

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Methionine aminopeptidase (Escherichia coli (strain K12)) | BDBM50129663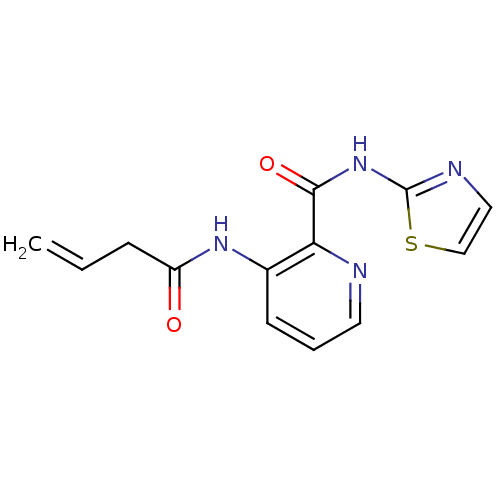 (3-But-3-enoylamino-pyridine-2-carboxylic acid thia...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibitory activity against Methionine aminopeptidase 1 from Escherichia coli | J Med Chem 46: 2631-40 (2003) Article DOI: 10.1021/jm0300532 BindingDB Entry DOI: 10.7270/Q2CF9QTC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 1 (Saccharomyces cerevisiae) | BDBM50129648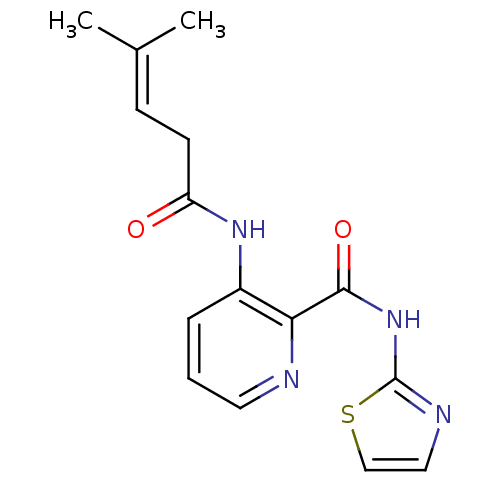 (3-(4-Methyl-pent-3-enoylamino)-pyridine-2-carboxyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibitory activity against type I methionine aminopeptidase from Saccharomyces cerevisiae | J Med Chem 46: 2631-40 (2003) Article DOI: 10.1021/jm0300532 BindingDB Entry DOI: 10.7270/Q2CF9QTC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase (Escherichia coli (strain K12)) | BDBM50129660 (3-(3-Methyl-but-3-enoylamino)-pyridine-2-carboxyli...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibitory activity against Methionine aminopeptidase 1 from Escherichia coli | J Med Chem 46: 2631-40 (2003) Article DOI: 10.1021/jm0300532 BindingDB Entry DOI: 10.7270/Q2CF9QTC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase (Escherichia coli (strain K12)) | BDBM17847 (CHEMBL327579 | pyridine-2-carboxylic acid inhibito...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibitory activity against Methionine aminopeptidase 1 from Escherichia coli | J Med Chem 46: 2631-40 (2003) Article DOI: 10.1021/jm0300532 BindingDB Entry DOI: 10.7270/Q2CF9QTC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 1 (Saccharomyces cerevisiae) | BDBM50129642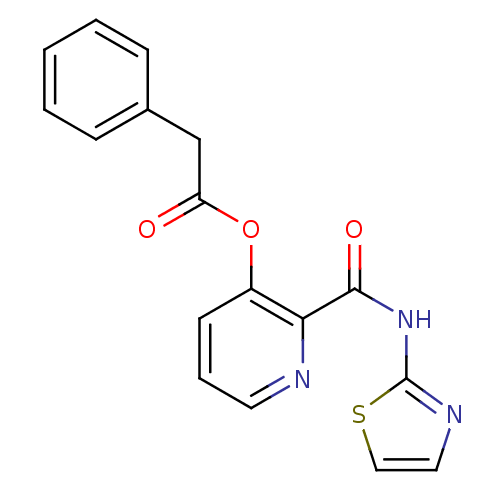 (CHEMBL86427 | Phenyl-acetic acid 2-(thiazol-2-ylca...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibitory activity against type I methionine aminopeptidase from Saccharomyces cerevisiae | J Med Chem 46: 2631-40 (2003) Article DOI: 10.1021/jm0300532 BindingDB Entry DOI: 10.7270/Q2CF9QTC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase (Escherichia coli (strain K12)) | BDBM50129640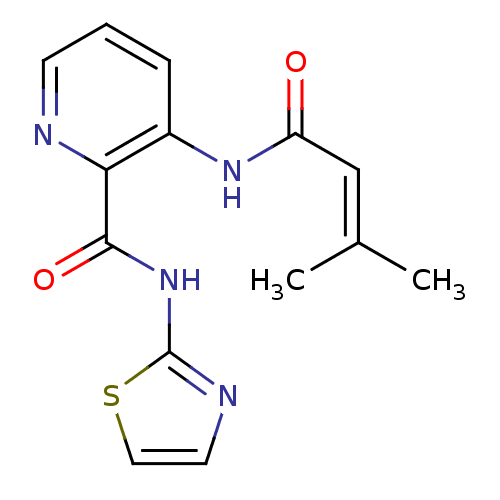 (3-(3-Methyl-but-2-enoylamino)-pyridine-2-carboxyli...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibitory activity against Methionine aminopeptidase 1 from Escherichia coli | J Med Chem 46: 2631-40 (2003) Article DOI: 10.1021/jm0300532 BindingDB Entry DOI: 10.7270/Q2CF9QTC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase (Escherichia coli (strain K12)) | BDBM50129648 (3-(4-Methyl-pent-3-enoylamino)-pyridine-2-carboxyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibitory activity against Methionine aminopeptidase 1 from Escherichia coli | J Med Chem 46: 2631-40 (2003) Article DOI: 10.1021/jm0300532 BindingDB Entry DOI: 10.7270/Q2CF9QTC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase (Escherichia coli (strain K12)) | BDBM50129672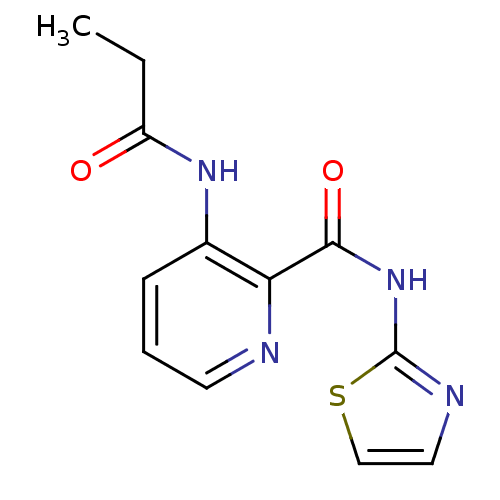 (3-Propionylamino-pyridine-2-carboxylic acid thiazo...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibitory concentration against Escherichia coli methionine aminopeptidase 1 | Bioorg Med Chem Lett 15: 635-8 (2005) Article DOI: 10.1016/j.bmcl.2004.11.034 BindingDB Entry DOI: 10.7270/Q2222T7R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase (Escherichia coli (strain K12)) | BDBM50129672 (3-Propionylamino-pyridine-2-carboxylic acid thiazo...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibitory activity against Methionine aminopeptidase 1 from Escherichia coli | J Med Chem 46: 2631-40 (2003) Article DOI: 10.1021/jm0300532 BindingDB Entry DOI: 10.7270/Q2CF9QTC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase (Escherichia coli (strain K12)) | BDBM50129674 (3-(2-Methyl-but-2-enoylamino)-pyridine-2-carboxyli...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 280 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibitory activity against Methionine aminopeptidase 1 from Escherichia coli | J Med Chem 46: 2631-40 (2003) Article DOI: 10.1021/jm0300532 BindingDB Entry DOI: 10.7270/Q2CF9QTC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase (Escherichia coli (strain K12)) | BDBM50129657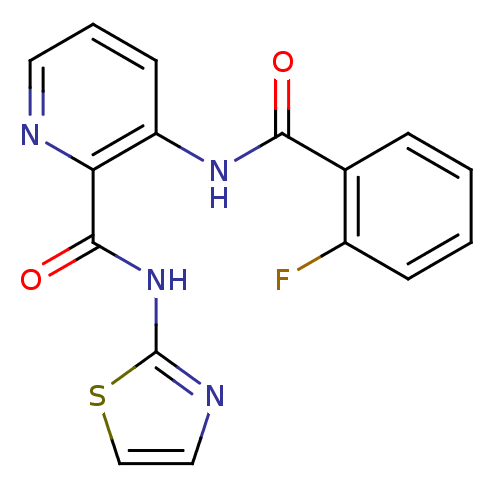 (3-(2-Fluoro-benzoylamino)-pyridine-2-carboxylic ac...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 280 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibitory activity against Methionine aminopeptidase 1 from Escherichia coli | J Med Chem 46: 2631-40 (2003) Article DOI: 10.1021/jm0300532 BindingDB Entry DOI: 10.7270/Q2CF9QTC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 1 (Saccharomyces cerevisiae) | BDBM50129669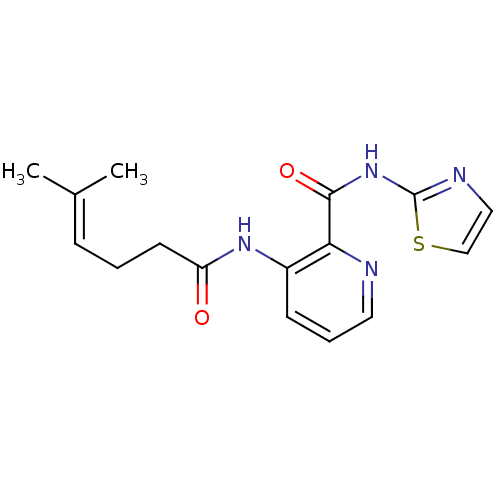 (3-(5-Methyl-hex-4-enoylamino)-pyridine-2-carboxyli...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 310 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibitory activity against type I methionine aminopeptidase from Saccharomyces cerevisiae | J Med Chem 46: 2631-40 (2003) Article DOI: 10.1021/jm0300532 BindingDB Entry DOI: 10.7270/Q2CF9QTC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 1 (Saccharomyces cerevisiae) | BDBM50129649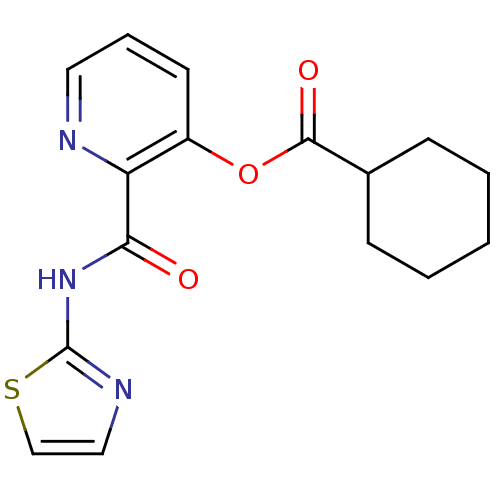 (CHEMBL315098 | Cyclohexanecarboxylic acid 2-(thiaz...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 320 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibitory activity against type I methionine aminopeptidase from Saccharomyces cerevisiae | J Med Chem 46: 2631-40 (2003) Article DOI: 10.1021/jm0300532 BindingDB Entry DOI: 10.7270/Q2CF9QTC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 1 (Saccharomyces cerevisiae) | BDBM50129635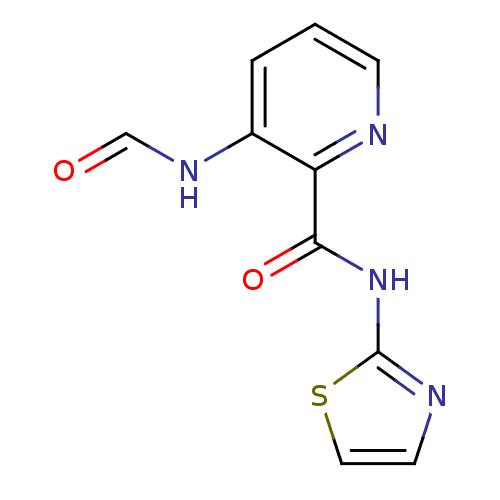 (3-Formylamino-pyridine-2-carboxylic acid thiazol-2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 320 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibitory activity against type I methionine aminopeptidase from Saccharomyces cerevisiae | J Med Chem 46: 2631-40 (2003) Article DOI: 10.1021/jm0300532 BindingDB Entry DOI: 10.7270/Q2CF9QTC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase (Escherichia coli (strain K12)) | BDBM50129635 (3-Formylamino-pyridine-2-carboxylic acid thiazol-2...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 330 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibitory activity against Methionine aminopeptidase 1 from Escherichia coli | J Med Chem 46: 2631-40 (2003) Article DOI: 10.1021/jm0300532 BindingDB Entry DOI: 10.7270/Q2CF9QTC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase (Escherichia coli (strain K12)) | BDBM50129670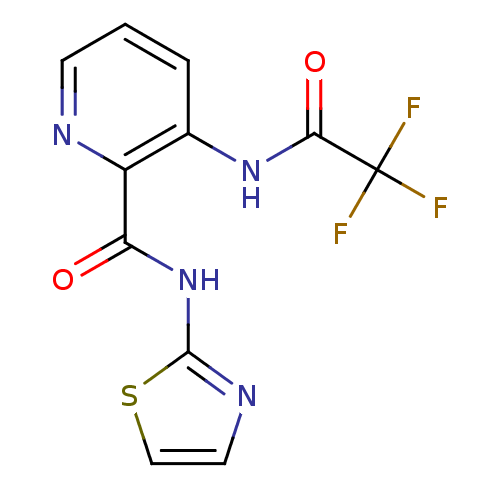 (3-(2,2,2-Trifluoro-acetylamino)-pyridine-2-carboxy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 340 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibitory activity against Methionine aminopeptidase 1 from Escherichia coli | J Med Chem 46: 2631-40 (2003) Article DOI: 10.1021/jm0300532 BindingDB Entry DOI: 10.7270/Q2CF9QTC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 1 (Saccharomyces cerevisiae) | BDBM17849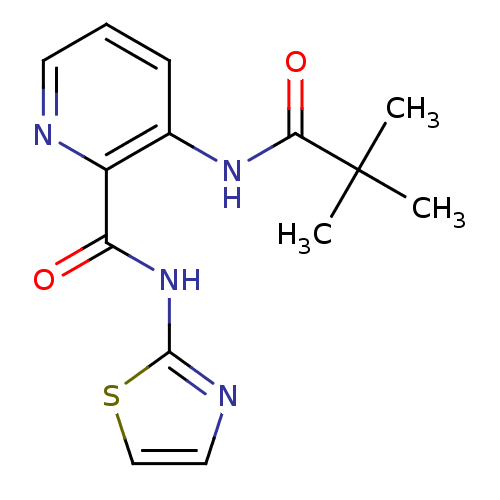 (3-(2,2-dimethylpropanamido)-N-(1,3-thiazol-2-yl)py...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 340 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibitory activity against type I methionine aminopeptidase from Saccharomyces cerevisiae | J Med Chem 46: 2631-40 (2003) Article DOI: 10.1021/jm0300532 BindingDB Entry DOI: 10.7270/Q2CF9QTC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 1 (Saccharomyces cerevisiae) | BDBM50129672 (3-Propionylamino-pyridine-2-carboxylic acid thiazo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 350 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibitory activity against type I methionine aminopeptidase from Saccharomyces cerevisiae | J Med Chem 46: 2631-40 (2003) Article DOI: 10.1021/jm0300532 BindingDB Entry DOI: 10.7270/Q2CF9QTC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 1 (Saccharomyces cerevisiae) | BDBM50129672 (3-Propionylamino-pyridine-2-carboxylic acid thiazo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 350 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibitory concentration against Saccharomyces cerevisiae methionine aminopeptidase 1 | Bioorg Med Chem Lett 15: 635-8 (2005) Article DOI: 10.1016/j.bmcl.2004.11.034 BindingDB Entry DOI: 10.7270/Q2222T7R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase (Escherichia coli (strain K12)) | BDBM50129656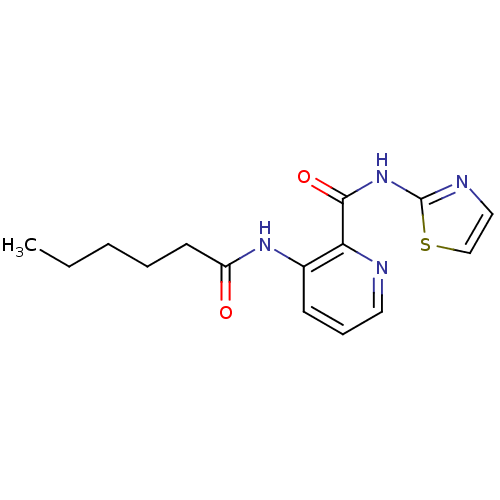 (3-Hexanoylamino-pyridine-2-carboxylic acid thiazol...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 380 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibitory activity against Methionine aminopeptidase 1 from Escherichia coli | J Med Chem 46: 2631-40 (2003) Article DOI: 10.1021/jm0300532 BindingDB Entry DOI: 10.7270/Q2CF9QTC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase (Escherichia coli (strain K12)) | BDBM50129656 (3-Hexanoylamino-pyridine-2-carboxylic acid thiazol...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 380 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibitory concentration against Escherichia coli methionine aminopeptidase 1 | Bioorg Med Chem Lett 15: 635-8 (2005) Article DOI: 10.1016/j.bmcl.2004.11.034 BindingDB Entry DOI: 10.7270/Q2222T7R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 1 (Saccharomyces cerevisiae) | BDBM50129663 (3-But-3-enoylamino-pyridine-2-carboxylic acid thia...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 380 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibitory activity against type I methionine aminopeptidase from Saccharomyces cerevisiae | J Med Chem 46: 2631-40 (2003) Article DOI: 10.1021/jm0300532 BindingDB Entry DOI: 10.7270/Q2CF9QTC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 1 (Saccharomyces cerevisiae) | BDBM50129650 (CHEMBL314448 | Propionic acid 2-(thiazol-2-ylcarba...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 380 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibitory activity against type I methionine aminopeptidase from Saccharomyces cerevisiae | J Med Chem 46: 2631-40 (2003) Article DOI: 10.1021/jm0300532 BindingDB Entry DOI: 10.7270/Q2CF9QTC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 1 (Saccharomyces cerevisiae) | BDBM50129641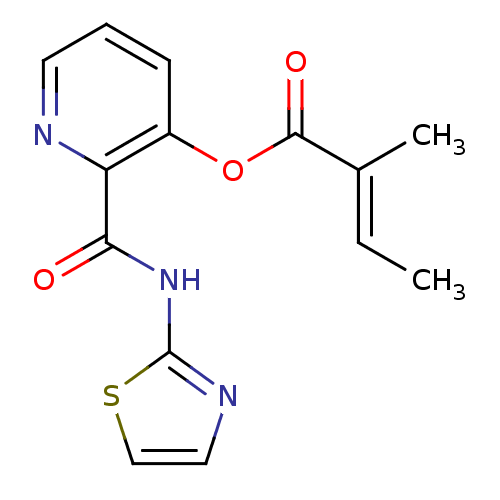 (2-Methyl-but-2-enoic acid 2-(thiazol-2-ylcarbamoyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 390 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibitory activity against type I methionine aminopeptidase from Saccharomyces cerevisiae | J Med Chem 46: 2631-40 (2003) Article DOI: 10.1021/jm0300532 BindingDB Entry DOI: 10.7270/Q2CF9QTC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase (Escherichia coli (strain K12)) | BDBM50129666 (3-Phenylacetylamino-pyridine-2-carboxylic acid thi...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibitory activity against Methionine aminopeptidase 1 from Escherichia coli | J Med Chem 46: 2631-40 (2003) Article DOI: 10.1021/jm0300532 BindingDB Entry DOI: 10.7270/Q2CF9QTC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 1 (Saccharomyces cerevisiae) | BDBM50129646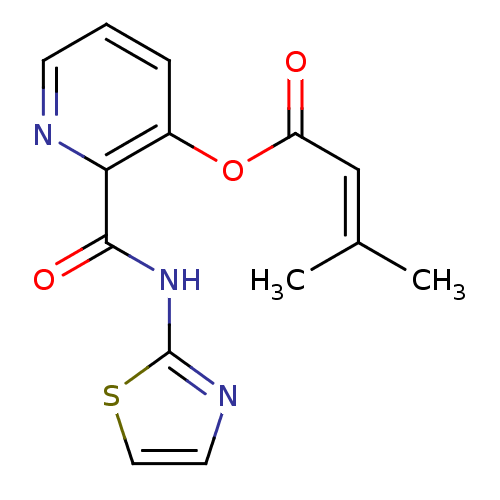 (3-Methyl-but-2-enoic acid 2-(thiazol-2-ylcarbamoyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 490 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibitory activity against type I methionine aminopeptidase from Saccharomyces cerevisiae | J Med Chem 46: 2631-40 (2003) Article DOI: 10.1021/jm0300532 BindingDB Entry DOI: 10.7270/Q2CF9QTC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase (Escherichia coli (strain K12)) | BDBM17849 (3-(2,2-dimethylpropanamido)-N-(1,3-thiazol-2-yl)py...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 540 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibitory activity against Methionine aminopeptidase 1 from Escherichia coli | J Med Chem 46: 2631-40 (2003) Article DOI: 10.1021/jm0300532 BindingDB Entry DOI: 10.7270/Q2CF9QTC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase (Escherichia coli (strain K12)) | BDBM50129642 (CHEMBL86427 | Phenyl-acetic acid 2-(thiazol-2-ylca...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 540 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibitory activity against Methionine aminopeptidase 1 from Escherichia coli | J Med Chem 46: 2631-40 (2003) Article DOI: 10.1021/jm0300532 BindingDB Entry DOI: 10.7270/Q2CF9QTC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 1 (Saccharomyces cerevisiae) | BDBM50129675 (2-Methoxy-benzoic acid 2-(thiazol-2-ylcarbamoyl)-p...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 550 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibitory activity against type I methionine aminopeptidase from Saccharomyces cerevisiae | J Med Chem 46: 2631-40 (2003) Article DOI: 10.1021/jm0300532 BindingDB Entry DOI: 10.7270/Q2CF9QTC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase (Escherichia coli (strain K12)) | BDBM50129638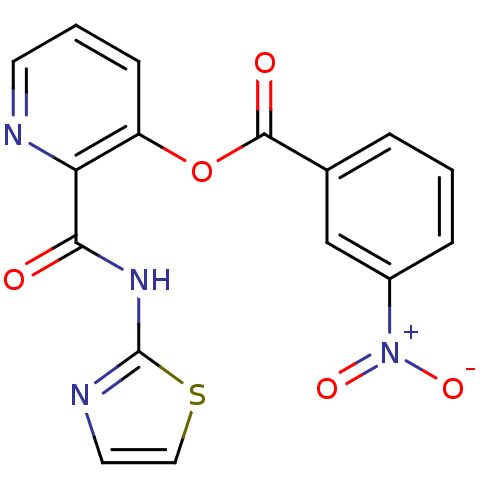 (3-Nitro-benzoic acid 2-(thiazol-2-ylcarbamoyl)-pyr...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibitory activity against Methionine aminopeptidase 1 from Escherichia coli | J Med Chem 46: 2631-40 (2003) Article DOI: 10.1021/jm0300532 BindingDB Entry DOI: 10.7270/Q2CF9QTC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase (Escherichia coli (strain K12)) | BDBM50129669 (3-(5-Methyl-hex-4-enoylamino)-pyridine-2-carboxyli...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 610 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibitory activity against Methionine aminopeptidase 1 from Escherichia coli | J Med Chem 46: 2631-40 (2003) Article DOI: 10.1021/jm0300532 BindingDB Entry DOI: 10.7270/Q2CF9QTC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 1 (Saccharomyces cerevisiae) | BDBM50129656 (3-Hexanoylamino-pyridine-2-carboxylic acid thiazol...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 620 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibitory concentration against Saccharomyces cerevisiae methionine aminopeptidase 1 | Bioorg Med Chem Lett 15: 635-8 (2005) Article DOI: 10.1016/j.bmcl.2004.11.034 BindingDB Entry DOI: 10.7270/Q2222T7R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 1 (Saccharomyces cerevisiae) | BDBM50129656 (3-Hexanoylamino-pyridine-2-carboxylic acid thiazol...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 620 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibitory activity against type I methionine aminopeptidase from Saccharomyces cerevisiae | J Med Chem 46: 2631-40 (2003) Article DOI: 10.1021/jm0300532 BindingDB Entry DOI: 10.7270/Q2CF9QTC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase (Escherichia coli (strain K12)) | BDBM50129636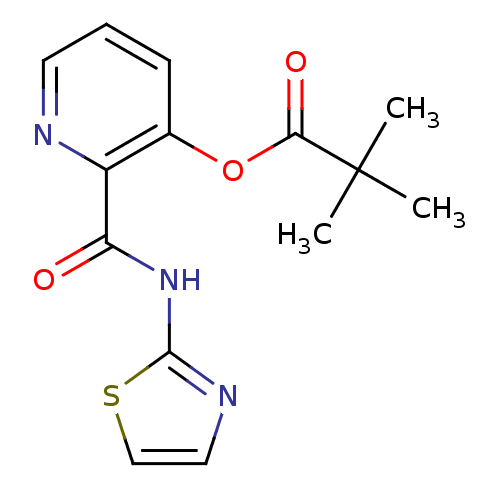 (2,2-Dimethyl-propionic acid 2-(thiazol-2-ylcarbamo...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 680 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibitory activity against Methionine aminopeptidase 1 from Escherichia coli | J Med Chem 46: 2631-40 (2003) Article DOI: 10.1021/jm0300532 BindingDB Entry DOI: 10.7270/Q2CF9QTC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase (Escherichia coli (strain K12)) | BDBM50129664 (3-(3-Fluoro-benzoylamino)-pyridine-2-carboxylic ac...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 710 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibitory activity against Methionine aminopeptidase 1 from Escherichia coli | J Med Chem 46: 2631-40 (2003) Article DOI: 10.1021/jm0300532 BindingDB Entry DOI: 10.7270/Q2CF9QTC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 1 (Saccharomyces cerevisiae) | BDBM50129640 (3-(3-Methyl-but-2-enoylamino)-pyridine-2-carboxyli...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 770 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibitory activity against type I methionine aminopeptidase from Saccharomyces cerevisiae | J Med Chem 46: 2631-40 (2003) Article DOI: 10.1021/jm0300532 BindingDB Entry DOI: 10.7270/Q2CF9QTC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 1 (Saccharomyces cerevisiae) | BDBM50160043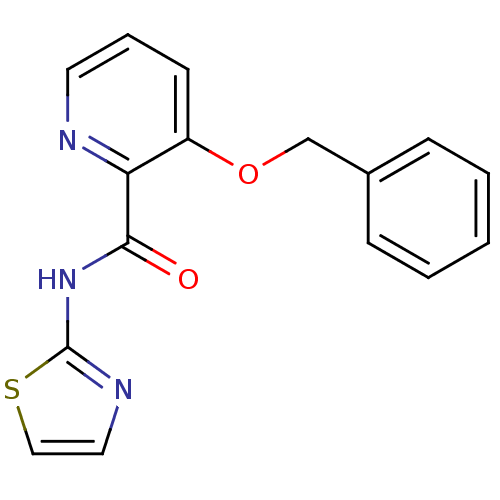 (3-Benzyloxy-pyridine-2-carboxylic acid thiazol-2-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 770 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibitory concentration against Saccharomyces cerevisiae methionine aminopeptidase 1 | Bioorg Med Chem Lett 15: 635-8 (2005) Article DOI: 10.1016/j.bmcl.2004.11.034 BindingDB Entry DOI: 10.7270/Q2222T7R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 1 (Saccharomyces cerevisiae) | BDBM50129636 (2,2-Dimethyl-propionic acid 2-(thiazol-2-ylcarbamo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 780 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibitory activity against type I methionine aminopeptidase from Saccharomyces cerevisiae | J Med Chem 46: 2631-40 (2003) Article DOI: 10.1021/jm0300532 BindingDB Entry DOI: 10.7270/Q2CF9QTC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 1 (Saccharomyces cerevisiae) | BDBM50129666 (3-Phenylacetylamino-pyridine-2-carboxylic acid thi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 820 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibitory activity against type I methionine aminopeptidase from Saccharomyces cerevisiae | J Med Chem 46: 2631-40 (2003) Article DOI: 10.1021/jm0300532 BindingDB Entry DOI: 10.7270/Q2CF9QTC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase (Escherichia coli (strain K12)) | BDBM50129655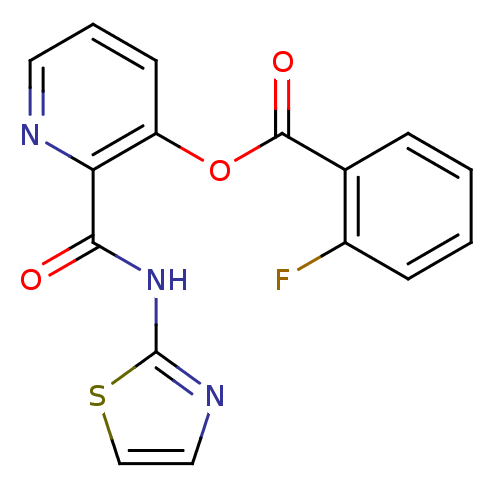 (2-Fluoro-benzoic acid 2-(thiazol-2-ylcarbamoyl)-py...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 830 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibitory activity against Methionine aminopeptidase 1 from Escherichia coli | J Med Chem 46: 2631-40 (2003) Article DOI: 10.1021/jm0300532 BindingDB Entry DOI: 10.7270/Q2CF9QTC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase (Escherichia coli (strain K12)) | BDBM50129659 (3-(Cyclohexanecarbonyl-amino)-pyridine-2-carboxyli...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 870 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibitory activity against Methionine aminopeptidase 1 from Escherichia coli | J Med Chem 46: 2631-40 (2003) Article DOI: 10.1021/jm0300532 BindingDB Entry DOI: 10.7270/Q2CF9QTC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 1 (Saccharomyces cerevisiae) | BDBM50129658 (2-Iodo-benzoic acid 2-(thiazol-2-ylcarbamoyl)-pyri...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 890 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibitory activity against type I methionine aminopeptidase from Saccharomyces cerevisiae | J Med Chem 46: 2631-40 (2003) Article DOI: 10.1021/jm0300532 BindingDB Entry DOI: 10.7270/Q2CF9QTC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase (Escherichia coli (strain K12)) | BDBM50129652 (3-Benzoylamino-pyridine-2-carboxylic acid thiazol-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 890 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibitory activity against Methionine aminopeptidase 1 from Escherichia coli | J Med Chem 46: 2631-40 (2003) Article DOI: 10.1021/jm0300532 BindingDB Entry DOI: 10.7270/Q2CF9QTC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase (Escherichia coli (strain K12)) | BDBM50129639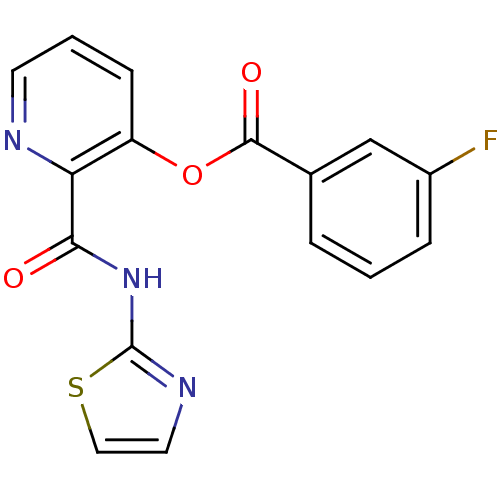 (3-Fluoro-benzoic acid 2-(thiazol-2-ylcarbamoyl)-py...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 950 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibitory activity against Methionine aminopeptidase 1 from Escherichia coli | J Med Chem 46: 2631-40 (2003) Article DOI: 10.1021/jm0300532 BindingDB Entry DOI: 10.7270/Q2CF9QTC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase (Escherichia coli (strain K12)) | BDBM50160054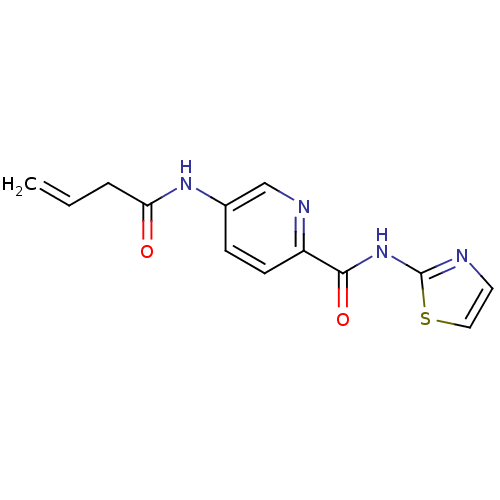 (5-But-3-enoylamino-pyridine-2-carboxylic acid thia...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.01E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibitory concentration against Escherichia coli methionine aminopeptidase 1 | Bioorg Med Chem Lett 15: 635-8 (2005) Article DOI: 10.1016/j.bmcl.2004.11.034 BindingDB Entry DOI: 10.7270/Q2222T7R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase (Escherichia coli (strain K12)) | BDBM50129637 (CHEMBL315903 | Hexanoic acid 2-(thiazol-2-ylcarbam...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.02E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibitory activity against Methionine aminopeptidase 1 from Escherichia coli | J Med Chem 46: 2631-40 (2003) Article DOI: 10.1021/jm0300532 BindingDB Entry DOI: 10.7270/Q2CF9QTC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 1 (Saccharomyces cerevisiae) | BDBM50129671 (Benzoic acid 2-(thiazol-2-ylcarbamoyl)-pyridin-3-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.03E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibitory activity against type I methionine aminopeptidase from Saccharomyces cerevisiae | J Med Chem 46: 2631-40 (2003) Article DOI: 10.1021/jm0300532 BindingDB Entry DOI: 10.7270/Q2CF9QTC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 1 (Saccharomyces cerevisiae) | BDBM50129671 (Benzoic acid 2-(thiazol-2-ylcarbamoyl)-pyridin-3-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.03E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibitory concentration against Saccharomyces cerevisiae methionine aminopeptidase 1 | Bioorg Med Chem Lett 15: 635-8 (2005) Article DOI: 10.1016/j.bmcl.2004.11.034 BindingDB Entry DOI: 10.7270/Q2222T7R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase (Escherichia coli (strain K12)) | BDBM50129644 (4-Fluoro-benzoic acid 2-(thiazol-2-ylcarbamoyl)-py...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibitory activity against Methionine aminopeptidase 1 from Escherichia coli | J Med Chem 46: 2631-40 (2003) Article DOI: 10.1021/jm0300532 BindingDB Entry DOI: 10.7270/Q2CF9QTC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase (Escherichia coli (strain K12)) | BDBM50129671 (Benzoic acid 2-(thiazol-2-ylcarbamoyl)-pyridin-3-y...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.22E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibitory activity against Methionine aminopeptidase 1 from Escherichia coli | J Med Chem 46: 2631-40 (2003) Article DOI: 10.1021/jm0300532 BindingDB Entry DOI: 10.7270/Q2CF9QTC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 127 total ) | Next | Last >> |