

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| cAMP-specific 3',5'-cyclic phosphodiesterase 4C (Rattus norvegicus) | BDBM14361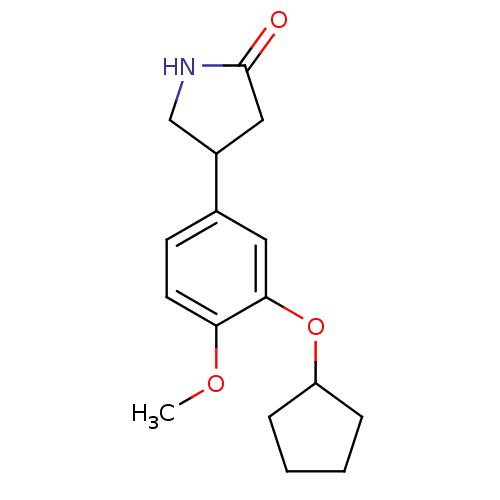 ((R,S)-Rolipram | 4-(3-cyclopentyloxy-4-methoxy-phe...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PubMed | 0.00230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
LEAD GENE CO., LTD Curated by ChEMBL | Assay Description Binding affinity towards rolipram binding site on phosphodiesterase 4 using [3H]rolipram as radioligand in crude rat brain homogenate | Bioorg Med Chem Lett 13: 2355-8 (2003) BindingDB Entry DOI: 10.7270/Q2CZ39CG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4C (Rattus norvegicus) | BDBM50220995 (CHEMBL77788) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
LEAD GENE CO., LTD Curated by ChEMBL | Assay Description Binding affinity towards rolipram binding site on phosphodiesterase 4 using [3H]rolipram as radioligand in crude rat brain homogenate | Bioorg Med Chem Lett 13: 2355-8 (2003) BindingDB Entry DOI: 10.7270/Q2CZ39CG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4C (Rattus norvegicus) | BDBM50221005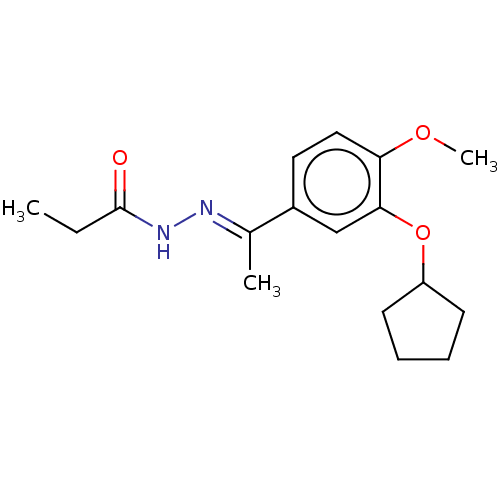 (CHEMBL75684) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
LEAD GENE CO., LTD Curated by ChEMBL | Assay Description Binding affinity towards rolipram binding site on phosphodiesterase 4 using [3H]rolipram as radioligand in crude rat brain homogenate | Bioorg Med Chem Lett 13: 2355-8 (2003) BindingDB Entry DOI: 10.7270/Q2CZ39CG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4C (Rattus norvegicus) | BDBM50220998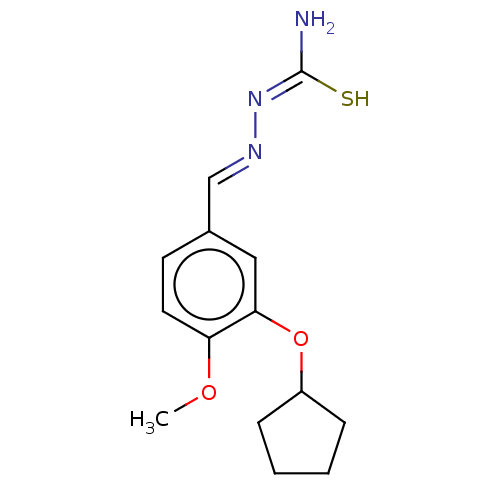 (CHEMBL76382) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
LEAD GENE CO., LTD Curated by ChEMBL | Assay Description Binding affinity towards rolipram binding site on phosphodiesterase 4 using [3H]rolipram as radioligand in crude rat brain homogenate | Bioorg Med Chem Lett 13: 2355-8 (2003) BindingDB Entry DOI: 10.7270/Q2CZ39CG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4C (Rattus norvegicus) | BDBM50221003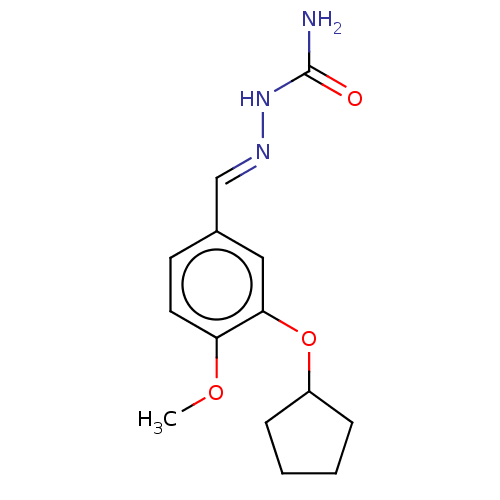 (CHEMBL432348) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0690 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
LEAD GENE CO., LTD Curated by ChEMBL | Assay Description Binding affinity towards rolipram binding site on phosphodiesterase 4 using [3H]rolipram as radioligand in crude rat brain homogenate | Bioorg Med Chem Lett 13: 2355-8 (2003) BindingDB Entry DOI: 10.7270/Q2CZ39CG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4C (Rattus norvegicus) | BDBM50220997 (CHEMBL78237) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0920 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
LEAD GENE CO., LTD Curated by ChEMBL | Assay Description Binding affinity towards rolipram binding site on phosphodiesterase 4 using [3H]rolipram as radioligand in crude rat brain homogenate | Bioorg Med Chem Lett 13: 2355-8 (2003) BindingDB Entry DOI: 10.7270/Q2CZ39CG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4C (Rattus norvegicus) | BDBM50221006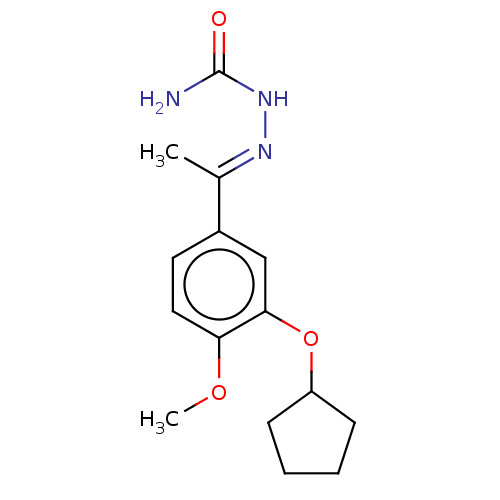 (CHEMBL77358) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.146 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
LEAD GENE CO., LTD Curated by ChEMBL | Assay Description Binding affinity towards rolipram binding site on phosphodiesterase 4 using [3H]rolipram as radioligand in crude rat brain homogenate | Bioorg Med Chem Lett 13: 2355-8 (2003) BindingDB Entry DOI: 10.7270/Q2CZ39CG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4C (Rattus norvegicus) | BDBM50220999 (CHEMBL77745) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.336 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
LEAD GENE CO., LTD Curated by ChEMBL | Assay Description Binding affinity towards rolipram binding site on phosphodiesterase 4 using [3H]rolipram as radioligand in crude rat brain homogenate | Bioorg Med Chem Lett 13: 2355-8 (2003) BindingDB Entry DOI: 10.7270/Q2CZ39CG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4C (Rattus norvegicus) | BDBM50220996 (CHEMBL76635) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
LEAD GENE CO., LTD Curated by ChEMBL | Assay Description Binding affinity towards rolipram binding site on phosphodiesterase 4 using [3H]rolipram as radioligand in crude rat brain homogenate | Bioorg Med Chem Lett 13: 2355-8 (2003) BindingDB Entry DOI: 10.7270/Q2CZ39CG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4C (Rattus norvegicus) | BDBM50221004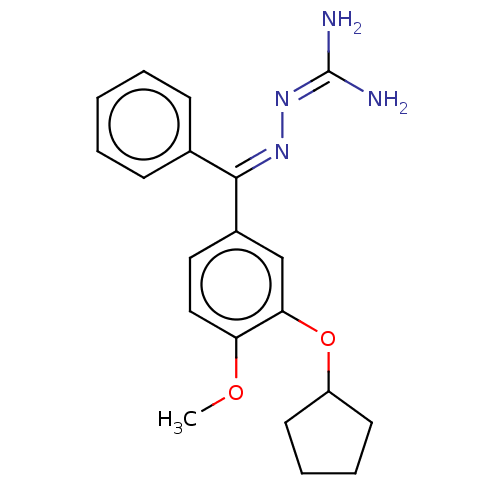 (CHEMBL77999) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
LEAD GENE CO., LTD Curated by ChEMBL | Assay Description Binding affinity towards rolipram binding site on phosphodiesterase 4 using [3H]rolipram as radioligand in crude rat brain homogenate | Bioorg Med Chem Lett 13: 2355-8 (2003) BindingDB Entry DOI: 10.7270/Q2CZ39CG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4C (Rattus norvegicus) | BDBM50221001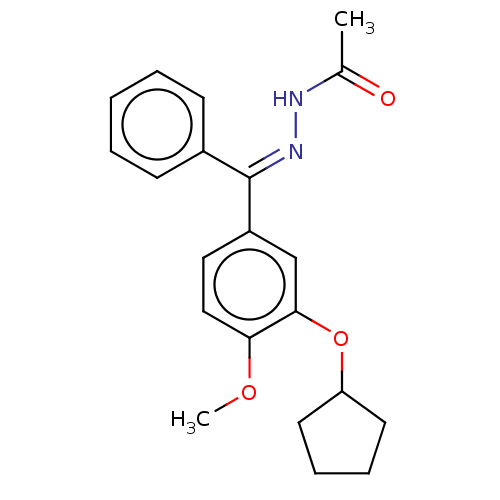 (CHEMBL76257) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
LEAD GENE CO., LTD Curated by ChEMBL | Assay Description Binding affinity towards rolipram binding site on phosphodiesterase 4 using [3H]rolipram as radioligand in crude rat brain homogenate | Bioorg Med Chem Lett 13: 2355-8 (2003) BindingDB Entry DOI: 10.7270/Q2CZ39CG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4C (Rattus norvegicus) | BDBM50220993 (CHEMBL78238) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
LEAD GENE CO., LTD Curated by ChEMBL | Assay Description Binding affinity towards rolipram binding site on phosphodiesterase 4 using [3H]rolipram as radioligand in crude rat brain homogenate | Bioorg Med Chem Lett 13: 2355-8 (2003) BindingDB Entry DOI: 10.7270/Q2CZ39CG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4C (Rattus norvegicus) | BDBM50221007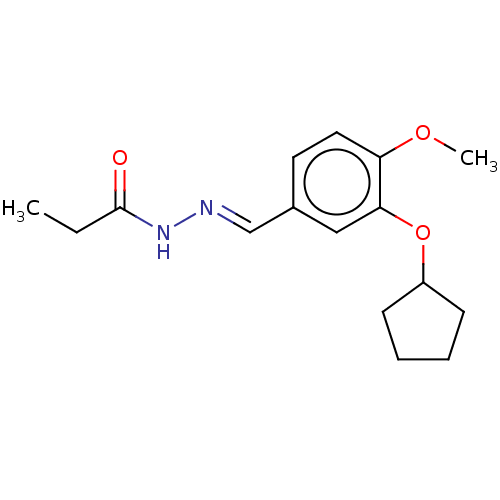 (CHEMBL80258) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
LEAD GENE CO., LTD Curated by ChEMBL | Assay Description Binding affinity towards rolipram binding site on phosphodiesterase 4 using [3H]rolipram as radioligand in crude rat brain homogenate | Bioorg Med Chem Lett 13: 2355-8 (2003) BindingDB Entry DOI: 10.7270/Q2CZ39CG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4C (Rattus norvegicus) | BDBM50221002 (CHEMBL306320) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
LEAD GENE CO., LTD Curated by ChEMBL | Assay Description Binding affinity towards rolipram binding site on phosphodiesterase 4 using [3H]rolipram as radioligand in crude rat brain homogenate | Bioorg Med Chem Lett 13: 2355-8 (2003) BindingDB Entry DOI: 10.7270/Q2CZ39CG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4C (Rattus norvegicus) | BDBM50220994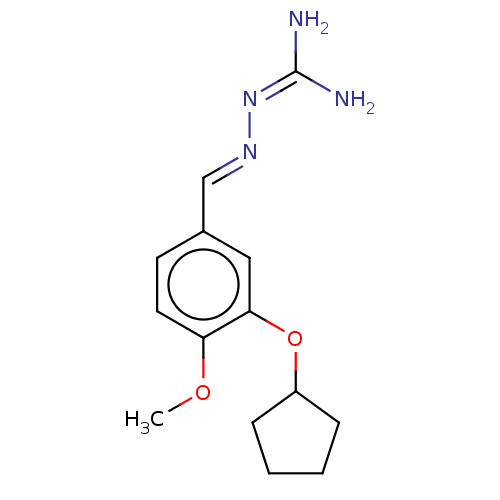 (CHEMBL77177) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
LEAD GENE CO., LTD Curated by ChEMBL | Assay Description Binding affinity towards rolipram binding site on phosphodiesterase 4 using [3H]rolipram as radioligand in crude rat brain homogenate | Bioorg Med Chem Lett 13: 2355-8 (2003) BindingDB Entry DOI: 10.7270/Q2CZ39CG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4C (Rattus norvegicus) | BDBM50221000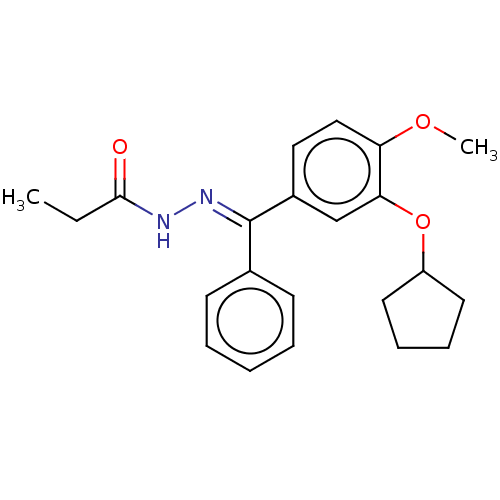 (CHEMBL431962) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
LEAD GENE CO., LTD Curated by ChEMBL | Assay Description Binding affinity towards rolipram binding site on phosphodiesterase 4 using [3H]rolipram as radioligand in crude rat brain homogenate | Bioorg Med Chem Lett 13: 2355-8 (2003) BindingDB Entry DOI: 10.7270/Q2CZ39CG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50164644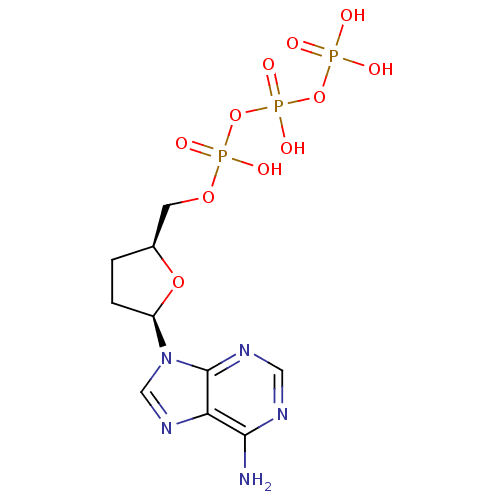 (2',3'-Dideoxyadenosine Triphosphate (Ddatp) | 2',3...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biota, Inc. Curated by ChEMBL | Assay Description Inhibitory constant against HIV-1 reverse transcriptase | J Med Chem 48: 2695-700 (2005) Article DOI: 10.1021/jm040101y BindingDB Entry DOI: 10.7270/Q2G73FG5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50164642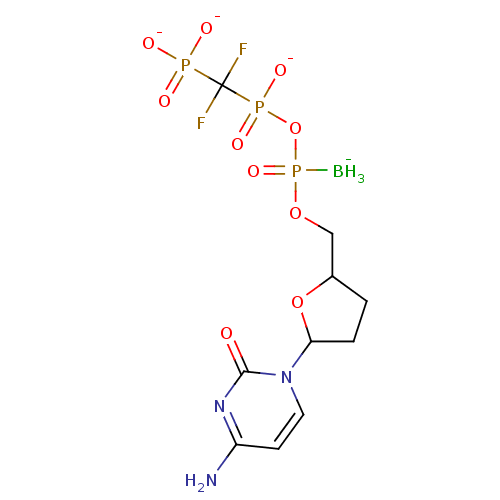 (2',3'-Dideoxynucleoside5'-alpha-P-Borano-beta,gamm...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biota, Inc. Curated by ChEMBL | Assay Description Inhibitory constant against HIV-1 reverse transcriptase | J Med Chem 48: 2695-700 (2005) Article DOI: 10.1021/jm040101y BindingDB Entry DOI: 10.7270/Q2G73FG5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50164639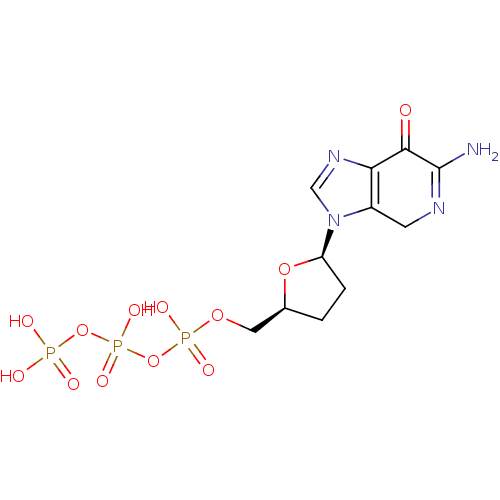 (2'-3'-dideoxy-7-deaza-guaninetriphosphate | CHEMBL...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem | Article PubMed | 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biota, Inc. Curated by ChEMBL | Assay Description Inhibitory constant against HIV-1 reverse transcriptase | J Med Chem 48: 2695-700 (2005) Article DOI: 10.1021/jm040101y BindingDB Entry DOI: 10.7270/Q2G73FG5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50164652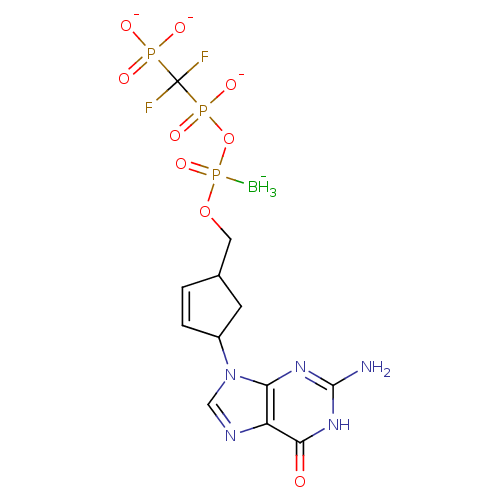 (2',3'-Dideoxynucleoside5'-alpha-P-Borano-beta,gamm...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 34 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biota, Inc. Curated by ChEMBL | Assay Description Inhibitory constant against HIV-1 reverse transcriptase | J Med Chem 48: 2695-700 (2005) Article DOI: 10.1021/jm040101y BindingDB Entry DOI: 10.7270/Q2G73FG5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50164654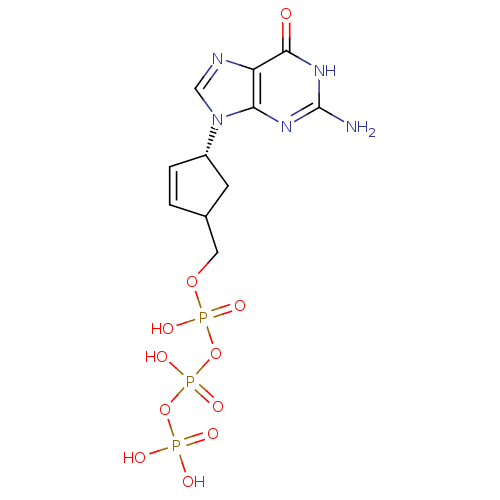 (CHEMBL370031 | [[[4-(2-amino-6-oxo-3,9-dihydropuri...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biota, Inc. Curated by ChEMBL | Assay Description Inhibitory constant against HIV-1 reverse transcriptase | J Med Chem 48: 2695-700 (2005) Article DOI: 10.1021/jm040101y BindingDB Entry DOI: 10.7270/Q2G73FG5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50164637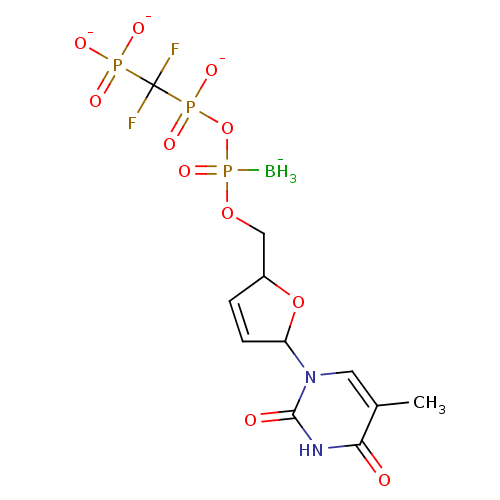 (2',3'-Dideoxynucleoside5'-alpha-P-Borano-beta,gamm...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 45 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biota, Inc. Curated by ChEMBL | Assay Description Inhibitory constant against HIV-1 reverse transcriptase | J Med Chem 48: 2695-700 (2005) Article DOI: 10.1021/jm040101y BindingDB Entry DOI: 10.7270/Q2G73FG5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50164638 (2',3'-Dideoxynucleoside5'-alpha-P-Borano-beta,gamm...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 46 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biota, Inc. Curated by ChEMBL | Assay Description Inhibitory constant against HIV-1 reverse transcriptase | J Med Chem 48: 2695-700 (2005) Article DOI: 10.1021/jm040101y BindingDB Entry DOI: 10.7270/Q2G73FG5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50164647 (2',3'-Dideoxynucleoside5'-alpha-P-Borano-beta,gamm...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 47 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biota, Inc. Curated by ChEMBL | Assay Description Inhibitory constant against HIV-1 reverse transcriptase | J Med Chem 48: 2695-700 (2005) Article DOI: 10.1021/jm040101y BindingDB Entry DOI: 10.7270/Q2G73FG5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50145605 (4-amino-1-[(2R,5S)-5-(hydroxymethyl)tetrahydrofura...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid UniChem Patents Similars | DrugBank Article PubMed | 51 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biota, Inc. Curated by ChEMBL | Assay Description Inhibitory constant against HIV-1 reverse transcriptase | J Med Chem 48: 2695-700 (2005) Article DOI: 10.1021/jm040101y BindingDB Entry DOI: 10.7270/Q2G73FG5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50370655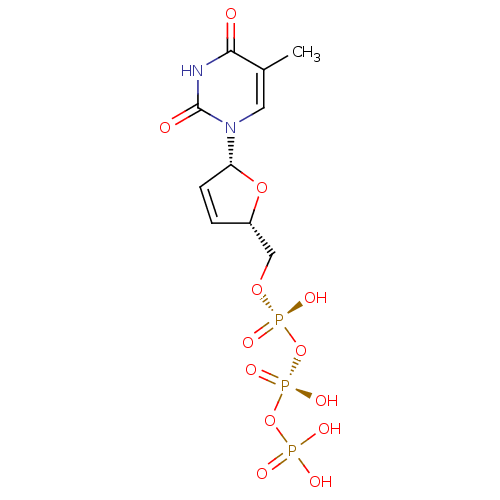 (CHEMBL485652) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 56 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biota, Inc. Curated by ChEMBL | Assay Description Inhibitory constant against HIV-1 reverse transcriptase | J Med Chem 48: 2695-700 (2005) Article DOI: 10.1021/jm040101y BindingDB Entry DOI: 10.7270/Q2G73FG5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50164648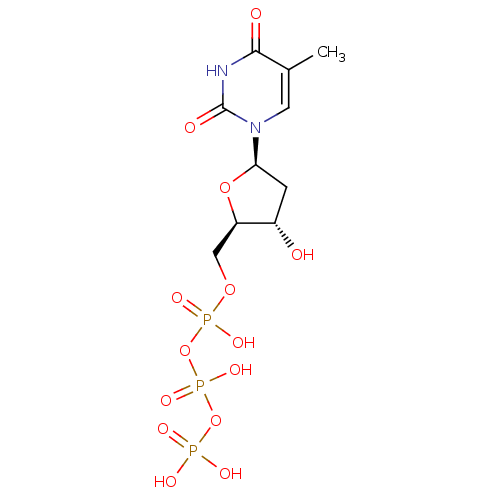 (2'-deoxythymidine triphosphate | 5'-TTP | CHEMBL36...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 61 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biota, Inc. Curated by ChEMBL | Assay Description Inhibitory constant against HIV-1 reverse transcriptase | J Med Chem 48: 2695-700 (2005) Article DOI: 10.1021/jm040101y BindingDB Entry DOI: 10.7270/Q2G73FG5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50164650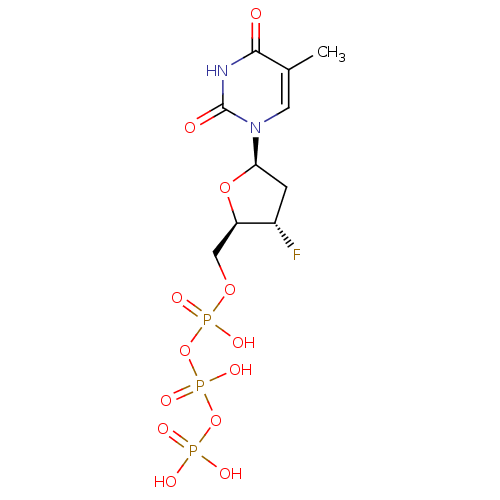 (CHEMBL191725 | [[[3-fluoro-5-(5-methyl-2,4-dioxo-1...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 71 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biota, Inc. Curated by ChEMBL | Assay Description Inhibitory constant against HIV-1 reverse transcriptase | J Med Chem 48: 2695-700 (2005) Article DOI: 10.1021/jm040101y BindingDB Entry DOI: 10.7270/Q2G73FG5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50164653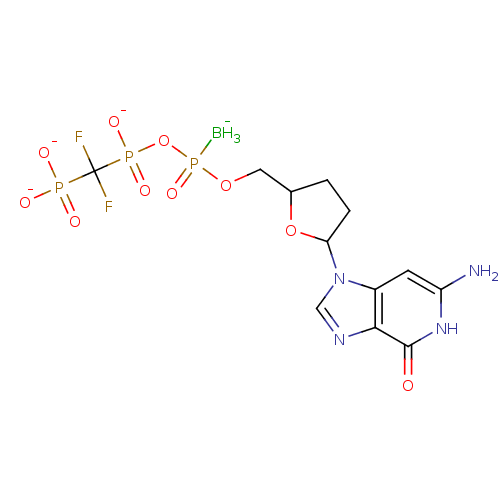 (2',3'-Dideoxynucleoside5'-alpha-P-Borano-beta,gamm...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 71 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biota, Inc. Curated by ChEMBL | Assay Description Inhibitory constant against HIV-1 reverse transcriptase | J Med Chem 48: 2695-700 (2005) Article DOI: 10.1021/jm040101y BindingDB Entry DOI: 10.7270/Q2G73FG5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50370476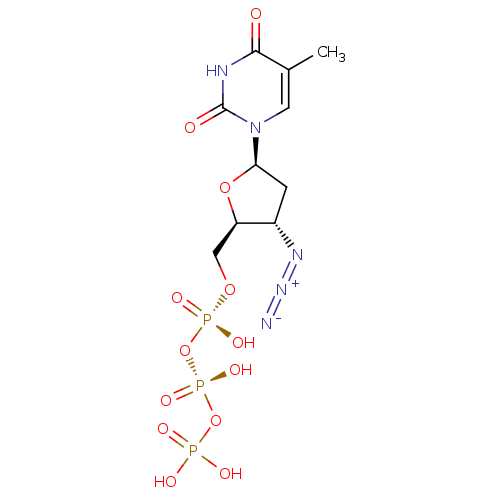 (Combivir | ZIDOVUDINE TRIPHOSPHATE) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 91 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biota, Inc. Curated by ChEMBL | Assay Description Inhibitory constant against HIV-1 reverse transcriptase | J Med Chem 48: 2695-700 (2005) Article DOI: 10.1021/jm040101y BindingDB Entry DOI: 10.7270/Q2G73FG5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50164640 (CHEMBL190609 | [[[[3-azido-5-(5-methyl-2,4-dioxo-1...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 94 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biota, Inc. Curated by ChEMBL | Assay Description Inhibitory constant against HIV-1 reverse transcriptase | J Med Chem 48: 2695-700 (2005) Article DOI: 10.1021/jm040101y BindingDB Entry DOI: 10.7270/Q2G73FG5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50138406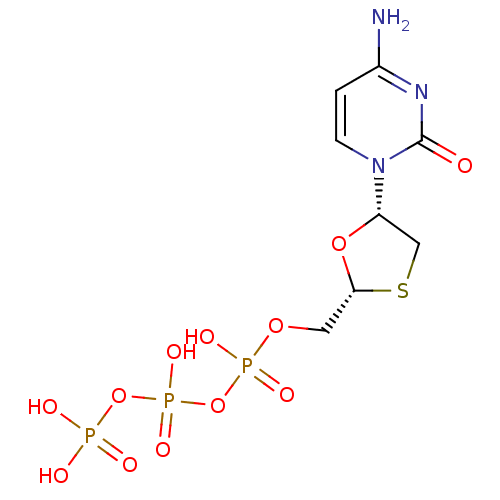 (3TC Triphosphate | CHEMBL1230 | LAMIVUDINE | Lamiv...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 188 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biota, Inc. Curated by ChEMBL | Assay Description Inhibitory constant against HIV-1 reverse transcriptase | J Med Chem 48: 2695-700 (2005) Article DOI: 10.1021/jm040101y BindingDB Entry DOI: 10.7270/Q2G73FG5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50164655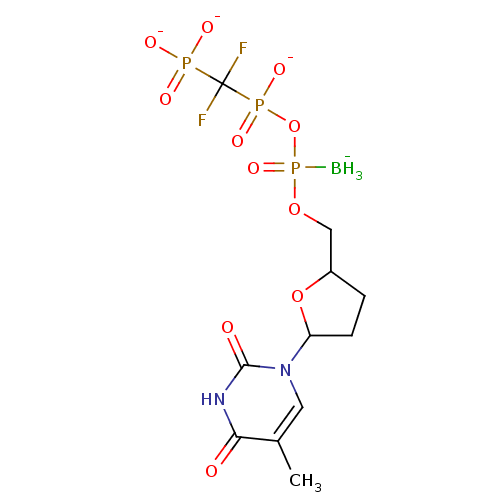 (2',3'-Dideoxynucleoside5'-alpha-P-Borano-beta,gamm...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 236 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biota, Inc. Curated by ChEMBL | Assay Description Inhibitory constant against HIV-1 reverse transcriptase | J Med Chem 48: 2695-700 (2005) Article DOI: 10.1021/jm040101y BindingDB Entry DOI: 10.7270/Q2G73FG5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50164651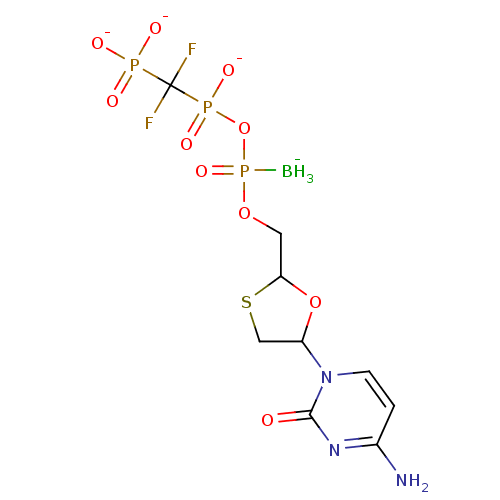 (2',3'-Dideoxynucleoside5'-alpha-P-Borano-beta,gamm...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 314 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biota, Inc. Curated by ChEMBL | Assay Description Inhibitory constant against HIV-1 reverse transcriptase | J Med Chem 48: 2695-700 (2005) Article DOI: 10.1021/jm040101y BindingDB Entry DOI: 10.7270/Q2G73FG5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50164656 (2',3'-Dideoxynucleoside5'-alpha-P-Borano-beta,gamm...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 438 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biota, Inc. Curated by ChEMBL | Assay Description Inhibitory constant against HIV-1 reverse transcriptase | J Med Chem 48: 2695-700 (2005) Article DOI: 10.1021/jm040101y BindingDB Entry DOI: 10.7270/Q2G73FG5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50164645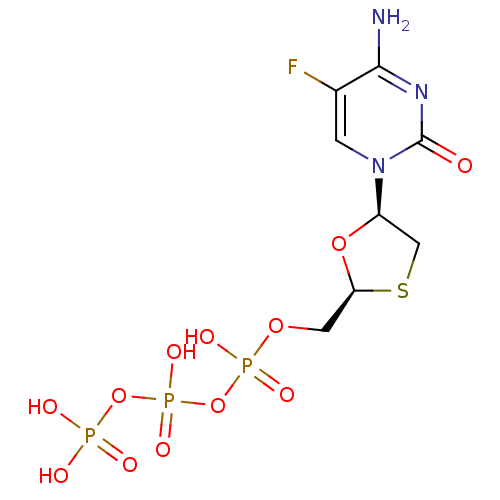 (CHEMBL192771 | [[[5-(4-amino-5-fluoro-2-oxo-1H-pyr...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid PDB UniChem Similars | Article PubMed | 501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biota, Inc. Curated by ChEMBL | Assay Description Inhibitory constant against HIV-1 reverse transcriptase | J Med Chem 48: 2695-700 (2005) Article DOI: 10.1021/jm040101y BindingDB Entry DOI: 10.7270/Q2G73FG5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50164646 (CHEMBL192371 | [[[5-(2,4-dioxo-1H-pyrimidin-1-yl)t...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 545 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biota, Inc. Curated by ChEMBL | Assay Description Inhibitory constant against HIV-1 reverse transcriptase | J Med Chem 48: 2695-700 (2005) Article DOI: 10.1021/jm040101y BindingDB Entry DOI: 10.7270/Q2G73FG5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM16436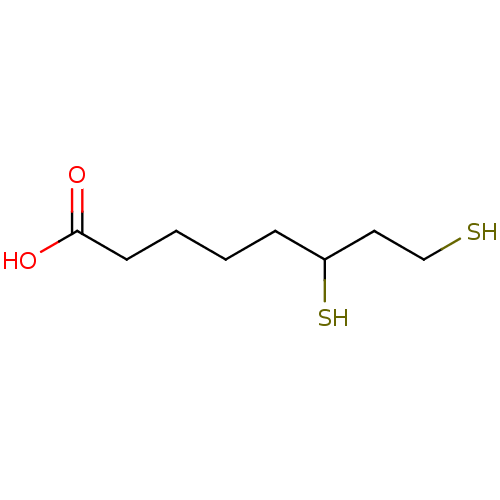 (6,8-disulfanyloctanoic acid | D,L-Dihydrolipoic ac...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 950 | -34.4 | n/a | n/a | n/a | n/a | n/a | 7.0 | 25 |
Medical College of Wiscosin | Assay Description The production of NADPH from NADP+ and benzyl alcohol and xylitol was monitored by an increase in NADPH fluorescence (ex: 340 nm; em: 460 nm) using a... | Bioorg Chem 34: 424-44 (2006) Article DOI: 10.1016/j.bioorg.2006.09.004 BindingDB Entry DOI: 10.7270/Q2SN076W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 [W220Y,C299A] (Homo sapiens (Human)) | BDBM16429 (2,6-Dichlorophenylacetic acid | 2-(2,6-dichlorophe...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical College of Wiscosin | Assay Description The production of NADPH from NADP+ and benzyl alcohol and xylitol was monitored by an increase in NADPH fluorescence (ex: 340 nm; em: 460 nm) using a... | Bioorg Chem 34: 424-44 (2006) Article DOI: 10.1016/j.bioorg.2006.09.004 BindingDB Entry DOI: 10.7270/Q2SN076W | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM16417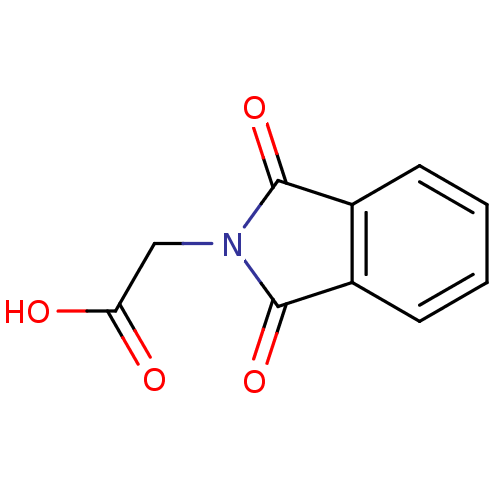 ((1,3-dioxo-1,3-dihydro-2H-isoindol-2-yl)acetic aci...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.50E+3 | -33.2 | n/a | n/a | n/a | n/a | n/a | 7.0 | 25 |
Medical College of Wiscosin | Assay Description The production of NADPH from NADP+ and benzyl alcohol and xylitol was monitored by an increase in NADPH fluorescence (ex: 340 nm; em: 460 nm) using a... | Bioorg Chem 34: 424-44 (2006) Article DOI: 10.1016/j.bioorg.2006.09.004 BindingDB Entry DOI: 10.7270/Q2SN076W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50164643 (({[({[5-(4-amino-5-fluoro-2-oxo-1,2-dihydropyrimid...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.77E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biota, Inc. Curated by ChEMBL | Assay Description Inhibitory constant against HIV-1 reverse transcriptase | J Med Chem 48: 2695-700 (2005) Article DOI: 10.1021/jm040101y BindingDB Entry DOI: 10.7270/Q2G73FG5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM16415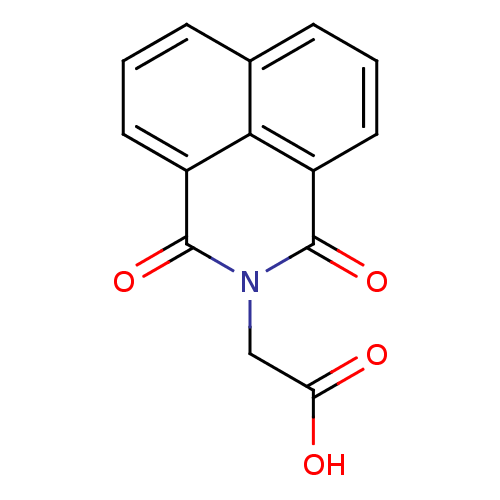 ((1,3-dioxo-1H-benzo[de]isoquinolin-2(3H)-yl)acetic...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 2.00E+3 | -32.5 | n/a | n/a | n/a | n/a | n/a | 7.0 | 25 |
Medical College of Wiscosin | Assay Description The production of NADPH from NADP+ and benzyl alcohol and xylitol was monitored by an increase in NADPH fluorescence (ex: 340 nm; em: 460 nm) using a... | Bioorg Chem 34: 424-44 (2006) Article DOI: 10.1016/j.bioorg.2006.09.004 BindingDB Entry DOI: 10.7270/Q2SN076W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM16439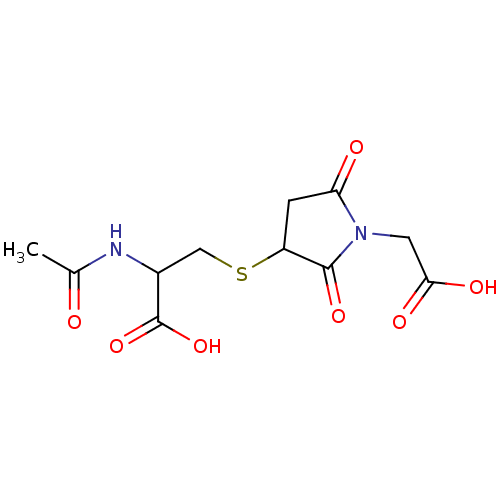 (3-{[1-(carboxymethyl)-2,5-dioxopyrrolidin-3-yl]sul...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical College of Wiscosin | Assay Description The production of NADPH from NADP+ and benzyl alcohol and xylitol was monitored by an increase in NADPH fluorescence (ex: 340 nm; em: 460 nm) using a... | Bioorg Chem 34: 424-44 (2006) Article DOI: 10.1016/j.bioorg.2006.09.004 BindingDB Entry DOI: 10.7270/Q2SN076W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM16428 (2,6-Difluorophenylacetic acid | 2-(2,6-difluorophe...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 2.50E+3 | -32.0 | n/a | n/a | n/a | n/a | n/a | 7.0 | 25 |
Medical College of Wiscosin | Assay Description The production of NADPH from NADP+ and benzyl alcohol and xylitol was monitored by an increase in NADPH fluorescence (ex: 340 nm; em: 460 nm) using a... | Bioorg Chem 34: 424-44 (2006) Article DOI: 10.1016/j.bioorg.2006.09.004 BindingDB Entry DOI: 10.7270/Q2SN076W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM16426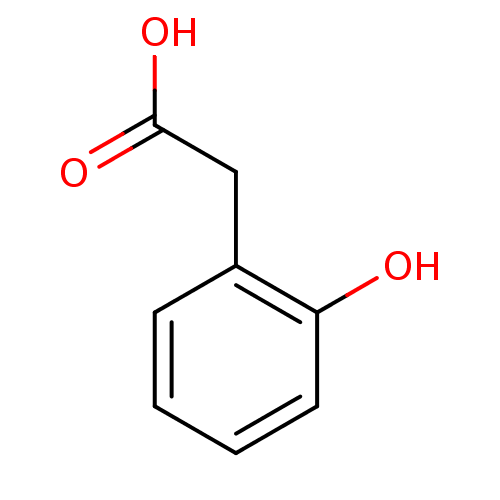 (2-(2-hydroxyphenyl)acetic acid | 2-Hydroxyphenylac...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents | PDB Article PubMed | 3.50E+3 | -31.1 | n/a | n/a | n/a | n/a | n/a | 7.0 | 25 |
Medical College of Wiscosin | Assay Description The production of NADPH from NADP+ and benzyl alcohol and xylitol was monitored by an increase in NADPH fluorescence (ex: 340 nm; em: 460 nm) using a... | Bioorg Chem 34: 424-44 (2006) Article DOI: 10.1016/j.bioorg.2006.09.004 BindingDB Entry DOI: 10.7270/Q2SN076W | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM16441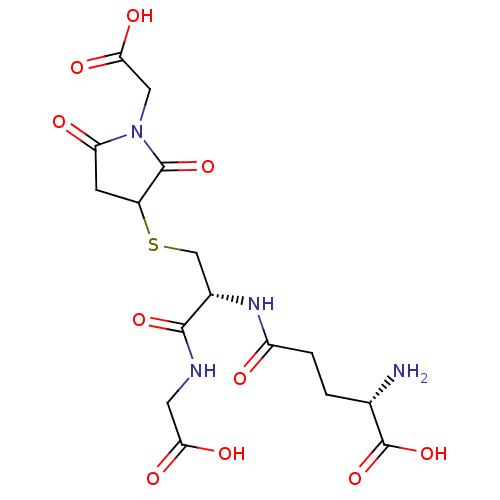 ((2S)-2-amino-4-{[(1R)-2-{[1-(carboxymethyl)-2,5-di...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | 3.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical College of Wiscosin | Assay Description The production of NADPH from NADP+ and benzyl alcohol and xylitol was monitored by an increase in NADPH fluorescence (ex: 340 nm; em: 460 nm) using a... | Bioorg Chem 34: 424-44 (2006) Article DOI: 10.1016/j.bioorg.2006.09.004 BindingDB Entry DOI: 10.7270/Q2SN076W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM16429 (2,6-Dichlorophenylacetic acid | 2-(2,6-dichlorophe...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 4.40E+3 | -30.6 | n/a | n/a | n/a | n/a | n/a | 7.0 | 25 |
Medical College of Wiscosin | Assay Description The production of NADPH from NADP+ and benzyl alcohol and xylitol was monitored by an increase in NADPH fluorescence (ex: 340 nm; em: 460 nm) using a... | Bioorg Chem 34: 424-44 (2006) Article DOI: 10.1016/j.bioorg.2006.09.004 BindingDB Entry DOI: 10.7270/Q2SN076W | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM16431 (2-(naphthalen-2-yl)acetic acid | 2-Napthylacetic a...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 6.20E+3 | -29.7 | n/a | n/a | n/a | n/a | n/a | 7.0 | 25 |
Medical College of Wiscosin | Assay Description The production of NADPH from NADP+ and benzyl alcohol and xylitol was monitored by an increase in NADPH fluorescence (ex: 340 nm; em: 460 nm) using a... | Bioorg Chem 34: 424-44 (2006) Article DOI: 10.1016/j.bioorg.2006.09.004 BindingDB Entry DOI: 10.7270/Q2SN076W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM16440 (2-{3-[(2-amino-3-methoxy-3-oxopropyl)sulfanyl]-2,5...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | 6.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical College of Wiscosin | Assay Description The production of NADPH from NADP+ and benzyl alcohol and xylitol was monitored by an increase in NADPH fluorescence (ex: 340 nm; em: 460 nm) using a... | Bioorg Chem 34: 424-44 (2006) Article DOI: 10.1016/j.bioorg.2006.09.004 BindingDB Entry DOI: 10.7270/Q2SN076W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 [W220Y,C299A] (Homo sapiens (Human)) | BDBM16415 ((1,3-dioxo-1H-benzo[de]isoquinolin-2(3H)-yl)acetic...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB Article PubMed | 7.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical College of Wiscosin | Assay Description The production of NADPH from NADP+ and benzyl alcohol and xylitol was monitored by an increase in NADPH fluorescence (ex: 340 nm; em: 460 nm) using a... | Bioorg Chem 34: 424-44 (2006) Article DOI: 10.1016/j.bioorg.2006.09.004 BindingDB Entry DOI: 10.7270/Q2SN076W | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Displayed 1 to 50 (of 429 total ) | Next | Last >> |