

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nociceptin receptor (RAT) | BDBM50087691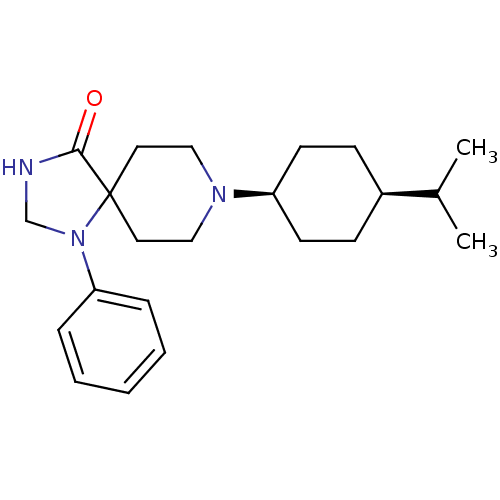 (8-(4-Isopropyl-cyclohexyl)-1-phenyl-1,3,8-triaza-s...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.0790 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Inhibition against binding of radioligand [leucyl-3H]-OFQ to membrane of human embryonic kidney 293 cells overexpressing rat Opioid receptor like 1 | Bioorg Med Chem Lett 10: 831-4 (2000) BindingDB Entry DOI: 10.7270/Q2N29XG2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (RAT) | BDBM50087692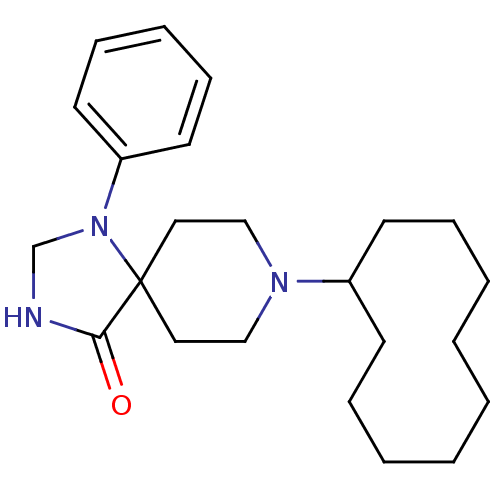 (8-Cyclodecyl-1-phenyl-1,3,8-triaza-spiro[4.5]decan...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.0820 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Inhibition against binding of radioligand [leucyl-3H]-OFQ to membrane of human embryonic kidney 293 cells overexpressing rat Opioid receptor like 1 | Bioorg Med Chem Lett 10: 831-4 (2000) BindingDB Entry DOI: 10.7270/Q2N29XG2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50027473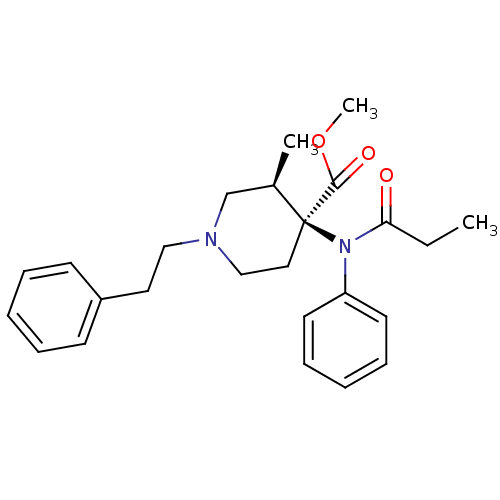 (3-Methyl-1-phenethyl-4-(phenyl-propionyl-amino)-pi...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL DrugBank PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Competitive binding displacement analyses was performed from permanently transfected HEK293 cells expressing Opioid receptor mu 1 | J Med Chem 43: 1329-38 (2001) BindingDB Entry DOI: 10.7270/Q2ZG6SZG | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50429823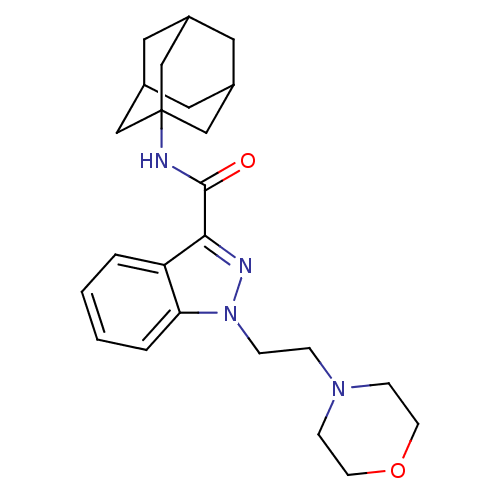 (CHEMBL2338173) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd Curated by ChEMBL | Assay Description Binding affinity to CB2 receptor (unknown origin) | Bioorg Med Chem Lett 23: 1177-81 (2013) Article DOI: 10.1016/j.bmcl.2013.01.044 BindingDB Entry DOI: 10.7270/Q2M32X3H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50057010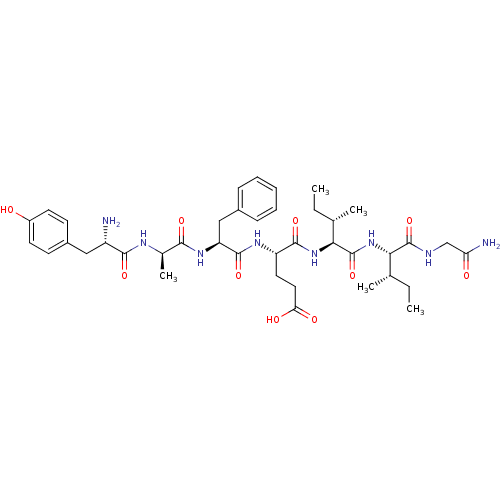 ((S)-4-((S)-2-{(R)-2-[(S)-2-Amino-3-(4-hydroxy-phen...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Inhibition against binding of radioligand [Ile5,6-3H]-deltorphin to membrane from baby hamster kidney cells infected with forest virus encoding the ... | Bioorg Med Chem Lett 10: 831-4 (2000) BindingDB Entry DOI: 10.7270/Q2N29XG2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50087697 (8-Cycloundecyl-1-phenyl-1,3,8-triaza-spiro[4.5]dec...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Inhibition against binding of radioligand [N-allyl-2-3-3H]-naloxone to membrane of baby hamster kidney cells infected with forest virus encoding the... | Bioorg Med Chem Lett 10: 831-4 (2000) BindingDB Entry DOI: 10.7270/Q2N29XG2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (RAT) | BDBM50087687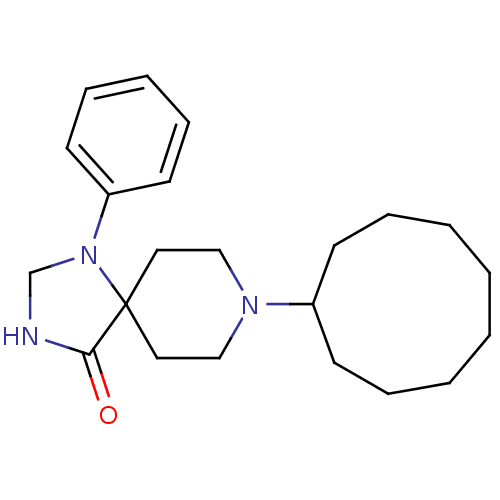 (8-Cyclononyl-1-phenyl-1,3,8-triaza-spiro[4.5]decan...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Inhibition against binding of radioligand [leucyl-3H]-OFQ to membrane of human embryonic kidney 293 cells overexpressing rat Opioid receptor like 1 | Bioorg Med Chem Lett 10: 831-4 (2000) BindingDB Entry DOI: 10.7270/Q2N29XG2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50087685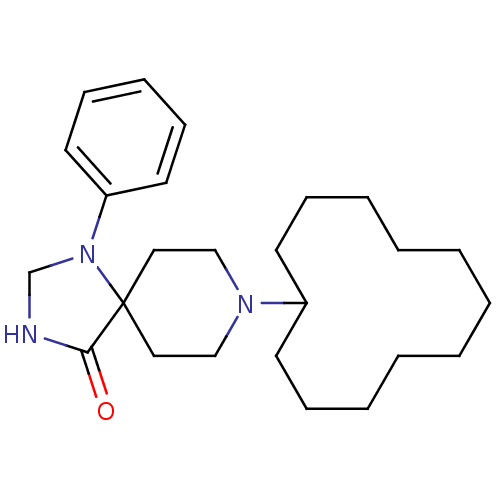 (8-Cyclododecyl-1-phenyl-1,3,8-triaza-spiro[4.5]dec...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Inhibition against binding of radioligand [N-allyl-2-3-3H]-naloxone to membrane of baby hamster kidney cells infected with forest virus encoding the... | Bioorg Med Chem Lett 10: 831-4 (2000) BindingDB Entry DOI: 10.7270/Q2N29XG2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM50169264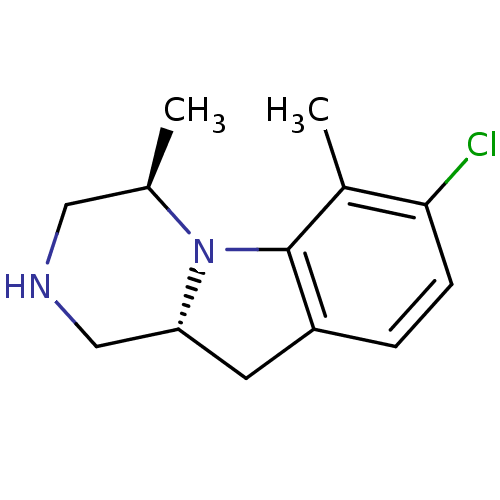 ((4R,10aR)-7-Chloro-4,6-dimethyl-1,2,3,4,10,10a-hex...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis Research Ltd. Curated by ChEMBL | Assay Description Binding affinity toward 5-HT2C receptor evaluated by displacement of [3H]-5-HT radioligand | Bioorg Med Chem Lett 15: 3604-8 (2005) Article DOI: 10.1016/j.bmcl.2005.05.074 BindingDB Entry DOI: 10.7270/Q2GQ6X87 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (RAT) | BDBM50087698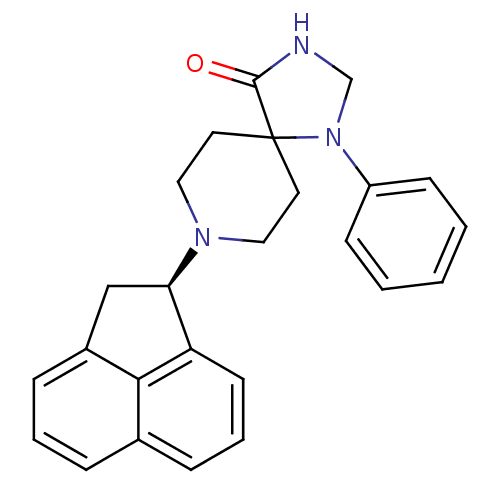 (8-(R)-Acenaphthen-1-yl-1-phenyl-1,3,8-triaza-spiro...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | 0.410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Inhibition against binding of radioligand [leucyl-3H]-OFQ to membrane of human embryonic kidney 293 cells overexpressing rat Opioid receptor like 1 | Bioorg Med Chem Lett 10: 831-4 (2000) BindingDB Entry DOI: 10.7270/Q2N29XG2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (RAT) | BDBM50087697 (8-Cycloundecyl-1-phenyl-1,3,8-triaza-spiro[4.5]dec...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Inhibition against binding of radioligand [leucyl-3H]-OFQ to membrane of human embryonic kidney 293 cells overexpressing rat Opioid receptor like 1 | Bioorg Med Chem Lett 10: 831-4 (2000) BindingDB Entry DOI: 10.7270/Q2N29XG2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50087016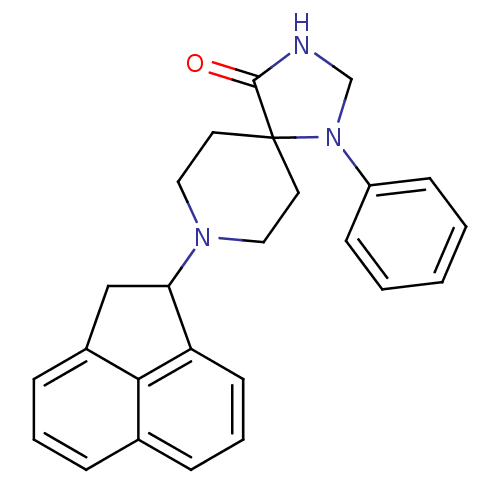 (8-Acenaphthen-1-yl-1-phenyl-1,3,8-triaza-spiro[4.5...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 0.520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Competitive binding affinity against transfected HEK293 cells expressing human Opioid receptor like 1 | J Med Chem 43: 1329-38 (2001) BindingDB Entry DOI: 10.7270/Q2ZG6SZG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50087692 (8-Cyclodecyl-1-phenyl-1,3,8-triaza-spiro[4.5]decan...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.660 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Inhibition against binding of radioligand [N-allyl-2-3-3H]-naloxone to membrane of baby hamster kidney cells infected with forest virus encoding the... | Bioorg Med Chem Lett 10: 831-4 (2000) BindingDB Entry DOI: 10.7270/Q2N29XG2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50087008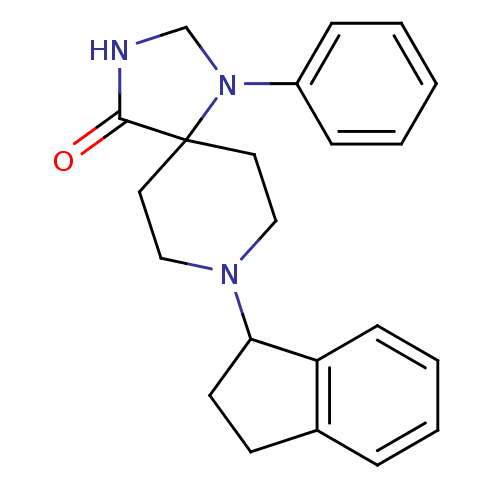 (8-indan-1-yl-1-phenyl-1,3,8-triaza-spiro[4.5]decan...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Competitive binding affinity against transfected HEK293 cells expressing Opioid receptor like 1 | J Med Chem 43: 1329-38 (2001) BindingDB Entry DOI: 10.7270/Q2ZG6SZG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Rattus norvegicus (rat)) | BDBM50087697 (8-Cycloundecyl-1-phenyl-1,3,8-triaza-spiro[4.5]dec...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.820 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Inhibition against binding of radioligand [N-allyl-2-3-3H]-naloxone to membrane of baby hamster kidney cells infected with forest virus encoding the... | Bioorg Med Chem Lett 10: 831-4 (2000) BindingDB Entry DOI: 10.7270/Q2N29XG2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (RAT) | BDBM50087685 (8-Cyclododecyl-1-phenyl-1,3,8-triaza-spiro[4.5]dec...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.950 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Inhibition against binding of radioligand [leucyl-3H]-OFQ to membrane of human embryonic kidney 293 cells overexpressing rat Opioid receptor like 1 | Bioorg Med Chem Lett 10: 831-4 (2000) BindingDB Entry DOI: 10.7270/Q2N29XG2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM50169263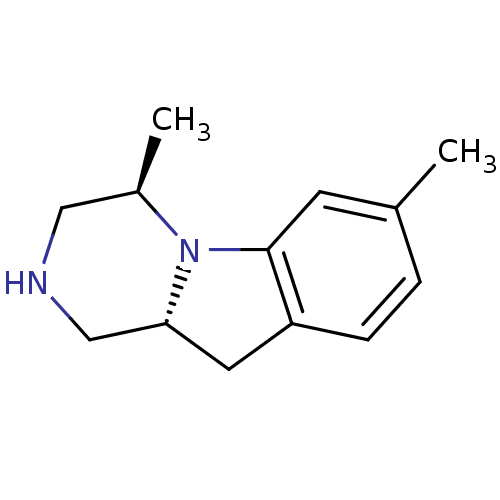 ((4R,10aR)-4,7-Dimethyl-1,2,3,4,10,10a-hexahydro-py...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis Research Ltd. Curated by ChEMBL | Assay Description Binding affinity toward 5-HT2C receptor evaluated by displacement of [3H]-5-HT radioligand | Bioorg Med Chem Lett 15: 3604-8 (2005) Article DOI: 10.1016/j.bmcl.2005.05.074 BindingDB Entry DOI: 10.7270/Q2GQ6X87 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM50169266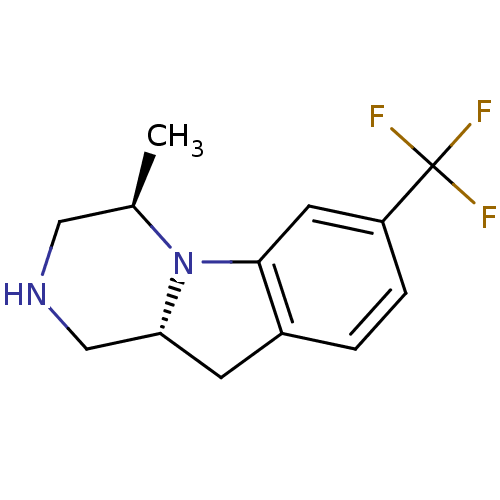 ((4R,10aR)-4-Methyl-7-trifluoromethyl-1,2,3,4,10,10...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis Research Ltd. Curated by ChEMBL | Assay Description Binding affinity toward 5-HT2C receptor evaluated by displacement of [3H]-5-HT radioligand | Bioorg Med Chem Lett 15: 3604-8 (2005) Article DOI: 10.1016/j.bmcl.2005.05.074 BindingDB Entry DOI: 10.7270/Q2GQ6X87 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50087011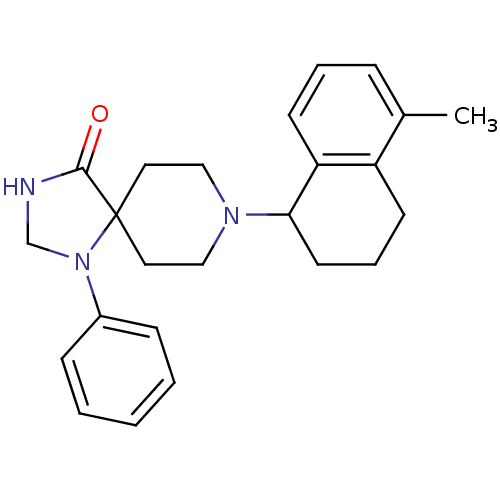 (8-(5-Methyl-1,2,3,4-tetrahydro-naphthalen-1-yl)-1-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Competitive binding affinity against transfected HEK293 cells expressing human Opioid receptor like 1 | J Med Chem 43: 1329-38 (2001) BindingDB Entry DOI: 10.7270/Q2ZG6SZG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (RAT) | BDBM50087690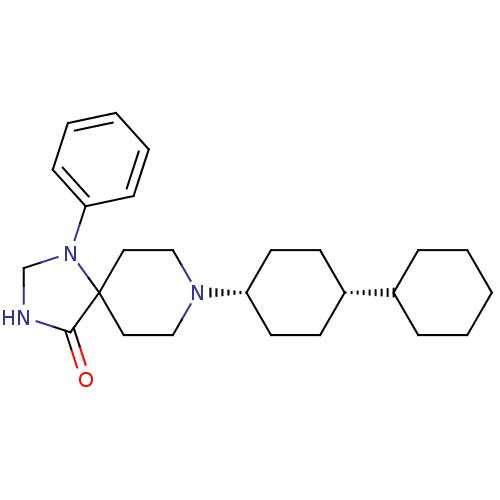 (8-Bicyclohexyl-4-yl-1-phenyl-1,3,8-triaza-spiro[4....) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Inhibition against binding of radioligand [leucyl-3H]-OFQ to membrane of human embryonic kidney 293 cells overexpressing rat Opioid receptor like 1 | Bioorg Med Chem Lett 10: 831-4 (2000) BindingDB Entry DOI: 10.7270/Q2N29XG2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50087690 (8-Bicyclohexyl-4-yl-1-phenyl-1,3,8-triaza-spiro[4....) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Inhibition against binding of radioligand [N-allyl-2-3-3H]-naloxone to membrane of baby hamster kidney cells infected with forest virus encoding the... | Bioorg Med Chem Lett 10: 831-4 (2000) BindingDB Entry DOI: 10.7270/Q2N29XG2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM50169267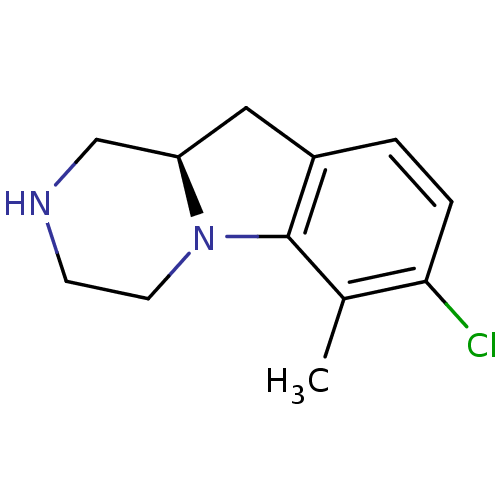 ((R)-7-Chloro-6-methyl-1,2,3,4,10,10a-hexahydro-pyr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis Research Ltd. Curated by ChEMBL | Assay Description Binding affinity toward 5-HT2C receptor evaluated by displacement of [3H]-5-HT radioligand | Bioorg Med Chem Lett 15: 3604-8 (2005) Article DOI: 10.1016/j.bmcl.2005.05.074 BindingDB Entry DOI: 10.7270/Q2GQ6X87 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM50169259 ((4R,10aR)-4,6,7-Trimethyl-1,2,3,4,10,10a-hexahydro...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis Research Ltd. Curated by ChEMBL | Assay Description Binding affinity toward 5-HT2C receptor evaluated by displacement of [3H]-5-HT radioligand | Bioorg Med Chem Lett 15: 3604-8 (2005) Article DOI: 10.1016/j.bmcl.2005.05.074 BindingDB Entry DOI: 10.7270/Q2GQ6X87 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM50169261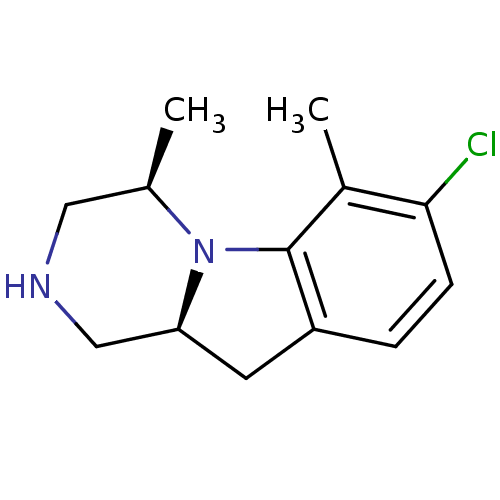 ((4R,10aS)-7-Chloro-4,6-dimethyl-1,2,3,4,10,10a-hex...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis Research Ltd. Curated by ChEMBL | Assay Description Binding affinity toward 5-HT2C receptor evaluated by displacement of [3H]-5-HT radioligand | Bioorg Med Chem Lett 15: 3604-8 (2005) Article DOI: 10.1016/j.bmcl.2005.05.074 BindingDB Entry DOI: 10.7270/Q2GQ6X87 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (RAT) | BDBM50087684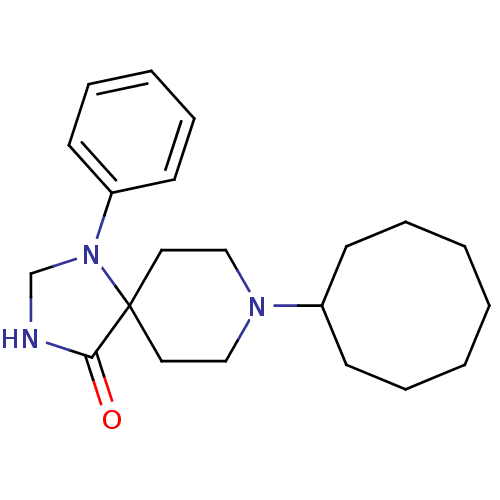 (8-Cyclooctyl-1-phenyl-1,3,8-triaza-spiro[4.5]decan...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Inhibition against binding of radioligand [leucyl-3H]-OFQ to membrane of human embryonic kidney 293 cells overexpressing rat Opioid receptor like 1 | Bioorg Med Chem Lett 10: 831-4 (2000) BindingDB Entry DOI: 10.7270/Q2N29XG2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (RAT) | BDBM50087693 (1-Phenyl-8-(4-propyl-cyclohexyl)-1,3,8-triaza-spir...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Inhibition against binding of radioligand [leucyl-3H]-OFQ to membrane of human embryonic kidney 293 cells overexpressing rat Opioid receptor like 1 | Bioorg Med Chem Lett 10: 831-4 (2000) BindingDB Entry DOI: 10.7270/Q2N29XG2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50087015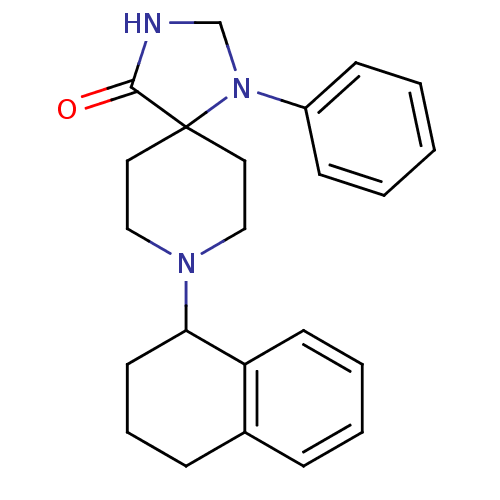 (1-Phenyl-8-(1,2,3,4-tetrahydro-naphthalen-1-yl)-1,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Competitive binding affinity against transfected HEK293 cells expressing Opioid receptor like 1 | J Med Chem 43: 1329-38 (2001) BindingDB Entry DOI: 10.7270/Q2ZG6SZG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Rattus norvegicus (rat)) | BDBM50087692 (8-Cyclodecyl-1-phenyl-1,3,8-triaza-spiro[4.5]decan...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Inhibition against binding of radioligand [N-allyl-2-3-3H]-naloxone to membrane of baby hamster kidney cells infected with forest virus encoding the... | Bioorg Med Chem Lett 10: 831-4 (2000) BindingDB Entry DOI: 10.7270/Q2N29XG2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50087002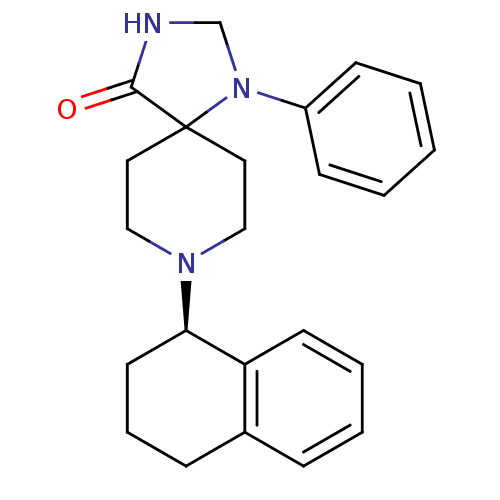 (1-Phenyl-8-(1,2,3,4-tetrahydro-naphthalen-1-yl)-1,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Competitive binding affinity against transfected HEK293 cells expressing Opioid receptor like 1 | J Med Chem 43: 1329-38 (2001) BindingDB Entry DOI: 10.7270/Q2ZG6SZG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50087022 (8-Indan-2-yl-1-phenyl-1,3,8-triaza-spiro[4.5]decan...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Competitive binding affinity against transfected HEK293 cells expressing human Opioid receptor like 1 | J Med Chem 43: 1329-38 (2001) BindingDB Entry DOI: 10.7270/Q2ZG6SZG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM50169268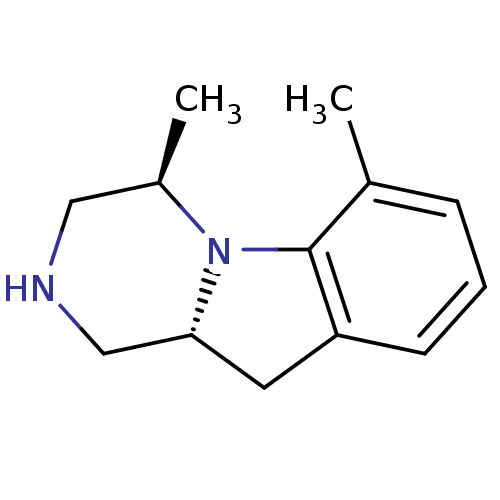 ((4R,10aR)-4,6-Dimethyl-1,2,3,4,10,10a-hexahydro-py...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis Research Ltd. Curated by ChEMBL | Assay Description Binding affinity toward 5-HT2C receptor evaluated by displacement of [3H]-5-HT radioligand | Bioorg Med Chem Lett 15: 3604-8 (2005) Article DOI: 10.1016/j.bmcl.2005.05.074 BindingDB Entry DOI: 10.7270/Q2GQ6X87 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50087004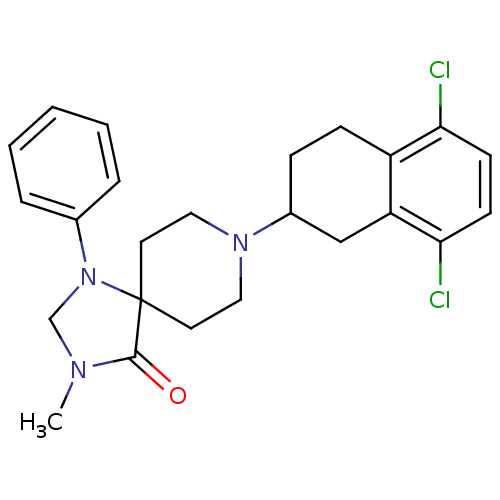 (8-(5,8-Dichloro-1,2,3,4-tetrahydro-naphthalen-2-yl...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Competitive binding displacement analyses was performed from permanently transfected HEK293 cells expressing human Opioid receptor mu 1 | J Med Chem 43: 1329-38 (2001) BindingDB Entry DOI: 10.7270/Q2ZG6SZG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50169264 ((4R,10aR)-7-Chloro-4,6-dimethyl-1,2,3,4,10,10a-hex...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis Research Ltd. Curated by ChEMBL | Assay Description Binding affinity toward 5-HT2A receptor evaluated by displacement of [125I]-DOI radioligand | Bioorg Med Chem Lett 15: 3604-8 (2005) Article DOI: 10.1016/j.bmcl.2005.05.074 BindingDB Entry DOI: 10.7270/Q2GQ6X87 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50087020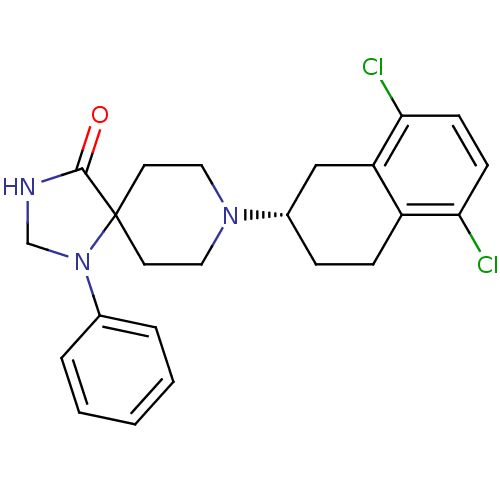 (8-(5,8-Dichloro-1,2,3,4-tetrahydro-naphthalen-2-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Effective concentration required to stimulate binding of GTPgammaS to Opioid receptor like 1 was determined using scintillation proximity assay | J Med Chem 43: 1329-38 (2001) BindingDB Entry DOI: 10.7270/Q2ZG6SZG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50087012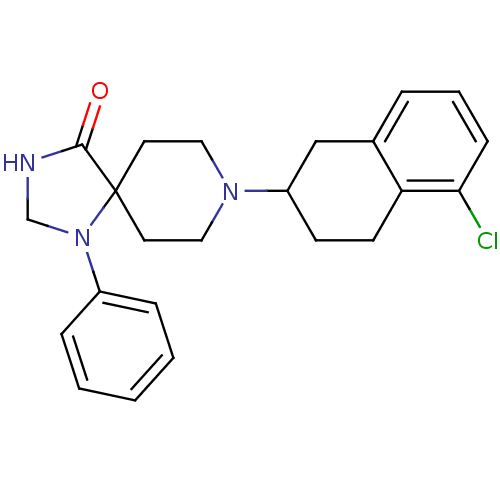 (8-(5-Chloro-1,2,3,4-tetrahydro-naphthalen-2-yl)-1-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Effective concentration required to stimulate binding of GTPgammaS to Opioid receptor like 1 was determined using scintillation proximity assay | J Med Chem 43: 1329-38 (2001) BindingDB Entry DOI: 10.7270/Q2ZG6SZG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Rattus norvegicus (rat)) | BDBM50087685 (8-Cyclododecyl-1-phenyl-1,3,8-triaza-spiro[4.5]dec...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Inhibition against binding of radioligand [N-allyl-2-3-3H]-naloxone to membrane of baby hamster kidney cells infected with forest virus encoding the... | Bioorg Med Chem Lett 10: 831-4 (2000) BindingDB Entry DOI: 10.7270/Q2N29XG2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50087691 (8-(4-Isopropyl-cyclohexyl)-1-phenyl-1,3,8-triaza-s...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Inhibition against binding of radioligand [N-allyl-2-3-3H]-naloxone to membrane of baby hamster kidney cells infected with forest virus encoding the... | Bioorg Med Chem Lett 10: 831-4 (2000) BindingDB Entry DOI: 10.7270/Q2N29XG2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50087687 (8-Cyclononyl-1-phenyl-1,3,8-triaza-spiro[4.5]decan...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Inhibition against binding of radioligand [N-allyl-2-3-3H]-naloxone to membrane of baby hamster kidney cells infected with forest virus encoding the... | Bioorg Med Chem Lett 10: 831-4 (2000) BindingDB Entry DOI: 10.7270/Q2N29XG2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2B (Homo sapiens (Human)) | BDBM50169264 ((4R,10aR)-7-Chloro-4,6-dimethyl-1,2,3,4,10,10a-hex...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis Research Ltd. Curated by ChEMBL | Assay Description Binding affinity toward 5-HT2B receptor evaluated by displacement of [3H]-5-HT radioligand | Bioorg Med Chem Lett 15: 3604-8 (2005) Article DOI: 10.1016/j.bmcl.2005.05.074 BindingDB Entry DOI: 10.7270/Q2GQ6X87 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (RAT) | BDBM50087683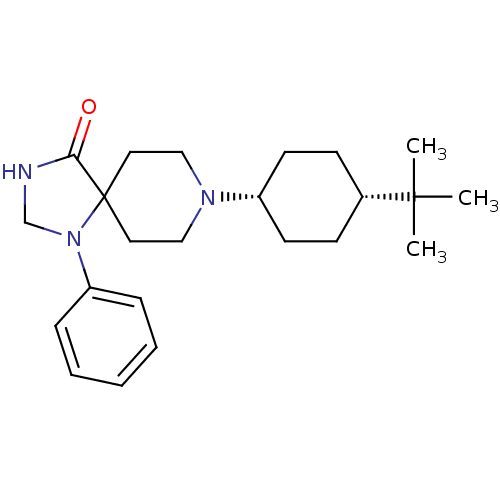 (8-(4-tert-Butyl-cyclohexyl)-1-phenyl-1,3,8-triaza-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Inhibition against binding of radioligand [leucyl-3H]-OFQ to membrane of human embryonic kidney 293 cells overexpressing rat Opioid receptor like 1 | Bioorg Med Chem Lett 10: 831-4 (2000) BindingDB Entry DOI: 10.7270/Q2N29XG2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50087004 (8-(5,8-Dichloro-1,2,3,4-tetrahydro-naphthalen-2-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Effective concentration required to stimulate binding of GTPgammaS to Opioid receptor like 1 was determined using scintillation proximity assay | J Med Chem 43: 1329-38 (2001) BindingDB Entry DOI: 10.7270/Q2ZG6SZG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50087003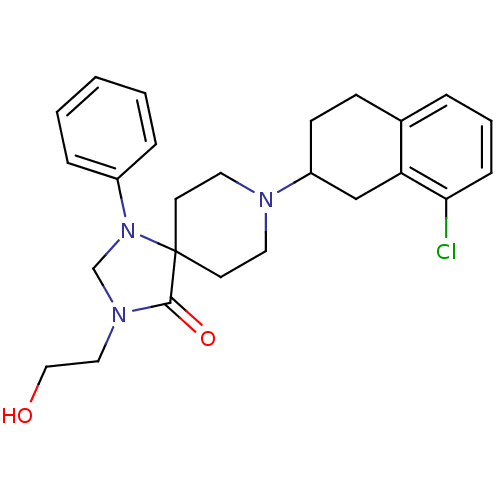 (8-(8-Chloro-1,2,3,4-tetrahydro-naphthalen-2-yl)-3-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Competitive binding displacement analyses was performed from permanently transfected HEK293 cells expressing human Opioid receptor mu 1 | J Med Chem 43: 1329-38 (2001) BindingDB Entry DOI: 10.7270/Q2ZG6SZG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50087008 (8-indan-1-yl-1-phenyl-1,3,8-triaza-spiro[4.5]decan...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Competitive binding displacement analyses was performed from permanently transfected HEK293 cells expressing Opioid receptor mu 1 | J Med Chem 43: 1329-38 (2001) BindingDB Entry DOI: 10.7270/Q2ZG6SZG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM50169258 ((4R,10aR)-4-Methyl-1,2,3,4,10,10a-hexahydro-pyrazi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis Research Ltd. Curated by ChEMBL | Assay Description Binding affinity toward 5-HT2C receptor evaluated by displacement of [3H]-5-HT radioligand | Bioorg Med Chem Lett 15: 3604-8 (2005) Article DOI: 10.1016/j.bmcl.2005.05.074 BindingDB Entry DOI: 10.7270/Q2GQ6X87 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Rattus norvegicus (rat)) | BDBM50087687 (8-Cyclononyl-1-phenyl-1,3,8-triaza-spiro[4.5]decan...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Inhibition against binding of radioligand [N-allyl-2-3-3H]-naloxone to membrane of baby hamster kidney cells infected with forest virus encoding the... | Bioorg Med Chem Lett 10: 831-4 (2000) BindingDB Entry DOI: 10.7270/Q2N29XG2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50087698 (8-(R)-Acenaphthen-1-yl-1-phenyl-1,3,8-triaza-spiro...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Inhibition against binding of radioligand [N-allyl-2-3-3H]-naloxone to membrane of baby hamster kidney cells infected with forest virus encoding the... | Bioorg Med Chem Lett 10: 831-4 (2000) BindingDB Entry DOI: 10.7270/Q2N29XG2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (RAT) | BDBM50087689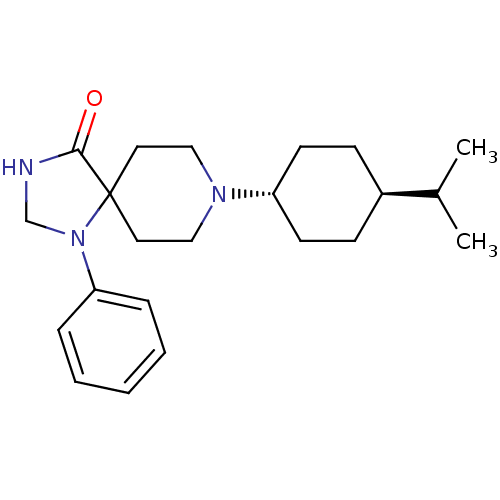 (8-(4-Isopropyl-cyclohexyl)-1-phenyl-1,3,8-triaza-s...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Inhibition against binding of radioligand [leucyl-3H]-OFQ to membrane of human embryonic kidney 293 cells overexpressing rat Opioid receptor like 1 | Bioorg Med Chem Lett 10: 831-4 (2000) BindingDB Entry DOI: 10.7270/Q2N29XG2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (RAT) | BDBM50087686 (8-Cycloheptyl-1-phenyl-1,3,8-triaza-spiro[4.5]deca...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Inhibition against binding of radioligand [leucyl-3H]-OFQ to membrane of human embryonic kidney 293 cells overexpressing rat Opioid receptor like 1 | Bioorg Med Chem Lett 10: 831-4 (2000) BindingDB Entry DOI: 10.7270/Q2N29XG2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kynurenine 3-monooxygenase (Rattus norvegicus) | BDBM50061916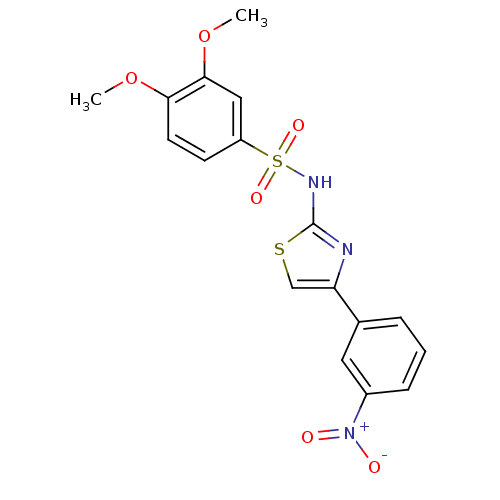 (3,4-Dimethoxy-N-[4-(3-nitro-phenyl)-thiazol-2-yl]-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Inhibitory constant of the compound against Kynurenine 3-hydroxylase | J Med Chem 40: 4378-85 (1998) Article DOI: 10.1021/jm970467t BindingDB Entry DOI: 10.7270/Q24B30DT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50087008 (8-indan-1-yl-1-phenyl-1,3,8-triaza-spiro[4.5]decan...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Competitive binding displacement analyses was performed from permanently transfected HEK293 cells expressing Opioid receptor kappa 1 | J Med Chem 43: 1329-38 (2001) BindingDB Entry DOI: 10.7270/Q2ZG6SZG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 403 total ) | Next | Last >> |