

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM106870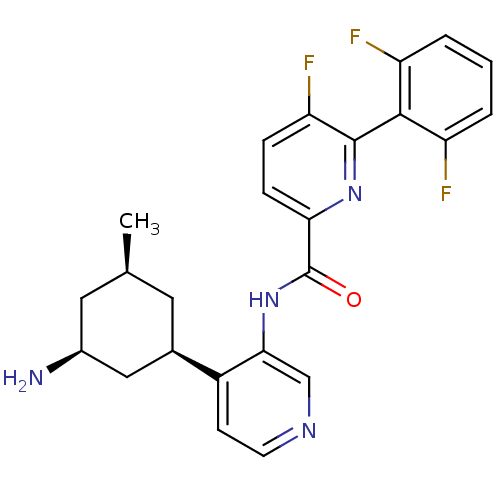 (US8592455, 70) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | Article PubMed | 0.00600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of PIM1 kinase (unknown origin) using Biotin-AGAGRSRHSSYPAGT-OH as substrate after 2 hrs by alphascreen assay | J Med Chem 58: 8373-86 (2015) Article DOI: 10.1021/acs.jmedchem.5b01275 BindingDB Entry DOI: 10.7270/Q2H41VGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-3 (Homo sapiens (Human)) | BDBM106870 (US8592455, 70) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | Article PubMed | 0.00900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of PIM3 kinase (unknown origin) using Biotin-AGAGRSRHSSYPAGT-OH as substrate after 2 hrs by alphascreen assay | J Med Chem 58: 8373-86 (2015) Article DOI: 10.1021/acs.jmedchem.5b01275 BindingDB Entry DOI: 10.7270/Q2H41VGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM50387298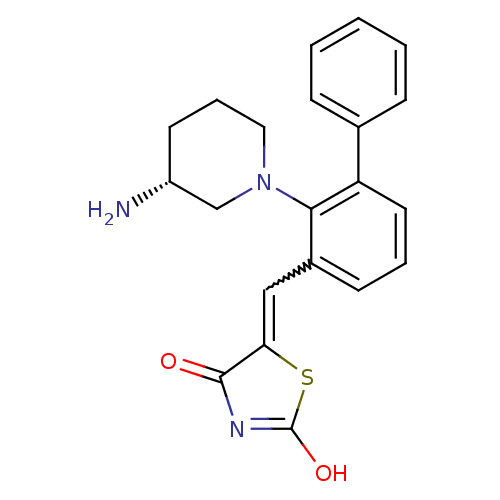 (CHEMBL2048872) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of PIM1 kinase (unknown origin) using Biotin-AGAGRSRHSSYPAGT-OH as substrate after 2 hrs by alphascreen assay | J Med Chem 58: 8373-86 (2015) Article DOI: 10.1021/acs.jmedchem.5b01275 BindingDB Entry DOI: 10.7270/Q2H41VGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-2 (Homo sapiens (Human)) | BDBM106870 (US8592455, 70) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | Article PubMed | 0.0180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of PIM2 kinase (unknown origin) using Biotin-AGAGRSRHSSYPAGT-OH as substrate after 2 hrs by alphascreen assay | J Med Chem 58: 8373-86 (2015) Article DOI: 10.1021/acs.jmedchem.5b01275 BindingDB Entry DOI: 10.7270/Q2H41VGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-2 (Homo sapiens (Human)) | BDBM50387298 (CHEMBL2048872) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of PIM2 kinase (unknown origin) using Biotin-AGAGRSRHSSYPAGT-OH as substrate after 2 hrs by alphascreen assay | J Med Chem 58: 8373-86 (2015) Article DOI: 10.1021/acs.jmedchem.5b01275 BindingDB Entry DOI: 10.7270/Q2H41VGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-3 (Homo sapiens (Human)) | BDBM50387298 (CHEMBL2048872) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of PIM3 kinase (unknown origin) using Biotin-AGAGRSRHSSYPAGT-OH as substrate after 2 hrs by alphascreen assay | J Med Chem 58: 8373-86 (2015) Article DOI: 10.1021/acs.jmedchem.5b01275 BindingDB Entry DOI: 10.7270/Q2H41VGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50076428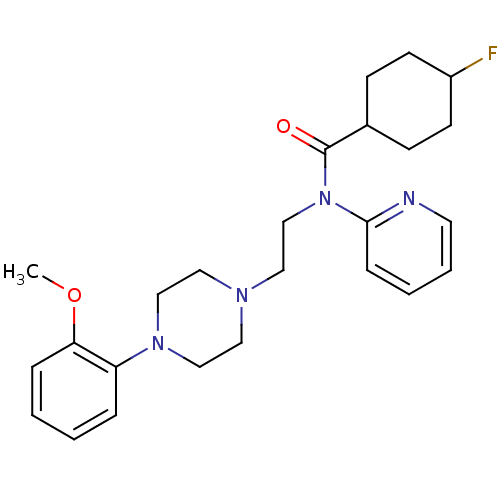 (4-Fluoro-cyclohexanecarboxylic acid {2-[4-(2-metho...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.247 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health Curated by ChEMBL | Assay Description In vitro inhibition of [3H]- 8-OH-DPAT binding to cloned cell line containing human 5-hydroxytryptamine 1A receptor by Panlabs assay | J Med Chem 42: 1576-86 (1999) Article DOI: 10.1021/jm980456f BindingDB Entry DOI: 10.7270/Q23R0S2J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Mus musculus (Mouse)) | BDBM86708 (CAS_146714-97-8 | CHEMBL31354 | CHEMBL514874 | CHE...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health Curated by ChEMBL | Assay Description Receptor-linked G protein activation at 5-hydroxytryptamine receptor was determined by measuring the stimulation of [35S]-GTP-gammaS, binding (Experi... | J Med Chem 42: 1576-86 (1999) Article DOI: 10.1021/jm980456f BindingDB Entry DOI: 10.7270/Q23R0S2J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50076428 (4-Fluoro-cyclohexanecarboxylic acid {2-[4-(2-metho...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.515 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health Curated by ChEMBL | Assay Description Compound was tested in vitro for the inhibition of [3H]- 8-OH-DPAT binding to cloned cell line containing human 5-hydroxytryptamine 1A receptor | J Med Chem 42: 1576-86 (1999) Article DOI: 10.1021/jm980456f BindingDB Entry DOI: 10.7270/Q23R0S2J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM86708 (CAS_146714-97-8 | CHEMBL31354 | CHEMBL514874 | CHE...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.590 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health Curated by ChEMBL | Assay Description Compound was tested in vitro for the inhibition of [3H]- 8-OH-DPAT binding to cloned cell line containing human 5-hydroxytryptamine 1A receptor | J Med Chem 42: 1576-86 (1999) Article DOI: 10.1021/jm980456f BindingDB Entry DOI: 10.7270/Q23R0S2J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50076429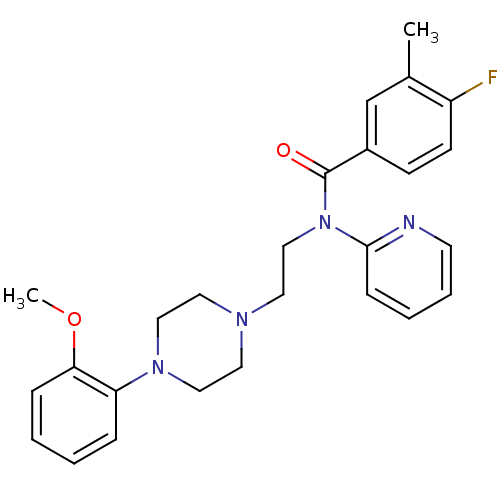 (4-Fluoro-N-{2-[4-(2-methoxy-phenyl)-piperazin-1-yl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.791 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health Curated by ChEMBL | Assay Description In vitro inhibition of [3H]- 8-OH-DPAT binding to cloned cell line containing human 5-hydroxytryptamine 1A receptor (Experiment 2) | J Med Chem 42: 1576-86 (1999) Article DOI: 10.1021/jm980456f BindingDB Entry DOI: 10.7270/Q23R0S2J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM106899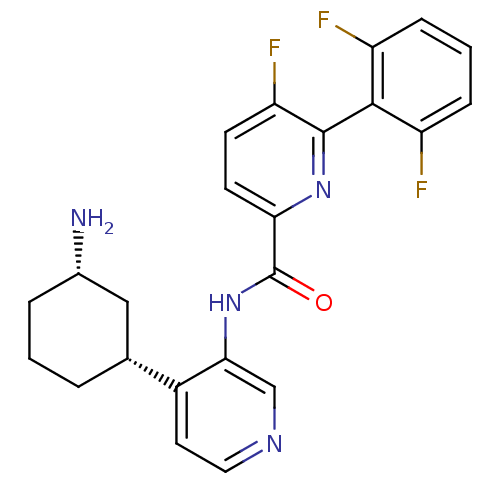 (US8592455, 99) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of PIM1 kinase (unknown origin) using NH2-AGAGRSRHSSYPAGT-OH as substrate by kinase-Glo assay | J Med Chem 58: 8373-86 (2015) Article DOI: 10.1021/acs.jmedchem.5b01275 BindingDB Entry DOI: 10.7270/Q2H41VGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM50500175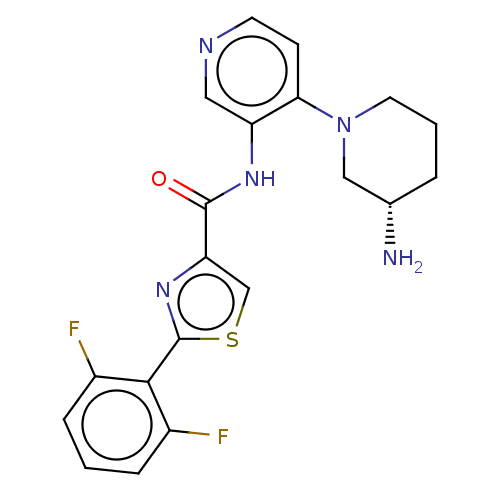 (CHEMBL3676258) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of PIM1 kinase (unknown origin) using NH2-AGAGRSRHSSYPAGT-OH as substrate by kinase-Glo assay | J Med Chem 58: 8373-86 (2015) Article DOI: 10.1021/acs.jmedchem.5b01275 BindingDB Entry DOI: 10.7270/Q2H41VGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM50500179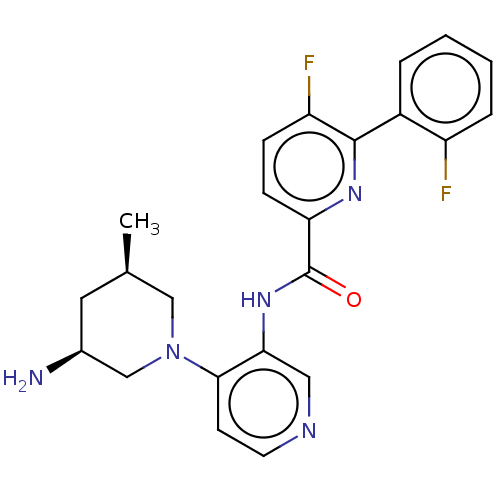 (CHEMBL3746909) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of PIM1 kinase (unknown origin) using NH2-AGAGRSRHSSYPAGT-OH as substrate by kinase-Glo assay | J Med Chem 58: 8373-86 (2015) Article DOI: 10.1021/acs.jmedchem.5b01275 BindingDB Entry DOI: 10.7270/Q2H41VGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM50500182 (CHEMBL3747545) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of PIM1 kinase (unknown origin) using NH2-AGAGRSRHSSYPAGT-OH as substrate by kinase-Glo assay | J Med Chem 58: 8373-86 (2015) Article DOI: 10.1021/acs.jmedchem.5b01275 BindingDB Entry DOI: 10.7270/Q2H41VGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM50500178 (CHEMBL3746984) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of PIM1 kinase (unknown origin) using NH2-AGAGRSRHSSYPAGT-OH as substrate by kinase-Glo assay | J Med Chem 58: 8373-86 (2015) Article DOI: 10.1021/acs.jmedchem.5b01275 BindingDB Entry DOI: 10.7270/Q2H41VGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM50500176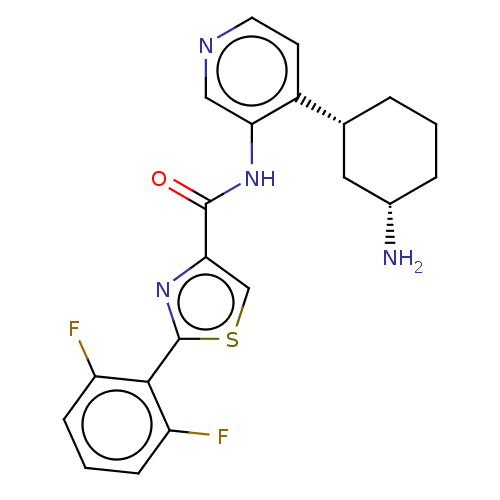 (CHEMBL3676290) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of PIM1 kinase (unknown origin) using NH2-AGAGRSRHSSYPAGT-OH as substrate by kinase-Glo assay | J Med Chem 58: 8373-86 (2015) Article DOI: 10.1021/acs.jmedchem.5b01275 BindingDB Entry DOI: 10.7270/Q2H41VGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM50500177 (CHEMBL3747207) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of PIM1 kinase (unknown origin) using NH2-AGAGRSRHSSYPAGT-OH as substrate by kinase-Glo assay | J Med Chem 58: 8373-86 (2015) Article DOI: 10.1021/acs.jmedchem.5b01275 BindingDB Entry DOI: 10.7270/Q2H41VGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM106857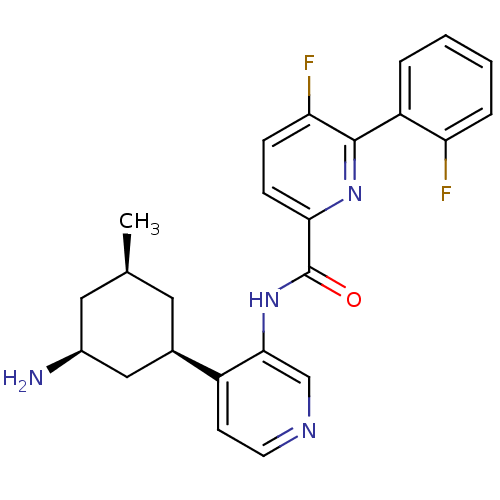 (US8592455, 57) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of PIM1 kinase (unknown origin) using NH2-AGAGRSRHSSYPAGT-OH as substrate by kinase-Glo assay | J Med Chem 58: 8373-86 (2015) Article DOI: 10.1021/acs.jmedchem.5b01275 BindingDB Entry DOI: 10.7270/Q2H41VGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM106850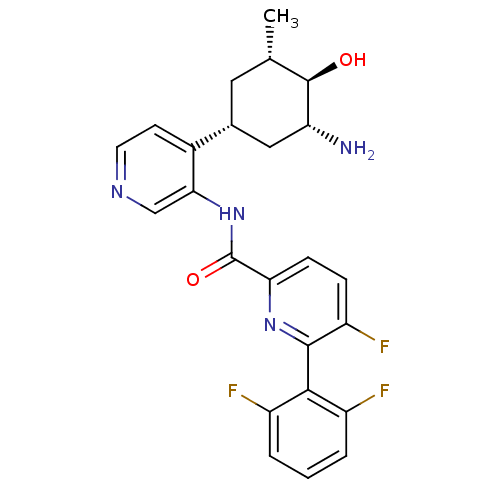 (US8592455, 50) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of PIM1 kinase (unknown origin) using NH2-AGAGRSRHSSYPAGT-OH as substrate by kinase-Glo assay | J Med Chem 58: 8373-86 (2015) Article DOI: 10.1021/acs.jmedchem.5b01275 BindingDB Entry DOI: 10.7270/Q2H41VGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM50559296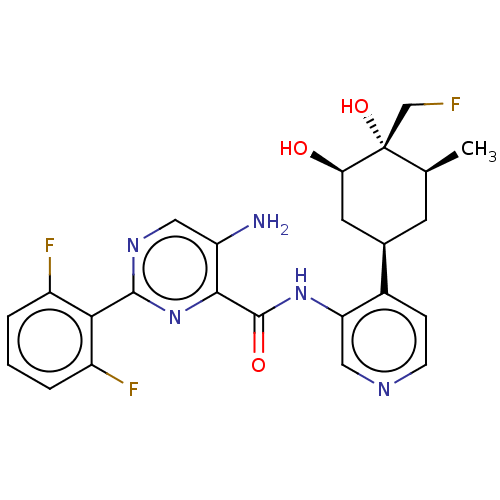 (CHEMBL4781892) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of PIM1 (unknown origin) using Bad peptide NH2-AGAGRSRHSSYPAGT-OH as substrate measured after 10 mins in presence of ATP by luciferase-luc... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01279 BindingDB Entry DOI: 10.7270/Q24X5CHB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM50559297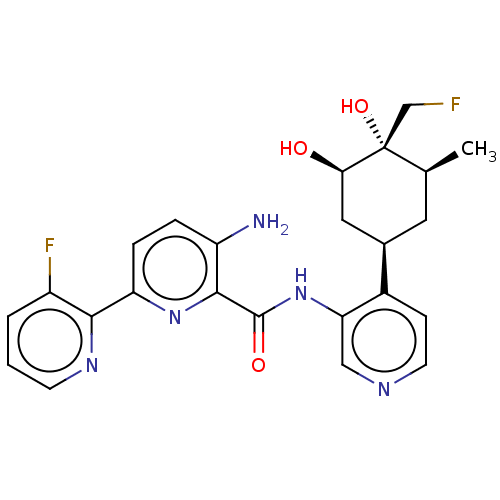 (CHEMBL4763702) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of PIM1 (unknown origin) using Bad peptide NH2-AGAGRSRHSSYPAGT-OH as substrate measured after 10 mins in presence of ATP by luciferase-luc... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01279 BindingDB Entry DOI: 10.7270/Q24X5CHB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM50559298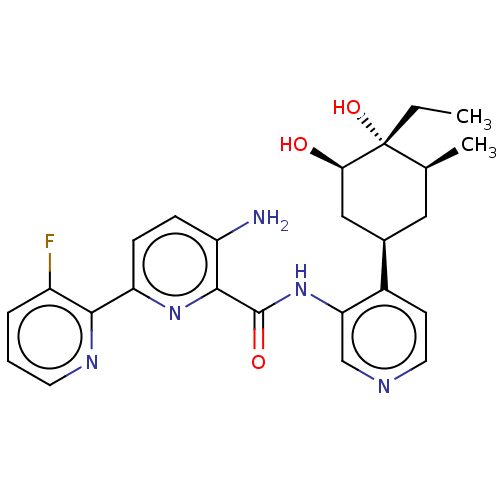 (CHEMBL4742957) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of PIM1 (unknown origin) using Bad peptide NH2-AGAGRSRHSSYPAGT-OH as substrate measured after 10 mins in presence of ATP by luciferase-luc... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01279 BindingDB Entry DOI: 10.7270/Q24X5CHB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM50559299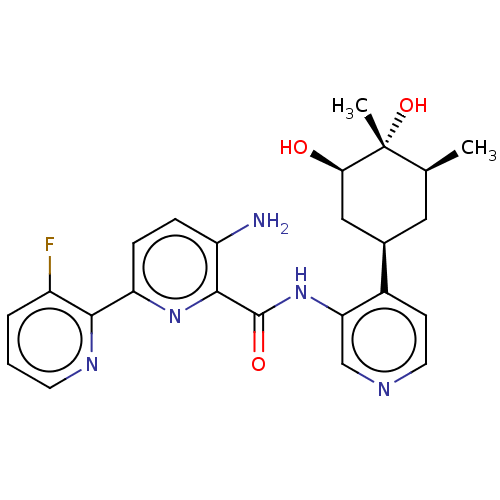 (CHEMBL4781112) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of PIM1 (unknown origin) using Bad peptide NH2-AGAGRSRHSSYPAGT-OH as substrate measured after 10 mins in presence of ATP by luciferase-luc... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01279 BindingDB Entry DOI: 10.7270/Q24X5CHB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM50559300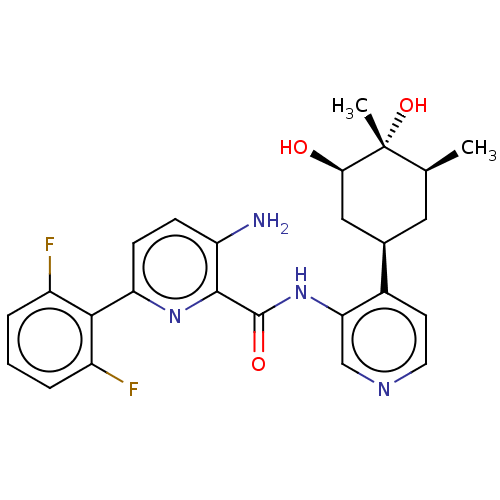 (CHEMBL4796070) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of PIM1 (unknown origin) using Bad peptide NH2-AGAGRSRHSSYPAGT-OH as substrate measured after 10 mins in presence of ATP by luciferase-luc... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01279 BindingDB Entry DOI: 10.7270/Q24X5CHB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM50559301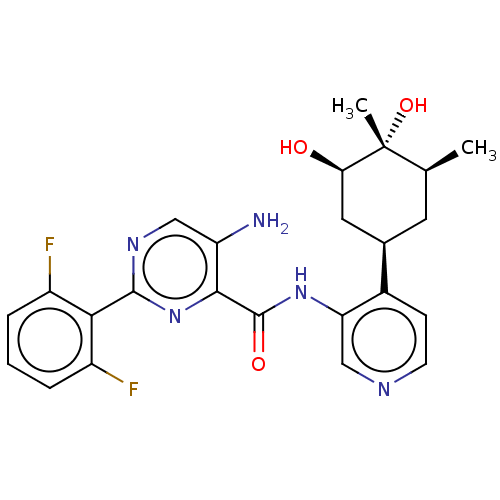 (CHEMBL4758068) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of PIM1 (unknown origin) using Bad peptide NH2-AGAGRSRHSSYPAGT-OH as substrate measured after 10 mins in presence of ATP by luciferase-luc... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01279 BindingDB Entry DOI: 10.7270/Q24X5CHB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM50559302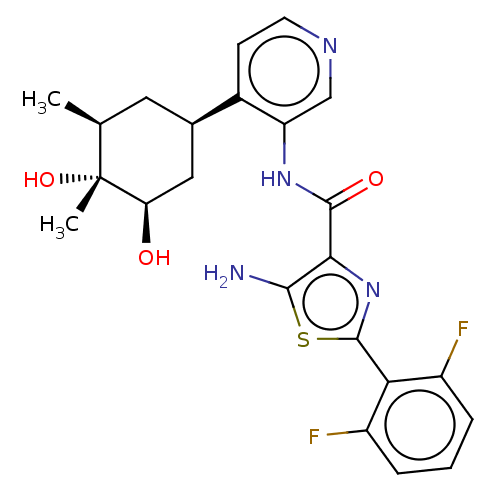 (CHEMBL4792066) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of PIM1 (unknown origin) using Bad peptide NH2-AGAGRSRHSSYPAGT-OH as substrate measured after 10 mins in presence of ATP by luciferase-luc... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01279 BindingDB Entry DOI: 10.7270/Q24X5CHB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM106896 (US8592455, 96) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | Article PubMed | <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of PIM1 (unknown origin) using Bad peptide NH2-AGAGRSRHSSYPAGT-OH as substrate measured after 10 mins in presence of ATP by luciferase-luc... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01279 BindingDB Entry DOI: 10.7270/Q24X5CHB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM106870 (US8592455, 70) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | Article PubMed | <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of PIM1 (unknown origin) using Bad peptide NH2-AGAGRSRHSSYPAGT-OH as substrate measured after 10 mins in presence of ATP by luciferase-luc... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01279 BindingDB Entry DOI: 10.7270/Q24X5CHB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM106801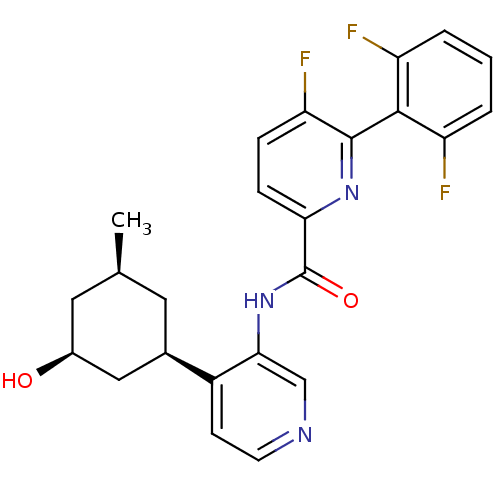 (US8592455, 1) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of PIM1 (unknown origin) using Bad peptide NH2-AGAGRSRHSSYPAGT-OH as substrate measured after 10 mins in presence of ATP by luciferase-luc... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01279 BindingDB Entry DOI: 10.7270/Q24X5CHB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM106917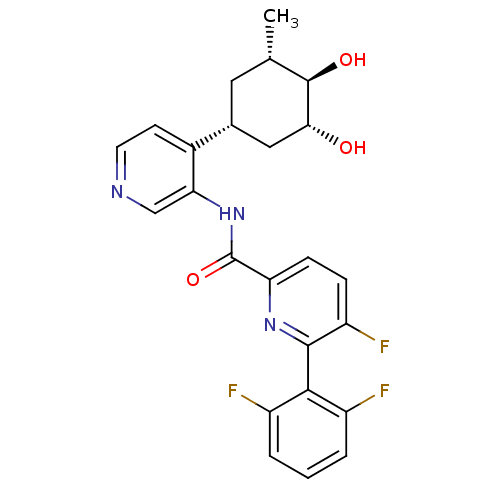 (US8592455, 117) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of PIM1 (unknown origin) using Bad peptide NH2-AGAGRSRHSSYPAGT-OH as substrate measured after 10 mins in presence of ATP by luciferase-luc... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01279 BindingDB Entry DOI: 10.7270/Q24X5CHB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM50559303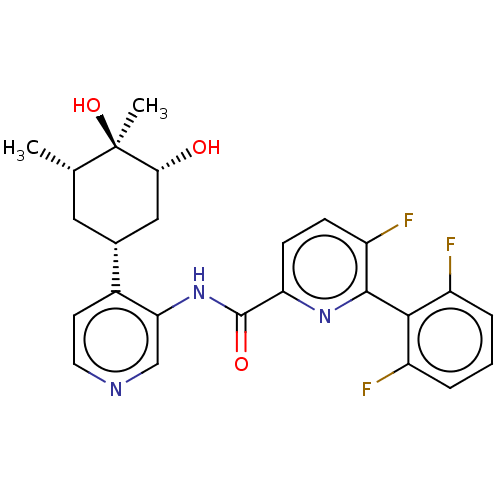 (CHEMBL4741855) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of PIM1 (unknown origin) using Bad peptide NH2-AGAGRSRHSSYPAGT-OH as substrate measured after 10 mins in presence of ATP by luciferase-luc... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01279 BindingDB Entry DOI: 10.7270/Q24X5CHB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM50559304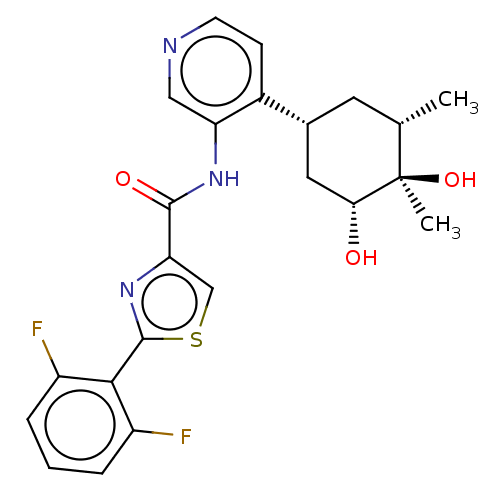 (CHEMBL4783935) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of PIM1 (unknown origin) using Bad peptide NH2-AGAGRSRHSSYPAGT-OH as substrate measured after 10 mins in presence of ATP by luciferase-luc... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01279 BindingDB Entry DOI: 10.7270/Q24X5CHB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM106870 (US8592455, 70) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | Article PubMed | <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of PIM1 kinase (unknown origin) using NH2-AGAGRSRHSSYPAGT-OH as substrate by kinase-Glo assay | J Med Chem 58: 8373-86 (2015) Article DOI: 10.1021/acs.jmedchem.5b01275 BindingDB Entry DOI: 10.7270/Q2H41VGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM50500180 (CHEMBL3676284) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of PIM1 kinase (unknown origin) using NH2-AGAGRSRHSSYPAGT-OH as substrate by kinase-Glo assay | J Med Chem 58: 8373-86 (2015) Article DOI: 10.1021/acs.jmedchem.5b01275 BindingDB Entry DOI: 10.7270/Q2H41VGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Mus musculus (Mouse)) | BDBM50076428 (4-Fluoro-cyclohexanecarboxylic acid {2-[4-(2-metho...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health Curated by ChEMBL | Assay Description Receptor-linked G protein activation at 5-hydroxytryptamine receptor was determined by measuring the stimulation of [35S]-GTP-gammaS, binding | J Med Chem 42: 1576-86 (1999) Article DOI: 10.1021/jm980456f BindingDB Entry DOI: 10.7270/Q23R0S2J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM50500181 (CHEMBL3676300) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of PIM1 kinase (unknown origin) using NH2-AGAGRSRHSSYPAGT-OH as substrate by kinase-Glo assay | J Med Chem 58: 8373-86 (2015) Article DOI: 10.1021/acs.jmedchem.5b01275 BindingDB Entry DOI: 10.7270/Q2H41VGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM106896 (US8592455, 96) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | Article PubMed | <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of PIM1 kinase (unknown origin) using NH2-AGAGRSRHSSYPAGT-OH as substrate by kinase-Glo assay | J Med Chem 58: 8373-86 (2015) Article DOI: 10.1021/acs.jmedchem.5b01275 BindingDB Entry DOI: 10.7270/Q2H41VGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C delta type (Homo sapiens (Human)) | BDBM50258529 (CHEMBL449158 | bryostatin 1) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Stanford University Curated by ChEMBL | Assay Description Inhibition of [3H]PDBu binding to recombinant full length human PKCdelta expressed in baculovirus expression system incubated for 5 mins by scintilla... | J Nat Prod 79: 680-4 (2016) Article DOI: 10.1021/acs.jnatprod.5b01017 BindingDB Entry DOI: 10.7270/Q2BK1GXV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Mus musculus (Mouse)) | BDBM86708 (CAS_146714-97-8 | CHEMBL31354 | CHEMBL514874 | CHE...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health Curated by ChEMBL | Assay Description Receptor-linked G protein activation at 5-hydroxytryptamine receptor was determined by measuring the stimulation of [35S]GTP-gamma-S, binding (Experi... | J Med Chem 42: 1576-86 (1999) Article DOI: 10.1021/jm980456f BindingDB Entry DOI: 10.7270/Q23R0S2J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50076429 (4-Fluoro-N-{2-[4-(2-methoxy-phenyl)-piperazin-1-yl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health Curated by ChEMBL | Assay Description Compound was tested in vitro for the inhibition of [3H]- 8-OH-DPAT binding to cloned cell line containing human 5-hydroxytryptamine 1A receptor (Expe... | J Med Chem 42: 1576-86 (1999) Article DOI: 10.1021/jm980456f BindingDB Entry DOI: 10.7270/Q23R0S2J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C delta type (Homo sapiens (Human)) | BDBM50535777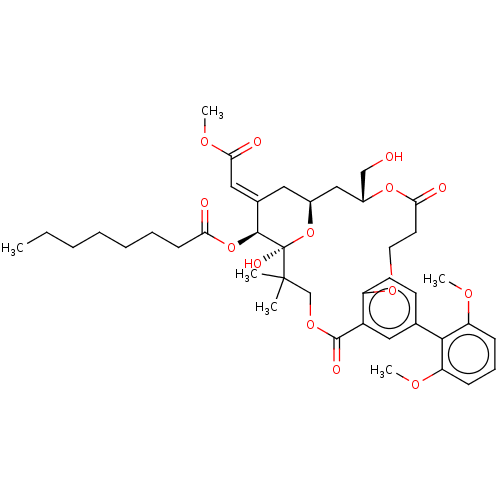 (CHEMBL4565484) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Stanford University Curated by ChEMBL | Assay Description Inhibition of [3H]PDBu binding to recombinant full length human PKCdelta expressed in baculovirus expression system incubated for 5 mins by scintilla... | J Nat Prod 79: 680-4 (2016) Article DOI: 10.1021/acs.jnatprod.5b01017 BindingDB Entry DOI: 10.7270/Q2BK1GXV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (RAT) | BDBM50035989 (1-Methyl-4-(4-propylsulfanyl-[1,2,5]thiadiazol-3-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health Curated by ChEMBL | Assay Description In vitro binding affinity towards Muscarinic acetylcholine receptor M2 was determined by measuring its ability to displace [3H]-AF-DX 384 from rat he... | J Med Chem 38: 5-8 (1995) BindingDB Entry DOI: 10.7270/Q2BC3XMW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (RAT) | BDBM50407329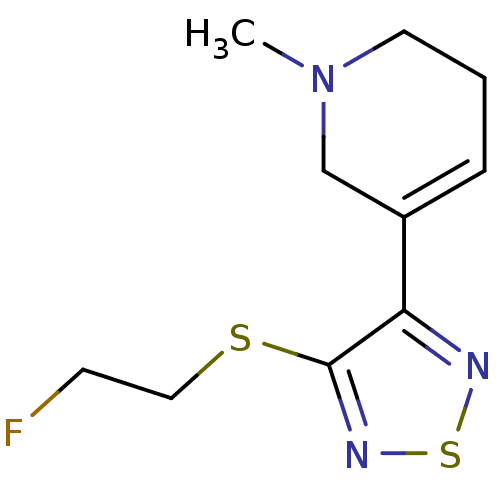 (CHEMBL2112938) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health Curated by ChEMBL | Assay Description In vitro binding affinity towards Muscarinic acetylcholine receptor M2 was determined by measuring its ability to displace [3H]-AF-DX 384 from rat he... | J Med Chem 38: 5-8 (1995) BindingDB Entry DOI: 10.7270/Q2BC3XMW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C delta type (Homo sapiens (Human)) | BDBM50535778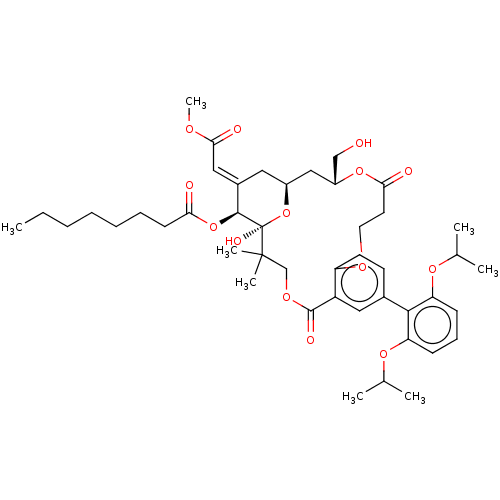 (CHEMBL4533216) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Stanford University Curated by ChEMBL | Assay Description Inhibition of [3H]PDBu binding to recombinant full length human PKCdelta expressed in baculovirus expression system incubated for 5 mins by scintilla... | J Nat Prod 79: 680-4 (2016) Article DOI: 10.1021/acs.jnatprod.5b01017 BindingDB Entry DOI: 10.7270/Q2BK1GXV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (RAT) | BDBM50407330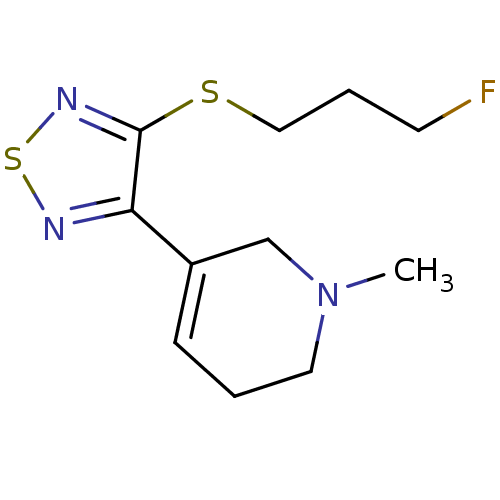 (CHEMBL2112939) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health Curated by ChEMBL | Assay Description In vitro binding affinity towards Muscarinic acetylcholine receptor M2 was determined by measuring its ability to displace [3H]-AF-DX 384 from rat he... | J Med Chem 38: 5-8 (1995) BindingDB Entry DOI: 10.7270/Q2BC3XMW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic receptor M1 (Bos taurus) | BDBM50407331 (CHEMBL2112940) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health Curated by ChEMBL | Assay Description In vitro binding affinity towards Muscarinic acetylcholine receptor M1 was determined by measuring its ability to displace [3H]-Pirenzepine from bovi... | J Med Chem 38: 5-8 (1995) BindingDB Entry DOI: 10.7270/Q2BC3XMW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C delta type (Homo sapiens (Human)) | BDBM50535789 (CHEMBL4574406) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Stanford University Curated by ChEMBL | Assay Description Inhibition of [3H]PDBu binding to recombinant full length human PKCdelta expressed in baculovirus expression system incubated for 5 mins by scintilla... | J Nat Prod 79: 680-4 (2016) Article DOI: 10.1021/acs.jnatprod.5b01017 BindingDB Entry DOI: 10.7270/Q2BK1GXV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C delta type (Homo sapiens (Human)) | BDBM50535791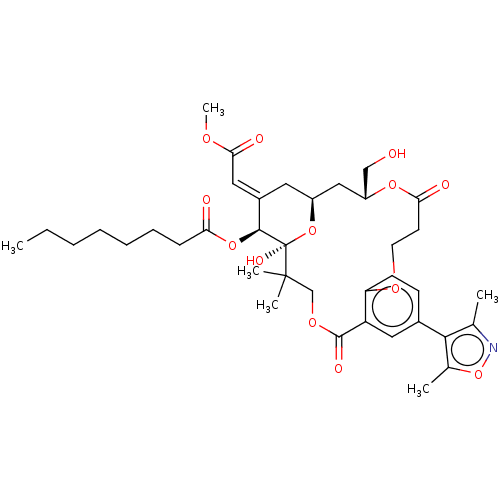 (CHEMBL4593581) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Stanford University Curated by ChEMBL | Assay Description Inhibition of [3H]PDBu binding to recombinant full length human PKCdelta expressed in baculovirus expression system incubated for 5 mins by scintilla... | J Nat Prod 79: 680-4 (2016) Article DOI: 10.1021/acs.jnatprod.5b01017 BindingDB Entry DOI: 10.7270/Q2BK1GXV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Mus musculus (Mouse)) | BDBM50076429 (4-Fluoro-N-{2-[4-(2-methoxy-phenyl)-piperazin-1-yl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health Curated by ChEMBL | Assay Description Receptor-linked G protein activation at 5-hydroxytryptamine receptor was determined by measuring the stimulation of [35S]-GTP-gammaS, binding (Experi... | J Med Chem 42: 1576-86 (1999) Article DOI: 10.1021/jm980456f BindingDB Entry DOI: 10.7270/Q23R0S2J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1353 total ) | Next | Last >> |