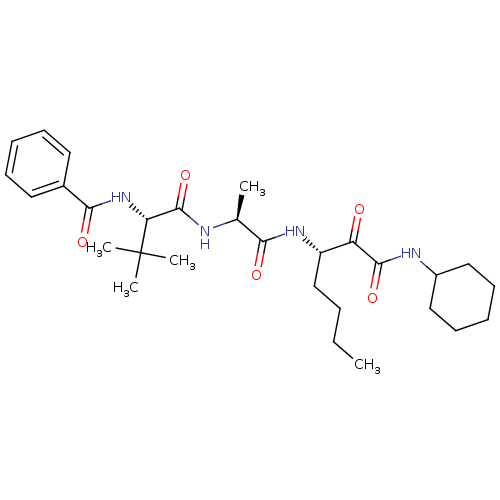
(Dermatophagoides pteronyssinus (European house dus...) | BDBM103023
(US8541363, PVA-039)Show SMILES CCCC[C@H](NC(=O)[C@H](C)NC(=O)[C@@H](NC(=O)c1ccncc1)C(C)(C)C)C(=O)C(=O)NC1CCCCC1 |r| Show InChI InChI=1S/C28H43N5O5/c1-6-7-13-21(22(34)26(37)31-20-11-9-8-10-12-20)32-24(35)18(2)30-27(38)23(28(3,4)5)33-25(36)19-14-16-29-17-15-19/h14-18,20-21,23H,6-13H2,1-5H3,(H,30,38)(H,31,37)(H,32,35)(H,33,36)/t18-,21-,23+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
St George's Hosptial Medical School; The University of Manchester
US Patent
| Assay Description
The fluorogenic substrate used for measuring Der p 1 proteolytic activity was 2-aminobenzoylvalylalanylnorleucylseryl-(3-nitro)tyrosinyl aspartamide.... |
US Patent US8541363 (2013)
BindingDB Entry DOI: 10.7270/Q2PC3105 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50264872

(2-(6-(6-methoxypyridazin-3-ylamino)-2H-benzo[b][1,...)Show SMILES COc1ccc(Nc2ccc3OCCN(c4nc5CC(C)(C)NC(=O)c5s4)c3c2)nn1 Show InChI InChI=1S/C21H22N6O3S/c1-21(2)11-13-18(19(28)24-21)31-20(23-13)27-8-9-30-15-5-4-12(10-14(15)27)22-16-6-7-17(29-3)26-25-16/h4-7,10H,8-9,11H2,1-3H3,(H,22,25)(H,24,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
UCB
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) |
Bioorg Med Chem Lett 18: 5299-302 (2008)
Article DOI: 10.1016/j.bmcl.2008.08.042
BindingDB Entry DOI: 10.7270/Q2WS8T3X |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50377186

(CHEMBL403433)Show InChI InChI=1S/C16H17N5O/c1-22-12-5-6-16-18-9-14(21(16)10-12)13-3-2-4-15(20-13)19-11-7-17-8-11/h2-6,9-11,17H,7-8H2,1H3,(H,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
UCB
Curated by ChEMBL
| Assay Description
Inhibition of IRAK4 |
Bioorg Med Chem Lett 18: 3656-60 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.042
BindingDB Entry DOI: 10.7270/Q2JH3N2N |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50377166
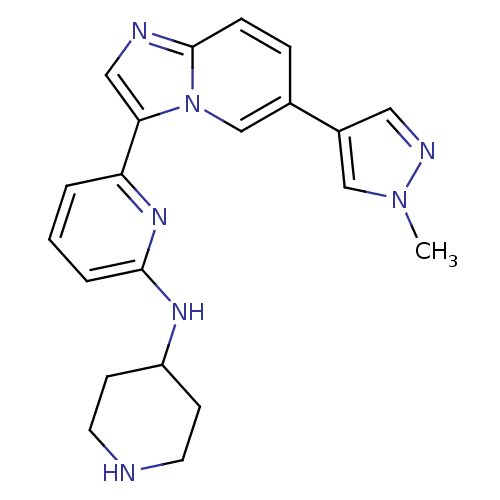
(CHEMBL255446)Show SMILES Cn1cc(cn1)-c1ccc2ncc(-c3cccc(NC4CCNCC4)n3)n2c1 Show InChI InChI=1S/C21H23N7/c1-27-13-16(11-24-27)15-5-6-21-23-12-19(28(21)14-15)18-3-2-4-20(26-18)25-17-7-9-22-10-8-17/h2-6,11-14,17,22H,7-10H2,1H3,(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
UCB
Curated by ChEMBL
| Assay Description
Inhibition of IRAK4 |
Bioorg Med Chem Lett 18: 3656-60 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.042
BindingDB Entry DOI: 10.7270/Q2JH3N2N |
More data for this
Ligand-Target Pair | |
Peptidase 1
(Dermatophagoides pteronyssinus (European house dus...) | BDBM50031668

(CHEMBL3359782)Show SMILES CC(C)[C@H](NC(=O)[C@H](C)NC(=O)[C@@H](N1Cc2ccccc2C1=O)C(C)(C)C)C(=O)C(=O)NCc1ccccc1 |r| Show InChI InChI=1S/C30H38N4O5/c1-18(2)23(24(35)27(37)31-16-20-12-8-7-9-13-20)33-26(36)19(3)32-28(38)25(30(4,5)6)34-17-21-14-10-11-15-22(21)29(34)39/h7-15,18-19,23,25H,16-17H2,1-6H3,(H,31,37)(H,32,38)(H,33,36)/t19-,23-,25+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Domainex Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of house dust mite Derp-1 after 20 mins |
J Med Chem 57: 9447-62 (2014)
Article DOI: 10.1021/jm501102h
BindingDB Entry DOI: 10.7270/Q2R212ZW |
More data for this
Ligand-Target Pair | |
Peptidase 1
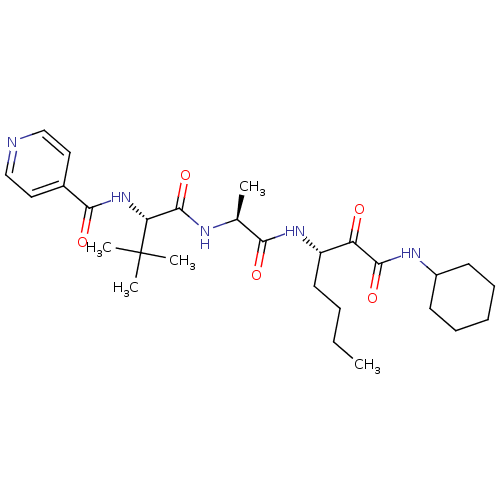
(Dermatophagoides pteronyssinus (European house dus...) | BDBM103022
(US8541363, PVA-037)Show SMILES CCCC[C@H](NC(=O)[C@H](C)NC(=O)[C@@H](NC(=O)c1ccccc1)C(C)(C)C)C(=O)C(=O)NC1CCCCC1 |r| Show InChI InChI=1S/C29H44N4O5/c1-6-7-18-22(23(34)27(37)31-21-16-12-9-13-17-21)32-25(35)19(2)30-28(38)24(29(3,4)5)33-26(36)20-14-10-8-11-15-20/h8,10-11,14-15,19,21-22,24H,6-7,9,12-13,16-18H2,1-5H3,(H,30,38)(H,31,37)(H,32,35)(H,33,36)/t19-,22-,24+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 7.85 | n/a | n/a | n/a | n/a | n/a | n/a |
St George's Hosptial Medical School; The University of Manchester
US Patent
| Assay Description
The fluorogenic substrate used for measuring Der p 1 proteolytic activity was 2-aminobenzoylvalylalanylnorleucylseryl-(3-nitro)tyrosinyl aspartamide.... |
US Patent US8541363 (2013)
BindingDB Entry DOI: 10.7270/Q2PC3105 |
More data for this
Ligand-Target Pair | |
Prolyl hydroxylase EGLN2
(Homo sapiens (Human)) | BDBM50239931
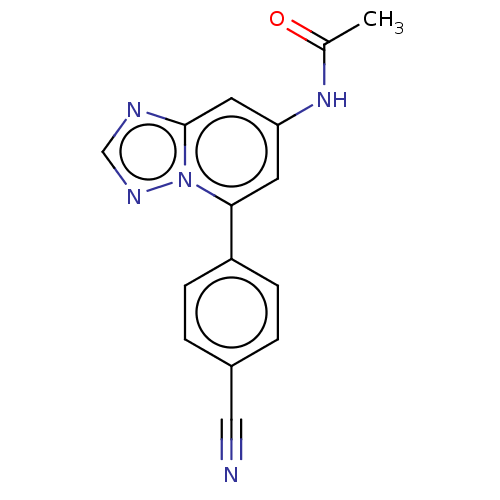
(CHEMBL4067995 | US10287286, Example 142)Show InChI InChI=1S/C15H11N5O/c1-10(21)19-13-6-14(20-15(7-13)17-9-18-20)12-4-2-11(8-16)3-5-12/h2-7,9H,1H3,(H,19,21) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi di Firenze
| Assay Description
The IC50 values for the PHD1 enzyme (residues 1-407) were determined by mixing increasing amounts of a compound of the invention with a fixed amount ... |
Bioorg Med Chem 16: 7424-8 (2008)
BindingDB Entry DOI: 10.7270/Q2QJ7KMD |
More data for this
Ligand-Target Pair | |
Prolyl hydroxylase EGLN2
(Homo sapiens (Human)) | BDBM50239931

(CHEMBL4067995 | US10287286, Example 142)Show InChI InChI=1S/C15H11N5O/c1-10(21)19-13-6-14(20-15(7-13)17-9-18-20)12-4-2-11(8-16)3-5-12/h2-7,9H,1H3,(H,19,21) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda California Inc.
Curated by ChEMBL
| Assay Description
Inhibition of full length HIF-PHD1 (unknown origin) expressed in baculovirus infected sf9 cells using DLDLEMLAPYIPMDDDFQL/2-Oxoglutarate as substrate... |
J Med Chem 60: 5663-5672 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00352
BindingDB Entry DOI: 10.7270/Q2GQ70XZ |
More data for this
Ligand-Target Pair | |
Prolyl hydroxylase EGLN2
(Homo sapiens (Human)) | BDBM385707
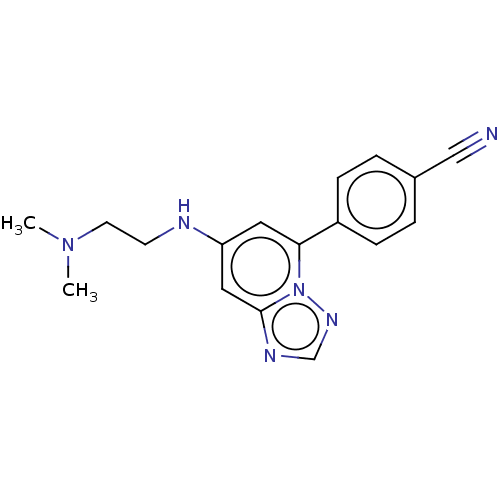
(4-(7-{[2- (dimethylamino) ethyl]amino}- [1,2,4]tri...)Show InChI InChI=1S/C17H18N6/c1-22(2)8-7-19-15-9-16(23-17(10-15)20-12-21-23)14-5-3-13(11-18)4-6-14/h3-6,9-10,12,19H,7-8H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi di Firenze
| Assay Description
The IC50 values for the PHD1 enzyme (residues 1-407) were determined by mixing increasing amounts of a compound of the invention with a fixed amount ... |
Bioorg Med Chem 16: 7424-8 (2008)
BindingDB Entry DOI: 10.7270/Q2QJ7KMD |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50377167
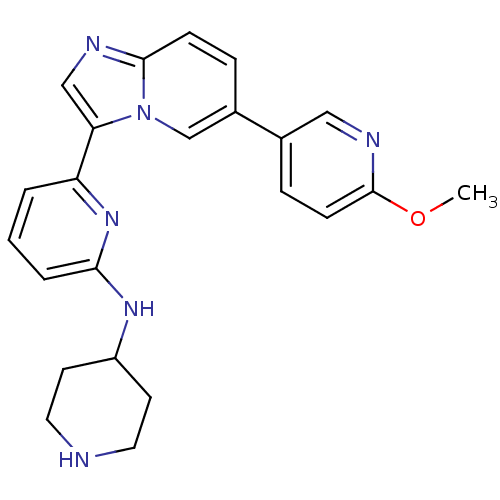
(CHEMBL402361)Show SMILES COc1ccc(cn1)-c1ccc2ncc(-c3cccc(NC4CCNCC4)n3)n2c1 Show InChI InChI=1S/C23H24N6O/c1-30-23-8-6-16(13-26-23)17-5-7-22-25-14-20(29(22)15-17)19-3-2-4-21(28-19)27-18-9-11-24-12-10-18/h2-8,13-15,18,24H,9-12H2,1H3,(H,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
UCB
Curated by ChEMBL
| Assay Description
Inhibition of IRAK4 |
Bioorg Med Chem Lett 18: 3656-60 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.042
BindingDB Entry DOI: 10.7270/Q2JH3N2N |
More data for this
Ligand-Target Pair | |
Peptidase 1
(Dermatophagoides pteronyssinus (European house dus...) | BDBM50031711
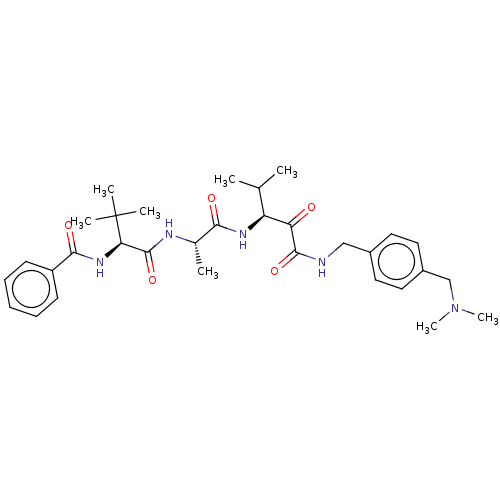
(CHEMBL3360284)Show SMILES CC(C)[C@H](NC(=O)[C@H](C)NC(=O)[C@@H](NC(=O)c1ccccc1)C(C)(C)C)C(=O)C(=O)NCc1ccc(CN(C)C)cc1 |r| Show InChI InChI=1S/C32H45N5O5/c1-20(2)25(26(38)30(41)33-18-22-14-16-23(17-15-22)19-37(7)8)35-28(39)21(3)34-31(42)27(32(4,5)6)36-29(40)24-12-10-9-11-13-24/h9-17,20-21,25,27H,18-19H2,1-8H3,(H,33,41)(H,34,42)(H,35,39)(H,36,40)/t21-,25-,27+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Domainex Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of house dust mite Derp-1 after 20 mins |
J Med Chem 57: 9447-62 (2014)
Article DOI: 10.1021/jm501102h
BindingDB Entry DOI: 10.7270/Q2R212ZW |
More data for this
Ligand-Target Pair | |
Peptidase 1
(Dermatophagoides pteronyssinus (European house dus...) | BDBM50031719
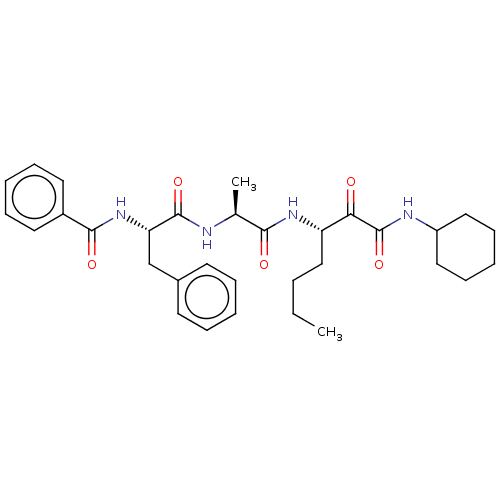
(CHEMBL3359772)Show SMILES CCCC[C@H](NC(=O)[C@H](C)NC(=O)[C@H](Cc1ccccc1)NC(=O)c1ccccc1)C(=O)C(=O)NC1CCCCC1 |r| Show InChI InChI=1S/C32H42N4O5/c1-3-4-20-26(28(37)32(41)34-25-18-12-7-13-19-25)35-29(38)22(2)33-31(40)27(21-23-14-8-5-9-15-23)36-30(39)24-16-10-6-11-17-24/h5-6,8-11,14-17,22,25-27H,3-4,7,12-13,18-21H2,1-2H3,(H,33,40)(H,34,41)(H,35,38)(H,36,39)/t22-,26-,27-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Domainex Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of house dust mite Derp-1 after 20 mins |
J Med Chem 57: 9447-62 (2014)
Article DOI: 10.1021/jm501102h
BindingDB Entry DOI: 10.7270/Q2R212ZW |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50265319
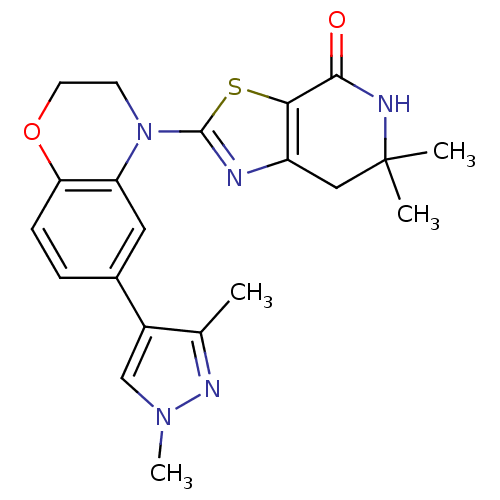
(2-(6-(1,3-dimethyl-1H-pyrazol-4-yl)-2H-benzo[b][1,...)Show SMILES Cc1nn(C)cc1-c1ccc2OCCN(c3nc4CC(C)(C)NC(=O)c4s3)c2c1 Show InChI InChI=1S/C21H23N5O2S/c1-12-14(11-25(4)24-12)13-5-6-17-16(9-13)26(7-8-28-17)20-22-15-10-21(2,3)23-19(27)18(15)29-20/h5-6,9,11H,7-8,10H2,1-4H3,(H,23,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
UCB
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) |
Bioorg Med Chem Lett 18: 5299-302 (2008)
Article DOI: 10.1016/j.bmcl.2008.08.042
BindingDB Entry DOI: 10.7270/Q2WS8T3X |
More data for this
Ligand-Target Pair | |
Prolyl hydroxylase EGLN2
(Homo sapiens (Human)) | BDBM385693
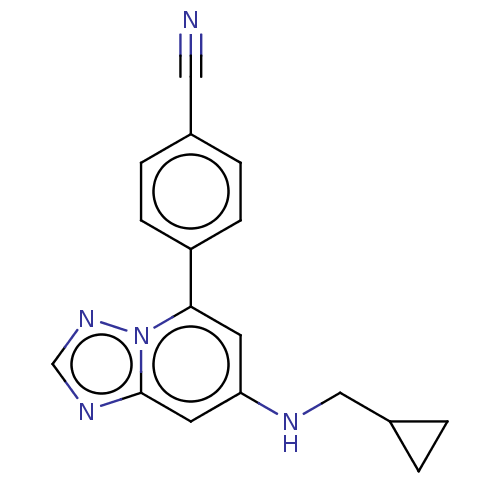
(4-{7- [(cyclopropyl- methyl) amino]- [1,2,4]triazo...)Show InChI InChI=1S/C17H15N5/c18-9-12-3-5-14(6-4-12)16-7-15(19-10-13-1-2-13)8-17-20-11-21-22(16)17/h3-8,11,13,19H,1-2,10H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi di Firenze
| Assay Description
The IC50 values for the PHD1 enzyme (residues 1-407) were determined by mixing increasing amounts of a compound of the invention with a fixed amount ... |
Bioorg Med Chem 16: 7424-8 (2008)
BindingDB Entry DOI: 10.7270/Q2QJ7KMD |
More data for this
Ligand-Target Pair | |
Prolyl hydroxylase EGLN2
(Homo sapiens (Human)) | BDBM385702
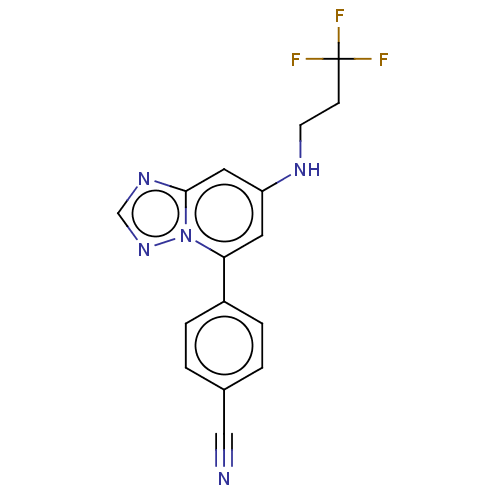
(4-{7-[(3,3,3- trifluoropropyl) amino]- [1,2,4]tria...)Show InChI InChI=1S/C16H12F3N5/c17-16(18,19)5-6-21-13-7-14(24-15(8-13)22-10-23-24)12-3-1-11(9-20)2-4-12/h1-4,7-8,10,21H,5-6H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi di Firenze
| Assay Description
The IC50 values for the PHD1 enzyme (residues 1-407) were determined by mixing increasing amounts of a compound of the invention with a fixed amount ... |
Bioorg Med Chem 16: 7424-8 (2008)
BindingDB Entry DOI: 10.7270/Q2QJ7KMD |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50264748
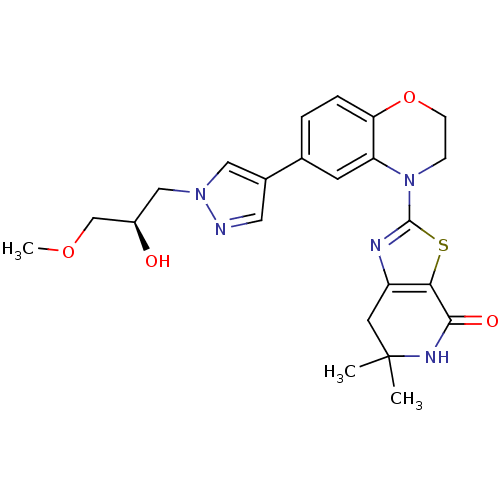
((R)-2-(6-(1-(2-hydroxy-3-methoxypropyl)-1H-pyrazol...)Show SMILES COC[C@H](O)Cn1cc(cn1)-c1ccc2OCCN(c3nc4CC(C)(C)NC(=O)c4s3)c2c1 |r| Show InChI InChI=1S/C23H27N5O4S/c1-23(2)9-17-20(21(30)26-23)33-22(25-17)28-6-7-32-19-5-4-14(8-18(19)28)15-10-24-27(11-15)12-16(29)13-31-3/h4-5,8,10-11,16,29H,6-7,9,12-13H2,1-3H3,(H,26,30)/t16-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
UCB
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) |
Bioorg Med Chem Lett 18: 5299-302 (2008)
Article DOI: 10.1016/j.bmcl.2008.08.042
BindingDB Entry DOI: 10.7270/Q2WS8T3X |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50377179
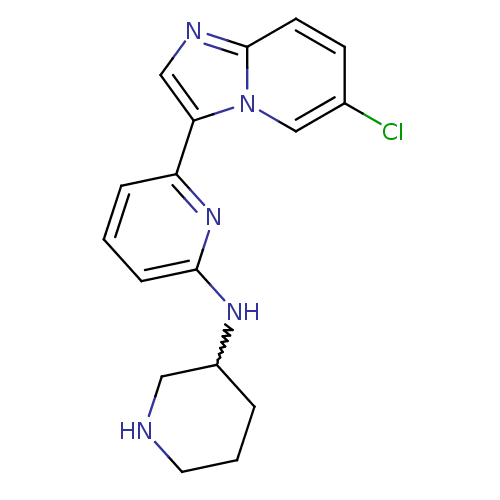
(CHEMBL256961)Show SMILES Clc1ccc2ncc(-c3cccc(NC4CCCNC4)n3)n2c1 |w:14.13| Show InChI InChI=1S/C17H18ClN5/c18-12-6-7-17-20-10-15(23(17)11-12)14-4-1-5-16(22-14)21-13-3-2-8-19-9-13/h1,4-7,10-11,13,19H,2-3,8-9H2,(H,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
UCB
Curated by ChEMBL
| Assay Description
Inhibition of IRAK4 |
Bioorg Med Chem Lett 18: 3656-60 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.042
BindingDB Entry DOI: 10.7270/Q2JH3N2N |
More data for this
Ligand-Target Pair | |
Peptidase 1
(Dermatophagoides pteronyssinus (European house dus...) | BDBM50031697

(CHEMBL3360290)Show SMILES CC(C)[C@H](NC(=O)[C@H](C)NC(=O)[C@H](Cc1ccccc1)NC(=O)c1ccccc1)C(=O)C(=O)NCC(=O)N1CCOCC1 |r| Show InChI InChI=1S/C31H39N5O7/c1-20(2)26(27(38)31(42)32-19-25(37)36-14-16-43-17-15-36)35-28(39)21(3)33-30(41)24(18-22-10-6-4-7-11-22)34-29(40)23-12-8-5-9-13-23/h4-13,20-21,24,26H,14-19H2,1-3H3,(H,32,42)(H,33,41)(H,34,40)(H,35,39)/t21-,24-,26-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Domainex Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of house dust mite Derp-1 after 20 mins |
J Med Chem 57: 9447-62 (2014)
Article DOI: 10.1021/jm501102h
BindingDB Entry DOI: 10.7270/Q2R212ZW |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data