 Found 2531 hits with Last Name = 'parmee' and Initial = 'e'
Found 2531 hits with Last Name = 'parmee' and Initial = 'e' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50137257
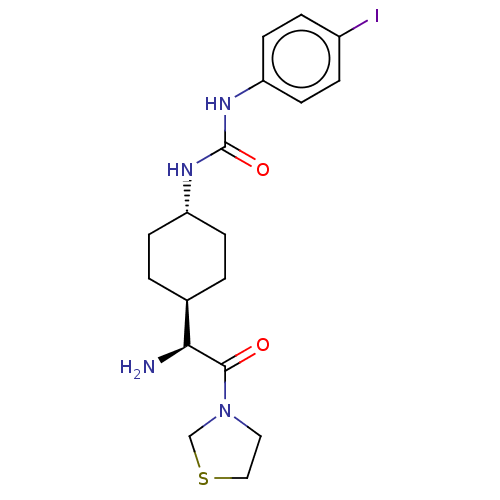
(1-[4-((S)-1-Amino-2-oxo-2-thiazolidin-3-yl-ethyl)-...)Show SMILES [H][C@@]1(CC[C@@H](CC1)NC(=O)Nc1ccc(I)cc1)[C@H](N)C(=O)N1CCSC1 |wU:4.7,1.0,wD:18.20,(1.73,2.3,;3.06,1.54,;1.75,.76,;1.75,-.78,;3.06,-1.54,;4.39,-.78,;4.39,.76,;3.06,-3.08,;4.41,-3.85,;5.74,-3.08,;4.41,-5.39,;5.74,-6.16,;5.74,-7.7,;7.07,-8.47,;8.41,-7.7,;9.74,-8.47,;8.4,-6.14,;7.07,-5.39,;3.06,3.08,;1.73,3.85,;4.41,3.87,;4.41,5.41,;5.74,3.08,;7.15,3.71,;8.17,2.57,;7.4,1.23,;5.9,1.56,)| Show InChI InChI=1S/C18H25IN4O2S/c19-13-3-7-15(8-4-13)22-18(25)21-14-5-1-12(2-6-14)16(20)17(24)23-9-10-26-11-23/h3-4,7-8,12,14,16H,1-2,5-6,9-11,20H2,(H2,21,22,25)/t12-,14-,16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Inc.
Curated by ChEMBL
| Assay Description
Binding affinity for Potassium channel HERG Kv11.1 |
Bioorg Med Chem Lett 14: 43-6 (2003)
BindingDB Entry DOI: 10.7270/Q26972ZM |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50137261
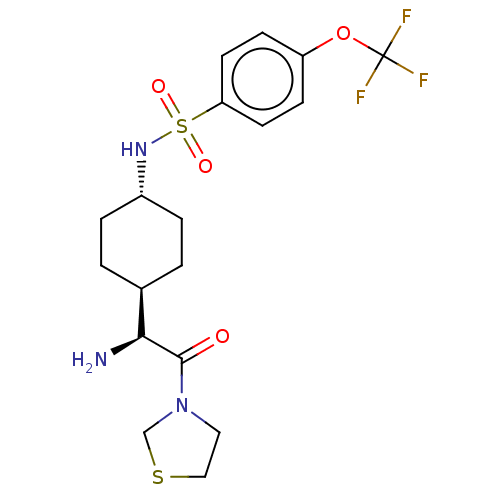
(CHEMBL25211 | N-[4-((S)-1-Amino-2-oxo-2-thiazolidi...)Show SMILES [H][C@@]1(CC[C@@H](CC1)NS(=O)(=O)c1ccc(OC(F)(F)F)cc1)[C@H](N)C(=O)N1CCSC1 |wU:22.24,4.7,1.0,(4.31,-7.01,;4.31,-5.47,;4.31,-3.92,;2.98,-3.15,;1.65,-3.92,;1.65,-5.47,;2.98,-6.24,;.31,-3.13,;-1.02,-3.9,;-.25,-5.25,;-2.37,-4.68,;-1.79,-2.56,;-1.02,-1.23,;-1.79,.11,;-3.34,.11,;-4.12,1.44,;-5.67,1.44,;-5.67,-.1,;-5.67,2.97,;-7.21,1.44,;-4.11,-1.23,;-3.34,-2.56,;5.64,-6.24,;5.64,-7.78,;6.97,-5.47,;6.97,-3.92,;8.3,-6.24,;9.64,-5.47,;10.79,-6.5,;10.15,-7.9,;8.62,-7.74,)| Show InChI InChI=1S/C18H24F3N3O4S2/c19-18(20,21)28-14-5-7-15(8-6-14)30(26,27)23-13-3-1-12(2-4-13)16(22)17(25)24-9-10-29-11-24/h5-8,12-13,16,23H,1-4,9-11,22H2/t12-,13-,16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Inc.
Curated by ChEMBL
| Assay Description
Binding affinity for Potassium channel HERG Kv11.1 |
Bioorg Med Chem Lett 14: 43-6 (2003)
BindingDB Entry DOI: 10.7270/Q26972ZM |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50140533
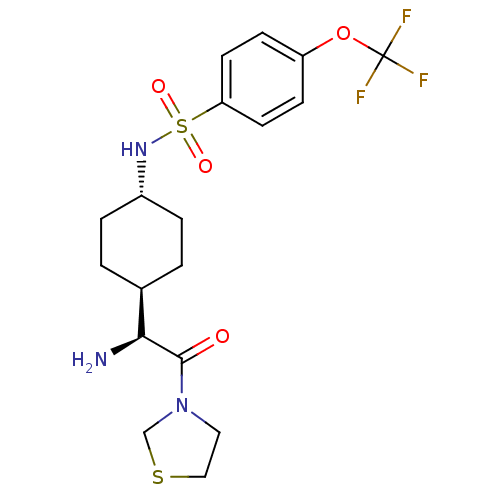
(CHEMBL25211 | N-[4-((S)-1-Amino-2-oxo-2-thiazolidi...)Show SMILES N[C@@H]([C@H]1CC[C@@H](CC1)NS(=O)(=O)c1ccc(OC(F)(F)F)cc1)C(=O)N1CCSC1 |wU:1.0,5.8,wD:2.7,(5.64,-7.78,;5.64,-6.24,;4.31,-5.47,;4.31,-3.92,;2.98,-3.15,;1.65,-3.92,;1.65,-5.47,;2.98,-6.24,;.31,-3.13,;-1.02,-3.9,;-.25,-5.25,;-2.37,-4.68,;-1.79,-2.56,;-1.02,-1.23,;-1.79,.11,;-3.34,.11,;-4.12,1.44,;-5.67,1.44,;-5.67,-.1,;-5.67,2.97,;-7.21,1.44,;-4.11,-1.23,;-3.34,-2.56,;6.97,-5.47,;6.97,-3.92,;8.3,-6.24,;9.64,-5.47,;10.79,-6.5,;10.15,-7.9,;8.62,-7.74,)| Show InChI InChI=1S/C18H24F3N3O4S2/c19-18(20,21)28-14-5-7-15(8-6-14)30(26,27)23-13-3-1-12(2-4-13)16(22)17(25)24-9-10-29-11-24/h5-8,12-13,16,23H,1-4,9-11,22H2/t12-,13-,16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity of the compound towards human ERG potassium ion channel was determined |
Bioorg Med Chem Lett 14: 1265-8 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.040
BindingDB Entry DOI: 10.7270/Q2G73D5Q |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50140529
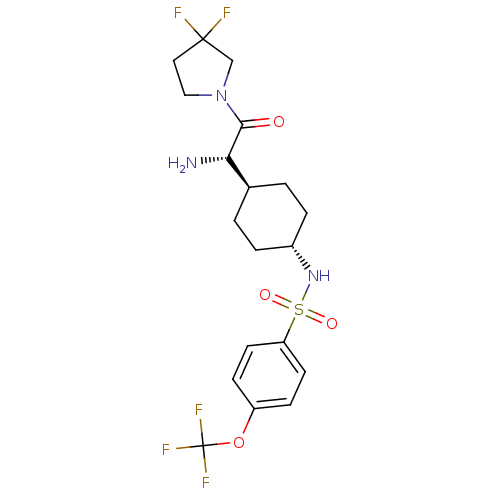
(CHEMBL423108 | N-{4-[(S)-1-Amino-2-(3,3-difluoro-p...)Show SMILES N[C@@H]([C@H]1CC[C@@H](CC1)NS(=O)(=O)c1ccc(OC(F)(F)F)cc1)C(=O)N1CCC(F)(F)C1 |wU:1.0,5.8,wD:2.2,(5.64,-7.78,;5.64,-6.24,;4.31,-5.47,;4.31,-3.92,;2.98,-3.15,;1.65,-3.92,;1.65,-5.47,;2.98,-6.24,;.31,-3.13,;-1.02,-3.9,;-.25,-5.25,;-2.37,-4.68,;-1.79,-2.56,;-1.02,-1.23,;-1.79,.11,;-3.34,.11,;-4.12,1.44,;-5.67,1.44,;-5.67,-.1,;-5.67,2.97,;-7.21,1.44,;-4.11,-1.23,;-3.34,-2.56,;6.97,-5.47,;6.97,-3.92,;8.3,-6.24,;9.64,-5.47,;10.79,-6.5,;10.15,-7.9,;10.85,-9.27,;9.67,-9.37,;8.62,-7.74,)| Show InChI InChI=1S/C19H24F5N3O4S/c20-18(21)9-10-27(11-18)17(28)16(25)12-1-3-13(4-2-12)26-32(29,30)15-7-5-14(6-8-15)31-19(22,23)24/h5-8,12-13,16,26H,1-4,9-11,25H2/t12-,13-,16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity of the compound towards human ERG potassium ion channel was determined |
Bioorg Med Chem Lett 14: 1265-8 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.040
BindingDB Entry DOI: 10.7270/Q2G73D5Q |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50140535
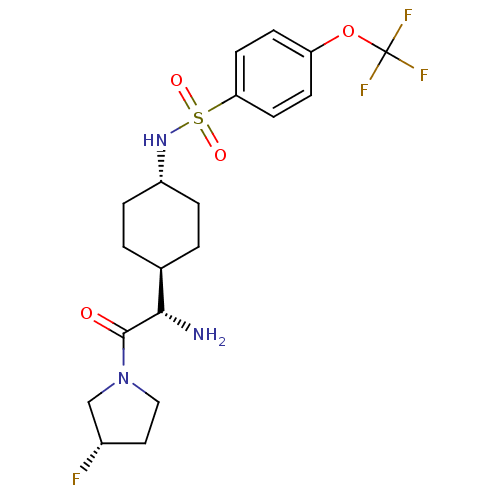
(CHEMBL281178 | N-{4-[(S)-1-Amino-2-((S)-3-fluoro-p...)Show SMILES N[C@@H]([C@H]1CC[C@@H](CC1)NS(=O)(=O)c1ccc(OC(F)(F)F)cc1)C(=O)N1CC[C@H](F)C1 |wU:1.0,5.8,wD:2.2,28.30,(5.64,-7.78,;5.64,-6.24,;4.31,-5.47,;4.31,-3.92,;2.98,-3.15,;1.65,-3.92,;1.65,-5.47,;2.98,-6.24,;.31,-3.13,;-1.02,-3.9,;-.25,-5.25,;-2.37,-4.68,;-1.79,-2.56,;-1.02,-1.23,;-1.79,.11,;-3.34,.11,;-4.12,1.44,;-5.67,1.44,;-5.67,-.1,;-5.67,2.97,;-7.21,1.44,;-4.11,-1.23,;-3.34,-2.56,;6.97,-5.47,;6.97,-3.92,;8.3,-6.24,;9.64,-5.47,;10.79,-6.5,;10.15,-7.9,;10.9,-9.23,;8.62,-7.74,)| Show InChI InChI=1S/C19H25F4N3O4S/c20-13-9-10-26(11-13)18(27)17(24)12-1-3-14(4-2-12)25-31(28,29)16-7-5-15(6-8-16)30-19(21,22)23/h5-8,12-14,17,25H,1-4,9-11,24H2/t12-,13-,14-,17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity of the compound towards human ERG potassium ion channel was determined |
Bioorg Med Chem Lett 14: 1265-8 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.040
BindingDB Entry DOI: 10.7270/Q2G73D5Q |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50140538
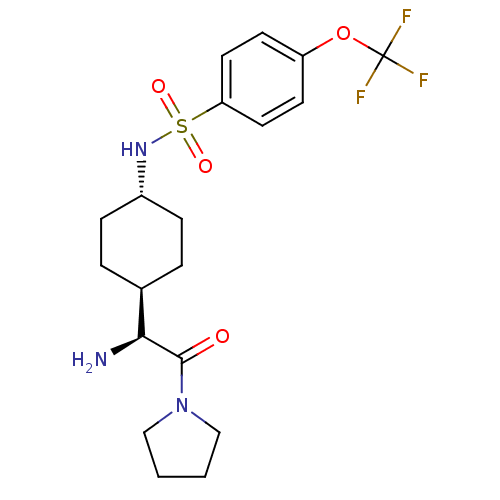
(CHEMBL23979 | N-[4-((S)-1-Amino-2-oxo-2-pyrrolidin...)Show SMILES N[C@@H]([C@H]1CC[C@@H](CC1)NS(=O)(=O)c1ccc(OC(F)(F)F)cc1)C(=O)N1CCCC1 |wU:1.0,5.8,wD:2.2,(5.64,-7.78,;5.64,-6.24,;4.31,-5.47,;4.31,-3.92,;2.98,-3.15,;1.65,-3.92,;1.65,-5.47,;2.98,-6.24,;.31,-3.13,;-1.02,-3.9,;-.25,-5.25,;-2.37,-4.68,;-1.79,-2.56,;-1.02,-1.23,;-1.79,.11,;-3.34,.11,;-4.12,1.44,;-5.67,1.44,;-5.67,-.1,;-5.67,2.97,;-7.21,1.44,;-4.11,-1.23,;-3.34,-2.56,;6.97,-5.47,;6.97,-3.92,;8.3,-6.24,;8.62,-7.74,;10.15,-7.9,;10.79,-6.5,;9.64,-5.47,)| Show InChI InChI=1S/C19H26F3N3O4S/c20-19(21,22)29-15-7-9-16(10-8-15)30(27,28)24-14-5-3-13(4-6-14)17(23)18(26)25-11-1-2-12-25/h7-10,13-14,17,24H,1-6,11-12,23H2/t13-,14-,17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity of the compound towards human ERG potassium ion channel was determined |
Bioorg Med Chem Lett 14: 1265-8 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.040
BindingDB Entry DOI: 10.7270/Q2G73D5Q |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50137271
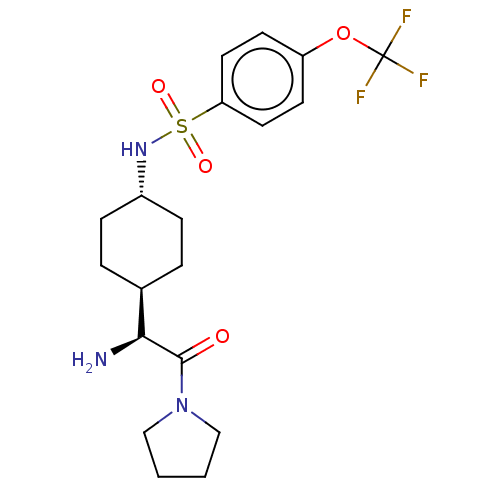
(CHEMBL23979 | N-[4-((S)-1-Amino-2-oxo-2-pyrrolidin...)Show SMILES [H][C@@]1(CC[C@@H](CC1)NS(=O)(=O)c1ccc(OC(F)(F)F)cc1)[C@H](N)C(=O)N1CCCC1 |wU:22.24,4.7,1.0,(4.31,-7.01,;4.31,-5.47,;4.31,-3.92,;2.98,-3.15,;1.65,-3.92,;1.65,-5.47,;2.98,-6.24,;.31,-3.13,;-1.02,-3.9,;-.25,-5.25,;-2.37,-4.68,;-1.79,-2.56,;-1.02,-1.23,;-1.79,.11,;-3.34,.11,;-4.12,1.44,;-5.67,1.44,;-5.67,-.1,;-5.67,2.97,;-7.21,1.44,;-4.11,-1.23,;-3.34,-2.56,;5.64,-6.24,;5.64,-7.78,;6.97,-5.47,;6.97,-3.92,;8.3,-6.24,;8.62,-7.74,;10.15,-7.9,;10.79,-6.5,;9.64,-5.47,)| Show InChI InChI=1S/C19H26F3N3O4S/c20-19(21,22)29-15-7-9-16(10-8-15)30(27,28)24-14-5-3-13(4-6-14)17(23)18(26)25-11-1-2-12-25/h7-10,13-14,17,24H,1-6,11-12,23H2/t13-,14-,17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 5.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Inc.
Curated by ChEMBL
| Assay Description
Binding affinity for Potassium channel HERG Kv11.1 |
Bioorg Med Chem Lett 14: 43-6 (2003)
BindingDB Entry DOI: 10.7270/Q26972ZM |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50137253
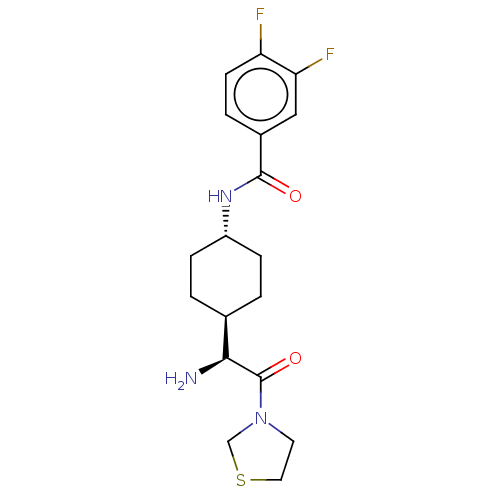
(CHEMBL22359 | N-[4-((S)-1-Amino-2-oxo-2-thiazolidi...)Show SMILES [H][C@@]1(CC[C@@H](CC1)NC(=O)c1ccc(F)c(F)c1)[C@H](N)C(=O)N1CCSC1 |wU:4.7,18.20,1.0,(2.09,-2.17,;2.09,-.63,;2.09,.91,;.75,1.67,;-.57,.91,;-.57,-.63,;.75,-1.4,;-1.9,1.69,;-3.23,.93,;-3.23,-.61,;-4.57,1.69,;-5.89,.93,;-7.24,1.69,;-7.24,3.24,;-8.57,4.01,;-5.89,4.01,;-5.87,5.54,;-4.57,3.24,;3.41,-1.4,;3.41,-2.93,;4.74,-.63,;4.74,.91,;6.07,-1.4,;7.39,-.63,;8.54,-1.67,;7.9,-3.05,;6.39,-2.9,)| Show InChI InChI=1S/C18H23F2N3O2S/c19-14-6-3-12(9-15(14)20)17(24)22-13-4-1-11(2-5-13)16(21)18(25)23-7-8-26-10-23/h3,6,9,11,13,16H,1-2,4-5,7-8,10,21H2,(H,22,24)/t11-,13-,16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 5.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Inc.
Curated by ChEMBL
| Assay Description
Binding affinity for Potassium channel HERG Kv11.1 |
Bioorg Med Chem Lett 14: 43-6 (2003)
BindingDB Entry DOI: 10.7270/Q26972ZM |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50140523
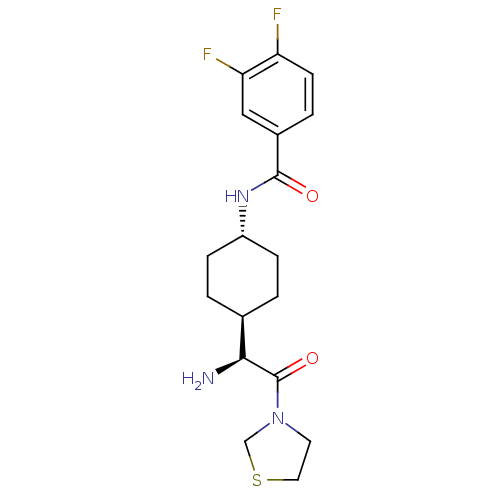
(CHEMBL22359 | N-[4-((S)-1-Amino-2-oxo-2-thiazolidi...)Show SMILES N[C@@H]([C@H]1CC[C@@H](CC1)NC(=O)c1ccc(F)c(F)c1)C(=O)N1CCSC1 |wU:5.8,1.0,wD:2.2,(3.41,-2.93,;3.41,-1.4,;2.09,-.63,;2.09,.91,;.75,1.67,;-.57,.91,;-.57,-.63,;.75,-1.4,;-1.9,1.69,;-3.23,.93,;-3.23,-.61,;-4.57,1.69,;-5.89,.93,;-7.24,1.69,;-7.24,3.24,;-8.57,4.01,;-5.89,4.01,;-5.87,5.54,;-4.57,3.24,;4.74,-.63,;4.74,.91,;6.07,-1.4,;7.39,-.63,;8.54,-1.67,;7.9,-3.05,;6.39,-2.9,)| Show InChI InChI=1S/C18H23F2N3O2S/c19-14-6-3-12(9-15(14)20)17(24)22-13-4-1-11(2-5-13)16(21)18(25)23-7-8-26-10-23/h3,6,9,11,13,16H,1-2,4-5,7-8,10,21H2,(H,22,24)/t11-,13-,16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity of the compound towards human ERG potassium ion channel was determined |
Bioorg Med Chem Lett 14: 1265-8 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.040
BindingDB Entry DOI: 10.7270/Q2G73D5Q |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50140525
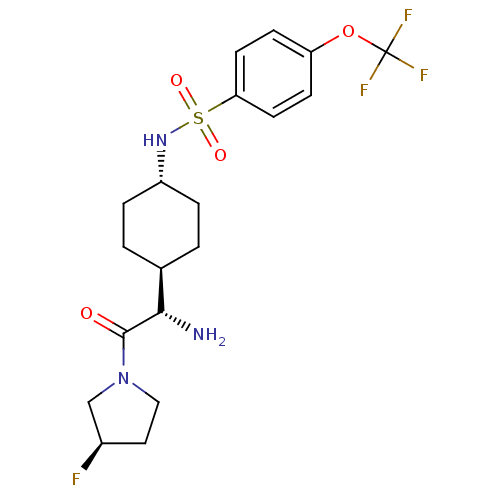
(CHEMBL281561 | N-{4-[(S)-1-Amino-2-((R)-3-fluoro-p...)Show SMILES N[C@@H]([C@H]1CC[C@@H](CC1)NS(=O)(=O)c1ccc(OC(F)(F)F)cc1)C(=O)N1CC[C@@H](F)C1 |wU:1.0,5.8,28.30,wD:2.2,(5.64,-7.78,;5.64,-6.24,;4.31,-5.47,;4.31,-3.92,;2.98,-3.15,;1.65,-3.92,;1.65,-5.47,;2.98,-6.24,;.31,-3.13,;-1.02,-3.9,;-.25,-5.25,;-2.37,-4.68,;-1.79,-2.56,;-1.02,-1.23,;-1.79,.11,;-3.34,.11,;-4.12,1.44,;-5.67,1.44,;-5.67,-.1,;-5.67,2.97,;-7.21,1.44,;-4.11,-1.23,;-3.34,-2.56,;6.97,-5.47,;6.97,-3.92,;8.3,-6.24,;9.64,-5.47,;10.79,-6.5,;10.15,-7.9,;10.9,-9.23,;8.62,-7.74,)| Show InChI InChI=1S/C19H25F4N3O4S/c20-13-9-10-26(11-13)18(27)17(24)12-1-3-14(4-2-12)25-31(28,29)16-7-5-15(6-8-16)30-19(21,22)23/h5-8,12-14,17,25H,1-4,9-11,24H2/t12-,13-,14-,17+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity of the compound towards human ERG potassium ion channel was determined |
Bioorg Med Chem Lett 14: 1265-8 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.040
BindingDB Entry DOI: 10.7270/Q2G73D5Q |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50140516
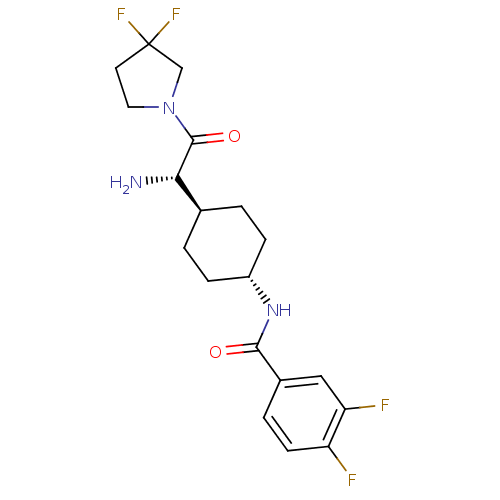
(CHEMBL23110 | N-{4-[(S)-1-Amino-2-(3,3-difluoro-py...)Show SMILES N[C@@H]([C@H]1CC[C@@H](CC1)NC(=O)c1ccc(F)c(F)c1)C(=O)N1CCC(F)(F)C1 |wU:5.8,1.0,wD:2.2,(3.86,-3.65,;3.86,-2.11,;2.53,-1.34,;2.53,.21,;1.2,.98,;-.13,.21,;-.13,-1.34,;1.2,-2.11,;-1.49,1,;-2.82,.23,;-2.82,-1.31,;-4.15,1,;-5.48,.23,;-6.83,1,;-6.83,2.56,;-8.18,3.33,;-5.48,3.33,;-5.46,4.87,;-4.15,2.56,;5.19,-1.34,;5.19,.21,;6.53,-2.11,;7.87,-1.34,;9.01,-2.38,;8.38,-3.76,;9.08,-5.14,;7.54,-5.07,;6.86,-3.62,)| Show InChI InChI=1S/C19H23F4N3O2/c20-14-6-3-12(9-15(14)21)17(27)25-13-4-1-11(2-5-13)16(24)18(28)26-8-7-19(22,23)10-26/h3,6,9,11,13,16H,1-2,4-5,7-8,10,24H2,(H,25,27)/t11-,13-,16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity of the compound towards human ERG potassium ion channel was determined |
Bioorg Med Chem Lett 14: 1265-8 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.040
BindingDB Entry DOI: 10.7270/Q2G73D5Q |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50137272
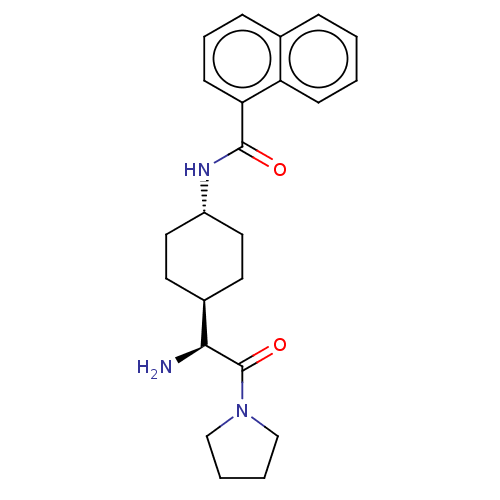
(CHEMBL3084944 | Naphthalene-1-carboxylic acid [4-(...)Show SMILES [H][C@@]1(CC[C@@H](CC1)NC(=O)c1cccc2ccccc12)[C@H](N)C(=O)N1CCCC1 |wU:4.7,1.0,wD:20.23,(12.92,.92,;11.58,.16,;10.25,-.63,;10.25,-2.17,;11.58,-2.92,;12.91,-2.17,;12.91,-.63,;11.58,-4.46,;12.91,-5.23,;14.24,-4.46,;12.91,-6.77,;11.58,-7.54,;11.58,-9.08,;12.91,-9.85,;14.24,-9.06,;15.57,-9.83,;16.89,-9.06,;16.88,-7.52,;15.55,-6.77,;14.24,-7.54,;11.58,1.7,;10.25,2.47,;12.91,2.47,;12.91,4.01,;14.24,1.7,;15.66,2.33,;16.69,1.18,;15.92,-.16,;14.4,.16,)| Show InChI InChI=1S/C23H29N3O2/c24-21(23(28)26-14-3-4-15-26)17-10-12-18(13-11-17)25-22(27)20-9-5-7-16-6-1-2-8-19(16)20/h1-2,5-9,17-18,21H,3-4,10-15,24H2,(H,25,27)/t17-,18-,21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Inc.
Curated by ChEMBL
| Assay Description
Binding affinity for Potassium channel HERG Kv11.1 |
Bioorg Med Chem Lett 14: 43-6 (2003)
BindingDB Entry DOI: 10.7270/Q26972ZM |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50140513
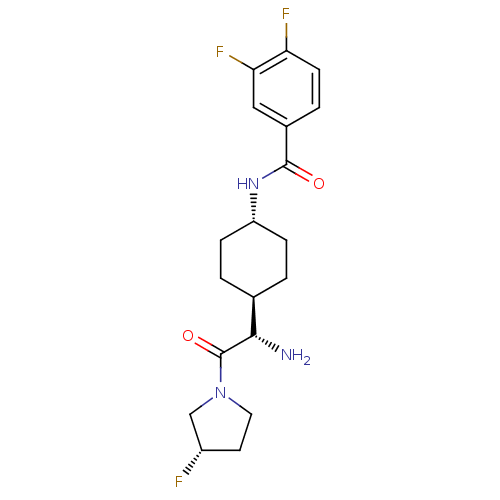
(CHEMBL23660 | N-{4-[(S)-1-Amino-2-((S)-3-fluoro-py...)Show SMILES N[C@@H]([C@H]1CC[C@@H](CC1)NC(=O)c1ccc(F)c(F)c1)C(=O)N1CC[C@H](F)C1 |wU:5.8,1.0,wD:2.7,24.26,(7.35,-11.7,;7.35,-10.16,;6.02,-9.4,;6.02,-7.85,;4.67,-7.08,;3.35,-7.85,;3.35,-9.4,;4.67,-10.16,;2.01,-7.06,;.68,-7.81,;.68,-9.37,;-.65,-7.06,;-2,-7.81,;-3.32,-7.06,;-3.32,-5.51,;-4.67,-4.74,;-1.98,-4.74,;-1.97,-3.2,;-.65,-5.51,;8.67,-9.4,;8.67,-7.85,;10,-10.16,;11.34,-9.4,;12.49,-10.44,;11.86,-11.82,;12.61,-13.15,;10.33,-11.67,)| Show InChI InChI=1S/C19H24F3N3O2/c20-13-7-8-25(10-13)19(27)17(23)11-1-4-14(5-2-11)24-18(26)12-3-6-15(21)16(22)9-12/h3,6,9,11,13-14,17H,1-2,4-5,7-8,10,23H2,(H,24,26)/t11-,13-,14-,17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity of the compound towards human ERG potassium ion channel was determined |
Bioorg Med Chem Lett 14: 1265-8 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.040
BindingDB Entry DOI: 10.7270/Q2G73D5Q |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50137264
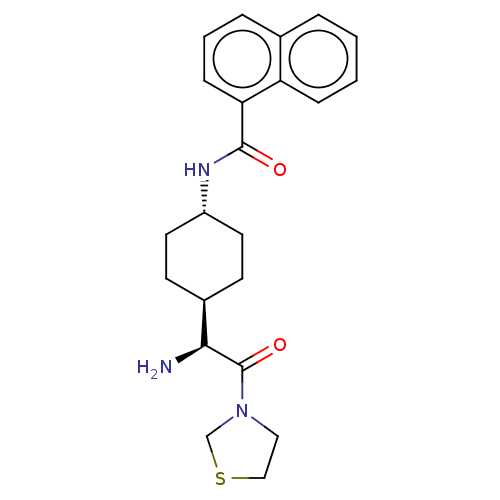
(CHEMBL3084949 | Naphthalene-1-carboxylic acid [4-(...)Show SMILES [H][C@@]1(CC[C@@H](CC1)NC(=O)c1cccc2ccccc12)[C@H](N)C(=O)N1CCSC1 |wU:4.7,1.0,wD:20.23,(1.73,2.3,;3.06,1.54,;1.75,.76,;1.75,-.78,;3.06,-1.54,;4.39,-.78,;4.39,.76,;3.06,-3.08,;4.41,-3.85,;5.74,-3.08,;4.41,-5.39,;5.72,-6.14,;5.74,-7.68,;4.39,-8.45,;3.06,-7.68,;1.75,-8.45,;.42,-7.68,;.42,-6.14,;1.75,-5.39,;3.06,-6.14,;3.06,3.08,;1.73,3.85,;4.41,3.87,;4.41,5.41,;5.74,3.08,;7.15,3.71,;8.17,2.57,;7.4,1.23,;5.9,1.56,)| Show InChI InChI=1S/C22H27N3O2S/c23-20(22(27)25-12-13-28-14-25)16-8-10-17(11-9-16)24-21(26)19-7-3-5-15-4-1-2-6-18(15)19/h1-7,16-17,20H,8-14,23H2,(H,24,26)/t16-,17-,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Inc.
Curated by ChEMBL
| Assay Description
Binding affinity for Potassium channel HERG Kv11.1 |
Bioorg Med Chem Lett 14: 43-6 (2003)
BindingDB Entry DOI: 10.7270/Q26972ZM |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50140536
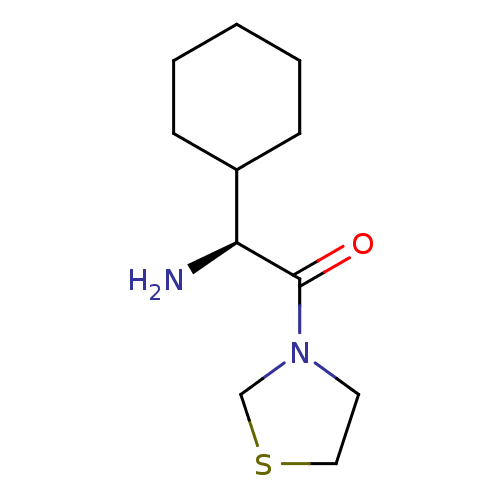
((S)-2-Amino-2-cyclohexyl-1-thiazolidin-3-yl-ethano...)Show InChI InChI=1S/C11H20N2OS/c12-10(9-4-2-1-3-5-9)11(14)13-6-7-15-8-13/h9-10H,1-8,12H2/t10-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity of the compound towards human ERG potassium ion channel was determined |
Bioorg Med Chem Lett 14: 1265-8 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.040
BindingDB Entry DOI: 10.7270/Q2G73D5Q |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50140526
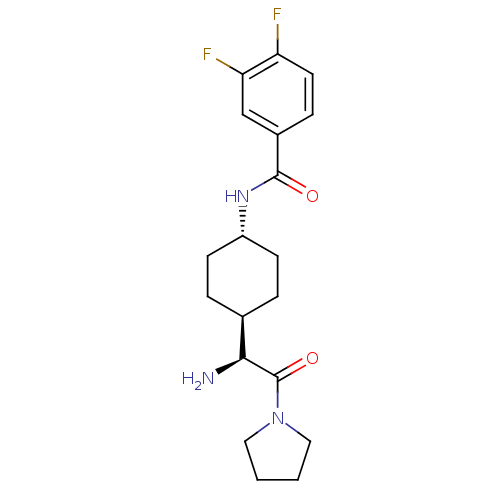
(CHEMBL278558 | N-[4-((S)-1-Amino-2-oxo-2-pyrrolidi...)Show SMILES N[C@@H]([C@H]1CC[C@@H](CC1)NC(=O)c1ccc(F)c(F)c1)C(=O)N1CCCC1 |wU:5.8,1.0,wD:2.2,(3.41,-2.93,;3.41,-1.4,;2.09,-.63,;2.09,.91,;.75,1.67,;-.57,.91,;-.57,-.63,;.75,-1.4,;-1.9,1.69,;-3.23,.93,;-3.23,-.61,;-4.57,1.69,;-5.89,.93,;-7.24,1.69,;-7.24,3.24,;-8.57,4.01,;-5.89,4.01,;-5.87,5.54,;-4.57,3.24,;4.74,-.63,;4.74,.91,;6.07,-1.4,;6.39,-2.9,;7.9,-3.05,;8.54,-1.67,;7.39,-.63,)| Show InChI InChI=1S/C19H25F2N3O2/c20-15-8-5-13(11-16(15)21)18(25)23-14-6-3-12(4-7-14)17(22)19(26)24-9-1-2-10-24/h5,8,11-12,14,17H,1-4,6-7,9-10,22H2,(H,23,25)/t12-,14-,17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity of the compound towards human ERG potassium ion channel was determined |
Bioorg Med Chem Lett 14: 1265-8 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.040
BindingDB Entry DOI: 10.7270/Q2G73D5Q |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50140521
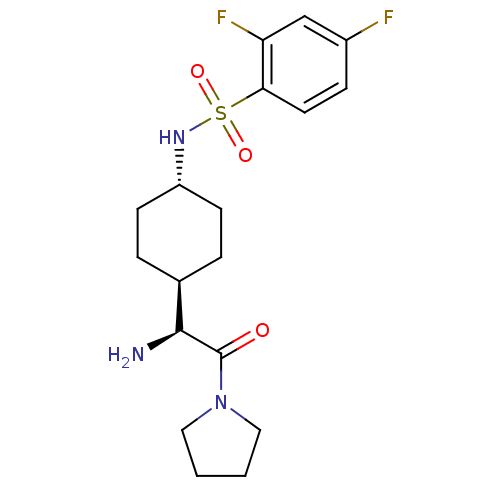
(CHEMBL25437 | N-[4-((S)-1-Amino-2-oxo-2-pyrrolidin...)Show SMILES N[C@@H]([C@H]1CC[C@@H](CC1)NS(=O)(=O)c1ccc(F)cc1F)C(=O)N1CCCC1 |wU:1.0,5.8,wD:2.7,(5.83,-7.06,;5.83,-5.5,;4.5,-4.73,;4.5,-3.19,;3.16,-2.42,;1.83,-3.19,;1.83,-4.73,;3.16,-5.5,;.48,-2.39,;-.86,-3.16,;-.07,-4.51,;-2.2,-3.94,;-1.63,-1.81,;-.86,-.48,;-1.63,.86,;-3.18,.86,;-3.97,2.2,;-3.95,-.48,;-3.18,-1.81,;-3.97,-3.14,;7.16,-4.73,;7.16,-3.19,;8.51,-5.5,;9.85,-4.73,;11,-5.78,;10.37,-7.18,;8.84,-7.02,)| Show InChI InChI=1S/C18H25F2N3O3S/c19-13-5-8-16(15(20)11-13)27(25,26)22-14-6-3-12(4-7-14)17(21)18(24)23-9-1-2-10-23/h5,8,11-12,14,17,22H,1-4,6-7,9-10,21H2/t12-,14-,17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity of the compound towards human ERG potassium ion channel was determined |
Bioorg Med Chem Lett 14: 1265-8 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.040
BindingDB Entry DOI: 10.7270/Q2G73D5Q |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50140521

(CHEMBL25437 | N-[4-((S)-1-Amino-2-oxo-2-pyrrolidin...)Show SMILES N[C@@H]([C@H]1CC[C@@H](CC1)NS(=O)(=O)c1ccc(F)cc1F)C(=O)N1CCCC1 |wU:1.0,5.8,wD:2.7,(5.83,-7.06,;5.83,-5.5,;4.5,-4.73,;4.5,-3.19,;3.16,-2.42,;1.83,-3.19,;1.83,-4.73,;3.16,-5.5,;.48,-2.39,;-.86,-3.16,;-.07,-4.51,;-2.2,-3.94,;-1.63,-1.81,;-.86,-.48,;-1.63,.86,;-3.18,.86,;-3.97,2.2,;-3.95,-.48,;-3.18,-1.81,;-3.97,-3.14,;7.16,-4.73,;7.16,-3.19,;8.51,-5.5,;9.85,-4.73,;11,-5.78,;10.37,-7.18,;8.84,-7.02,)| Show InChI InChI=1S/C18H25F2N3O3S/c19-13-5-8-16(15(20)11-13)27(25,26)22-14-6-3-12(4-7-14)17(21)18(24)23-9-1-2-10-23/h5,8,11-12,14,17,22H,1-4,6-7,9-10,21H2/t12-,14-,17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Inc.
Curated by ChEMBL
| Assay Description
Binding affinity for Potassium channel HERG Kv11.1 |
Bioorg Med Chem Lett 14: 43-6 (2003)
BindingDB Entry DOI: 10.7270/Q26972ZM |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50140512
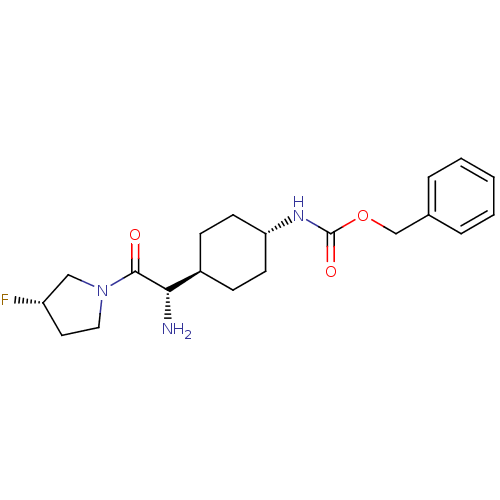
(CHEMBL23891 | {4-[(S)-1-Amino-2-((S)-3-fluoro-pyrr...)Show SMILES N[C@@H]([C@H]1CC[C@@H](CC1)NC(=O)OCc1ccccc1)C(=O)N1CC[C@H](F)C1 |wU:5.8,1.0,wD:2.2,24.26,(5.79,-6.89,;5.79,-5.34,;4.46,-4.57,;4.46,-3.02,;3.12,-2.25,;1.8,-3.02,;1.8,-4.57,;3.12,-5.34,;.45,-2.22,;-.88,-2.99,;-.88,-4.55,;-2.22,-2.22,;-3.56,-2.99,;-4.89,-2.22,;-4.89,-.68,;-6.22,.09,;-7.57,-.68,;-7.57,-2.22,;-6.23,-2.99,;7.12,-4.57,;7.12,-3.02,;8.45,-5.34,;9.79,-4.57,;10.94,-5.6,;10.31,-7,;11.08,-8.36,;8.78,-6.84,)| Show InChI InChI=1S/C20H28FN3O3/c21-16-10-11-24(12-16)19(25)18(22)15-6-8-17(9-7-15)23-20(26)27-13-14-4-2-1-3-5-14/h1-5,15-18H,6-13,22H2,(H,23,26)/t15-,16-,17-,18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity of the compound towards human ERG potassium ion channel was determined |
Bioorg Med Chem Lett 14: 1265-8 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.040
BindingDB Entry DOI: 10.7270/Q2G73D5Q |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50140517
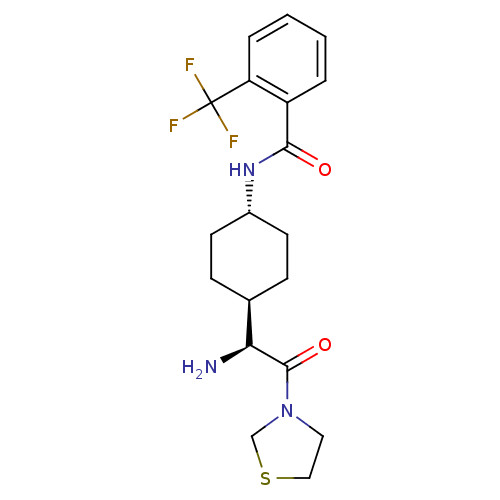
(CHEMBL283368 | N-[4-((S)-1-Amino-2-oxo-2-thiazolid...)Show SMILES N[C@@H]([C@H]1CC[C@@H](CC1)NC(=O)c1ccccc1C(F)(F)F)C(=O)N1CCSC1 |wU:5.8,1.0,wD:2.2,(3.86,-3.65,;3.86,-2.11,;2.53,-1.34,;2.53,.21,;1.2,.98,;-.13,.21,;-.13,-1.34,;1.2,-2.11,;-1.49,1,;-2.82,.23,;-2.82,-1.31,;-4.15,1,;-4.15,2.56,;-5.48,3.33,;-6.83,2.56,;-6.83,1,;-5.48,.23,;-5.48,-1.31,;-7.02,-1.31,;-3.94,-1.31,;-5.48,-2.85,;5.19,-1.34,;5.19,.21,;6.53,-2.11,;7.87,-1.34,;9.01,-2.38,;8.38,-3.76,;6.86,-3.62,)| Show InChI InChI=1S/C19H24F3N3O2S/c20-19(21,22)15-4-2-1-3-14(15)17(26)24-13-7-5-12(6-8-13)16(23)18(27)25-9-10-28-11-25/h1-4,12-13,16H,5-11,23H2,(H,24,26)/t12-,13-,16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 3.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity of the compound towards human ERG potassium ion channel was determined |
Bioorg Med Chem Lett 14: 1265-8 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.040
BindingDB Entry DOI: 10.7270/Q2G73D5Q |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50140515
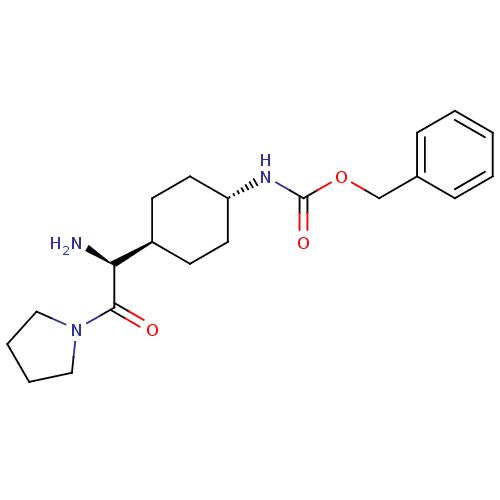
(CHEMBL22679 | [4-((S)-1-Amino-2-oxo-2-pyrrolidin-1...)Show SMILES N[C@@H]([C@H]1CC[C@@H](CC1)NC(=O)OCc1ccccc1)C(=O)N1CCCC1 |wU:5.8,1.0,wD:2.7,(4.17,-4.08,;4.17,-2.53,;2.84,-1.76,;2.84,-.21,;1.5,.56,;.17,-.21,;.17,-1.76,;1.5,-2.53,;-1.18,.58,;-2.52,-.17,;-2.52,-1.74,;-3.85,.58,;-5.19,-.17,;-6.54,.58,;-7.87,-.17,;-9.21,.58,;-9.21,2.14,;-7.87,2.91,;-6.54,2.14,;5.5,-1.76,;5.5,-.21,;6.85,-2.53,;8.19,-1.76,;9.34,-2.81,;8.71,-4.19,;7.18,-4.05,)| Show InChI InChI=1S/C20H29N3O3/c21-18(19(24)23-12-4-5-13-23)16-8-10-17(11-9-16)22-20(25)26-14-15-6-2-1-3-7-15/h1-3,6-7,16-18H,4-5,8-14,21H2,(H,22,25)/t16-,17-,18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity of the compound towards human ERG potassium ion channel was determined |
Bioorg Med Chem Lett 14: 1265-8 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.040
BindingDB Entry DOI: 10.7270/Q2G73D5Q |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50137260

(CHEMBL22679 | [4-((S)-1-Amino-2-oxo-2-pyrrolidin-1...)Show SMILES [H][C@@]1(CC[C@@H](CC1)NC(=O)OCc1ccccc1)[C@H](N)C(=O)N1CCCC1 |wU:4.7,18.20,1.0,(2.84,-3.3,;2.84,-1.76,;2.84,-.21,;1.5,.56,;.17,-.21,;.17,-1.76,;1.5,-2.53,;-1.18,.58,;-2.52,-.17,;-2.52,-1.74,;-3.85,.58,;-5.19,-.17,;-6.54,.58,;-7.87,-.17,;-9.21,.58,;-9.21,2.14,;-7.87,2.91,;-6.54,2.14,;4.17,-2.53,;4.17,-4.08,;5.5,-1.76,;5.5,-.21,;6.85,-2.53,;8.19,-1.76,;9.34,-2.81,;8.71,-4.19,;7.18,-4.05,)| Show InChI InChI=1S/C20H29N3O3/c21-18(19(24)23-12-4-5-13-23)16-8-10-17(11-9-16)22-20(25)26-14-15-6-2-1-3-7-15/h1-3,6-7,16-18H,4-5,8-14,21H2,(H,22,25)/t16-,17-,18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Inc.
Curated by ChEMBL
| Assay Description
Binding affinity for Potassium channel HERG Kv11.1 |
Bioorg Med Chem Lett 14: 43-6 (2003)
BindingDB Entry DOI: 10.7270/Q26972ZM |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50140518
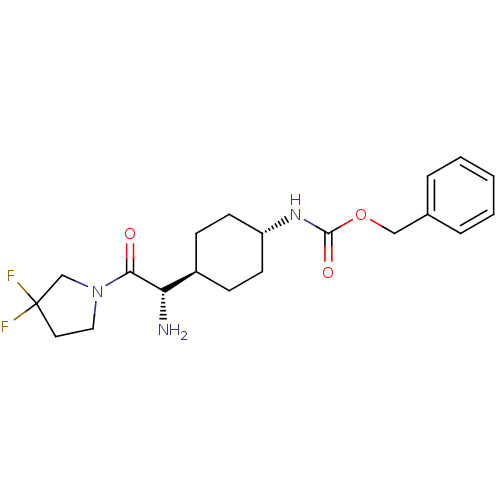
(CHEMBL279944 | {4-[(S)-1-Amino-2-(3,3-difluoro-pyr...)Show SMILES N[C@@H]([C@H]1CC[C@@H](CC1)NC(=O)OCc1ccccc1)C(=O)N1CCC(F)(F)C1 |wU:5.8,1.0,wD:2.7,(4.17,-4.08,;4.17,-2.53,;2.84,-1.76,;2.84,-.21,;1.5,.56,;.17,-.21,;.17,-1.76,;1.5,-2.53,;-1.18,.58,;-2.52,-.17,;-2.52,-1.74,;-3.85,.58,;-5.19,-.17,;-6.54,.58,;-7.87,-.17,;-9.21,.58,;-9.21,2.14,;-7.87,2.91,;-6.54,2.14,;5.5,-1.76,;5.5,-.21,;6.85,-2.53,;8.19,-1.76,;9.34,-2.81,;8.71,-4.19,;8.68,-5.74,;10.11,-4.85,;7.18,-4.05,)| Show InChI InChI=1S/C20H27F2N3O3/c21-20(22)10-11-25(13-20)18(26)17(23)15-6-8-16(9-7-15)24-19(27)28-12-14-4-2-1-3-5-14/h1-5,15-17H,6-13,23H2,(H,24,27)/t15-,16-,17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity of the compound towards human ERG potassium ion channel was determined |
Bioorg Med Chem Lett 14: 1265-8 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.040
BindingDB Entry DOI: 10.7270/Q2G73D5Q |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50140531
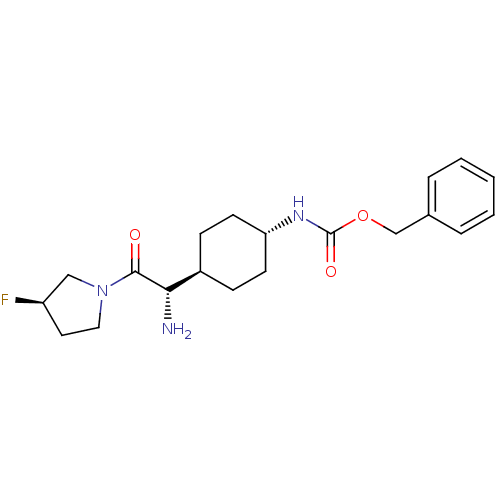
(CHEMBL277693 | {4-[(S)-1-Amino-2-((R)-3-fluoro-pyr...)Show SMILES N[C@@H]([C@H]1CC[C@@H](CC1)NC(=O)OCc1ccccc1)C(=O)N1CC[C@@H](F)C1 |wU:5.8,1.0,24.26,wD:2.2,(5.79,-6.89,;5.79,-5.34,;4.46,-4.57,;4.46,-3.02,;3.12,-2.25,;1.8,-3.02,;1.8,-4.57,;3.12,-5.34,;.45,-2.22,;-.88,-2.99,;-.88,-4.55,;-2.22,-2.22,;-3.56,-2.99,;-4.89,-2.22,;-4.89,-.68,;-6.22,.09,;-7.57,-.68,;-7.57,-2.22,;-6.23,-2.99,;7.12,-4.57,;7.12,-3.02,;8.45,-5.34,;9.79,-4.57,;10.94,-5.6,;10.31,-7,;11.08,-8.36,;8.78,-6.84,)| Show InChI InChI=1S/C20H28FN3O3/c21-16-10-11-24(12-16)19(25)18(22)15-6-8-17(9-7-15)23-20(26)27-13-14-4-2-1-3-5-14/h1-5,15-18H,6-13,22H2,(H,23,26)/t15-,16-,17-,18+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity of the compound towards human ERG potassium ion channel was determined |
Bioorg Med Chem Lett 14: 1265-8 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.040
BindingDB Entry DOI: 10.7270/Q2G73D5Q |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM11463
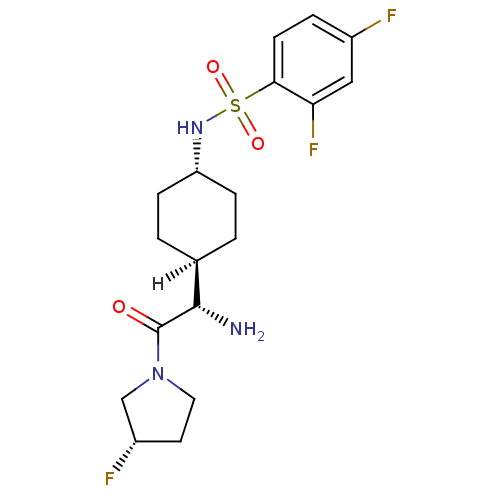
(CHEMBL22310 | N-{4-[(1S)-1-amino-2-[(3S)-3-fluorop...)Show SMILES [H][C@@]1(CC[C@@H](CC1)NS(=O)(=O)c1ccc(F)cc1F)[C@H](N)C(=O)N1CC[C@H](F)C1 |r,wU:4.7,19.21,1.0,wD:1.1,26.28,(1.34,.92,;-.05,.24,;-.05,1.79,;-1.38,2.56,;-2.72,1.79,;-2.72,.24,;-1.38,-.53,;-4.05,2.56,;-5.38,1.78,;-6.47,.7,;-4.29,.7,;-6.72,2.55,;-6.72,4.1,;-8.05,4.87,;-9.38,4.1,;-10.72,4.87,;-9.38,2.55,;-8.05,1.78,;-8.05,.24,;1.29,-.53,;1.29,-2.07,;2.62,.24,;2.62,1.78,;3.95,-.53,;5.2,.38,;6.44,-.53,;5.97,-1.99,;6.87,-3.24,;4.43,-1.99,)| Show InChI InChI=1S/C18H24F3N3O3S/c19-12-3-6-16(15(21)9-12)28(26,27)23-14-4-1-11(2-5-14)17(22)18(25)24-8-7-13(20)10-24/h3,6,9,11,13-14,17,23H,1-2,4-5,7-8,10,22H2/t11-,13-,14-,17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity of the compound towards human ERG potassium ion channel was determined |
Bioorg Med Chem Lett 14: 1265-8 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.040
BindingDB Entry DOI: 10.7270/Q2G73D5Q |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50140522
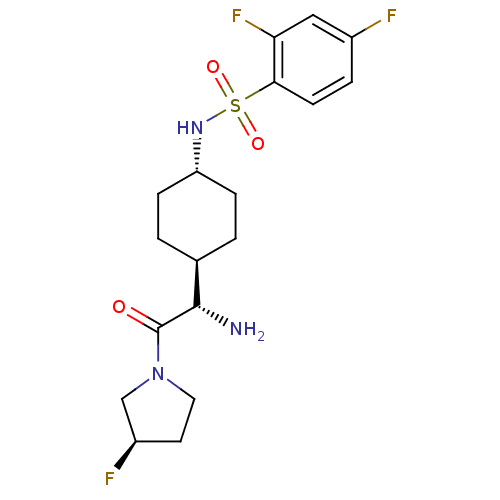
(CHEMBL283309 | N-{4-[(S)-1-Amino-2-((R)-3-fluoro-p...)Show SMILES N[C@@H]([C@H]1CC[C@@H](CC1)NS(=O)(=O)c1ccc(F)cc1F)C(=O)N1CC[C@@H](F)C1 |wU:1.0,5.8,25.27,wD:2.7,(5.89,-7.16,;5.89,-5.61,;4.56,-4.84,;4.56,-3.3,;3.22,-2.53,;1.89,-3.3,;1.89,-4.84,;3.22,-5.61,;.54,-2.5,;-.79,-3.27,;-.02,-4.62,;-2.14,-4.04,;-1.56,-1.92,;-.79,-.59,;-1.56,.74,;-3.12,.74,;-3.89,2.08,;-3.89,-.59,;-3.12,-1.92,;-3.89,-3.25,;7.22,-4.84,;7.22,-3.3,;8.55,-5.61,;9.89,-4.84,;11.04,-5.89,;10.41,-7.28,;11.16,-8.61,;8.87,-7.12,)| Show InChI InChI=1S/C18H24F3N3O3S/c19-12-3-6-16(15(21)9-12)28(26,27)23-14-4-1-11(2-5-14)17(22)18(25)24-8-7-13(20)10-24/h3,6,9,11,13-14,17,23H,1-2,4-5,7-8,10,22H2/t11-,13-,14-,17+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity of the compound towards human ERG potassium ion channel was determined |
Bioorg Med Chem Lett 14: 1265-8 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.040
BindingDB Entry DOI: 10.7270/Q2G73D5Q |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50140524
(CHEMBL23924 | N-{4-[(S)-1-Amino-2-((R)-3-fluoro-py...)Show SMILES N[C@@H]([C@H]1CC[C@@H](CC1)NC(=O)c1ccc(F)c(F)c1)C(=O)N1CC[C@@H](F)C1 |wU:5.8,1.0,24.26,wD:2.7,(7.35,-11.7,;7.35,-10.16,;6.02,-9.4,;6.02,-7.85,;4.67,-7.08,;3.35,-7.85,;3.35,-9.4,;4.67,-10.16,;2.01,-7.06,;.68,-7.81,;.68,-9.37,;-.65,-7.06,;-2,-7.81,;-3.32,-7.06,;-3.32,-5.51,;-4.67,-4.74,;-1.98,-4.74,;-1.97,-3.2,;-.65,-5.51,;8.67,-9.4,;8.67,-7.85,;10,-10.16,;11.34,-9.4,;12.49,-10.44,;11.86,-11.82,;12.61,-13.15,;10.33,-11.67,)| Show InChI InChI=1S/C19H24F3N3O2/c20-13-7-8-25(10-13)19(27)17(23)11-1-4-14(5-2-11)24-18(26)12-3-6-15(21)16(22)9-12/h3,6,9,11,13-14,17H,1-2,4-5,7-8,10,23H2,(H,24,26)/t11-,13-,14-,17+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity of the compound towards human ERG potassium ion channel was determined |
Bioorg Med Chem Lett 14: 1265-8 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.040
BindingDB Entry DOI: 10.7270/Q2G73D5Q |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50140527
((S)-2-Amino-2-cyclohexyl-1-((S)-3-fluoro-pyrrolidi...)Show InChI InChI=1S/C12H21FN2O/c13-10-6-7-15(8-10)12(16)11(14)9-4-2-1-3-5-9/h9-11H,1-8,14H2/t10-,11-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >9.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity of the compound towards human ERG potassium ion channel was determined |
Bioorg Med Chem Lett 14: 1265-8 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.040
BindingDB Entry DOI: 10.7270/Q2G73D5Q |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50140539
((S)-2-Amino-2-cyclohexyl-1-((R)-3-fluoro-pyrrolidi...)Show InChI InChI=1S/C12H21FN2O/c13-10-6-7-15(8-10)12(16)11(14)9-4-2-1-3-5-9/h9-11H,1-8,14H2/t10-,11+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >9.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity of the compound towards human ERG potassium ion channel was determined |
Bioorg Med Chem Lett 14: 1265-8 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.040
BindingDB Entry DOI: 10.7270/Q2G73D5Q |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50140541
((S)-2-Amino-2-cyclohexyl-1-(3,3-difluoro-pyrrolidi...)Show InChI InChI=1S/C12H20F2N2O/c13-12(14)6-7-16(8-12)11(17)10(15)9-4-2-1-3-5-9/h9-10H,1-8,15H2/t10-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >9.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity of the compound towards human ERG potassium ion channel was determined |
Bioorg Med Chem Lett 14: 1265-8 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.040
BindingDB Entry DOI: 10.7270/Q2G73D5Q |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50137270

(CHEMBL3084938 | N-[4-((S)-1-Amino-2-oxo-2-pyrrolid...)Show SMILES [H][C@@]1(CC[C@@H](CC1)NS(=O)(=O)c1ccc(NC(=O)N(C)C)cc1)[C@H](N)C(=O)N1CCCC1 |wU:4.7,1.0,wD:23.25,(10.79,7.46,;12.14,6.7,;10.81,5.91,;10.81,4.36,;12.14,3.6,;13.47,4.36,;13.47,5.91,;12.14,2.06,;13.48,1.29,;13.48,-.26,;14.81,2.06,;14.81,.51,;16.12,1.29,;17.45,.53,;17.45,-1.03,;18.8,-1.8,;20.13,-1.03,;20.13,.51,;21.47,-1.8,;21.46,-3.34,;22.81,-1.03,;16.12,-1.78,;14.79,-1.01,;12.14,8.23,;10.8,9,;13.48,9.01,;13.48,10.55,;14.81,8.24,;14.97,6.7,;16.48,6.38,;17.26,7.72,;16.21,8.85,)| Show InChI InChI=1S/C21H33N5O4S/c1-25(2)21(28)23-16-9-11-18(12-10-16)31(29,30)24-17-7-5-15(6-8-17)19(22)20(27)26-13-3-4-14-26/h9-12,15,17,19,24H,3-8,13-14,22H2,1-2H3,(H,23,28)/t15-,17-,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Inc.
Curated by ChEMBL
| Assay Description
Binding affinity for Potassium channel HERG Kv11.1 |
Bioorg Med Chem Lett 14: 43-6 (2003)
BindingDB Entry DOI: 10.7270/Q26972ZM |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50265158

(CHEMBL497992 | N-((trans)-4-((S)-1-amino-2-oxo-2-(...)Show SMILES N[C@@H]([C@H]1CC[C@@H](CC1)NS(=O)(=O)c1ccc(NS(=O)(=O)CC(F)(F)F)cc1)C(=O)N1CCCC1 |r,wU:5.8,wD:2.1,1.0,(13.27,5.92,;14.6,5.15,;14.6,3.61,;13.27,2.83,;13.27,1.28,;14.6,.51,;15.95,1.28,;15.95,2.83,;14.6,-1.03,;13.27,-1.79,;14.03,-3.13,;12.5,-.46,;11.93,-2.55,;10.6,-1.76,;9.26,-2.51,;9.25,-4.05,;7.91,-4.81,;7.89,-6.35,;6.35,-6.34,;9.43,-6.37,;7.88,-7.89,;6.54,-8.65,;5.2,-9.4,;7.3,-9.99,;5.78,-7.31,;10.58,-4.84,;11.91,-4.08,;15.93,5.92,;15.93,7.46,;17.27,5.15,;17.43,3.63,;18.93,3.31,;19.7,4.64,;18.67,5.79,)| Show InChI InChI=1S/C20H29F3N4O5S2/c21-20(22,23)13-33(29,30)25-15-7-9-17(10-8-15)34(31,32)26-16-5-3-14(4-6-16)18(24)19(28)27-11-1-2-12-27/h7-10,14,16,18,25-26H,1-6,11-13,24H2/t14-,16-,18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Inc.
Curated by ChEMBL
| Assay Description
Binding affinity for Potassium channel HERG Kv11.1 |
Bioorg Med Chem Lett 14: 43-6 (2003)
BindingDB Entry DOI: 10.7270/Q26972ZM |
More data for this
Ligand-Target Pair | |
cGMP-inhibited 3',5'-cyclic phosphodiesterase 3B [387-1112]
(Homo sapiens (Human)) | BDBM15311

((5R)-5-methyl-6-{4-[(2-{[3-(3-nitrophenyl)phenyl]m...)Show SMILES C[C@@H]1CC(=O)NN=C1c1ccc(NC2=C(Cc3cccc(c3)-c3cccc(c3)[N+]([O-])=O)C(=O)CCC2)cc1 |r,c:6,14| Show InChI InChI=1S/C30H28N4O4/c1-19-15-29(36)32-33-30(19)21-11-13-24(14-12-21)31-27-9-4-10-28(35)26(27)17-20-5-2-6-22(16-20)23-7-3-8-25(18-23)34(37)38/h2-3,5-8,11-14,16,18-19,31H,4,9-10,15,17H2,1H3,(H,32,36)/t19-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0490 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Merck Research Laboratories
| Assay Description
PDE activity was monitored by measuring the hydrolysis of [3H]-cAMP to [3H]-AMP using scintillation proximity assay (SPA). [3H]-AMP was captured by t... |
Bioorg Med Chem Lett 13: 3983-7 (2003)
Article DOI: 10.1016/j.bmcl.2003.08.056
BindingDB Entry DOI: 10.7270/Q2JD4V11 |
More data for this
Ligand-Target Pair | |
cGMP-inhibited 3',5'-cyclic phosphodiesterase 3B [387-1112]
(Homo sapiens (Human)) | BDBM15310
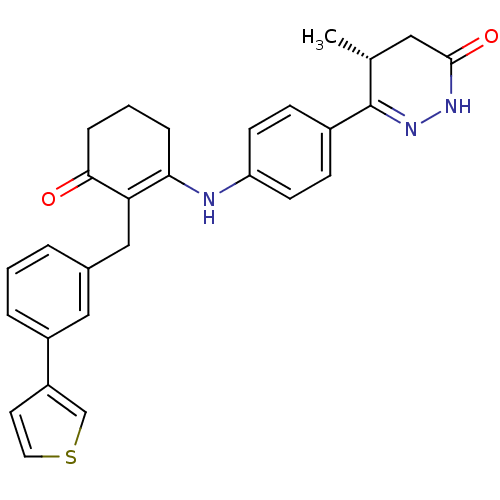
((5R)-5-methyl-6-{4-[(3-oxo-2-{[3-(thiophen-3-yl)ph...)Show SMILES C[C@@H]1CC(=O)NN=C1c1ccc(NC2=C(Cc3cccc(c3)-c3ccsc3)C(=O)CCC2)cc1 |r,c:6,14| Show InChI InChI=1S/C28H27N3O2S/c1-18-14-27(33)30-31-28(18)20-8-10-23(11-9-20)29-25-6-3-7-26(32)24(25)16-19-4-2-5-21(15-19)22-12-13-34-17-22/h2,4-5,8-13,15,17-18,29H,3,6-7,14,16H2,1H3,(H,30,33)/t18-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0680 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Merck Research Laboratories
| Assay Description
PDE activity was monitored by measuring the hydrolysis of [3H]-cAMP to [3H]-AMP using scintillation proximity assay (SPA). [3H]-AMP was captured by t... |
Bioorg Med Chem Lett 13: 3983-7 (2003)
Article DOI: 10.1016/j.bmcl.2003.08.056
BindingDB Entry DOI: 10.7270/Q2JD4V11 |
More data for this
Ligand-Target Pair | |
Glucagon receptor
(Homo sapiens (Human)) | BDBM235136

(US9359339, 25)Show SMILES CCC[C@@H](C(c1csc2c(cc(Cl)cc12)C#N)c1ccc(OC(F)(F)F)cc1)c1ccc(cc1)C(=O)NCCC(O)=O Show InChI InChI=1S/C31H26ClF3N2O4S/c1-2-3-24(18-4-6-20(7-5-18)30(40)37-13-12-27(38)39)28(19-8-10-23(11-9-19)41-31(33,34)35)26-17-42-29-21(16-36)14-22(32)15-25(26)29/h4-11,14-15,17,24,28H,2-3,12-13H2,1H3,(H,37,40)(H,38,39)/t24-,28?/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
A stable CHO (Chinese hamster ovary) cell line expressing cloned human glucagon receptor was maintained as described (Chicchi, et. al. J Biol Chem 27... |
US Patent US9359339 (2016)
BindingDB Entry DOI: 10.7270/Q2FQ9VHC |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50151798
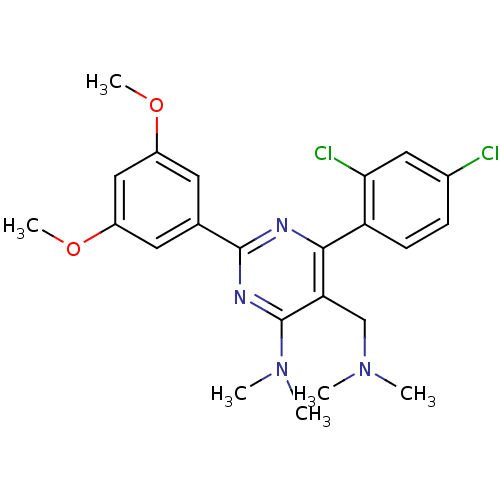
(CHEMBL186877 | [6-(2,4-Dichloro-phenyl)-2-(3,5-dim...)Show SMILES COc1cc(OC)cc(c1)-c1nc(N(C)C)c(CN(C)C)c(n1)-c1ccc(Cl)cc1Cl Show InChI InChI=1S/C23H26Cl2N4O2/c1-28(2)13-19-21(18-8-7-15(24)11-20(18)25)26-22(27-23(19)29(3)4)14-9-16(30-5)12-17(10-14)31-6/h7-12H,13H2,1-6H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Dipeptidyl peptidase IV |
Bioorg Med Chem Lett 14: 4759-62 (2004)
Article DOI: 10.1016/j.bmcl.2004.06.099
BindingDB Entry DOI: 10.7270/Q2XK8F1Q |
More data for this
Ligand-Target Pair | |
cGMP-inhibited 3',5'-cyclic phosphodiesterase 3A [388-1112]
(Homo sapiens (Human)) | BDBM15311

((5R)-5-methyl-6-{4-[(2-{[3-(3-nitrophenyl)phenyl]m...)Show SMILES C[C@@H]1CC(=O)NN=C1c1ccc(NC2=C(Cc3cccc(c3)-c3cccc(c3)[N+]([O-])=O)C(=O)CCC2)cc1 |r,c:6,14| Show InChI InChI=1S/C30H28N4O4/c1-19-15-29(36)32-33-30(19)21-11-13-24(14-12-21)31-27-9-4-10-28(35)26(27)17-20-5-2-6-22(16-20)23-7-3-8-25(18-23)34(37)38/h2-3,5-8,11-14,16,18-19,31H,4,9-10,15,17H2,1H3,(H,32,36)/t19-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
| Assay Description
PDE activity was monitored by measuring the hydrolysis of [3H]-cAMP to [3H]-AMP using scintillation proximity assay (SPA). [3H]-AMP was captured by t... |
Bioorg Med Chem Lett 13: 3983-7 (2003)
Article DOI: 10.1016/j.bmcl.2003.08.056
BindingDB Entry DOI: 10.7270/Q2JD4V11 |
More data for this
Ligand-Target Pair | |
cGMP-inhibited 3',5'-cyclic phosphodiesterase 3A [388-1112]
(Homo sapiens (Human)) | BDBM15310

((5R)-5-methyl-6-{4-[(3-oxo-2-{[3-(thiophen-3-yl)ph...)Show SMILES C[C@@H]1CC(=O)NN=C1c1ccc(NC2=C(Cc3cccc(c3)-c3ccsc3)C(=O)CCC2)cc1 |r,c:6,14| Show InChI InChI=1S/C28H27N3O2S/c1-18-14-27(33)30-31-28(18)20-8-10-23(11-9-20)29-25-6-3-7-26(32)24(25)16-19-4-2-5-21(15-19)22-12-13-34-17-22/h2,4-5,8-13,15,17-18,29H,3,6-7,14,16H2,1H3,(H,30,33)/t18-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
| Assay Description
PDE activity was monitored by measuring the hydrolysis of [3H]-cAMP to [3H]-AMP using scintillation proximity assay (SPA). [3H]-AMP was captured by t... |
Bioorg Med Chem Lett 13: 3983-7 (2003)
Article DOI: 10.1016/j.bmcl.2003.08.056
BindingDB Entry DOI: 10.7270/Q2JD4V11 |
More data for this
Ligand-Target Pair | |
cGMP-inhibited 3',5'-cyclic phosphodiesterase 3B [387-1112]
(Homo sapiens (Human)) | BDBM15337
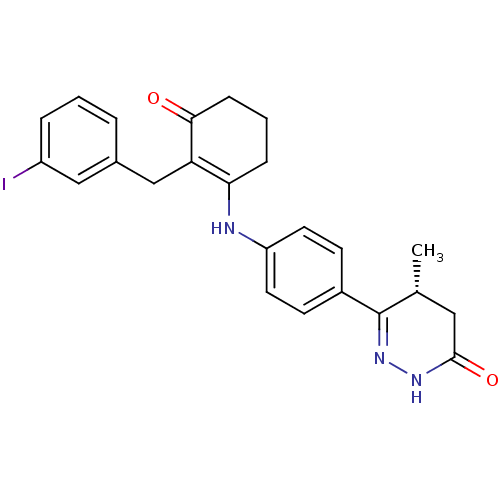
((5R)-6-(4-{[2-(3-iodobenzyl)-3-oxocyclohex-1-en-1-...)Show SMILES C[C@@H]1CC(=O)NN=C1c1ccc(NC2=C(Cc3cccc(I)c3)C(=O)CCC2)cc1 |r,c:6,14| Show InChI InChI=1S/C24H24IN3O2/c1-15-12-23(30)27-28-24(15)17-8-10-19(11-9-17)26-21-6-3-7-22(29)20(21)14-16-4-2-5-18(25)13-16/h2,4-5,8-11,13,15,26H,3,6-7,12,14H2,1H3,(H,27,30)/t15-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.110 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Merck Research Laboratories
| Assay Description
PDE activity was monitored by measuring the hydrolysis of [3H]-cAMP to [3H]-AMP using scintillation proximity assay (SPA). [3H]-AMP was captured by t... |
Bioorg Med Chem Lett 13: 3983-7 (2003)
Article DOI: 10.1016/j.bmcl.2003.08.056
BindingDB Entry DOI: 10.7270/Q2JD4V11 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
cGMP-inhibited 3',5'-cyclic phosphodiesterase 3B [387-1112]
(Homo sapiens (Human)) | BDBM15329

(3-[4-({2-[(2,6-dichlorophenyl)methyl]-3-oxocyclohe...)Show SMILES CC1(C)C(=O)NN=C1c1ccc(NC2=C(Cc3c(Cl)cccc3Cl)C(=O)CCC2)cc1F |c:6,14| Show InChI InChI=1S/C24H22Cl2FN3O2/c1-24(2)22(29-30-23(24)32)14-10-9-13(11-19(14)27)28-20-7-4-8-21(31)16(20)12-15-17(25)5-3-6-18(15)26/h3,5-6,9-11,28H,4,7-8,12H2,1-2H3,(H,30,32) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
| Assay Description
PDE activity was monitored by measuring the hydrolysis of [3H]-cAMP to [3H]-AMP using scintillation proximity assay (SPA). [3H]-AMP was captured by t... |
Bioorg Med Chem Lett 13: 3983-7 (2003)
Article DOI: 10.1016/j.bmcl.2003.08.056
BindingDB Entry DOI: 10.7270/Q2JD4V11 |
More data for this
Ligand-Target Pair | |
cGMP-inhibited 3',5'-cyclic phosphodiesterase 3B [387-1112]
(Homo sapiens (Human)) | BDBM15304
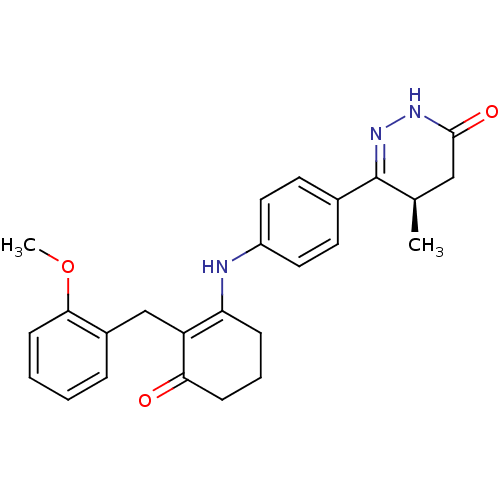
((5R)-6-[4-({2-[(2-methoxyphenyl)methyl]-3-oxocyclo...)Show SMILES COc1ccccc1CC1=C(CCCC1=O)Nc1ccc(cc1)C1=NNC(=O)C[C@H]1C |r,t:10,26| Show InChI InChI=1S/C25H27N3O3/c1-16-14-24(30)27-28-25(16)17-10-12-19(13-11-17)26-21-7-5-8-22(29)20(21)15-18-6-3-4-9-23(18)31-2/h3-4,6,9-13,16,26H,5,7-8,14-15H2,1-2H3,(H,27,30)/t16-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.130 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Merck Research Laboratories
| Assay Description
PDE activity was monitored by measuring the hydrolysis of [3H]-cAMP to [3H]-AMP using scintillation proximity assay (SPA). [3H]-AMP was captured by t... |
Bioorg Med Chem Lett 13: 3983-7 (2003)
Article DOI: 10.1016/j.bmcl.2003.08.056
BindingDB Entry DOI: 10.7270/Q2JD4V11 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50371255

(CHEMBL1203953)Show SMILES N[C@@H](CC(=O)N1CCn2c(nnc2C(F)(F)F)[C@H]1Cc1ccc(F)cc1)Cc1cc(F)c(F)cc1F |r| Show InChI InChI=1S/C23H20F7N5O/c24-14-3-1-12(2-4-14)7-19-21-32-33-22(23(28,29)30)35(21)6-5-34(19)20(36)10-15(31)8-13-9-17(26)18(27)11-16(13)25/h1-4,9,11,15,19H,5-8,10,31H2/t15-,19-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 |
J Med Chem 51: 589-602 (2008)
Article DOI: 10.1021/jm070330v
BindingDB Entry DOI: 10.7270/Q2B27W4M |
More data for this
Ligand-Target Pair | |
cGMP-inhibited 3',5'-cyclic phosphodiesterase 3B [387-1112]
(Homo sapiens (Human)) | BDBM15302
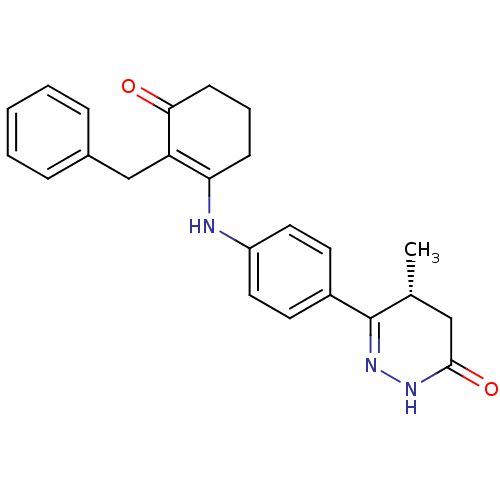
((5R)-6-{4-[(2-benzyl-3-oxocyclohex-1-en-1-yl)amino...)Show SMILES C[C@@H]1CC(=O)NN=C1c1ccc(NC2=C(Cc3ccccc3)C(=O)CCC2)cc1 |r,c:6,14| Show InChI InChI=1S/C24H25N3O2/c1-16-14-23(29)26-27-24(16)18-10-12-19(13-11-18)25-21-8-5-9-22(28)20(21)15-17-6-3-2-4-7-17/h2-4,6-7,10-13,16,25H,5,8-9,14-15H2,1H3,(H,26,29)/t16-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.190 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Merck Research Laboratories
| Assay Description
PDE activity was monitored by measuring the hydrolysis of [3H]-cAMP to [3H]-AMP using scintillation proximity assay (SPA). [3H]-AMP was captured by t... |
Bioorg Med Chem Lett 13: 3983-7 (2003)
Article DOI: 10.1016/j.bmcl.2003.08.056
BindingDB Entry DOI: 10.7270/Q2JD4V11 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Stimulator of interferon genes protein
(Human) | BDBM50604556
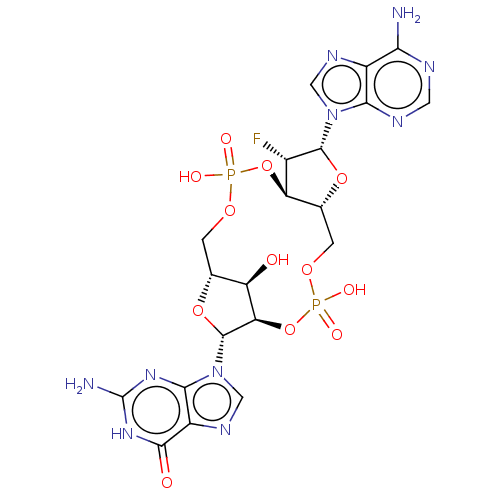
(CHEMBL5175703)Show SMILES N.N.[H][C@@]12COP(O)(=O)O[C@@]3([H])[C@H](F)[C@@H](O[C@]3([H])COP(O)(=O)O[C@]([H])([C@@H]1O)[C@@H](O2)n1cnc2c1nc(N)[nH]c2=O)n1cnc2c(N)ncnc12 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02197
BindingDB Entry DOI: 10.7270/Q2028WM5 |
More data for this
Ligand-Target Pair | |
Glucagon receptor
(Homo sapiens (Human)) | BDBM235137
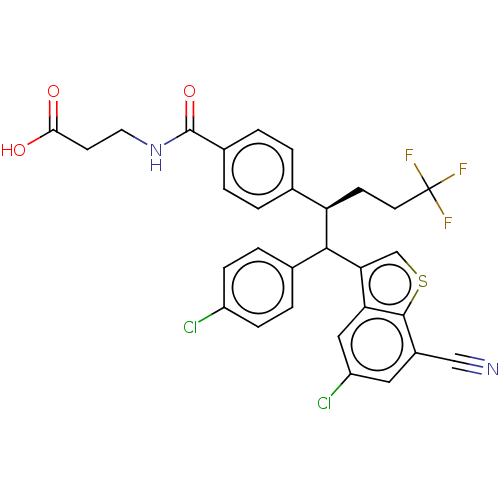
(US9359339, 27)Show SMILES OC(=O)CCNC(=O)c1ccc(cc1)[C@@H](CCC(F)(F)F)C(c1csc2c(cc(Cl)cc12)C#N)c1ccc(Cl)cc1 Show InChI InChI=1S/C30H23Cl2F3N2O3S/c31-21-7-5-18(6-8-21)27(25-16-41-28-20(15-36)13-22(32)14-24(25)28)23(9-11-30(33,34)35)17-1-3-19(4-2-17)29(40)37-12-10-26(38)39/h1-8,13-14,16,23,27H,9-12H2,(H,37,40)(H,38,39)/t23-,27?/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
A stable CHO (Chinese hamster ovary) cell line expressing cloned human glucagon receptor was maintained as described (Chicchi, et. al. J Biol Chem 27... |
US Patent US9359339 (2016)
BindingDB Entry DOI: 10.7270/Q2FQ9VHC |
More data for this
Ligand-Target Pair | |
Glucagon receptor
(Homo sapiens (Human)) | BDBM235135

(US9359339, 23)Show SMILES CCC[C@@H](C(c1csc2c(cc(C)cc12)C#N)c1ccc(OC(F)(F)F)cc1)c1ccc(cc1)C(=O)NCCC(O)=O Show InChI InChI=1S/C32H29F3N2O4S/c1-3-4-25(20-5-7-22(8-6-20)31(40)37-14-13-28(38)39)29(21-9-11-24(12-10-21)41-32(33,34)35)27-18-42-30-23(17-36)15-19(2)16-26(27)30/h5-12,15-16,18,25,29H,3-4,13-14H2,1-2H3,(H,37,40)(H,38,39)/t25-,29?/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
A stable CHO (Chinese hamster ovary) cell line expressing cloned human glucagon receptor was maintained as described (Chicchi, et. al. J Biol Chem 27... |
US Patent US9359339 (2016)
BindingDB Entry DOI: 10.7270/Q2FQ9VHC |
More data for this
Ligand-Target Pair | |
cGMP-inhibited 3',5'-cyclic phosphodiesterase 3B [387-1112]
(Homo sapiens (Human)) | BDBM15318

(6-{4-[(2-benzyl-3-oxocyclohex-1-en-1-yl)amino]-2-f...)Show SMILES CC1CC(=O)NN=C1c1ccc(NC2=C(Cc3ccccc3)C(=O)CCC2)cc1F |c:6,14| Show InChI InChI=1S/C24H24FN3O2/c1-15-12-23(30)27-28-24(15)18-11-10-17(14-20(18)25)26-21-8-5-9-22(29)19(21)13-16-6-3-2-4-7-16/h2-4,6-7,10-11,14-15,26H,5,8-9,12-13H2,1H3,(H,27,30) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
| Assay Description
PDE activity was monitored by measuring the hydrolysis of [3H]-cAMP to [3H]-AMP using scintillation proximity assay (SPA). [3H]-AMP was captured by t... |
Bioorg Med Chem Lett 13: 3983-7 (2003)
Article DOI: 10.1016/j.bmcl.2003.08.056
BindingDB Entry DOI: 10.7270/Q2JD4V11 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
cGMP-inhibited 3',5'-cyclic phosphodiesterase 3B [387-1112]
(Homo sapiens (Human)) | BDBM15319
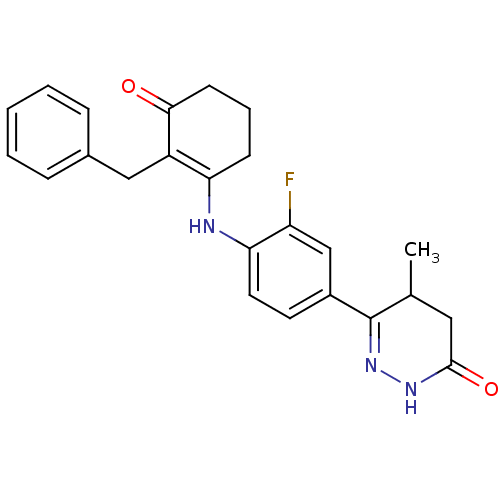
(6-{4-[(2-benzyl-3-oxocyclohex-1-en-1-yl)amino]-3-f...)Show SMILES CC1CC(=O)NN=C1c1ccc(NC2=C(Cc3ccccc3)C(=O)CCC2)c(F)c1 |c:6,14| Show InChI InChI=1S/C24H24FN3O2/c1-15-12-23(30)27-28-24(15)17-10-11-21(19(25)14-17)26-20-8-5-9-22(29)18(20)13-16-6-3-2-4-7-16/h2-4,6-7,10-11,14-15,26H,5,8-9,12-13H2,1H3,(H,27,30) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
| Assay Description
PDE activity was monitored by measuring the hydrolysis of [3H]-cAMP to [3H]-AMP using scintillation proximity assay (SPA). [3H]-AMP was captured by t... |
Bioorg Med Chem Lett 13: 3983-7 (2003)
Article DOI: 10.1016/j.bmcl.2003.08.056
BindingDB Entry DOI: 10.7270/Q2JD4V11 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
cGMP-inhibited 3',5'-cyclic phosphodiesterase 3B [654-1073]
(Homo sapiens (Human)) | BDBM15337

((5R)-6-(4-{[2-(3-iodobenzyl)-3-oxocyclohex-1-en-1-...)Show SMILES C[C@@H]1CC(=O)NN=C1c1ccc(NC2=C(Cc3cccc(I)c3)C(=O)CCC2)cc1 |r,c:6,14| Show InChI InChI=1S/C24H24IN3O2/c1-15-12-23(30)27-28-24(15)17-8-10-19(11-9-17)26-21-6-3-7-22(29)20(21)14-16-4-2-5-18(25)13-16/h2,4-5,8-11,13,15,26H,3,6-7,12,14H2,1H3,(H,27,30)/t15-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.270 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Merck Research Laboratories
| Assay Description
PDE activity was monitored by measuring the hydrolysis of [3H]-cAMP to [3H]-AMP using scintillation proximity assay (SPA). [3H]-AMP was captured by t... |
Biochemistry 43: 6091-100 (2004)
Article DOI: 10.1021/bi049868i
BindingDB Entry DOI: 10.7270/Q2DR2SQM |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Stimulator of interferon genes protein
(Human) | BDBM50509399
(CHEMBL4468010)Show SMILES Nc1nc2n(cnc2c(=O)[nH]1)[C@@H]1O[C@@H]2COP(O)(=O)O[C@@H]3[C@@H](COP(O)(=O)O[C@@H]1[C@@H]2F)O[C@H]([C@@H]3F)n1cnc2c(N)ncnc12 |r| Show InChI InChI=1S/C20H22F2N10O11P2/c21-8-6-1-38-44(34,35)42-12-7(41-18(9(12)22)31-4-27-10-14(23)25-3-26-15(10)31)2-39-45(36,37)43-13(8)19(40-6)32-5-28-11-16(32)29-20(24)30-17(11)33/h3-9,12-13,18-19H,1-2H2,(H,34,35)(H,36,37)(H2,23,25,26)(H3,24,29,30,33)/t6-,7-,8-,9-,12-,13-,18-,19-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02197
BindingDB Entry DOI: 10.7270/Q2028WM5 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data