 Found 6020 hits with Last Name = 'lo' and Initial = 'jr'
Found 6020 hits with Last Name = 'lo' and Initial = 'jr' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50334730
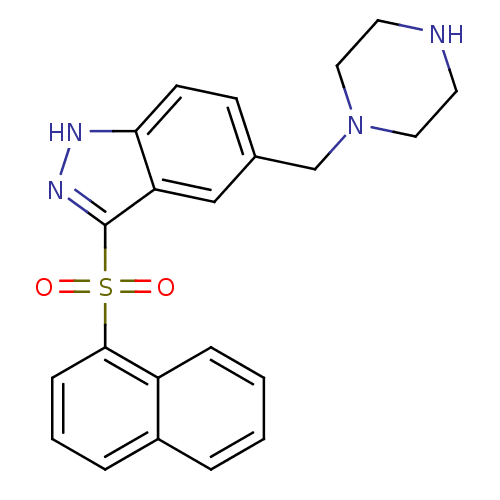
(3-(Naphthalen-1-ylsulfonyl)-5-(piperazin-1-ylmethy...)Show SMILES O=S(=O)(c1n[nH]c2ccc(CN3CCNCC3)cc12)c1cccc2ccccc12 Show InChI InChI=1S/C22H22N4O2S/c27-29(28,21-7-3-5-17-4-1-2-6-18(17)21)22-19-14-16(8-9-20(19)24-25-22)15-26-12-10-23-11-13-26/h1-9,14,23H,10-13,15H2,(H,24,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]-LSD from cloned human 5-HT6 receptor expressed in human HeLa cells |
Bioorg Med Chem 19: 650-62 (2011)
Article DOI: 10.1016/j.bmc.2010.10.033
BindingDB Entry DOI: 10.7270/Q29S1R9V |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 5
(Rat 6B) | BDBM82276
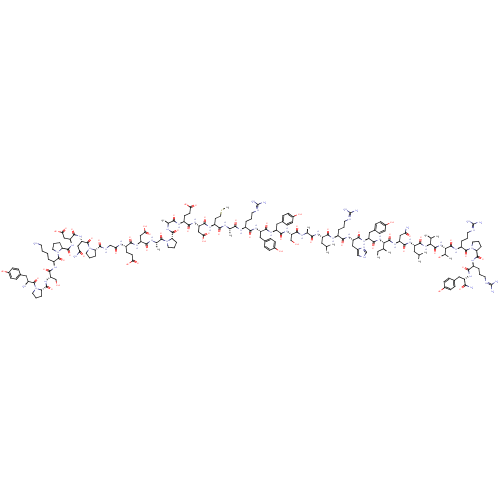
(L31,P34-NPY,human | NPY Leu31, Pro34, human, rat |...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](Cc1c[nH]cn1)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](C)NC(=O)[C@H](CO)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](C)NC(=O)[C@H](CCSC)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](C)NC(=O)[C@@H]1CCCN1C(=O)[C@H](C)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)CNC(=O)[C@@H]1CCCN1C(=O)[C@H](CC(N)=O)NC(=O)[C@H](CC(O)=O)NC(=O)[C@@H]1CCCN1C(=O)[C@H](CCCCN)NC(=O)[C@H](CO)NC(=O)[C@@H]1CCCN1C(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc1ccc(O)cc1)C(N)=O Show InChI InChI=1S/C188H282N54O56S/c1-15-94(8)148(178(292)231-127(81-139(191)251)165(279)223-122(74-92(4)5)168(282)235-147(93(6)7)177(291)237-149(99(13)245)179(293)220-119(31-21-66-206-188(200)201)183(297)241-70-25-35-137(241)174(288)218-114(30-20-65-205-187(198)199)155(269)221-120(150(193)264)76-101-39-49-107(247)50-40-101)236-169(283)125(79-104-45-55-110(250)56-46-104)226-164(278)126(80-105-86-202-90-208-105)227-157(271)113(29-19-64-204-186(196)197)216-161(275)121(73-91(2)3)222-153(267)96(10)210-170(284)132(88-243)233-163(277)124(78-103-43-53-109(249)54-44-103)225-162(276)123(77-102-41-51-108(248)52-42-102)224-156(270)112(28-18-63-203-185(194)195)214-151(265)95(9)209-154(268)117(61-72-299-14)217-166(280)129(84-145(260)261)229-159(273)116(58-60-143(256)257)215-152(266)97(11)211-173(287)135-33-23-67-238(135)180(294)98(12)212-160(274)128(83-144(258)259)228-158(272)115(57-59-142(254)255)213-141(253)87-207-172(286)134-32-22-69-240(134)184(298)131(82-140(192)252)232-167(281)130(85-146(262)263)230-175(289)138-36-26-71-242(138)182(296)118(27-16-17-62-189)219-171(285)133(89-244)234-176(290)136-34-24-68-239(136)181(295)111(190)75-100-37-47-106(246)48-38-100/h37-56,86,90-99,111-138,147-149,243-250H,15-36,57-85,87-89,189-190H2,1-14H3,(H2,191,251)(H2,192,252)(H2,193,264)(H,202,208)(H,207,286)(H,209,268)(H,210,284)(H,211,287)(H,212,274)(H,213,253)(H,214,265)(H,215,266)(H,216,275)(H,217,280)(H,218,288)(H,219,285)(H,220,293)(H,221,269)(H,222,267)(H,223,279)(H,224,270)(H,225,276)(H,226,278)(H,227,271)(H,228,272)(H,229,273)(H,230,289)(H,231,292)(H,232,281)(H,233,277)(H,234,290)(H,235,282)(H,236,283)(H,237,291)(H,254,255)(H,256,257)(H,258,259)(H,260,261)(H,262,263)(H4,194,195,203)(H4,196,197,204)(H4,198,199,205)(H4,200,201,206)/t94-,95-,96-,97-,98-,99+,111-,112-,113-,114-,115-,116-,117-,118-,119-,120-,121-,122-,123-,124-,125-,126-,127-,128-,129-,130-,131-,132-,133-,134-,135-,136-,137-,138-,147-,148-,149-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Corporation
Curated by PDSP Ki Database
| |
J Biol Chem 271: 26315-9 (1996)
Article DOI: 10.1074/jbc.271.42.26315
BindingDB Entry DOI: 10.7270/Q20G3HPR |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 5
(Rat 6B) | BDBM50091652
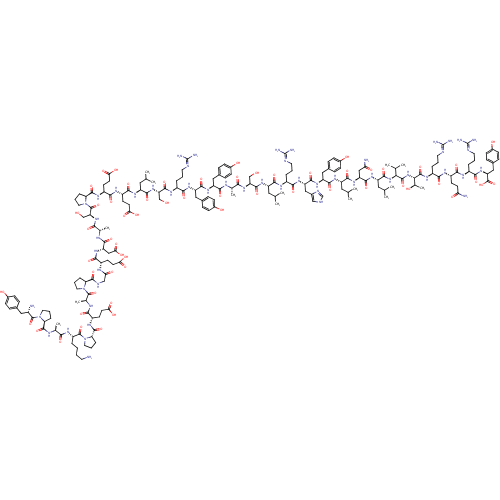
(CHEMBL269503 | PYY | PYY, rat | Peptide YY(PYY)(YP...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@@H]1CCCN1C(=O)[C@H](CO)NC(=O)[C@H](C)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)CNC(=O)[C@@H]1CCCN1C(=O)[C@H](C)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@@H]1CCCN1C(=O)[C@H](CCCCN)NC(=O)[C@H](C)NC(=O)[C@@H]1CCCN1C(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](CO)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](C)C(=O)N[C@@H](CO)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc1c[nH]cn1)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc1ccc(O)cc1)C(O)=O Show InChI InChI=1S/C190H287N53O58/c1-92(2)74-124(166(280)216-114(27-18-66-204-187(195)196)158(272)231-131(83-107-86-203-91-209-107)171(285)230-130(81-105-41-51-111(251)52-42-105)169(283)225-125(75-93(3)4)167(281)232-132(84-143(194)254)172(286)226-127(77-95(7)8)173(287)238-150(96(9)10)180(294)239-151(101(15)247)181(295)222-117(30-21-69-207-190(201)202)156(270)218-119(55-60-142(193)253)161(275)215-116(29-20-68-206-189(199)200)159(273)234-134(186(300)301)82-106-43-53-112(252)54-44-106)227-175(289)135(88-244)235-153(267)97(11)210-164(278)128(79-103-37-47-109(249)48-38-103)229-170(284)129(80-104-39-49-110(250)50-40-104)228-157(271)115(28-19-67-205-188(197)198)217-174(288)136(89-245)236-168(282)126(76-94(5)6)224-163(277)121(58-63-147(260)261)219-162(276)122(59-64-148(262)263)221-179(293)141-34-25-73-243(141)185(299)137(90-246)237-154(268)98(12)211-165(279)133(85-149(264)265)233-160(274)118(56-61-145(256)257)214-144(255)87-208-176(290)138-31-22-70-240(138)182(296)100(14)213-155(269)120(57-62-146(258)259)220-178(292)140-33-24-72-242(140)184(298)123(26-16-17-65-191)223-152(266)99(13)212-177(291)139-32-23-71-241(139)183(297)113(192)78-102-35-45-108(248)46-36-102/h35-54,86,91-101,113-141,150-151,244-252H,16-34,55-85,87-90,191-192H2,1-15H3,(H2,193,253)(H2,194,254)(H,203,209)(H,208,290)(H,210,278)(H,211,279)(H,212,291)(H,213,269)(H,214,255)(H,215,275)(H,216,280)(H,217,288)(H,218,270)(H,219,276)(H,220,292)(H,221,293)(H,222,295)(H,223,266)(H,224,277)(H,225,283)(H,226,286)(H,227,289)(H,228,271)(H,229,284)(H,230,285)(H,231,272)(H,232,281)(H,233,274)(H,234,273)(H,235,267)(H,236,282)(H,237,268)(H,238,287)(H,239,294)(H,256,257)(H,258,259)(H,260,261)(H,262,263)(H,264,265)(H,300,301)(H4,195,196,204)(H4,197,198,205)(H4,199,200,206)(H4,201,202,207)/t97-,98-,99-,100-,101+,113-,114-,115-,116-,117-,118-,119-,120-,121-,122-,123-,124-,125-,126-,127-,128-,129-,130-,131-,132-,133-,134-,135-,136-,137-,138-,139-,140-,141-,150-,151-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Corporation
Curated by PDSP Ki Database
| |
J Biol Chem 271: 26315-9 (1996)
Article DOI: 10.1074/jbc.271.42.26315
BindingDB Entry DOI: 10.7270/Q20G3HPR |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 5
(Rat 6B) | BDBM50015490
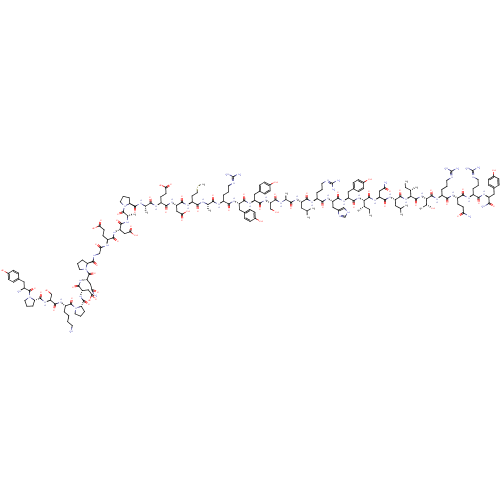
(CHEMBL438945 | H-YPSKPDNPGEDAPAEDMARYYSALRHYINLITR...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](Cc1c[nH]cn1)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](C)NC(=O)[C@H](CO)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](C)NC(=O)[C@H](CCSC)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](C)NC(=O)[C@@H]1CCCN1C(=O)[C@H](C)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)CNC(=O)[C@@H]1CCCN1C(=O)[C@H](CC(N)=O)NC(=O)[C@H](CC(O)=O)NC(=O)[C@@H]1CCCN1C(=O)[C@H](CCCCN)NC(=O)[C@H](CO)NC(=O)[C@@H]1CCCN1C(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc1ccc(O)cc1)C(N)=O Show InChI InChI=1S/C189H285N55O57S/c1-15-93(7)148(179(295)234-128(81-140(193)254)168(284)226-123(74-92(5)6)171(287)239-149(94(8)16-2)180(296)240-150(99(13)247)181(297)222-115(31-22-67-208-189(202)203)156(272)220-117(56-59-139(192)253)161(277)218-113(29-20-65-206-187(198)199)157(273)224-121(151(195)267)76-101-38-48-107(249)49-39-101)238-172(288)126(79-104-44-54-110(252)55-45-104)229-167(283)127(80-105-86-204-90-210-105)230-159(275)114(30-21-66-207-188(200)201)219-164(280)122(73-91(3)4)225-154(270)96(10)212-173(289)133(88-245)236-166(282)125(78-103-42-52-109(251)53-43-103)228-165(281)124(77-102-40-50-108(250)51-41-102)227-158(274)112(28-19-64-205-186(196)197)216-152(268)95(9)211-155(271)119(62-72-302-14)221-169(285)130(84-146(263)264)232-162(278)118(58-61-144(259)260)217-153(269)97(11)213-176(292)136-33-24-68-241(136)182(298)98(12)214-163(279)129(83-145(261)262)231-160(276)116(57-60-143(257)258)215-142(256)87-209-175(291)135-32-23-70-243(135)185(301)132(82-141(194)255)235-170(286)131(85-147(265)266)233-177(293)138-35-26-71-244(138)184(300)120(27-17-18-63-190)223-174(290)134(89-246)237-178(294)137-34-25-69-242(137)183(299)111(191)75-100-36-46-106(248)47-37-100/h36-55,86,90-99,111-138,148-150,245-252H,15-35,56-85,87-89,190-191H2,1-14H3,(H2,192,253)(H2,193,254)(H2,194,255)(H2,195,267)(H,204,210)(H,209,291)(H,211,271)(H,212,289)(H,213,292)(H,214,279)(H,215,256)(H,216,268)(H,217,269)(H,218,277)(H,219,280)(H,220,272)(H,221,285)(H,222,297)(H,223,290)(H,224,273)(H,225,270)(H,226,284)(H,227,274)(H,228,281)(H,229,283)(H,230,275)(H,231,276)(H,232,278)(H,233,293)(H,234,295)(H,235,286)(H,236,282)(H,237,294)(H,238,288)(H,239,287)(H,240,296)(H,257,258)(H,259,260)(H,261,262)(H,263,264)(H,265,266)(H4,196,197,205)(H4,198,199,206)(H4,200,201,207)(H4,202,203,208)/t93-,94-,95-,96-,97-,98-,99+,111-,112-,113-,114-,115-,116-,117-,118-,119-,120-,121-,122-,123-,124-,125-,126-,127-,128-,129-,130-,131-,132-,133-,134-,135-,136-,137-,138-,148-,149-,150-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Corporation
Curated by PDSP Ki Database
| |
J Biol Chem 271: 26315-9 (1996)
Article DOI: 10.1074/jbc.271.42.26315
BindingDB Entry DOI: 10.7270/Q20G3HPR |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM28574
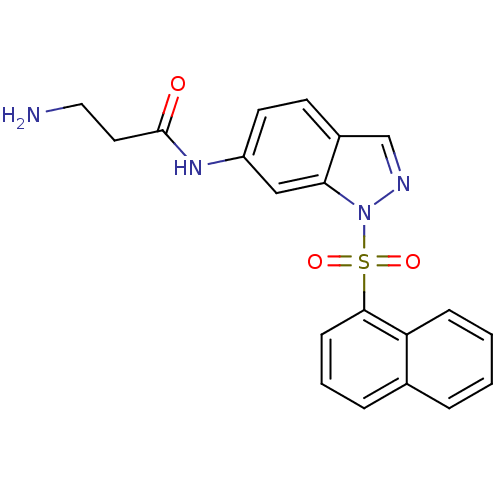
(1-sulfonylindazole, 6c | 3-amino-N-[1-(naphthalene...)Show SMILES NCCC(=O)Nc1ccc2cnn(c2c1)S(=O)(=O)c1cccc2ccccc12 Show InChI InChI=1S/C20H18N4O3S/c21-11-10-20(25)23-16-9-8-15-13-22-24(18(15)12-16)28(26,27)19-7-3-5-14-4-1-2-6-17(14)19/h1-9,12-13H,10-11,21H2,(H,23,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.5 | -52.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Wyeth Research
| Assay Description
Competition experiments were performed in the presence radioligand with membrane protein (obtained from cells expressing the receptor) and test compo... |
Bioorg Med Chem Lett 19: 2413-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.071
BindingDB Entry DOI: 10.7270/Q28K77CF |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 5
(Rat 6B) | BDBM50015490

(CHEMBL438945 | H-YPSKPDNPGEDAPAEDMARYYSALRHYINLITR...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](Cc1c[nH]cn1)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](C)NC(=O)[C@H](CO)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](C)NC(=O)[C@H](CCSC)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](C)NC(=O)[C@@H]1CCCN1C(=O)[C@H](C)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)CNC(=O)[C@@H]1CCCN1C(=O)[C@H](CC(N)=O)NC(=O)[C@H](CC(O)=O)NC(=O)[C@@H]1CCCN1C(=O)[C@H](CCCCN)NC(=O)[C@H](CO)NC(=O)[C@@H]1CCCN1C(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc1ccc(O)cc1)C(N)=O Show InChI InChI=1S/C189H285N55O57S/c1-15-93(7)148(179(295)234-128(81-140(193)254)168(284)226-123(74-92(5)6)171(287)239-149(94(8)16-2)180(296)240-150(99(13)247)181(297)222-115(31-22-67-208-189(202)203)156(272)220-117(56-59-139(192)253)161(277)218-113(29-20-65-206-187(198)199)157(273)224-121(151(195)267)76-101-38-48-107(249)49-39-101)238-172(288)126(79-104-44-54-110(252)55-45-104)229-167(283)127(80-105-86-204-90-210-105)230-159(275)114(30-21-66-207-188(200)201)219-164(280)122(73-91(3)4)225-154(270)96(10)212-173(289)133(88-245)236-166(282)125(78-103-42-52-109(251)53-43-103)228-165(281)124(77-102-40-50-108(250)51-41-102)227-158(274)112(28-19-64-205-186(196)197)216-152(268)95(9)211-155(271)119(62-72-302-14)221-169(285)130(84-146(263)264)232-162(278)118(58-61-144(259)260)217-153(269)97(11)213-176(292)136-33-24-68-241(136)182(298)98(12)214-163(279)129(83-145(261)262)231-160(276)116(57-60-143(257)258)215-142(256)87-209-175(291)135-32-23-70-243(135)185(301)132(82-141(194)255)235-170(286)131(85-147(265)266)233-177(293)138-35-26-71-244(138)184(300)120(27-17-18-63-190)223-174(290)134(89-246)237-178(294)137-34-25-69-242(137)183(299)111(191)75-100-36-46-106(248)47-37-100/h36-55,86,90-99,111-138,148-150,245-252H,15-35,56-85,87-89,190-191H2,1-14H3,(H2,192,253)(H2,193,254)(H2,194,255)(H2,195,267)(H,204,210)(H,209,291)(H,211,271)(H,212,289)(H,213,292)(H,214,279)(H,215,256)(H,216,268)(H,217,269)(H,218,277)(H,219,280)(H,220,272)(H,221,285)(H,222,297)(H,223,290)(H,224,273)(H,225,270)(H,226,284)(H,227,274)(H,228,281)(H,229,283)(H,230,275)(H,231,276)(H,232,278)(H,233,293)(H,234,295)(H,235,286)(H,236,282)(H,237,294)(H,238,288)(H,239,287)(H,240,296)(H,257,258)(H,259,260)(H,261,262)(H,263,264)(H,265,266)(H4,196,197,205)(H4,198,199,206)(H4,200,201,207)(H4,202,203,208)/t93-,94-,95-,96-,97-,98-,99+,111-,112-,113-,114-,115-,116-,117-,118-,119-,120-,121-,122-,123-,124-,125-,126-,127-,128-,129-,130-,131-,132-,133-,134-,135-,136-,137-,138-,148-,149-,150-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Corporation
Curated by PDSP Ki Database
| |
J Biol Chem 271: 26315-9 (1996)
Article DOI: 10.1074/jbc.271.42.26315
BindingDB Entry DOI: 10.7270/Q20G3HPR |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 5
(Rat 6B) | BDBM84999
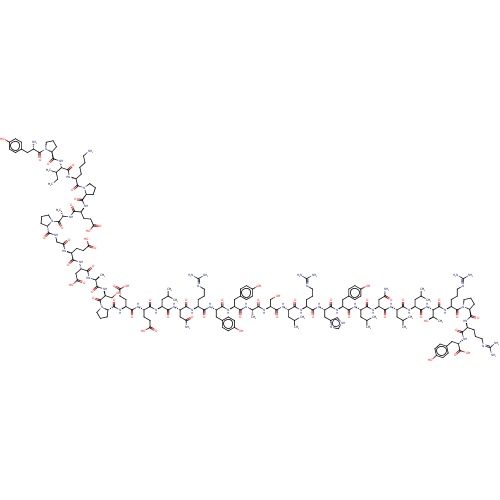
(PYY,[leu31,Pro34], human)Show SMILES CCC(C)[C@H](NC(=O)[C@@H]1CCCN1C(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](CCCCN)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](C)C(=O)N1CCC[C@H]1C(=O)NCC(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](C)C(=O)N[C@@H](CO)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](C)C(=O)N[C@@H](CO)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc1c[nH]cn1)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc1ccc(O)cc1)C(O)=O Show InChI InChI=1S/C194H295N53O58/c1-17-101(12)155(241-182(294)144-37-25-73-244(144)187(299)117(196)82-106-39-49-112(251)50-40-106)184(296)224-125(29-18-19-67-195)188(300)245-74-27-36-143(245)181(293)222-122(60-64-151(261)262)159(271)216-104(15)186(298)243-72-24-34-141(243)179(291)212-92-148(258)217-121(59-63-150(259)260)163(275)237-138(90-154(267)268)167(279)215-103(14)158(270)240-149(94-249)305(304)247-76-28-38-145(247)183(295)223-124(62-66-153(265)266)164(276)220-123(61-65-152(263)264)165(277)226-128(78-97(4)5)170(282)235-136(88-146(197)256)175(287)219-118(30-20-68-208-191(199)200)160(272)231-133(84-108-43-53-114(253)54-44-108)173(285)232-132(83-107-41-51-113(252)52-42-107)166(278)214-102(13)157(269)239-140(93-248)178(290)230-127(77-96(2)3)168(280)218-119(31-21-69-209-192(201)202)161(273)234-135(87-111-91-207-95-213-111)174(286)233-134(85-109-45-55-115(254)56-46-109)172(284)227-130(80-99(8)9)171(283)236-137(89-147(198)257)176(288)228-129(79-98(6)7)169(281)229-131(81-100(10)11)177(289)242-156(105(16)250)185(297)225-126(33-23-71-211-194(205)206)189(301)246-75-26-35-142(246)180(292)221-120(32-22-70-210-193(203)204)162(274)238-139(190(302)303)86-110-47-57-116(255)58-48-110/h39-58,91,95-105,117-145,149,155-156,248-255H,17-38,59-90,92-94,195-196H2,1-16H3,(H2,197,256)(H2,198,257)(H,207,213)(H,212,291)(H,214,278)(H,215,279)(H,216,271)(H,217,258)(H,218,280)(H,219,287)(H,220,276)(H,221,292)(H,222,293)(H,223,295)(H,224,296)(H,225,297)(H,226,277)(H,227,284)(H,228,288)(H,229,281)(H,230,290)(H,231,272)(H,232,285)(H,233,286)(H,234,273)(H,235,282)(H,236,283)(H,237,275)(H,238,274)(H,239,269)(H,240,270)(H,241,294)(H,242,289)(H,259,260)(H,261,262)(H,263,264)(H,265,266)(H,267,268)(H,302,303)(H4,199,200,208)(H4,201,202,209)(H4,203,204,210)(H4,205,206,211)/t101?,102-,103-,104-,105+,117-,118-,119-,120-,121-,122-,123-,124-,125-,126-,127-,128-,129-,130-,131-,132-,133-,134-,135-,136-,137-,138-,139-,140-,141-,142-,143-,144-,145-,149+,155-,156-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.570 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Corporation
Curated by PDSP Ki Database
| |
J Biol Chem 271: 26315-9 (1996)
Article DOI: 10.1074/jbc.271.42.26315
BindingDB Entry DOI: 10.7270/Q20G3HPR |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50334734
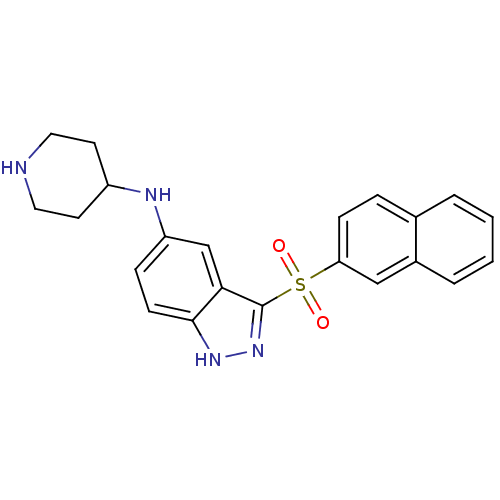
(3-(Naphthalen-2-ylsulfonyl)-N-(piperidin-4-yl)-1H-...)Show SMILES O=S(=O)(c1n[nH]c2ccc(NC3CCNCC3)cc12)c1ccc2ccccc2c1 Show InChI InChI=1S/C22H22N4O2S/c27-29(28,19-7-5-15-3-1-2-4-16(15)13-19)22-20-14-18(6-8-21(20)25-26-22)24-17-9-11-23-12-10-17/h1-8,13-14,17,23-24H,9-12H2,(H,25,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]-LSD from cloned human 5-HT6 receptor expressed in human HeLa cells |
Bioorg Med Chem 19: 650-62 (2011)
Article DOI: 10.1016/j.bmc.2010.10.033
BindingDB Entry DOI: 10.7270/Q29S1R9V |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 5
(Rat 6B) | BDBM82276

(L31,P34-NPY,human | NPY Leu31, Pro34, human, rat |...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](Cc1c[nH]cn1)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](C)NC(=O)[C@H](CO)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](C)NC(=O)[C@H](CCSC)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](C)NC(=O)[C@@H]1CCCN1C(=O)[C@H](C)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)CNC(=O)[C@@H]1CCCN1C(=O)[C@H](CC(N)=O)NC(=O)[C@H](CC(O)=O)NC(=O)[C@@H]1CCCN1C(=O)[C@H](CCCCN)NC(=O)[C@H](CO)NC(=O)[C@@H]1CCCN1C(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc1ccc(O)cc1)C(N)=O Show InChI InChI=1S/C188H282N54O56S/c1-15-94(8)148(178(292)231-127(81-139(191)251)165(279)223-122(74-92(4)5)168(282)235-147(93(6)7)177(291)237-149(99(13)245)179(293)220-119(31-21-66-206-188(200)201)183(297)241-70-25-35-137(241)174(288)218-114(30-20-65-205-187(198)199)155(269)221-120(150(193)264)76-101-39-49-107(247)50-40-101)236-169(283)125(79-104-45-55-110(250)56-46-104)226-164(278)126(80-105-86-202-90-208-105)227-157(271)113(29-19-64-204-186(196)197)216-161(275)121(73-91(2)3)222-153(267)96(10)210-170(284)132(88-243)233-163(277)124(78-103-43-53-109(249)54-44-103)225-162(276)123(77-102-41-51-108(248)52-42-102)224-156(270)112(28-18-63-203-185(194)195)214-151(265)95(9)209-154(268)117(61-72-299-14)217-166(280)129(84-145(260)261)229-159(273)116(58-60-143(256)257)215-152(266)97(11)211-173(287)135-33-23-67-238(135)180(294)98(12)212-160(274)128(83-144(258)259)228-158(272)115(57-59-142(254)255)213-141(253)87-207-172(286)134-32-22-69-240(134)184(298)131(82-140(192)252)232-167(281)130(85-146(262)263)230-175(289)138-36-26-71-242(138)182(296)118(27-16-17-62-189)219-171(285)133(89-244)234-176(290)136-34-24-68-239(136)181(295)111(190)75-100-37-47-106(246)48-38-100/h37-56,86,90-99,111-138,147-149,243-250H,15-36,57-85,87-89,189-190H2,1-14H3,(H2,191,251)(H2,192,252)(H2,193,264)(H,202,208)(H,207,286)(H,209,268)(H,210,284)(H,211,287)(H,212,274)(H,213,253)(H,214,265)(H,215,266)(H,216,275)(H,217,280)(H,218,288)(H,219,285)(H,220,293)(H,221,269)(H,222,267)(H,223,279)(H,224,270)(H,225,276)(H,226,278)(H,227,271)(H,228,272)(H,229,273)(H,230,289)(H,231,292)(H,232,281)(H,233,277)(H,234,290)(H,235,282)(H,236,283)(H,237,291)(H,254,255)(H,256,257)(H,258,259)(H,260,261)(H,262,263)(H4,194,195,203)(H4,196,197,204)(H4,198,199,205)(H4,200,201,206)/t94-,95-,96-,97-,98-,99+,111-,112-,113-,114-,115-,116-,117-,118-,119-,120-,121-,122-,123-,124-,125-,126-,127-,128-,129-,130-,131-,132-,133-,134-,135-,136-,137-,138-,147-,148-,149-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.640 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Corporation
Curated by PDSP Ki Database
| |
J Biol Chem 271: 26315-9 (1996)
Article DOI: 10.1074/jbc.271.42.26315
BindingDB Entry DOI: 10.7270/Q20G3HPR |
More data for this
Ligand-Target Pair | |
Protein kinase C gamma type
(Homo sapiens (Human)) | BDBM50587724
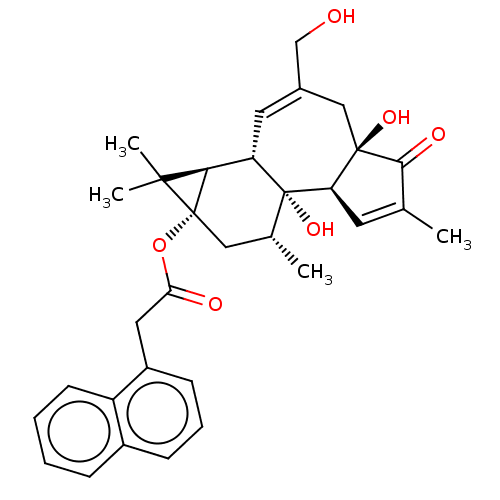
(CHEMBL5175742)Show SMILES [H][C@]12[C@]3([H])C=C(CO)C[C@]4(O)C(=O)C(C)=C[C@@]4([H])[C@@]3(O)[C@H](C)C[C@@]1(OC(=O)Cc1cccc3ccccc13)C2(C)C |r,c:14,t:4| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113213
BindingDB Entry DOI: 10.7270/Q26Q2260 |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 5
(Rat 6B) | BDBM82277
(NPY2-36, human | NPY2-36, rat, human)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](Cc1c[nH]cn1)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](C)NC(=O)[C@H](CO)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](C)NC(=O)[C@H](CCSC)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](C)NC(=O)[C@@H]1CCCN1C(=O)[C@H](C)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)CNC(=O)[C@@H]1CCCN1C(=O)[C@H](CC(N)=O)NC(=O)[C@H](CC(O)=O)NC(=O)[C@@H]1CCCN1C(=O)[C@H](CCCCN)NC(=O)[C@H](CO)NC(=O)[C@@H]1CCCN1)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc1ccc(O)cc1)C(N)=O Show InChI InChI=1S/C180H276N54O55S/c1-15-88(7)140(171(284)225-121(76-132(183)243)161(274)217-116(70-87(5)6)164(277)230-141(89(8)16-2)172(285)231-142(94(13)237)173(286)213-108(32-23-64-199-180(192)193)149(262)211-110(52-55-131(182)242)154(267)209-106(30-21-62-197-178(188)189)150(263)215-114(143(185)256)71-95-36-44-100(238)45-37-95)229-165(278)119(74-98-42-50-103(241)51-43-98)220-160(273)120(75-99-81-194-85-201-99)221-152(265)107(31-22-63-198-179(190)191)210-157(270)115(69-86(3)4)216-146(259)91(10)203-166(279)126(83-235)228-159(272)118(73-97-40-48-102(240)49-41-97)219-158(271)117(72-96-38-46-101(239)47-39-96)218-151(264)105(29-20-61-196-177(186)187)207-144(257)90(9)202-148(261)112(58-68-290-14)212-162(275)123(79-138(252)253)223-155(268)111(54-57-136(248)249)208-145(258)92(11)204-169(282)129-34-25-65-232(129)174(287)93(12)205-156(269)122(78-137(250)251)222-153(266)109(53-56-135(246)247)206-134(245)82-200-168(281)128-33-24-66-233(128)176(289)125(77-133(184)244)226-163(276)124(80-139(254)255)224-170(283)130-35-26-67-234(130)175(288)113(27-17-18-59-181)214-167(280)127(84-236)227-147(260)104-28-19-60-195-104/h36-51,81,85-94,104-130,140-142,195,235-241H,15-35,52-80,82-84,181H2,1-14H3,(H2,182,242)(H2,183,243)(H2,184,244)(H2,185,256)(H,194,201)(H,200,281)(H,202,261)(H,203,279)(H,204,282)(H,205,269)(H,206,245)(H,207,257)(H,208,258)(H,209,267)(H,210,270)(H,211,262)(H,212,275)(H,213,286)(H,214,280)(H,215,263)(H,216,259)(H,217,274)(H,218,264)(H,219,271)(H,220,273)(H,221,265)(H,222,266)(H,223,268)(H,224,283)(H,225,284)(H,226,276)(H,227,260)(H,228,272)(H,229,278)(H,230,277)(H,231,285)(H,246,247)(H,248,249)(H,250,251)(H,252,253)(H,254,255)(H4,186,187,196)(H4,188,189,197)(H4,190,191,198)(H4,192,193,199)/t88-,89-,90-,91-,92-,93-,94+,104-,105-,106-,107-,108-,109-,110-,111-,112-,113-,114-,115-,116-,117-,118-,119-,120-,121-,122-,123-,124-,125-,126-,127-,128-,129-,130-,140-,141-,142-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Corporation
Curated by PDSP Ki Database
| |
J Biol Chem 271: 26315-9 (1996)
Article DOI: 10.1074/jbc.271.42.26315
BindingDB Entry DOI: 10.7270/Q20G3HPR |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50334731
(CHEMBL1642851 | [3-(Naphthalen-1-sulfonyl)-1H-inda...)Show SMILES O=S(=O)(c1n[nH]c2ccc(NC3CCNCC3)cc12)c1cccc2ccccc12 Show InChI InChI=1S/C22H22N4O2S/c27-29(28,21-7-3-5-15-4-1-2-6-18(15)21)22-19-14-17(8-9-20(19)25-26-22)24-16-10-12-23-13-11-16/h1-9,14,16,23-24H,10-13H2,(H,25,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]-LSD from cloned human 5-HT6 receptor expressed in human HeLa cells |
Bioorg Med Chem 19: 650-62 (2011)
Article DOI: 10.1016/j.bmc.2010.10.033
BindingDB Entry DOI: 10.7270/Q29S1R9V |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 5
(Homo sapiens (Human)) | BDBM50015490

(CHEMBL438945 | H-YPSKPDNPGEDAPAEDMARYYSALRHYINLITR...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](Cc1c[nH]cn1)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](C)NC(=O)[C@H](CO)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](C)NC(=O)[C@H](CCSC)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](C)NC(=O)[C@@H]1CCCN1C(=O)[C@H](C)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)CNC(=O)[C@@H]1CCCN1C(=O)[C@H](CC(N)=O)NC(=O)[C@H](CC(O)=O)NC(=O)[C@@H]1CCCN1C(=O)[C@H](CCCCN)NC(=O)[C@H](CO)NC(=O)[C@@H]1CCCN1C(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc1ccc(O)cc1)C(N)=O Show InChI InChI=1S/C189H285N55O57S/c1-15-93(7)148(179(295)234-128(81-140(193)254)168(284)226-123(74-92(5)6)171(287)239-149(94(8)16-2)180(296)240-150(99(13)247)181(297)222-115(31-22-67-208-189(202)203)156(272)220-117(56-59-139(192)253)161(277)218-113(29-20-65-206-187(198)199)157(273)224-121(151(195)267)76-101-38-48-107(249)49-39-101)238-172(288)126(79-104-44-54-110(252)55-45-104)229-167(283)127(80-105-86-204-90-210-105)230-159(275)114(30-21-66-207-188(200)201)219-164(280)122(73-91(3)4)225-154(270)96(10)212-173(289)133(88-245)236-166(282)125(78-103-42-52-109(251)53-43-103)228-165(281)124(77-102-40-50-108(250)51-41-102)227-158(274)112(28-19-64-205-186(196)197)216-152(268)95(9)211-155(271)119(62-72-302-14)221-169(285)130(84-146(263)264)232-162(278)118(58-61-144(259)260)217-153(269)97(11)213-176(292)136-33-24-68-241(136)182(298)98(12)214-163(279)129(83-145(261)262)231-160(276)116(57-60-143(257)258)215-142(256)87-209-175(291)135-32-23-70-243(135)185(301)132(82-141(194)255)235-170(286)131(85-147(265)266)233-177(293)138-35-26-71-244(138)184(300)120(27-17-18-63-190)223-174(290)134(89-246)237-178(294)137-34-25-69-242(137)183(299)111(191)75-100-36-46-106(248)47-37-100/h36-55,86,90-99,111-138,148-150,245-252H,15-35,56-85,87-89,190-191H2,1-14H3,(H2,192,253)(H2,193,254)(H2,194,255)(H2,195,267)(H,204,210)(H,209,291)(H,211,271)(H,212,289)(H,213,292)(H,214,279)(H,215,256)(H,216,268)(H,217,269)(H,218,277)(H,219,280)(H,220,272)(H,221,285)(H,222,297)(H,223,290)(H,224,273)(H,225,270)(H,226,284)(H,227,274)(H,228,281)(H,229,283)(H,230,275)(H,231,276)(H,232,278)(H,233,293)(H,234,295)(H,235,286)(H,236,282)(H,237,294)(H,238,288)(H,239,287)(H,240,296)(H,257,258)(H,259,260)(H,261,262)(H,263,264)(H,265,266)(H4,196,197,205)(H4,198,199,206)(H4,200,201,207)(H4,202,203,208)/t93-,94-,95-,96-,97-,98-,99+,111-,112-,113-,114-,115-,116-,117-,118-,119-,120-,121-,122-,123-,124-,125-,126-,127-,128-,129-,130-,131-,132-,133-,134-,135-,136-,137-,138-,148-,149-,150-/m0/s1 | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.730 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Corporation
Curated by PDSP Ki Database
| |
J Biol Chem 271: 26315-9 (1996)
Article DOI: 10.1074/jbc.271.42.26315
BindingDB Entry DOI: 10.7270/Q20G3HPR |
More data for this
Ligand-Target Pair | |
Sodium-dependent dopamine transporter
(Rattus norvegicus (rat)) | BDBM22170

((2S)-1-(4-{2-[bis(4-fluorophenyl)methoxy]ethyl}pip...)Show SMILES O[C@H](CN1CCN(CCOC(c2ccc(F)cc2)c2ccc(F)cc2)CC1)Cc1ccccc1 |r| Show InChI InChI=1S/C28H32F2N2O2/c29-25-10-6-23(7-11-25)28(24-8-12-26(30)13-9-24)34-19-18-31-14-16-32(17-15-31)21-27(33)20-22-4-2-1-3-5-22/h1-13,27-28,33H,14-21H2/t27-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases
Curated by ChEMBL
| Assay Description
Binding affinity for dopamine transporter |
J Med Chem 45: 1321-9 (2002)
BindingDB Entry DOI: 10.7270/Q2K073KD |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50334754
(CHEMBL1642883 | N-(Piperidin-4-yl)-3-(m-tolylsulfo...)Show SMILES Cc1cccc(c1)S(=O)(=O)c1n[nH]c2ccc(NC3CCNCC3)cc12 Show InChI InChI=1S/C19H22N4O2S/c1-13-3-2-4-16(11-13)26(24,25)19-17-12-15(5-6-18(17)22-23-19)21-14-7-9-20-10-8-14/h2-6,11-12,14,20-21H,7-10H2,1H3,(H,22,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]-LSD from cloned human 5-HT6 receptor expressed in human HeLa cells |
Bioorg Med Chem 19: 650-62 (2011)
Article DOI: 10.1016/j.bmc.2010.10.033
BindingDB Entry DOI: 10.7270/Q29S1R9V |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 5
(Homo sapiens (Human)) | BDBM82276

(L31,P34-NPY,human | NPY Leu31, Pro34, human, rat |...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](Cc1c[nH]cn1)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](C)NC(=O)[C@H](CO)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](C)NC(=O)[C@H](CCSC)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](C)NC(=O)[C@@H]1CCCN1C(=O)[C@H](C)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)CNC(=O)[C@@H]1CCCN1C(=O)[C@H](CC(N)=O)NC(=O)[C@H](CC(O)=O)NC(=O)[C@@H]1CCCN1C(=O)[C@H](CCCCN)NC(=O)[C@H](CO)NC(=O)[C@@H]1CCCN1C(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc1ccc(O)cc1)C(N)=O Show InChI InChI=1S/C188H282N54O56S/c1-15-94(8)148(178(292)231-127(81-139(191)251)165(279)223-122(74-92(4)5)168(282)235-147(93(6)7)177(291)237-149(99(13)245)179(293)220-119(31-21-66-206-188(200)201)183(297)241-70-25-35-137(241)174(288)218-114(30-20-65-205-187(198)199)155(269)221-120(150(193)264)76-101-39-49-107(247)50-40-101)236-169(283)125(79-104-45-55-110(250)56-46-104)226-164(278)126(80-105-86-202-90-208-105)227-157(271)113(29-19-64-204-186(196)197)216-161(275)121(73-91(2)3)222-153(267)96(10)210-170(284)132(88-243)233-163(277)124(78-103-43-53-109(249)54-44-103)225-162(276)123(77-102-41-51-108(248)52-42-102)224-156(270)112(28-18-63-203-185(194)195)214-151(265)95(9)209-154(268)117(61-72-299-14)217-166(280)129(84-145(260)261)229-159(273)116(58-60-143(256)257)215-152(266)97(11)211-173(287)135-33-23-67-238(135)180(294)98(12)212-160(274)128(83-144(258)259)228-158(272)115(57-59-142(254)255)213-141(253)87-207-172(286)134-32-22-69-240(134)184(298)131(82-140(192)252)232-167(281)130(85-146(262)263)230-175(289)138-36-26-71-242(138)182(296)118(27-16-17-62-189)219-171(285)133(89-244)234-176(290)136-34-24-68-239(136)181(295)111(190)75-100-37-47-106(246)48-38-100/h37-56,86,90-99,111-138,147-149,243-250H,15-36,57-85,87-89,189-190H2,1-14H3,(H2,191,251)(H2,192,252)(H2,193,264)(H,202,208)(H,207,286)(H,209,268)(H,210,284)(H,211,287)(H,212,274)(H,213,253)(H,214,265)(H,215,266)(H,216,275)(H,217,280)(H,218,288)(H,219,285)(H,220,293)(H,221,269)(H,222,267)(H,223,279)(H,224,270)(H,225,276)(H,226,278)(H,227,271)(H,228,272)(H,229,273)(H,230,289)(H,231,292)(H,232,281)(H,233,277)(H,234,290)(H,235,282)(H,236,283)(H,237,291)(H,254,255)(H,256,257)(H,258,259)(H,260,261)(H,262,263)(H4,194,195,203)(H4,196,197,204)(H4,198,199,205)(H4,200,201,206)/t94-,95-,96-,97-,98-,99+,111-,112-,113-,114-,115-,116-,117-,118-,119-,120-,121-,122-,123-,124-,125-,126-,127-,128-,129-,130-,131-,132-,133-,134-,135-,136-,137-,138-,147-,148-,149-/m0/s1 | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.880 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Corporation
Curated by PDSP Ki Database
| |
J Biol Chem 271: 26315-9 (1996)
Article DOI: 10.1074/jbc.271.42.26315
BindingDB Entry DOI: 10.7270/Q20G3HPR |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50330507
(5-(4-Methylpiperazin-1-yl)-3-(m-tolylsulfonyl)-1H-...)Show SMILES CN1CCN(CC1)c1ccc2[nH]nc(c2c1)S(=O)(=O)c1cccc(C)c1 Show InChI InChI=1S/C19H22N4O2S/c1-14-4-3-5-16(12-14)26(24,25)19-17-13-15(6-7-18(17)20-21-19)23-10-8-22(2)9-11-23/h3-7,12-13H,8-11H2,1-2H3,(H,20,21) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human cloned 5HT6 receptor expressed in human HeLa cells |
J Med Chem 53: 7639-46 (2010)
Article DOI: 10.1021/jm1007825
BindingDB Entry DOI: 10.7270/Q2GQ6XZ6 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50507371
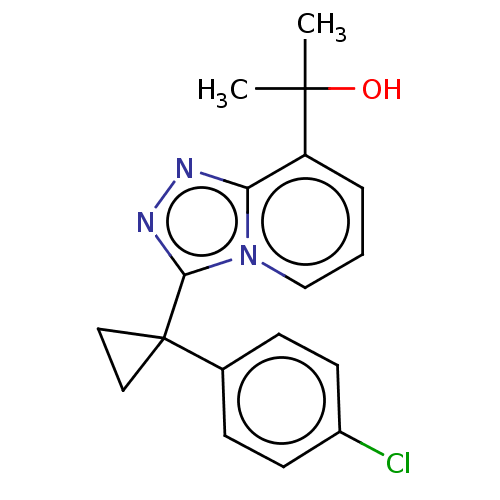
(BMS-823778)Show InChI InChI=1S/C18H18ClN3O/c1-17(2,23)14-4-3-11-22-15(14)20-21-16(22)18(9-10-18)12-5-7-13(19)8-6-12/h3-8,11,23H,9-10H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human 11beta-HSD1 expressed in HEK293 cell microsomes using [3H]cortisone as substrate after 4 hrs by homogeneous immuno-ra... |
ACS Med Chem Lett 9: 1170-1174 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00307
BindingDB Entry DOI: 10.7270/Q20R9SP3 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Sodium-dependent dopamine transporter
(Rattus norvegicus (rat)) | BDBM22199
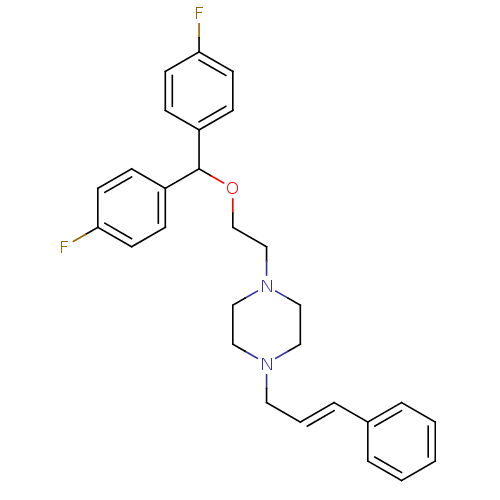
(1-{2-[bis(4-fluorophenyl)methoxy]ethyl}-4-[(2E)-3-...)Show SMILES Fc1ccc(cc1)C(OCCN1CCN(C\C=C\c2ccccc2)CC1)c1ccc(F)cc1 Show InChI InChI=1S/C28H30F2N2O/c29-26-12-8-24(9-13-26)28(25-10-14-27(30)15-11-25)33-22-21-32-19-17-31(18-20-32)16-4-7-23-5-2-1-3-6-23/h1-15,28H,16-22H2/b7-4+ | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases
Curated by ChEMBL
| Assay Description
Binding affinity against dopamine transporter labelled with [125I]- RTI-55 in rat. |
J Med Chem 42: 5029-42 (2000)
BindingDB Entry DOI: 10.7270/Q2R49PZ2 |
More data for this
Ligand-Target Pair | |
Sodium-dependent dopamine transporter
(Rattus norvegicus (rat)) | BDBM50083214
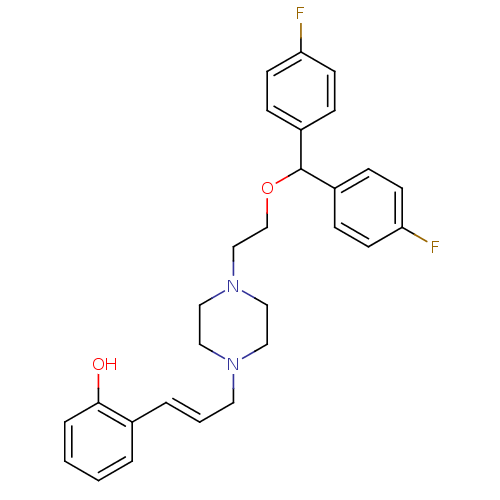
(2-[(E)-3-(4-{2-[Bis-(4-fluoro-phenyl)-methoxy]-eth...)Show SMILES Oc1ccccc1\C=C\CN1CCN(CCOC(c2ccc(F)cc2)c2ccc(F)cc2)CC1 Show InChI InChI=1S/C28H30F2N2O2/c29-25-11-7-23(8-12-25)28(24-9-13-26(30)14-10-24)34-21-20-32-18-16-31(17-19-32)15-3-5-22-4-1-2-6-27(22)33/h1-14,28,33H,15-21H2/b5-3+ | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.920 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases
Curated by ChEMBL
| Assay Description
Binding affinity against dopamine transporter labelled with [125I]- RTI-55 in rat. |
J Med Chem 42: 5029-42 (2000)
BindingDB Entry DOI: 10.7270/Q2R49PZ2 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50334733
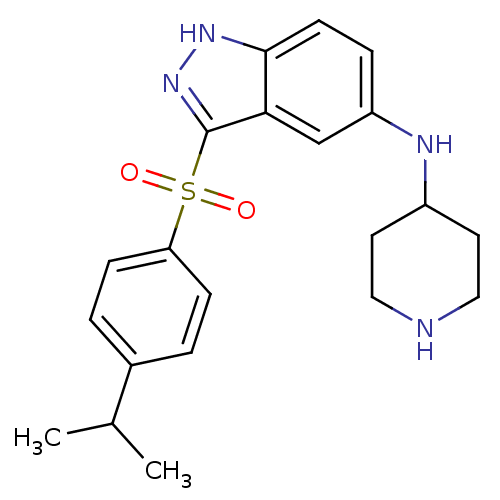
(3-(4-Isopropylphenylsulfonyl)-N-(piperidin-4-yl)-1...)Show SMILES CC(C)c1ccc(cc1)S(=O)(=O)c1n[nH]c2ccc(NC3CCNCC3)cc12 Show InChI InChI=1S/C21H26N4O2S/c1-14(2)15-3-6-18(7-4-15)28(26,27)21-19-13-17(5-8-20(19)24-25-21)23-16-9-11-22-12-10-16/h3-8,13-14,16,22-23H,9-12H2,1-2H3,(H,24,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]-LSD from cloned human 5-HT6 receptor expressed in human HeLa cells |
Bioorg Med Chem 19: 650-62 (2011)
Article DOI: 10.1016/j.bmc.2010.10.033
BindingDB Entry DOI: 10.7270/Q29S1R9V |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 5
(Rat 6B) | BDBM50091652

(CHEMBL269503 | PYY | PYY, rat | Peptide YY(PYY)(YP...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@@H]1CCCN1C(=O)[C@H](CO)NC(=O)[C@H](C)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)CNC(=O)[C@@H]1CCCN1C(=O)[C@H](C)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@@H]1CCCN1C(=O)[C@H](CCCCN)NC(=O)[C@H](C)NC(=O)[C@@H]1CCCN1C(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](CO)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](C)C(=O)N[C@@H](CO)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc1c[nH]cn1)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc1ccc(O)cc1)C(O)=O Show InChI InChI=1S/C190H287N53O58/c1-92(2)74-124(166(280)216-114(27-18-66-204-187(195)196)158(272)231-131(83-107-86-203-91-209-107)171(285)230-130(81-105-41-51-111(251)52-42-105)169(283)225-125(75-93(3)4)167(281)232-132(84-143(194)254)172(286)226-127(77-95(7)8)173(287)238-150(96(9)10)180(294)239-151(101(15)247)181(295)222-117(30-21-69-207-190(201)202)156(270)218-119(55-60-142(193)253)161(275)215-116(29-20-68-206-189(199)200)159(273)234-134(186(300)301)82-106-43-53-112(252)54-44-106)227-175(289)135(88-244)235-153(267)97(11)210-164(278)128(79-103-37-47-109(249)48-38-103)229-170(284)129(80-104-39-49-110(250)50-40-104)228-157(271)115(28-19-67-205-188(197)198)217-174(288)136(89-245)236-168(282)126(76-94(5)6)224-163(277)121(58-63-147(260)261)219-162(276)122(59-64-148(262)263)221-179(293)141-34-25-73-243(141)185(299)137(90-246)237-154(268)98(12)211-165(279)133(85-149(264)265)233-160(274)118(56-61-145(256)257)214-144(255)87-208-176(290)138-31-22-70-240(138)182(296)100(14)213-155(269)120(57-62-146(258)259)220-178(292)140-33-24-72-242(140)184(298)123(26-16-17-65-191)223-152(266)99(13)212-177(291)139-32-23-71-241(139)183(297)113(192)78-102-35-45-108(248)46-36-102/h35-54,86,91-101,113-141,150-151,244-252H,16-34,55-85,87-90,191-192H2,1-15H3,(H2,193,253)(H2,194,254)(H,203,209)(H,208,290)(H,210,278)(H,211,279)(H,212,291)(H,213,269)(H,214,255)(H,215,275)(H,216,280)(H,217,288)(H,218,270)(H,219,276)(H,220,292)(H,221,293)(H,222,295)(H,223,266)(H,224,277)(H,225,283)(H,226,286)(H,227,289)(H,228,271)(H,229,284)(H,230,285)(H,231,272)(H,232,281)(H,233,274)(H,234,273)(H,235,267)(H,236,282)(H,237,268)(H,238,287)(H,239,294)(H,256,257)(H,258,259)(H,260,261)(H,262,263)(H,264,265)(H,300,301)(H4,195,196,204)(H4,197,198,205)(H4,199,200,206)(H4,201,202,207)/t97-,98-,99-,100-,101+,113-,114-,115-,116-,117-,118-,119-,120-,121-,122-,123-,124-,125-,126-,127-,128-,129-,130-,131-,132-,133-,134-,135-,136-,137-,138-,139-,140-,141-,150-,151-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Corporation
Curated by PDSP Ki Database
| |
J Biol Chem 271: 26315-9 (1996)
Article DOI: 10.1074/jbc.271.42.26315
BindingDB Entry DOI: 10.7270/Q20G3HPR |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50334732
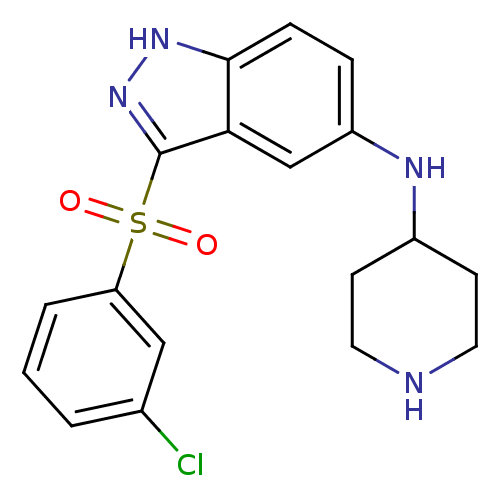
(3-(3-Chlorophenylsulfonyl)-N-(piperidin-4-yl)-1H-i...)Show SMILES Clc1cccc(c1)S(=O)(=O)c1n[nH]c2ccc(NC3CCNCC3)cc12 Show InChI InChI=1S/C18H19ClN4O2S/c19-12-2-1-3-15(10-12)26(24,25)18-16-11-14(4-5-17(16)22-23-18)21-13-6-8-20-9-7-13/h1-5,10-11,13,20-21H,6-9H2,(H,22,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]-LSD from cloned human 5-HT6 receptor expressed in human HeLa cells |
Bioorg Med Chem 19: 650-62 (2011)
Article DOI: 10.1016/j.bmc.2010.10.033
BindingDB Entry DOI: 10.7270/Q29S1R9V |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50330538
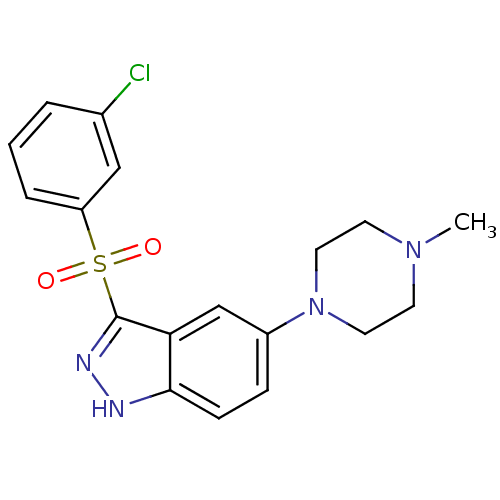
(3-(3-Chlorophenylsulfonyl)-5-(4-methylpiperazin-1-...)Show SMILES CN1CCN(CC1)c1ccc2[nH]nc(c2c1)S(=O)(=O)c1cccc(Cl)c1 Show InChI InChI=1S/C18H19ClN4O2S/c1-22-7-9-23(10-8-22)14-5-6-17-16(12-14)18(21-20-17)26(24,25)15-4-2-3-13(19)11-15/h2-6,11-12H,7-10H2,1H3,(H,20,21) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human cloned 5HT6 receptor expressed in human HeLa cells |
J Med Chem 53: 7639-46 (2010)
Article DOI: 10.1021/jm1007825
BindingDB Entry DOI: 10.7270/Q2GQ6XZ6 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50334725
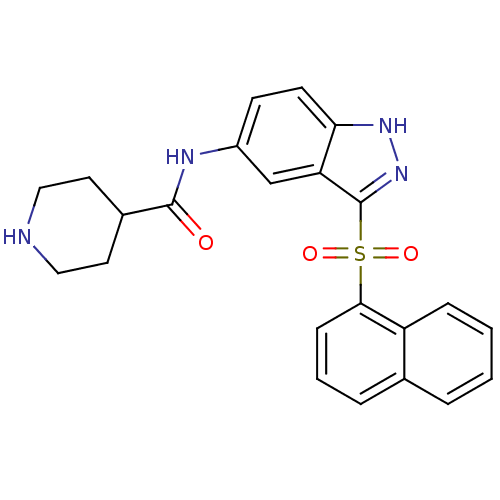
(CHEMBL1642866 | N-(3-(Naphthalen-1-ylsulfonyl)-1H-...)Show SMILES O=C(Nc1ccc2[nH]nc(c2c1)S(=O)(=O)c1cccc2ccccc12)C1CCNCC1 Show InChI InChI=1S/C23H22N4O3S/c28-22(16-10-12-24-13-11-16)25-17-8-9-20-19(14-17)23(27-26-20)31(29,30)21-7-3-5-15-4-1-2-6-18(15)21/h1-9,14,16,24H,10-13H2,(H,25,28)(H,26,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]-LSD from cloned human 5-HT6 receptor expressed in human HeLa cells |
Bioorg Med Chem 19: 650-62 (2011)
Article DOI: 10.1016/j.bmc.2010.10.033
BindingDB Entry DOI: 10.7270/Q29S1R9V |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50330505
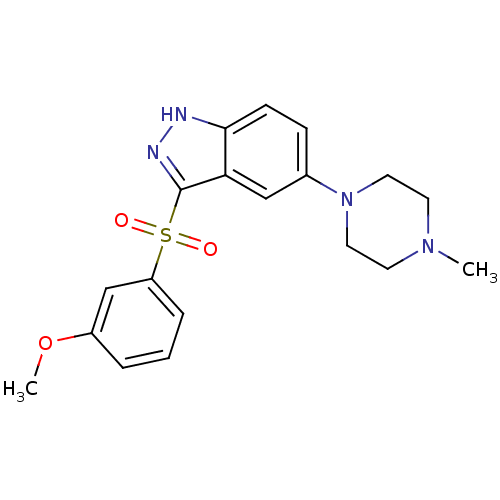
(3-(3-Methoxyphenylsulfonyl)-5-(4-methylpiperazin-1...)Show SMILES COc1cccc(c1)S(=O)(=O)c1n[nH]c2ccc(cc12)N1CCN(C)CC1 Show InChI InChI=1S/C19H22N4O3S/c1-22-8-10-23(11-9-22)14-6-7-18-17(12-14)19(21-20-18)27(24,25)16-5-3-4-15(13-16)26-2/h3-7,12-13H,8-11H2,1-2H3,(H,20,21) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human cloned 5HT6 receptor expressed in human HeLa cells |
J Med Chem 53: 7639-46 (2010)
Article DOI: 10.1021/jm1007825
BindingDB Entry DOI: 10.7270/Q2GQ6XZ6 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50330512
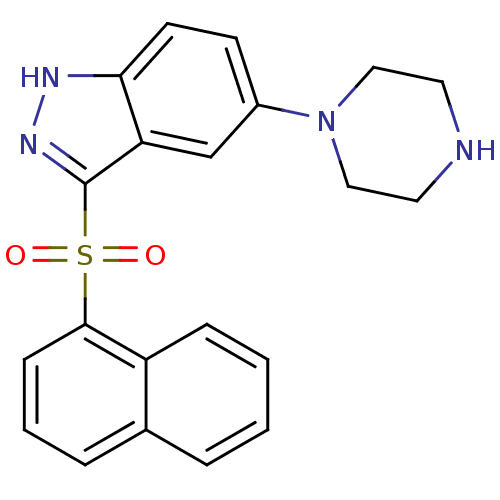
(3-(Naphthalen-1-ylsulfonyl)-5-(piperazin-1-yl)-1H-...)Show SMILES O=S(=O)(c1n[nH]c2ccc(cc12)N1CCNCC1)c1cccc2ccccc12 Show InChI InChI=1S/C21H20N4O2S/c26-28(27,20-7-3-5-15-4-1-2-6-17(15)20)21-18-14-16(8-9-19(18)23-24-21)25-12-10-22-11-13-25/h1-9,14,22H,10-13H2,(H,23,24) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human cloned 5HT6 receptor expressed in human HeLa cells |
J Med Chem 53: 7639-46 (2010)
Article DOI: 10.1021/jm1007825
BindingDB Entry DOI: 10.7270/Q2GQ6XZ6 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM28575
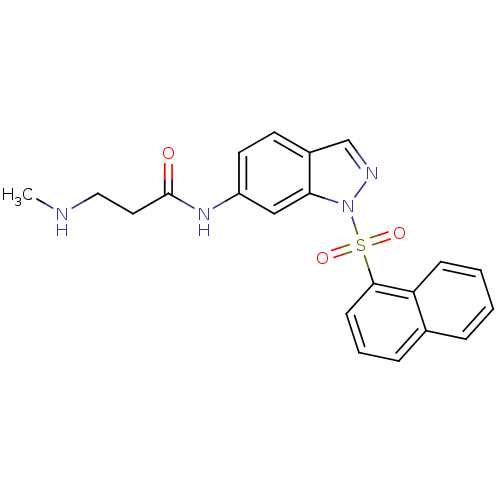
(1-sulfonylindazole, 6d | 3-(methylamino)-N-[1-(nap...)Show SMILES CNCCC(=O)Nc1ccc2cnn(c2c1)S(=O)(=O)c1cccc2ccccc12 Show InChI InChI=1S/C21H20N4O3S/c1-22-12-11-21(26)24-17-10-9-16-14-23-25(19(16)13-17)29(27,28)20-8-4-6-15-5-2-3-7-18(15)20/h2-10,13-14,22H,11-12H2,1H3,(H,24,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.20 | -50.4 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Wyeth Research
| Assay Description
Competition experiments were performed in the presence radioligand with membrane protein (obtained from cells expressing the receptor) and test compo... |
Bioorg Med Chem Lett 19: 2413-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.071
BindingDB Entry DOI: 10.7270/Q28K77CF |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 5
(Rat 6B) | BDBM82277

(NPY2-36, human | NPY2-36, rat, human)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](Cc1c[nH]cn1)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](C)NC(=O)[C@H](CO)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](C)NC(=O)[C@H](CCSC)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](C)NC(=O)[C@@H]1CCCN1C(=O)[C@H](C)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)CNC(=O)[C@@H]1CCCN1C(=O)[C@H](CC(N)=O)NC(=O)[C@H](CC(O)=O)NC(=O)[C@@H]1CCCN1C(=O)[C@H](CCCCN)NC(=O)[C@H](CO)NC(=O)[C@@H]1CCCN1)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc1ccc(O)cc1)C(N)=O Show InChI InChI=1S/C180H276N54O55S/c1-15-88(7)140(171(284)225-121(76-132(183)243)161(274)217-116(70-87(5)6)164(277)230-141(89(8)16-2)172(285)231-142(94(13)237)173(286)213-108(32-23-64-199-180(192)193)149(262)211-110(52-55-131(182)242)154(267)209-106(30-21-62-197-178(188)189)150(263)215-114(143(185)256)71-95-36-44-100(238)45-37-95)229-165(278)119(74-98-42-50-103(241)51-43-98)220-160(273)120(75-99-81-194-85-201-99)221-152(265)107(31-22-63-198-179(190)191)210-157(270)115(69-86(3)4)216-146(259)91(10)203-166(279)126(83-235)228-159(272)118(73-97-40-48-102(240)49-41-97)219-158(271)117(72-96-38-46-101(239)47-39-96)218-151(264)105(29-20-61-196-177(186)187)207-144(257)90(9)202-148(261)112(58-68-290-14)212-162(275)123(79-138(252)253)223-155(268)111(54-57-136(248)249)208-145(258)92(11)204-169(282)129-34-25-65-232(129)174(287)93(12)205-156(269)122(78-137(250)251)222-153(266)109(53-56-135(246)247)206-134(245)82-200-168(281)128-33-24-66-233(128)176(289)125(77-133(184)244)226-163(276)124(80-139(254)255)224-170(283)130-35-26-67-234(130)175(288)113(27-17-18-59-181)214-167(280)127(84-236)227-147(260)104-28-19-60-195-104/h36-51,81,85-94,104-130,140-142,195,235-241H,15-35,52-80,82-84,181H2,1-14H3,(H2,182,242)(H2,183,243)(H2,184,244)(H2,185,256)(H,194,201)(H,200,281)(H,202,261)(H,203,279)(H,204,282)(H,205,269)(H,206,245)(H,207,257)(H,208,258)(H,209,267)(H,210,270)(H,211,262)(H,212,275)(H,213,286)(H,214,280)(H,215,263)(H,216,259)(H,217,274)(H,218,264)(H,219,271)(H,220,273)(H,221,265)(H,222,266)(H,223,268)(H,224,283)(H,225,284)(H,226,276)(H,227,260)(H,228,272)(H,229,278)(H,230,277)(H,231,285)(H,246,247)(H,248,249)(H,250,251)(H,252,253)(H,254,255)(H4,186,187,196)(H4,188,189,197)(H4,190,191,198)(H4,192,193,199)/t88-,89-,90-,91-,92-,93-,94+,104-,105-,106-,107-,108-,109-,110-,111-,112-,113-,114-,115-,116-,117-,118-,119-,120-,121-,122-,123-,124-,125-,126-,127-,128-,129-,130-,140-,141-,142-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Corporation
Curated by PDSP Ki Database
| |
J Biol Chem 271: 26315-9 (1996)
Article DOI: 10.1074/jbc.271.42.26315
BindingDB Entry DOI: 10.7270/Q20G3HPR |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 5
(Homo sapiens (Human)) | BDBM82277

(NPY2-36, human | NPY2-36, rat, human)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](Cc1c[nH]cn1)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](C)NC(=O)[C@H](CO)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](C)NC(=O)[C@H](CCSC)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](C)NC(=O)[C@@H]1CCCN1C(=O)[C@H](C)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)CNC(=O)[C@@H]1CCCN1C(=O)[C@H](CC(N)=O)NC(=O)[C@H](CC(O)=O)NC(=O)[C@@H]1CCCN1C(=O)[C@H](CCCCN)NC(=O)[C@H](CO)NC(=O)[C@@H]1CCCN1)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc1ccc(O)cc1)C(N)=O Show InChI InChI=1S/C180H276N54O55S/c1-15-88(7)140(171(284)225-121(76-132(183)243)161(274)217-116(70-87(5)6)164(277)230-141(89(8)16-2)172(285)231-142(94(13)237)173(286)213-108(32-23-64-199-180(192)193)149(262)211-110(52-55-131(182)242)154(267)209-106(30-21-62-197-178(188)189)150(263)215-114(143(185)256)71-95-36-44-100(238)45-37-95)229-165(278)119(74-98-42-50-103(241)51-43-98)220-160(273)120(75-99-81-194-85-201-99)221-152(265)107(31-22-63-198-179(190)191)210-157(270)115(69-86(3)4)216-146(259)91(10)203-166(279)126(83-235)228-159(272)118(73-97-40-48-102(240)49-41-97)219-158(271)117(72-96-38-46-101(239)47-39-96)218-151(264)105(29-20-61-196-177(186)187)207-144(257)90(9)202-148(261)112(58-68-290-14)212-162(275)123(79-138(252)253)223-155(268)111(54-57-136(248)249)208-145(258)92(11)204-169(282)129-34-25-65-232(129)174(287)93(12)205-156(269)122(78-137(250)251)222-153(266)109(53-56-135(246)247)206-134(245)82-200-168(281)128-33-24-66-233(128)176(289)125(77-133(184)244)226-163(276)124(80-139(254)255)224-170(283)130-35-26-67-234(130)175(288)113(27-17-18-59-181)214-167(280)127(84-236)227-147(260)104-28-19-60-195-104/h36-51,81,85-94,104-130,140-142,195,235-241H,15-35,52-80,82-84,181H2,1-14H3,(H2,182,242)(H2,183,243)(H2,184,244)(H2,185,256)(H,194,201)(H,200,281)(H,202,261)(H,203,279)(H,204,282)(H,205,269)(H,206,245)(H,207,257)(H,208,258)(H,209,267)(H,210,270)(H,211,262)(H,212,275)(H,213,286)(H,214,280)(H,215,263)(H,216,259)(H,217,274)(H,218,264)(H,219,271)(H,220,273)(H,221,265)(H,222,266)(H,223,268)(H,224,283)(H,225,284)(H,226,276)(H,227,260)(H,228,272)(H,229,278)(H,230,277)(H,231,285)(H,246,247)(H,248,249)(H,250,251)(H,252,253)(H,254,255)(H4,186,187,196)(H4,188,189,197)(H4,190,191,198)(H4,192,193,199)/t88-,89-,90-,91-,92-,93-,94+,104-,105-,106-,107-,108-,109-,110-,111-,112-,113-,114-,115-,116-,117-,118-,119-,120-,121-,122-,123-,124-,125-,126-,127-,128-,129-,130-,140-,141-,142-/m0/s1 | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Corporation
Curated by PDSP Ki Database
| |
J Biol Chem 271: 26315-9 (1996)
Article DOI: 10.1074/jbc.271.42.26315
BindingDB Entry DOI: 10.7270/Q20G3HPR |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 5
(Homo sapiens (Human)) | BDBM50091652

(CHEMBL269503 | PYY | PYY, rat | Peptide YY(PYY)(YP...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@@H]1CCCN1C(=O)[C@H](CO)NC(=O)[C@H](C)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)CNC(=O)[C@@H]1CCCN1C(=O)[C@H](C)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@@H]1CCCN1C(=O)[C@H](CCCCN)NC(=O)[C@H](C)NC(=O)[C@@H]1CCCN1C(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](CO)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](C)C(=O)N[C@@H](CO)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc1c[nH]cn1)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc1ccc(O)cc1)C(O)=O Show InChI InChI=1S/C190H287N53O58/c1-92(2)74-124(166(280)216-114(27-18-66-204-187(195)196)158(272)231-131(83-107-86-203-91-209-107)171(285)230-130(81-105-41-51-111(251)52-42-105)169(283)225-125(75-93(3)4)167(281)232-132(84-143(194)254)172(286)226-127(77-95(7)8)173(287)238-150(96(9)10)180(294)239-151(101(15)247)181(295)222-117(30-21-69-207-190(201)202)156(270)218-119(55-60-142(193)253)161(275)215-116(29-20-68-206-189(199)200)159(273)234-134(186(300)301)82-106-43-53-112(252)54-44-106)227-175(289)135(88-244)235-153(267)97(11)210-164(278)128(79-103-37-47-109(249)48-38-103)229-170(284)129(80-104-39-49-110(250)50-40-104)228-157(271)115(28-19-67-205-188(197)198)217-174(288)136(89-245)236-168(282)126(76-94(5)6)224-163(277)121(58-63-147(260)261)219-162(276)122(59-64-148(262)263)221-179(293)141-34-25-73-243(141)185(299)137(90-246)237-154(268)98(12)211-165(279)133(85-149(264)265)233-160(274)118(56-61-145(256)257)214-144(255)87-208-176(290)138-31-22-70-240(138)182(296)100(14)213-155(269)120(57-62-146(258)259)220-178(292)140-33-24-72-242(140)184(298)123(26-16-17-65-191)223-152(266)99(13)212-177(291)139-32-23-71-241(139)183(297)113(192)78-102-35-45-108(248)46-36-102/h35-54,86,91-101,113-141,150-151,244-252H,16-34,55-85,87-90,191-192H2,1-15H3,(H2,193,253)(H2,194,254)(H,203,209)(H,208,290)(H,210,278)(H,211,279)(H,212,291)(H,213,269)(H,214,255)(H,215,275)(H,216,280)(H,217,288)(H,218,270)(H,219,276)(H,220,292)(H,221,293)(H,222,295)(H,223,266)(H,224,277)(H,225,283)(H,226,286)(H,227,289)(H,228,271)(H,229,284)(H,230,285)(H,231,272)(H,232,281)(H,233,274)(H,234,273)(H,235,267)(H,236,282)(H,237,268)(H,238,287)(H,239,294)(H,256,257)(H,258,259)(H,260,261)(H,262,263)(H,264,265)(H,300,301)(H4,195,196,204)(H4,197,198,205)(H4,199,200,206)(H4,201,202,207)/t97-,98-,99-,100-,101+,113-,114-,115-,116-,117-,118-,119-,120-,121-,122-,123-,124-,125-,126-,127-,128-,129-,130-,131-,132-,133-,134-,135-,136-,137-,138-,139-,140-,141-,150-,151-/m0/s1 | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Corporation
Curated by PDSP Ki Database
| |
J Biol Chem 271: 26315-9 (1996)
Article DOI: 10.1074/jbc.271.42.26315
BindingDB Entry DOI: 10.7270/Q20G3HPR |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM28583
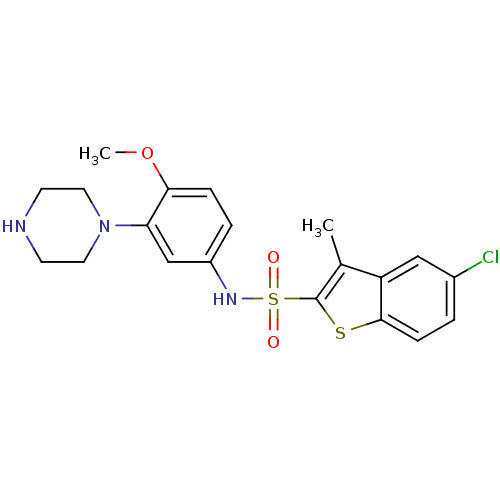
(5-chloro-N-[4-methoxy-3-(piperazin-1-yl)phenyl]-3-...)Show SMILES COc1ccc(NS(=O)(=O)c2sc3ccc(Cl)cc3c2C)cc1N1CCNCC1 Show InChI InChI=1S/C20H22ClN3O3S2/c1-13-16-11-14(21)3-6-19(16)28-20(13)29(25,26)23-15-4-5-18(27-2)17(12-15)24-9-7-22-8-10-24/h3-6,11-12,22-23H,7-10H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5HT6 receptor expressed in HeLa cells |
Bioorg Med Chem Lett 19: 3214-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.04.108
BindingDB Entry DOI: 10.7270/Q2377927 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM28580
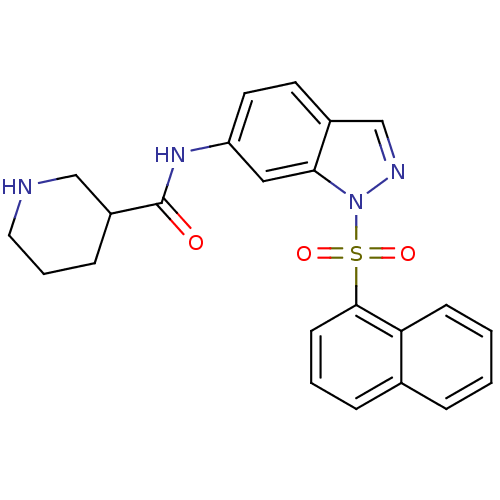
(1-sulfonylindazole, 7b | N-[1-(naphthalene-1-sulfo...)Show SMILES O=C(Nc1ccc2cnn(c2c1)S(=O)(=O)c1cccc2ccccc12)C1CCCNC1 Show InChI InChI=1S/C23H22N4O3S/c28-23(18-7-4-12-24-14-18)26-19-11-10-17-15-25-27(21(17)13-19)31(29,30)22-9-3-6-16-5-1-2-8-20(16)22/h1-3,5-6,8-11,13,15,18,24H,4,7,12,14H2,(H,26,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30 | -50.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Wyeth Research
| Assay Description
Competition experiments were performed in the presence radioligand with membrane protein (obtained from cells expressing the receptor) and test compo... |
Bioorg Med Chem Lett 19: 2413-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.071
BindingDB Entry DOI: 10.7270/Q28K77CF |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50330523
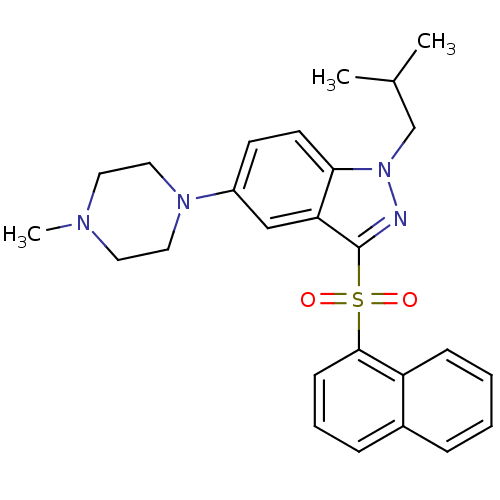
(1-isobutyl-5-(4-methylpiperazin-1-yl)-3-(naphthale...)Show SMILES CC(C)Cn1nc(c2cc(ccc12)N1CCN(C)CC1)S(=O)(=O)c1cccc2ccccc12 Show InChI InChI=1S/C26H30N4O2S/c1-19(2)18-30-24-12-11-21(29-15-13-28(3)14-16-29)17-23(24)26(27-30)33(31,32)25-10-6-8-20-7-4-5-9-22(20)25/h4-12,17,19H,13-16,18H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human cloned 5HT6 receptor expressed in human HeLa cells |
J Med Chem 53: 7639-46 (2010)
Article DOI: 10.1021/jm1007825
BindingDB Entry DOI: 10.7270/Q2GQ6XZ6 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50334747
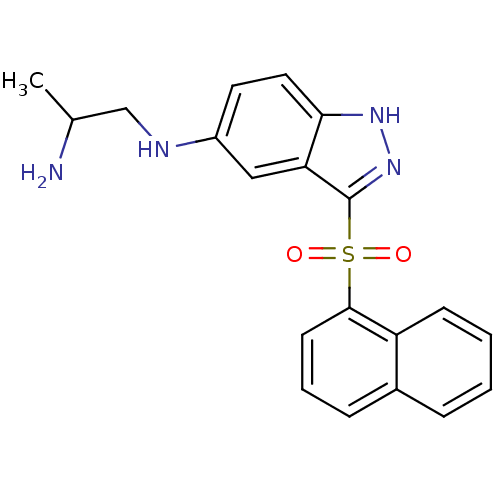
(CHEMBL1642849 | N1-(3-(Naphthalen-1-ylsulfonyl)-1H...)Show SMILES CC(N)CNc1ccc2[nH]nc(c2c1)S(=O)(=O)c1cccc2ccccc12 Show InChI InChI=1S/C20H20N4O2S/c1-13(21)12-22-15-9-10-18-17(11-15)20(24-23-18)27(25,26)19-8-4-6-14-5-2-3-7-16(14)19/h2-11,13,22H,12,21H2,1H3,(H,23,24) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]-LSD from cloned human 5-HT6 receptor expressed in human HeLa cells |
Bioorg Med Chem 19: 650-62 (2011)
Article DOI: 10.1016/j.bmc.2010.10.033
BindingDB Entry DOI: 10.7270/Q29S1R9V |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 5
(Rat 6B) | BDBM82290
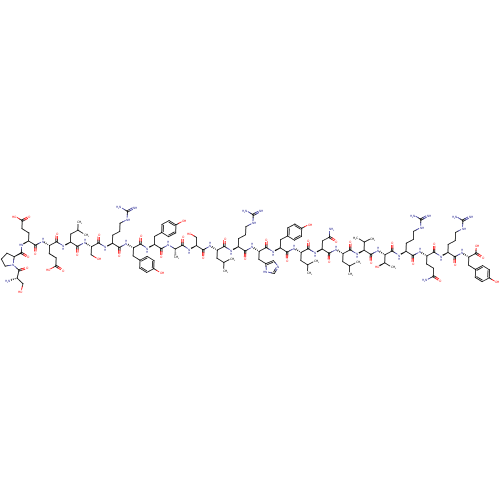
(PYY 3-36, rat | PYY13-36, porcine)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@@H]1CCCN1C(=O)[C@@H](N)CO)C(=O)N[C@@H](CO)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](C)C(=O)N[C@@H](CO)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1ccc(O)cc1)C(O)=O Show InChI InChI=1S/C135H208N40O39/c1-65(2)50-89(164-126(208)99(62-177)171-108(190)70(11)153-116(198)93(54-72-23-31-77(180)32-24-72)166-121(203)94(55-73-25-33-78(181)34-26-73)165-110(192)83(19-14-46-149-133(141)142)156-125(207)100(63-178)172-119(201)91(52-67(5)6)161-115(197)87(40-43-104(186)187)158-114(196)88(41-44-105(188)189)159-127(209)101-22-17-49-175(101)130(212)81(136)61-176)117(199)155-82(18-13-45-148-132(139)140)111(193)168-96(58-76-60-147-64-152-76)122(204)167-95(56-74-27-35-79(182)36-28-74)120(202)162-90(51-66(3)4)118(200)169-97(59-103(138)185)123(205)163-92(53-68(7)8)124(206)173-106(69(9)10)128(210)174-107(71(12)179)129(211)160-85(21-16-48-151-135(145)146)109(191)157-86(39-42-102(137)184)113(195)154-84(20-15-47-150-134(143)144)112(194)170-98(131(213)214)57-75-29-37-80(183)38-30-75/h23-38,60,64-71,81-101,106-107,176-183H,13-22,39-59,61-63,136H2,1-12H3,(H2,137,184)(H2,138,185)(H,147,152)(H,153,198)(H,154,195)(H,155,199)(H,156,207)(H,157,191)(H,158,196)(H,159,209)(H,160,211)(H,161,197)(H,162,202)(H,163,205)(H,164,208)(H,165,192)(H,166,203)(H,167,204)(H,168,193)(H,169,200)(H,170,194)(H,171,190)(H,172,201)(H,173,206)(H,174,210)(H,186,187)(H,188,189)(H,213,214)(H4,139,140,148)(H4,141,142,149)(H4,143,144,150)(H4,145,146,151)/t70-,71+,81-,82-,83-,84-,85-,86-,87-,88-,89-,90-,91-,92-,93-,94-,95-,96-,97-,98-,99-,100-,101-,106-,107-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Corporation
Curated by PDSP Ki Database
| |
J Biol Chem 271: 26315-9 (1996)
Article DOI: 10.1074/jbc.271.42.26315
BindingDB Entry DOI: 10.7270/Q20G3HPR |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50334720
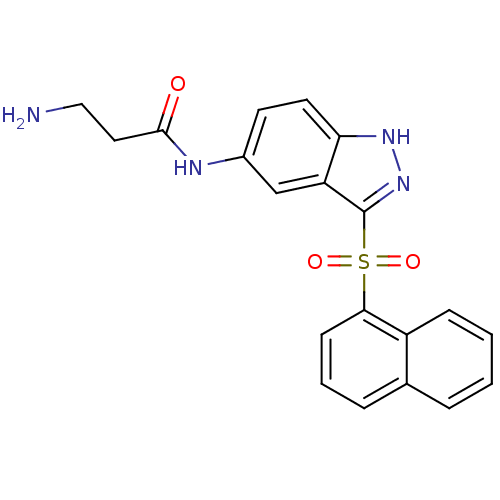
(3-Amino-N-[3-(naphthalen-1-sulfonyl)-1H-indazol-5-...)Show SMILES NCCC(=O)Nc1ccc2[nH]nc(c2c1)S(=O)(=O)c1cccc2ccccc12 Show InChI InChI=1S/C20H18N4O3S/c21-11-10-19(25)22-14-8-9-17-16(12-14)20(24-23-17)28(26,27)18-7-3-5-13-4-1-2-6-15(13)18/h1-9,12H,10-11,21H2,(H,22,25)(H,23,24) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]-LSD from cloned human 5-HT6 receptor expressed in human HeLa cells |
Bioorg Med Chem 19: 650-62 (2011)
Article DOI: 10.1016/j.bmc.2010.10.033
BindingDB Entry DOI: 10.7270/Q29S1R9V |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50334757
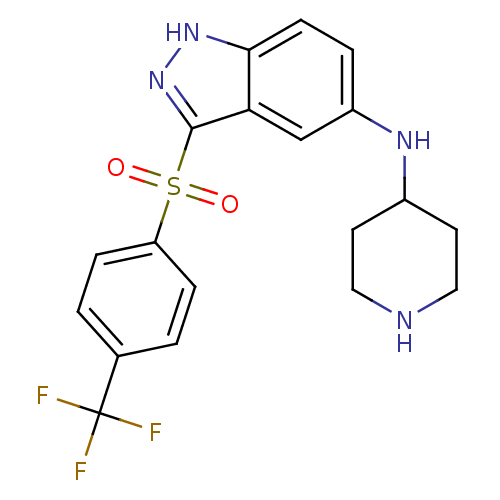
(CHEMBL1642887 | N-(Piperidin-4-yl)-3-(4-(trifluoro...)Show SMILES FC(F)(F)c1ccc(cc1)S(=O)(=O)c1n[nH]c2ccc(NC3CCNCC3)cc12 Show InChI InChI=1S/C19H19F3N4O2S/c20-19(21,22)12-1-4-15(5-2-12)29(27,28)18-16-11-14(3-6-17(16)25-26-18)24-13-7-9-23-10-8-13/h1-6,11,13,23-24H,7-10H2,(H,25,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]-LSD from cloned human 5-HT6 receptor expressed in human HeLa cells |
Bioorg Med Chem 19: 650-62 (2011)
Article DOI: 10.1016/j.bmc.2010.10.033
BindingDB Entry DOI: 10.7270/Q29S1R9V |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50330511
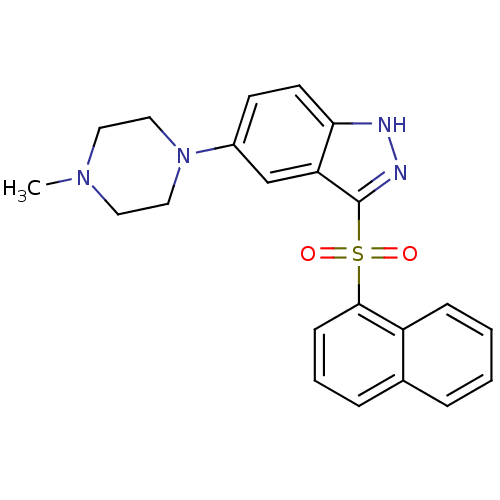
(5-(4-Methylpiperazin-1-yl)-3-(1-naphthalenesulfony...)Show SMILES CN1CCN(CC1)c1ccc2[nH]nc(c2c1)S(=O)(=O)c1cccc2ccccc12 Show InChI InChI=1S/C22H22N4O2S/c1-25-11-13-26(14-12-25)17-9-10-20-19(15-17)22(24-23-20)29(27,28)21-8-4-6-16-5-2-3-7-18(16)21/h2-10,15H,11-14H2,1H3,(H,23,24) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human cloned 5HT6 receptor expressed in human HeLa cells |
J Med Chem 53: 7639-46 (2010)
Article DOI: 10.1021/jm1007825
BindingDB Entry DOI: 10.7270/Q2GQ6XZ6 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50124895
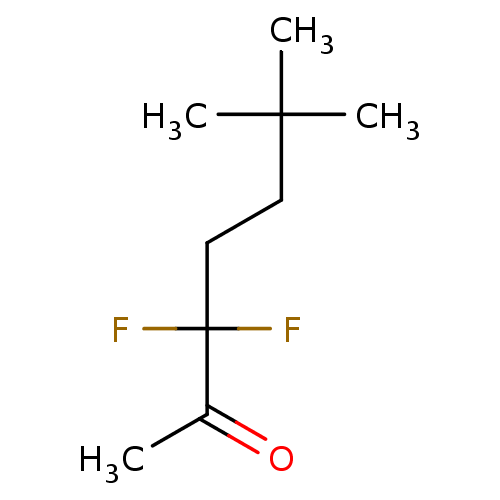
(CHEMBL3623568)Show InChI InChI=1S/C9H16F2O/c1-7(12)9(10,11)6-5-8(2,3)4/h5-6H2,1-4H3 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Tech
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE |
Bioorg Med Chem Lett 25: 4405-11 (2015)
Article DOI: 10.1016/j.bmcl.2015.09.019
BindingDB Entry DOI: 10.7270/Q2Z60QV8 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50330516
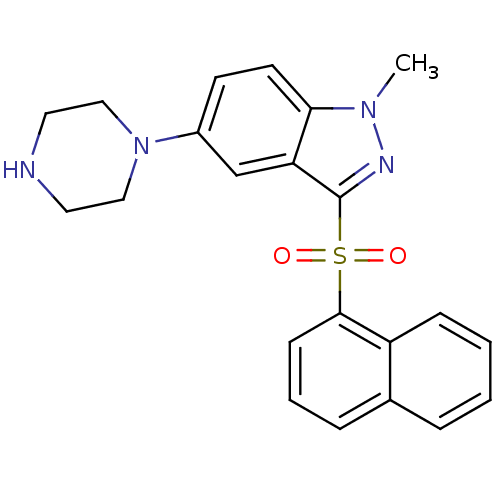
(1-methyl-3-(naphthalen-1-ylsulfonyl)-5-(piperazin-...)Show SMILES Cn1nc(c2cc(ccc12)N1CCNCC1)S(=O)(=O)c1cccc2ccccc12 Show InChI InChI=1S/C22H22N4O2S/c1-25-20-10-9-17(26-13-11-23-12-14-26)15-19(20)22(24-25)29(27,28)21-8-4-6-16-5-2-3-7-18(16)21/h2-10,15,23H,11-14H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human cloned 5HT6 receptor expressed in human HeLa cells |
J Med Chem 53: 7639-46 (2010)
Article DOI: 10.1021/jm1007825
BindingDB Entry DOI: 10.7270/Q2GQ6XZ6 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50330517
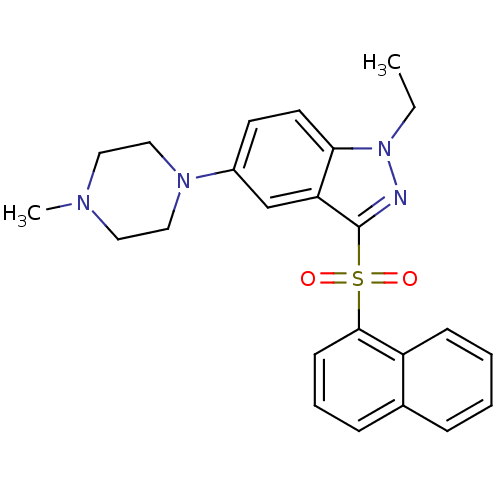
(1-ethyl-5-(4-methylpiperazin-1-yl)-3-(naphthalen-1...)Show SMILES CCn1nc(c2cc(ccc12)N1CCN(C)CC1)S(=O)(=O)c1cccc2ccccc12 Show InChI InChI=1S/C24H26N4O2S/c1-3-28-22-12-11-19(27-15-13-26(2)14-16-27)17-21(22)24(25-28)31(29,30)23-10-6-8-18-7-4-5-9-20(18)23/h4-12,17H,3,13-16H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human cloned 5HT6 receptor expressed in human HeLa cells |
J Med Chem 53: 7639-46 (2010)
Article DOI: 10.1021/jm1007825
BindingDB Entry DOI: 10.7270/Q2GQ6XZ6 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50330515
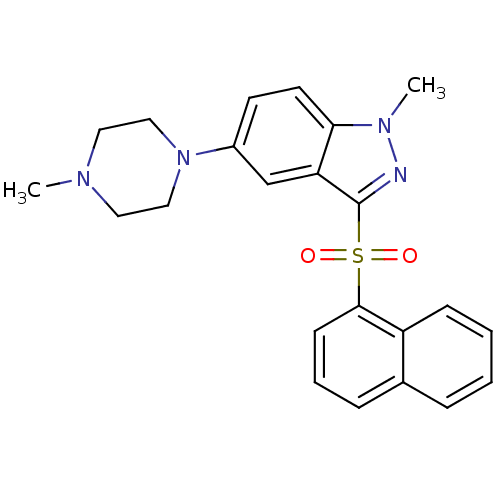
(1-methyl-5-(4-methylpiperazin-1-yl)-3-(naphthalen-...)Show SMILES CN1CCN(CC1)c1ccc2n(C)nc(c2c1)S(=O)(=O)c1cccc2ccccc12 Show InChI InChI=1S/C23H24N4O2S/c1-25-12-14-27(15-13-25)18-10-11-21-20(16-18)23(24-26(21)2)30(28,29)22-9-5-7-17-6-3-4-8-19(17)22/h3-11,16H,12-15H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human cloned 5HT6 receptor expressed in human HeLa cells |
J Med Chem 53: 7639-46 (2010)
Article DOI: 10.1021/jm1007825
BindingDB Entry DOI: 10.7270/Q2GQ6XZ6 |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 5
(Homo sapiens (Human)) | BDBM84999

(PYY,[leu31,Pro34], human)Show SMILES CCC(C)[C@H](NC(=O)[C@@H]1CCCN1C(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](CCCCN)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](C)C(=O)N1CCC[C@H]1C(=O)NCC(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](C)C(=O)N[C@@H](CO)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](C)C(=O)N[C@@H](CO)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc1c[nH]cn1)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc1ccc(O)cc1)C(O)=O Show InChI InChI=1S/C194H295N53O58/c1-17-101(12)155(241-182(294)144-37-25-73-244(144)187(299)117(196)82-106-39-49-112(251)50-40-106)184(296)224-125(29-18-19-67-195)188(300)245-74-27-36-143(245)181(293)222-122(60-64-151(261)262)159(271)216-104(15)186(298)243-72-24-34-141(243)179(291)212-92-148(258)217-121(59-63-150(259)260)163(275)237-138(90-154(267)268)167(279)215-103(14)158(270)240-149(94-249)305(304)247-76-28-38-145(247)183(295)223-124(62-66-153(265)266)164(276)220-123(61-65-152(263)264)165(277)226-128(78-97(4)5)170(282)235-136(88-146(197)256)175(287)219-118(30-20-68-208-191(199)200)160(272)231-133(84-108-43-53-114(253)54-44-108)173(285)232-132(83-107-41-51-113(252)52-42-107)166(278)214-102(13)157(269)239-140(93-248)178(290)230-127(77-96(2)3)168(280)218-119(31-21-69-209-192(201)202)161(273)234-135(87-111-91-207-95-213-111)174(286)233-134(85-109-45-55-115(254)56-46-109)172(284)227-130(80-99(8)9)171(283)236-137(89-147(198)257)176(288)228-129(79-98(6)7)169(281)229-131(81-100(10)11)177(289)242-156(105(16)250)185(297)225-126(33-23-71-211-194(205)206)189(301)246-75-26-35-142(246)180(292)221-120(32-22-70-210-193(203)204)162(274)238-139(190(302)303)86-110-47-57-116(255)58-48-110/h39-58,91,95-105,117-145,149,155-156,248-255H,17-38,59-90,92-94,195-196H2,1-16H3,(H2,197,256)(H2,198,257)(H,207,213)(H,212,291)(H,214,278)(H,215,279)(H,216,271)(H,217,258)(H,218,280)(H,219,287)(H,220,276)(H,221,292)(H,222,293)(H,223,295)(H,224,296)(H,225,297)(H,226,277)(H,227,284)(H,228,288)(H,229,281)(H,230,290)(H,231,272)(H,232,285)(H,233,286)(H,234,273)(H,235,282)(H,236,283)(H,237,275)(H,238,274)(H,239,269)(H,240,270)(H,241,294)(H,242,289)(H,259,260)(H,261,262)(H,263,264)(H,265,266)(H,267,268)(H,302,303)(H4,199,200,208)(H4,201,202,209)(H4,203,204,210)(H4,205,206,211)/t101?,102-,103-,104-,105+,117-,118-,119-,120-,121-,122-,123-,124-,125-,126-,127-,128-,129-,130-,131-,132-,133-,134-,135-,136-,137-,138-,139-,140-,141-,142-,143-,144-,145-,149+,155-,156-/m0/s1 | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Corporation
Curated by PDSP Ki Database
| |
J Biol Chem 271: 26315-9 (1996)
Article DOI: 10.1074/jbc.271.42.26315
BindingDB Entry DOI: 10.7270/Q20G3HPR |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50330535
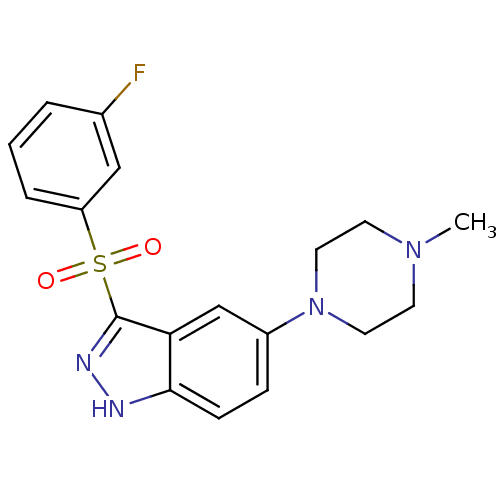
(3-(3-Fluorophenylsulfonyl)-5-(4-methylpiperazin-1-...)Show SMILES CN1CCN(CC1)c1ccc2[nH]nc(c2c1)S(=O)(=O)c1cccc(F)c1 Show InChI InChI=1S/C18H19FN4O2S/c1-22-7-9-23(10-8-22)14-5-6-17-16(12-14)18(21-20-17)26(24,25)15-4-2-3-13(19)11-15/h2-6,11-12H,7-10H2,1H3,(H,20,21) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human cloned 5HT6 receptor expressed in human HeLa cells |
J Med Chem 53: 7639-46 (2010)
Article DOI: 10.1021/jm1007825
BindingDB Entry DOI: 10.7270/Q2GQ6XZ6 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50334716
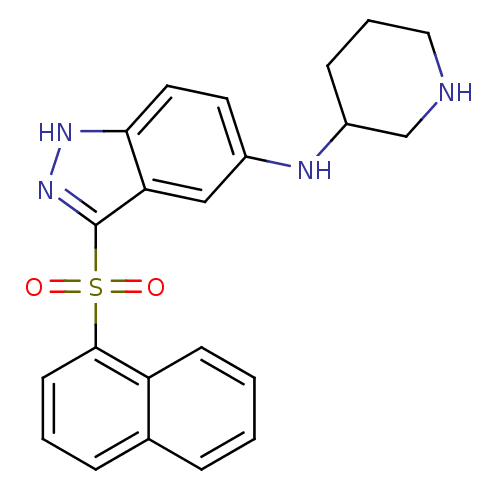
(3-(Naphthalen-1-ylsulfonyl)-N-(piperidin-5-yl)-1H-...)Show SMILES O=S(=O)(c1n[nH]c2ccc(NC3CCCNC3)cc12)c1cccc2ccccc12 Show InChI InChI=1S/C22H22N4O2S/c27-29(28,21-9-3-6-15-5-1-2-8-18(15)21)22-19-13-16(10-11-20(19)25-26-22)24-17-7-4-12-23-14-17/h1-3,5-6,8-11,13,17,23-24H,4,7,12,14H2,(H,25,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]-LSD from cloned human 5-HT6 receptor expressed in human HeLa cells |
Bioorg Med Chem 19: 650-62 (2011)
Article DOI: 10.1016/j.bmc.2010.10.033
BindingDB Entry DOI: 10.7270/Q29S1R9V |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50083225

(CHEMBL341692 | Decanoic acid 3-(4-{2-[bis-(4-fluor...)Show SMILES CCCCCCCCCC(=O)OC(CCN1CCN(CCOC(c2ccc(F)cc2)c2ccc(F)cc2)CC1)c1ccccc1 Show InChI InChI=1S/C38H50F2N2O3/c1-2-3-4-5-6-7-11-14-37(43)45-36(31-12-9-8-10-13-31)23-24-41-25-27-42(28-26-41)29-30-44-38(32-15-19-34(39)20-16-32)33-17-21-35(40)22-18-33/h8-10,12-13,15-22,36,38H,2-7,11,14,23-30H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 1.73 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases
Curated by ChEMBL
| Assay Description
Binding affinity against Serotonin transporter (SERT) labelled with [125I]-RTI-55 in rat. |
J Med Chem 42: 5029-42 (2000)
BindingDB Entry DOI: 10.7270/Q2R49PZ2 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50330521
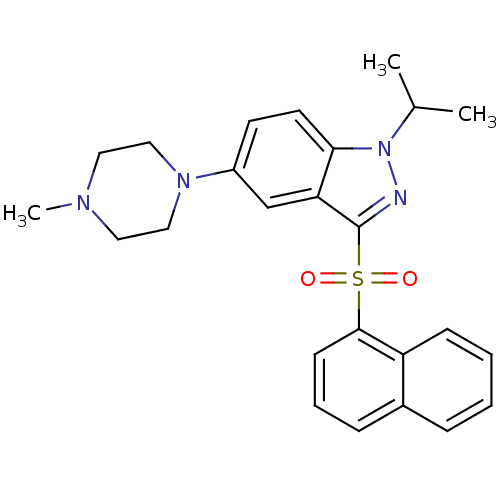
(1-isopropyl-5-(4-methylpiperazin-1-yl)-3-(naphthal...)Show SMILES CC(C)n1nc(c2cc(ccc12)N1CCN(C)CC1)S(=O)(=O)c1cccc2ccccc12 Show InChI InChI=1S/C25H28N4O2S/c1-18(2)29-23-12-11-20(28-15-13-27(3)14-16-28)17-22(23)25(26-29)32(30,31)24-10-6-8-19-7-4-5-9-21(19)24/h4-12,17-18H,13-16H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human cloned 5HT6 receptor expressed in human HeLa cells |
J Med Chem 53: 7639-46 (2010)
Article DOI: 10.1021/jm1007825
BindingDB Entry DOI: 10.7270/Q2GQ6XZ6 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50330519
(5-(4-methylpiperazin-1-yl)-3-(naphthalen-1-ylsulfo...)Show SMILES CCCn1nc(c2cc(ccc12)N1CCN(C)CC1)S(=O)(=O)c1cccc2ccccc12 Show InChI InChI=1S/C25H28N4O2S/c1-3-13-29-23-12-11-20(28-16-14-27(2)15-17-28)18-22(23)25(26-29)32(30,31)24-10-6-8-19-7-4-5-9-21(19)24/h4-12,18H,3,13-17H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human cloned 5HT6 receptor expressed in human HeLa cells |
J Med Chem 53: 7639-46 (2010)
Article DOI: 10.1021/jm1007825
BindingDB Entry DOI: 10.7270/Q2GQ6XZ6 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM28576
(1-sulfonylindazole, 6e | 3-(dimethylamino)-N-[1-(n...)Show SMILES CN(C)CCC(=O)Nc1ccc2cnn(c2c1)S(=O)(=O)c1cccc2ccccc12 Show InChI InChI=1S/C22H22N4O3S/c1-25(2)13-12-22(27)24-18-11-10-17-15-23-26(20(17)14-18)30(28,29)21-9-5-7-16-6-3-4-8-19(16)21/h3-11,14-15H,12-13H2,1-2H3,(H,24,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.80 | -49.4 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Wyeth Research
| Assay Description
Competition experiments were performed in the presence radioligand with membrane protein (obtained from cells expressing the receptor) and test compo... |
Bioorg Med Chem Lett 19: 2413-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.071
BindingDB Entry DOI: 10.7270/Q28K77CF |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data