

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Serine/threonine-protein kinase PAK 4 (Homo sapiens (Human)) | BDBM101546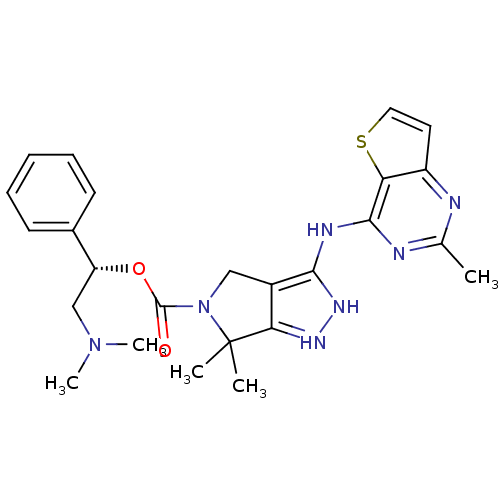 (US8530494, 211 | US8530652, 125 | US8530652, 73) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1.60 | n/a | n/a | n/a | <3.90 | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc.; Pfizer Inc. US Patent | Assay Description The enzymatic activity of PAK4 KD was measured by its ability to catalyzed the transfer of a phosphate residue from a nucleoside triphosphate to an a... | US Patent US8530652 (2013) BindingDB Entry DOI: 10.7270/Q2F18XC4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PAK 4 (Homo sapiens (Human)) | BDBM101542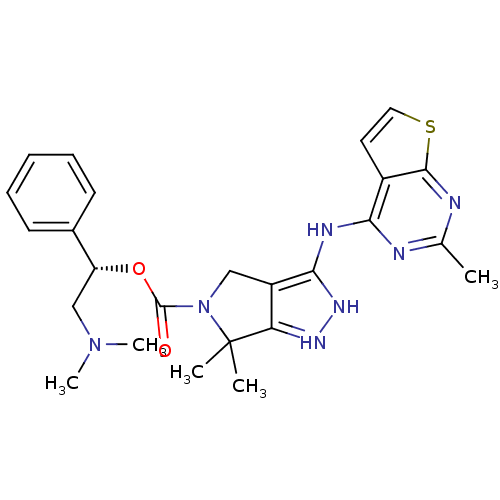 (US8530494, 207 | US8530652, 121 | US8530652, 69) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1.90 | n/a | n/a | n/a | 3 | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc.; Pfizer Inc. US Patent | Assay Description The enzymatic activity of PAK4 KD was measured by its ability to catalyzed the transfer of a phosphate residue from a nucleoside triphosphate to an a... | US Patent US8530652 (2013) BindingDB Entry DOI: 10.7270/Q2F18XC4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PAK 4 (Homo sapiens (Human)) | BDBM101479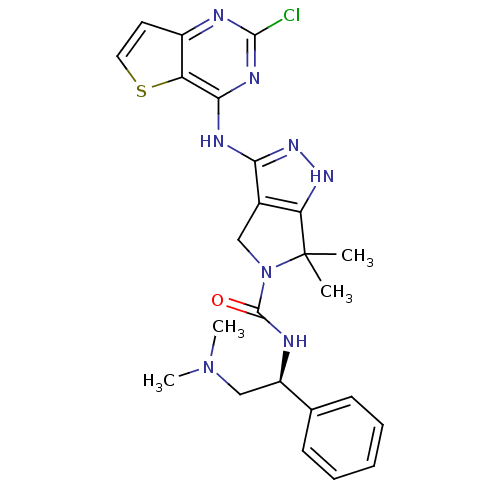 (US8524710, 56 | US8530652, 50 | US8530652, 6) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 2.80 | n/a | n/a | n/a | 0.940 | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc.; Pfizer Inc. US Patent | Assay Description The enzymatic activity of PAK4 KD was measured by its ability to catalyzed the transfer of a phosphate residue from a nucleoside triphosphate to an a... | US Patent US8530652 (2013) BindingDB Entry DOI: 10.7270/Q2F18XC4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PAK 4 (Homo sapiens (Human)) | BDBM50389112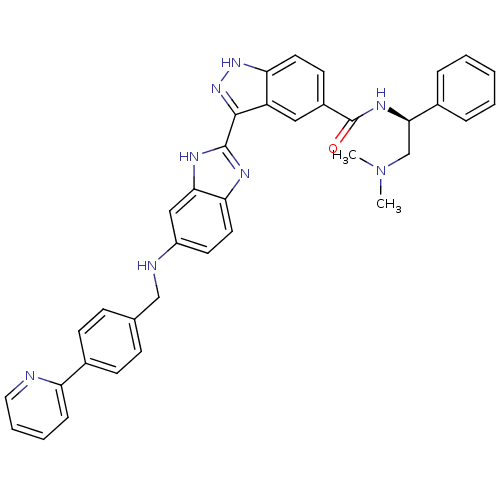 (CHEMBL2064556) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of PAK4 | J Med Chem 55: 4728-39 (2012) Article DOI: 10.1021/jm300204j BindingDB Entry DOI: 10.7270/Q2BZ673W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PAK 4 (Homo sapiens (Human)) | BDBM101537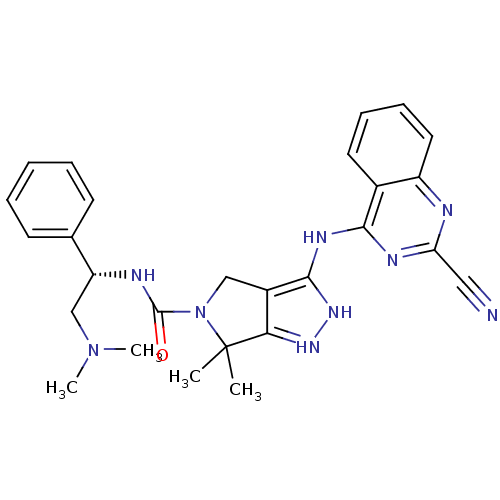 (US8530494, 204 | US8530652, 115 | US8530652, 64) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 3 | n/a | n/a | n/a | <3.90 | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc.; Pfizer Inc. US Patent | Assay Description The enzymatic activity of PAK4 KD was measured by its ability to catalyzed the transfer of a phosphate residue from a nucleoside triphosphate to an a... | US Patent US8530652 (2013) BindingDB Entry DOI: 10.7270/Q2F18XC4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM50389111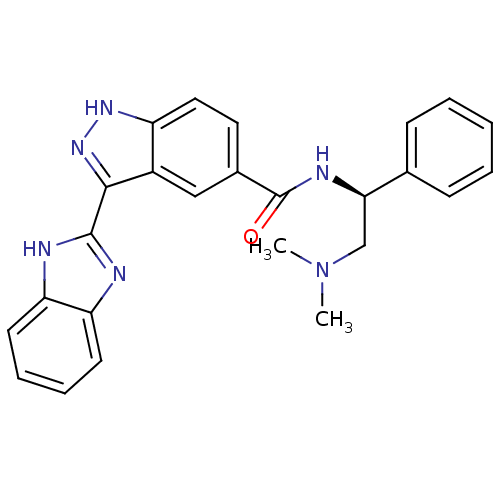 (CHEMBL2064555) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of CDK2 | J Med Chem 55: 4728-39 (2012) Article DOI: 10.1021/jm300204j BindingDB Entry DOI: 10.7270/Q2BZ673W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PAK 4 (Homo sapiens (Human)) | BDBM4838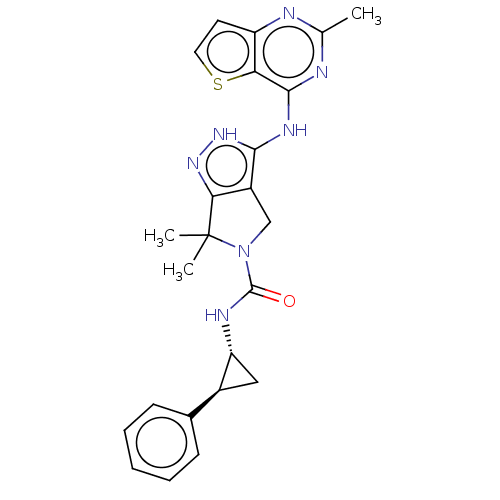 (US8524710, 83 | US8530494, 13 | US8530652, 52) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 3.5 | n/a | n/a | n/a | 19 | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc.; Pfizer Inc. US Patent | Assay Description The enzymatic activity of PAK4 KD was measured by its ability to catalyzed the transfer of a phosphate residue from a nucleoside triphosphate to an a... | US Patent US8530652 (2013) BindingDB Entry DOI: 10.7270/Q2F18XC4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PAK 4 (Homo sapiens (Human)) | BDBM4838 (US8524710, 83 | US8530494, 13 | US8530652, 52) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 3.5 | n/a | n/a | n/a | 19 | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc.; Pfizer Inc. US Patent | Assay Description The enzymatic activity of PAK4 KD was measured by its ability to catalyzed the transfer of a phosphate residue from a nucleoside triphosphate to an a... | US Patent US8530652 (2013) BindingDB Entry DOI: 10.7270/Q2F18XC4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PAK 4 (Homo sapiens (Human)) | BDBM101547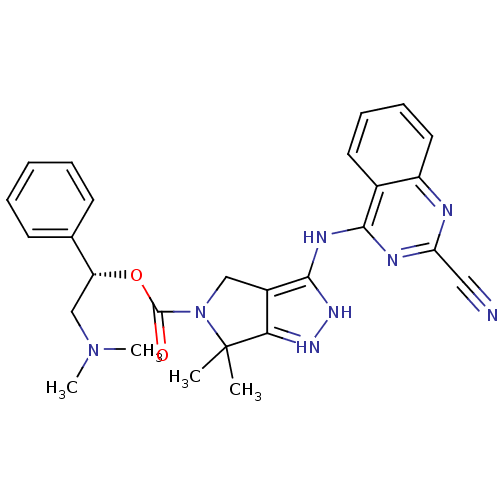 (US8530494, 212 | US8530652, 126 | US8530652, 74) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 3.60 | n/a | n/a | n/a | <3.90 | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc.; Pfizer Inc. US Patent | Assay Description The enzymatic activity of PAK4 KD was measured by its ability to catalyzed the transfer of a phosphate residue from a nucleoside triphosphate to an a... | US Patent US8530652 (2013) BindingDB Entry DOI: 10.7270/Q2F18XC4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM50122777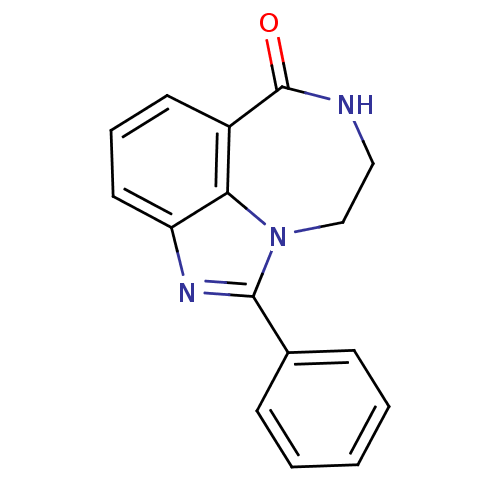 (1-Phenyl-8,9-dihydro-7H-2,7,9a-triaza-benzo[cd]azu...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global R&D Curated by ChEMBL | Assay Description Inhibitory activity of the compound against human full length Poly (ADP-ribose) polymerase 1 | J Med Chem 46: 210-3 (2003) Article DOI: 10.1021/jm0255769 BindingDB Entry DOI: 10.7270/Q2154GDB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM50122775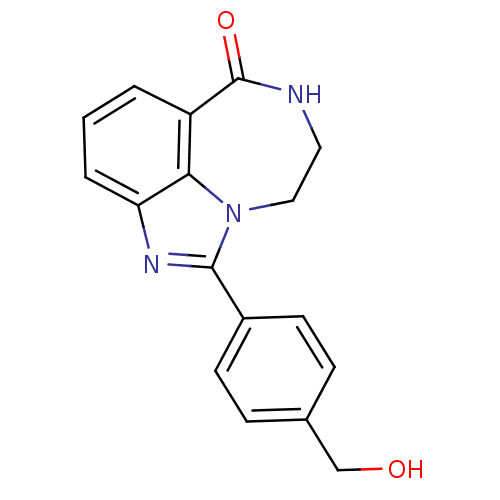 (1-(4-Hydroxymethyl-phenyl)-8,9-dihydro-7H-2,7,9a-t...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global R&D Curated by ChEMBL | Assay Description Inhibitory activity of the compound against human full length Poly (ADP-ribose) polymerase 1 | J Med Chem 46: 210-3 (2003) Article DOI: 10.1021/jm0255769 BindingDB Entry DOI: 10.7270/Q2154GDB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM50122781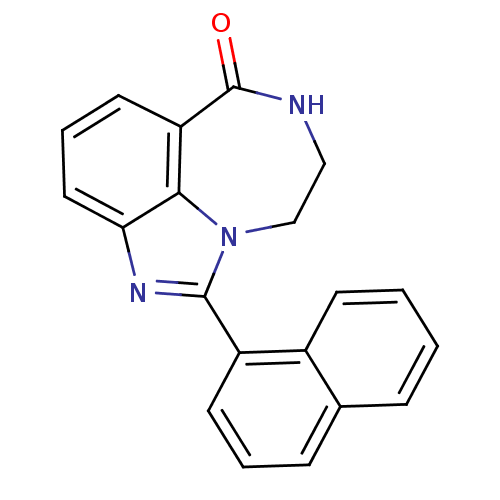 (1-Naphthalen-1-yl-8,9-dihydro-7H-2,7,9a-triaza-ben...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global R&D Curated by ChEMBL | Assay Description Inhibitory activity of the compound against human full length Poly (ADP-ribose) polymerase 1 | J Med Chem 46: 210-3 (2003) Article DOI: 10.1021/jm0255769 BindingDB Entry DOI: 10.7270/Q2154GDB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PAK 4 (Homo sapiens (Human)) | BDBM101544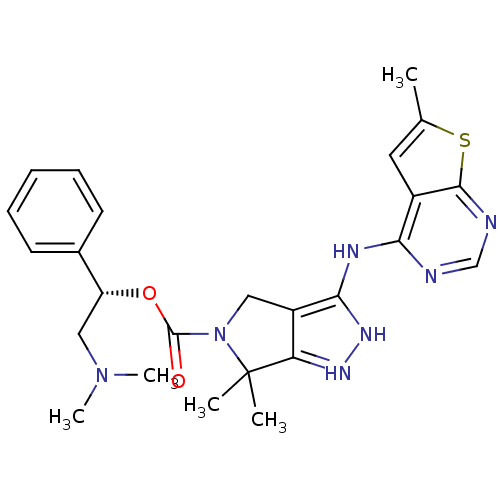 (US8530494, 105 | US8530652, 123 | US8530652, 71) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc.; Pfizer Inc. US Patent | Assay Description The enzymatic activity of PAK4 KD was measured by its ability to catalyzed the transfer of a phosphate residue from a nucleoside triphosphate to an a... | US Patent US8530652 (2013) BindingDB Entry DOI: 10.7270/Q2F18XC4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PAK 4 (Homo sapiens (Human)) | BDBM101548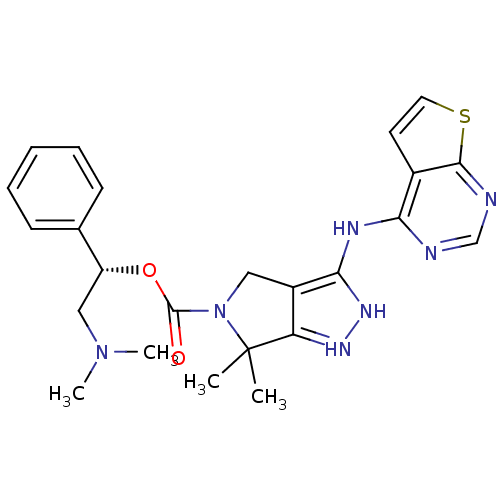 (US8530494, 108 | US8530652, 127 | US8530652, 75) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 5.20 | n/a | n/a | n/a | <3.90 | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc.; Pfizer Inc. US Patent | Assay Description The enzymatic activity of PAK4 KD was measured by its ability to catalyzed the transfer of a phosphate residue from a nucleoside triphosphate to an a... | US Patent US8530652 (2013) BindingDB Entry DOI: 10.7270/Q2F18XC4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM50122779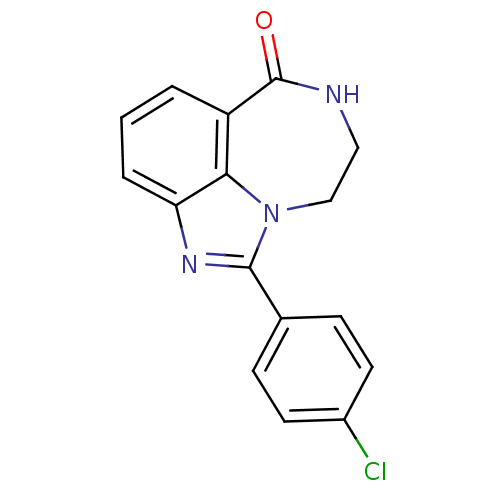 (1-(4-Chloro-phenyl)-8,9-dihydro-7H-2,7,9a-triaza-b...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global R&D Curated by ChEMBL | Assay Description Inhibitory activity of the compound against human full length Poly (ADP-ribose) polymerase 1 | J Med Chem 46: 210-3 (2003) Article DOI: 10.1021/jm0255769 BindingDB Entry DOI: 10.7270/Q2154GDB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM50122776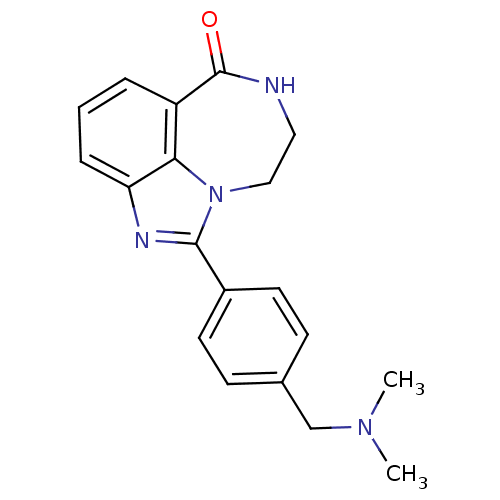 (1-(4-Dimethylaminomethyl-phenyl)-8,9-dihydro-7H-2,...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 5.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global R&D Curated by ChEMBL | Assay Description Inhibitory activity of the compound against human full length Poly (ADP-ribose) polymerase 1 | J Med Chem 46: 210-3 (2003) Article DOI: 10.1021/jm0255769 BindingDB Entry DOI: 10.7270/Q2154GDB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase NIMA-interacting 1 (Homo sapiens (Human)) | BDBM34012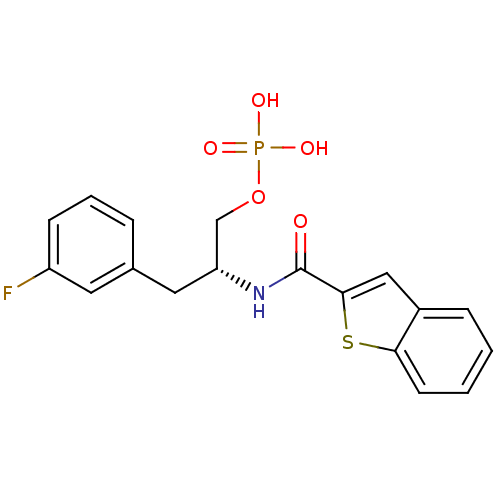 (3-fluorophenylalanine derivative, 21b) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 6 | -45.4 | n/a | n/a | n/a | n/a | n/a | 7.5 | 15 |
Pfizer | Assay Description The cis/trans conversion of cis-Suc-AEPFpNA peptide by the PPIase led to the cleavage of para nitroaniline by subtilisin which was monitored at 390 n... | Bioorg Med Chem Lett 19: 5613-6 (2009) Article DOI: 10.1016/j.bmcl.2009.08.034 BindingDB Entry DOI: 10.7270/Q2XD100H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PAK 4 (Homo sapiens (Human)) | BDBM50389111 (CHEMBL2064555) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of PAK4 | J Med Chem 55: 4728-39 (2012) Article DOI: 10.1021/jm300204j BindingDB Entry DOI: 10.7270/Q2BZ673W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase NIMA-interacting 1 (Homo sapiens (Human)) | BDBM50314714 ((R)-N-(1-(3-fluorophenyl)-3-hydroxypropan-2-yl)ben...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of WFYpSPFLE from human Pin1 catalytic domain after 10-20 mins by fluorescence polarization assay | Bioorg Med Chem Lett 20: 2210-4 (2010) Article DOI: 10.1016/j.bmcl.2010.02.033 BindingDB Entry DOI: 10.7270/Q2GX4BPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM50122772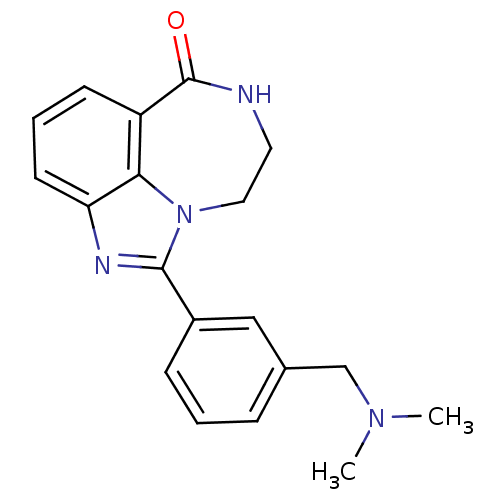 (1-(3-Dimethylaminomethyl-phenyl)-8,9-dihydro-7H-2,...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global R&D Curated by ChEMBL | Assay Description Inhibitory activity of the compound against human full length Poly (ADP-ribose) polymerase 1 | J Med Chem 46: 210-3 (2003) Article DOI: 10.1021/jm0255769 BindingDB Entry DOI: 10.7270/Q2154GDB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PAK 4 (Homo sapiens (Human)) | BDBM101541 (US8530494, 104 | US8530652, 120 | US8530652, 68) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 6.70 | n/a | n/a | n/a | 20 | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc.; Pfizer Inc. US Patent | Assay Description The enzymatic activity of PAK4 KD was measured by its ability to catalyzed the transfer of a phosphate residue from a nucleoside triphosphate to an a... | US Patent US8530652 (2013) BindingDB Entry DOI: 10.7270/Q2F18XC4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PAK 4 (Homo sapiens (Human)) | BDBM101504 (US8524699, 91 | US8524710, 82 | US8530652, 31) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 7.20 | n/a | n/a | n/a | 21 | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc.; Pfizer Inc. US Patent | Assay Description The enzymatic activity of PAK4 KD was measured by its ability to catalyzed the transfer of a phosphate residue from a nucleoside triphosphate to an a... | US Patent US8530652 (2013) BindingDB Entry DOI: 10.7270/Q2F18XC4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM50122774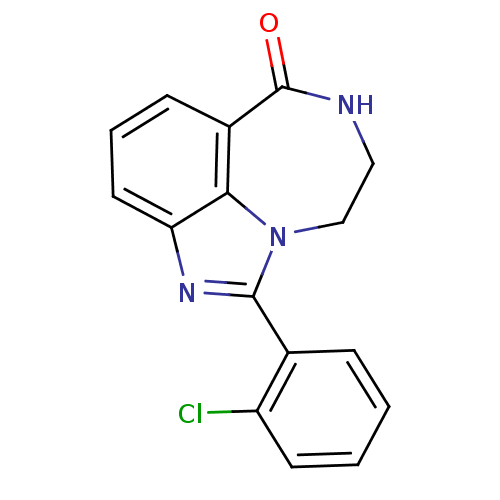 (1-(2-Chloro-phenyl)-8,9-dihydro-7H-2,7,9a-triaza-b...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 7.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global R&D Curated by ChEMBL | Assay Description Inhibitory activity of the compound against human full length Poly (ADP-ribose) polymerase 1 | J Med Chem 46: 210-3 (2003) Article DOI: 10.1021/jm0255769 BindingDB Entry DOI: 10.7270/Q2154GDB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase NIMA-interacting 1 (Homo sapiens (Human)) | BDBM34011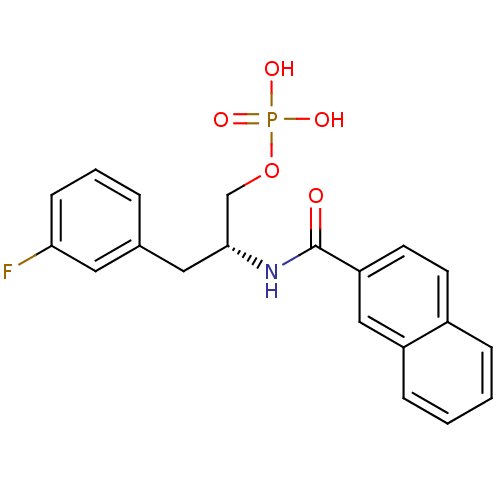 (3-fluorophenylalanine derivative, 21a) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 8 | -44.7 | n/a | n/a | n/a | n/a | n/a | 7.5 | 15 |
Pfizer | Assay Description The cis/trans conversion of cis-Suc-AEPFpNA peptide by the PPIase led to the cleavage of para nitroaniline by subtilisin which was monitored at 390 n... | Bioorg Med Chem Lett 19: 5613-6 (2009) Article DOI: 10.1016/j.bmcl.2009.08.034 BindingDB Entry DOI: 10.7270/Q2XD100H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PAK 4 (Homo sapiens (Human)) | BDBM101549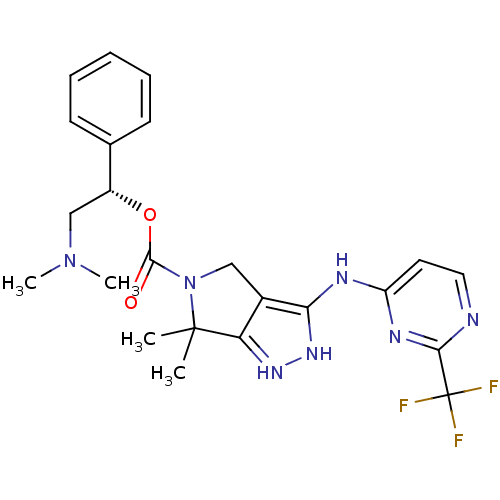 (US8530494, 109 | US8530652, 128 | US8530652, 76) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 8.30 | n/a | n/a | n/a | <3.90 | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc.; Pfizer Inc. US Patent | Assay Description The enzymatic activity of PAK4 KD was measured by its ability to catalyzed the transfer of a phosphate residue from a nucleoside triphosphate to an a... | US Patent US8530652 (2013) BindingDB Entry DOI: 10.7270/Q2F18XC4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM50122780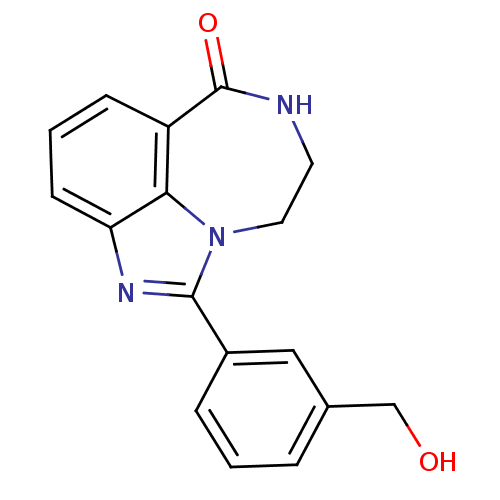 (1-(3-Hydroxymethyl-phenyl)-8,9-dihydro-7H-2,7,9a-t...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 8.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global R&D Curated by ChEMBL | Assay Description Inhibitory activity of the compound against human full length Poly (ADP-ribose) polymerase 1 | J Med Chem 46: 210-3 (2003) Article DOI: 10.1021/jm0255769 BindingDB Entry DOI: 10.7270/Q2154GDB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PAK 4 (Homo sapiens (Human)) | BDBM101543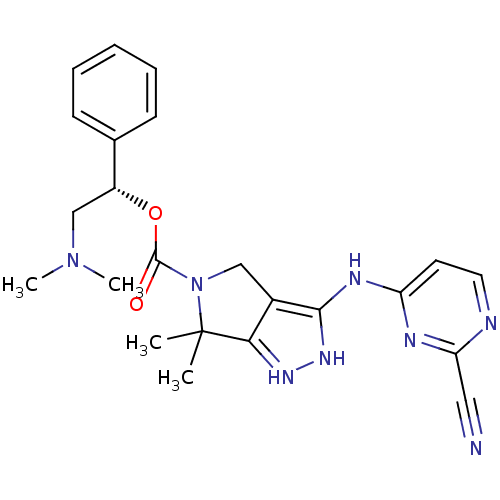 (US8530494, 206 | US8530652, 122 | US8530652, 70) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 8.80 | n/a | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc.; Pfizer Inc. US Patent | Assay Description The enzymatic activity of PAK4 KD was measured by its ability to catalyzed the transfer of a phosphate residue from a nucleoside triphosphate to an a... | US Patent US8530652 (2013) BindingDB Entry DOI: 10.7270/Q2F18XC4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PAK 4 (Homo sapiens (Human)) | BDBM101476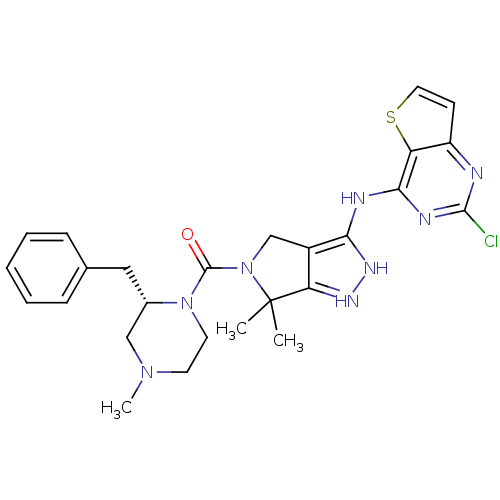 (US8524710, 53 | US8530652, 3 | US8530652, 47) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 11 | n/a | n/a | n/a | 16 | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc.; Pfizer Inc. US Patent | Assay Description The enzymatic activity of PAK4 KD was measured by its ability to catalyzed the transfer of a phosphate residue from a nucleoside triphosphate to an a... | US Patent US8530652 (2013) BindingDB Entry DOI: 10.7270/Q2F18XC4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM50122773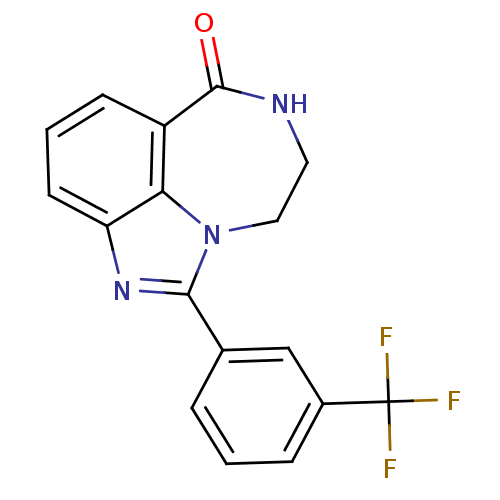 (1-(3-Trifluoromethyl-phenyl)-8,9-dihydro-7H-2,7,9a...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 11.2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global R&D Curated by ChEMBL | Assay Description Inhibitory activity of the compound against human full length Poly (ADP-ribose) polymerase 1 | J Med Chem 46: 210-3 (2003) Article DOI: 10.1021/jm0255769 BindingDB Entry DOI: 10.7270/Q2154GDB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PAK 4 (Homo sapiens (Human)) | BDBM50389116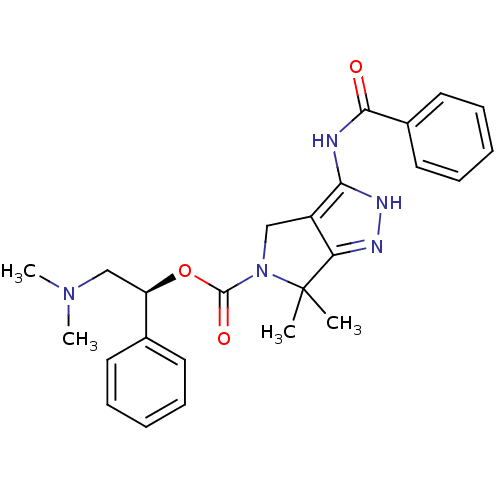 (CHEMBL2064561) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of PAK4 | J Med Chem 55: 4728-39 (2012) Article DOI: 10.1021/jm300204j BindingDB Entry DOI: 10.7270/Q2BZ673W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase NIMA-interacting 1 (Homo sapiens (Human)) | BDBM50056217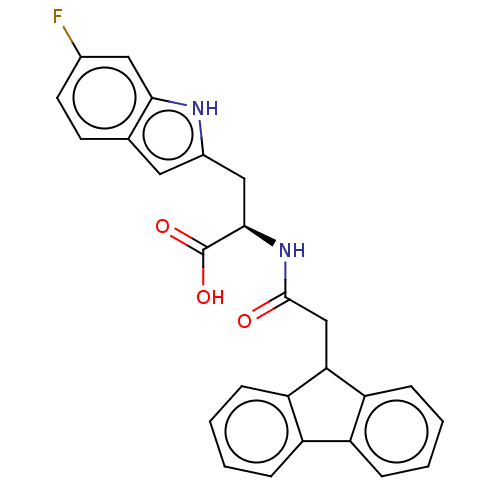 (CHEMBL3353369) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of Pin1 (unknown origin) | Bioorg Med Chem Lett 24: 4187-91 (2014) Article DOI: 10.1016/j.bmcl.2014.07.044 BindingDB Entry DOI: 10.7270/Q2QJ7JX1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PAK 4 (Homo sapiens (Human)) | BDBM50389117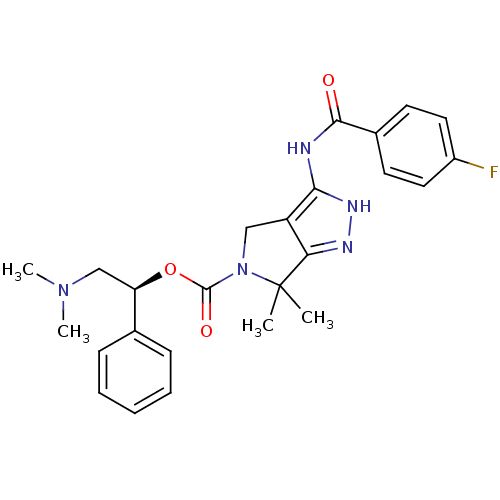 (CHEMBL2064562) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of PAK4 | J Med Chem 55: 4728-39 (2012) Article DOI: 10.1021/jm300204j BindingDB Entry DOI: 10.7270/Q2BZ673W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PAK 4 (Homo sapiens (Human)) | BDBM101475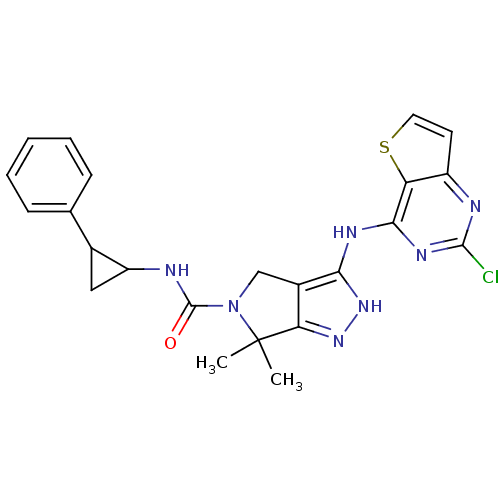 (US8524710, 52 | US8530652, 2 | US8530652, 46) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 14 | n/a | n/a | n/a | 36 | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc.; Pfizer Inc. US Patent | Assay Description The enzymatic activity of PAK4 KD was measured by its ability to catalyzed the transfer of a phosphate residue from a nucleoside triphosphate to an a... | US Patent US8530652 (2013) BindingDB Entry DOI: 10.7270/Q2F18XC4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PAK 4 (Homo sapiens (Human)) | BDBM101530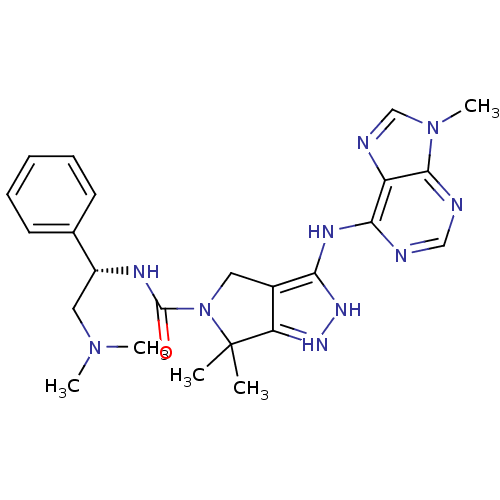 (US8530494, 29 | US8530494, 7 | US8530652, 57) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 14 | n/a | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc.; Pfizer Inc. US Patent | Assay Description The enzymatic activity of PAK4 KD was measured by its ability to catalyzed the transfer of a phosphate residue from a nucleoside triphosphate to an a... | US Patent US8530652 (2013) BindingDB Entry DOI: 10.7270/Q2F18XC4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PAK 4 (Homo sapiens (Human)) | BDBM101535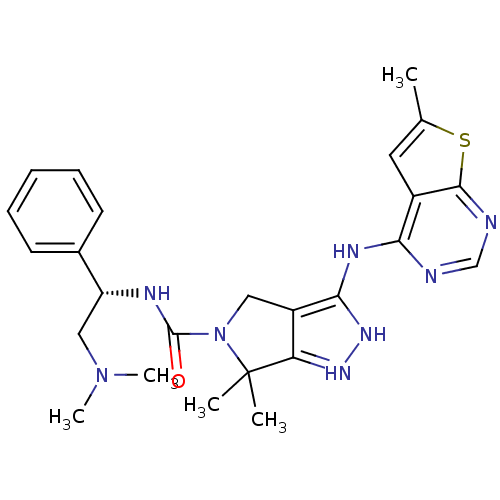 (US8530494, 104 | US8530652, 113 | US8530652, 62) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 15 | n/a | n/a | n/a | 15 | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc.; Pfizer Inc. US Patent | Assay Description The enzymatic activity of PAK4 KD was measured by its ability to catalyzed the transfer of a phosphate residue from a nucleoside triphosphate to an a... | US Patent US8530652 (2013) BindingDB Entry DOI: 10.7270/Q2F18XC4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PAK 4 (Homo sapiens (Human)) | BDBM101515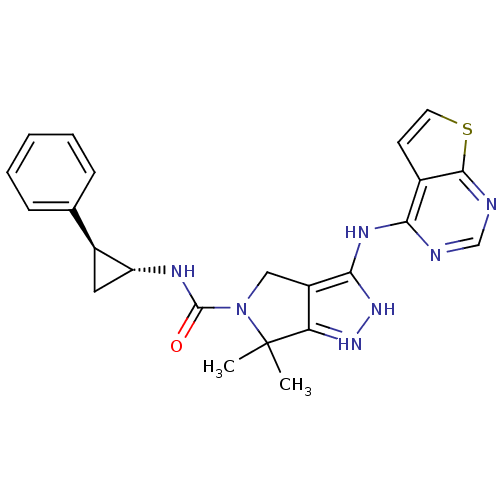 (US8524699, 26 | US8530652, 42 | US8530670, 31) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 16 | n/a | n/a | n/a | 340 | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc.; Pfizer Inc. US Patent | Assay Description The enzymatic activity of PAK4 KD was measured by its ability to catalyzed the transfer of a phosphate residue from a nucleoside triphosphate to an a... | US Patent US8530652 (2013) BindingDB Entry DOI: 10.7270/Q2F18XC4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PAK 4 (Homo sapiens (Human)) | BDBM101545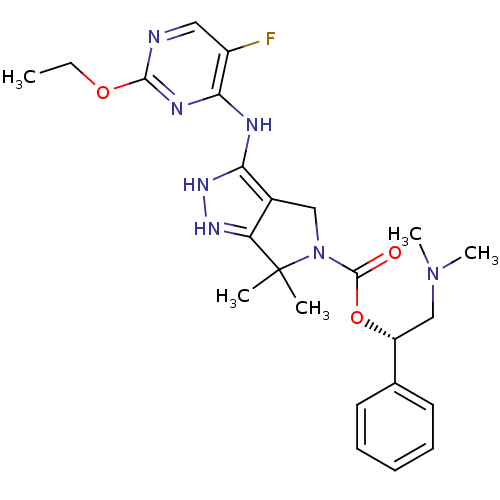 (US8530494, 107 | US8530652, 124 | US8530652, 72) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 16 | n/a | n/a | n/a | 4.40 | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc.; Pfizer Inc. US Patent | Assay Description The enzymatic activity of PAK4 KD was measured by its ability to catalyzed the transfer of a phosphate residue from a nucleoside triphosphate to an a... | US Patent US8530652 (2013) BindingDB Entry DOI: 10.7270/Q2F18XC4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PAK 4 (Homo sapiens (Human)) | BDBM101513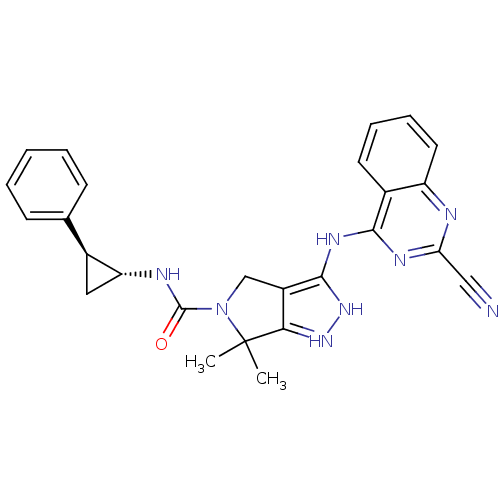 (US8524699, 18 | US8530652, 40 | US8530652, 85) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 18 | n/a | n/a | n/a | 98 | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc.; Pfizer Inc. US Patent | Assay Description The enzymatic activity of PAK4 KD was measured by its ability to catalyzed the transfer of a phosphate residue from a nucleoside triphosphate to an a... | US Patent US8530652 (2013) BindingDB Entry DOI: 10.7270/Q2F18XC4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PAK 4 (Homo sapiens (Human)) | BDBM101524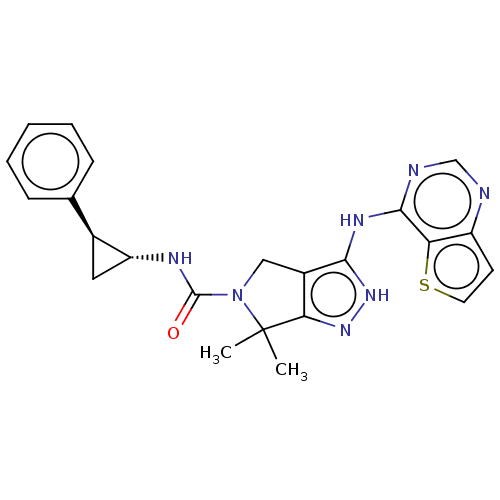 (US8530652, 51 | US8530652, 96) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 18 | n/a | n/a | n/a | 31 | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc.; Pfizer Inc. US Patent | Assay Description The enzymatic activity of PAK4 KD was measured by its ability to catalyzed the transfer of a phosphate residue from a nucleoside triphosphate to an a... | US Patent US8530652 (2013) BindingDB Entry DOI: 10.7270/Q2F18XC4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PAK 4 (Homo sapiens (Human)) | BDBM101504 (US8524699, 91 | US8524710, 82 | US8530652, 31) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 20 | n/a | n/a | n/a | 103 | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc.; Pfizer Inc. US Patent | Assay Description The enzymatic activity of PAK4 KD was measured by its ability to catalyzed the transfer of a phosphate residue from a nucleoside triphosphate to an a... | US Patent US8530652 (2013) BindingDB Entry DOI: 10.7270/Q2F18XC4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PAK 4 (Homo sapiens (Human)) | BDBM101536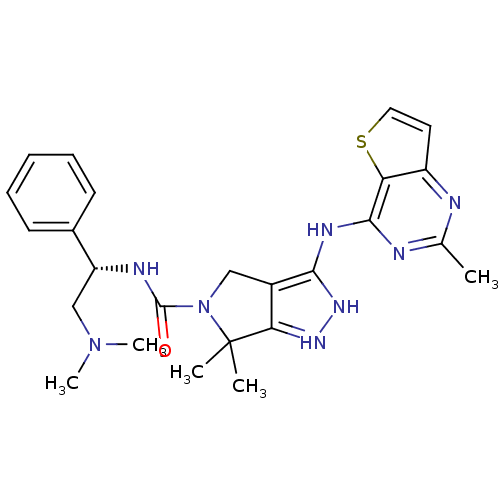 (CHEMBL3128043 | US8530494, 203 | US8530652, 114 | ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | US Patent | 20 | n/a | n/a | n/a | <3.90 | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc.; Pfizer Inc. US Patent | Assay Description The enzymatic activity of PAK4 KD was measured by its ability to catalyzed the transfer of a phosphate residue from a nucleoside triphosphate to an a... | US Patent US8530652 (2013) BindingDB Entry DOI: 10.7270/Q2F18XC4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PAK 4 (Homo sapiens (Human)) | BDBM101507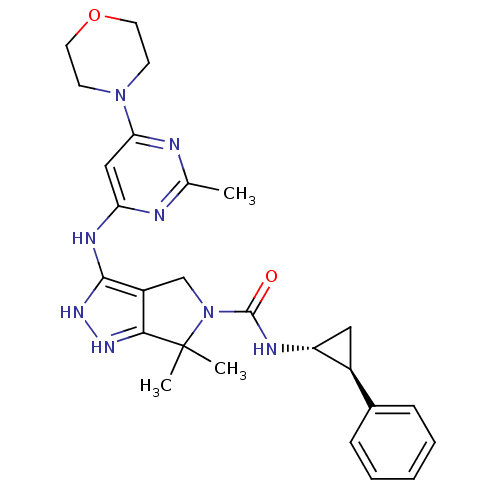 (US8524710, 86 | US8530652, 34 | US8530652, 79) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 21 | n/a | n/a | n/a | 778 | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc.; Pfizer Inc. US Patent | Assay Description The enzymatic activity of PAK4 KD was measured by its ability to catalyzed the transfer of a phosphate residue from a nucleoside triphosphate to an a... | US Patent US8530652 (2013) BindingDB Entry DOI: 10.7270/Q2F18XC4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PAK 4 (Homo sapiens (Human)) | BDBM101534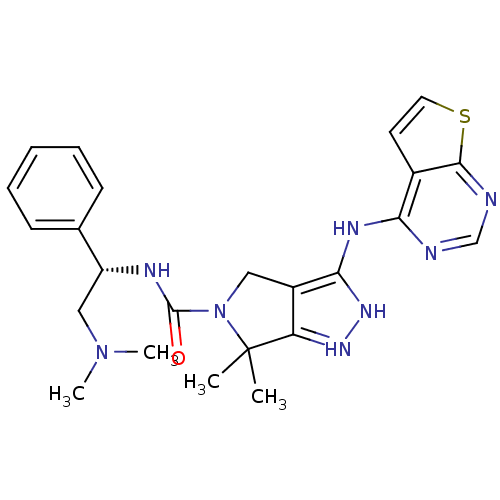 (US8530494, 202 | US8530652, 112 | US8530652, 61) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 22 | n/a | n/a | n/a | 19 | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc.; Pfizer Inc. US Patent | Assay Description The enzymatic activity of PAK4 KD was measured by its ability to catalyzed the transfer of a phosphate residue from a nucleoside triphosphate to an a... | US Patent US8530652 (2013) BindingDB Entry DOI: 10.7270/Q2F18XC4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PAK 4 (Homo sapiens (Human)) | BDBM101514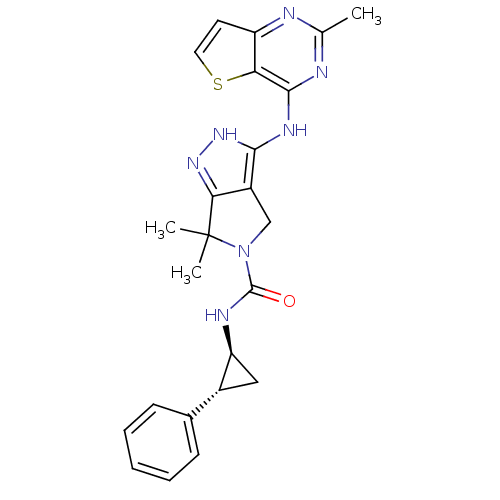 (US8524699, 22 | US8530652, 41 | US8530652, 86) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 24 | n/a | n/a | n/a | 147 | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc.; Pfizer Inc. US Patent | Assay Description The enzymatic activity of PAK4 KD was measured by its ability to catalyzed the transfer of a phosphate residue from a nucleoside triphosphate to an a... | US Patent US8530652 (2013) BindingDB Entry DOI: 10.7270/Q2F18XC4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PAK 4 (Homo sapiens (Human)) | BDBM101518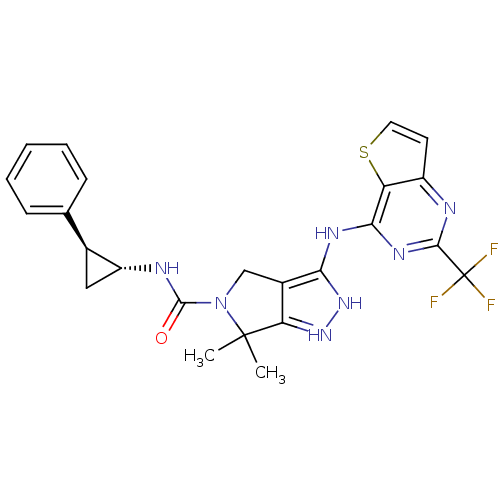 (US8524699, 46 | US8530652, 45 | US8530652, 90) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 25 | n/a | n/a | n/a | 32 | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc.; Pfizer Inc. US Patent | Assay Description The enzymatic activity of PAK4 KD was measured by its ability to catalyzed the transfer of a phosphate residue from a nucleoside triphosphate to an a... | US Patent US8530652 (2013) BindingDB Entry DOI: 10.7270/Q2F18XC4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PAK 4 (Homo sapiens (Human)) | BDBM50389113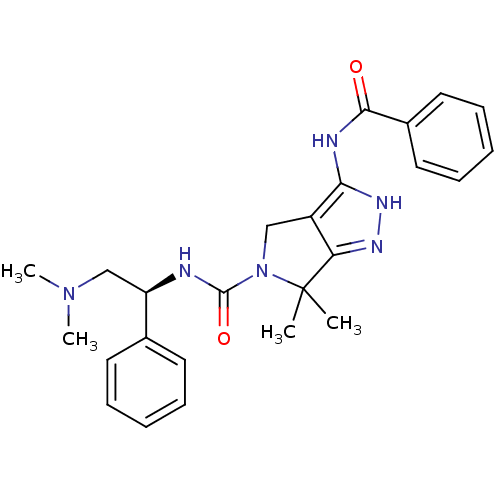 (CHEMBL2064558) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of PAK4 | J Med Chem 55: 4728-39 (2012) Article DOI: 10.1021/jm300204j BindingDB Entry DOI: 10.7270/Q2BZ673W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50065621 (CHEMBL94688 | [(S)-1-((S)-1-{(S)-3-Carbamoyl-1-[2-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Catalytic rate constant (Kobs/[I]) of the compound was evaluated against human rhinovirus (HRV) serotype 14 3C Protease (3CP) | J Med Chem 41: 2806-18 (1998) Article DOI: 10.1021/jm980068d BindingDB Entry DOI: 10.7270/Q29G5KZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PAK 4 (Homo sapiens (Human)) | BDBM50389120 (CHEMBL2064565) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of PAK4 | J Med Chem 55: 4728-39 (2012) Article DOI: 10.1021/jm300204j BindingDB Entry DOI: 10.7270/Q2BZ673W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase NIMA-interacting 1 (Homo sapiens (Human)) | BDBM34013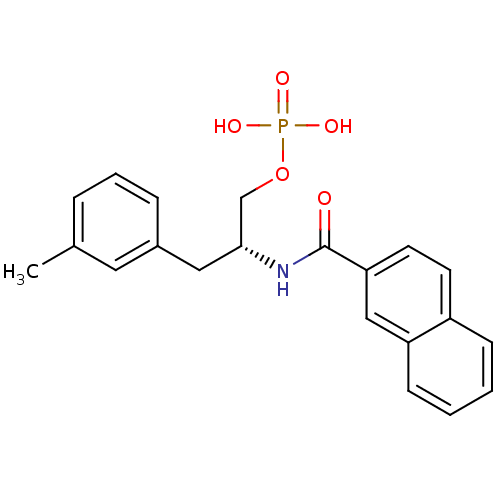 (3-methylphenylalanine derivative, 22a) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 32 | -41.3 | n/a | n/a | n/a | n/a | n/a | 7.5 | 15 |
Pfizer | Assay Description The cis/trans conversion of cis-Suc-AEPFpNA peptide by the PPIase led to the cleavage of para nitroaniline by subtilisin which was monitored at 390 n... | Bioorg Med Chem Lett 19: 5613-6 (2009) Article DOI: 10.1016/j.bmcl.2009.08.034 BindingDB Entry DOI: 10.7270/Q2XD100H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PAK 4 (Homo sapiens (Human)) | BDBM101639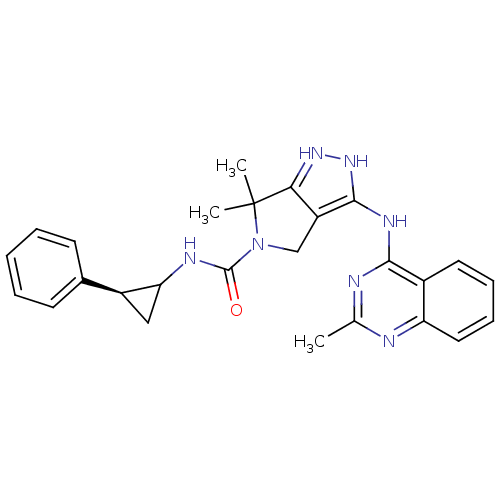 (US8530652, 84) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 34 | n/a | n/a | n/a | 455 | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc.; Pfizer Inc. US Patent | Assay Description The enzymatic activity of PAK4 KD was measured by its ability to catalyzed the transfer of a phosphate residue from a nucleoside triphosphate to an a... | US Patent US8530652 (2013) BindingDB Entry DOI: 10.7270/Q2F18XC4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 4281 total ) | Next | Last >> |