

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50443101 (Cariprazine | RGH-188) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem Similars | Article PubMed | 0.0850 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]Spiperone from dopamine D3 receptor (unknown origin) | J Med Chem 60: 6480-6515 (2017) Article DOI: 10.1021/acs.jmedchem.7b00010 BindingDB Entry DOI: 10.7270/Q2PK0JDP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50443101 (Cariprazine | RGH-188) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem Similars | DrugBank Article PubMed | 0.490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]Spiperone from human dopamine D2L receptor expressed in CHO cells | J Med Chem 60: 6480-6515 (2017) Article DOI: 10.1021/acs.jmedchem.7b00010 BindingDB Entry DOI: 10.7270/Q2PK0JDP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50443101 (Cariprazine | RGH-188) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem Similars | DrugBank Article PubMed | 0.690 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]Spiperone from human D2S receptor expressed in CHO cells | J Med Chem 60: 6480-6515 (2017) Article DOI: 10.1021/acs.jmedchem.7b00010 BindingDB Entry DOI: 10.7270/Q2PK0JDP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50309873 (4-(6-Chlorooxazolo[4,5-b]pyridin-2-yl)-1,4-diazabi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [125I]BTX from rat alpha7 nicotinic acetylcholine receptor expressed in GH4C1 cells | J Med Chem 53: 1222-37 (2010) Article DOI: 10.1021/jm9015075 BindingDB Entry DOI: 10.7270/Q2QN66W3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50333450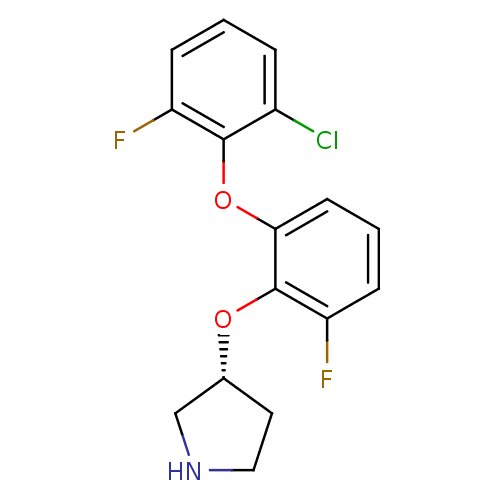 ((R)-3-(2-(2-chloro-6-fluorophenoxy)-6-fluorophenox...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from human cloned 5HT1A receptor by liquid scintillation spectrophotometry | Bioorg Med Chem Lett 21: 865-8 (2011) Article DOI: 10.1016/j.bmcl.2010.11.066 BindingDB Entry DOI: 10.7270/Q2HT2PK6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50333433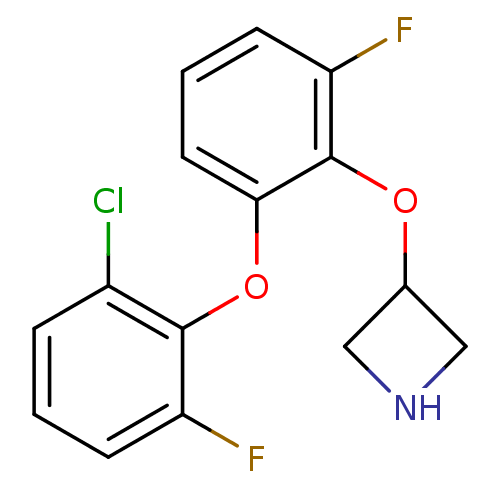 (3-(2-(2-chloro-6-fluorophenoxy)-6-fluorophenoxy)az...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from human cloned 5HT1A receptor by liquid scintillation spectrophotometry | Bioorg Med Chem Lett 21: 865-8 (2011) Article DOI: 10.1016/j.bmcl.2010.11.066 BindingDB Entry DOI: 10.7270/Q2HT2PK6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/glucose cotransporter 2 (Homo sapiens (Human)) | BDBM50315426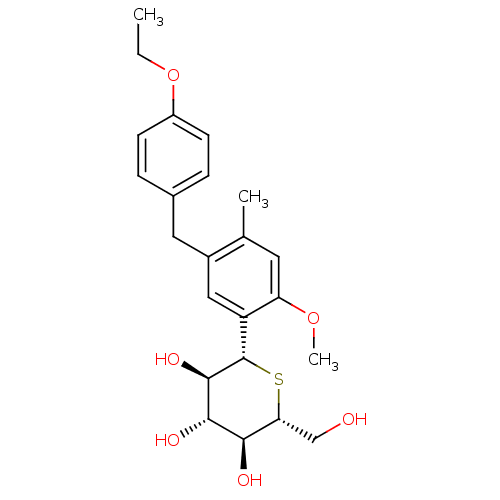 ((1S)-1,5-Anhydro-1-[5-(4-ethoxybenzyl)-2-methoxy-4...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Competitive inhibition of human SGLT2 expressed in CHO-K1 cells assessed as inhibition of [14C]-alpha-methylglucoside uptake | Bioorg Med Chem 24: 1937-80 (2016) Article DOI: 10.1016/j.bmc.2016.03.004 BindingDB Entry DOI: 10.7270/Q22B90WR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Mus musculus (house mouse)) | BDBM50309864 (4-(5-Chlorobenzoxazol-2-yl)-1,4-diazabicyclo[3.2.2...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]LY278584 from mouse 5HT3 receptor expressed in HEK293 cells | J Med Chem 53: 1222-37 (2010) Article DOI: 10.1021/jm9015075 BindingDB Entry DOI: 10.7270/Q2QN66W3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50309897 (2-(1,4-Diazabicyclo[3.2.2]nonan-4-yl)-5-(pyrrolidi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.91 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [125I]BTX from rat alpha7 nicotinic acetylcholine receptor expressed in GH4C1 cells | J Med Chem 53: 1222-37 (2010) Article DOI: 10.1021/jm9015075 BindingDB Entry DOI: 10.7270/Q2QN66W3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50309864 (4-(5-Chlorobenzoxazol-2-yl)-1,4-diazabicyclo[3.2.2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.92 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [125I]BTX from rat alpha7 nicotinic acetylcholine receptor expressed in GH4C1 cells | J Med Chem 53: 1222-37 (2010) Article DOI: 10.1021/jm9015075 BindingDB Entry DOI: 10.7270/Q2QN66W3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50309872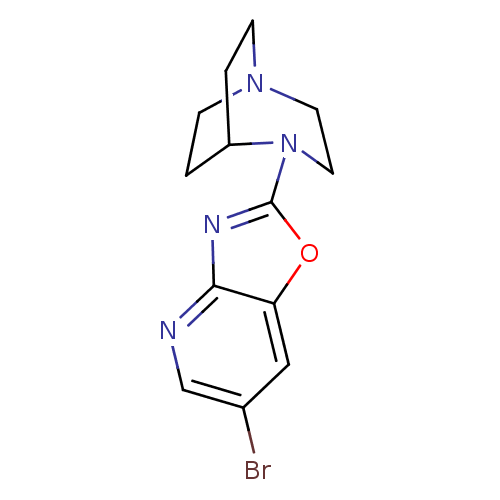 (4-(6-Bromooxazolo[4,5-b]pyridin-2-yl)-1,4-diazabic...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.08 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [125I]BTX from rat alpha7 nicotinic acetylcholine receptor expressed in GH4C1 cells | J Med Chem 53: 1222-37 (2010) Article DOI: 10.1021/jm9015075 BindingDB Entry DOI: 10.7270/Q2QN66W3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50309865 (4-(5-Bromobenzoxazol-2-yl)-1,4-diazabicyclo[3.2.2]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [125I]BTX from rat alpha7 nicotinic acetylcholine receptor expressed in GH4C1 cells | J Med Chem 53: 1222-37 (2010) Article DOI: 10.1021/jm9015075 BindingDB Entry DOI: 10.7270/Q2QN66W3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50309880 (4-(6-Phenyloxazolo[5,4-b]pyridin-2-yl)-1,4-diazabi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 2.31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [125I]BTX from rat alpha7 nicotinic acetylcholine receptor expressed in GH4C1 cells | J Med Chem 53: 1222-37 (2010) Article DOI: 10.1021/jm9015075 BindingDB Entry DOI: 10.7270/Q2QN66W3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50309879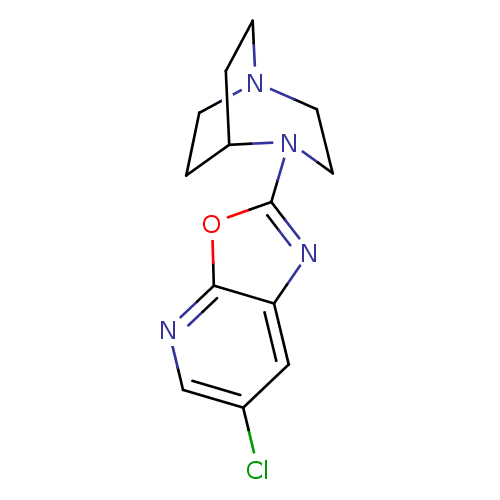 (2-(6-Chlorooxazolo[5,4-b]pyridin-2-yl)-2,5-diazabi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.59 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [125I]BTX from rat alpha7 nicotinic acetylcholine receptor expressed in GH4C1 cells | J Med Chem 53: 1222-37 (2010) Article DOI: 10.1021/jm9015075 BindingDB Entry DOI: 10.7270/Q2QN66W3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50333434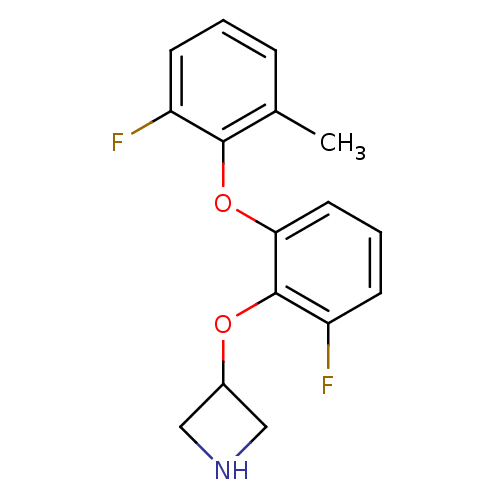 (3-(2-fluoro-6-(2-fluoro-6-methylphenoxy)phenoxy)az...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from human cloned 5HT1A receptor by liquid scintillation spectrophotometry | Bioorg Med Chem Lett 21: 865-8 (2011) Article DOI: 10.1016/j.bmcl.2010.11.066 BindingDB Entry DOI: 10.7270/Q2HT2PK6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50333448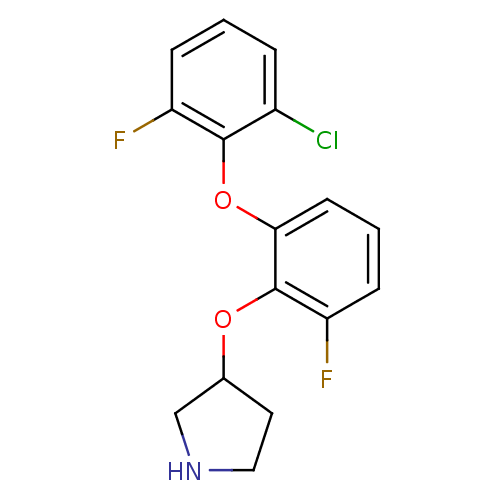 (3-(2-(2-chloro-6-fluorophenoxy)-6-fluorophenoxy)py...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from human cloned 5HT1A receptor by liquid scintillation spectrophotometry | Bioorg Med Chem Lett 21: 865-8 (2011) Article DOI: 10.1016/j.bmcl.2010.11.066 BindingDB Entry DOI: 10.7270/Q2HT2PK6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50309888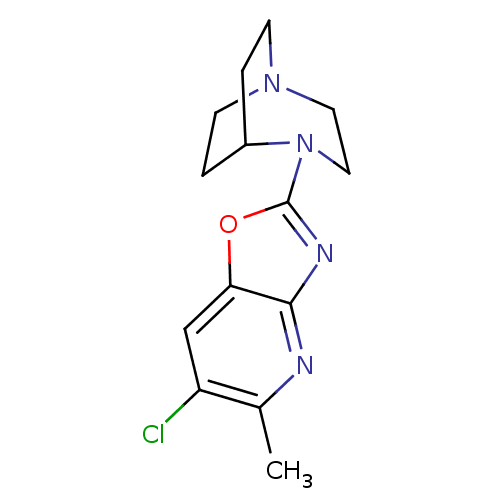 (4-(6-Chloro-5-methyloxazolo[4,5-b]pyridin-2-yl)-1,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [125I]BTX from rat alpha7 nicotinic acetylcholine receptor expressed in GH4C1 cells | J Med Chem 53: 1222-37 (2010) Article DOI: 10.1021/jm9015075 BindingDB Entry DOI: 10.7270/Q2QN66W3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50309892 (2-(5-Phenyloxazolo[4,5-b]pyridin-2-yl)-2,5-diazabi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [125I]BTX from rat alpha7 nicotinic acetylcholine receptor expressed in GH4C1 cells | J Med Chem 53: 1222-37 (2010) Article DOI: 10.1021/jm9015075 BindingDB Entry DOI: 10.7270/Q2QN66W3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Mus musculus (house mouse)) | BDBM50309866 (4-(5-Methylbenzoxazol-2-yl)-1,4-diazabicyclo[3.2.2...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]LY278584 from mouse 5HT3 receptor expressed in HEK293 cells | J Med Chem 53: 1222-37 (2010) Article DOI: 10.1021/jm9015075 BindingDB Entry DOI: 10.7270/Q2QN66W3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50309893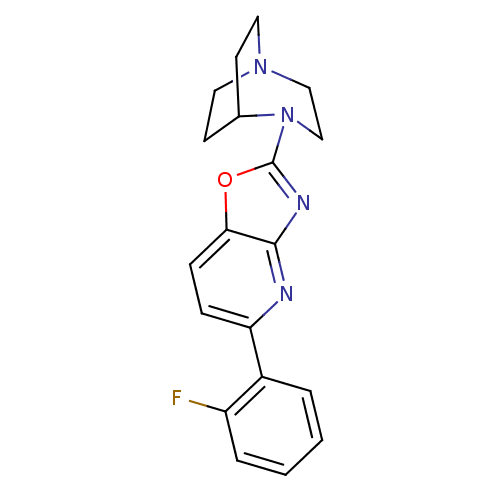 (2-(5-(2-Fluorophenyl)oxazolo[4,5-b]pyridin-2-yl)-2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.45 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [125I]BTX from rat alpha7 nicotinic acetylcholine receptor expressed in GH4C1 cells | J Med Chem 53: 1222-37 (2010) Article DOI: 10.1021/jm9015075 BindingDB Entry DOI: 10.7270/Q2QN66W3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Mus musculus (house mouse)) | BDBM50309863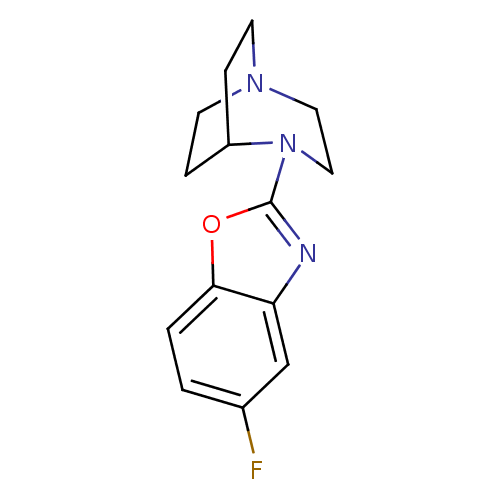 (4-(5-Fluorobenzoxazol-2-yl)-1,4-diazabicyclo[3.2.2...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]LY278584 from mouse 5HT3 receptor expressed in HEK293 cells | J Med Chem 53: 1222-37 (2010) Article DOI: 10.1021/jm9015075 BindingDB Entry DOI: 10.7270/Q2QN66W3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50333442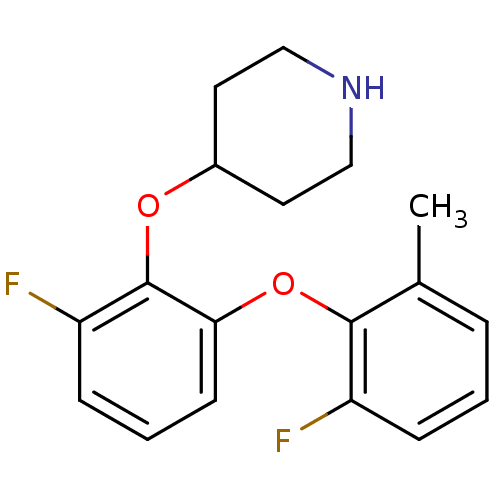 (4-(2-fluoro-6-(2-fluoro-6-methylphenoxy)phenoxy)pi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from human cloned 5HT1A receptor by liquid scintillation spectrophotometry | Bioorg Med Chem Lett 21: 865-8 (2011) Article DOI: 10.1016/j.bmcl.2010.11.066 BindingDB Entry DOI: 10.7270/Q2HT2PK6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50333447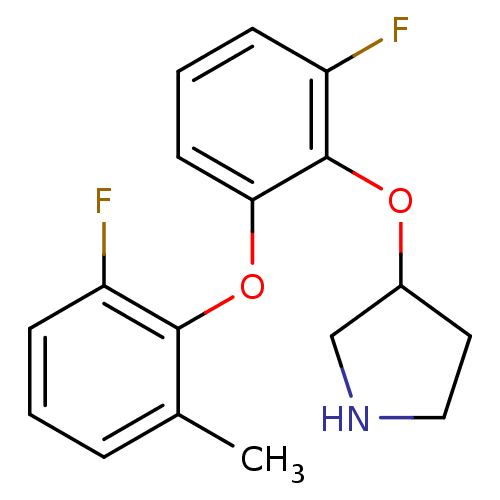 (3-(2-fluoro-6-(2-fluoro-6-methylphenoxy)phenoxy)py...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from human cloned 5HT1A receptor by liquid scintillation spectrophotometry | Bioorg Med Chem Lett 21: 865-8 (2011) Article DOI: 10.1016/j.bmcl.2010.11.066 BindingDB Entry DOI: 10.7270/Q2HT2PK6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50309886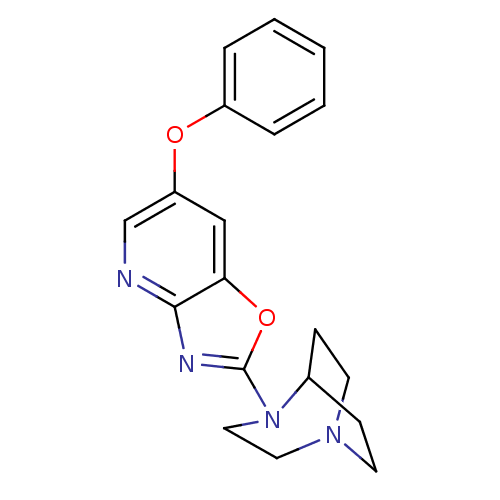 (2-(6-Phenoxyoxazolo[4,5-b]pyridin-2-yl)-2,5-diazab...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 4.16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [125I]BTX from rat alpha7 nicotinic acetylcholine receptor expressed in GH4C1 cells | J Med Chem 53: 1222-37 (2010) Article DOI: 10.1021/jm9015075 BindingDB Entry DOI: 10.7270/Q2QN66W3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-dependent 3',5'-cyclic phosphodiesterase (Rattus norvegicus) | BDBM107767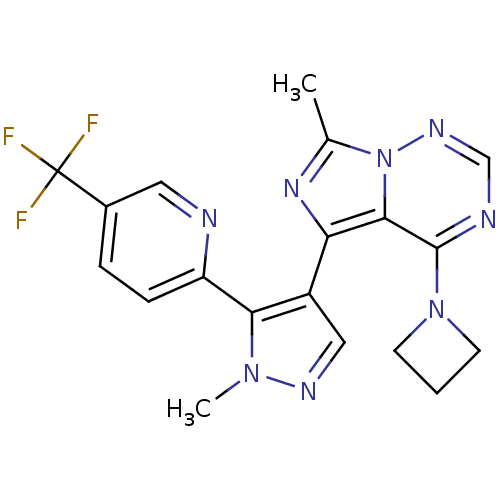 (US11419874, PF-05180999 | US8598155, 2) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of radiolabeled 4-(azetidin-1-yl)-3-[5-[4-(trifluoromethyl)phenyl]-1H-pyrazol-4-yl]-1-(tritritiomethyl)pyrazolo[3,4-d]pyrimidine from PD... | J Med Chem 61: 1001-1018 (2018) Article DOI: 10.1021/acs.jmedchem.7b01466 BindingDB Entry DOI: 10.7270/Q2ZS2ZZ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50309505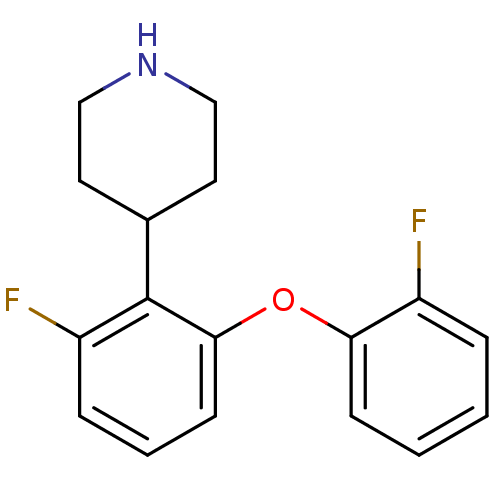 (4-(2-fluoro-6-(2-fluorophenoxy)phenyl)piperidine |...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from human cloned 5HT1A receptor by liquid scintillation spectrophotometry | Bioorg Med Chem Lett 21: 865-8 (2011) Article DOI: 10.1016/j.bmcl.2010.11.066 BindingDB Entry DOI: 10.7270/Q2HT2PK6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50309891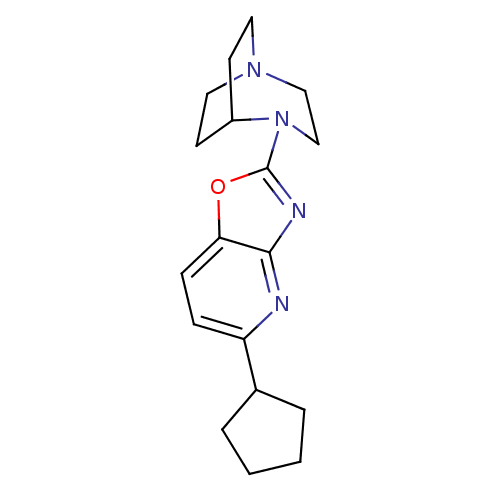 (2-(5-Cyclopentyloxazolo[4,5-b]pyridin-2-yl)-2,5-di...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [125I]BTX from rat alpha7 nicotinic acetylcholine receptor expressed in GH4C1 cells | J Med Chem 53: 1222-37 (2010) Article DOI: 10.1021/jm9015075 BindingDB Entry DOI: 10.7270/Q2QN66W3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50333436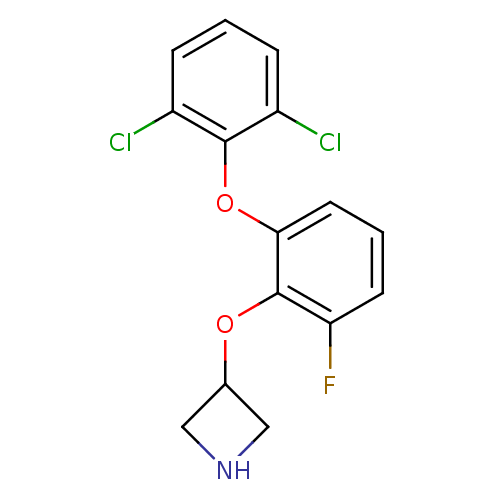 (3-(2-(2,6-dichlorophenoxy)-6-fluorophenoxy)azetidi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from human cloned 5HT1A receptor by liquid scintillation spectrophotometry | Bioorg Med Chem Lett 21: 865-8 (2011) Article DOI: 10.1016/j.bmcl.2010.11.066 BindingDB Entry DOI: 10.7270/Q2HT2PK6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50309882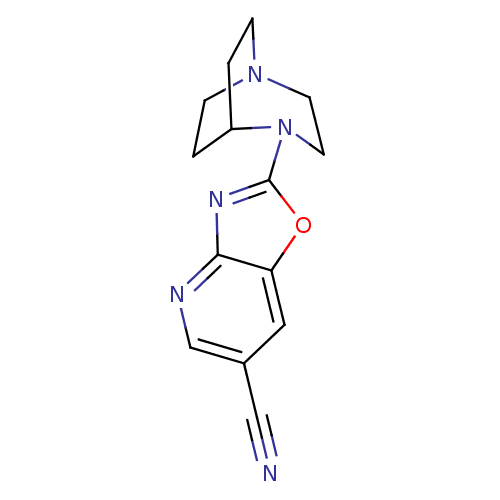 (4-(6-Cyanooxazolo[4,5-b]pyridin-2-yl)-1,4-diazabic...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [125I]BTX from rat alpha7 nicotinic acetylcholine receptor expressed in GH4C1 cells | J Med Chem 53: 1222-37 (2010) Article DOI: 10.1021/jm9015075 BindingDB Entry DOI: 10.7270/Q2QN66W3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50309875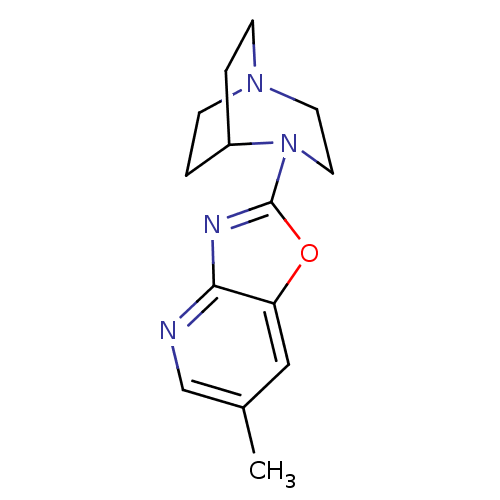 (4-(6-Methyloxazolo[4,5-b]pyridin-2-yl)-1,4-diazabi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.66 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [125I]BTX from rat alpha7 nicotinic acetylcholine receptor expressed in GH4C1 cells | J Med Chem 53: 1222-37 (2010) Article DOI: 10.1021/jm9015075 BindingDB Entry DOI: 10.7270/Q2QN66W3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50309878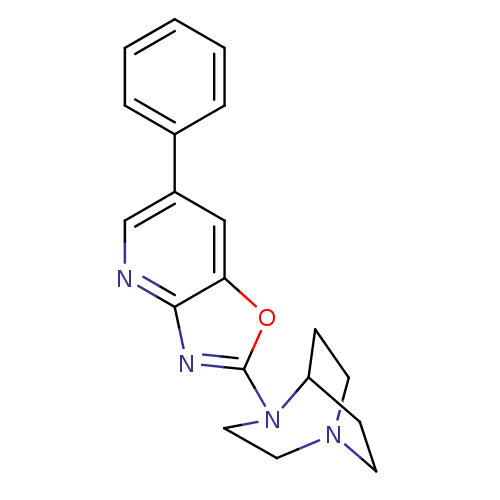 (4-(6-Phenyloxazolo[4,5-b]pyridin-2-yl)-1,4-diazabi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.04 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [125I]BTX from rat alpha7 nicotinic acetylcholine receptor expressed in GH4C1 cells | J Med Chem 53: 1222-37 (2010) Article DOI: 10.1021/jm9015075 BindingDB Entry DOI: 10.7270/Q2QN66W3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50333445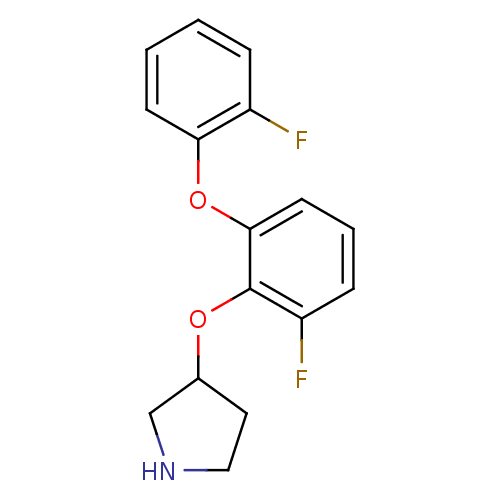 (3-(2-fluoro-6-(2-fluorophenoxy)phenoxy)pyrrolidine...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from human cloned 5HT1A receptor by liquid scintillation spectrophotometry | Bioorg Med Chem Lett 21: 865-8 (2011) Article DOI: 10.1016/j.bmcl.2010.11.066 BindingDB Entry DOI: 10.7270/Q2HT2PK6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50333441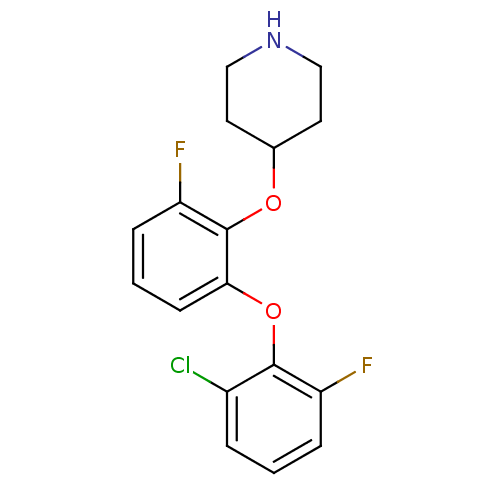 (4-(2-(2-chloro-6-fluorophenoxy)-6-fluorophenoxy)pi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from human cloned 5HT1A receptor by liquid scintillation spectrophotometry | Bioorg Med Chem Lett 21: 865-8 (2011) Article DOI: 10.1016/j.bmcl.2010.11.066 BindingDB Entry DOI: 10.7270/Q2HT2PK6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50296715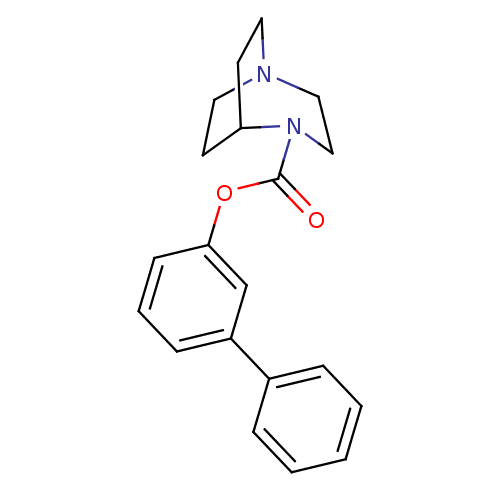 (CHEMBL552005 | biphenyl-3-yl 1,4-diazabicyclo[3.2....) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research& Development Curated by ChEMBL | Assay Description Displacement of [125I]BTX from rat alpha7 nAChR expressed in rat GH4C1 cells | Bioorg Med Chem Lett 19: 4747-51 (2009) Article DOI: 10.1016/j.bmcl.2009.06.059 BindingDB Entry DOI: 10.7270/Q21G0N69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50309890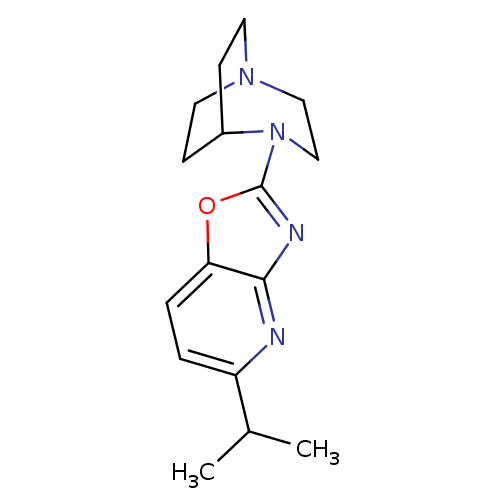 (2-(5-Isopropyloxazolo[4,5-b]pyridin-2-yl)-2,5-diaz...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 9.05 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [125I]BTX from rat alpha7 nicotinic acetylcholine receptor expressed in GH4C1 cells | J Med Chem 53: 1222-37 (2010) Article DOI: 10.1021/jm9015075 BindingDB Entry DOI: 10.7270/Q2QN66W3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50296724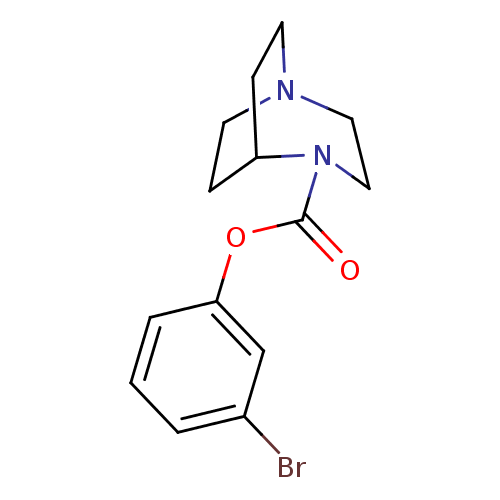 (3-bromophenyl 1,4-diazabicyclo[3.2.2]nonane-4-carb...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 9.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research& Development Curated by ChEMBL | Assay Description Displacement of [125I]BTX from rat alpha7 nAChR expressed in rat GH4C1 cells | Bioorg Med Chem Lett 19: 4747-51 (2009) Article DOI: 10.1016/j.bmcl.2009.06.059 BindingDB Entry DOI: 10.7270/Q21G0N69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50296717 (3-benzoylphenyl 1,4-diazabicyclo[3.2.2]nonane-4-ca...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research& Development Curated by ChEMBL | Assay Description Displacement of [125I]BTX from rat alpha7 nAChR expressed in rat GH4C1 cells | Bioorg Med Chem Lett 19: 4747-51 (2009) Article DOI: 10.1016/j.bmcl.2009.06.059 BindingDB Entry DOI: 10.7270/Q21G0N69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50309519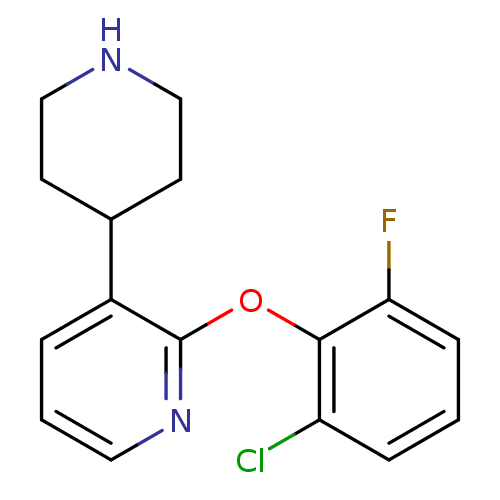 (2-(2-chloro-6-fluorophenoxy)-3-(piperidin-4-yl)pyr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from human cloned 5HT1A receptor by liquid scintillation spectrophotometry | Bioorg Med Chem Lett 21: 865-8 (2011) Article DOI: 10.1016/j.bmcl.2010.11.066 BindingDB Entry DOI: 10.7270/Q2HT2PK6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/glucose cotransporter 2 (Homo sapiens (Human)) | BDBM50559516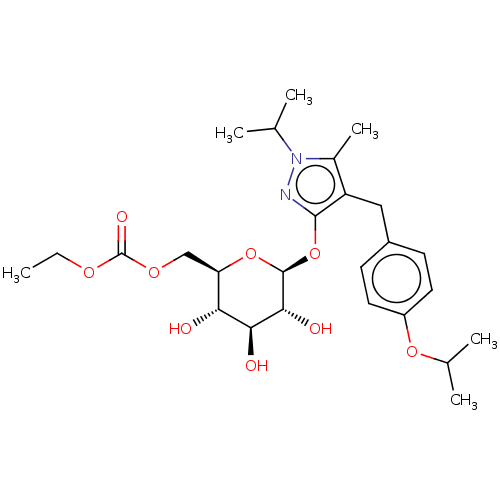 (GSK-189075 | GSK-189075A | GSK189075A | Remogliflo...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Antagonist potency against adenosine A2B receptor of guinea pig thoracic aortic smooth muscle | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50333431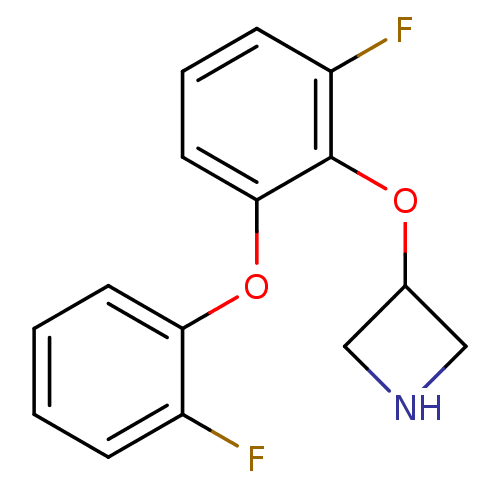 (3-(2-fluoro-6-(2-fluorophenoxy)phenoxy)azetidine |...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from human cloned 5HT1A receptor by liquid scintillation spectrophotometry | Bioorg Med Chem Lett 21: 865-8 (2011) Article DOI: 10.1016/j.bmcl.2010.11.066 BindingDB Entry DOI: 10.7270/Q2HT2PK6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Mus musculus (house mouse)) | BDBM50309862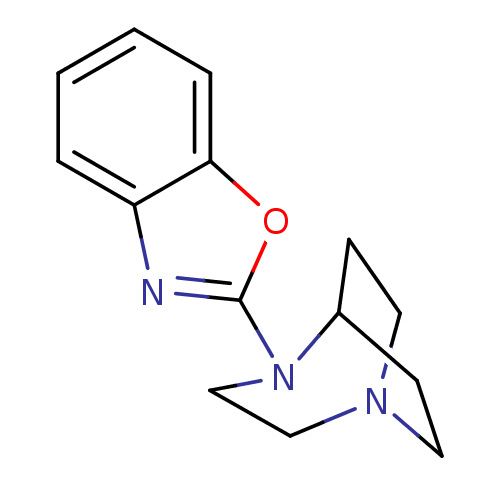 (4-Benzoxazo-2-yl-1,4-diazabicyclo[3.2.2]nonane | C...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]LY278584 from mouse 5HT3 receptor expressed in HEK293 cells | J Med Chem 53: 1222-37 (2010) Article DOI: 10.1021/jm9015075 BindingDB Entry DOI: 10.7270/Q2QN66W3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50296713 (3-phenoxyphenyl 1,4-diazabicyclo[3.2.2]nonane-4-ca...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research& Development Curated by ChEMBL | Assay Description Displacement of [125I]BTX from rat alpha7 nAChR expressed in rat GH4C1 cells | Bioorg Med Chem Lett 19: 4747-51 (2009) Article DOI: 10.1016/j.bmcl.2009.06.059 BindingDB Entry DOI: 10.7270/Q21G0N69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50333449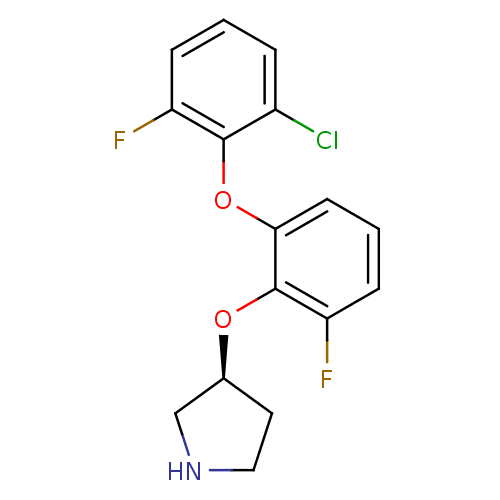 ((S)-3-(2-(2-chloro-6-fluorophenoxy)-6-fluorophenox...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from human cloned 5HT1A receptor by liquid scintillation spectrophotometry | Bioorg Med Chem Lett 21: 865-8 (2011) Article DOI: 10.1016/j.bmcl.2010.11.066 BindingDB Entry DOI: 10.7270/Q2HT2PK6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50309861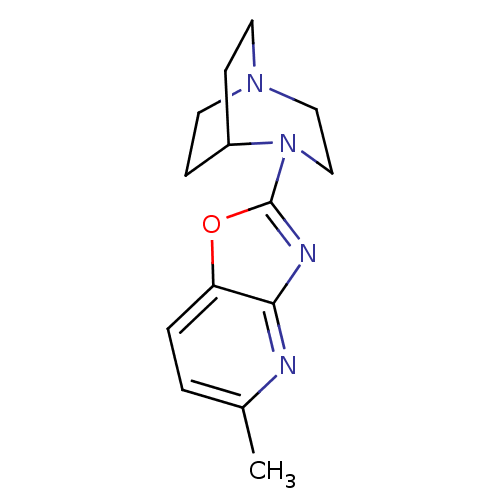 (4-(5-Methyloxazolo[4,5-b]pyridin-2-yl)-1,4-diazabi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 13.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [125I]BTX from rat alpha7 nicotinic acetylcholine receptor expressed in GH4C1 cells | J Med Chem 53: 1222-37 (2010) Article DOI: 10.1021/jm9015075 BindingDB Entry DOI: 10.7270/Q2QN66W3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50333432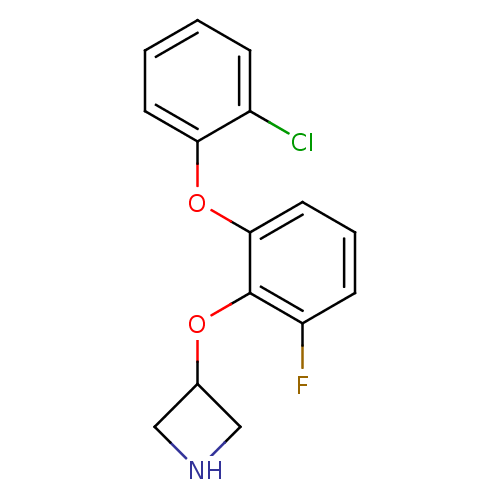 (3-(2-(2-chlorophenoxy)-6-fluorophenoxy)azetidine |...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from human cloned 5HT1A receptor by liquid scintillation spectrophotometry | Bioorg Med Chem Lett 21: 865-8 (2011) Article DOI: 10.1016/j.bmcl.2010.11.066 BindingDB Entry DOI: 10.7270/Q2HT2PK6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50309887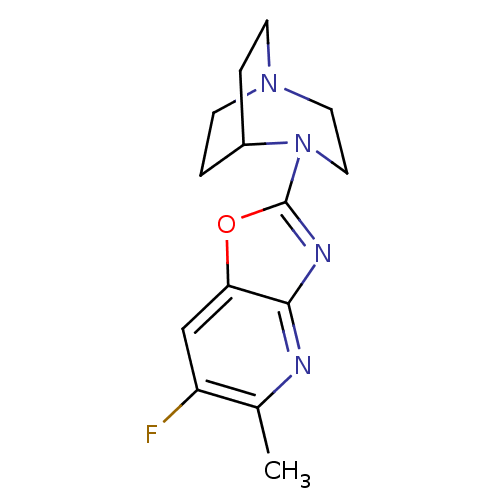 (4-(6-Fluoro-5-methyloxazolo[4,5-b]pyridin-2-yl)-1,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 15.3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [125I]BTX from rat alpha7 nicotinic acetylcholine receptor expressed in GH4C1 cells | J Med Chem 53: 1222-37 (2010) Article DOI: 10.1021/jm9015075 BindingDB Entry DOI: 10.7270/Q2QN66W3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50296747 (3-benzoyl-4-bromophenyl 1,4-diazabicyclo[3.2.2]non...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research& Development Curated by ChEMBL | Assay Description Displacement of [125I]BTX from rat alpha7 nAChR expressed in rat GH4C1 cells | Bioorg Med Chem Lett 19: 4747-51 (2009) Article DOI: 10.1016/j.bmcl.2009.06.059 BindingDB Entry DOI: 10.7270/Q21G0N69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50333438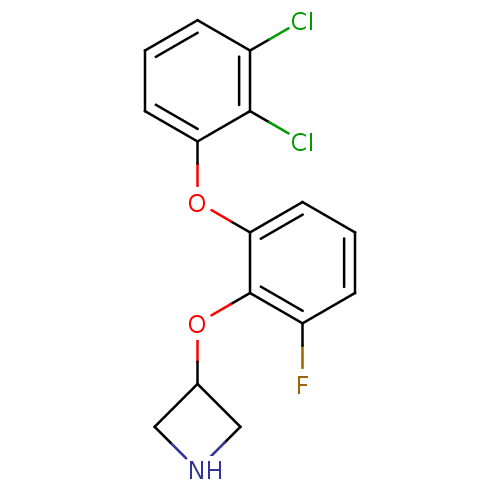 (3-(2-(2,3-dichlorophenoxy)-6-fluorophenoxy)azetidi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from human cloned 5HT1A receptor by liquid scintillation spectrophotometry | Bioorg Med Chem Lett 21: 865-8 (2011) Article DOI: 10.1016/j.bmcl.2010.11.066 BindingDB Entry DOI: 10.7270/Q2HT2PK6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50333437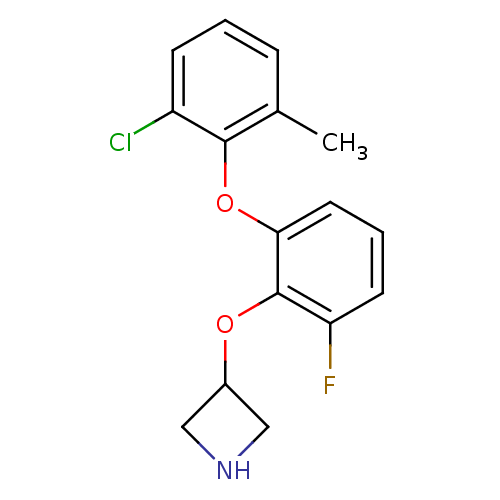 (3-(2-(2-chloro-6-methylphenoxy)-6-fluorophenoxy)az...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from human cloned 5HT1A receptor by liquid scintillation spectrophotometry | Bioorg Med Chem Lett 21: 865-8 (2011) Article DOI: 10.1016/j.bmcl.2010.11.066 BindingDB Entry DOI: 10.7270/Q2HT2PK6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Homo sapiens (Human)) | BDBM50309861 (4-(5-Methyloxazolo[4,5-b]pyridin-2-yl)-1,4-diazabi...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 16.4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [125I]BTX from human alpha7 nicotinic acetylcholine receptor expressed in IMR32 cells | J Med Chem 53: 1222-37 (2010) Article DOI: 10.1021/jm9015075 BindingDB Entry DOI: 10.7270/Q2QN66W3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 454 total ) | Next | Last >> |