

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 7-dehydrocholesterol reductase (Rattus norvegicus) | BDBM50406621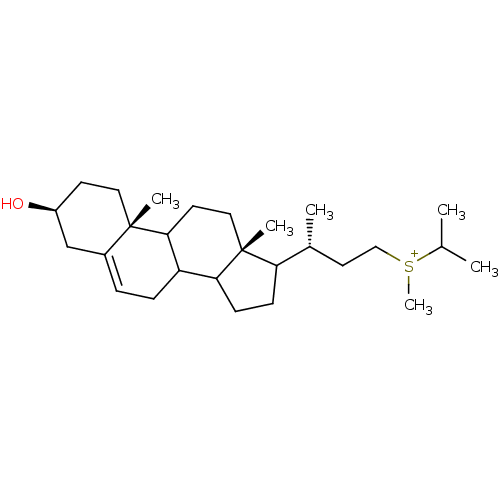 (CHEMBL9820) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Compound was tested for the inhibition of Delta-(24)-sterol reductase | J Med Chem 35: 100-6 (1992) BindingDB Entry DOI: 10.7270/Q2C82BHD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dopamine beta-hydroxylase (Bos taurus) | BDBM50014978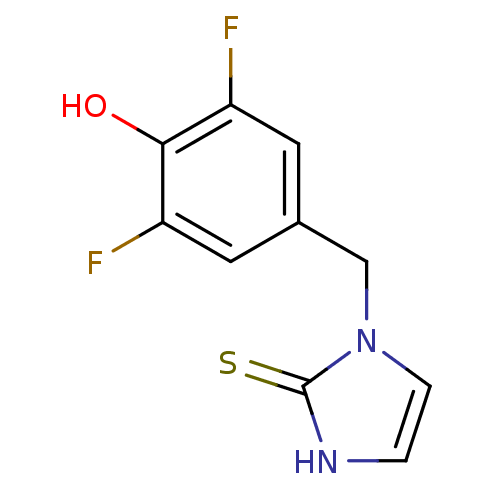 (1-(3,5-Difluoro-4-hydroxy-benzyl)-1,3-dihydro-imid...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a | 4.5 | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity towards Dopamine beta hydroxylase using tyramine substrate at pH 4.5 in the absence of fumarate | J Med Chem 29: 887-9 (1986) BindingDB Entry DOI: 10.7270/Q2JH3K55 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 7-dehydrocholesterol reductase (Rattus norvegicus) | BDBM50406617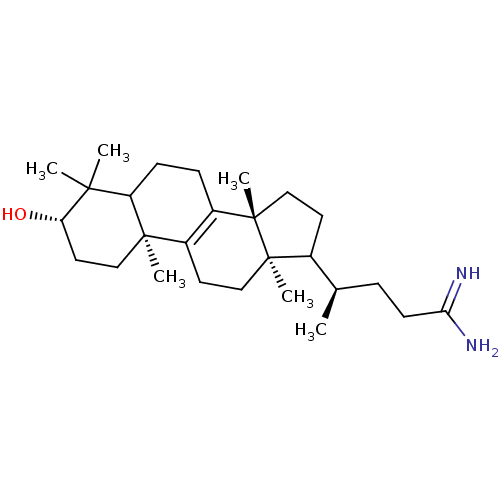 (CHEMBL276388) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Compound was tested for the inhibition of Delta-(24)-sterol reductase | J Med Chem 35: 100-6 (1992) BindingDB Entry DOI: 10.7270/Q2C82BHD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 7-dehydrocholesterol reductase (Rattus norvegicus) | BDBM50406618 (CHEMBL9875) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Compound was tested for the inhibition of delta24-sterol reductase | J Med Chem 35: 100-6 (1992) BindingDB Entry DOI: 10.7270/Q2C82BHD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 7-dehydrocholesterol reductase (Rattus norvegicus) | BDBM50406620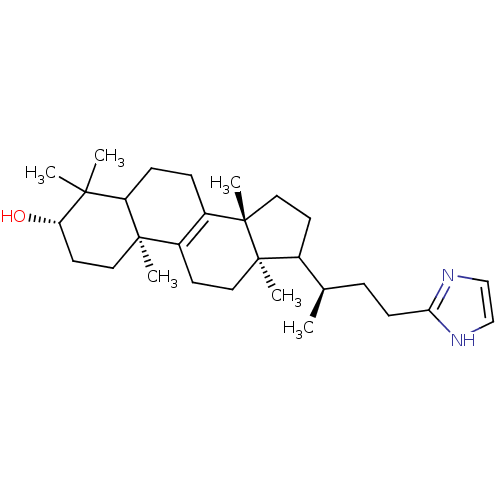 (CHEMBL9864) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Compound was tested for the inhibition of delta24-sterol reductase | J Med Chem 35: 100-6 (1992) BindingDB Entry DOI: 10.7270/Q2C82BHD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dopamine beta-hydroxylase (Bos taurus) | BDBM50014983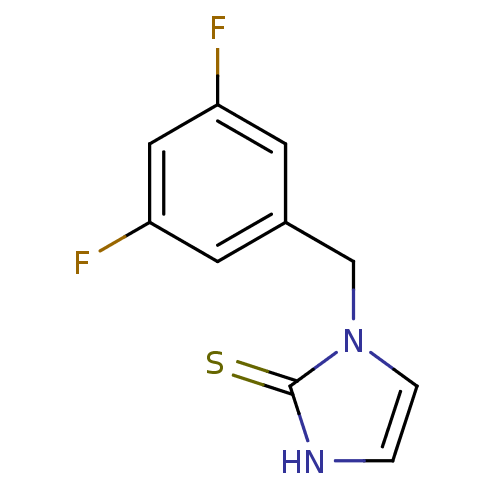 (1-(3,5-Difluoro-benzyl)-1,3-dihydro-imidazole-2-th...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 41 | n/a | n/a | n/a | n/a | n/a | n/a | 4.5 | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity towards Dopamine beta hydroxylase using tyramine substrate at pH 4.5 in the absence of fumarate | J Med Chem 29: 887-9 (1986) BindingDB Entry DOI: 10.7270/Q2JH3K55 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 7-dehydrocholesterol reductase (Rattus norvegicus) | BDBM50406616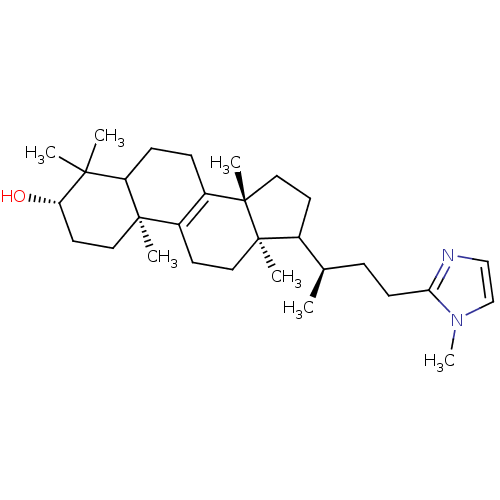 (CHEMBL268041) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 48 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Compound was tested for the inhibition of delta24-sterol reductase | J Med Chem 35: 100-6 (1992) BindingDB Entry DOI: 10.7270/Q2C82BHD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dopamine beta-hydroxylase (Bos taurus) | BDBM50014968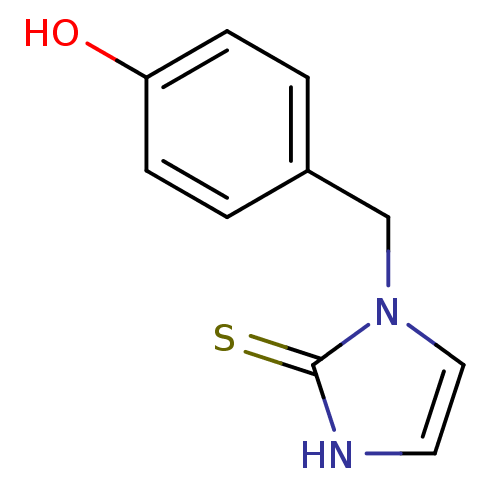 (1-(4-Hydroxy-benzyl)-1,3-dihydro-imidazole-2-thion...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 55 | n/a | n/a | n/a | n/a | n/a | n/a | 4.5 | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition of Dopamine beta hydroxylase at pH 4.5 | J Med Chem 29: 2465-72 (1987) BindingDB Entry DOI: 10.7270/Q2BR8R5P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dopamine beta-hydroxylase (Bos taurus) | BDBM50014968 (1-(4-Hydroxy-benzyl)-1,3-dihydro-imidazole-2-thion...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 55 | n/a | n/a | n/a | n/a | n/a | n/a | 4.5 | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity towards Dopamine beta hydroxylase using tyramine substrate at pH 4.5 in the absence of fumarate | J Med Chem 29: 887-9 (1986) BindingDB Entry DOI: 10.7270/Q2JH3K55 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dopamine beta-hydroxylase (Bos taurus) | BDBM50000439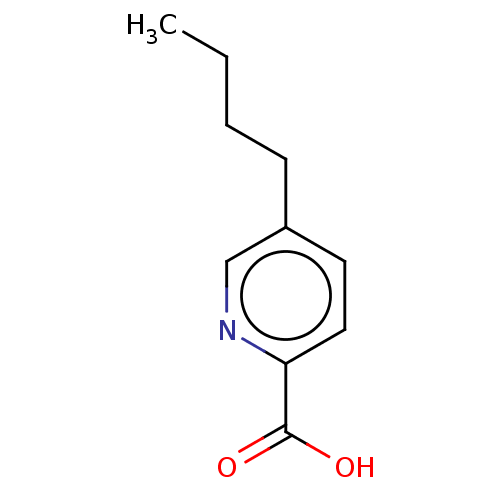 (5-Butyl-pyridine-2-carboxylic acid | 5-Butyl-pyrid...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents | PubMed | 149 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition of Dopamine beta hydroxylase at pH 4.5 | J Med Chem 29: 2465-72 (1987) BindingDB Entry DOI: 10.7270/Q2BR8R5P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dopamine beta-hydroxylase (Bos taurus) | BDBM50000439 (5-Butyl-pyridine-2-carboxylic acid | 5-Butyl-pyrid...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents | PubMed | 149 | n/a | n/a | n/a | n/a | n/a | n/a | 4.5 | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity towards Dopamine beta hydroxylase using tyramine substrate at pH 4.5 in the absence of fumarate | J Med Chem 29: 887-9 (1986) BindingDB Entry DOI: 10.7270/Q2JH3K55 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ATP-citrate synthase (Rattus norvegicus) | BDBM50036210 ((2S,3S)-3-Carboxy-2,3-dihydroxy-pentanedioic acid ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Similars | PDB PubMed | 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Ltd. Curated by ChEMBL | Assay Description Reversible binding potential for rat ATP-Citrate Lyase as carbon-carbon bond cleavage activity in citrate substrate | J Med Chem 38: 537-43 (1995) BindingDB Entry DOI: 10.7270/Q21Z43GM | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dopamine beta-hydroxylase (Bos taurus) | BDBM50014968 (1-(4-Hydroxy-benzyl)-1,3-dihydro-imidazole-2-thion...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 344 | n/a | n/a | n/a | n/a | n/a | n/a | 6.6 | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition of Dopamine beta hydroxylase at pH 6.6 | J Med Chem 29: 2465-72 (1987) BindingDB Entry DOI: 10.7270/Q2BR8R5P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 7-dehydrocholesterol reductase (Rattus norvegicus) | BDBM50406619 (CHEMBL9808) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Compound was tested for the inhibition of Delta-(24)-sterol reductase | J Med Chem 35: 100-6 (1992) BindingDB Entry DOI: 10.7270/Q2C82BHD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ATP-citrate synthase (Rattus norvegicus) | BDBM50036215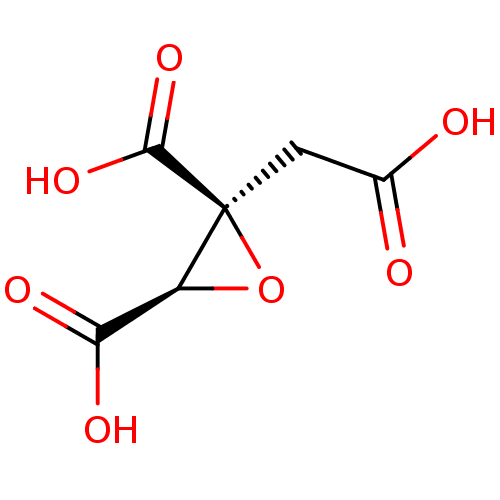 ((2S,3R)-2-Carboxymethyl-oxirane-2,3-dicarboxylic a...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Similars | PubMed | 1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Ltd. Curated by ChEMBL | Assay Description Reversible binding potential for rat ATP-Citrate Lyase as carbon-carbon bond cleavage activity in citrate substrate | J Med Chem 38: 537-43 (1995) BindingDB Entry DOI: 10.7270/Q21Z43GM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ATP-citrate synthase (Rattus norvegicus) | BDBM50036214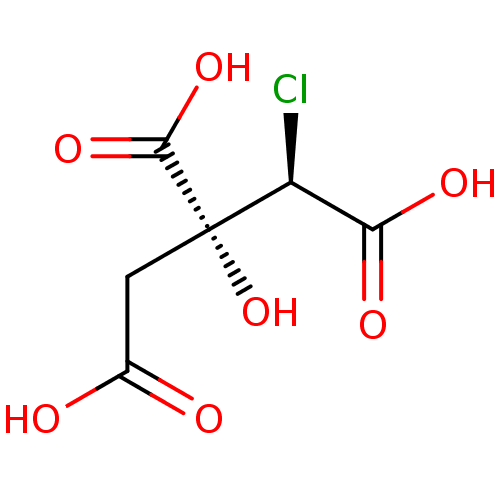 ((2R,3R)-3-Carboxy-2-chloro-3-hydroxy-pentanedioic ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem | PubMed | 2.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Ltd. Curated by ChEMBL | Assay Description Reversible binding potential for rat ATP-Citrate Lyase as carbon-carbon bond cleavage activity in citrate substrate | J Med Chem 38: 537-43 (1995) BindingDB Entry DOI: 10.7270/Q21Z43GM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ATP-citrate synthase (Rattus norvegicus) | BDBM50036217 ((2S,3R)-3-Carboxy-3-hydroxy-2-methylsulfanyl-penta...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 3.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Ltd. Curated by ChEMBL | Assay Description Reversible binding potential for rat ATP-Citrate Lyase as carbon-carbon bond cleavage activity in citrate substrate | J Med Chem 38: 537-43 (1995) BindingDB Entry DOI: 10.7270/Q21Z43GM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dopamine beta-hydroxylase (Bos taurus) | BDBM50023370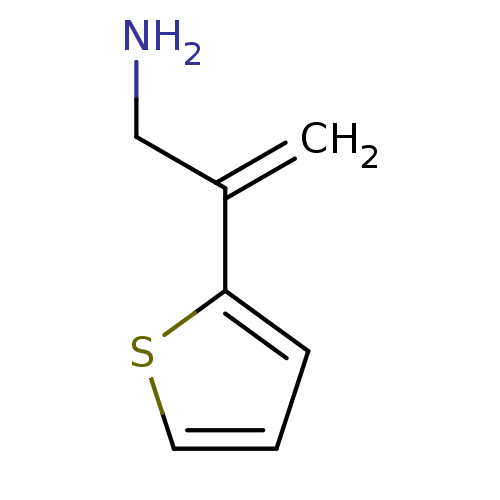 (2-Thiophen-2-yl-allylamine | CHEMBL110604) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 3.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline & French Laboratories Curated by ChEMBL | Assay Description KI value was determined from plots of 1/kinact(observed) vs 1/[inhibitor] | J Med Chem 31: 704-6 (1988) BindingDB Entry DOI: 10.7270/Q26972KP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dopamine beta-hydroxylase (Bos taurus) | BDBM50241361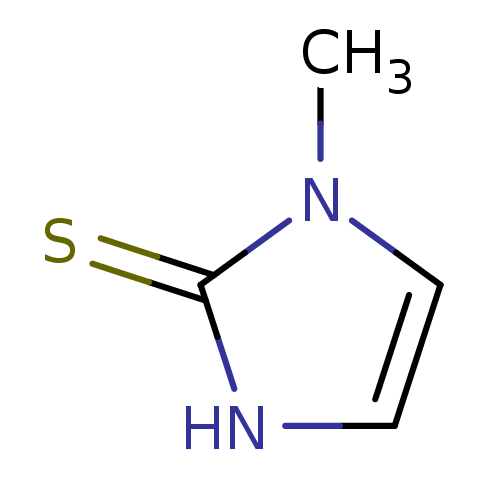 (CHEMBL1515 | METHIMAZOLE | US9138393, Methimazole ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents | PubMed | 4.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 4.5 | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition of Dopamine beta hydroxylase at pH 6.6 | J Med Chem 29: 2465-72 (1987) BindingDB Entry DOI: 10.7270/Q2BR8R5P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dopamine beta-hydroxylase (Bos taurus) | BDBM50017834 (4-(1-Aminomethyl-prop-2-ynyl)-phenol | CHEMBL16312...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | 5.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline & French Laboratories Curated by ChEMBL | Assay Description KI value was determined from plots of 1/kinact(observed) vs 1/[inhibitor] | J Med Chem 31: 704-6 (1988) BindingDB Entry DOI: 10.7270/Q26972KP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dopamine beta-hydroxylase (Bos taurus) | BDBM50017834 (4-(1-Aminomethyl-prop-2-ynyl)-phenol | CHEMBL16312...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | 5.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline & French Laboratories Curated by ChEMBL | Assay Description KI value was determined from plots of 1/kinact(observed) vs 1/[inhibitor] | J Med Chem 31: 704-6 (1988) BindingDB Entry DOI: 10.7270/Q26972KP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ATP-citrate synthase (Rattus norvegicus) | BDBM50036219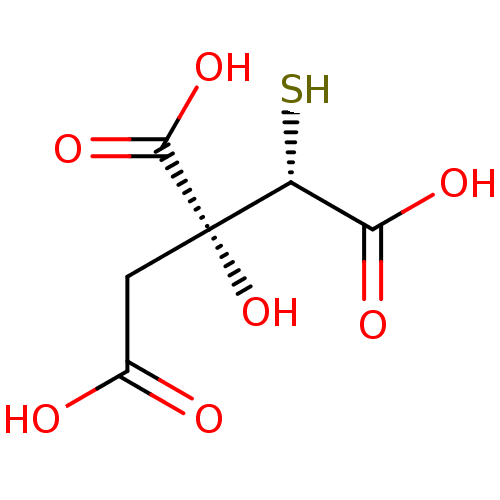 ((2S,3R)-3-Carboxy-3-hydroxy-2-mercapto-pentanedioi...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 5.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Ltd. Curated by ChEMBL | Assay Description Reversible binding potential for rat ATP-Citrate Lyase as carbon-carbon bond cleavage activity in citrate substrate | J Med Chem 38: 537-43 (1995) BindingDB Entry DOI: 10.7270/Q21Z43GM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ATP-citrate synthase (Rattus norvegicus) | BDBM50036221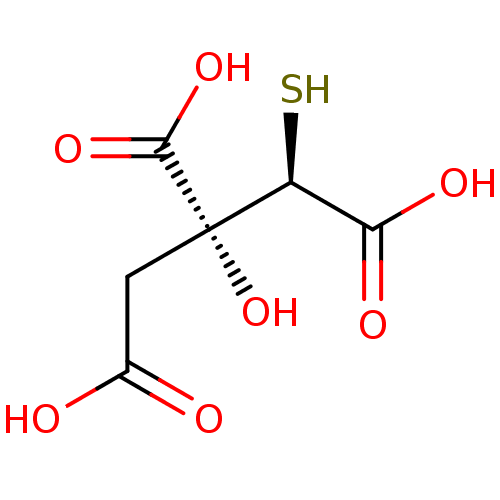 ((2R,3R)-3-Carboxy-3-hydroxy-2-mercapto-pentanedioi...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 1.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Ltd. Curated by ChEMBL | Assay Description Reversible binding potential for rat ATP-Citrate Lyase as carbon-carbon bond cleavage activity in citrate substrate | J Med Chem 38: 537-43 (1995) BindingDB Entry DOI: 10.7270/Q21Z43GM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ATP-citrate synthase (Rattus norvegicus) | BDBM50036218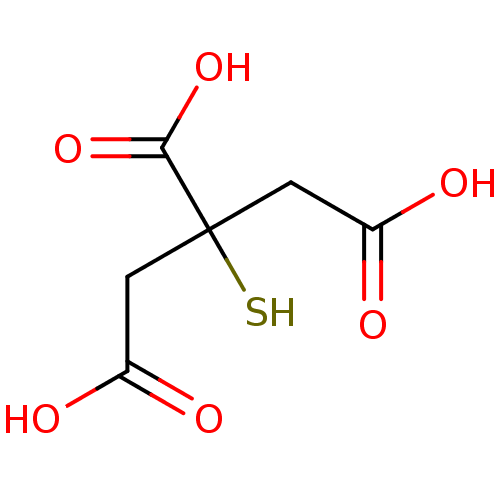 (3-Carboxy-3-mercapto-pentanedioic acid | CHEMBL151...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | 2.95E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Ltd. Curated by ChEMBL | Assay Description Reversible binding potential for rat ATP-Citrate Lyase as carbon-carbon bond cleavage activity in citrate substrate | J Med Chem 38: 537-43 (1995) BindingDB Entry DOI: 10.7270/Q21Z43GM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ATP-citrate synthase (Rattus norvegicus) | BDBM50036213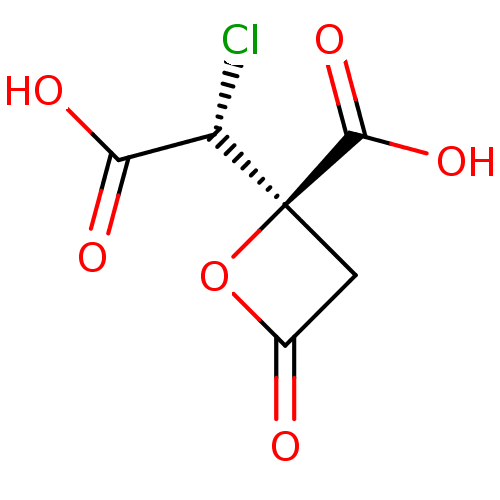 ((R)-2-((R)-Carboxy-chloro-methyl)-4-oxo-oxetane-2-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 3.40E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Ltd. Curated by ChEMBL | Assay Description Reversible binding potential for rat ATP-Citrate Lyase as carbon-carbon bond cleavage activity in citrate substrate | J Med Chem 38: 537-43 (1995) BindingDB Entry DOI: 10.7270/Q21Z43GM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ATP-citrate synthase (Rattus norvegicus) | BDBM50036220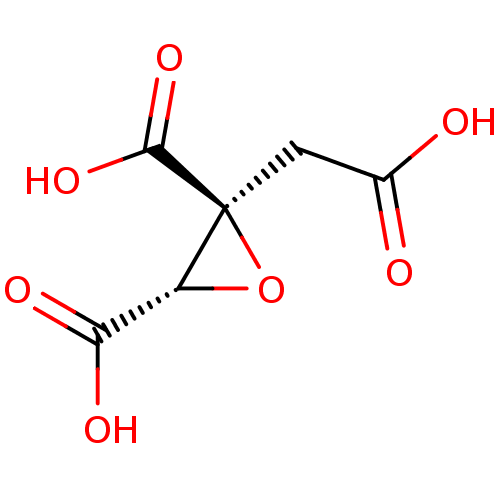 ((2S,3S)-2-Carboxymethyl-oxirane-2,3-dicarboxylic a...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Ltd. Curated by ChEMBL | Assay Description Reversible binding potential for rat ATP-Citrate Lyase as carbon-carbon bond cleavage activity in citrate substrate | J Med Chem 38: 537-43 (1995) BindingDB Entry DOI: 10.7270/Q21Z43GM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ATP-citrate synthase (Rattus norvegicus) | BDBM50036211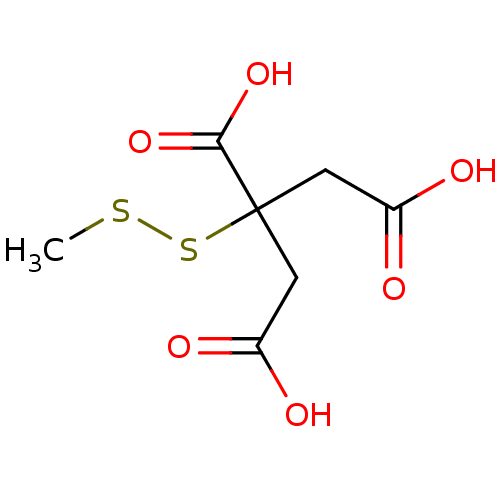 (3-Carboxy-3-methyldisulfanyl-pentanedioic acid | C...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem | PubMed | 4.80E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Ltd. Curated by ChEMBL | Assay Description Reversible binding potential for rat ATP-Citrate Lyase as carbon-carbon bond cleavage activity in citrate substrate | J Med Chem 38: 537-43 (1995) BindingDB Entry DOI: 10.7270/Q21Z43GM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dopamine beta-hydroxylase (Bos taurus) | BDBM50017827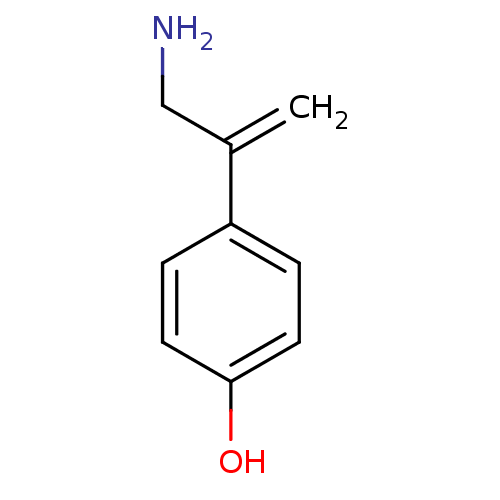 (4-(1-Aminomethyl-vinyl)-phenol | CHEMBL330118) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.20E+5 | n/a | n/a | n/a | n/a | n/a | n/a | 5.0 | n/a |
Smith Kline & French Laboratories Curated by ChEMBL | Assay Description KI value was determined from plots of 1/kinact(observed) vs 1/[inhibitor]; Apparent values at pH 5.0, 0.24 mM O2 | J Med Chem 31: 704-6 (1988) BindingDB Entry DOI: 10.7270/Q26972KP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dopamine beta-hydroxylase (Bos taurus) | BDBM50241361 (CHEMBL1515 | METHIMAZOLE | US9138393, Methimazole ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents | PubMed | 7.16E+5 | n/a | n/a | n/a | n/a | n/a | n/a | 6.6 | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition of Dopamine beta hydroxylase at pH 4.5 | J Med Chem 29: 2465-72 (1987) BindingDB Entry DOI: 10.7270/Q2BR8R5P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ATP-citrate synthase (Rattus norvegicus) | BDBM50036212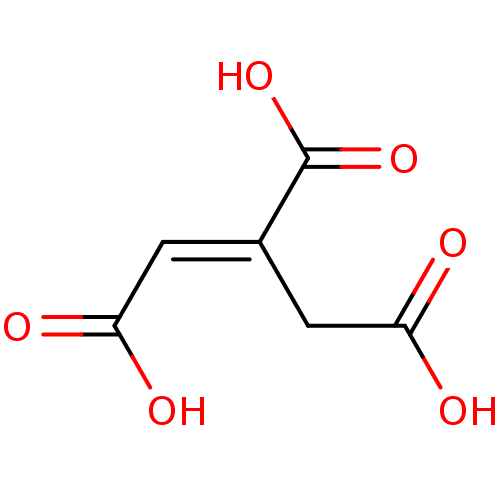 ((1E)-1-propene-1,2,3-tricarboxylic acid | (1E)-pro...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid UniChem Patents Similars | PubMed | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Ltd. Curated by ChEMBL | Assay Description Reversible binding potential for rat ATP-Citrate Lyase as carbon-carbon bond cleavage activity in citrate substrate | J Med Chem 38: 537-43 (1995) BindingDB Entry DOI: 10.7270/Q21Z43GM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ATP-citrate synthase (Rattus norvegicus) | BDBM50036222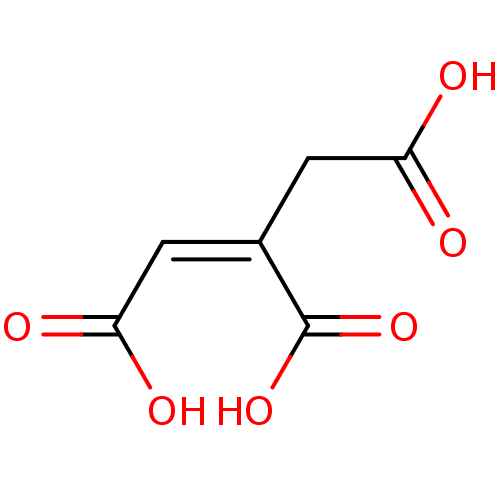 ((1Z)-prop-1-ene-1,2,3-tricarboxylic acid | (Z)-1-p...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | PubMed | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Ltd. Curated by ChEMBL | Assay Description Reversible binding potential for rat ATP-Citrate Lyase as carbon-carbon bond cleavage activity in citrate substrate | J Med Chem 38: 537-43 (1995) BindingDB Entry DOI: 10.7270/Q21Z43GM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ATP-citrate synthase (Rattus norvegicus) | BDBM50036216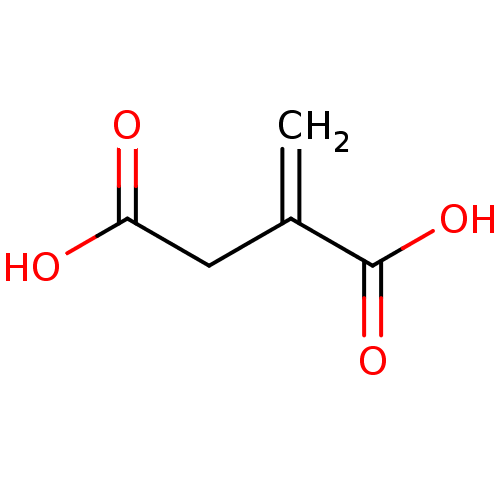 (2-methylenesuccinic acid | 2-methylidenebutanedioi...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents | PubMed | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Ltd. Curated by ChEMBL | Assay Description Reversible binding potential for rat ATP-Citrate Lyase as carbon-carbon bond cleavage activity in citrate substrate | J Med Chem 38: 537-43 (1995) BindingDB Entry DOI: 10.7270/Q21Z43GM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dopamine beta-hydroxylase (Bos taurus) | BDBM50023371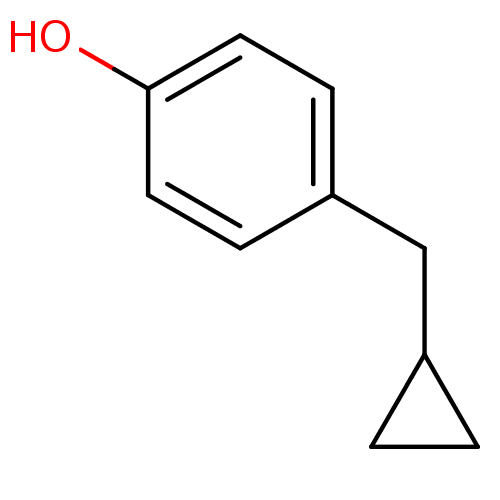 (4-Cyclopropylmethyl-phenol | CHEMBL162030) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.20E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline & French Laboratories Curated by ChEMBL | Assay Description KI value was determined from plots of 1/kinact(observed) vs 1/[inhibitor] | J Med Chem 31: 704-6 (1988) BindingDB Entry DOI: 10.7270/Q26972KP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dopamine beta-hydroxylase (Bos taurus) | BDBM50014970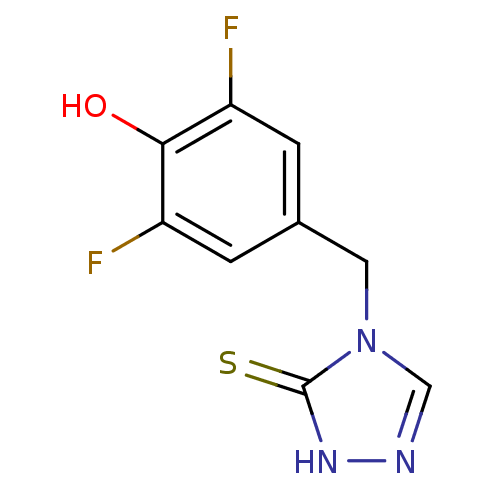 (4-(3,5-Difluoro-4-hydroxy-benzyl)-2,4-dihydro-[1,2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline & French Laboratories Curated by ChEMBL | Assay Description Inhibitory activity was determined against bovine dopamine beta-hydroxylase(DBH) | J Med Chem 33: 781-9 (1990) BindingDB Entry DOI: 10.7270/Q2DZ078G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dopamine beta-hydroxylase (Bos taurus) | BDBM50014978 (1-(3,5-Difluoro-4-hydroxy-benzyl)-1,3-dihydro-imid...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 74 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory Concentration against bovine Dopamine beta hydroxylase (DBH); Range is between (0.060-0.0877). | J Med Chem 30: 486-94 (1987) BindingDB Entry DOI: 10.7270/Q2FJ2FSM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dopamine beta-hydroxylase (Bos taurus) | BDBM50014978 (1-(3,5-Difluoro-4-hydroxy-benzyl)-1,3-dihydro-imid...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 74 | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline & French Laboratories Curated by ChEMBL | Assay Description Inhibitory activity was determined against bovine dopamine beta-hydroxylase(DBH) | J Med Chem 33: 781-9 (1990) BindingDB Entry DOI: 10.7270/Q2DZ078G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dopamine beta-hydroxylase (Bos taurus) | BDBM50014963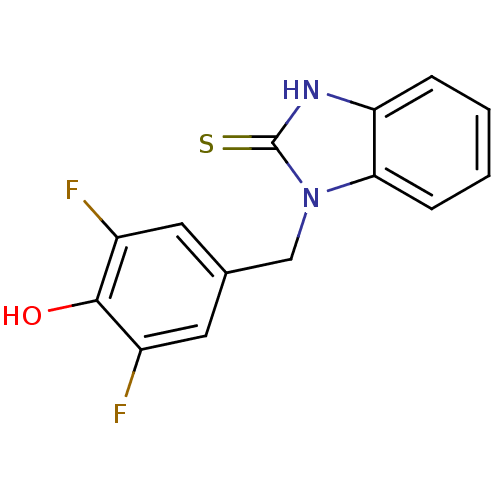 (1-(3,5-Difluoro-4-hydroxy-benzyl)-1,3-dihydro-benz...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 660 | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline & French Laboratories Curated by ChEMBL | Assay Description Inhibitory activity was determined against bovine dopamine beta-hydroxylase(DBH) | J Med Chem 33: 781-9 (1990) BindingDB Entry DOI: 10.7270/Q2DZ078G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dopamine beta-hydroxylase (Bos taurus) | BDBM50025811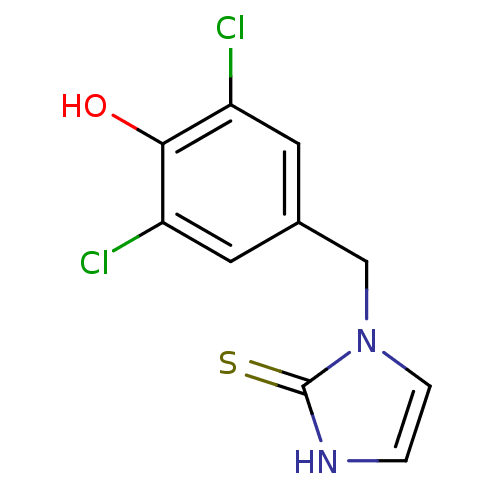 (1-(3,5-Dichloro-4-hydroxy-benzyl)-1,3-dihydro-imid...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 680 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory Concentration against bovine Dopamine beta hydroxylase (DBH); Range is between (0.42-1.1). | J Med Chem 30: 486-94 (1987) BindingDB Entry DOI: 10.7270/Q2FJ2FSM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dopamine beta-hydroxylase (Rattus norvegicus) | BDBM50000439 (5-Butyl-pyridine-2-carboxylic acid | 5-Butyl-pyrid...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents | PubMed | n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Dopamine beta hydroxylase in spontaneously hypertensive rats; Value ranges from 0.4-1.1 | J Med Chem 30: 1309-13 (1987) BindingDB Entry DOI: 10.7270/Q2MC8Z0M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tubulin alpha-1A chain (Sus scrofa (Pig)) | BDBM50020475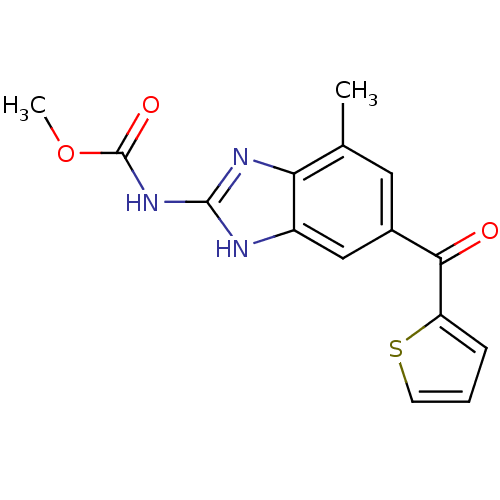 (CHEMBL341192 | [7-Methyl-5-(thiophene-2-carbonyl)-...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 860 | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline & French Laboratories Curated by ChEMBL | Assay Description Displacement of 5.8 uM [3H]-oncodazole from homogeneous tubulin. | J Med Chem 32: 409-17 (1989) BindingDB Entry DOI: 10.7270/Q2125RN4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tubulin alpha-1A chain (Sus scrofa (Pig)) | BDBM50020464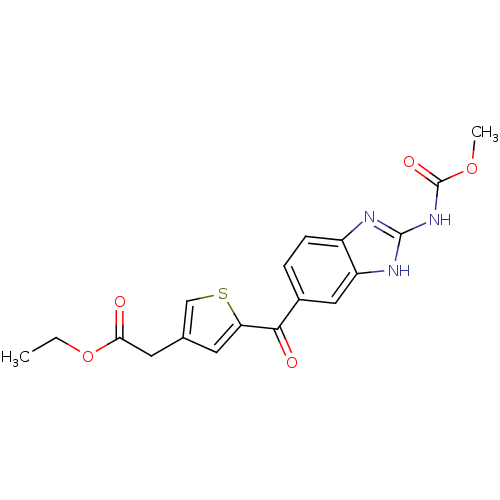 (CHEMBL135161 | [5-(2-Methoxycarbonylamino-1H-benzo...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 880 | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline & French Laboratories Curated by ChEMBL | Assay Description Displacement of 5.8 uM [3H]-oncodazole from homogeneous tubulin. | J Med Chem 32: 409-17 (1989) BindingDB Entry DOI: 10.7270/Q2125RN4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dopamine beta-hydroxylase (Bos taurus) | BDBM50014984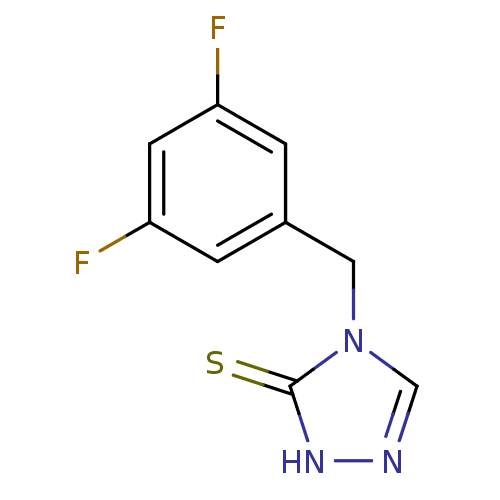 (4-(3,5-Difluoro-benzyl)-2,4-dihydro-[1,2,4]triazol...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline & French Laboratories Curated by ChEMBL | Assay Description Inhibitory activity was determined against bovine dopamine beta-hydroxylase(DBH) | J Med Chem 33: 781-9 (1990) BindingDB Entry DOI: 10.7270/Q2DZ078G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dopamine beta-hydroxylase (Bos taurus) | BDBM50014983 (1-(3,5-Difluoro-benzyl)-1,3-dihydro-imidazole-2-th...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline & French Laboratories Curated by ChEMBL | Assay Description Inhibitory activity was determined against bovine dopamine beta-hydroxylase(DBH) | J Med Chem 33: 781-9 (1990) BindingDB Entry DOI: 10.7270/Q2DZ078G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dopamine beta-hydroxylase (Rattus norvegicus) | BDBM50014983 (1-(3,5-Difluoro-benzyl)-1,3-dihydro-imidazole-2-th...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Dopamine beta hydroxylase in spontaneously hypertensive rats; Value ranges from 1.1-1.4 | J Med Chem 30: 1309-13 (1987) BindingDB Entry DOI: 10.7270/Q2MC8Z0M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dopamine beta-hydroxylase (Bos taurus) | BDBM50014983 (1-(3,5-Difluoro-benzyl)-1,3-dihydro-imidazole-2-th...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory Concentration against bovine Dopamine beta hydroxylase (DBH); Range is between (1.0-1.9) | J Med Chem 30: 486-94 (1987) BindingDB Entry DOI: 10.7270/Q2FJ2FSM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dopamine beta-hydroxylase (Bos taurus) | BDBM50014971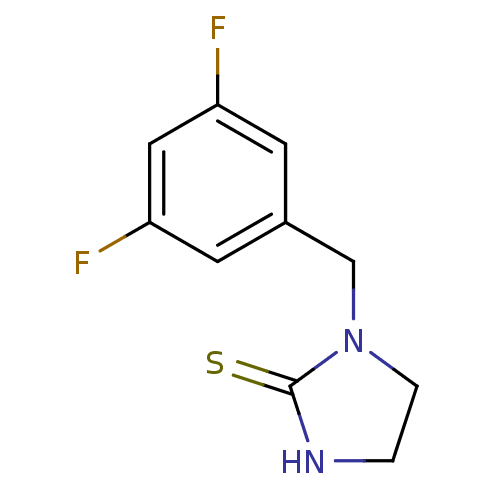 (1-(3,5-Difluoro-benzyl)-imidazolidine-2-thione | C...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline & French Laboratories Curated by ChEMBL | Assay Description Inhibitory activity was determined against bovine dopamine beta-hydroxylase(DBH) | J Med Chem 33: 781-9 (1990) BindingDB Entry DOI: 10.7270/Q2DZ078G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dopamine beta-hydroxylase (Bos taurus) | BDBM50025795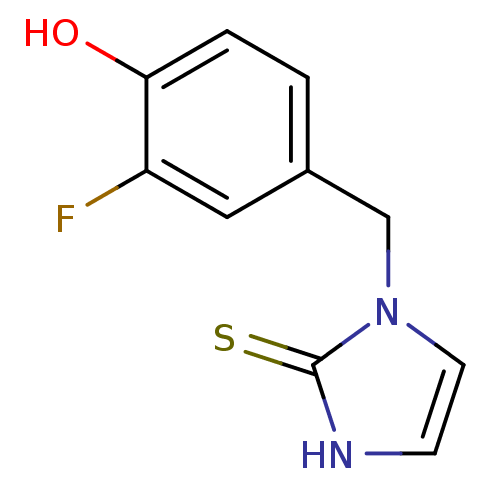 (1-(3-Fluoro-4-hydroxy-benzyl)-1,3-dihydro-imidazol...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory Concentration against bovine Dopamine beta hydroxylase (DBH); Range is between (0.9-2.2) | J Med Chem 30: 486-94 (1987) BindingDB Entry DOI: 10.7270/Q2FJ2FSM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dopamine beta-hydroxylase (Bos taurus) | BDBM50014967 (1-(3,5-Difluoro-benzyl)-1H-tetrazole-5-thiol | CHE...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 1.54E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline & French Laboratories Curated by ChEMBL | Assay Description Inhibitory activity was determined against bovine dopamine beta-hydroxylase(DBH) | J Med Chem 33: 781-9 (1990) BindingDB Entry DOI: 10.7270/Q2DZ078G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dopamine beta-hydroxylase (Bos taurus) | BDBM50014979 (4-(3-Fluoro-benzyl)-2,4-dihydro-[1,2,4]triazole-3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline & French Laboratories Curated by ChEMBL | Assay Description Inhibitory activity was determined against bovine dopamine beta-hydroxylase(DBH) | J Med Chem 33: 781-9 (1990) BindingDB Entry DOI: 10.7270/Q2DZ078G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tubulin alpha-1A chain (Sus scrofa (Pig)) | BDBM50020463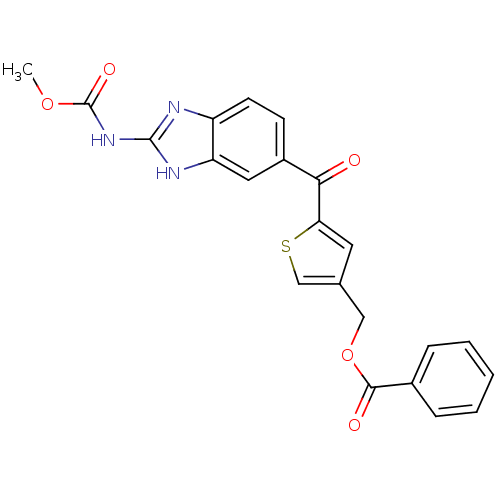 (Benzoic acid 5-(2-methoxycarbonylamino-1H-benzoimi...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.95E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline & French Laboratories Curated by ChEMBL | Assay Description Displacement of 5.8 uM [3H]-oncodazole from homogeneous tubulin. | J Med Chem 32: 409-17 (1989) BindingDB Entry DOI: 10.7270/Q2125RN4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 137 total ) | Next | Last >> |