 Found 166 hits with Last Name = 'reszka' and Initial = 'ap'
Found 166 hits with Last Name = 'reszka' and Initial = 'ap' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50068324
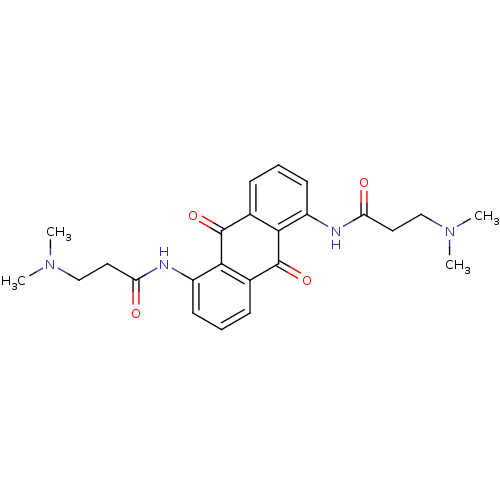
(3-Dimethylamino-N-[5-(3-dimethylamino-propionylami...)Show SMILES CN(C)CCC(=O)Nc1cccc2C(=O)c3c(NC(=O)CCN(C)C)cccc3C(=O)c12 Show InChI InChI=1S/C24H28N4O4/c1-27(2)13-11-19(29)25-17-9-5-7-15-21(17)23(31)16-8-6-10-18(22(16)24(15)32)26-20(30)12-14-28(3)4/h5-10H,11-14H2,1-4H3,(H,25,29)(H,26,30) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Inhibitory activity of telomerase was measured using the TRAP assay. |
J Med Chem 41: 4873-84 (1998)
Article DOI: 10.1021/jm981067o
BindingDB Entry DOI: 10.7270/Q2WW7GSD |
More data for this
Ligand-Target Pair | |
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50068304
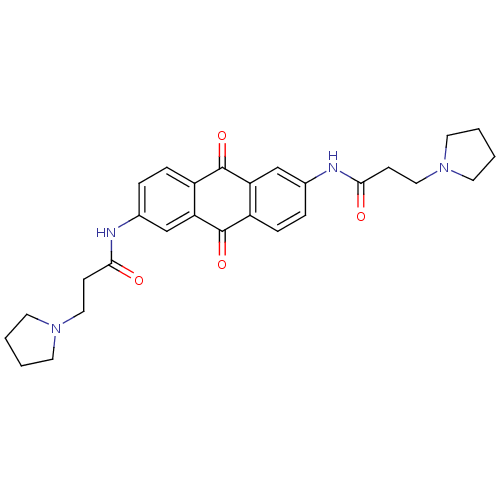
(CHEMBL343445 | N-[9,10-Dioxo-6-(3-pyrrolidin-1-yl-...)Show SMILES O=C(CCN1CCCC1)Nc1ccc2C(=O)c3cc(NC(=O)CCN4CCCC4)ccc3C(=O)c2c1 Show InChI InChI=1S/C28H32N4O4/c33-25(9-15-31-11-1-2-12-31)29-19-5-7-21-23(17-19)27(35)22-8-6-20(18-24(22)28(21)36)30-26(34)10-16-32-13-3-4-14-32/h5-8,17-18H,1-4,9-16H2,(H,29,33)(H,30,34) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Inhibitory activity of telomerase was measured using the TRAP assay. |
J Med Chem 41: 4873-84 (1998)
Article DOI: 10.1021/jm981067o
BindingDB Entry DOI: 10.7270/Q2WW7GSD |
More data for this
Ligand-Target Pair | |
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50068320

(2,7-Bis[3-(pyrrolidino)propionamido]anthraquinone ...)Show SMILES O=C(CCN1CCCC1)Nc1ccc2C(=O)c3ccc(NC(=O)CCN4CCCC4)cc3C(=O)c2c1 Show InChI InChI=1S/C28H32N4O4/c33-25(9-15-31-11-1-2-12-31)29-19-5-7-21-23(17-19)28(36)24-18-20(6-8-22(24)27(21)35)30-26(34)10-16-32-13-3-4-14-32/h5-8,17-18H,1-4,9-16H2,(H,29,33)(H,30,34) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Inhibitory activity of telomerase was measured using the TRAP assay. |
J Med Chem 41: 4873-84 (1998)
Article DOI: 10.1021/jm981067o
BindingDB Entry DOI: 10.7270/Q2WW7GSD |
More data for this
Ligand-Target Pair | |
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50068330

(CHEMBL144848 | N-[9,10-Dioxo-5-(3-piperidin-1-yl-p...)Show SMILES O=C(CCN1CCCCC1)Nc1cccc2C(=O)c3c(NC(=O)CCN4CCCCC4)cccc3C(=O)c12 Show InChI InChI=1S/C30H36N4O4/c35-25(13-19-33-15-3-1-4-16-33)31-23-11-7-9-21-27(23)29(37)22-10-8-12-24(28(22)30(21)38)32-26(36)14-20-34-17-5-2-6-18-34/h7-12H,1-6,13-20H2,(H,31,35)(H,32,36) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Inhibitory activity of telomerase was measured using the TRAP assay. |
J Med Chem 41: 4873-84 (1998)
Article DOI: 10.1021/jm981067o
BindingDB Entry DOI: 10.7270/Q2WW7GSD |
More data for this
Ligand-Target Pair | |
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50068303
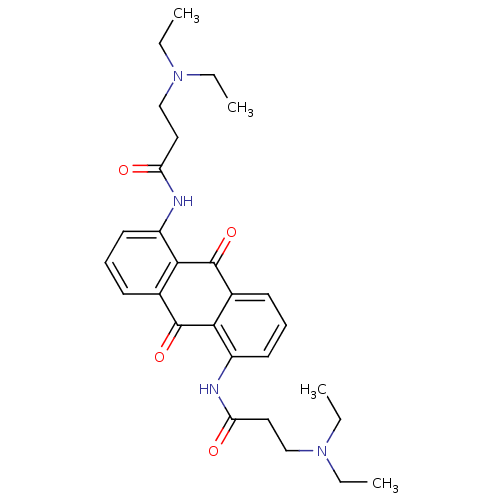
(1,5-BIS[3-(DIETHYLAMINO)PROPIONAMIDO]ANTHRACENE-9,...)Show SMILES CCN(CC)CCC(=O)Nc1cccc2C(=O)c3c(NC(=O)CCN(CC)CC)cccc3C(=O)c12 Show InChI InChI=1S/C28H36N4O4/c1-5-31(6-2)17-15-23(33)29-21-13-9-11-19-25(21)27(35)20-12-10-14-22(26(20)28(19)36)30-24(34)16-18-32(7-3)8-4/h9-14H,5-8,15-18H2,1-4H3,(H,29,33)(H,30,34) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Inhibitory activity of telomerase was measured using the TRAP assay. |
J Med Chem 41: 4873-84 (1998)
Article DOI: 10.1021/jm981067o
BindingDB Entry DOI: 10.7270/Q2WW7GSD |
More data for this
Ligand-Target Pair | |
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50068321

(2,7-Bis[3-(piperidino)propionamido]anthraquinone |...)Show SMILES O=C(CCN1CCCCC1)Nc1ccc2C(=O)c3ccc(NC(=O)CCN4CCCCC4)cc3C(=O)c2c1 Show InChI InChI=1S/C30H36N4O4/c35-27(11-17-33-13-3-1-4-14-33)31-21-7-9-23-25(19-21)30(38)26-20-22(8-10-24(26)29(23)37)32-28(36)12-18-34-15-5-2-6-16-34/h7-10,19-20H,1-6,11-18H2,(H,31,35)(H,32,36) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Inhibitory activity of telomerase was measured using the TRAP assay. |
J Med Chem 41: 4873-84 (1998)
Article DOI: 10.1021/jm981067o
BindingDB Entry DOI: 10.7270/Q2WW7GSD |
More data for this
Ligand-Target Pair | |
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50068321

(2,7-Bis[3-(piperidino)propionamido]anthraquinone |...)Show SMILES O=C(CCN1CCCCC1)Nc1ccc2C(=O)c3ccc(NC(=O)CCN4CCCCC4)cc3C(=O)c2c1 Show InChI InChI=1S/C30H36N4O4/c35-27(11-17-33-13-3-1-4-14-33)31-21-7-9-23-25(19-21)30(38)26-20-22(8-10-24(26)29(23)37)32-28(36)12-18-34-15-5-2-6-16-34/h7-10,19-20H,1-6,11-18H2,(H,31,35)(H,32,36) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against telomerase |
J Med Chem 42: 2679-84 (1999)
Article DOI: 10.1021/jm990084q
BindingDB Entry DOI: 10.7270/Q2DF6RX9 |
More data for this
Ligand-Target Pair | |
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50068328
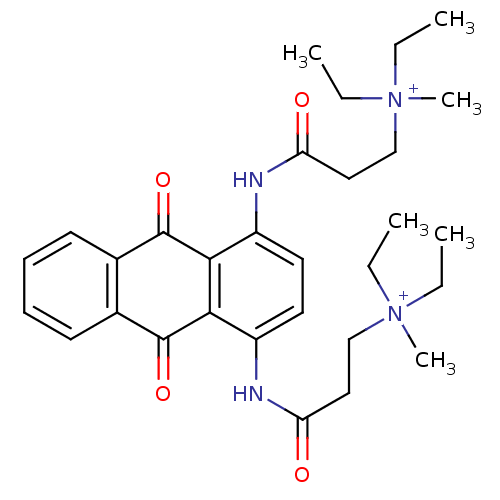
(CHEMBL144303 | N-[9,10-Dioxo-4-(3-(N,N-diethyl-N-m...)Show SMILES CC[N+](C)(CC)CCC(=O)Nc1ccc(NC(=O)CC[N+](C)(CC)CC)c2C(=O)c3ccccc3C(=O)c12 Show InChI InChI=1S/C30H40N4O4/c1-7-33(5,8-2)19-17-25(35)31-23-15-16-24(32-26(36)18-20-34(6,9-3)10-4)28-27(23)29(37)21-13-11-12-14-22(21)30(28)38/h11-16H,7-10,17-20H2,1-6H3/p+2 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Inhibitory activity of telomerase was measured using the TRAP assay. |
J Med Chem 41: 4873-84 (1998)
Article DOI: 10.1021/jm981067o
BindingDB Entry DOI: 10.7270/Q2WW7GSD |
More data for this
Ligand-Target Pair | |
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50005750
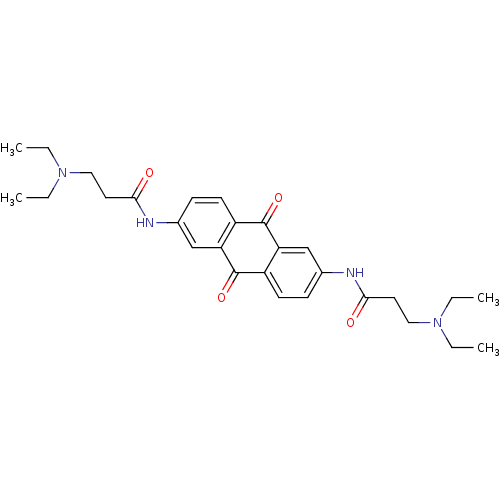
(3-Diethylamino-N-[6-(3-diethylamino-propionylamino...)Show SMILES CCN(CC)CCC(=O)Nc1ccc2C(=O)c3cc(NC(=O)CCN(CC)CC)ccc3C(=O)c2c1 Show InChI InChI=1S/C28H36N4O4/c1-5-31(6-2)15-13-25(33)29-19-9-11-21-23(17-19)27(35)22-12-10-20(18-24(22)28(21)36)30-26(34)14-16-32(7-3)8-4/h9-12,17-18H,5-8,13-16H2,1-4H3,(H,29,33)(H,30,34) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Inhibitory activity of telomerase was measured using the TRAP assay. |
J Med Chem 41: 4873-84 (1998)
Article DOI: 10.1021/jm981067o
BindingDB Entry DOI: 10.7270/Q2WW7GSD |
More data for this
Ligand-Target Pair | |
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50068332
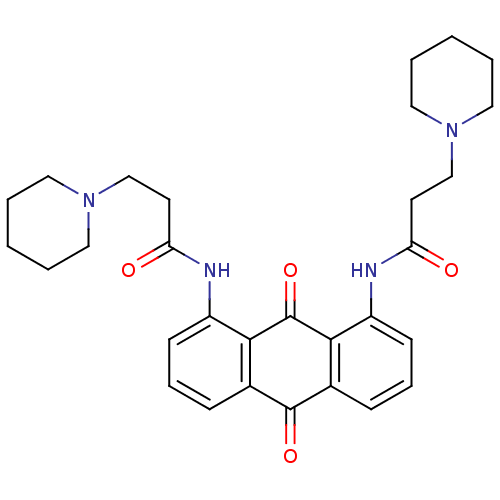
(CHEMBL144386 | N-[9,10-Dioxo-8-(3-piperidin-1-yl-p...)Show SMILES O=C(CCN1CCCCC1)Nc1cccc2C(=O)c3cccc(NC(=O)CCN4CCCCC4)c3C(=O)c12 Show InChI InChI=1S/C30H36N4O4/c35-25(13-19-33-15-3-1-4-16-33)31-23-11-7-9-21-27(23)30(38)28-22(29(21)37)10-8-12-24(28)32-26(36)14-20-34-17-5-2-6-18-34/h7-12H,1-6,13-20H2,(H,31,35)(H,32,36) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Inhibitory activity of telomerase was measured using the TRAP assay. |
J Med Chem 41: 4873-84 (1998)
Article DOI: 10.1021/jm981067o
BindingDB Entry DOI: 10.7270/Q2WW7GSD |
More data for this
Ligand-Target Pair | |
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50068311
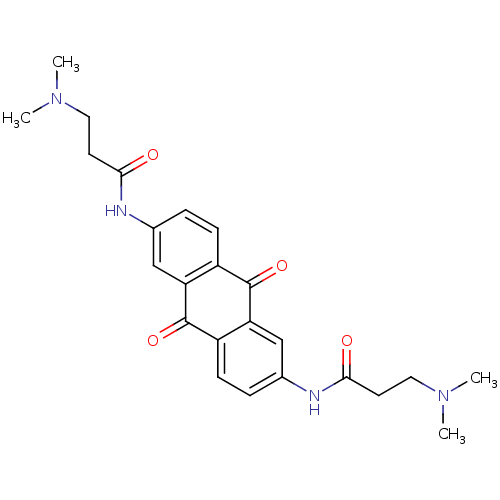
(3-Dimethylamino-N-[6-(3-dimethylamino-propionylami...)Show SMILES CN(C)CCC(=O)Nc1ccc2C(=O)c3cc(NC(=O)CCN(C)C)ccc3C(=O)c2c1 Show InChI InChI=1S/C24H28N4O4/c1-27(2)11-9-21(29)25-15-5-7-17-19(13-15)23(31)18-8-6-16(14-20(18)24(17)32)26-22(30)10-12-28(3)4/h5-8,13-14H,9-12H2,1-4H3,(H,25,29)(H,26,30) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Inhibitory activity of telomerase was measured using the TRAP assay. |
J Med Chem 41: 4873-84 (1998)
Article DOI: 10.1021/jm981067o
BindingDB Entry DOI: 10.7270/Q2WW7GSD |
More data for this
Ligand-Target Pair | |
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50068315

(3-Diethylamino-N-[8-(3-diethylamino-propionylamino...)Show SMILES CCN(CC)CCC(=O)Nc1cccc2C(=O)c3cccc(NC(=O)CCN(CC)CC)c3C(=O)c12 Show InChI InChI=1S/C28H36N4O4/c1-5-31(6-2)17-15-23(33)29-21-13-9-11-19-25(21)28(36)26-20(27(19)35)12-10-14-22(26)30-24(34)16-18-32(7-3)8-4/h9-14H,5-8,15-18H2,1-4H3,(H,29,33)(H,30,34) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Inhibitory activity of telomerase was measured using the TRAP assay. |
J Med Chem 41: 4873-84 (1998)
Article DOI: 10.1021/jm981067o
BindingDB Entry DOI: 10.7270/Q2WW7GSD |
More data for this
Ligand-Target Pair | |
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50068312

(2,7-Bis[3-(diethylamino)propionamido]anthraquinone...)Show SMILES CCN(CC)CCC(=O)Nc1ccc2C(=O)c3ccc(NC(=O)CCN(CC)CC)cc3C(=O)c2c1 Show InChI InChI=1S/C28H36N4O4/c1-5-31(6-2)15-13-25(33)29-19-9-11-21-23(17-19)28(36)24-18-20(10-12-22(24)27(21)35)30-26(34)14-16-32(7-3)8-4/h9-12,17-18H,5-8,13-16H2,1-4H3,(H,29,33)(H,30,34) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against telomerase |
J Med Chem 42: 2679-84 (1999)
Article DOI: 10.1021/jm990084q
BindingDB Entry DOI: 10.7270/Q2DF6RX9 |
More data for this
Ligand-Target Pair | |
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50068312

(2,7-Bis[3-(diethylamino)propionamido]anthraquinone...)Show SMILES CCN(CC)CCC(=O)Nc1ccc2C(=O)c3ccc(NC(=O)CCN(CC)CC)cc3C(=O)c2c1 Show InChI InChI=1S/C28H36N4O4/c1-5-31(6-2)15-13-25(33)29-19-9-11-21-23(17-19)28(36)24-18-20(10-12-22(24)27(21)35)30-26(34)14-16-32(7-3)8-4/h9-12,17-18H,5-8,13-16H2,1-4H3,(H,29,33)(H,30,34) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Inhibitory activity of telomerase was measured using the TRAP assay. |
J Med Chem 41: 4873-84 (1998)
Article DOI: 10.1021/jm981067o
BindingDB Entry DOI: 10.7270/Q2WW7GSD |
More data for this
Ligand-Target Pair | |
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50068335
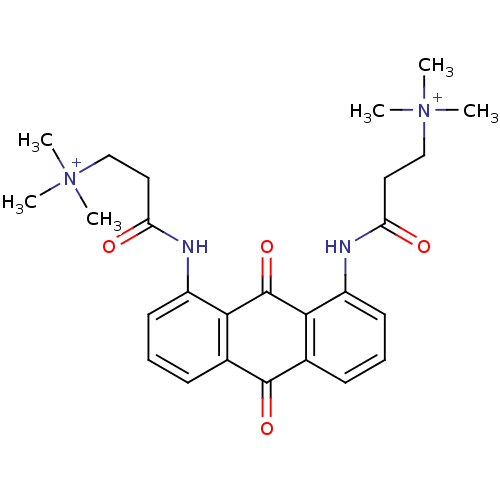
(CHEMBL422120 | N-[9,10-Dioxo-8-(3-(N,N,N-trimethyl...)Show SMILES C[N+](C)(C)CCC(=O)Nc1cccc2C(=O)c3cccc(NC(=O)CC[N+](C)(C)C)c3C(=O)c12 Show InChI InChI=1S/C26H32N4O4/c1-29(2,3)15-13-21(31)27-19-11-7-9-17-23(19)26(34)24-18(25(17)33)10-8-12-20(24)28-22(32)14-16-30(4,5)6/h7-12H,13-16H2,1-6H3/p+2 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Inhibitory activity of telomerase was measured using the TRAP assay. |
J Med Chem 41: 4873-84 (1998)
Article DOI: 10.1021/jm981067o
BindingDB Entry DOI: 10.7270/Q2WW7GSD |
More data for this
Ligand-Target Pair | |
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50005746
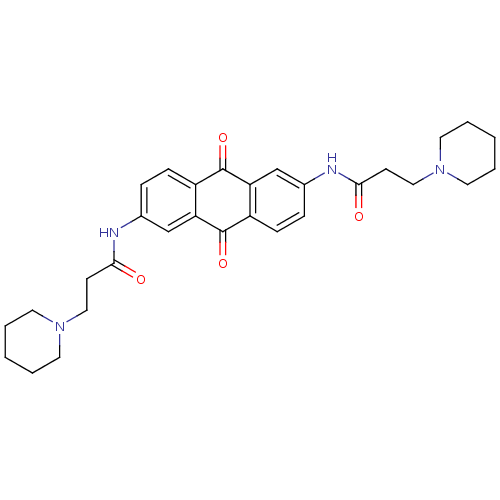
(CHEMBL109382 | CHEMBL33618 | N-[9,10-Dioxo-6-(3-pi...)Show SMILES O=C(CCN1CCCCC1)Nc1ccc2C(=O)c3cc(NC(=O)CCN4CCCCC4)ccc3C(=O)c2c1 Show InChI InChI=1S/C30H36N4O4/c35-27(11-17-33-13-3-1-4-14-33)31-21-7-9-23-25(19-21)29(37)24-10-8-22(20-26(24)30(23)38)32-28(36)12-18-34-15-5-2-6-16-34/h7-10,19-20H,1-6,11-18H2,(H,31,35)(H,32,36) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Inhibitory activity of telomerase was measured using the TRAP assay. |
J Med Chem 41: 4873-84 (1998)
Article DOI: 10.1021/jm981067o
BindingDB Entry DOI: 10.7270/Q2WW7GSD |
More data for this
Ligand-Target Pair | |
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50005746

(CHEMBL109382 | CHEMBL33618 | N-[9,10-Dioxo-6-(3-pi...)Show SMILES O=C(CCN1CCCCC1)Nc1ccc2C(=O)c3cc(NC(=O)CCN4CCCCC4)ccc3C(=O)c2c1 Show InChI InChI=1S/C30H36N4O4/c35-27(11-17-33-13-3-1-4-14-33)31-21-7-9-23-25(19-21)29(37)24-10-8-22(20-26(24)30(23)38)32-28(36)12-18-34-15-5-2-6-16-34/h7-10,19-20H,1-6,11-18H2,(H,31,35)(H,32,36) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Inhibition of telomerase activity |
J Med Chem 41: 3253-60 (1998)
Article DOI: 10.1021/jm9801105
BindingDB Entry DOI: 10.7270/Q2TX3G1R |
More data for this
Ligand-Target Pair | |
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50068336
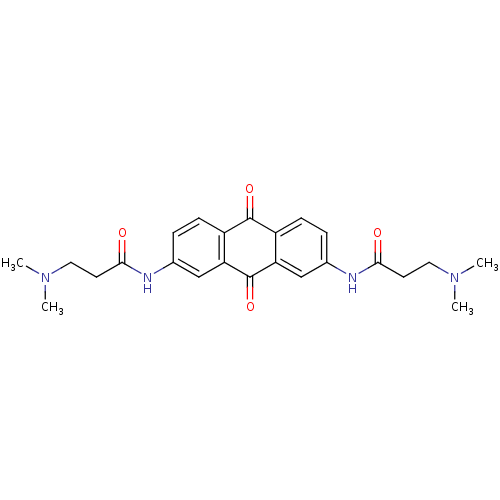
(2,7-Bis[3-(dimethylamino)propionamido]anthraquinon...)Show SMILES CN(C)CCC(=O)Nc1ccc2C(=O)c3ccc(NC(=O)CCN(C)C)cc3C(=O)c2c1 Show InChI InChI=1S/C24H28N4O4/c1-27(2)11-9-21(29)25-15-5-7-17-19(13-15)24(32)20-14-16(6-8-18(20)23(17)31)26-22(30)10-12-28(3)4/h5-8,13-14H,9-12H2,1-4H3,(H,25,29)(H,26,30) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Inhibitory activity of telomerase was measured using the TRAP assay. |
J Med Chem 41: 4873-84 (1998)
Article DOI: 10.1021/jm981067o
BindingDB Entry DOI: 10.7270/Q2WW7GSD |
More data for this
Ligand-Target Pair | |
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50068336

(2,7-Bis[3-(dimethylamino)propionamido]anthraquinon...)Show SMILES CN(C)CCC(=O)Nc1ccc2C(=O)c3ccc(NC(=O)CCN(C)C)cc3C(=O)c2c1 Show InChI InChI=1S/C24H28N4O4/c1-27(2)11-9-21(29)25-15-5-7-17-19(13-15)24(32)20-14-16(6-8-18(20)23(17)31)26-22(30)10-12-28(3)4/h5-8,13-14H,9-12H2,1-4H3,(H,25,29)(H,26,30) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against telomerase |
J Med Chem 42: 2679-84 (1999)
Article DOI: 10.1021/jm990084q
BindingDB Entry DOI: 10.7270/Q2DF6RX9 |
More data for this
Ligand-Target Pair | |
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50068313
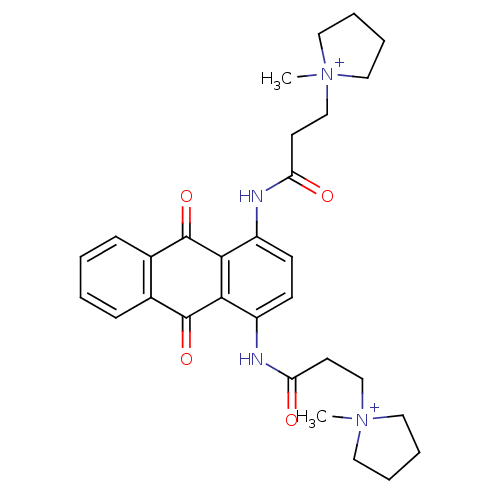
(CHEMBL143452 | N-[9,10-Dioxo-4-(3-(N-methylpyrroli...)Show SMILES C[N+]1(CCC(=O)Nc2ccc(NC(=O)CC[N+]3(C)CCCC3)c3C(=O)c4ccccc4C(=O)c23)CCCC1 Show InChI InChI=1S/C30H36N4O4/c1-33(15-5-6-16-33)19-13-25(35)31-23-11-12-24(32-26(36)14-20-34(2)17-7-8-18-34)28-27(23)29(37)21-9-3-4-10-22(21)30(28)38/h3-4,9-12H,5-8,13-20H2,1-2H3/p+2 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Inhibitory activity of telomerase was measured using the TRAP assay. |
J Med Chem 41: 4873-84 (1998)
Article DOI: 10.1021/jm981067o
BindingDB Entry DOI: 10.7270/Q2WW7GSD |
More data for this
Ligand-Target Pair | |
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50068323
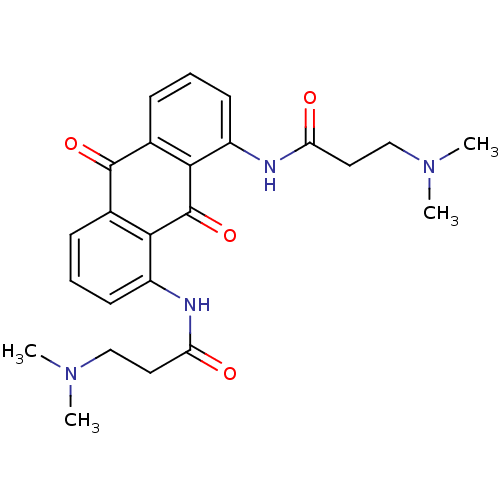
(3-Dimethylamino-N-[8-(3-dimethylamino-propionylami...)Show SMILES CN(C)CCC(=O)Nc1cccc2C(=O)c3cccc(NC(=O)CCN(C)C)c3C(=O)c12 Show InChI InChI=1S/C24H28N4O4/c1-27(2)13-11-19(29)25-17-9-5-7-15-21(17)24(32)22-16(23(15)31)8-6-10-18(22)26-20(30)12-14-28(3)4/h5-10H,11-14H2,1-4H3,(H,25,29)(H,26,30) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Inhibitory activity of telomerase was measured using the TRAP assay. |
J Med Chem 41: 4873-84 (1998)
Article DOI: 10.1021/jm981067o
BindingDB Entry DOI: 10.7270/Q2WW7GSD |
More data for this
Ligand-Target Pair | |
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50066349
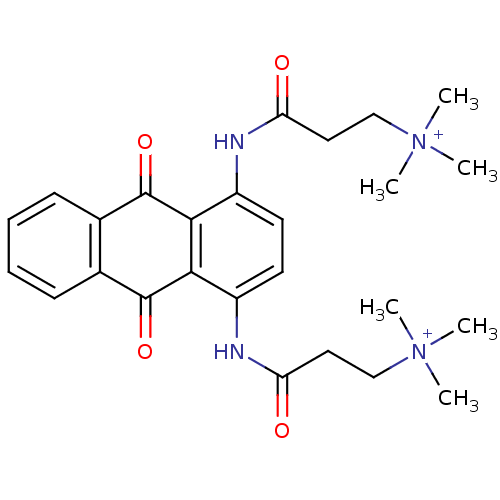
(3-Trimethylammonium-N-[4-(3-dimethylammonium-propi...)Show SMILES C[N+](C)(C)CCC(=O)Nc1ccc(NC(=O)CC[N+](C)(C)C)c2C(=O)c3ccccc3C(=O)c12 Show InChI InChI=1S/C26H32N4O4/c1-29(2,3)15-13-21(31)27-19-11-12-20(28-22(32)14-16-30(4,5)6)24-23(19)25(33)17-9-7-8-10-18(17)26(24)34/h7-12H,13-16H2,1-6H3/p+2 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Inhibitory activity of telomerase was measured using the TRAP assay. |
J Med Chem 41: 4873-84 (1998)
Article DOI: 10.1021/jm981067o
BindingDB Entry DOI: 10.7270/Q2WW7GSD |
More data for this
Ligand-Target Pair | |
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50068318
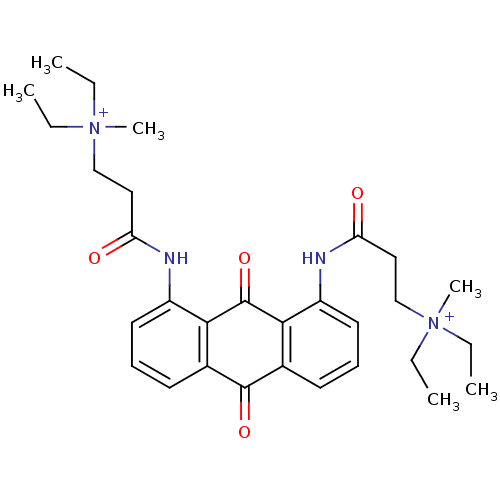
(CHEMBL145311 | N-[9,10-Dioxo-8-(3-(N,N-diethyl-N-m...)Show SMILES CC[N+](C)(CC)CCC(=O)Nc1cccc2C(=O)c3cccc(NC(=O)CC[N+](C)(CC)CC)c3C(=O)c12 Show InChI InChI=1S/C30H40N4O4/c1-7-33(5,8-2)19-17-25(35)31-23-15-11-13-21-27(23)30(38)28-22(29(21)37)14-12-16-24(28)32-26(36)18-20-34(6,9-3)10-4/h11-16H,7-10,17-20H2,1-6H3/p+2 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Inhibitory activity of telomerase was measured using the TRAP assay. |
J Med Chem 41: 4873-84 (1998)
Article DOI: 10.1021/jm981067o
BindingDB Entry DOI: 10.7270/Q2WW7GSD |
More data for this
Ligand-Target Pair | |
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50068337

(CHEMBL343238 | N-[9,10-Dioxo-8-(3-(N-methylpiperid...)Show SMILES C[N+]1(CCC(=O)Nc2cccc3C(=O)c4cccc(NC(=O)CC[N+]5(C)CCCCC5)c4C(=O)c23)CCCCC1 Show InChI InChI=1S/C32H40N4O4/c1-35(17-5-3-6-18-35)21-15-27(37)33-25-13-9-11-23-29(25)32(40)30-24(31(23)39)12-10-14-26(30)34-28(38)16-22-36(2)19-7-4-8-20-36/h9-14H,3-8,15-22H2,1-2H3/p+2 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Inhibitory activity of telomerase was measured using the TRAP assay. |
J Med Chem 41: 4873-84 (1998)
Article DOI: 10.1021/jm981067o
BindingDB Entry DOI: 10.7270/Q2WW7GSD |
More data for this
Ligand-Target Pair | |
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50068308
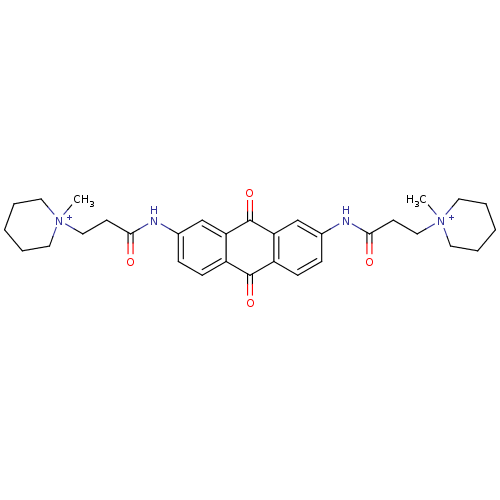
(CHEMBL14832 | CHEMBL90901 | N-[9,10-Dioxo-7-(3-(N-...)Show SMILES C[N+]1(CCC(=O)Nc2ccc3C(=O)c4ccc(NC(=O)CC[N+]5(C)CCCCC5)cc4C(=O)c3c2)CCCCC1 Show InChI InChI=1S/C32H40N4O4/c1-35(15-5-3-6-16-35)19-13-29(37)33-23-9-11-25-27(21-23)32(40)28-22-24(10-12-26(28)31(25)39)34-30(38)14-20-36(2)17-7-4-8-18-36/h9-12,21-22H,3-8,13-20H2,1-2H3/p+2 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Inhibitory activity of telomerase was measured using the TRAP assay. |
J Med Chem 41: 4873-84 (1998)
Article DOI: 10.1021/jm981067o
BindingDB Entry DOI: 10.7270/Q2WW7GSD |
More data for this
Ligand-Target Pair | |
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50068308

(CHEMBL14832 | CHEMBL90901 | N-[9,10-Dioxo-7-(3-(N-...)Show SMILES C[N+]1(CCC(=O)Nc2ccc3C(=O)c4ccc(NC(=O)CC[N+]5(C)CCCCC5)cc4C(=O)c3c2)CCCCC1 Show InChI InChI=1S/C32H40N4O4/c1-35(15-5-3-6-16-35)19-13-29(37)33-23-9-11-25-27(21-23)32(40)28-22-24(10-12-26(28)31(25)39)34-30(38)14-20-36(2)17-7-4-8-18-36/h9-12,21-22H,3-8,13-20H2,1-2H3/p+2 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against telomerase |
J Med Chem 42: 2679-84 (1999)
Article DOI: 10.1021/jm990084q
BindingDB Entry DOI: 10.7270/Q2DF6RX9 |
More data for this
Ligand-Target Pair | |
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50079139
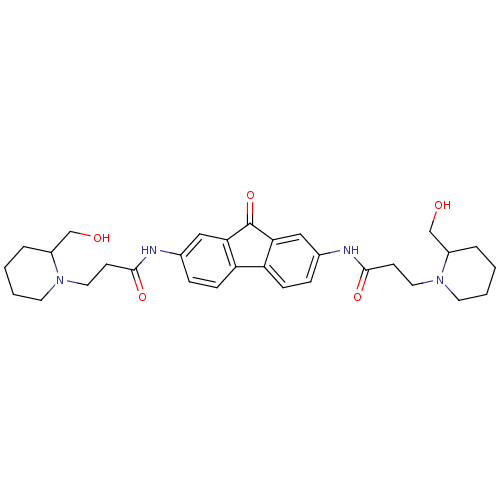
(3-(2-Hydroxymethyl-piperidin-1-yl)-N-{7-[3-(2-hydr...)Show SMILES OCC1CCCCN1CCC(=O)Nc1ccc2-c3ccc(NC(=O)CCN4CCCCC4CO)cc3C(=O)c2c1 Show InChI InChI=1S/C31H40N4O5/c36-19-23-5-1-3-13-34(23)15-11-29(38)32-21-7-9-25-26-10-8-22(18-28(26)31(40)27(25)17-21)33-30(39)12-16-35-14-4-2-6-24(35)20-37/h7-10,17-18,23-24,36-37H,1-6,11-16,19-20H2,(H,32,38)(H,33,39) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against telomerase |
J Med Chem 42: 2679-84 (1999)
Article DOI: 10.1021/jm990084q
BindingDB Entry DOI: 10.7270/Q2DF6RX9 |
More data for this
Ligand-Target Pair | |
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50068322
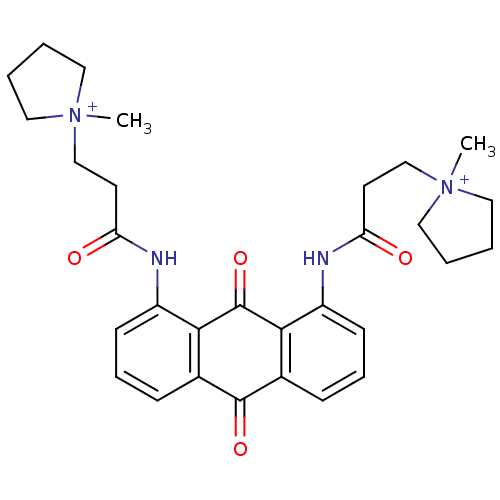
(CHEMBL144219 | N-[9,10-Dioxo-8-(3-(N-methylpyrroli...)Show SMILES C[N+]1(CCC(=O)Nc2cccc3C(=O)c4cccc(NC(=O)CC[N+]5(C)CCCC5)c4C(=O)c23)CCCC1 Show InChI InChI=1S/C30H36N4O4/c1-33(15-3-4-16-33)19-13-25(35)31-23-11-7-9-21-27(23)30(38)28-22(29(21)37)10-8-12-24(28)32-26(36)14-20-34(2)17-5-6-18-34/h7-12H,3-6,13-20H2,1-2H3/p+2 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Inhibitory activity of telomerase was measured using the TRAP assay. |
J Med Chem 41: 4873-84 (1998)
Article DOI: 10.1021/jm981067o
BindingDB Entry DOI: 10.7270/Q2WW7GSD |
More data for this
Ligand-Target Pair | |
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50369390
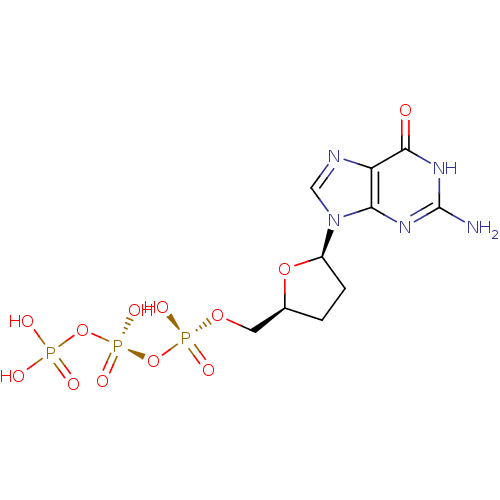
(CHEMBL54224)Show SMILES Nc1nc2n(cnc2c(=O)[nH]1)[C@H]1CC[C@@H](CO[P@](O)(=O)O[P@@](O)(=O)OP(O)(O)=O)O1 |r| Show InChI InChI=1S/C10H16N5O12P3/c11-10-13-8-7(9(16)14-10)12-4-15(8)6-2-1-5(25-6)3-24-29(20,21)27-30(22,23)26-28(17,18)19/h4-6H,1-3H2,(H,20,21)(H,22,23)(H2,17,18,19)(H3,11,13,14,16)/t5-,6+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Concentration required to inhibit telomerase activity |
J Med Chem 41: 3253-60 (1998)
Article DOI: 10.1021/jm9801105
BindingDB Entry DOI: 10.7270/Q2TX3G1R |
More data for this
Ligand-Target Pair | |
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50068305
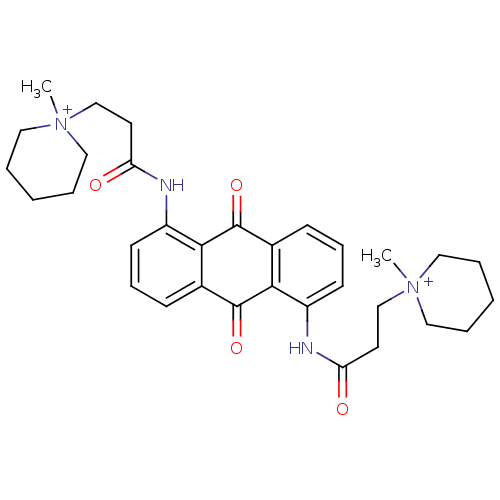
(CHEMBL144334 | N-[9,10-Dioxo-5-(3-(N-methylpiperid...)Show SMILES C[N+]1(CCC(=O)Nc2cccc3C(=O)c4c(NC(=O)CC[N+]5(C)CCCCC5)cccc4C(=O)c23)CCCCC1 Show InChI InChI=1S/C32H40N4O4/c1-35(17-5-3-6-18-35)21-15-27(37)33-25-13-9-11-23-29(25)31(39)24-12-10-14-26(30(24)32(23)40)34-28(38)16-22-36(2)19-7-4-8-20-36/h9-14H,3-8,15-22H2,1-2H3/p+2 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Inhibitory activity of telomerase was measured using the TRAP assay. |
J Med Chem 41: 4873-84 (1998)
Article DOI: 10.1021/jm981067o
BindingDB Entry DOI: 10.7270/Q2WW7GSD |
More data for this
Ligand-Target Pair | |
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50068310
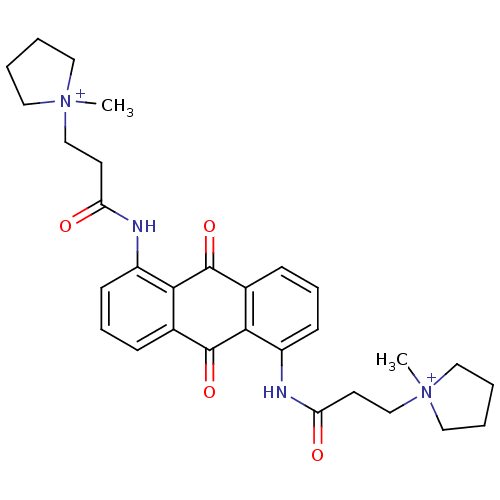
(CHEMBL344072 | N-[9,10-Dioxo-5-(3-(N-methylpyrroli...)Show SMILES C[N+]1(CCC(=O)Nc2cccc3C(=O)c4c(NC(=O)CC[N+]5(C)CCCC5)cccc4C(=O)c23)CCCC1 Show InChI InChI=1S/C30H36N4O4/c1-33(15-3-4-16-33)19-13-25(35)31-23-11-7-9-21-27(23)29(37)22-10-8-12-24(28(22)30(21)38)32-26(36)14-20-34(2)17-5-6-18-34/h7-12H,3-6,13-20H2,1-2H3/p+2 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Inhibitory activity of telomerase was measured using the TRAP assay. |
J Med Chem 41: 4873-84 (1998)
Article DOI: 10.1021/jm981067o
BindingDB Entry DOI: 10.7270/Q2WW7GSD |
More data for this
Ligand-Target Pair | |
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50079130
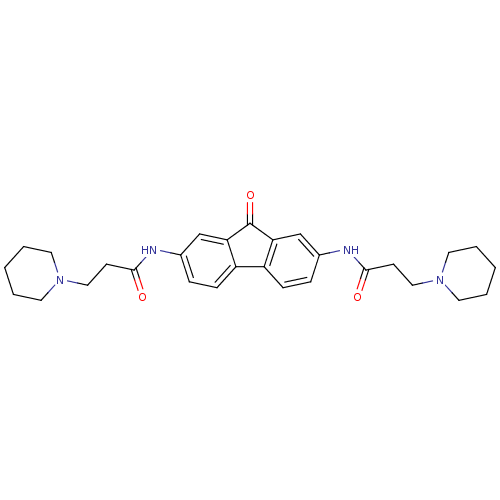
(CHEMBL91935 | N-[9-Oxo-7-(3-piperidin-1-yl-propion...)Show SMILES O=C(CCN1CCCCC1)Nc1ccc2-c3ccc(NC(=O)CCN4CCCCC4)cc3C(=O)c2c1 Show InChI InChI=1S/C29H36N4O3/c34-27(11-17-32-13-3-1-4-14-32)30-21-7-9-23-24-10-8-22(20-26(24)29(36)25(23)19-21)31-28(35)12-18-33-15-5-2-6-16-33/h7-10,19-20H,1-6,11-18H2,(H,30,34)(H,31,35) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against telomerase |
J Med Chem 42: 2679-84 (1999)
Article DOI: 10.1021/jm990084q
BindingDB Entry DOI: 10.7270/Q2DF6RX9 |
More data for this
Ligand-Target Pair | |
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50066353
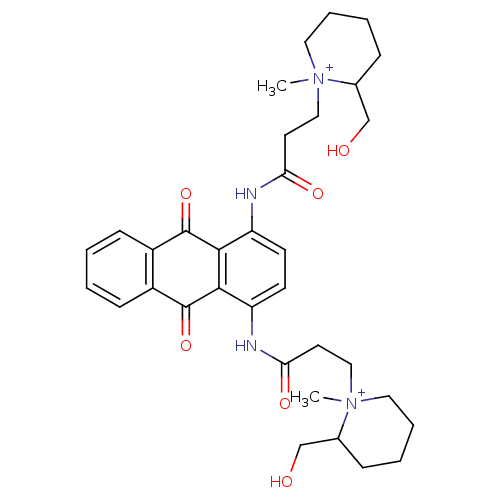
(3-(2-Hydroxymethyl-1-methyl-piperidinium-1-yl)-N-{...)Show SMILES C[N+]1(CCC(=O)Nc2ccc(NC(=O)CC[N+]3(C)CCCCC3CO)c3C(=O)c4ccccc4C(=O)c23)CCCCC1CO Show InChI InChI=1S/C34H44N4O6/c1-37(17-7-5-9-23(37)21-39)19-15-29(41)35-27-13-14-28(32-31(27)33(43)25-11-3-4-12-26(25)34(32)44)36-30(42)16-20-38(2)18-8-6-10-24(38)22-40/h3-4,11-14,23-24,39-40H,5-10,15-22H2,1-2H3/p+2 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Inhibition of telomerase activity |
J Med Chem 41: 3253-60 (1998)
Article DOI: 10.1021/jm9801105
BindingDB Entry DOI: 10.7270/Q2TX3G1R |
More data for this
Ligand-Target Pair | |
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50068329
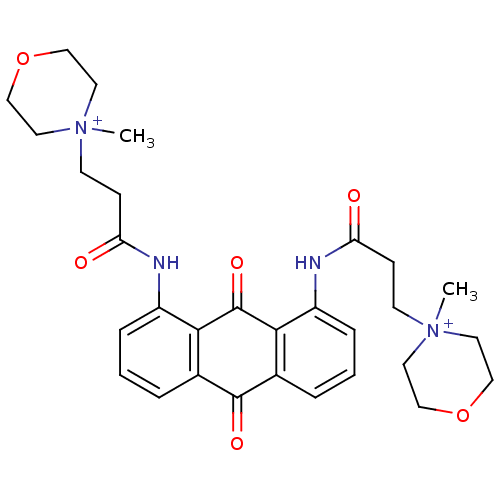
(CHEMBL144617 | N-[9,10-Dioxo-8-(3-(N-methylmorphol...)Show SMILES C[N+]1(CCC(=O)Nc2cccc3C(=O)c4cccc(NC(=O)CC[N+]5(C)CCOCC5)c4C(=O)c23)CCOCC1 Show InChI InChI=1S/C30H36N4O6/c1-33(13-17-39-18-14-33)11-9-25(35)31-23-7-3-5-21-27(23)30(38)28-22(29(21)37)6-4-8-24(28)32-26(36)10-12-34(2)15-19-40-20-16-34/h3-8H,9-20H2,1-2H3/p+2 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Inhibitory activity of telomerase was measured using the TRAP assay. |
J Med Chem 41: 4873-84 (1998)
Article DOI: 10.1021/jm981067o
BindingDB Entry DOI: 10.7270/Q2WW7GSD |
More data for this
Ligand-Target Pair | |
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50066357
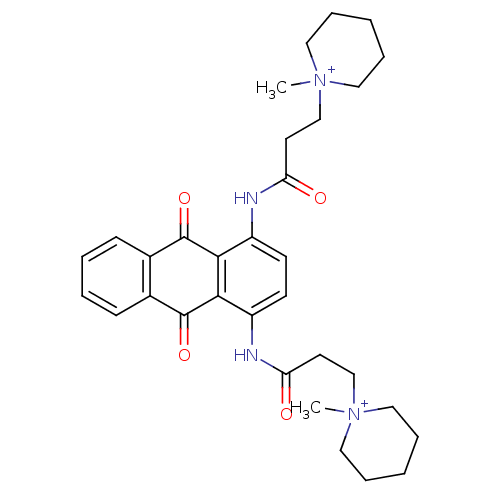
(3-(1-Methyl-piperidinium-1-yl)-N-{4-[3-(1-methyl-p...)Show SMILES C[N+]1(CCC(=O)Nc2ccc(NC(=O)CC[N+]3(C)CCCCC3)c3C(=O)c4ccccc4C(=O)c23)CCCCC1 Show InChI InChI=1S/C32H40N4O4/c1-35(17-7-3-8-18-35)21-15-27(37)33-25-13-14-26(34-28(38)16-22-36(2)19-9-4-10-20-36)30-29(25)31(39)23-11-5-6-12-24(23)32(30)40/h5-6,11-14H,3-4,7-10,15-22H2,1-2H3/p+2 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.11E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Inhibition of telomerase activity |
J Med Chem 41: 3253-60 (1998)
Article DOI: 10.1021/jm9801105
BindingDB Entry DOI: 10.7270/Q2TX3G1R |
More data for this
Ligand-Target Pair | |
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50066357

(3-(1-Methyl-piperidinium-1-yl)-N-{4-[3-(1-methyl-p...)Show SMILES C[N+]1(CCC(=O)Nc2ccc(NC(=O)CC[N+]3(C)CCCCC3)c3C(=O)c4ccccc4C(=O)c23)CCCCC1 Show InChI InChI=1S/C32H40N4O4/c1-35(17-7-3-8-18-35)21-15-27(37)33-25-13-14-26(34-28(38)16-22-36(2)19-9-4-10-20-36)30-29(25)31(39)23-11-5-6-12-24(23)32(30)40/h5-6,11-14H,3-4,7-10,15-22H2,1-2H3/p+2 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.11E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Inhibitory activity of telomerase was measured using the TRAP assay. |
J Med Chem 41: 4873-84 (1998)
Article DOI: 10.1021/jm981067o
BindingDB Entry DOI: 10.7270/Q2WW7GSD |
More data for this
Ligand-Target Pair | |
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50079129
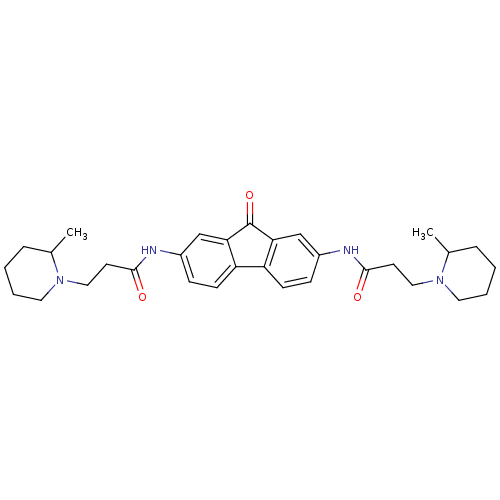
(3-(2-Methyl-piperidin-1-yl)-N-{7-[3-(2-methyl-pipe...)Show SMILES CC1CCCCN1CCC(=O)Nc1ccc2-c3ccc(NC(=O)CCN4CCCCC4C)cc3C(=O)c2c1 Show InChI InChI=1S/C31H40N4O3/c1-21-7-3-5-15-34(21)17-13-29(36)32-23-9-11-25-26-12-10-24(20-28(26)31(38)27(25)19-23)33-30(37)14-18-35-16-6-4-8-22(35)2/h9-12,19-22H,3-8,13-18H2,1-2H3,(H,32,36)(H,33,37) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against telomerase |
J Med Chem 42: 2679-84 (1999)
Article DOI: 10.1021/jm990084q
BindingDB Entry DOI: 10.7270/Q2DF6RX9 |
More data for this
Ligand-Target Pair | |
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50068326

(CHEMBL142900 | N-[9,10-Dioxo-5-(3-(N-methylmorphol...)Show SMILES C[N+]1(CCC(=O)Nc2cccc3C(=O)c4c(NC(=O)CC[N+]5(C)CCOCC5)cccc4C(=O)c23)CCOCC1 Show InChI InChI=1S/C30H36N4O6/c1-33(13-17-39-18-14-33)11-9-25(35)31-23-7-3-5-21-27(23)29(37)22-6-4-8-24(28(22)30(21)38)32-26(36)10-12-34(2)15-19-40-20-16-34/h3-8H,9-20H2,1-2H3/p+2 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.32E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Inhibitory activity of telomerase was measured using the TRAP assay. |
J Med Chem 41: 4873-84 (1998)
Article DOI: 10.1021/jm981067o
BindingDB Entry DOI: 10.7270/Q2WW7GSD |
More data for this
Ligand-Target Pair | |
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50068314
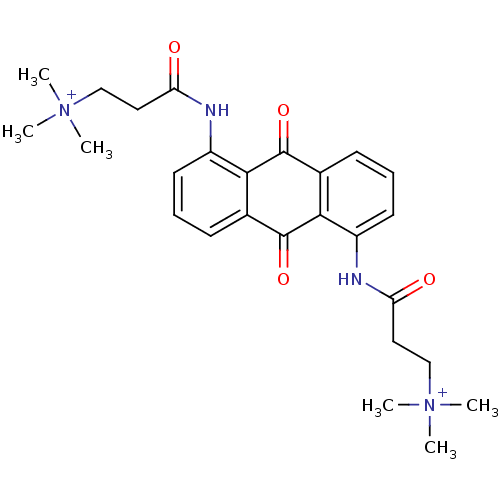
(CHEMBL145490 | N-[9,10-Dioxo-5-(3-(N,N,N-trimethyl...)Show SMILES C[N+](C)(C)CCC(=O)Nc1cccc2C(=O)c3c(NC(=O)CC[N+](C)(C)C)cccc3C(=O)c12 Show InChI InChI=1S/C26H32N4O4/c1-29(2,3)15-13-21(31)27-19-11-7-9-17-23(19)25(33)18-10-8-12-20(24(18)26(17)34)28-22(32)14-16-30(4,5)6/h7-12H,13-16H2,1-6H3/p+2 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.32E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Inhibitory activity of telomerase was measured using the TRAP assay. |
J Med Chem 41: 4873-84 (1998)
Article DOI: 10.1021/jm981067o
BindingDB Entry DOI: 10.7270/Q2WW7GSD |
More data for this
Ligand-Target Pair | |
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50079131

(3-(2-Ethyl-piperidin-1-yl)-N-{7-[3-(2-ethyl-piperi...)Show SMILES CCC1CCCCN1CCC(=O)Nc1ccc2-c3ccc(NC(=O)CCN4CCCCC4CC)cc3C(=O)c2c1 Show InChI InChI=1S/C33H44N4O3/c1-3-25-9-5-7-17-36(25)19-15-31(38)34-23-11-13-27-28-14-12-24(22-30(28)33(40)29(27)21-23)35-32(39)16-20-37-18-8-6-10-26(37)4-2/h11-14,21-22,25-26H,3-10,15-20H2,1-2H3,(H,34,38)(H,35,39) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against telomerase |
J Med Chem 42: 2679-84 (1999)
Article DOI: 10.1021/jm990084q
BindingDB Entry DOI: 10.7270/Q2DF6RX9 |
More data for this
Ligand-Target Pair | |
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50068309
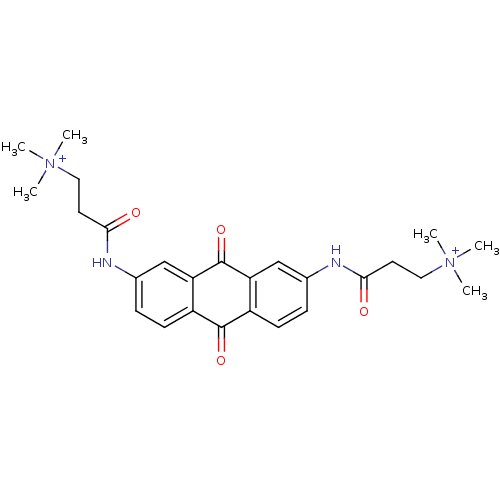
(CHEMBL144756 | CHEMBL91398 | N-[9,10-Dioxo-7-(3-(N...)Show SMILES C[N+](C)(C)CCC(=O)Nc1ccc2C(=O)c3ccc(NC(=O)CC[N+](C)(C)C)cc3C(=O)c2c1 Show InChI InChI=1S/C26H32N4O4/c1-29(2,3)13-11-23(31)27-17-7-9-19-21(15-17)26(34)22-16-18(8-10-20(22)25(19)33)28-24(32)12-14-30(4,5)6/h7-10,15-16H,11-14H2,1-6H3/p+2 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.45E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Inhibitory activity of telomerase was measured using the TRAP assay. |
J Med Chem 41: 4873-84 (1998)
Article DOI: 10.1021/jm981067o
BindingDB Entry DOI: 10.7270/Q2WW7GSD |
More data for this
Ligand-Target Pair | |
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50068309

(CHEMBL144756 | CHEMBL91398 | N-[9,10-Dioxo-7-(3-(N...)Show SMILES C[N+](C)(C)CCC(=O)Nc1ccc2C(=O)c3ccc(NC(=O)CC[N+](C)(C)C)cc3C(=O)c2c1 Show InChI InChI=1S/C26H32N4O4/c1-29(2,3)13-11-23(31)27-17-7-9-19-21(15-17)26(34)22-16-18(8-10-20(22)25(19)33)28-24(32)12-14-30(4,5)6/h7-10,15-16H,11-14H2,1-6H3/p+2 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.45E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against telomerase |
J Med Chem 42: 2679-84 (1999)
Article DOI: 10.1021/jm990084q
BindingDB Entry DOI: 10.7270/Q2DF6RX9 |
More data for this
Ligand-Target Pair | |
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50079125

(3-Diethylamino-N-[7-(3-diethylamino-propionylamino...)Show SMILES CCN(CC)CCC(=O)Nc1ccc2-c3ccc(NC(=O)CCN(CC)CC)cc3C(=O)c2c1 Show InChI InChI=1S/C27H36N4O3/c1-5-30(6-2)15-13-25(32)28-19-9-11-21-22-12-10-20(18-24(22)27(34)23(21)17-19)29-26(33)14-16-31(7-3)8-4/h9-12,17-18H,5-8,13-16H2,1-4H3,(H,28,32)(H,29,33) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.55E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against telomerase |
J Med Chem 42: 2679-84 (1999)
Article DOI: 10.1021/jm990084q
BindingDB Entry DOI: 10.7270/Q2DF6RX9 |
More data for this
Ligand-Target Pair | |
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50068325
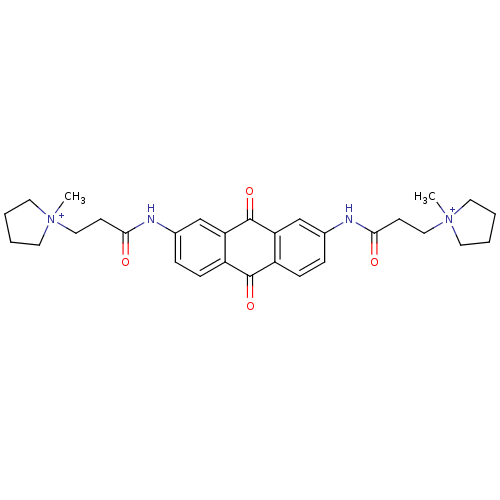
(CHEMBL146602 | N-[9,10-Dioxo-7-(3-(N-methylpyrroli...)Show SMILES C[N+]1(CCC(=O)Nc2ccc3C(=O)c4ccc(NC(=O)CC[N+]5(C)CCCC5)cc4C(=O)c3c2)CCCC1 Show InChI InChI=1S/C30H36N4O4/c1-33(13-3-4-14-33)17-11-27(35)31-21-7-9-23-25(19-21)30(38)26-20-22(8-10-24(26)29(23)37)32-28(36)12-18-34(2)15-5-6-16-34/h7-10,19-20H,3-6,11-18H2,1-2H3/p+2 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Inhibitory activity of telomerase was measured using the TRAP assay. |
J Med Chem 41: 4873-84 (1998)
Article DOI: 10.1021/jm981067o
BindingDB Entry DOI: 10.7270/Q2WW7GSD |
More data for this
Ligand-Target Pair | |
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50079146

(3-Dimethylamino-N-[7-(3-dimethylamino-propionylami...)Show SMILES CN(C)CCC(=O)Nc1ccc2-c3ccc(NC(=O)CCN(C)C)cc3C(=O)c2c1 Show InChI InChI=1S/C23H28N4O3/c1-26(2)11-9-21(28)24-15-5-7-17-18-8-6-16(25-22(29)10-12-27(3)4)14-20(18)23(30)19(17)13-15/h5-8,13-14H,9-12H2,1-4H3,(H,24,28)(H,25,29) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.62E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against telomerase |
J Med Chem 42: 2679-84 (1999)
Article DOI: 10.1021/jm990084q
BindingDB Entry DOI: 10.7270/Q2DF6RX9 |
More data for this
Ligand-Target Pair | |
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50079143
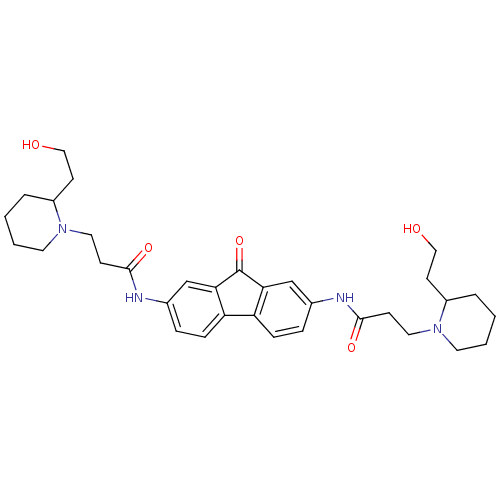
(3-[2-(2-Hydroxy-ethyl)-piperidin-1-yl]-N-(7-{3-[2-...)Show SMILES OCCC1CCCCN1CCC(=O)Nc1ccc2-c3ccc(NC(=O)CCN4CCCCC4CCO)cc3C(=O)c2c1 Show InChI InChI=1S/C33H44N4O5/c38-19-13-25-5-1-3-15-36(25)17-11-31(40)34-23-7-9-27-28-10-8-24(22-30(28)33(42)29(27)21-23)35-32(41)12-18-37-16-4-2-6-26(37)14-20-39/h7-10,21-22,25-26,38-39H,1-6,11-20H2,(H,34,40)(H,35,41) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.65E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against telomerase |
J Med Chem 42: 2679-84 (1999)
Article DOI: 10.1021/jm990084q
BindingDB Entry DOI: 10.7270/Q2DF6RX9 |
More data for this
Ligand-Target Pair | |
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50068327
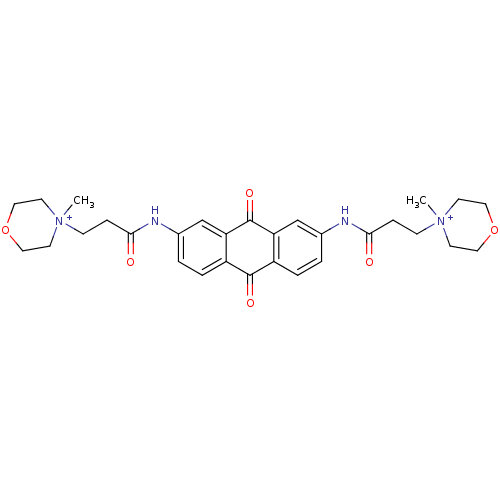
(CHEMBL144999 | CHEMBL92265 | N-[9,10-Dioxo-7-(3-(N...)Show SMILES C[N+]1(CCC(=O)Nc2ccc3C(=O)c4ccc(NC(=O)CC[N+]5(C)CCOCC5)cc4C(=O)c3c2)CCOCC1 Show InChI InChI=1S/C30H36N4O6/c1-33(11-15-39-16-12-33)9-7-27(35)31-21-3-5-23-25(19-21)30(38)26-20-22(4-6-24(26)29(23)37)32-28(36)8-10-34(2)13-17-40-18-14-34/h3-6,19-20H,7-18H2,1-2H3/p+2 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.65E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Inhibitory activity of telomerase was measured using the TRAP assay. |
J Med Chem 41: 4873-84 (1998)
Article DOI: 10.1021/jm981067o
BindingDB Entry DOI: 10.7270/Q2WW7GSD |
More data for this
Ligand-Target Pair | |
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50068327

(CHEMBL144999 | CHEMBL92265 | N-[9,10-Dioxo-7-(3-(N...)Show SMILES C[N+]1(CCC(=O)Nc2ccc3C(=O)c4ccc(NC(=O)CC[N+]5(C)CCOCC5)cc4C(=O)c3c2)CCOCC1 Show InChI InChI=1S/C30H36N4O6/c1-33(11-15-39-16-12-33)9-7-27(35)31-21-3-5-23-25(19-21)30(38)26-20-22(4-6-24(26)29(23)37)32-28(36)8-10-34(2)13-17-40-18-14-34/h3-6,19-20H,7-18H2,1-2H3/p+2 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.65E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against telomerase |
J Med Chem 42: 2679-84 (1999)
Article DOI: 10.1021/jm990084q
BindingDB Entry DOI: 10.7270/Q2DF6RX9 |
More data for this
Ligand-Target Pair | |
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50066361
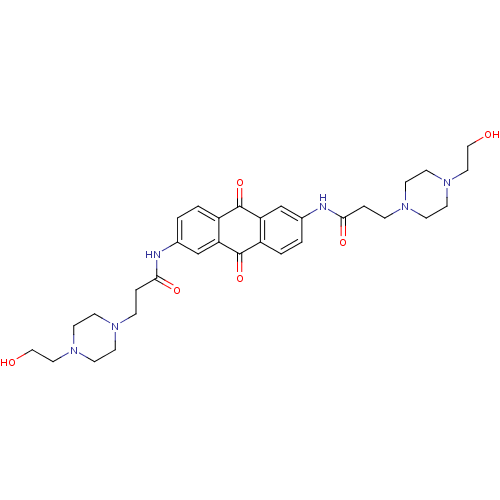
(3-[4-(2-Hydroxy-ethyl)-piperazin-1-yl]-N-(6-{3-[4-...)Show SMILES OCCN1CCN(CCC(=O)Nc2ccc3C(=O)c4cc(NC(=O)CCN5CCN(CCO)CC5)ccc4C(=O)c3c2)CC1 Show InChI InChI=1S/C32H42N6O6/c39-19-17-37-13-9-35(10-14-37)7-5-29(41)33-23-1-3-25-27(21-23)32(44)26-4-2-24(22-28(26)31(25)43)34-30(42)6-8-36-11-15-38(16-12-36)18-20-40/h1-4,21-22,39-40H,5-20H2,(H,33,41)(H,34,42) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.65E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Inhibition of telomerase activity |
J Med Chem 41: 3253-60 (1998)
Article DOI: 10.1021/jm9801105
BindingDB Entry DOI: 10.7270/Q2TX3G1R |
More data for this
Ligand-Target Pair | |
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50068307
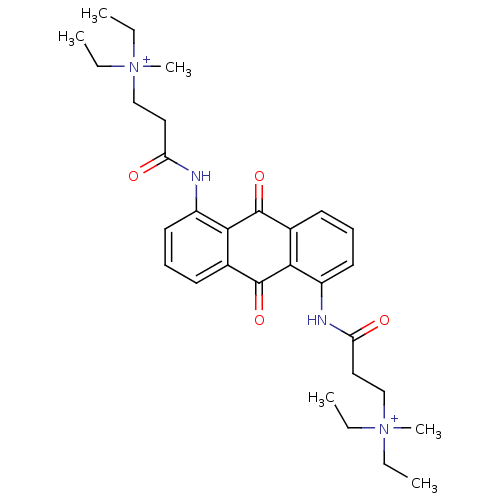
(CHEMBL344274 | N-[9,10-Dioxo-5-(3-(N,N-diethyl-N-m...)Show SMILES CC[N+](C)(CC)CCC(=O)Nc1cccc2C(=O)c3c(NC(=O)CC[N+](C)(CC)CC)cccc3C(=O)c12 Show InChI InChI=1S/C30H40N4O4/c1-7-33(5,8-2)19-17-25(35)31-23-15-11-13-21-27(23)29(37)22-14-12-16-24(28(22)30(21)38)32-26(36)18-20-34(6,9-3)10-4/h11-16H,7-10,17-20H2,1-6H3/p+2 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.68E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Inhibitory activity of telomerase was measured using the TRAP assay. |
J Med Chem 41: 4873-84 (1998)
Article DOI: 10.1021/jm981067o
BindingDB Entry DOI: 10.7270/Q2WW7GSD |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data