

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM35723 (CHEMBL344159 | N-[4-(7-Chloro-5-hydroxy-2,3,4,5-te...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Second Tokushima Institute of New Drug Research Curated by PDSP Ki Database | J Pharmacol Exp Ther 287: 860-7 (1998) BindingDB Entry DOI: 10.7270/Q2S46QGC | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1b receptor (Homo sapiens (Human)) | BDBM35667 (AVP | CHEMBL373742 | US10131692, 44 (AVP) | [3H]Ar...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid UniChem Patents Similars | PubMed | 0.590 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Second Tokushima Institute of New Drug Research Curated by PDSP Ki Database | J Pharmacol Exp Ther 287: 860-7 (1998) BindingDB Entry DOI: 10.7270/Q2S46QGC | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM35667 (AVP | CHEMBL373742 | US10131692, 44 (AVP) | [3H]Ar...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid UniChem Patents Similars | PubMed | 0.780 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Second Tokushima Institute of New Drug Research Curated by PDSP Ki Database | J Pharmacol Exp Ther 287: 860-7 (1998) BindingDB Entry DOI: 10.7270/Q2S46QGC | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Rattus norvegicus (Rat)) | BDBM35667 (AVP | CHEMBL373742 | US10131692, 44 (AVP) | [3H]Ar...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid UniChem Patents Similars | PubMed | 0.950 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Second Tokushima Institute of New Drug Research Curated by PDSP Ki Database | J Pharmacol Exp Ther 287: 860-7 (1998) BindingDB Entry DOI: 10.7270/Q2S46QGC | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Rattus norvegicus (Rat)) | BDBM35723 (CHEMBL344159 | N-[4-(7-Chloro-5-hydroxy-2,3,4,5-te...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 1.33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Second Tokushima Institute of New Drug Research Curated by PDSP Ki Database | J Pharmacol Exp Ther 287: 860-7 (1998) BindingDB Entry DOI: 10.7270/Q2S46QGC | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (RAT) | BDBM35667 (AVP | CHEMBL373742 | US10131692, 44 (AVP) | [3H]Ar...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid UniChem Patents Similars | PubMed | 1.45 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Second Tokushima Institute of New Drug Research Curated by PDSP Ki Database | J Pharmacol Exp Ther 287: 860-7 (1998) BindingDB Entry DOI: 10.7270/Q2S46QGC | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Rattus norvegicus (Rat)) | BDBM35714 (CHEMBL420762 | Mozavaptan | N-[4-(5-Dimethylamino-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 6.36 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Second Tokushima Institute of New Drug Research Curated by PDSP Ki Database | J Pharmacol Exp Ther 287: 860-7 (1998) BindingDB Entry DOI: 10.7270/Q2S46QGC | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM35714 (CHEMBL420762 | Mozavaptan | N-[4-(5-Dimethylamino-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 9.42 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Second Tokushima Institute of New Drug Research Curated by PDSP Ki Database | J Pharmacol Exp Ther 287: 860-7 (1998) BindingDB Entry DOI: 10.7270/Q2S46QGC | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (Homo sapiens (Human)) | BDBM35723 (CHEMBL344159 | N-[4-(7-Chloro-5-hydroxy-2,3,4,5-te...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 12.3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Second Tokushima Institute of New Drug Research Curated by PDSP Ki Database | J Pharmacol Exp Ther 287: 860-7 (1998) BindingDB Entry DOI: 10.7270/Q2S46QGC | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (Homo sapiens (Human)) | BDBM35714 (CHEMBL420762 | Mozavaptan | N-[4-(5-Dimethylamino-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Second Tokushima Institute of New Drug Research Curated by PDSP Ki Database | J Pharmacol Exp Ther 287: 860-7 (1998) BindingDB Entry DOI: 10.7270/Q2S46QGC | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Deoxyuridine 5'-triphosphate nucleotidohydrolase, mitochondrial (Homo sapiens (Human)) | BDBM50386618 (CHEMBL2048443) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Taiho Pharmaceutical Co. Ltd. Curated by ChEMBL | Assay Description Inhibition of human dUTPase | J Med Chem 55: 2960-9 (2012) Article DOI: 10.1021/jm201627n BindingDB Entry DOI: 10.7270/Q2WQ04VS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (RAT) | BDBM35723 (CHEMBL344159 | N-[4-(7-Chloro-5-hydroxy-2,3,4,5-te...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 325 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Second Tokushima Institute of New Drug Research Curated by PDSP Ki Database | J Pharmacol Exp Ther 287: 860-7 (1998) BindingDB Entry DOI: 10.7270/Q2S46QGC | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (RAT) | BDBM35714 (CHEMBL420762 | Mozavaptan | N-[4-(5-Dimethylamino-...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 524 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Second Tokushima Institute of New Drug Research Curated by PDSP Ki Database | J Pharmacol Exp Ther 287: 860-7 (1998) BindingDB Entry DOI: 10.7270/Q2S46QGC | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Solute carrier organic anion transporter family member 1A4 (Rattus norvegicus) | BDBM18957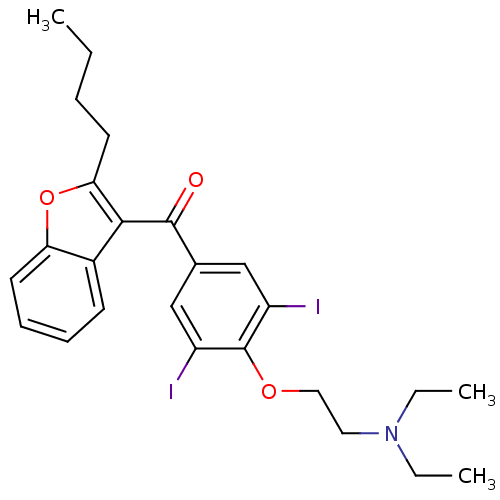 ((2-{4-[(2-butyl-1-benzofuran-3-yl)carbonyl]-2,6-di...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku University School of Medicine Curated by ChEMBL | Assay Description TP_TRANSPORTER: inhibition of Digoxin uptake in Xenopus laevis oocytes | Endocrinology 142: 2005-12 (2001) Article DOI: 10.1210/endo.142.5.8115 BindingDB Entry DOI: 10.7270/Q2GF0XB3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1b receptor (Homo sapiens (Human)) | BDBM35714 (CHEMBL420762 | Mozavaptan | N-[4-(5-Dimethylamino-...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Second Tokushima Institute of New Drug Research Curated by PDSP Ki Database | J Pharmacol Exp Ther 287: 860-7 (1998) BindingDB Entry DOI: 10.7270/Q2S46QGC | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1b receptor (Homo sapiens (Human)) | BDBM35723 (CHEMBL344159 | N-[4-(7-Chloro-5-hydroxy-2,3,4,5-te...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Second Tokushima Institute of New Drug Research Curated by PDSP Ki Database | J Pharmacol Exp Ther 287: 860-7 (1998) BindingDB Entry DOI: 10.7270/Q2S46QGC | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Deoxyuridine 5'-triphosphate nucleotidohydrolase, mitochondrial (Homo sapiens (Human)) | BDBM50173539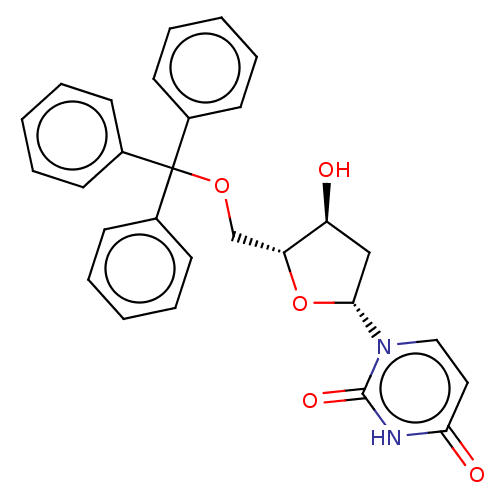 (1-(4-Hydroxy-5-trityloxymethyl-tetrahydro-furan-2-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Taiho Pharmaceutical Co. Ltd. Curated by ChEMBL | Assay Description Inhibition of human dUTPase assessed as production of [5-3H]dUMP from [5-3H]dUTP after 15 mins measured by HPLC analysis | J Med Chem 55: 2970-80 (2012) Article DOI: 10.1021/jm201628y BindingDB Entry DOI: 10.7270/Q2C82BB5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM456483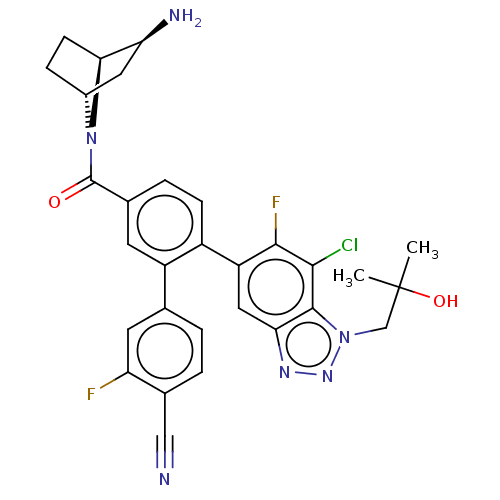 (US10723742, Example 209 | US10723742, Example 213 ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a |
Taiho Pharmaceutical Co., Ltd. US Patent | Assay Description The conditions for measuring inhibitory activity of compounds against LSD1 activity were determined with reference to a document available from the w... | US Patent US10723742 (2020) BindingDB Entry DOI: 10.7270/Q2TF01DB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM456491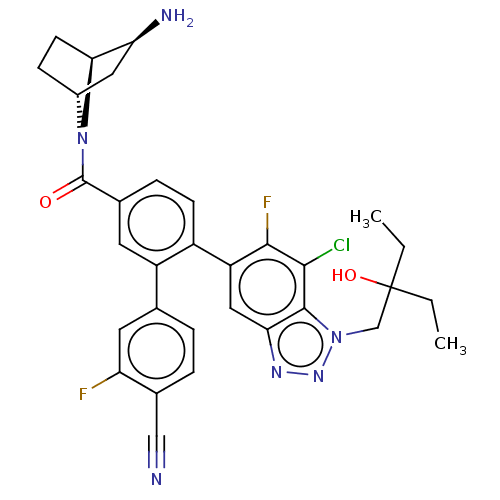 (US10723742, Example 218 | US10723742, Example 221 ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a |
Taiho Pharmaceutical Co., Ltd. US Patent | Assay Description The conditions for measuring inhibitory activity of compounds against LSD1 activity were determined with reference to a document available from the w... | US Patent US10723742 (2020) BindingDB Entry DOI: 10.7270/Q2TF01DB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM456476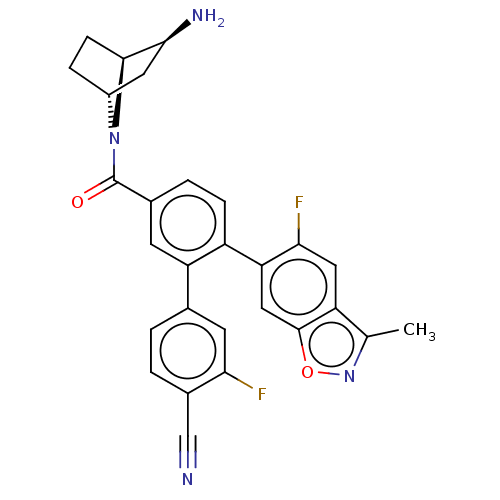 (US10723742, Example 202 | US11510915, Example 202) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a |
Taiho Pharmaceutical Co., Ltd. US Patent | Assay Description The conditions for measuring inhibitory activity of compounds against LSD1 activity were determined with reference to a document available from the w... | US Patent US10723742 (2020) BindingDB Entry DOI: 10.7270/Q2TF01DB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM456492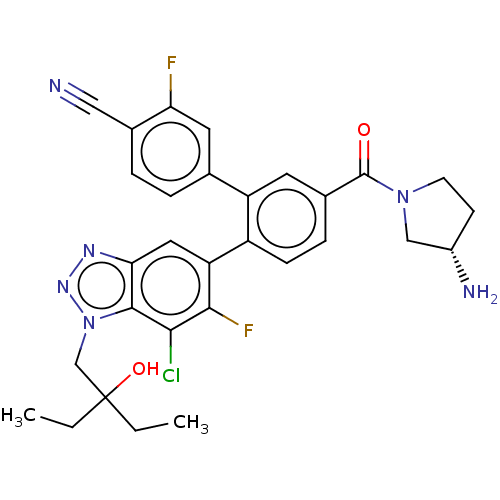 (US10723742, Example 219 | US11510915, Example 219) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Taiho Pharmaceutical Co., Ltd. US Patent | Assay Description The conditions for measuring inhibitory activity of compounds against LSD1 activity were determined with reference to a document available from the w... | US Patent US10723742 (2020) BindingDB Entry DOI: 10.7270/Q2TF01DB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM456487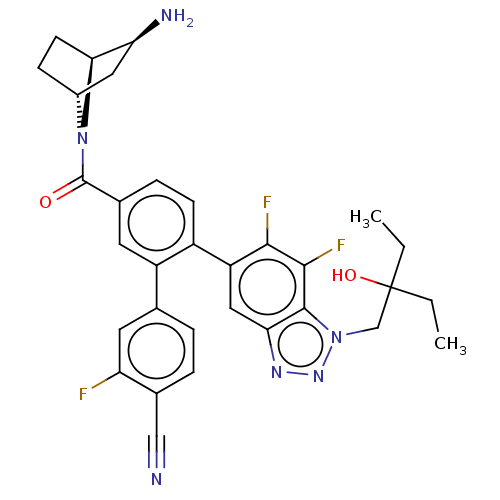 (US10723742, Example 214 | US10723742, Example 217 ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Taiho Pharmaceutical Co., Ltd. US Patent | Assay Description The conditions for measuring inhibitory activity of compounds against LSD1 activity were determined with reference to a document available from the w... | US Patent US10723742 (2020) BindingDB Entry DOI: 10.7270/Q2TF01DB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM456471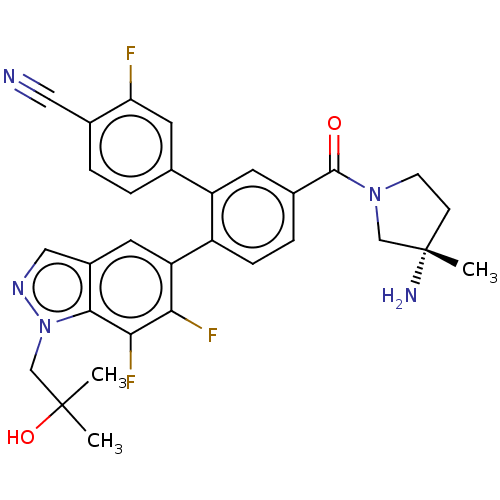 (US10723742, Example 197 | US11510915, Example 197) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a |
Taiho Pharmaceutical Co., Ltd. US Patent | Assay Description The conditions for measuring inhibitory activity of compounds against LSD1 activity were determined with reference to a document available from the w... | US Patent US10723742 (2020) BindingDB Entry DOI: 10.7270/Q2TF01DB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM456500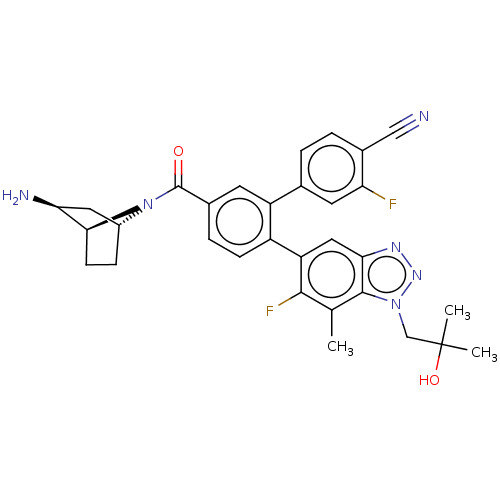 (US10723742, Example 228 | US10723742, Example 241 ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a |
Taiho Pharmaceutical Co., Ltd. US Patent | Assay Description The conditions for measuring inhibitory activity of compounds against LSD1 activity were determined with reference to a document available from the w... | US Patent US10723742 (2020) BindingDB Entry DOI: 10.7270/Q2TF01DB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM456493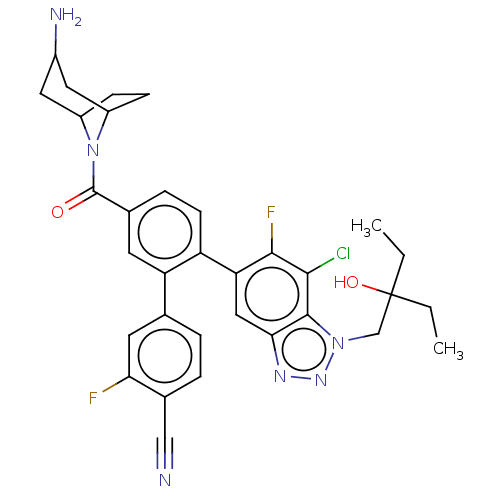 (US10723742, Example 220 | US11510915, Example 220) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a |
Taiho Pharmaceutical Co., Ltd. US Patent | Assay Description The conditions for measuring inhibitory activity of compounds against LSD1 activity were determined with reference to a document available from the w... | US Patent US10723742 (2020) BindingDB Entry DOI: 10.7270/Q2TF01DB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM456528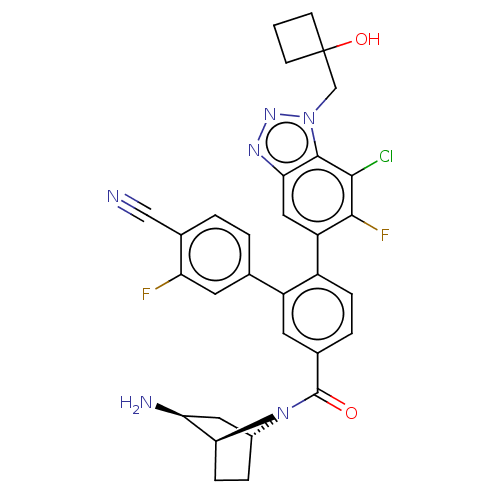 (US10723742, Example 257 | US10723742, Example 269 ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a |
Taiho Pharmaceutical Co., Ltd. US Patent | Assay Description The conditions for measuring inhibitory activity of compounds against LSD1 activity were determined with reference to a document available from the w... | US Patent US10723742 (2020) BindingDB Entry DOI: 10.7270/Q2TF01DB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM456304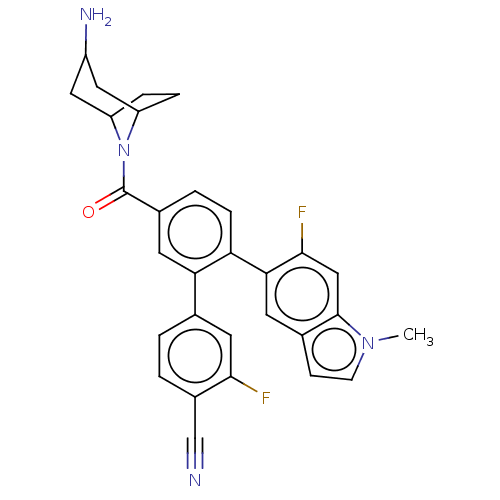 (US10723742, Example 123 | US10723742, Example 23 |...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a |
Taiho Pharmaceutical Co., Ltd. US Patent | Assay Description The conditions for measuring inhibitory activity of compounds against LSD1 activity were determined with reference to a document available from the w... | US Patent US10723742 (2020) BindingDB Entry DOI: 10.7270/Q2TF01DB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM456529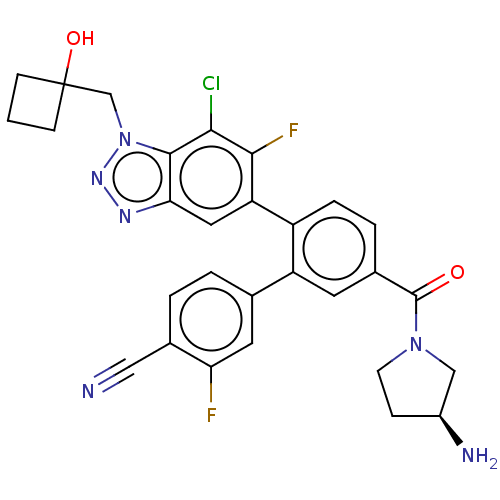 (US10723742, Example 258 | US11510915, Example 258) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a |
Taiho Pharmaceutical Co., Ltd. US Patent | Assay Description The conditions for measuring inhibitory activity of compounds against LSD1 activity were determined with reference to a document available from the w... | US Patent US10723742 (2020) BindingDB Entry DOI: 10.7270/Q2TF01DB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM456525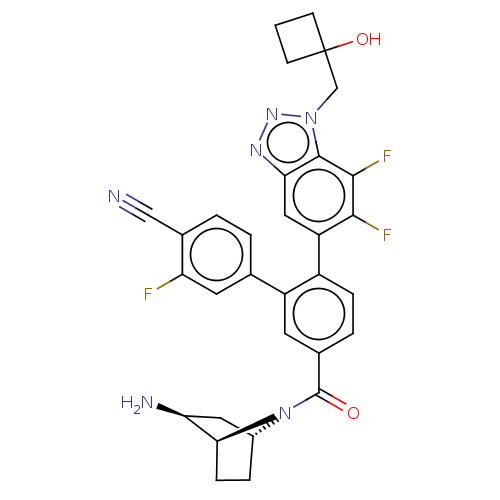 (US10723742, Example 254 | US10723742, Example 268 ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a |
Taiho Pharmaceutical Co., Ltd. US Patent | Assay Description The conditions for measuring inhibitory activity of compounds against LSD1 activity were determined with reference to a document available from the w... | US Patent US10723742 (2020) BindingDB Entry DOI: 10.7270/Q2TF01DB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM456485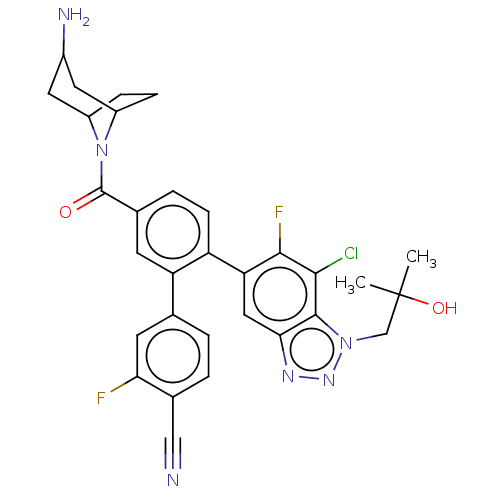 (US10723742, Example 211 | US11510915, Example 211) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a |
Taiho Pharmaceutical Co., Ltd. US Patent | Assay Description The conditions for measuring inhibitory activity of compounds against LSD1 activity were determined with reference to a document available from the w... | US Patent US10723742 (2020) BindingDB Entry DOI: 10.7270/Q2TF01DB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM456530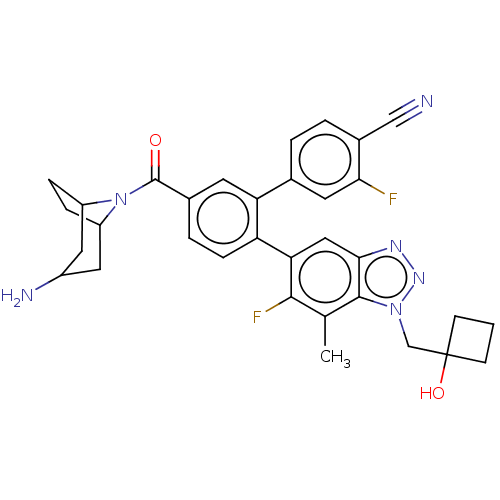 (US10723742, Example 259 | US10723742, Example 272 ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a |
Taiho Pharmaceutical Co., Ltd. US Patent | Assay Description The conditions for measuring inhibitory activity of compounds against LSD1 activity were determined with reference to a document available from the w... | US Patent US10723742 (2020) BindingDB Entry DOI: 10.7270/Q2TF01DB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM456470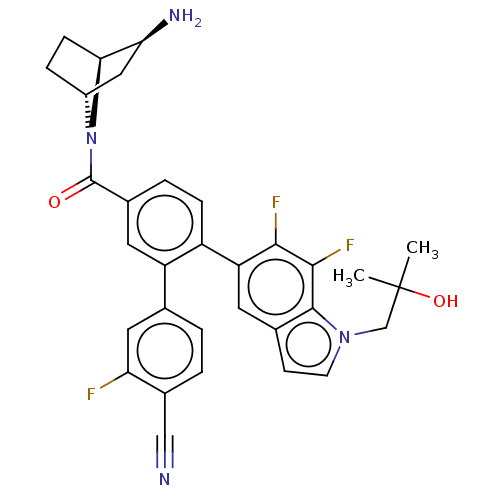 (US10723742, Example 196 | US11510915, Example 196) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a |
Taiho Pharmaceutical Co., Ltd. US Patent | Assay Description The conditions for measuring inhibitory activity of compounds against LSD1 activity were determined with reference to a document available from the w... | US Patent US10723742 (2020) BindingDB Entry DOI: 10.7270/Q2TF01DB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM456449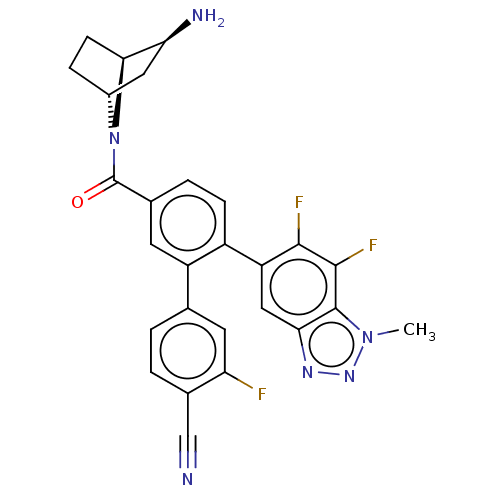 (US10723742, Example 175 | US10723742, Example 185 ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a |
Taiho Pharmaceutical Co., Ltd. US Patent | Assay Description The conditions for measuring inhibitory activity of compounds against LSD1 activity were determined with reference to a document available from the w... | US Patent US10723742 (2020) BindingDB Entry DOI: 10.7270/Q2TF01DB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM456531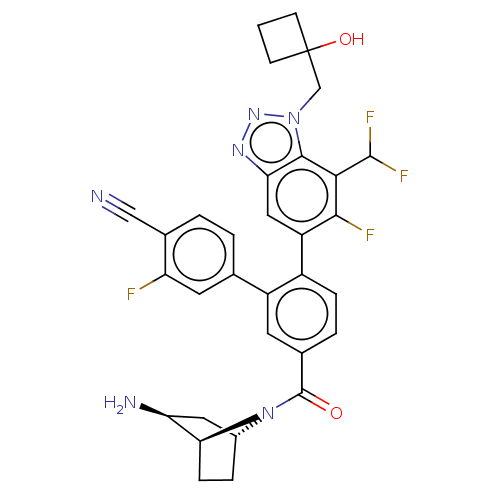 (US10723742, Example 260 | US10723742, Example 263 ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a |
Taiho Pharmaceutical Co., Ltd. US Patent | Assay Description The conditions for measuring inhibitory activity of compounds against LSD1 activity were determined with reference to a document available from the w... | US Patent US10723742 (2020) BindingDB Entry DOI: 10.7270/Q2TF01DB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM456541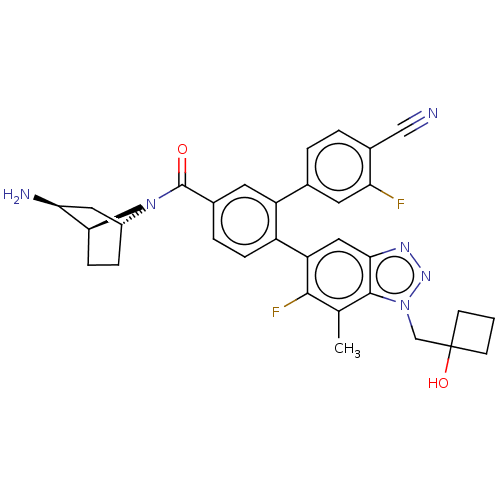 (US10723742, Example 270 | US10723742, Example 276 ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a |
Taiho Pharmaceutical Co., Ltd. US Patent | Assay Description The conditions for measuring inhibitory activity of compounds against LSD1 activity were determined with reference to a document available from the w... | US Patent US10723742 (2020) BindingDB Entry DOI: 10.7270/Q2TF01DB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM456483 (US10723742, Example 209 | US10723742, Example 213 ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a |
Taiho Pharmaceutical Co., Ltd. US Patent | Assay Description The conditions for measuring inhibitory activity of compounds against LSD1 activity were determined with reference to a document available from the w... | US Patent US10723742 (2020) BindingDB Entry DOI: 10.7270/Q2TF01DB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM456464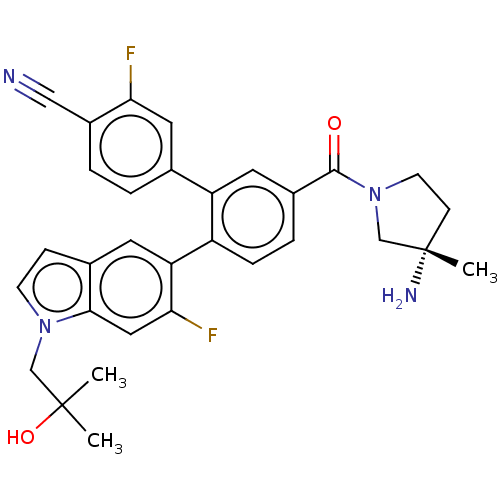 (US10723742, Example 190 | US11510915, Example 190) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a |
Taiho Pharmaceutical Co., Ltd. US Patent | Assay Description The conditions for measuring inhibitory activity of compounds against LSD1 activity were determined with reference to a document available from the w... | US Patent US10723742 (2020) BindingDB Entry DOI: 10.7270/Q2TF01DB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM456484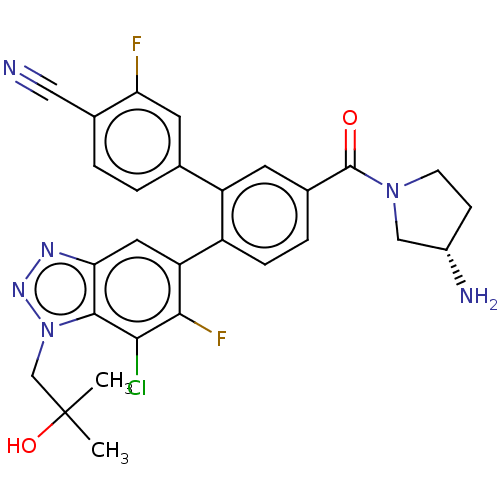 (US10723742, Example 210 | US11510915, Example 210) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a |
Taiho Pharmaceutical Co., Ltd. US Patent | Assay Description The conditions for measuring inhibitory activity of compounds against LSD1 activity were determined with reference to a document available from the w... | US Patent US10723742 (2020) BindingDB Entry DOI: 10.7270/Q2TF01DB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM456517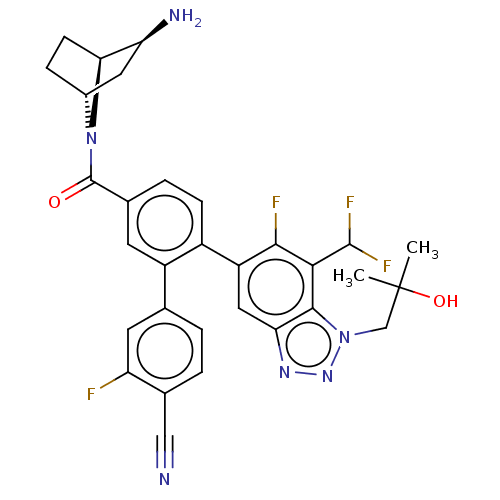 (US10723742, Example 246 | US10723742, Example 252 ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a |
Taiho Pharmaceutical Co., Ltd. US Patent | Assay Description The conditions for measuring inhibitory activity of compounds against LSD1 activity were determined with reference to a document available from the w... | US Patent US10723742 (2020) BindingDB Entry DOI: 10.7270/Q2TF01DB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 4 (Homo sapiens (Human)) | BDBM50264847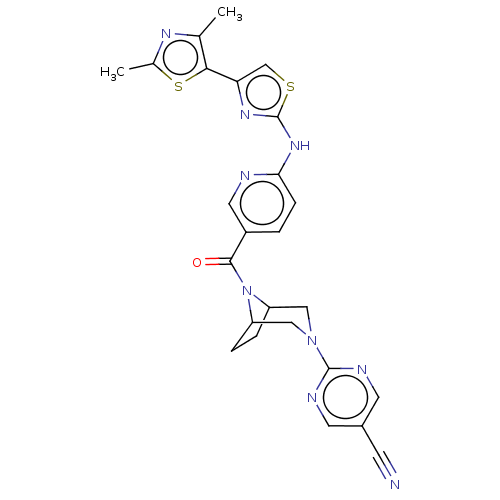 (CHEMBL4096902) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi& Co., Ltd Curated by ChEMBL | Assay Description Antagonist activity at recombinant human TRPV4 expressed in CHOK1 cells assessed as inhibition of 4alphaPDD-induced activation pretreated for 5 mins ... | Bioorg Med Chem 25: 2177-2190 (2017) Article DOI: 10.1016/j.bmc.2017.02.047 BindingDB Entry DOI: 10.7270/Q2N58PT9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM456472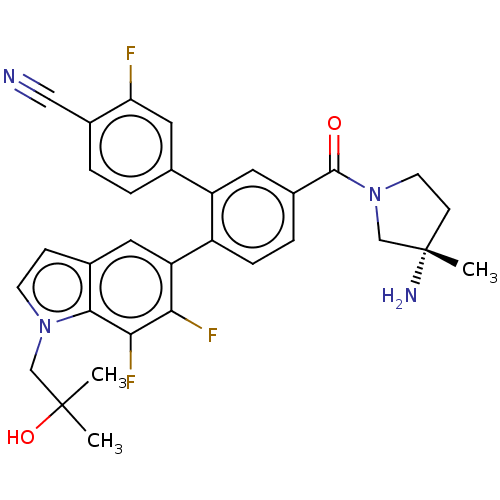 (US10723742, Example 198 | US11510915, Example 198) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a |
Taiho Pharmaceutical Co., Ltd. US Patent | Assay Description The conditions for measuring inhibitory activity of compounds against LSD1 activity were determined with reference to a document available from the w... | US Patent US10723742 (2020) BindingDB Entry DOI: 10.7270/Q2TF01DB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM456304 (US10723742, Example 123 | US10723742, Example 23 |...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a |
Taiho Pharmaceutical Co., Ltd. US Patent | Assay Description The conditions for measuring inhibitory activity of compounds against LSD1 activity were determined with reference to a document available from the w... | US Patent US10723742 (2020) BindingDB Entry DOI: 10.7270/Q2TF01DB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM456544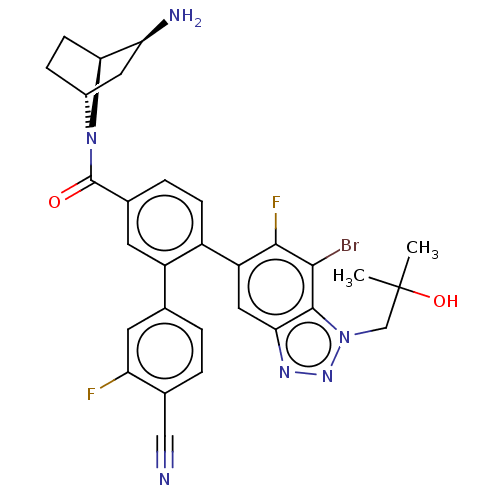 (US10723742, Example 273 | US10723742, Example 277 ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a |
Taiho Pharmaceutical Co., Ltd. US Patent | Assay Description The conditions for measuring inhibitory activity of compounds against LSD1 activity were determined with reference to a document available from the w... | US Patent US10723742 (2020) BindingDB Entry DOI: 10.7270/Q2TF01DB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 4 (Homo sapiens (Human)) | BDBM50256209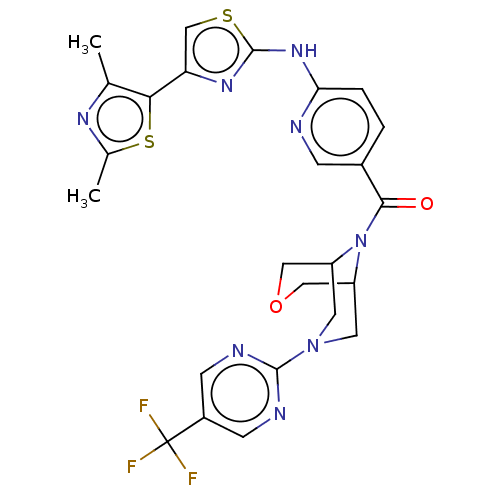 (CHEMBL4101768) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi& Co., Ltd Curated by ChEMBL | Assay Description Antagonist activity at recombinant human TRPV4 expressed in CHOK1 cells assessed as inhibition of 4alphaPDD-induced activation pretreated for 5 mins ... | Bioorg Med Chem 25: 2177-2190 (2017) Article DOI: 10.1016/j.bmc.2017.02.047 BindingDB Entry DOI: 10.7270/Q2N58PT9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM456491 (US10723742, Example 218 | US10723742, Example 221 ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Taiho Pharmaceutical Co., Ltd. US Patent | Assay Description The conditions for measuring inhibitory activity of compounds against LSD1 activity were determined with reference to a document available from the w... | US Patent US10723742 (2020) BindingDB Entry DOI: 10.7270/Q2TF01DB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM456469 (US10723742, Example 195 | US11510915, Example 195) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Taiho Pharmaceutical Co., Ltd. US Patent | Assay Description The conditions for measuring inhibitory activity of compounds against LSD1 activity were determined with reference to a document available from the w... | US Patent US10723742 (2020) BindingDB Entry DOI: 10.7270/Q2TF01DB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM456508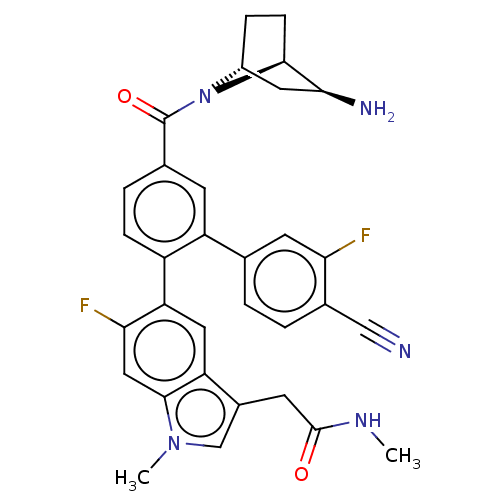 (US10723742, Example 237 | US10723742, Example 243 ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Taiho Pharmaceutical Co., Ltd. US Patent | Assay Description The conditions for measuring inhibitory activity of compounds against LSD1 activity were determined with reference to a document available from the w... | US Patent US10723742 (2020) BindingDB Entry DOI: 10.7270/Q2TF01DB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM456480 (US10723742, Example 206 | US10723742, Example 225 ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Taiho Pharmaceutical Co., Ltd. US Patent | Assay Description The conditions for measuring inhibitory activity of compounds against LSD1 activity were determined with reference to a document available from the w... | US Patent US10723742 (2020) BindingDB Entry DOI: 10.7270/Q2TF01DB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 4 (Homo sapiens (Human)) | BDBM50264846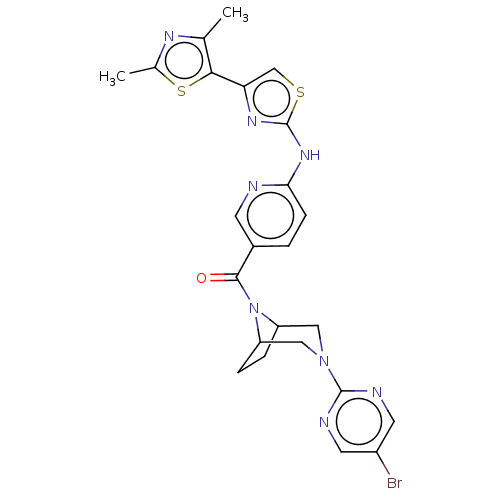 (CHEMBL4066531) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi& Co., Ltd Curated by ChEMBL | Assay Description Antagonist activity at recombinant human TRPV4 expressed in CHOK1 cells assessed as inhibition of 4alphaPDD-induced activation pretreated for 5 mins ... | Bioorg Med Chem 25: 2177-2190 (2017) Article DOI: 10.1016/j.bmc.2017.02.047 BindingDB Entry DOI: 10.7270/Q2N58PT9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM456487 (US10723742, Example 214 | US10723742, Example 217 ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a |
Taiho Pharmaceutical Co., Ltd. US Patent | Assay Description The conditions for measuring inhibitory activity of compounds against LSD1 activity were determined with reference to a document available from the w... | US Patent US10723742 (2020) BindingDB Entry DOI: 10.7270/Q2TF01DB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 3464 total ) | Next | Last >> |