

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50218166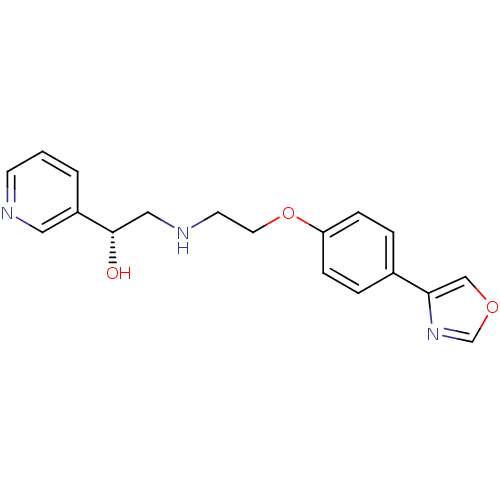 ((R)-2-(2-(4-(oxazol-4-yl)phenoxy)ethylamino)-1-(py...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [125I]iodocyanopindolol from human adrenergic beta-2 receptor expressed in CHO cells | Bioorg Med Chem Lett 17: 5245-50 (2007) Article DOI: 10.1016/j.bmcl.2007.06.072 BindingDB Entry DOI: 10.7270/Q2MK6CMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50218165 ((R)-1-(pyridin-3-yl)-2-(2-(4-(thiazol-4-yl)phenoxy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [125I]iodocyanopindolol from human adrenergic beta-2 receptor expressed in CHO cells | Bioorg Med Chem Lett 17: 5245-50 (2007) Article DOI: 10.1016/j.bmcl.2007.06.072 BindingDB Entry DOI: 10.7270/Q2MK6CMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50218173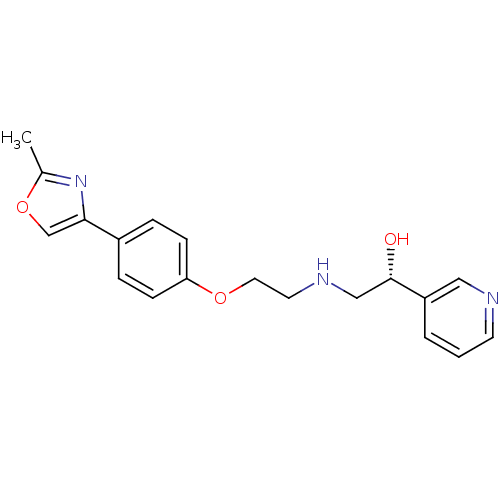 ((R)-2-(2-(4-(2-methyloxazol-4-yl)phenoxy)ethylamin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [125I]iodocyanopindolol from human adrenergic beta-2 receptor expressed in CHO cells | Bioorg Med Chem Lett 17: 5245-50 (2007) Article DOI: 10.1016/j.bmcl.2007.06.072 BindingDB Entry DOI: 10.7270/Q2MK6CMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50218170 ((R)-2-(2-(4-(2-(hydroxymethyl)oxazol-4-yl)phenoxy)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [125I]iodocyanopindolol from human adrenergic beta-2 receptor expressed in CHO cells | Bioorg Med Chem Lett 17: 5245-50 (2007) Article DOI: 10.1016/j.bmcl.2007.06.072 BindingDB Entry DOI: 10.7270/Q2MK6CMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-1 adrenergic receptor (Homo sapiens (Human)) | BDBM50218180 ((R)-2-(2-(4-(2-ethylthiazol-4-yl)phenoxy)ethylamin...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [125I]iodocyanopindolol from human adrenergic beta-1 receptor expressed in CHO cells | Bioorg Med Chem Lett 17: 5245-50 (2007) Article DOI: 10.1016/j.bmcl.2007.06.072 BindingDB Entry DOI: 10.7270/Q2MK6CMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50218168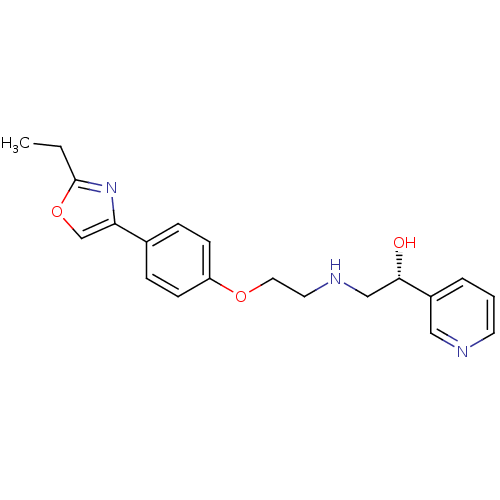 ((R)-2-(2-(4-(2-ethyloxazol-4-yl)phenoxy)ethylamino...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [125I]iodocyanopindolol from human adrenergic beta-2 receptor expressed in CHO cells | Bioorg Med Chem Lett 17: 5245-50 (2007) Article DOI: 10.1016/j.bmcl.2007.06.072 BindingDB Entry DOI: 10.7270/Q2MK6CMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50218167 ((R)-2-(2-(4-(2-isopropyloxazol-4-yl)phenoxy)ethyla...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 49 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [125I]iodocyanopindolol from human adrenergic beta-2 receptor expressed in CHO cells | Bioorg Med Chem Lett 17: 5245-50 (2007) Article DOI: 10.1016/j.bmcl.2007.06.072 BindingDB Entry DOI: 10.7270/Q2MK6CMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-1 adrenergic receptor (Homo sapiens (Human)) | BDBM50218177 ((R)-2-(2-(4-(2-(benzyloxymethyl)oxazol-4-yl)phenox...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [125I]iodocyanopindolol from human adrenergic beta-1 receptor expressed in CHO cells | Bioorg Med Chem Lett 17: 5245-50 (2007) Article DOI: 10.1016/j.bmcl.2007.06.072 BindingDB Entry DOI: 10.7270/Q2MK6CMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-1 adrenergic receptor (Homo sapiens (Human)) | BDBM50218176 ((R)-1-(pyridin-3-yl)-2-(2-(4-(2-(pyridin-4-yl)thia...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 97 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [125I]iodocyanopindolol from human adrenergic beta-1 receptor expressed in CHO cells | Bioorg Med Chem Lett 17: 5245-50 (2007) Article DOI: 10.1016/j.bmcl.2007.06.072 BindingDB Entry DOI: 10.7270/Q2MK6CMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-1 adrenergic receptor (Homo sapiens (Human)) | BDBM50218164 ((R)-2-(2-(4-(2-methylthiazol-4-yl)phenoxy)ethylami...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [125I]iodocyanopindolol from human adrenergic beta-1 receptor expressed in CHO cells | Bioorg Med Chem Lett 17: 5245-50 (2007) Article DOI: 10.1016/j.bmcl.2007.06.072 BindingDB Entry DOI: 10.7270/Q2MK6CMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-1 adrenergic receptor (Homo sapiens (Human)) | BDBM50218182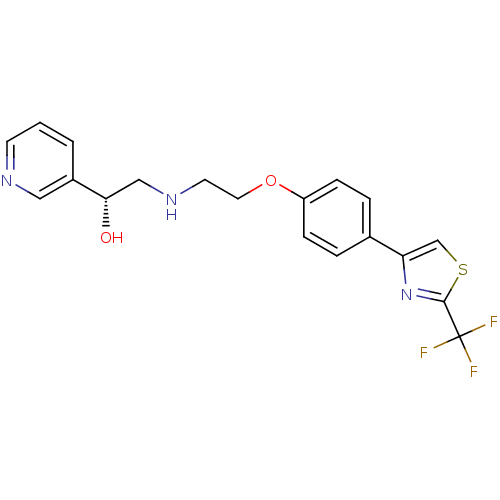 ((R)-1-(pyridin-3-yl)-2-(2-(4-(2-(trifluoromethyl)t...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [125I]iodocyanopindolol from human adrenergic beta-1 receptor expressed in CHO cells | Bioorg Med Chem Lett 17: 5245-50 (2007) Article DOI: 10.1016/j.bmcl.2007.06.072 BindingDB Entry DOI: 10.7270/Q2MK6CMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-1 adrenergic receptor (Homo sapiens (Human)) | BDBM50218185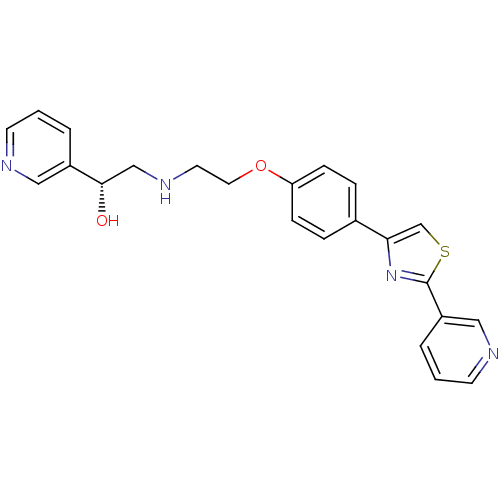 ((1R)-1-(pyridin-3-yl)-2-(2-(4-(2-(pyridin-3-yl)thi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [125I]iodocyanopindolol from human adrenergic beta-1 receptor expressed in CHO cells | Bioorg Med Chem Lett 17: 5245-50 (2007) Article DOI: 10.1016/j.bmcl.2007.06.072 BindingDB Entry DOI: 10.7270/Q2MK6CMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50218164 ((R)-2-(2-(4-(2-methylthiazol-4-yl)phenoxy)ethylami...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [125I]iodocyanopindolol from human adrenergic beta-2 receptor expressed in CHO cells | Bioorg Med Chem Lett 17: 5245-50 (2007) Article DOI: 10.1016/j.bmcl.2007.06.072 BindingDB Entry DOI: 10.7270/Q2MK6CMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-1 adrenergic receptor (Homo sapiens (Human)) | BDBM50218172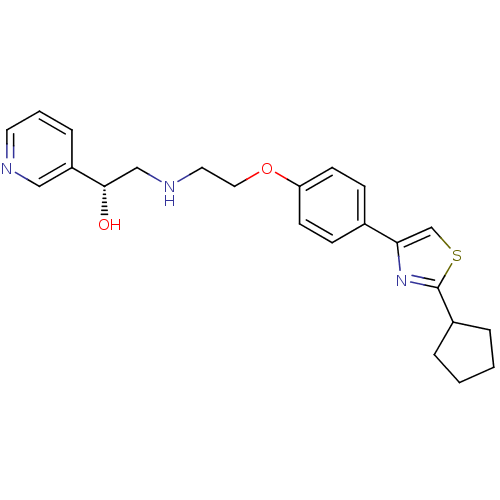 ((R)-2-(2-(4-(2-cyclopentylthiazol-4-yl)phenoxy)eth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [125I]iodocyanopindolol from human adrenergic beta-1 receptor expressed in CHO cells | Bioorg Med Chem Lett 17: 5245-50 (2007) Article DOI: 10.1016/j.bmcl.2007.06.072 BindingDB Entry DOI: 10.7270/Q2MK6CMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-1 adrenergic receptor (Homo sapiens (Human)) | BDBM50218165 ((R)-1-(pyridin-3-yl)-2-(2-(4-(thiazol-4-yl)phenoxy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [125I]iodocyanopindolol from human adrenergic beta-1 receptor expressed in CHO cells | Bioorg Med Chem Lett 17: 5245-50 (2007) Article DOI: 10.1016/j.bmcl.2007.06.072 BindingDB Entry DOI: 10.7270/Q2MK6CMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-1 adrenergic receptor (Homo sapiens (Human)) | BDBM50218174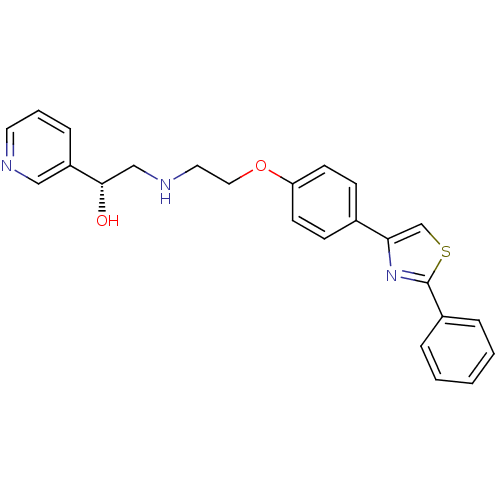 ((R)-2-(2-(4-(2-phenylthiazol-4-yl)phenoxy)ethylami...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [125I]iodocyanopindolol from human adrenergic beta-1 receptor expressed in CHO cells | Bioorg Med Chem Lett 17: 5245-50 (2007) Article DOI: 10.1016/j.bmcl.2007.06.072 BindingDB Entry DOI: 10.7270/Q2MK6CMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-1 adrenergic receptor (Homo sapiens (Human)) | BDBM50218181 ((R)-2-(2-(4-(5-methyloxazol-4-yl)phenoxy)ethylamin...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [125I]iodocyanopindolol from human adrenergic beta-1 receptor expressed in CHO cells | Bioorg Med Chem Lett 17: 5245-50 (2007) Article DOI: 10.1016/j.bmcl.2007.06.072 BindingDB Entry DOI: 10.7270/Q2MK6CMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-1 adrenergic receptor (Homo sapiens (Human)) | BDBM50218166 ((R)-2-(2-(4-(oxazol-4-yl)phenoxy)ethylamino)-1-(py...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [125I]iodocyanopindolol from human adrenergic beta-1 receptor expressed in CHO cells | Bioorg Med Chem Lett 17: 5245-50 (2007) Article DOI: 10.1016/j.bmcl.2007.06.072 BindingDB Entry DOI: 10.7270/Q2MK6CMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-1 adrenergic receptor (Homo sapiens (Human)) | BDBM50218171 ((R)-2-(2-(4-(2-(methoxymethyl)oxazol-4-yl)phenoxy)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [125I]iodocyanopindolol from human adrenergic beta-1 receptor expressed in CHO cells | Bioorg Med Chem Lett 17: 5245-50 (2007) Article DOI: 10.1016/j.bmcl.2007.06.072 BindingDB Entry DOI: 10.7270/Q2MK6CMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-1 adrenergic receptor (Homo sapiens (Human)) | BDBM50218170 ((R)-2-(2-(4-(2-(hydroxymethyl)oxazol-4-yl)phenoxy)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [125I]iodocyanopindolol from human adrenergic beta-1 receptor expressed in CHO cells | Bioorg Med Chem Lett 17: 5245-50 (2007) Article DOI: 10.1016/j.bmcl.2007.06.072 BindingDB Entry DOI: 10.7270/Q2MK6CMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-1 adrenergic receptor (Homo sapiens (Human)) | BDBM50218167 ((R)-2-(2-(4-(2-isopropyloxazol-4-yl)phenoxy)ethyla...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [125I]iodocyanopindolol from human adrenergic beta-1 receptor expressed in CHO cells | Bioorg Med Chem Lett 17: 5245-50 (2007) Article DOI: 10.1016/j.bmcl.2007.06.072 BindingDB Entry DOI: 10.7270/Q2MK6CMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-1 adrenergic receptor (Homo sapiens (Human)) | BDBM50218168 ((R)-2-(2-(4-(2-ethyloxazol-4-yl)phenoxy)ethylamino...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [125I]iodocyanopindolol from human adrenergic beta-1 receptor expressed in CHO cells | Bioorg Med Chem Lett 17: 5245-50 (2007) Article DOI: 10.1016/j.bmcl.2007.06.072 BindingDB Entry DOI: 10.7270/Q2MK6CMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-1 adrenergic receptor (Homo sapiens (Human)) | BDBM50218173 ((R)-2-(2-(4-(2-methyloxazol-4-yl)phenoxy)ethylamin...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [125I]iodocyanopindolol from human adrenergic beta-1 receptor expressed in CHO cells | Bioorg Med Chem Lett 17: 5245-50 (2007) Article DOI: 10.1016/j.bmcl.2007.06.072 BindingDB Entry DOI: 10.7270/Q2MK6CMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50218174 ((R)-2-(2-(4-(2-phenylthiazol-4-yl)phenoxy)ethylami...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [125I]iodocyanopindolol from human adrenergic beta-2 receptor expressed in CHO cells | Bioorg Med Chem Lett 17: 5245-50 (2007) Article DOI: 10.1016/j.bmcl.2007.06.072 BindingDB Entry DOI: 10.7270/Q2MK6CMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-1 adrenergic receptor (Homo sapiens (Human)) | BDBM50218179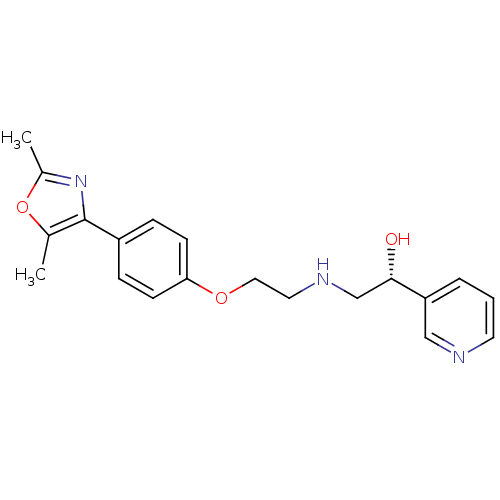 ((R)-2-(2-(4-(2,5-dimethyloxazol-4-yl)phenoxy)ethyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.55E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [125I]iodocyanopindolol from human adrenergic beta-1 receptor expressed in CHO cells | Bioorg Med Chem Lett 17: 5245-50 (2007) Article DOI: 10.1016/j.bmcl.2007.06.072 BindingDB Entry DOI: 10.7270/Q2MK6CMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM16315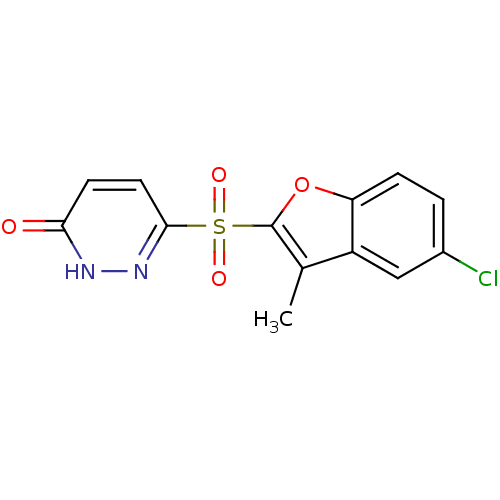 (6-[(5-chloro-3-methyl-1-benzofuran-2-)sulfonyl]-2,...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 0.840 | n/a | n/a | n/a | n/a | 7.0 | 24 |
Pfizer | Assay Description The activity of the enzyme was determined spectrophotometrically by monitoring the change in absorbance at 340 nm, which is due to the disappearance ... | J Med Chem 46: 2283-6 (2003) Article DOI: 10.1021/jm034065z BindingDB Entry DOI: 10.7270/Q2NV9GGM | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM16315 (6-[(5-chloro-3-methyl-1-benzofuran-2-)sulfonyl]-2,...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer | Assay Description The activity of the enzyme was determined spectrophotometrically by monitoring the change in absorbance at 340 nm, which is due to the disappearance ... | J Med Chem 48: 6326-39 (2005) Article DOI: 10.1021/jm050462t BindingDB Entry DOI: 10.7270/Q2RN363Z | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM16633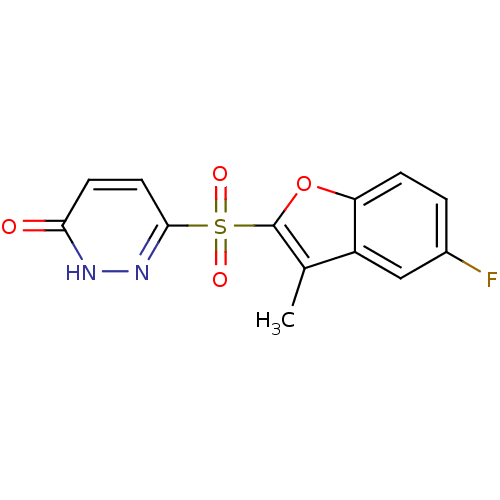 (6-[(5-fluoro-3-methyl-1-benzofuran-2-)sulfonyl]-2,...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer | Assay Description The activity of the enzyme was determined spectrophotometrically by monitoring the change in absorbance at 340 nm, which is due to the disappearance ... | J Med Chem 48: 6326-39 (2005) Article DOI: 10.1021/jm050462t BindingDB Entry DOI: 10.7270/Q2RN363Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Menin (Homo sapiens (Human)) | BDBM50455873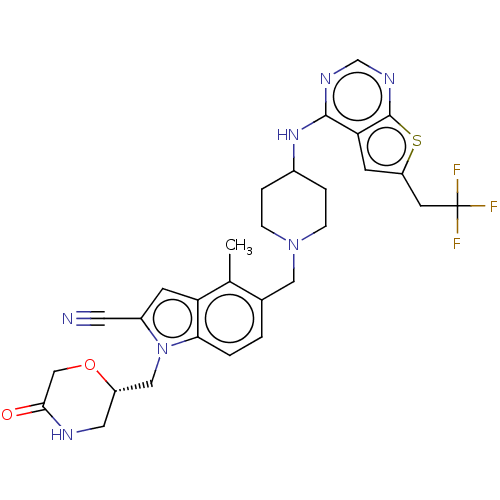 (CHEMBL4216801) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Displacement of fluorescein-labeled MLL4-43 from full length human menin expressed in Escherichia coli BL21 (DE3) cells after 3 hrs by fluorescence p... | J Med Chem 61: 4832-4850 (2018) Article DOI: 10.1021/acs.jmedchem.8b00071 BindingDB Entry DOI: 10.7270/Q2Z03BRF | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM16452 ((4-oxo-3-{[5-(trifluoromethyl)-1,3-benzothiazol-2-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | 7.0 | 24 |
Pfizer | Assay Description The activity of the enzyme was determined spectrophotometrically by monitoring the change in absorbance at 340 nm, which is due to the disappearance ... | J Med Chem 46: 2283-6 (2003) Article DOI: 10.1021/jm034065z BindingDB Entry DOI: 10.7270/Q2NV9GGM | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Sorbitol dehydrogenase (Homo sapiens (Human)) | BDBM50118706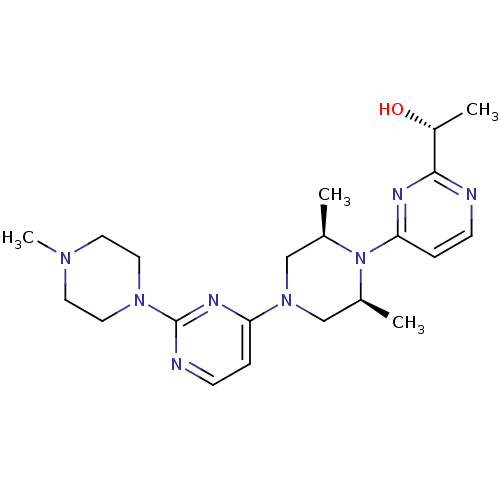 ((S)-1-(4-{2,6-Dimethyl-4-[2-(4-methyl-piperazin-1-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Concentration required for 50% for in vitro activity against human SDH (sorbitol dehydrogenase) | J Med Chem 45: 4398-401 (2002) BindingDB Entry DOI: 10.7270/Q2V69HZM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sorbitol dehydrogenase (Homo sapiens (Human)) | BDBM50118710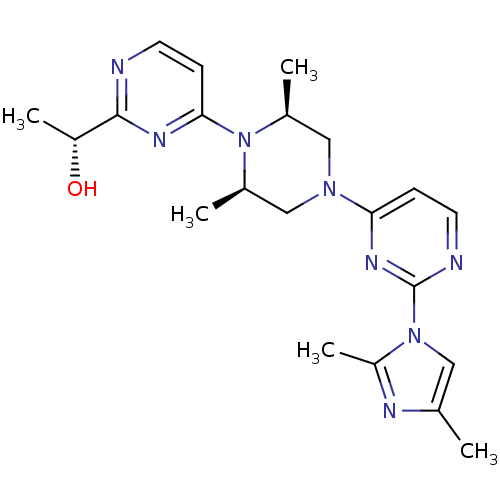 (1-(4-{4-[2-(2,4-Dimethyl-imidazol-1-yl)-pyrimidin-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Concentration required for 50% for in vitro activity against human SDH (sorbitol dehydrogenase) | J Med Chem 45: 4398-401 (2002) BindingDB Entry DOI: 10.7270/Q2V69HZM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM16634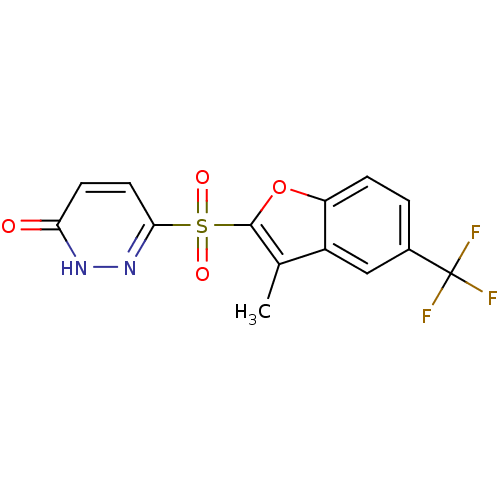 (6-{[3-methyl-5-(trifluoromethyl)-1-benzofuran-2-]s...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer | Assay Description The activity of the enzyme was determined spectrophotometrically by monitoring the change in absorbance at 340 nm, which is due to the disappearance ... | J Med Chem 48: 6326-39 (2005) Article DOI: 10.1021/jm050462t BindingDB Entry DOI: 10.7270/Q2RN363Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sorbitol dehydrogenase (Rattus norvegicus) | BDBM50118709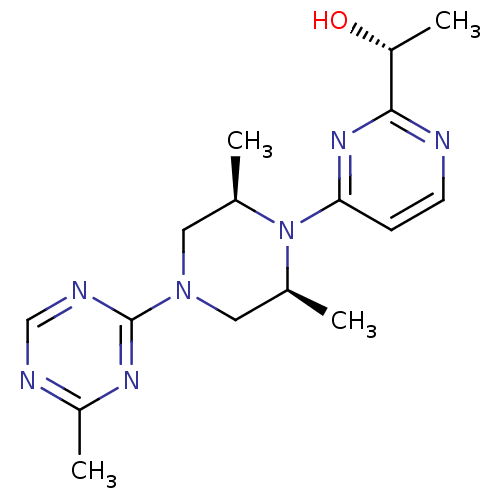 (1-{4-[2,6-Dimethyl-4-(4-methyl-[1,3,5]triazin-2-yl...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Concentration required for 50% in vitro activity against rat SDH (sorbitol dehydrogenase) | J Med Chem 45: 4398-401 (2002) BindingDB Entry DOI: 10.7270/Q2V69HZM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM16636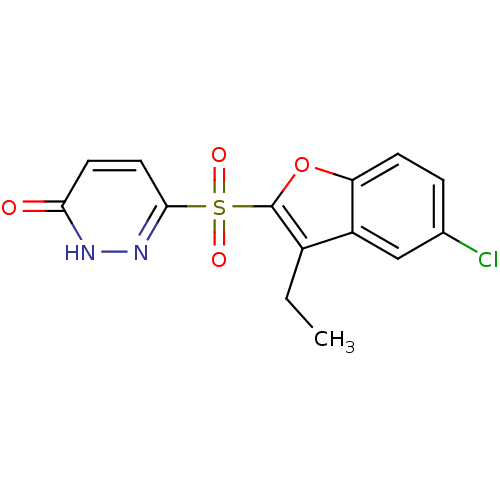 (6-[(5-chloro-3-ethyl-1-benzofuran-2-)sulfonyl]-2,3...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer | Assay Description The activity of the enzyme was determined spectrophotometrically by monitoring the change in absorbance at 340 nm, which is due to the disappearance ... | J Med Chem 48: 6326-39 (2005) Article DOI: 10.1021/jm050462t BindingDB Entry DOI: 10.7270/Q2RN363Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sorbitol dehydrogenase (Rattus norvegicus) | BDBM50118706 ((S)-1-(4-{2,6-Dimethyl-4-[2-(4-methyl-piperazin-1-...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Concentration required for 50% in vitro activity against rat SDH (sorbitol dehydrogenase) | J Med Chem 45: 4398-401 (2002) BindingDB Entry DOI: 10.7270/Q2V69HZM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sorbitol dehydrogenase (Rattus norvegicus) | BDBM50118710 (1-(4-{4-[2-(2,4-Dimethyl-imidazol-1-yl)-pyrimidin-...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Concentration required for 50% in vitro activity against rat SDH (sorbitol dehydrogenase) | J Med Chem 45: 4398-401 (2002) BindingDB Entry DOI: 10.7270/Q2V69HZM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sorbitol dehydrogenase (Homo sapiens (Human)) | BDBM50118708 (1-{4-[2,6-Dimethyl-4-(4-phenyl-[1,3,5]triazin-2-yl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Concentration required for 50% for in vitro activity against human SDH (sorbitol dehydrogenase) | J Med Chem 45: 4398-401 (2002) BindingDB Entry DOI: 10.7270/Q2V69HZM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sorbitol dehydrogenase (Homo sapiens (Human)) | BDBM50118709 (1-{4-[2,6-Dimethyl-4-(4-methyl-[1,3,5]triazin-2-yl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Concentration required for 50% for in vitro activity against human SDH (sorbitol dehydrogenase) | J Med Chem 45: 4398-401 (2002) BindingDB Entry DOI: 10.7270/Q2V69HZM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Menin (Homo sapiens (Human)) | BDBM50455856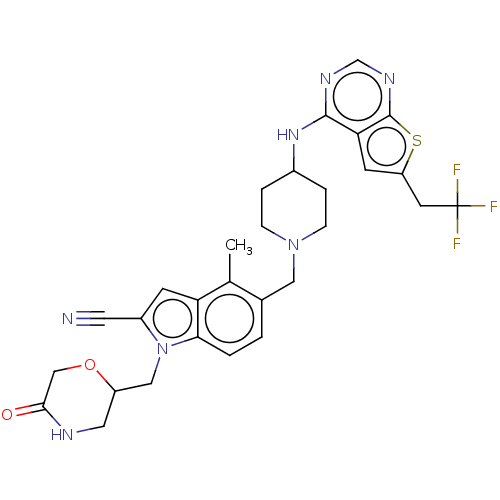 (CHEMBL4218724) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 7.5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Displacement of fluorescein-labeled MLL4-43 from full length human menin expressed in Escherichia coli BL21 (DE3) cells after 3 hrs by fluorescence p... | J Med Chem 61: 4832-4850 (2018) Article DOI: 10.1021/acs.jmedchem.8b00071 BindingDB Entry DOI: 10.7270/Q2Z03BRF | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Sorbitol dehydrogenase (Rattus norvegicus) | BDBM50118711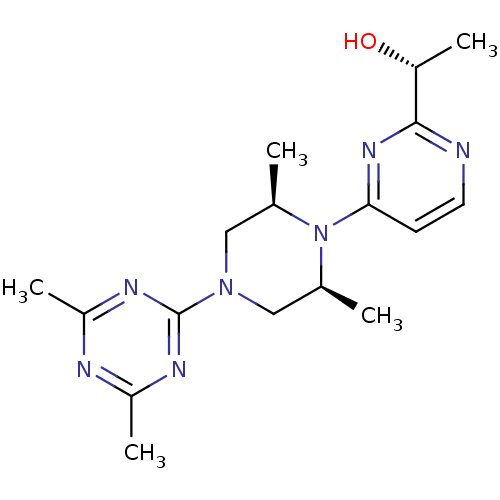 (1-{4-[4-(4,6-Dimethyl-[1,3,5]triazin-2-yl)-2,6-dim...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Concentration required for 50% in vitro activity against rat SDH (sorbitol dehydrogenase) | J Med Chem 45: 4398-401 (2002) BindingDB Entry DOI: 10.7270/Q2V69HZM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sorbitol dehydrogenase (Rattus norvegicus) | BDBM50118711 (1-{4-[4-(4,6-Dimethyl-[1,3,5]triazin-2-yl)-2,6-dim...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Concentration required for 50% in vitro activity against rat SDH (sorbitol dehydrogenase) | J Med Chem 45: 4398-401 (2002) BindingDB Entry DOI: 10.7270/Q2V69HZM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Menin (Homo sapiens (Human)) | BDBM50455865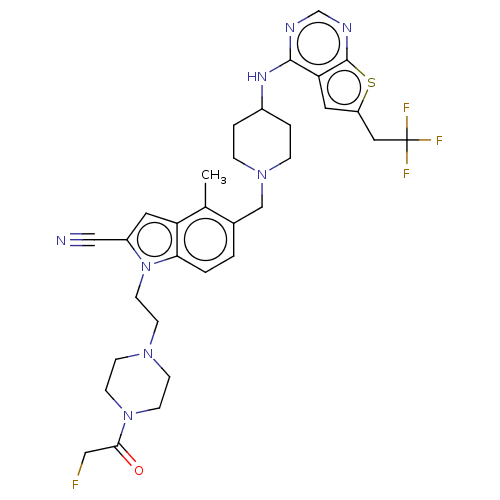 (CHEMBL4211318) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Displacement of fluorescein-labeled MLL4-43 from full length human menin expressed in Escherichia coli BL21 (DE3) cells after 3 hrs by fluorescence p... | J Med Chem 61: 4832-4850 (2018) Article DOI: 10.1021/acs.jmedchem.8b00071 BindingDB Entry DOI: 10.7270/Q2Z03BRF | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Sorbitol dehydrogenase (Homo sapiens (Human)) | BDBM50113496 ((S)-1-(4-{(3S,5R)-4-[2-((R)-1-Hydroxy-ethyl)-pyrim...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Concentration required for 50% in vitro activity against human SDH (sorbitol dehydrogenase) | J Med Chem 45: 4398-401 (2002) BindingDB Entry DOI: 10.7270/Q2V69HZM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sorbitol dehydrogenase (Rattus norvegicus) | BDBM50118708 (1-{4-[2,6-Dimethyl-4-(4-phenyl-[1,3,5]triazin-2-yl...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Concentration required for 50% in vitro activity against rat SDH (sorbitol dehydrogenase) | J Med Chem 45: 4398-401 (2002) BindingDB Entry DOI: 10.7270/Q2V69HZM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sorbitol dehydrogenase (Rattus norvegicus) | BDBM50113496 ((S)-1-(4-{(3S,5R)-4-[2-((R)-1-Hydroxy-ethyl)-pyrim...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Concentration required for 50% in vitro activity against rat SDH (sorbitol dehydrogenase) | J Med Chem 45: 4398-401 (2002) BindingDB Entry DOI: 10.7270/Q2V69HZM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sorbitol dehydrogenase (Rattus norvegicus) | BDBM50118712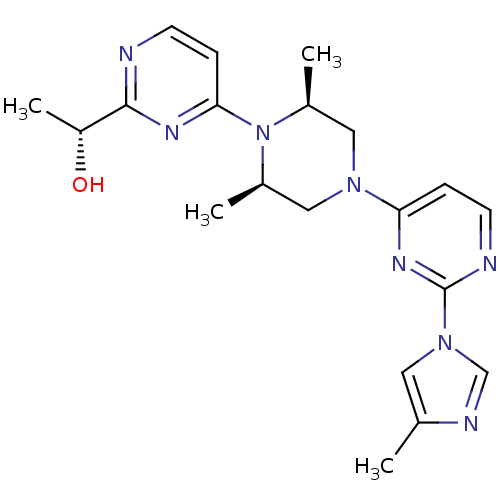 (1-(4-{2,6-Dimethyl-4-[2-(4-methyl-imidazol-1-yl)-p...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Concentration required for 50% in vitro activity against rat SDH (sorbitol dehydrogenase) | J Med Chem 45: 4398-401 (2002) BindingDB Entry DOI: 10.7270/Q2V69HZM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sorbitol dehydrogenase (Homo sapiens (Human)) | BDBM50118712 (1-(4-{2,6-Dimethyl-4-[2-(4-methyl-imidazol-1-yl)-p...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Concentration required for 50% for in vitro activity against human SDH (sorbitol dehydrogenase) | J Med Chem 45: 4398-401 (2002) BindingDB Entry DOI: 10.7270/Q2V69HZM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM16635 (6-[(3,5-dimethyl-1-benzofuran-2-)sulfonyl]-2,3-dih...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer | Assay Description The activity of the enzyme was determined spectrophotometrically by monitoring the change in absorbance at 340 nm, which is due to the disappearance ... | J Med Chem 48: 6326-39 (2005) Article DOI: 10.1021/jm050462t BindingDB Entry DOI: 10.7270/Q2RN363Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Menin (Homo sapiens (Human)) | BDBM50455883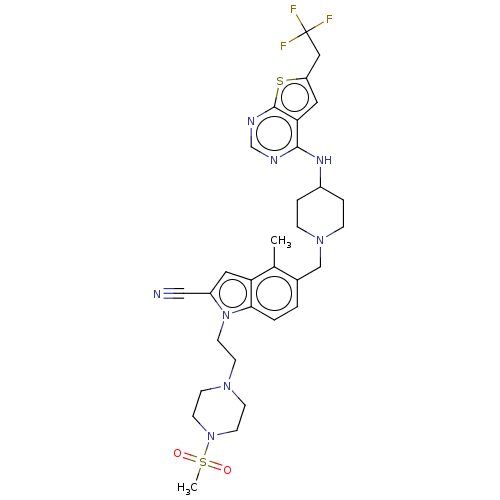 (CHEMBL4207749) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Displacement of fluorescein-labeled MLL4-43 from full length human menin expressed in Escherichia coli BL21 (DE3) cells after 3 hrs by fluorescence p... | J Med Chem 61: 4832-4850 (2018) Article DOI: 10.1021/acs.jmedchem.8b00071 BindingDB Entry DOI: 10.7270/Q2Z03BRF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 203 total ) | Next | Last >> |