 Found 151 hits with Last Name = 'park' and Initial = 'mj'
Found 151 hits with Last Name = 'park' and Initial = 'mj' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
neuronal acetylcholine receptor subunit alpha-2
(Xenopus) | BDBM86041
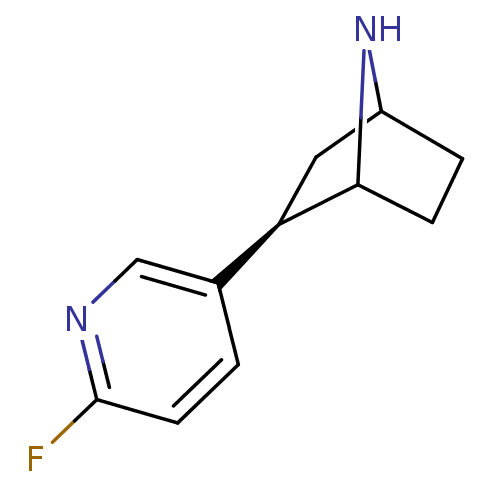
(NFEP)Show SMILES Fc1ccc(cn1)[C@H]1CC2CCC1N2 |r,TLB:4:7:10.11:13| Show InChI InChI=1S/C11H13FN2/c12-11-4-1-7(6-13-11)9-5-8-2-3-10(9)14-8/h1,4,6,8-10,14H,2-3,5H2/t8?,9-,10?/m1/s1 | PDB
Reactome pathway
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Miami
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 302: 1246-52 (2002)
Article DOI: 10.1124/jpet.102.035899
BindingDB Entry DOI: 10.7270/Q2HQ3XG3 |
More data for this
Ligand-Target Pair | |
cholinergic receptor, nicotinic, alpha 1 (Muscle) isoform X1
(Xenopus) | BDBM86042

(NEP)Show InChI InChI=1S/C11H14N2/c1-2-8(7-12-5-1)10-6-9-3-4-11(10)13-9/h1-2,5,7,9-11,13H,3-4,6H2/t9?,10-,11?/m1/s1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Miami
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 302: 1246-52 (2002)
Article DOI: 10.1124/jpet.102.035899
BindingDB Entry DOI: 10.7270/Q2HQ3XG3 |
More data for this
Ligand-Target Pair | |
cholinergic receptor, nicotinic, alpha 1 (Muscle) isoform X1
(Xenopus) | BDBM86041

(NFEP)Show SMILES Fc1ccc(cn1)[C@H]1CC2CCC1N2 |r,TLB:4:7:10.11:13| Show InChI InChI=1S/C11H13FN2/c12-11-4-1-7(6-13-11)9-5-8-2-3-10(9)14-8/h1,4,6,8-10,14H,2-3,5H2/t8?,9-,10?/m1/s1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Miami
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 302: 1246-52 (2002)
Article DOI: 10.1124/jpet.102.035899
BindingDB Entry DOI: 10.7270/Q2HQ3XG3 |
More data for this
Ligand-Target Pair | |
cholinergic receptor, nicotinic, alpha 1 (Muscle) isoform X1
(Xenopus) | BDBM86040
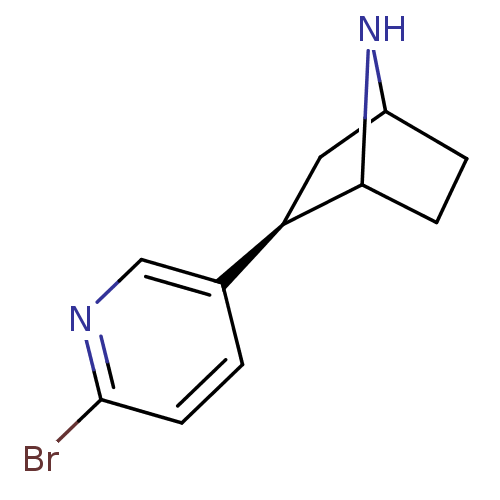
(NBEP)Show SMILES Brc1ccc(cn1)[C@H]1CC2CCC1N2 |r,TLB:4:7:10.11:13| Show InChI InChI=1S/C11H13BrN2/c12-11-4-1-7(6-13-11)9-5-8-2-3-10(9)14-8/h1,4,6,8-10,14H,2-3,5H2/t8?,9-,10?/m1/s1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Miami
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 302: 1246-52 (2002)
Article DOI: 10.1124/jpet.102.035899
BindingDB Entry DOI: 10.7270/Q2HQ3XG3 |
More data for this
Ligand-Target Pair | |
neuronal acetylcholine receptor subunit alpha-4
(Xenopus) | BDBM86042

(NEP)Show InChI InChI=1S/C11H14N2/c1-2-8(7-12-5-1)10-6-9-3-4-11(10)13-9/h1-2,5,7,9-11,13H,3-4,6H2/t9?,10-,11?/m1/s1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00850 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Miami
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 302: 1246-52 (2002)
Article DOI: 10.1124/jpet.102.035899
BindingDB Entry DOI: 10.7270/Q2HQ3XG3 |
More data for this
Ligand-Target Pair | |
neuronal acetylcholine receptor subunit alpha-4
(Xenopus) | BDBM86041

(NFEP)Show SMILES Fc1ccc(cn1)[C@H]1CC2CCC1N2 |r,TLB:4:7:10.11:13| Show InChI InChI=1S/C11H13FN2/c12-11-4-1-7(6-13-11)9-5-8-2-3-10(9)14-8/h1,4,6,8-10,14H,2-3,5H2/t8?,9-,10?/m1/s1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00920 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Miami
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 302: 1246-52 (2002)
Article DOI: 10.1124/jpet.102.035899
BindingDB Entry DOI: 10.7270/Q2HQ3XG3 |
More data for this
Ligand-Target Pair | |
neuronal acetylcholine receptor subunit alpha-4
(Xenopus) | BDBM86040

(NBEP)Show SMILES Brc1ccc(cn1)[C@H]1CC2CCC1N2 |r,TLB:4:7:10.11:13| Show InChI InChI=1S/C11H13BrN2/c12-11-4-1-7(6-13-11)9-5-8-2-3-10(9)14-8/h1,4,6,8-10,14H,2-3,5H2/t8?,9-,10?/m1/s1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Miami
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 302: 1246-52 (2002)
Article DOI: 10.1124/jpet.102.035899
BindingDB Entry DOI: 10.7270/Q2HQ3XG3 |
More data for this
Ligand-Target Pair | |
neuronal acetylcholine receptor subunit alpha-2
(Xenopus) | BDBM86040

(NBEP)Show SMILES Brc1ccc(cn1)[C@H]1CC2CCC1N2 |r,TLB:4:7:10.11:13| Show InChI InChI=1S/C11H13BrN2/c12-11-4-1-7(6-13-11)9-5-8-2-3-10(9)14-8/h1,4,6,8-10,14H,2-3,5H2/t8?,9-,10?/m1/s1 | PDB
Reactome pathway
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Miami
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 302: 1246-52 (2002)
Article DOI: 10.1124/jpet.102.035899
BindingDB Entry DOI: 10.7270/Q2HQ3XG3 |
More data for this
Ligand-Target Pair | |
neuronal acetylcholine receptor subunit alpha-2
(Xenopus) | BDBM86042

(NEP)Show InChI InChI=1S/C11H14N2/c1-2-8(7-12-5-1)10-6-9-3-4-11(10)13-9/h1-2,5,7,9-11,13H,3-4,6H2/t9?,10-,11?/m1/s1 | PDB
Reactome pathway
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Miami
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 302: 1246-52 (2002)
Article DOI: 10.1124/jpet.102.035899
BindingDB Entry DOI: 10.7270/Q2HQ3XG3 |
More data for this
Ligand-Target Pair | |
Cholinergic receptor, nicotinic alpha 1
(Xenopus) | BDBM86040

(NBEP)Show SMILES Brc1ccc(cn1)[C@H]1CC2CCC1N2 |r,TLB:4:7:10.11:13| Show InChI InChI=1S/C11H13BrN2/c12-11-4-1-7(6-13-11)9-5-8-2-3-10(9)14-8/h1,4,6,8-10,14H,2-3,5H2/t8?,9-,10?/m1/s1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Miami
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 302: 1246-52 (2002)
Article DOI: 10.1124/jpet.102.035899
BindingDB Entry DOI: 10.7270/Q2HQ3XG3 |
More data for this
Ligand-Target Pair | |
neuronal acetylcholine receptor subunit alpha-2
(Xenopus) | BDBM86040

(NBEP)Show SMILES Brc1ccc(cn1)[C@H]1CC2CCC1N2 |r,TLB:4:7:10.11:13| Show InChI InChI=1S/C11H13BrN2/c12-11-4-1-7(6-13-11)9-5-8-2-3-10(9)14-8/h1,4,6,8-10,14H,2-3,5H2/t8?,9-,10?/m1/s1 | PDB
Reactome pathway
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Miami
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 302: 1246-52 (2002)
Article DOI: 10.1124/jpet.102.035899
BindingDB Entry DOI: 10.7270/Q2HQ3XG3 |
More data for this
Ligand-Target Pair | |
neuronal acetylcholine receptor subunit alpha-2
(Xenopus) | BDBM86045
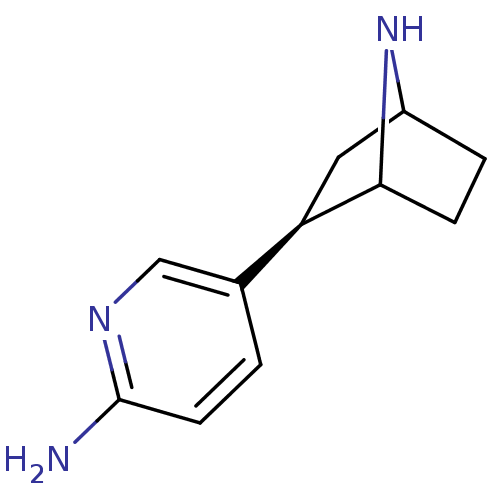
(NNEP)Show SMILES Nc1ccc(cn1)[C@H]1CC2CCC1N2 |r,TLB:4:7:10.11:13| Show InChI InChI=1S/C11H15N3/c12-11-4-1-7(6-13-11)9-5-8-2-3-10(9)14-8/h1,4,6,8-10,14H,2-3,5H2,(H2,12,13)/t8?,9-,10?/m1/s1 | PDB
Reactome pathway
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0620 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Miami
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 302: 1246-52 (2002)
Article DOI: 10.1124/jpet.102.035899
BindingDB Entry DOI: 10.7270/Q2HQ3XG3 |
More data for this
Ligand-Target Pair | |
cholinergic receptor, nicotinic, alpha 1 (Muscle) isoform X1
(Xenopus) | BDBM86045

(NNEP)Show SMILES Nc1ccc(cn1)[C@H]1CC2CCC1N2 |r,TLB:4:7:10.11:13| Show InChI InChI=1S/C11H15N3/c12-11-4-1-7(6-13-11)9-5-8-2-3-10(9)14-8/h1,4,6,8-10,14H,2-3,5H2,(H2,12,13)/t8?,9-,10?/m1/s1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0770 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Miami
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 302: 1246-52 (2002)
Article DOI: 10.1124/jpet.102.035899
BindingDB Entry DOI: 10.7270/Q2HQ3XG3 |
More data for this
Ligand-Target Pair | |
Cholinergic receptor nicotinic alpha 5 subunit
(Xenopus) | BDBM86040

(NBEP)Show SMILES Brc1ccc(cn1)[C@H]1CC2CCC1N2 |r,TLB:4:7:10.11:13| Show InChI InChI=1S/C11H13BrN2/c12-11-4-1-7(6-13-11)9-5-8-2-3-10(9)14-8/h1,4,6,8-10,14H,2-3,5H2/t8?,9-,10?/m1/s1 | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Miami
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 302: 1246-52 (2002)
Article DOI: 10.1124/jpet.102.035899
BindingDB Entry DOI: 10.7270/Q2HQ3XG3 |
More data for this
Ligand-Target Pair | |
neuronal acetylcholine receptor subunit alpha-4
(Xenopus) | BDBM86045

(NNEP)Show SMILES Nc1ccc(cn1)[C@H]1CC2CCC1N2 |r,TLB:4:7:10.11:13| Show InChI InChI=1S/C11H15N3/c12-11-4-1-7(6-13-11)9-5-8-2-3-10(9)14-8/h1,4,6,8-10,14H,2-3,5H2,(H2,12,13)/t8?,9-,10?/m1/s1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Miami
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 302: 1246-52 (2002)
Article DOI: 10.1124/jpet.102.035899
BindingDB Entry DOI: 10.7270/Q2HQ3XG3 |
More data for this
Ligand-Target Pair | |
Cholinergic receptor, nicotinic alpha 1
(Xenopus) | BDBM86041

(NFEP)Show SMILES Fc1ccc(cn1)[C@H]1CC2CCC1N2 |r,TLB:4:7:10.11:13| Show InChI InChI=1S/C11H13FN2/c12-11-4-1-7(6-13-11)9-5-8-2-3-10(9)14-8/h1,4,6,8-10,14H,2-3,5H2/t8?,9-,10?/m1/s1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Miami
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 302: 1246-52 (2002)
Article DOI: 10.1124/jpet.102.035899
BindingDB Entry DOI: 10.7270/Q2HQ3XG3 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50367249

(CHEMBL309601)Show SMILES C[C@H](NP(O)(=O)CCc1ccccc1)C(=O)N1CCC[C@H]1C(O)=O Show InChI InChI=1S/C16H23N2O5P/c1-12(15(19)18-10-5-8-14(18)16(20)21)17-24(22,23)11-9-13-6-3-2-4-7-13/h2-4,6-7,12,14H,5,8-11H2,1H3,(H,20,21)(H2,17,22,23)/t12-,14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for the inhibition of Angiotensin I converting enzyme |
J Med Chem 28: 393-9 (1985)
BindingDB Entry DOI: 10.7270/Q28W3DWT |
More data for this
Ligand-Target Pair | |
neuronal acetylcholine receptor subunit alpha-2
(Xenopus) | BDBM86041

(NFEP)Show SMILES Fc1ccc(cn1)[C@H]1CC2CCC1N2 |r,TLB:4:7:10.11:13| Show InChI InChI=1S/C11H13FN2/c12-11-4-1-7(6-13-11)9-5-8-2-3-10(9)14-8/h1,4,6,8-10,14H,2-3,5H2/t8?,9-,10?/m1/s1 | PDB
Reactome pathway
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Miami
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 302: 1246-52 (2002)
Article DOI: 10.1124/jpet.102.035899
BindingDB Entry DOI: 10.7270/Q2HQ3XG3 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50020843

(2-[(2S)-2-carboxypyrrolidin-1-yl]-1-methyl-2-oxoet...)Show SMILES C[C@@H](NP([O-])([O-])=O)C(=O)N1CCC[C@H]1C(O)=O Show InChI InChI=1S/C8H15N2O6P/c1-5(9-17(14,15)16)7(11)10-4-2-3-6(10)8(12)13/h5-6H,2-4H2,1H3,(H,12,13)(H3,9,14,15,16)/p-2/t5?,6-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for the inhibition of Angiotensin I converting enzyme |
J Med Chem 28: 393-9 (1985)
BindingDB Entry DOI: 10.7270/Q28W3DWT |
More data for this
Ligand-Target Pair | |
Cholinergic receptor nicotinic alpha 5 subunit
(Xenopus) | BDBM86041

(NFEP)Show SMILES Fc1ccc(cn1)[C@H]1CC2CCC1N2 |r,TLB:4:7:10.11:13| Show InChI InChI=1S/C11H13FN2/c12-11-4-1-7(6-13-11)9-5-8-2-3-10(9)14-8/h1,4,6,8-10,14H,2-3,5H2/t8?,9-,10?/m1/s1 | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Miami
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 302: 1246-52 (2002)
Article DOI: 10.1124/jpet.102.035899
BindingDB Entry DOI: 10.7270/Q2HQ3XG3 |
More data for this
Ligand-Target Pair | |
Cholinergic receptor, nicotinic alpha 1
(Xenopus) | BDBM86042

(NEP)Show InChI InChI=1S/C11H14N2/c1-2-8(7-12-5-1)10-6-9-3-4-11(10)13-9/h1-2,5,7,9-11,13H,3-4,6H2/t9?,10-,11?/m1/s1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Miami
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 302: 1246-52 (2002)
Article DOI: 10.1124/jpet.102.035899
BindingDB Entry DOI: 10.7270/Q2HQ3XG3 |
More data for this
Ligand-Target Pair | |
neuronal acetylcholine receptor subunit alpha-2
(Xenopus) | BDBM86042

(NEP)Show InChI InChI=1S/C11H14N2/c1-2-8(7-12-5-1)10-6-9-3-4-11(10)13-9/h1-2,5,7,9-11,13H,3-4,6H2/t9?,10-,11?/m1/s1 | PDB
Reactome pathway
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Miami
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 302: 1246-52 (2002)
Article DOI: 10.1124/jpet.102.035899
BindingDB Entry DOI: 10.7270/Q2HQ3XG3 |
More data for this
Ligand-Target Pair | |
neuronal acetylcholine receptor subunit alpha-2
(Xenopus) | BDBM86045

(NNEP)Show SMILES Nc1ccc(cn1)[C@H]1CC2CCC1N2 |r,TLB:4:7:10.11:13| Show InChI InChI=1S/C11H15N3/c12-11-4-1-7(6-13-11)9-5-8-2-3-10(9)14-8/h1,4,6,8-10,14H,2-3,5H2,(H2,12,13)/t8?,9-,10?/m1/s1 | PDB
Reactome pathway
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Miami
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 302: 1246-52 (2002)
Article DOI: 10.1124/jpet.102.035899
BindingDB Entry DOI: 10.7270/Q2HQ3XG3 |
More data for this
Ligand-Target Pair | |
Cholinergic receptor, nicotinic alpha 1
(Xenopus) | BDBM86045

(NNEP)Show SMILES Nc1ccc(cn1)[C@H]1CC2CCC1N2 |r,TLB:4:7:10.11:13| Show InChI InChI=1S/C11H15N3/c12-11-4-1-7(6-13-11)9-5-8-2-3-10(9)14-8/h1,4,6,8-10,14H,2-3,5H2,(H2,12,13)/t8?,9-,10?/m1/s1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Miami
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 302: 1246-52 (2002)
Article DOI: 10.1124/jpet.102.035899
BindingDB Entry DOI: 10.7270/Q2HQ3XG3 |
More data for this
Ligand-Target Pair | |
Cholinergic receptor nicotinic alpha 5 subunit
(Xenopus) | BDBM86045

(NNEP)Show SMILES Nc1ccc(cn1)[C@H]1CC2CCC1N2 |r,TLB:4:7:10.11:13| Show InChI InChI=1S/C11H15N3/c12-11-4-1-7(6-13-11)9-5-8-2-3-10(9)14-8/h1,4,6,8-10,14H,2-3,5H2,(H2,12,13)/t8?,9-,10?/m1/s1 | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Miami
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 302: 1246-52 (2002)
Article DOI: 10.1124/jpet.102.035899
BindingDB Entry DOI: 10.7270/Q2HQ3XG3 |
More data for this
Ligand-Target Pair | |
neuronal acetylcholine receptor subunit alpha-2
(Xenopus) | BDBM86044
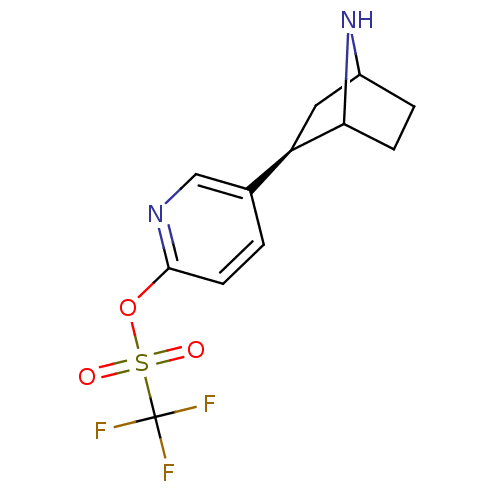
(NTEP)Show SMILES FC(F)(F)S(=O)(=O)Oc1ccc(cn1)[C@H]1CC2CCC1N2 |r,TLB:11:14:17.18:20| Show InChI InChI=1S/C12H13F3N2O3S/c13-12(14,15)21(18,19)20-11-4-1-7(6-16-11)9-5-8-2-3-10(9)17-8/h1,4,6,8-10,17H,2-3,5H2/t8?,9-,10?/m1/s1 | PDB
Reactome pathway
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Miami
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 302: 1246-52 (2002)
Article DOI: 10.1124/jpet.102.035899
BindingDB Entry DOI: 10.7270/Q2HQ3XG3 |
More data for this
Ligand-Target Pair | |
Cholinergic receptor nicotinic alpha 5 subunit
(Xenopus) | BDBM86042

(NEP)Show InChI InChI=1S/C11H14N2/c1-2-8(7-12-5-1)10-6-9-3-4-11(10)13-9/h1-2,5,7,9-11,13H,3-4,6H2/t9?,10-,11?/m1/s1 | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Miami
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 302: 1246-52 (2002)
Article DOI: 10.1124/jpet.102.035899
BindingDB Entry DOI: 10.7270/Q2HQ3XG3 |
More data for this
Ligand-Target Pair | |
cholinergic receptor, nicotinic, alpha 1 (Muscle) isoform X1
(Xenopus) | BDBM50063218
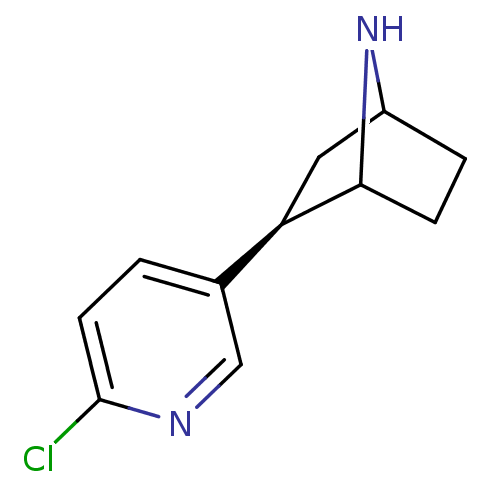
((R)-2-(6-Chloro-pyridin-3-yl)-7-aza-bicyclo[2.2.1]...)Show SMILES Clc1ccc(cn1)[C@H]1CC2CCC1N2 |THB:4:7:13:11.10| Show InChI InChI=1S/C11H13ClN2/c12-11-4-1-7(6-13-11)9-5-8-2-3-10(9)14-8/h1,4,6,8-10,14H,2-3,5H2/t8?,9-,10?/m1/s1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Miami
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 302: 1246-52 (2002)
Article DOI: 10.1124/jpet.102.035899
BindingDB Entry DOI: 10.7270/Q2HQ3XG3 |
More data for this
Ligand-Target Pair | |
neuronal acetylcholine receptor subunit alpha-4
(Xenopus) | BDBM50063218

((R)-2-(6-Chloro-pyridin-3-yl)-7-aza-bicyclo[2.2.1]...)Show SMILES Clc1ccc(cn1)[C@H]1CC2CCC1N2 |THB:4:7:13:11.10| Show InChI InChI=1S/C11H13ClN2/c12-11-4-1-7(6-13-11)9-5-8-2-3-10(9)14-8/h1,4,6,8-10,14H,2-3,5H2/t8?,9-,10?/m1/s1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Miami
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 302: 1246-52 (2002)
Article DOI: 10.1124/jpet.102.035899
BindingDB Entry DOI: 10.7270/Q2HQ3XG3 |
More data for this
Ligand-Target Pair | |
Cholinergic receptor, nicotinic alpha 1
(Xenopus) | BDBM50063218

((R)-2-(6-Chloro-pyridin-3-yl)-7-aza-bicyclo[2.2.1]...)Show SMILES Clc1ccc(cn1)[C@H]1CC2CCC1N2 |THB:4:7:13:11.10| Show InChI InChI=1S/C11H13ClN2/c12-11-4-1-7(6-13-11)9-5-8-2-3-10(9)14-8/h1,4,6,8-10,14H,2-3,5H2/t8?,9-,10?/m1/s1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 85 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Miami
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 302: 1246-52 (2002)
Article DOI: 10.1124/jpet.102.035899
BindingDB Entry DOI: 10.7270/Q2HQ3XG3 |
More data for this
Ligand-Target Pair | |
neuronal acetylcholine receptor subunit alpha-2
(Xenopus) | BDBM50063218

((R)-2-(6-Chloro-pyridin-3-yl)-7-aza-bicyclo[2.2.1]...)Show SMILES Clc1ccc(cn1)[C@H]1CC2CCC1N2 |THB:4:7:13:11.10| Show InChI InChI=1S/C11H13ClN2/c12-11-4-1-7(6-13-11)9-5-8-2-3-10(9)14-8/h1,4,6,8-10,14H,2-3,5H2/t8?,9-,10?/m1/s1 | PDB
Reactome pathway
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 87 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Miami
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 302: 1246-52 (2002)
Article DOI: 10.1124/jpet.102.035899
BindingDB Entry DOI: 10.7270/Q2HQ3XG3 |
More data for this
Ligand-Target Pair | |
neuronal acetylcholine receptor subunit alpha-4
(Xenopus) | BDBM86044

(NTEP)Show SMILES FC(F)(F)S(=O)(=O)Oc1ccc(cn1)[C@H]1CC2CCC1N2 |r,TLB:11:14:17.18:20| Show InChI InChI=1S/C12H13F3N2O3S/c13-12(14,15)21(18,19)20-11-4-1-7(6-16-11)9-5-8-2-3-10(9)17-8/h1,4,6,8-10,17H,2-3,5H2/t8?,9-,10?/m1/s1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Miami
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 302: 1246-52 (2002)
Article DOI: 10.1124/jpet.102.035899
BindingDB Entry DOI: 10.7270/Q2HQ3XG3 |
More data for this
Ligand-Target Pair | |
neuronal acetylcholine receptor subunit alpha-4
(Xenopus) | BDBM50119631
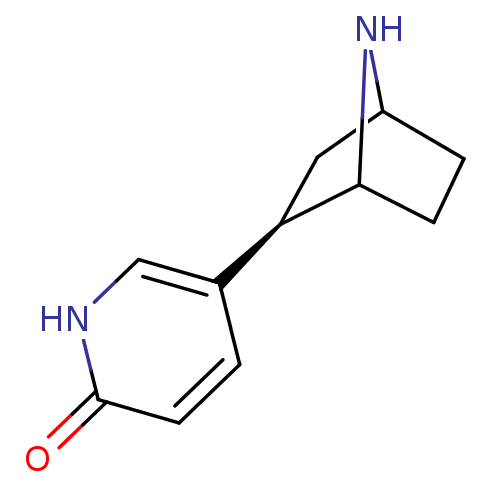
(5-(7-Aza-bicyclo[2.2.1]hept-2-yl)-pyridin-2-ol | C...)Show SMILES O=c1ccc(c[nH]1)[C@H]1CC2CCC1N2 |THB:4:7:13:11.10| Show InChI InChI=1S/C11H14N2O/c14-11-4-1-7(6-12-11)9-5-8-2-3-10(9)13-8/h1,4,6,8-10,13H,2-3,5H2,(H,12,14)/t8?,9-,10?/m1/s1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Miami
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 302: 1246-52 (2002)
Article DOI: 10.1124/jpet.102.035899
BindingDB Entry DOI: 10.7270/Q2HQ3XG3 |
More data for this
Ligand-Target Pair | |
neuronal acetylcholine receptor subunit alpha-4
(Xenopus) | BDBM86043

(NDMNEP)Show SMILES CN(C)c1ccc(cn1)[C@H]1CC2CCC1N2 |r,TLB:6:9:12.13:15| Show InChI InChI=1S/C13H19N3/c1-16(2)13-6-3-9(8-14-13)11-7-10-4-5-12(11)15-10/h3,6,8,10-12,15H,4-5,7H2,1-2H3/t10?,11-,12?/m1/s1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Miami
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 302: 1246-52 (2002)
Article DOI: 10.1124/jpet.102.035899
BindingDB Entry DOI: 10.7270/Q2HQ3XG3 |
More data for this
Ligand-Target Pair | |
cholinergic receptor, nicotinic, alpha 1 (Muscle) isoform X1
(Xenopus) | BDBM86044

(NTEP)Show SMILES FC(F)(F)S(=O)(=O)Oc1ccc(cn1)[C@H]1CC2CCC1N2 |r,TLB:11:14:17.18:20| Show InChI InChI=1S/C12H13F3N2O3S/c13-12(14,15)21(18,19)20-11-4-1-7(6-16-11)9-5-8-2-3-10(9)17-8/h1,4,6,8-10,17H,2-3,5H2/t8?,9-,10?/m1/s1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Miami
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 302: 1246-52 (2002)
Article DOI: 10.1124/jpet.102.035899
BindingDB Entry DOI: 10.7270/Q2HQ3XG3 |
More data for this
Ligand-Target Pair | |
cholinergic receptor, nicotinic, alpha 1 (Muscle) isoform X1
(Xenopus) | BDBM50119631

(5-(7-Aza-bicyclo[2.2.1]hept-2-yl)-pyridin-2-ol | C...)Show SMILES O=c1ccc(c[nH]1)[C@H]1CC2CCC1N2 |THB:4:7:13:11.10| Show InChI InChI=1S/C11H14N2O/c14-11-4-1-7(6-12-11)9-5-8-2-3-10(9)13-8/h1,4,6,8-10,13H,2-3,5H2,(H,12,14)/t8?,9-,10?/m1/s1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Miami
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 302: 1246-52 (2002)
Article DOI: 10.1124/jpet.102.035899
BindingDB Entry DOI: 10.7270/Q2HQ3XG3 |
More data for this
Ligand-Target Pair | |
cholinergic receptor, nicotinic, alpha 1 (Muscle) isoform X1
(Xenopus) | BDBM86043

(NDMNEP)Show SMILES CN(C)c1ccc(cn1)[C@H]1CC2CCC1N2 |r,TLB:6:9:12.13:15| Show InChI InChI=1S/C13H19N3/c1-16(2)13-6-3-9(8-14-13)11-7-10-4-5-12(11)15-10/h3,6,8,10-12,15H,4-5,7H2,1-2H3/t10?,11-,12?/m1/s1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Miami
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 302: 1246-52 (2002)
Article DOI: 10.1124/jpet.102.035899
BindingDB Entry DOI: 10.7270/Q2HQ3XG3 |
More data for this
Ligand-Target Pair | |
neuronal acetylcholine receptor subunit alpha-2
(Xenopus) | BDBM50119631

(5-(7-Aza-bicyclo[2.2.1]hept-2-yl)-pyridin-2-ol | C...)Show SMILES O=c1ccc(c[nH]1)[C@H]1CC2CCC1N2 |THB:4:7:13:11.10| Show InChI InChI=1S/C11H14N2O/c14-11-4-1-7(6-12-11)9-5-8-2-3-10(9)13-8/h1,4,6,8-10,13H,2-3,5H2,(H,12,14)/t8?,9-,10?/m1/s1 | PDB
Reactome pathway
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Miami
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 302: 1246-52 (2002)
Article DOI: 10.1124/jpet.102.035899
BindingDB Entry DOI: 10.7270/Q2HQ3XG3 |
More data for this
Ligand-Target Pair | |
neuronal acetylcholine receptor subunit alpha-2
(Xenopus) | BDBM86043

(NDMNEP)Show SMILES CN(C)c1ccc(cn1)[C@H]1CC2CCC1N2 |r,TLB:6:9:12.13:15| Show InChI InChI=1S/C13H19N3/c1-16(2)13-6-3-9(8-14-13)11-7-10-4-5-12(11)15-10/h3,6,8,10-12,15H,4-5,7H2,1-2H3/t10?,11-,12?/m1/s1 | PDB
Reactome pathway
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Miami
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 302: 1246-52 (2002)
Article DOI: 10.1124/jpet.102.035899
BindingDB Entry DOI: 10.7270/Q2HQ3XG3 |
More data for this
Ligand-Target Pair | |
Cholinergic receptor nicotinic alpha 5 subunit
(Xenopus) | BDBM50063218

((R)-2-(6-Chloro-pyridin-3-yl)-7-aza-bicyclo[2.2.1]...)Show SMILES Clc1ccc(cn1)[C@H]1CC2CCC1N2 |THB:4:7:13:11.10| Show InChI InChI=1S/C11H13ClN2/c12-11-4-1-7(6-13-11)9-5-8-2-3-10(9)14-8/h1,4,6,8-10,14H,2-3,5H2/t8?,9-,10?/m1/s1 | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Miami
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 302: 1246-52 (2002)
Article DOI: 10.1124/jpet.102.035899
BindingDB Entry DOI: 10.7270/Q2HQ3XG3 |
More data for this
Ligand-Target Pair | |
ALK tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM50344664
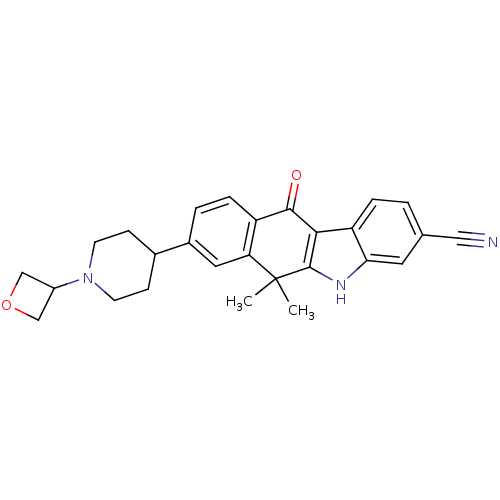
(6,6-dimethyl-8-(1-(oxetan-3-yl)piperidin-4-yl)-11-...)Show SMILES CC1(C)c2[nH]c3cc(ccc3c2C(=O)c2ccc(cc12)C1CCN(CC1)C1COC1)C#N Show InChI InChI=1S/C27H27N3O2/c1-27(2)22-12-18(17-7-9-30(10-8-17)19-14-32-15-19)4-6-20(22)25(31)24-21-5-3-16(13-28)11-23(21)29-26(24)27/h3-6,11-12,17,19,29H,7-10,14-15H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Chugai Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of ALK activity by TR-FRET assay |
J Med Chem 54: 6286-94 (2011)
Article DOI: 10.1021/jm200652u
BindingDB Entry DOI: 10.7270/Q2P55NWZ |
More data for this
Ligand-Target Pair | |
Diacylglycerol O-acyltransferase 1
(Mus musculus (mouse)) | BDBM50117151

(CHEMBL3613351)Show SMILES Cc1ccccc1-c1ccc(cc1)C(=O)NCCNC(=O)c1ccc(O[C@H]2C3CC4CC2C[C@](C4)(C3)C(O)=O)cc1 |r,wU:26.27,wD:33.40,TLB:26:27:34:30.31.32,25:26:34.29.30:32,THB:28:29:32:35.27.26,28:27:34.29.30:32,26:31:34:35.28.27,(-17.57,11.45,;-18.54,10.69,;-19.97,11.26,;-21.18,10.31,;-20.96,8.79,;-19.53,8.21,;-18.32,9.17,;-16.89,8.59,;-15.68,9.54,;-14.25,8.97,;-14.03,7.44,;-15.25,6.49,;-16.67,7.07,;-12.61,6.87,;-11.63,7.62,;-12.39,5.34,;-10.96,4.76,;-10.75,3.24,;-9.32,2.66,;-9.1,1.13,;-10.07,.37,;-7.68,.56,;-7.46,-.97,;-6.03,-1.54,;-4.82,-.59,;-3.39,-1.17,;-2.19,-.22,;-1.2,1.02,;-1.2,2.69,;.12,3.07,;-1,2.05,;-.95,.32,;.56,-.22,;1.56,1.02,;1.56,2.59,;.34,.44,;3.07,1.15,;3.59,2.27,;3.78,.15,;-5.03,.93,;-6.46,1.51,)| Show InChI InChI=1S/C34H36N2O5/c1-21-4-2-3-5-29(21)23-6-8-24(9-7-23)31(37)35-14-15-36-32(38)25-10-12-28(13-11-25)41-30-26-16-22-17-27(30)20-34(18-22,19-26)33(39)40/h2-13,22,26-27,30H,14-20H2,1H3,(H,35,37)(H,36,38)(H,39,40)/t22?,26?,27?,30-,34- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology
Curated by ChEMBL
| Assay Description
Inhibition of mouse DGAT1 incubated for 60 mins using didecanoyl glycerol and [14C]decanoyl-CoA substrate by liquid scintillation counting and lumino... |
Eur J Med Chem 101: 716-35 (2015)
Article DOI: 10.1016/j.ejmech.2015.06.043
BindingDB Entry DOI: 10.7270/Q2B859WR |
More data for this
Ligand-Target Pair | |
ALK tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM50352764
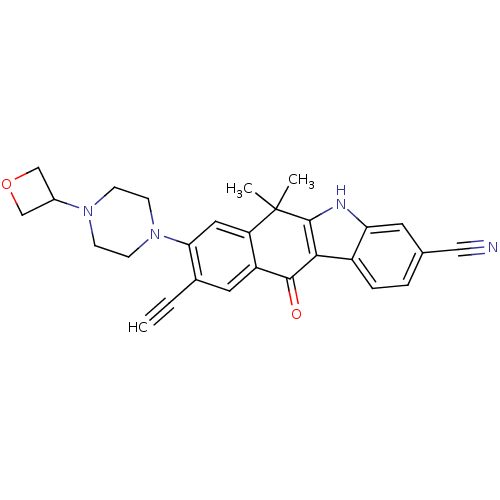
(CHEMBL1823220)Show SMILES CC1(C)c2[nH]c3cc(ccc3c2C(=O)c2cc(C#C)c(cc12)N1CCN(CC1)C1COC1)C#N Show InChI InChI=1S/C28H26N4O2/c1-4-18-12-21-22(13-24(18)32-9-7-31(8-10-32)19-15-34-16-19)28(2,3)27-25(26(21)33)20-6-5-17(14-29)11-23(20)30-27/h1,5-6,11-13,19,30H,7-10,15-16H2,2-3H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Chugai Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of ALK activity by TR-FRET assay |
J Med Chem 54: 6286-94 (2011)
Article DOI: 10.1021/jm200652u
BindingDB Entry DOI: 10.7270/Q2P55NWZ |
More data for this
Ligand-Target Pair | |
ALK tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM50352760
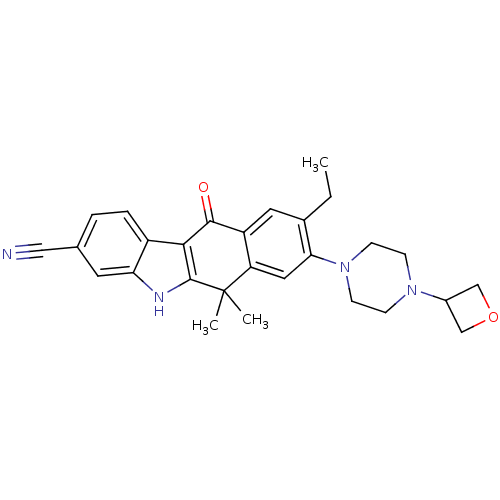
(CHEMBL1823221 | US9126931, 350)Show SMILES CCc1cc2C(=O)c3c([nH]c4cc(ccc34)C#N)C(C)(C)c2cc1N1CCN(CC1)C1COC1 Show InChI InChI=1S/C28H30N4O2/c1-4-18-12-21-22(13-24(18)32-9-7-31(8-10-32)19-15-34-16-19)28(2,3)27-25(26(21)33)20-6-5-17(14-29)11-23(20)30-27/h5-6,11-13,19,30H,4,7-10,15-16H2,1-3H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Chugai Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of ALK activity by TR-FRET assay |
J Med Chem 54: 6286-94 (2011)
Article DOI: 10.1021/jm200652u
BindingDB Entry DOI: 10.7270/Q2P55NWZ |
More data for this
Ligand-Target Pair | |
Diacylglycerol O-acyltransferase 1
(Mus musculus (mouse)) | BDBM50117187
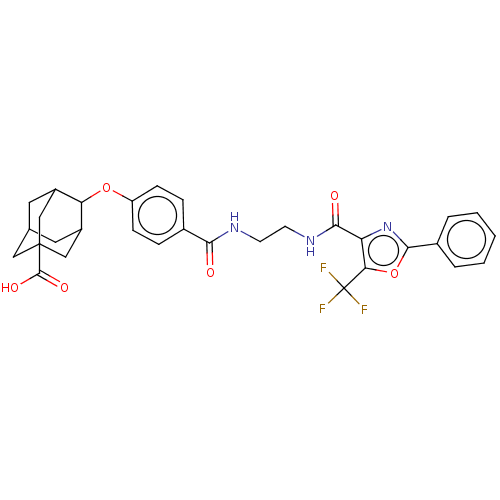
(CHEMBL3613341)Show SMILES OC(=O)C12CC3CC(C1)C(Oc1ccc(cc1)C(=O)NCCNC(=O)c1nc(oc1C(F)(F)F)-c1ccccc1)C(C3)C2 |TLB:6:5:42:8.7.9,6:7:4.5.41:42,THB:9:7:4:41.40.42,9:40:4:8.6.7,10:9:4.5.41:42,(3.79,.17,;3.07,1.17,;3.58,2.29,;1.56,1.02,;1.56,2.59,;.12,3.07,;-1.2,2.69,;-1.2,1.02,;.34,.44,;-2.19,-.22,;-3.39,-1.16,;-3.17,-2.69,;-4.38,-3.64,;-4.16,-5.17,;-2.73,-5.74,;-1.52,-4.78,;-1.74,-3.26,;-2.5,-7.26,;-3.47,-8.03,;-1.07,-7.83,;-.85,-9.36,;.59,-9.92,;.81,-11.45,;2.24,-12.02,;3.21,-11.25,;2.47,-13.54,;1.36,-14.59,;2.04,-15.98,;3.57,-15.75,;3.83,-14.24,;5.2,-13.55,;5.27,-12.32,;6.23,-14.22,;6.3,-12.99,;1.32,-17.34,;2.04,-18.7,;1.23,-20,;-.31,-19.94,;-1.03,-18.58,;-.22,-17.28,;-.95,.32,;-1,2.05,;.56,-.22,)| Show InChI InChI=1S/C31H30F3N3O6/c32-31(33,34)25-23(37-28(43-25)19-4-2-1-3-5-19)27(39)36-11-10-35-26(38)18-6-8-22(9-7-18)42-24-20-12-17-13-21(24)16-30(14-17,15-20)29(40)41/h1-9,17,20-21,24H,10-16H2,(H,35,38)(H,36,39)(H,40,41) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology
Curated by ChEMBL
| Assay Description
Inhibition of mouse DGAT1 incubated for 60 mins using didecanoyl glycerol and [14C]decanoyl-CoA substrate by liquid scintillation counting and lumino... |
Eur J Med Chem 101: 716-35 (2015)
Article DOI: 10.1016/j.ejmech.2015.06.043
BindingDB Entry DOI: 10.7270/Q2B859WR |
More data for this
Ligand-Target Pair | |
Diacylglycerol O-acyltransferase 1
(Mus musculus (mouse)) | BDBM50117149
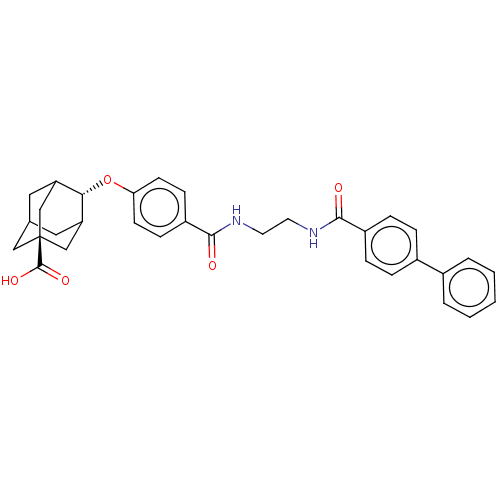
(CHEMBL3613349)Show SMILES OC(=O)[C@@]12CC3CC(C1)[C@H](Oc1ccc(cc1)C(=O)NCCNC(=O)c1ccc(cc1)-c1ccccc1)C(C3)C2 |r,wU:9.10,wD:3.2,TLB:6:5:39:8.7.9,6:7:4.5.38:39,THB:9:7:4:38.37.39,9:37:4:8.6.7,10:9:4.5.38:39,(3.59,2.27,;3.07,1.15,;3.78,.15,;1.56,1.02,;1.56,2.59,;.12,3.07,;-1.2,2.69,;-1.2,1.02,;.34,.44,;-2.19,-.22,;-3.39,-1.17,;-4.82,-.59,;-6.03,-1.54,;-7.46,-.97,;-7.68,.56,;-6.46,1.51,;-5.03,.93,;-9.1,1.13,;-10.07,.37,;-9.32,2.66,;-10.75,3.24,;-10.96,4.76,;-12.39,5.34,;-12.61,6.87,;-11.63,7.62,;-14.03,7.44,;-14.25,8.97,;-15.68,9.54,;-16.89,8.59,;-16.67,7.07,;-15.25,6.49,;-18.32,9.17,;-18.54,10.69,;-19.97,11.26,;-21.18,10.31,;-20.96,8.79,;-19.53,8.21,;-.95,.32,;-1,2.05,;.56,-.22,)| Show InChI InChI=1S/C33H34N2O5/c36-30(24-8-6-23(7-9-24)22-4-2-1-3-5-22)34-14-15-35-31(37)25-10-12-28(13-11-25)40-29-26-16-21-17-27(29)20-33(18-21,19-26)32(38)39/h1-13,21,26-27,29H,14-20H2,(H,34,36)(H,35,37)(H,38,39)/t21?,26?,27?,29-,33- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology
Curated by ChEMBL
| Assay Description
Inhibition of mouse DGAT1 incubated for 60 mins using didecanoyl glycerol and [14C]decanoyl-CoA substrate by liquid scintillation counting and lumino... |
Eur J Med Chem 101: 716-35 (2015)
Article DOI: 10.1016/j.ejmech.2015.06.043
BindingDB Entry DOI: 10.7270/Q2B859WR |
More data for this
Ligand-Target Pair | |
Diacylglycerol O-acyltransferase 1
(Homo sapiens (Human)) | BDBM50117149

(CHEMBL3613349)Show SMILES OC(=O)[C@@]12CC3CC(C1)[C@H](Oc1ccc(cc1)C(=O)NCCNC(=O)c1ccc(cc1)-c1ccccc1)C(C3)C2 |r,wU:9.10,wD:3.2,TLB:6:5:39:8.7.9,6:7:4.5.38:39,THB:9:7:4:38.37.39,9:37:4:8.6.7,10:9:4.5.38:39,(3.59,2.27,;3.07,1.15,;3.78,.15,;1.56,1.02,;1.56,2.59,;.12,3.07,;-1.2,2.69,;-1.2,1.02,;.34,.44,;-2.19,-.22,;-3.39,-1.17,;-4.82,-.59,;-6.03,-1.54,;-7.46,-.97,;-7.68,.56,;-6.46,1.51,;-5.03,.93,;-9.1,1.13,;-10.07,.37,;-9.32,2.66,;-10.75,3.24,;-10.96,4.76,;-12.39,5.34,;-12.61,6.87,;-11.63,7.62,;-14.03,7.44,;-14.25,8.97,;-15.68,9.54,;-16.89,8.59,;-16.67,7.07,;-15.25,6.49,;-18.32,9.17,;-18.54,10.69,;-19.97,11.26,;-21.18,10.31,;-20.96,8.79,;-19.53,8.21,;-.95,.32,;-1,2.05,;.56,-.22,)| Show InChI InChI=1S/C33H34N2O5/c36-30(24-8-6-23(7-9-24)22-4-2-1-3-5-22)34-14-15-35-31(37)25-10-12-28(13-11-25)40-29-26-16-21-17-27(29)20-33(18-21,19-26)32(38)39/h1-13,21,26-27,29H,14-20H2,(H,34,36)(H,35,37)(H,38,39)/t21?,26?,27?,29-,33- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology
Curated by ChEMBL
| Assay Description
Inhibition of human DGAT1 expressed in human Hep3B cells incubated for 60 mins using didecanoyl glycerol and [14C]decanoyl-CoA substrate by liquid sc... |
Eur J Med Chem 101: 716-35 (2015)
Article DOI: 10.1016/j.ejmech.2015.06.043
BindingDB Entry DOI: 10.7270/Q2B859WR |
More data for this
Ligand-Target Pair | |
Diacylglycerol O-acyltransferase 1
(Mus musculus (mouse)) | BDBM50117150

(CHEMBL3613355)Show SMILES Cc1cccc(C)c1-c1ccc(cc1)C(=O)NCCNC(=O)c1ccc(O[C@H]2C3CC4CC2C[C@](C4)(C3)C(O)=O)cc1 |r,wU:27.28,wD:34.41,TLB:27:28:35:31.32.33,26:27:35.30.31:33,THB:29:30:33:36.28.27,29:28:35.30.31:33,27:32:35:36.29.28,(-19.36,6.99,;-19.53,8.21,;-20.96,8.79,;-21.18,10.31,;-19.97,11.26,;-18.54,10.69,;-17.57,11.45,;-18.32,9.17,;-16.89,8.59,;-15.68,9.54,;-14.25,8.97,;-14.03,7.44,;-15.25,6.49,;-16.67,7.07,;-12.61,6.87,;-11.63,7.62,;-12.39,5.34,;-10.96,4.76,;-10.75,3.24,;-9.32,2.66,;-9.1,1.13,;-10.07,.37,;-7.68,.56,;-7.46,-.97,;-6.03,-1.54,;-4.82,-.59,;-3.39,-1.17,;-2.19,-.22,;-1.2,1.02,;-1.2,2.69,;.12,3.07,;-1,2.05,;-.95,.32,;.56,-.22,;1.56,1.02,;1.56,2.59,;.34,.44,;3.07,1.15,;3.59,2.27,;3.78,.15,;-5.03,.93,;-6.46,1.51,)| Show InChI InChI=1S/C35H38N2O5/c1-21-4-3-5-22(2)30(21)24-6-8-25(9-7-24)32(38)36-14-15-37-33(39)26-10-12-29(13-11-26)42-31-27-16-23-17-28(31)20-35(18-23,19-27)34(40)41/h3-13,23,27-28,31H,14-20H2,1-2H3,(H,36,38)(H,37,39)(H,40,41)/t23?,27?,28?,31-,35- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology
Curated by ChEMBL
| Assay Description
Inhibition of mouse DGAT1 incubated for 60 mins using didecanoyl glycerol and [14C]decanoyl-CoA substrate by liquid scintillation counting and lumino... |
Eur J Med Chem 101: 716-35 (2015)
Article DOI: 10.1016/j.ejmech.2015.06.043
BindingDB Entry DOI: 10.7270/Q2B859WR |
More data for this
Ligand-Target Pair | |
Diacylglycerol O-acyltransferase 1
(Mus musculus (mouse)) | BDBM50117184

(CHEMBL3613347)Show SMILES OC(=O)[C@@]12CC3CC(C1)[C@H](Oc1ccc(cc1)C(=O)NCCNC(=O)c1ccc4ccccc4c1)C(C3)C2 |r,wU:3.2,wD:9.10,TLB:8:3:6.7.9:36,10:9:4.37.3:36,THB:1:3:6.7.9:36,8:7:4.37.3:36,9:7:4:37.36.35,9:35:4:6.7.8,(3.58,2.29,;3.07,1.17,;3.79,.17,;1.56,1.02,;1.56,2.59,;.12,3.07,;-1,2.05,;-.95,.32,;.56,-.22,;-2.19,-.22,;-3.39,-1.17,;-4.82,-.59,;-6.03,-1.54,;-7.46,-.97,;-7.68,.56,;-6.46,1.51,;-5.03,.93,;-9.11,1.13,;-9.28,2.35,;-10.32,.17,;-11.75,.75,;-12.96,-.21,;-14.39,.37,;-15.6,-.59,;-15.43,-1.81,;-17.03,-.02,;-17.25,1.51,;-18.69,2.08,;-19.9,1.13,;-21.33,1.7,;-22.54,.75,;-22.32,-.77,;-20.89,-1.35,;-19.68,-.4,;-18.26,-.97,;-1.2,1.02,;-1.2,2.69,;.34,.44,)| Show InChI InChI=1S/C31H32N2O5/c34-28(32-11-12-33-29(35)23-6-5-20-3-1-2-4-22(20)15-23)21-7-9-26(10-8-21)38-27-24-13-19-14-25(27)18-31(16-19,17-24)30(36)37/h1-10,15,19,24-25,27H,11-14,16-18H2,(H,32,34)(H,33,35)(H,36,37)/t19?,24?,25?,27-,31- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology
Curated by ChEMBL
| Assay Description
Inhibition of mouse DGAT1 incubated for 60 mins using didecanoyl glycerol and [14C]decanoyl-CoA substrate by liquid scintillation counting and lumino... |
Eur J Med Chem 101: 716-35 (2015)
Article DOI: 10.1016/j.ejmech.2015.06.043
BindingDB Entry DOI: 10.7270/Q2B859WR |
More data for this
Ligand-Target Pair | |
Diacylglycerol O-acyltransferase 1
(Mus musculus (mouse)) | BDBM50117189

(CHEMBL3613340)Show SMILES OC(=O)C12CC3CC(C1)C(Oc1ccc(cc1)C(=O)NCCNC(=O)c1ccc(cc1)-c1ccccc1)C(C3)C2 |TLB:6:5:39:8.7.9,6:7:4.5.38:39,THB:9:7:4:38.37.39,9:37:4:8.6.7,10:9:4.5.38:39,(3.79,.17,;3.07,1.17,;3.58,2.29,;1.56,1.02,;1.56,2.59,;.12,3.07,;-1.2,2.69,;-1.2,1.02,;.34,.44,;-2.19,-.22,;-3.39,-1.16,;-3.17,-2.69,;-4.38,-3.64,;-4.16,-5.17,;-2.73,-5.74,;-1.52,-4.78,;-1.74,-3.26,;-2.5,-7.26,;-3.47,-8.03,;-1.07,-7.83,;-.85,-9.36,;.59,-9.92,;.81,-11.45,;2.24,-12.02,;3.21,-11.25,;2.47,-13.54,;3.9,-14.11,;4.12,-15.64,;2.91,-16.59,;1.48,-16.02,;1.26,-14.5,;3.13,-18.12,;4.56,-18.69,;4.78,-20.21,;3.57,-21.16,;2.14,-20.59,;1.92,-19.07,;-.95,.32,;-1,2.05,;.56,-.22,)| Show InChI InChI=1S/C33H34N2O5/c36-30(24-8-6-23(7-9-24)22-4-2-1-3-5-22)34-14-15-35-31(37)25-10-12-28(13-11-25)40-29-26-16-21-17-27(29)20-33(18-21,19-26)32(38)39/h1-13,21,26-27,29H,14-20H2,(H,34,36)(H,35,37)(H,38,39) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology
Curated by ChEMBL
| Assay Description
Inhibition of mouse DGAT1 incubated for 60 mins using didecanoyl glycerol and [14C]decanoyl-CoA substrate by liquid scintillation counting and lumino... |
Eur J Med Chem 101: 716-35 (2015)
Article DOI: 10.1016/j.ejmech.2015.06.043
BindingDB Entry DOI: 10.7270/Q2B859WR |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data