 Found 114 hits with Last Name = 'romero' and Initial = 'r'
Found 114 hits with Last Name = 'romero' and Initial = 'r' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cathepsin K
(Homo sapiens (Human)) | BDBM19807
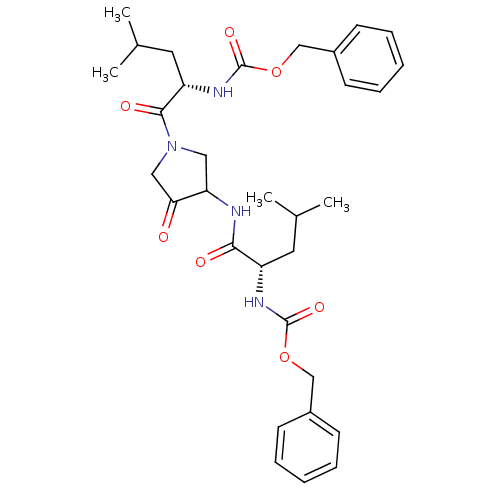
(CHEMBL100563 | benzyl N-[(1S)-1-({1-[(2S)-2-{[(ben...)Show SMILES CC(C)C[C@H](NC(=O)OCc1ccccc1)C(=O)NC1CN(CC1=O)C(=O)[C@H](CC(C)C)NC(=O)OCc1ccccc1 |r| Show InChI InChI=1S/C32H42N4O7/c1-21(2)15-25(34-31(40)42-19-23-11-7-5-8-12-23)29(38)33-27-17-36(18-28(27)37)30(39)26(16-22(3)4)35-32(41)43-20-24-13-9-6-10-14-24/h5-14,21-22,25-27H,15-20H2,1-4H3,(H,33,38)(H,34,40)(H,35,41)/t25-,26-,27?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Research Center
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against Cathepsin K |
Bioorg Med Chem Lett 11: 2951-4 (2001)
BindingDB Entry DOI: 10.7270/Q23R0S5W |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Cathepsin K
(Homo sapiens (Human)) | BDBM50106075

((S)-1-[2-Oxo-2-(3,4,5-trimethoxy-phenyl)-ethyl]-pi...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@@H]1CCCCN1CC(=O)c1cc(OC)c(OC)c(OC)c1)\C=C\c1ccccc1 Show InChI InChI=1S/C30H40N2O5/c1-6-21(2)24(16-15-22-12-8-7-9-13-22)31-30(34)25-14-10-11-17-32(25)20-26(33)23-18-27(35-3)29(37-5)28(19-23)36-4/h7-9,12-13,15-16,18-19,21,24-25H,6,10-11,14,17,20H2,1-5H3,(H,31,34)/b16-15+/t21-,24+,25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 790 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Research Center
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against cathepsin K |
Bioorg Med Chem Lett 11: 2951-4 (2001)
BindingDB Entry DOI: 10.7270/Q23R0S5W |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50114250
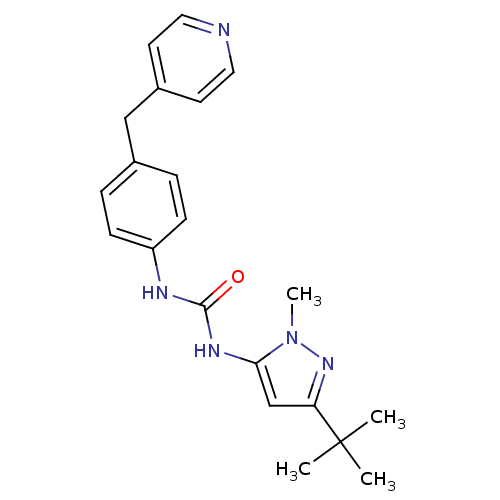
(1-(5-tert-Butyl-2-methyl-2H-pyrazol-3-yl)-3-(4-pyr...)Show SMILES Cn1nc(cc1NC(=O)Nc1ccc(Cc2ccncc2)cc1)C(C)(C)C Show InChI InChI=1S/C21H25N5O/c1-21(2,3)18-14-19(26(4)25-18)24-20(27)23-17-7-5-15(6-8-17)13-16-9-11-22-12-10-16/h5-12,14H,13H2,1-4H3,(H2,23,24,27) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Research Center
Curated by ChEMBL
| Assay Description
Inhibition of human epidermal growth factor receptor-2 |
Bioorg Med Chem Lett 12: 1559-62 (2002)
BindingDB Entry DOI: 10.7270/Q2CV4H28 |
More data for this
Ligand-Target Pair | |
Glucagon receptor
(Homo sapiens (Human)) | BDBM50121047

(5-Fluoro-2-[3-(1-hydroxy-ethyl)-2,6-diisopropyl-5-...)Show SMILES CCCc1c(nc(C(C)C)c(C(C)O)c1-c1ccc(F)cc1O)C(C)C |(2.39,-.22,;1.05,-.99,;-.28,-.22,;-1.6,-.98,;-1.6,-2.53,;-2.94,-3.3,;-4.27,-2.53,;-5.62,-3.3,;-6.95,-2.53,;-5.62,-4.84,;-4.27,-.98,;-5.6,-.22,;-6.95,-.99,;-5.6,1.32,;-2.94,-.21,;-2.96,1.33,;-4.27,2.1,;-4.29,3.62,;-2.96,4.4,;-2.96,5.94,;-1.63,3.63,;-1.63,2.09,;-.28,1.32,;-.27,-3.3,;-.27,-4.84,;1.06,-2.52,)| Show InChI InChI=1S/C22H30FNO2/c1-7-8-17-20(16-10-9-15(23)11-18(16)26)19(14(6)25)22(13(4)5)24-21(17)12(2)3/h9-14,25-26H,7-8H2,1-6H3 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Research Center
Curated by ChEMBL
| Assay Description
Binding affinity of second enantiomer (E2) of the compound against human glucagon receptor was determined |
Bioorg Med Chem Lett 12: 3421-4 (2002)
BindingDB Entry DOI: 10.7270/Q24B30NZ |
More data for this
Ligand-Target Pair | |
Glucagon receptor
(Homo sapiens (Human)) | BDBM50121049

(5-Fluoro-2-[3-(1-hydroxy-ethyl)-2,6-diisopropyl-5-...)Show SMILES CCCCCc1c(nc(C(C)C)c(C(C)O)c1-c1ccc(F)cc1O)C(C)C |(5.05,-.22,;3.72,-.99,;2.39,-.22,;1.05,-.99,;-.28,-.22,;-1.6,-.98,;-1.6,-2.53,;-2.94,-3.3,;-4.27,-2.53,;-5.62,-3.3,;-6.95,-2.53,;-5.62,-4.84,;-4.27,-.98,;-5.6,-.22,;-6.95,-.99,;-5.6,1.32,;-2.94,-.21,;-2.96,1.33,;-4.27,2.1,;-4.29,3.62,;-2.96,4.4,;-2.96,5.94,;-1.63,3.63,;-1.63,2.09,;-.28,1.32,;-.27,-3.3,;-.27,-4.84,;1.06,-2.52,)| Show InChI InChI=1S/C24H34FNO2/c1-7-8-9-10-19-22(18-12-11-17(25)13-20(18)28)21(16(6)27)24(15(4)5)26-23(19)14(2)3/h11-16,27-28H,7-10H2,1-6H3 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Research Center
Curated by ChEMBL
| Assay Description
Binding affinity of first enantiomer (E1) of the compound against human glucagon receptor was determined |
Bioorg Med Chem Lett 12: 3421-4 (2002)
BindingDB Entry DOI: 10.7270/Q24B30NZ |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50114245
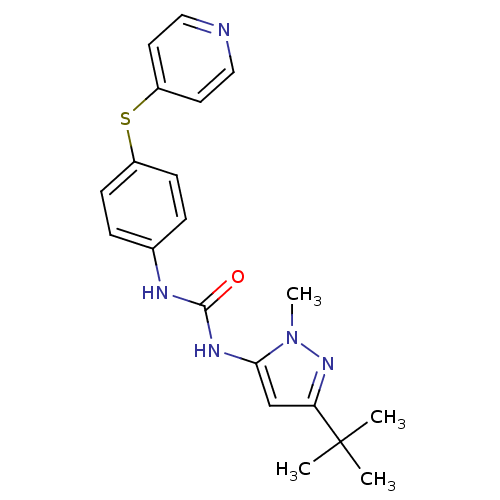
(1-(5-tert-Butyl-2-methyl-2H-pyrazol-3-yl)-3-[4-(py...)Show SMILES Cn1nc(cc1NC(=O)Nc1ccc(Sc2ccncc2)cc1)C(C)(C)C Show InChI InChI=1S/C20H23N5OS/c1-20(2,3)17-13-18(25(4)24-17)23-19(26)22-14-5-7-15(8-6-14)27-16-9-11-21-12-10-16/h5-13H,1-4H3,(H2,22,23,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Research Center
Curated by ChEMBL
| Assay Description
Inhibitory activity against Mitogen-activated protein kinase p38 alpha 2 |
Bioorg Med Chem Lett 12: 1559-62 (2002)
BindingDB Entry DOI: 10.7270/Q2CV4H28 |
More data for this
Ligand-Target Pair | |
Glucagon receptor
(Homo sapiens (Human)) | BDBM50121045

(2-[3-Ethyl-5-(1-hydroxy-ethyl)-2,6-diisopropyl-pyr...)Show SMILES CCc1c(nc(C(C)C)c(C(C)O)c1-c1ccc(F)cc1O)C(C)C |(1.05,-.99,;-.28,-.22,;-1.6,-.98,;-1.6,-2.53,;-2.94,-3.3,;-4.27,-2.53,;-5.62,-3.3,;-6.95,-2.53,;-5.62,-4.84,;-4.27,-.98,;-5.6,-.22,;-6.95,-.99,;-5.6,1.32,;-2.94,-.21,;-2.96,1.33,;-4.27,2.1,;-4.29,3.62,;-2.96,4.4,;-2.96,5.94,;-1.63,3.63,;-1.63,2.09,;-.28,1.32,;-.27,-3.3,;-.27,-4.84,;1.06,-2.52,)| Show InChI InChI=1S/C21H28FNO2/c1-7-15-19(16-9-8-14(22)10-17(16)25)18(13(6)24)21(12(4)5)23-20(15)11(2)3/h8-13,24-25H,7H2,1-6H3 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Research Center
Curated by ChEMBL
| Assay Description
Binding affinity of second enantiomer (E2) of the compound against human glucagon receptor was determined |
Bioorg Med Chem Lett 12: 3421-4 (2002)
BindingDB Entry DOI: 10.7270/Q24B30NZ |
More data for this
Ligand-Target Pair | |
Glucagon receptor
(Homo sapiens (Human)) | BDBM50121045

(2-[3-Ethyl-5-(1-hydroxy-ethyl)-2,6-diisopropyl-pyr...)Show SMILES CCc1c(nc(C(C)C)c(C(C)O)c1-c1ccc(F)cc1O)C(C)C |(1.05,-.99,;-.28,-.22,;-1.6,-.98,;-1.6,-2.53,;-2.94,-3.3,;-4.27,-2.53,;-5.62,-3.3,;-6.95,-2.53,;-5.62,-4.84,;-4.27,-.98,;-5.6,-.22,;-6.95,-.99,;-5.6,1.32,;-2.94,-.21,;-2.96,1.33,;-4.27,2.1,;-4.29,3.62,;-2.96,4.4,;-2.96,5.94,;-1.63,3.63,;-1.63,2.09,;-.28,1.32,;-.27,-3.3,;-.27,-4.84,;1.06,-2.52,)| Show InChI InChI=1S/C21H28FNO2/c1-7-15-19(16-9-8-14(22)10-17(16)25)18(13(6)24)21(12(4)5)23-20(15)11(2)3/h8-13,24-25H,7H2,1-6H3 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Research Center
Curated by ChEMBL
| Assay Description
Binding affinity of first diastereomer (D1) of the compound against human glucagon receptor was determined |
Bioorg Med Chem Lett 12: 3421-4 (2002)
BindingDB Entry DOI: 10.7270/Q24B30NZ |
More data for this
Ligand-Target Pair | |
Glucagon receptor
(Homo sapiens (Human)) | BDBM50121047

(5-Fluoro-2-[3-(1-hydroxy-ethyl)-2,6-diisopropyl-5-...)Show SMILES CCCc1c(nc(C(C)C)c(C(C)O)c1-c1ccc(F)cc1O)C(C)C |(2.39,-.22,;1.05,-.99,;-.28,-.22,;-1.6,-.98,;-1.6,-2.53,;-2.94,-3.3,;-4.27,-2.53,;-5.62,-3.3,;-6.95,-2.53,;-5.62,-4.84,;-4.27,-.98,;-5.6,-.22,;-6.95,-.99,;-5.6,1.32,;-2.94,-.21,;-2.96,1.33,;-4.27,2.1,;-4.29,3.62,;-2.96,4.4,;-2.96,5.94,;-1.63,3.63,;-1.63,2.09,;-.28,1.32,;-.27,-3.3,;-.27,-4.84,;1.06,-2.52,)| Show InChI InChI=1S/C22H30FNO2/c1-7-8-17-20(16-10-9-15(23)11-18(16)26)19(14(6)25)22(13(4)5)24-21(17)12(2)3/h9-14,25-26H,7-8H2,1-6H3 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Research Center
Curated by ChEMBL
| Assay Description
Binding affinity of second enantiomer (E2) of the compound against human glucagon receptor was determined |
Bioorg Med Chem Lett 12: 3421-4 (2002)
BindingDB Entry DOI: 10.7270/Q24B30NZ |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM13336
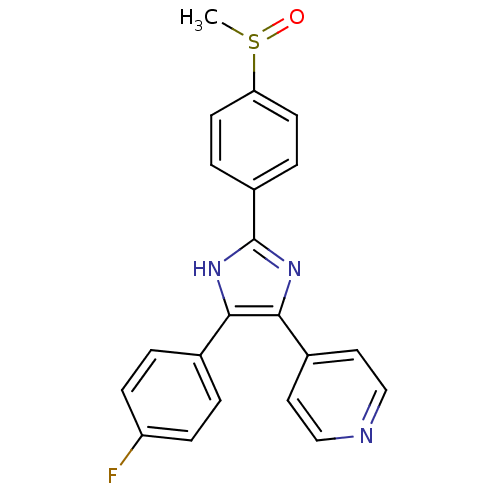
(4-[4-(4-fluorophenyl)-2-(4-methanesulfinylphenyl)-...)Show SMILES CS(=O)c1ccc(cc1)-c1nc(c([nH]1)-c1ccc(F)cc1)-c1ccncc1 Show InChI InChI=1S/C21H16FN3OS/c1-27(26)18-8-4-16(5-9-18)21-24-19(14-2-6-17(22)7-3-14)20(25-21)15-10-12-23-13-11-15/h2-13H,1H3,(H,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Research Center
Curated by ChEMBL
| Assay Description
Inhibitory activity against Mitogen-activated protein kinase p38 alpha 2 |
Bioorg Med Chem Lett 12: 1559-62 (2002)
BindingDB Entry DOI: 10.7270/Q2CV4H28 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Glucagon receptor
(Homo sapiens (Human)) | BDBM50121045

(2-[3-Ethyl-5-(1-hydroxy-ethyl)-2,6-diisopropyl-pyr...)Show SMILES CCc1c(nc(C(C)C)c(C(C)O)c1-c1ccc(F)cc1O)C(C)C |(1.05,-.99,;-.28,-.22,;-1.6,-.98,;-1.6,-2.53,;-2.94,-3.3,;-4.27,-2.53,;-5.62,-3.3,;-6.95,-2.53,;-5.62,-4.84,;-4.27,-.98,;-5.6,-.22,;-6.95,-.99,;-5.6,1.32,;-2.94,-.21,;-2.96,1.33,;-4.27,2.1,;-4.29,3.62,;-2.96,4.4,;-2.96,5.94,;-1.63,3.63,;-1.63,2.09,;-.28,1.32,;-.27,-3.3,;-.27,-4.84,;1.06,-2.52,)| Show InChI InChI=1S/C21H28FNO2/c1-7-15-19(16-9-8-14(22)10-17(16)25)18(13(6)24)21(12(4)5)23-20(15)11(2)3/h8-13,24-25H,7H2,1-6H3 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Research Center
Curated by ChEMBL
| Assay Description
Binding affinity of second diastereomer (D2) of the compound against human glucagon receptor was determined |
Bioorg Med Chem Lett 12: 3421-4 (2002)
BindingDB Entry DOI: 10.7270/Q24B30NZ |
More data for this
Ligand-Target Pair | |
Glucagon receptor
(Homo sapiens (Human)) | BDBM50121046
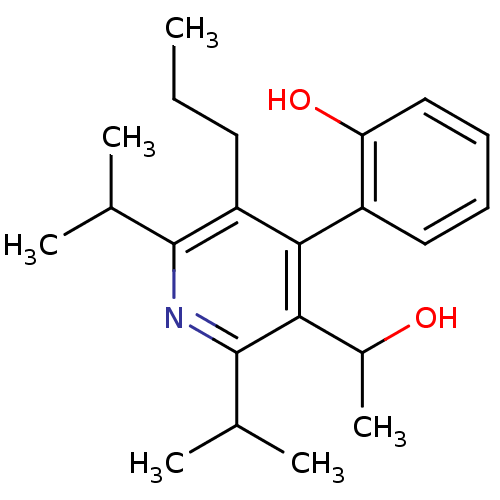
(2-[3-(1-Hydroxy-ethyl)-2,6-diisopropyl-5-propyl-py...)Show SMILES CCCc1c(nc(C(C)C)c(C(C)O)c1-c1ccccc1O)C(C)C |(2.39,-.22,;1.05,-.99,;-.28,-.22,;-1.6,-.98,;-1.6,-2.53,;-2.94,-3.3,;-4.27,-2.53,;-5.62,-3.3,;-5.62,-4.84,;-6.95,-2.53,;-4.27,-.98,;-5.6,-.22,;-6.95,-.99,;-5.6,1.32,;-2.94,-.21,;-2.96,1.33,;-4.27,2.1,;-4.29,3.62,;-2.96,4.4,;-1.63,3.63,;-1.63,2.09,;-.28,1.32,;-.27,-3.3,;1.06,-2.52,;-.27,-4.84,)| Show InChI InChI=1S/C22H31NO2/c1-7-10-17-20(16-11-8-9-12-18(16)25)19(15(6)24)22(14(4)5)23-21(17)13(2)3/h8-9,11-15,24-25H,7,10H2,1-6H3 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Research Center
Curated by ChEMBL
| Assay Description
Binding affinity of first diastereomer (D1) of the compound against human glucagon receptor was determined |
Bioorg Med Chem Lett 12: 3421-4 (2002)
BindingDB Entry DOI: 10.7270/Q24B30NZ |
More data for this
Ligand-Target Pair | |
Glucagon receptor
(Homo sapiens (Human)) | BDBM50121048

(2-[3-Butyl-5-(1-hydroxy-ethyl)-2,6-diisopropyl-pyr...)Show SMILES CCCCc1c(nc(C(C)C)c(C(C)O)c1-c1ccc(F)cc1O)C(C)C |(3.72,-.99,;2.39,-.22,;1.05,-.99,;-.28,-.22,;-1.6,-.98,;-1.6,-2.53,;-2.94,-3.3,;-4.27,-2.53,;-5.62,-3.3,;-6.95,-2.53,;-5.62,-4.84,;-4.27,-.98,;-5.6,-.22,;-6.95,-.99,;-5.6,1.32,;-2.94,-.21,;-2.96,1.33,;-4.27,2.1,;-4.29,3.62,;-2.96,4.4,;-2.96,5.94,;-1.63,3.63,;-1.63,2.09,;-.28,1.32,;-.27,-3.3,;-.27,-4.84,;1.06,-2.52,)| Show InChI InChI=1S/C23H32FNO2/c1-7-8-9-18-21(17-11-10-16(24)12-19(17)27)20(15(6)26)23(14(4)5)25-22(18)13(2)3/h10-15,26-27H,7-9H2,1-6H3 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Research Center
Curated by ChEMBL
| Assay Description
Binding affinity of second enantiomer (E2) of the compound against human glucagon receptor was determined |
Bioorg Med Chem Lett 12: 3421-4 (2002)
BindingDB Entry DOI: 10.7270/Q24B30NZ |
More data for this
Ligand-Target Pair | |
Glucagon receptor
(Homo sapiens (Human)) | BDBM50121049

(5-Fluoro-2-[3-(1-hydroxy-ethyl)-2,6-diisopropyl-5-...)Show SMILES CCCCCc1c(nc(C(C)C)c(C(C)O)c1-c1ccc(F)cc1O)C(C)C |(5.05,-.22,;3.72,-.99,;2.39,-.22,;1.05,-.99,;-.28,-.22,;-1.6,-.98,;-1.6,-2.53,;-2.94,-3.3,;-4.27,-2.53,;-5.62,-3.3,;-6.95,-2.53,;-5.62,-4.84,;-4.27,-.98,;-5.6,-.22,;-6.95,-.99,;-5.6,1.32,;-2.94,-.21,;-2.96,1.33,;-4.27,2.1,;-4.29,3.62,;-2.96,4.4,;-2.96,5.94,;-1.63,3.63,;-1.63,2.09,;-.28,1.32,;-.27,-3.3,;-.27,-4.84,;1.06,-2.52,)| Show InChI InChI=1S/C24H34FNO2/c1-7-8-9-10-19-22(18-12-11-17(25)13-20(18)28)21(16(6)27)24(15(4)5)26-23(19)14(2)3/h11-16,27-28H,7-10H2,1-6H3 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Research Center
Curated by ChEMBL
| Assay Description
Binding affinity of first enantiomer (E1) of the compound against human glucagon receptor was determined |
Bioorg Med Chem Lett 12: 3421-4 (2002)
BindingDB Entry DOI: 10.7270/Q24B30NZ |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50114231
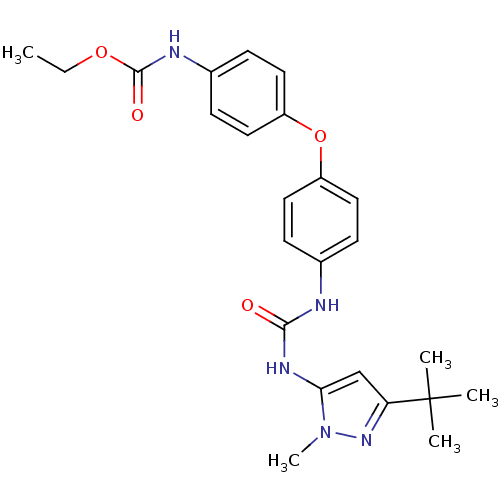
((4-{4-[3-(5-tert-Butyl-2-methyl-2H-pyrazol-3-yl)-u...)Show SMILES CCOC(=O)Nc1ccc(Oc2ccc(NC(=O)Nc3cc(nn3C)C(C)(C)C)cc2)cc1 Show InChI InChI=1S/C24H29N5O4/c1-6-32-23(31)26-17-9-13-19(14-10-17)33-18-11-7-16(8-12-18)25-22(30)27-21-15-20(24(2,3)4)28-29(21)5/h7-15H,6H2,1-5H3,(H,26,31)(H2,25,27,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Research Center
Curated by ChEMBL
| Assay Description
Inhibitory activity against Mitogen-activated protein kinase p38 alpha 2 |
Bioorg Med Chem Lett 12: 1559-62 (2002)
BindingDB Entry DOI: 10.7270/Q2CV4H28 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50114235
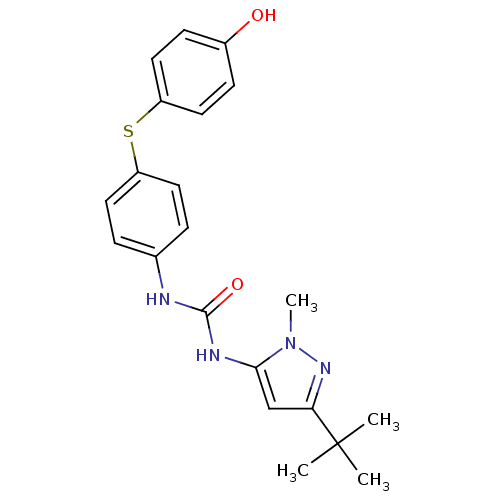
(1-(5-tert-Butyl-2-methyl-2H-pyrazol-3-yl)-3-[4-(4-...)Show SMILES Cn1nc(cc1NC(=O)Nc1ccc(Sc2ccc(O)cc2)cc1)C(C)(C)C Show InChI InChI=1S/C21H24N4O2S/c1-21(2,3)18-13-19(25(4)24-18)23-20(27)22-14-5-9-16(10-6-14)28-17-11-7-15(26)8-12-17/h5-13,26H,1-4H3,(H2,22,23,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Research Center
Curated by ChEMBL
| Assay Description
Inhibitory activity against Mitogen-activated protein kinase p38 alpha 2 |
Bioorg Med Chem Lett 12: 1559-62 (2002)
BindingDB Entry DOI: 10.7270/Q2CV4H28 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50114247
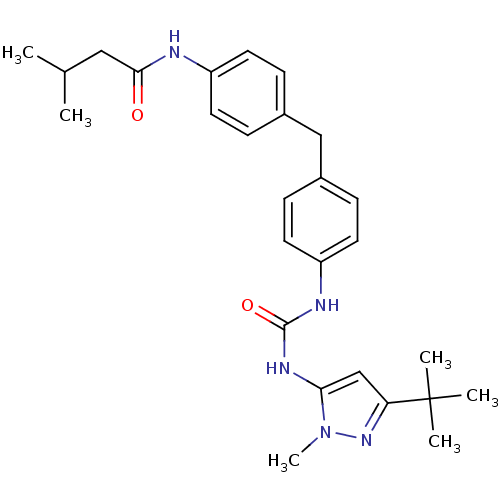
(CHEMBL415679 | N-(4-{4-[3-(5-tert-Butyl-2-methyl-2...)Show SMILES CC(C)CC(=O)Nc1ccc(Cc2ccc(NC(=O)Nc3cc(nn3C)C(C)(C)C)cc2)cc1 Show InChI InChI=1S/C27H35N5O2/c1-18(2)15-25(33)28-21-11-7-19(8-12-21)16-20-9-13-22(14-10-20)29-26(34)30-24-17-23(27(3,4)5)31-32(24)6/h7-14,17-18H,15-16H2,1-6H3,(H,28,33)(H2,29,30,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Research Center
Curated by ChEMBL
| Assay Description
Inhibitory activity against Mitogen-activated protein kinase p38 alpha 2 |
Bioorg Med Chem Lett 12: 1559-62 (2002)
BindingDB Entry DOI: 10.7270/Q2CV4H28 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50114240
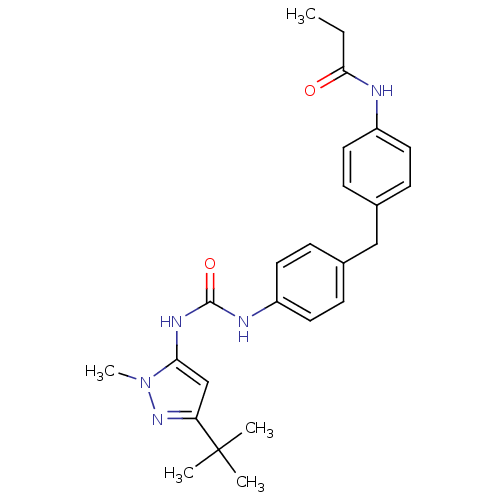
(CHEMBL290982 | N-(4-{4-[3-(5-tert-Butyl-2-methyl-2...)Show SMILES CCC(=O)Nc1ccc(Cc2ccc(NC(=O)Nc3cc(nn3C)C(C)(C)C)cc2)cc1 Show InChI InChI=1S/C25H31N5O2/c1-6-23(31)26-19-11-7-17(8-12-19)15-18-9-13-20(14-10-18)27-24(32)28-22-16-21(25(2,3)4)29-30(22)5/h7-14,16H,6,15H2,1-5H3,(H,26,31)(H2,27,28,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Research Center
Curated by ChEMBL
| Assay Description
Inhibitory activity against Mitogen-activated protein kinase p38 alpha 2 |
Bioorg Med Chem Lett 12: 1559-62 (2002)
BindingDB Entry DOI: 10.7270/Q2CV4H28 |
More data for this
Ligand-Target Pair | |
Glucagon receptor
(Homo sapiens (Human)) | BDBM50121046

(2-[3-(1-Hydroxy-ethyl)-2,6-diisopropyl-5-propyl-py...)Show SMILES CCCc1c(nc(C(C)C)c(C(C)O)c1-c1ccccc1O)C(C)C |(2.39,-.22,;1.05,-.99,;-.28,-.22,;-1.6,-.98,;-1.6,-2.53,;-2.94,-3.3,;-4.27,-2.53,;-5.62,-3.3,;-5.62,-4.84,;-6.95,-2.53,;-4.27,-.98,;-5.6,-.22,;-6.95,-.99,;-5.6,1.32,;-2.94,-.21,;-2.96,1.33,;-4.27,2.1,;-4.29,3.62,;-2.96,4.4,;-1.63,3.63,;-1.63,2.09,;-.28,1.32,;-.27,-3.3,;1.06,-2.52,;-.27,-4.84,)| Show InChI InChI=1S/C22H31NO2/c1-7-10-17-20(16-11-8-9-12-18(16)25)19(15(6)24)22(14(4)5)23-21(17)13(2)3/h8-9,11-15,24-25H,7,10H2,1-6H3 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Research Center
Curated by ChEMBL
| Assay Description
Binding affinity of first diastereomer (D1) of the compound against human glucagon receptor was determined |
Bioorg Med Chem Lett 12: 3421-4 (2002)
BindingDB Entry DOI: 10.7270/Q24B30NZ |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50114250

(1-(5-tert-Butyl-2-methyl-2H-pyrazol-3-yl)-3-(4-pyr...)Show SMILES Cn1nc(cc1NC(=O)Nc1ccc(Cc2ccncc2)cc1)C(C)(C)C Show InChI InChI=1S/C21H25N5O/c1-21(2,3)18-14-19(26(4)25-18)24-20(27)23-17-7-5-15(6-8-17)13-16-9-11-22-12-10-16/h5-12,14H,13H2,1-4H3,(H2,23,24,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Research Center
Curated by ChEMBL
| Assay Description
Inhibitory activity against Mitogen-activated protein kinase p38 alpha 2 |
Bioorg Med Chem Lett 12: 1559-62 (2002)
BindingDB Entry DOI: 10.7270/Q2CV4H28 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50114244
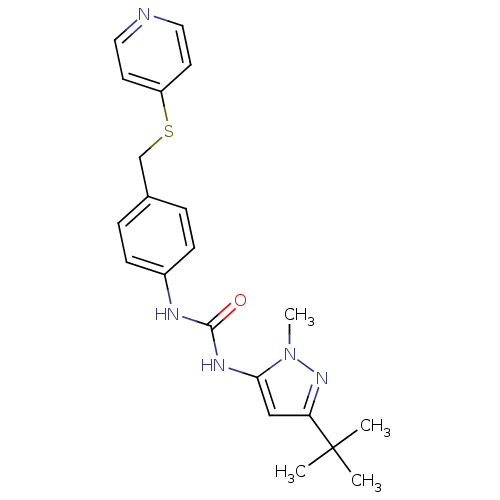
(1-(5-tert-Butyl-2-methyl-2H-pyrazol-3-yl)-3-[4-(py...)Show SMILES Cn1nc(cc1NC(=O)Nc1ccc(CSc2ccncc2)cc1)C(C)(C)C Show InChI InChI=1S/C21H25N5OS/c1-21(2,3)18-13-19(26(4)25-18)24-20(27)23-16-7-5-15(6-8-16)14-28-17-9-11-22-12-10-17/h5-13H,14H2,1-4H3,(H2,23,24,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Research Center
Curated by ChEMBL
| Assay Description
Inhibitory activity against Mitogen-activated protein kinase p38 alpha 2 |
Bioorg Med Chem Lett 12: 1559-62 (2002)
BindingDB Entry DOI: 10.7270/Q2CV4H28 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50114239
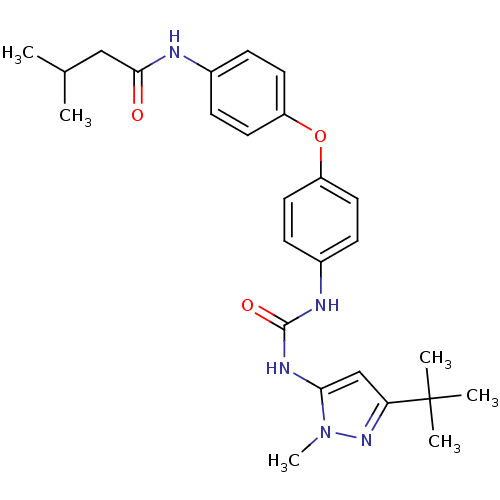
(CHEMBL40166 | N-(4-{4-[3-(5-tert-Butyl-2-methyl-2H...)Show SMILES CC(C)CC(=O)Nc1ccc(Oc2ccc(NC(=O)Nc3cc(nn3C)C(C)(C)C)cc2)cc1 Show InChI InChI=1S/C26H33N5O3/c1-17(2)15-24(32)27-18-7-11-20(12-8-18)34-21-13-9-19(10-14-21)28-25(33)29-23-16-22(26(3,4)5)30-31(23)6/h7-14,16-17H,15H2,1-6H3,(H,27,32)(H2,28,29,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Research Center
Curated by ChEMBL
| Assay Description
Inhibitory activity against Mitogen-activated protein kinase p38 alpha 2 |
Bioorg Med Chem Lett 12: 1559-62 (2002)
BindingDB Entry DOI: 10.7270/Q2CV4H28 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50091923
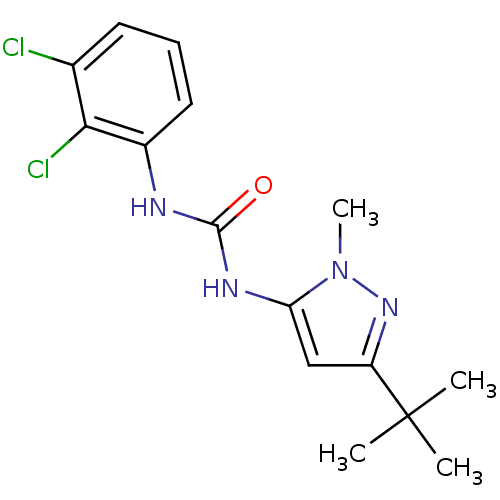
(1-(5-tert-Butyl-2-methyl-2H-pyrazol-3-yl)-3-(2,3-d...)Show InChI InChI=1S/C15H18Cl2N4O/c1-15(2,3)11-8-12(21(4)20-11)19-14(22)18-10-7-5-6-9(16)13(10)17/h5-8H,1-4H3,(H2,18,19,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 53 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Research Center
Curated by ChEMBL
| Assay Description
Inhibitory activity against Mitogen-activated protein kinase p38 alpha 2 |
Bioorg Med Chem Lett 12: 1559-62 (2002)
BindingDB Entry DOI: 10.7270/Q2CV4H28 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50114242
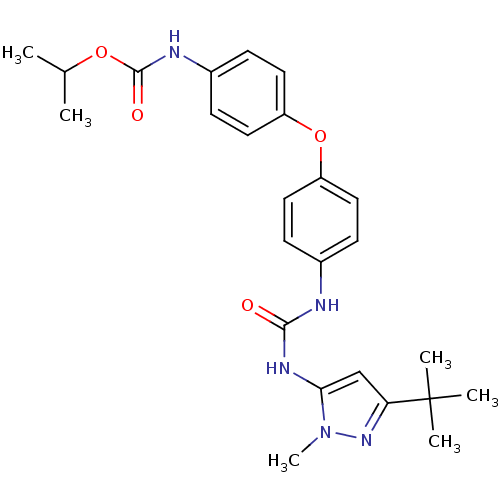
((4-{4-[3-(5-tert-Butyl-2-methyl-2H-pyrazol-3-yl)-u...)Show SMILES CC(C)OC(=O)Nc1ccc(Oc2ccc(NC(=O)Nc3cc(nn3C)C(C)(C)C)cc2)cc1 Show InChI InChI=1S/C25H31N5O4/c1-16(2)33-24(32)27-18-9-13-20(14-10-18)34-19-11-7-17(8-12-19)26-23(31)28-22-15-21(25(3,4)5)29-30(22)6/h7-16H,1-6H3,(H,27,32)(H2,26,28,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 54 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Research Center
Curated by ChEMBL
| Assay Description
Inhibitory activity against Mitogen-activated protein kinase p38 alpha 2 |
Bioorg Med Chem Lett 12: 1559-62 (2002)
BindingDB Entry DOI: 10.7270/Q2CV4H28 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50114238
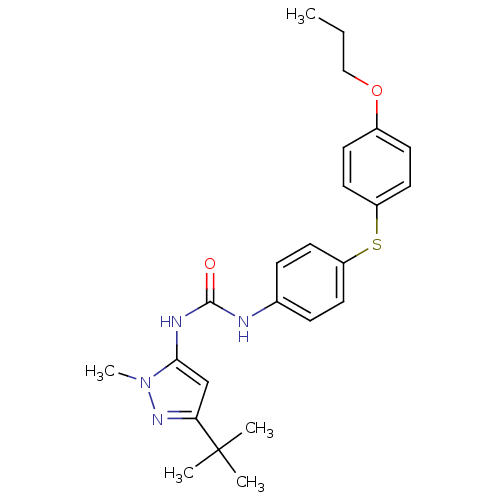
(1-(5-tert-Butyl-2-methyl-2H-pyrazol-3-yl)-3-[4-(4-...)Show SMILES CCCOc1ccc(Sc2ccc(NC(=O)Nc3cc(nn3C)C(C)(C)C)cc2)cc1 Show InChI InChI=1S/C24H30N4O2S/c1-6-15-30-18-9-13-20(14-10-18)31-19-11-7-17(8-12-19)25-23(29)26-22-16-21(24(2,3)4)27-28(22)5/h7-14,16H,6,15H2,1-5H3,(H2,25,26,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 57 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Research Center
Curated by ChEMBL
| Assay Description
Inhibitory activity against Mitogen-activated protein kinase p38 alpha 2 |
Bioorg Med Chem Lett 12: 1559-62 (2002)
BindingDB Entry DOI: 10.7270/Q2CV4H28 |
More data for this
Ligand-Target Pair | |
Glucagon receptor
(Homo sapiens (Human)) | BDBM50109326

((R)-1-[4-(4-Fluoro-phenyl)-2,6-diisopropyl-5-propy...)Show SMILES CCCc1c(nc(C(C)C)c([C@@H](C)O)c1-c1ccc(F)cc1)C(C)C Show InChI InChI=1S/C22H30FNO/c1-7-8-18-20(16-9-11-17(23)12-10-16)19(15(6)25)22(14(4)5)24-21(18)13(2)3/h9-15,25H,7-8H2,1-6H3/t15-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 65 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Research Center
Curated by ChEMBL
| Assay Description
Tested for its ability to inhibit cAMP production in human glucagon receptor expressed CHO cells |
Bioorg Med Chem Lett 12: 1303-6 (2002)
BindingDB Entry DOI: 10.7270/Q25H7FK0 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50114251
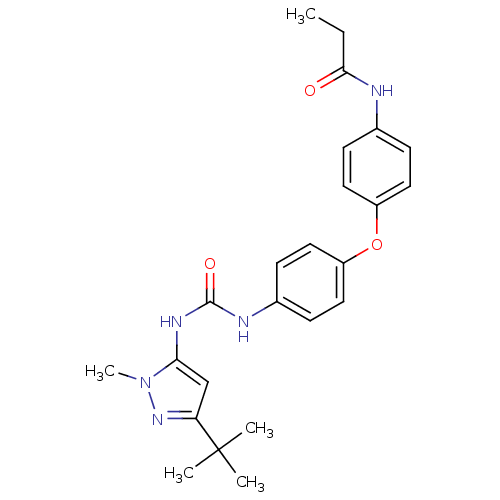
(CHEMBL288889 | N-(4-{4-[3-(5-tert-Butyl-2-methyl-2...)Show SMILES CCC(=O)Nc1ccc(Oc2ccc(NC(=O)Nc3cc(nn3C)C(C)(C)C)cc2)cc1 Show InChI InChI=1S/C24H29N5O3/c1-6-22(30)25-16-7-11-18(12-8-16)32-19-13-9-17(10-14-19)26-23(31)27-21-15-20(24(2,3)4)28-29(21)5/h7-15H,6H2,1-5H3,(H,25,30)(H2,26,27,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 69 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Research Center
Curated by ChEMBL
| Assay Description
Inhibitory activity against Mitogen-activated protein kinase p38 alpha 2 |
Bioorg Med Chem Lett 12: 1559-62 (2002)
BindingDB Entry DOI: 10.7270/Q2CV4H28 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50114233
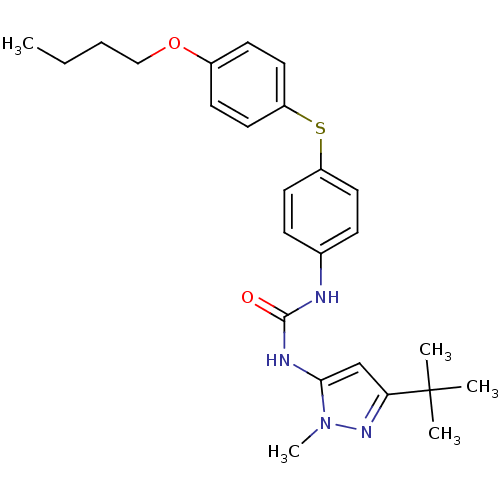
(1-[4-(4-Butoxy-phenylsulfanyl)-phenyl]-3-(5-tert-b...)Show SMILES CCCCOc1ccc(Sc2ccc(NC(=O)Nc3cc(nn3C)C(C)(C)C)cc2)cc1 Show InChI InChI=1S/C25H32N4O2S/c1-6-7-16-31-19-10-14-21(15-11-19)32-20-12-8-18(9-13-20)26-24(30)27-23-17-22(25(2,3)4)28-29(23)5/h8-15,17H,6-7,16H2,1-5H3,(H2,26,27,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 69 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Research Center
Curated by ChEMBL
| Assay Description
Inhibitory activity against Mitogen-activated protein kinase p38 alpha 2 |
Bioorg Med Chem Lett 12: 1559-62 (2002)
BindingDB Entry DOI: 10.7270/Q2CV4H28 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50114253
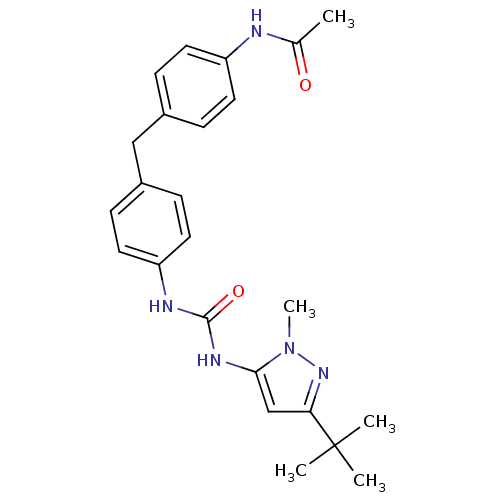
(CHEMBL416425 | N-(4-{4-[3-(5-tert-Butyl-2-methyl-2...)Show SMILES CC(=O)Nc1ccc(Cc2ccc(NC(=O)Nc3cc(nn3C)C(C)(C)C)cc2)cc1 Show InChI InChI=1S/C24H29N5O2/c1-16(30)25-19-10-6-17(7-11-19)14-18-8-12-20(13-9-18)26-23(31)27-22-15-21(24(2,3)4)28-29(22)5/h6-13,15H,14H2,1-5H3,(H,25,30)(H2,26,27,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Research Center
Curated by ChEMBL
| Assay Description
Inhibitory activity against Mitogen-activated protein kinase p38 alpha 2 |
Bioorg Med Chem Lett 12: 1559-62 (2002)
BindingDB Entry DOI: 10.7270/Q2CV4H28 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50114248
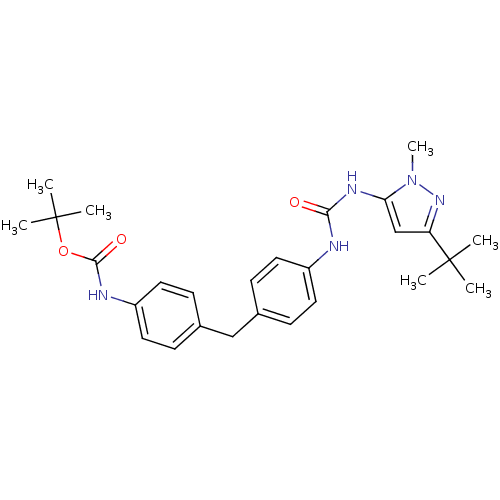
((4-{4-[3-(5-tert-Butyl-2-methyl-2H-pyrazol-3-yl)-u...)Show SMILES Cn1nc(cc1NC(=O)Nc1ccc(Cc2ccc(NC(=O)OC(C)(C)C)cc2)cc1)C(C)(C)C Show InChI InChI=1S/C27H35N5O3/c1-26(2,3)22-17-23(32(7)31-22)30-24(33)28-20-12-8-18(9-13-20)16-19-10-14-21(15-11-19)29-25(34)35-27(4,5)6/h8-15,17H,16H2,1-7H3,(H,29,34)(H2,28,30,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 87 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Research Center
Curated by ChEMBL
| Assay Description
Inhibitory activity against Mitogen-activated protein kinase p38 alpha 2 |
Bioorg Med Chem Lett 12: 1559-62 (2002)
BindingDB Entry DOI: 10.7270/Q2CV4H28 |
More data for this
Ligand-Target Pair | |
Glucagon receptor
(Homo sapiens (Human)) | BDBM50109326

((R)-1-[4-(4-Fluoro-phenyl)-2,6-diisopropyl-5-propy...)Show SMILES CCCc1c(nc(C(C)C)c([C@@H](C)O)c1-c1ccc(F)cc1)C(C)C Show InChI InChI=1S/C22H30FNO/c1-7-8-18-20(16-9-11-17(23)12-10-16)19(15(6)25)22(14(4)5)24-21(18)13(2)3/h9-15,25H,7-8H2,1-6H3/t15-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Research Center
Curated by ChEMBL
| Assay Description
Tested for its inhibitory activity against human glucagon receptor (hGR) expressed in CHO cells |
Bioorg Med Chem Lett 12: 1303-6 (2002)
BindingDB Entry DOI: 10.7270/Q25H7FK0 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 11
(Homo sapiens (Human)) | BDBM50114250

(1-(5-tert-Butyl-2-methyl-2H-pyrazol-3-yl)-3-(4-pyr...)Show SMILES Cn1nc(cc1NC(=O)Nc1ccc(Cc2ccncc2)cc1)C(C)(C)C Show InChI InChI=1S/C21H25N5O/c1-21(2,3)18-14-19(26(4)25-18)24-20(27)23-17-7-5-15(6-8-17)13-16-9-11-22-12-10-16/h5-12,14H,13H2,1-4H3,(H2,23,24,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Research Center
Curated by ChEMBL
| Assay Description
Inhibition of Mitogen-activated protein kinase p38 beta |
Bioorg Med Chem Lett 12: 1559-62 (2002)
BindingDB Entry DOI: 10.7270/Q2CV4H28 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50114232
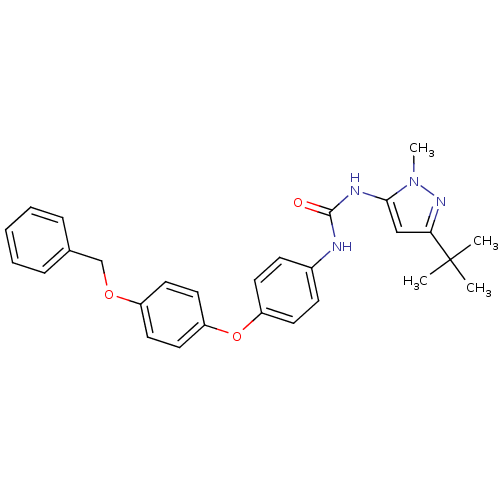
(1-[4-(4-Benzyloxy-phenoxy)-phenyl]-3-(5-tert-butyl...)Show SMILES Cn1nc(cc1NC(=O)Nc1ccc(Oc2ccc(OCc3ccccc3)cc2)cc1)C(C)(C)C Show InChI InChI=1S/C28H30N4O3/c1-28(2,3)25-18-26(32(4)31-25)30-27(33)29-21-10-12-23(13-11-21)35-24-16-14-22(15-17-24)34-19-20-8-6-5-7-9-20/h5-18H,19H2,1-4H3,(H2,29,30,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Research Center
Curated by ChEMBL
| Assay Description
Inhibitory activity against Mitogen-activated protein kinase p38 alpha 2 |
Bioorg Med Chem Lett 12: 1559-62 (2002)
BindingDB Entry DOI: 10.7270/Q2CV4H28 |
More data for this
Ligand-Target Pair | |
Glucagon receptor
(Homo sapiens (Human)) | BDBM50109326

((R)-1-[4-(4-Fluoro-phenyl)-2,6-diisopropyl-5-propy...)Show SMILES CCCc1c(nc(C(C)C)c([C@@H](C)O)c1-c1ccc(F)cc1)C(C)C Show InChI InChI=1S/C22H30FNO/c1-7-8-18-20(16-9-11-17(23)12-10-16)19(15(6)25)22(14(4)5)24-21(18)13(2)3/h9-15,25H,7-8H2,1-6H3/t15-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Research Center
Curated by ChEMBL
| Assay Description
Binding affinity of the compound against human glucagon receptor was determined |
Bioorg Med Chem Lett 12: 3421-4 (2002)
BindingDB Entry DOI: 10.7270/Q24B30NZ |
More data for this
Ligand-Target Pair | |
Glucagon receptor
(Homo sapiens (Human)) | BDBM50112476

(5-Fluoro-2-(3-hydroxymethyl-2,6-diisopropyl-5-pent...)Show SMILES CCCCCc1c(nc(C(C)C)c(CO)c1-c1ccc(F)cc1O)C(C)C |(13.61,-8.26,;12.28,-9.04,;10.94,-8.27,;9.62,-9.05,;8.27,-8.31,;6.95,-9.08,;6.95,-10.62,;5.61,-11.39,;4.28,-10.62,;2.95,-11.39,;2.95,-12.93,;1.62,-10.62,;4.28,-9.08,;2.95,-8.31,;1.62,-9.08,;5.61,-8.29,;5.61,-6.77,;4.28,-6,;4.25,-4.48,;5.6,-3.69,;5.58,-2.15,;6.94,-4.46,;6.93,-6,;8.27,-6.77,;8.29,-11.39,;9.62,-10.59,;8.29,-12.93,)| Show InChI InChI=1S/C23H32FNO2/c1-6-7-8-9-18-21(17-11-10-16(24)12-20(17)27)19(13-26)23(15(4)5)25-22(18)14(2)3/h10-12,14-15,26-27H,6-9,13H2,1-5H3 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Research Center
Curated by ChEMBL
| Assay Description
Tested for its ability to inhibit cAMP production in human glucagon receptor expressed CHO cells |
Bioorg Med Chem Lett 12: 1303-6 (2002)
BindingDB Entry DOI: 10.7270/Q25H7FK0 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50114252
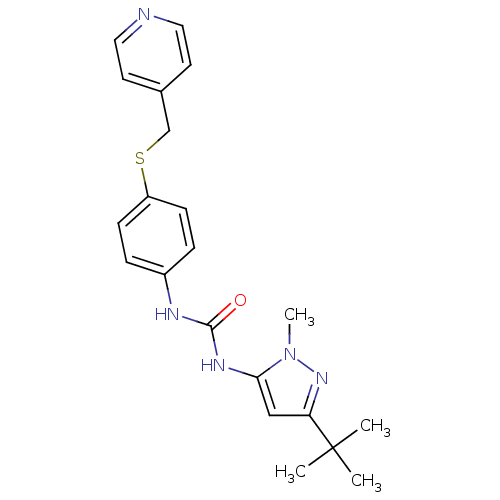
(1-(5-tert-Butyl-2-methyl-2H-pyrazol-3-yl)-3-[4-(py...)Show SMILES Cn1nc(cc1NC(=O)Nc1ccc(SCc2ccncc2)cc1)C(C)(C)C Show InChI InChI=1S/C21H25N5OS/c1-21(2,3)18-13-19(26(4)25-18)24-20(27)23-16-5-7-17(8-6-16)28-14-15-9-11-22-12-10-15/h5-13H,14H2,1-4H3,(H2,23,24,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Research Center
Curated by ChEMBL
| Assay Description
Inhibitory activity against Mitogen-activated protein kinase p38 alpha 2 |
Bioorg Med Chem Lett 12: 1559-62 (2002)
BindingDB Entry DOI: 10.7270/Q2CV4H28 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50114249
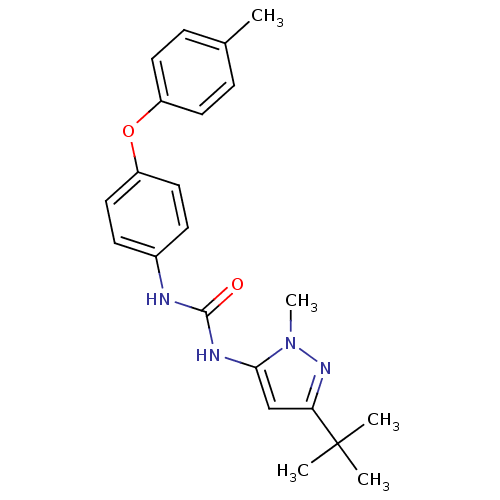
(1-(5-tert-Butyl-2-methyl-2H-pyrazol-3-yl)-3-(4-p-t...)Show SMILES Cc1ccc(Oc2ccc(NC(=O)Nc3cc(nn3C)C(C)(C)C)cc2)cc1 Show InChI InChI=1S/C22H26N4O2/c1-15-6-10-17(11-7-15)28-18-12-8-16(9-13-18)23-21(27)24-20-14-19(22(2,3)4)25-26(20)5/h6-14H,1-5H3,(H2,23,24,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Research Center
Curated by ChEMBL
| Assay Description
Inhibitory activity against Mitogen-activated protein kinase p38 alpha 2 |
Bioorg Med Chem Lett 12: 1559-62 (2002)
BindingDB Entry DOI: 10.7270/Q2CV4H28 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50114236
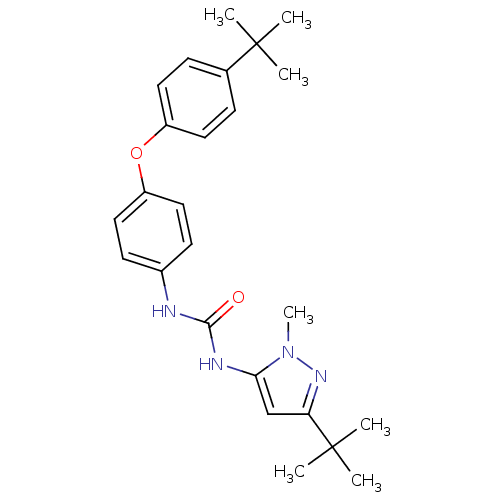
(1-(5-tert-Butyl-2-methyl-2H-pyrazol-3-yl)-3-[4-(4-...)Show SMILES Cn1nc(cc1NC(=O)Nc1ccc(Oc2ccc(cc2)C(C)(C)C)cc1)C(C)(C)C Show InChI InChI=1S/C25H32N4O2/c1-24(2,3)17-8-12-19(13-9-17)31-20-14-10-18(11-15-20)26-23(30)27-22-16-21(25(4,5)6)28-29(22)7/h8-16H,1-7H3,(H2,26,27,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 183 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Research Center
Curated by ChEMBL
| Assay Description
Inhibitory activity against Mitogen-activated protein kinase p38 alpha 2 |
Bioorg Med Chem Lett 12: 1559-62 (2002)
BindingDB Entry DOI: 10.7270/Q2CV4H28 |
More data for this
Ligand-Target Pair | |
Glucagon receptor
(Homo sapiens (Human)) | BDBM50112476

(5-Fluoro-2-(3-hydroxymethyl-2,6-diisopropyl-5-pent...)Show SMILES CCCCCc1c(nc(C(C)C)c(CO)c1-c1ccc(F)cc1O)C(C)C |(13.61,-8.26,;12.28,-9.04,;10.94,-8.27,;9.62,-9.05,;8.27,-8.31,;6.95,-9.08,;6.95,-10.62,;5.61,-11.39,;4.28,-10.62,;2.95,-11.39,;2.95,-12.93,;1.62,-10.62,;4.28,-9.08,;2.95,-8.31,;1.62,-9.08,;5.61,-8.29,;5.61,-6.77,;4.28,-6,;4.25,-4.48,;5.6,-3.69,;5.58,-2.15,;6.94,-4.46,;6.93,-6,;8.27,-6.77,;8.29,-11.39,;9.62,-10.59,;8.29,-12.93,)| Show InChI InChI=1S/C23H32FNO2/c1-6-7-8-9-18-21(17-11-10-16(24)12-20(17)27)19(13-26)23(15(4)5)25-22(18)14(2)3/h10-12,14-15,26-27H,6-9,13H2,1-5H3 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Research Center
Curated by ChEMBL
| Assay Description
Tested for its inhibitory activity against human glucagon receptor (hGR) expressed in CHO cells |
Bioorg Med Chem Lett 12: 1303-6 (2002)
BindingDB Entry DOI: 10.7270/Q25H7FK0 |
More data for this
Ligand-Target Pair | |
Glucagon receptor
(Homo sapiens (Human)) | BDBM50112476

(5-Fluoro-2-(3-hydroxymethyl-2,6-diisopropyl-5-pent...)Show SMILES CCCCCc1c(nc(C(C)C)c(CO)c1-c1ccc(F)cc1O)C(C)C |(13.61,-8.26,;12.28,-9.04,;10.94,-8.27,;9.62,-9.05,;8.27,-8.31,;6.95,-9.08,;6.95,-10.62,;5.61,-11.39,;4.28,-10.62,;2.95,-11.39,;2.95,-12.93,;1.62,-10.62,;4.28,-9.08,;2.95,-8.31,;1.62,-9.08,;5.61,-8.29,;5.61,-6.77,;4.28,-6,;4.25,-4.48,;5.6,-3.69,;5.58,-2.15,;6.94,-4.46,;6.93,-6,;8.27,-6.77,;8.29,-11.39,;9.62,-10.59,;8.29,-12.93,)| Show InChI InChI=1S/C23H32FNO2/c1-6-7-8-9-18-21(17-11-10-16(24)12-20(17)27)19(13-26)23(15(4)5)25-22(18)14(2)3/h10-12,14-15,26-27H,6-9,13H2,1-5H3 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Research Center
Curated by ChEMBL
| Assay Description
Binding affinity of the compound against human glucagon receptor was determined |
Bioorg Med Chem Lett 12: 3421-4 (2002)
BindingDB Entry DOI: 10.7270/Q24B30NZ |
More data for this
Ligand-Target Pair | |
Glucagon receptor
(Homo sapiens (Human)) | BDBM50121046

(2-[3-(1-Hydroxy-ethyl)-2,6-diisopropyl-5-propyl-py...)Show SMILES CCCc1c(nc(C(C)C)c(C(C)O)c1-c1ccccc1O)C(C)C |(2.39,-.22,;1.05,-.99,;-.28,-.22,;-1.6,-.98,;-1.6,-2.53,;-2.94,-3.3,;-4.27,-2.53,;-5.62,-3.3,;-5.62,-4.84,;-6.95,-2.53,;-4.27,-.98,;-5.6,-.22,;-6.95,-.99,;-5.6,1.32,;-2.94,-.21,;-2.96,1.33,;-4.27,2.1,;-4.29,3.62,;-2.96,4.4,;-1.63,3.63,;-1.63,2.09,;-.28,1.32,;-.27,-3.3,;1.06,-2.52,;-.27,-4.84,)| Show InChI InChI=1S/C22H31NO2/c1-7-10-17-20(16-11-8-9-12-18(16)25)19(15(6)24)22(14(4)5)23-21(17)13(2)3/h8-9,11-15,24-25H,7,10H2,1-6H3 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Research Center
Curated by ChEMBL
| Assay Description
Binding affinity of first enantiomer (E1) of the compound against human glucagon receptor was determined |
Bioorg Med Chem Lett 12: 3421-4 (2002)
BindingDB Entry DOI: 10.7270/Q24B30NZ |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50114246
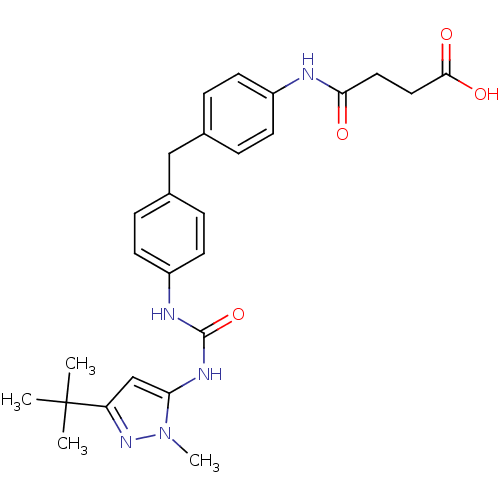
(CHEMBL40020 | N-(4-{4-[3-(5-tert-Butyl-2-methyl-2H...)Show SMILES Cn1nc(cc1NC(=O)Nc1ccc(Cc2ccc(NC(=O)CCC(O)=O)cc2)cc1)C(C)(C)C Show InChI InChI=1S/C26H31N5O4/c1-26(2,3)21-16-22(31(4)30-21)29-25(35)28-20-11-7-18(8-12-20)15-17-5-9-19(10-6-17)27-23(32)13-14-24(33)34/h5-12,16H,13-15H2,1-4H3,(H,27,32)(H,33,34)(H2,28,29,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Research Center
Curated by ChEMBL
| Assay Description
Inhibitory activity against Mitogen-activated protein kinase p38 alpha 2 |
Bioorg Med Chem Lett 12: 1559-62 (2002)
BindingDB Entry DOI: 10.7270/Q2CV4H28 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50106079
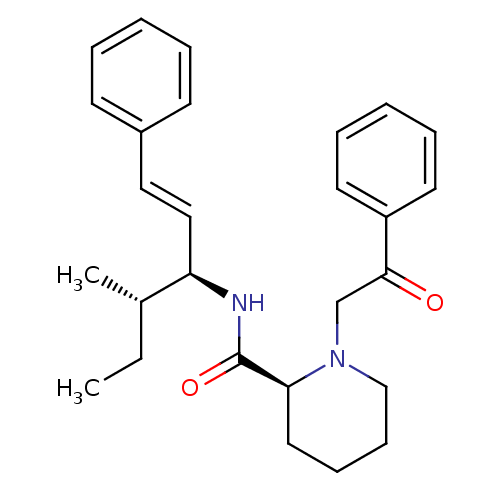
((S)-1-(2-Oxo-2-phenyl-ethyl)-piperidine-2-carboxyl...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@@H]1CCCCN1CC(=O)c1ccccc1)\C=C\c1ccccc1 Show InChI InChI=1S/C27H34N2O2/c1-3-21(2)24(18-17-22-12-6-4-7-13-22)28-27(31)25-16-10-11-19-29(25)20-26(30)23-14-8-5-9-15-23/h4-9,12-15,17-18,21,24-25H,3,10-11,16,19-20H2,1-2H3,(H,28,31)/b18-17+/t21-,24+,25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Research Center
Curated by ChEMBL
| Assay Description
Inhibitory activity against cathepsin K |
Bioorg Med Chem Lett 11: 2951-4 (2001)
BindingDB Entry DOI: 10.7270/Q23R0S5W |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50114243
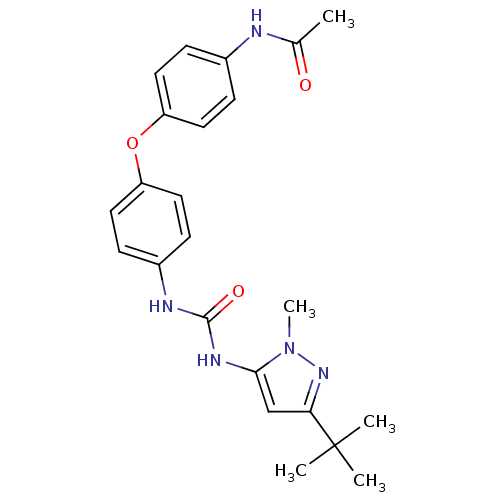
(CHEMBL289155 | N-(4-{4-[3-(5-tert-Butyl-2-methyl-2...)Show SMILES CC(=O)Nc1ccc(Oc2ccc(NC(=O)Nc3cc(nn3C)C(C)(C)C)cc2)cc1 Show InChI InChI=1S/C23H27N5O3/c1-15(29)24-16-6-10-18(11-7-16)31-19-12-8-17(9-13-19)25-22(30)26-21-14-20(23(2,3)4)27-28(21)5/h6-14H,1-5H3,(H,24,29)(H2,25,26,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 235 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Research Center
Curated by ChEMBL
| Assay Description
Inhibitory activity against Mitogen-activated protein kinase p38 alpha 2 |
Bioorg Med Chem Lett 12: 1559-62 (2002)
BindingDB Entry DOI: 10.7270/Q2CV4H28 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50114237
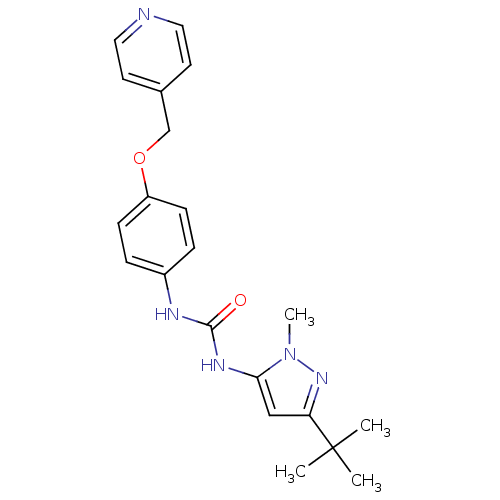
(1-(5-tert-Butyl-2-methyl-2H-pyrazol-3-yl)-3-[4-(py...)Show SMILES Cn1nc(cc1NC(=O)Nc1ccc(OCc2ccncc2)cc1)C(C)(C)C Show InChI InChI=1S/C21H25N5O2/c1-21(2,3)18-13-19(26(4)25-18)24-20(27)23-16-5-7-17(8-6-16)28-14-15-9-11-22-12-10-15/h5-13H,14H2,1-4H3,(H2,23,24,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Research Center
Curated by ChEMBL
| Assay Description
Inhibitory activity against Mitogen-activated protein kinase p38 alpha 2 |
Bioorg Med Chem Lett 12: 1559-62 (2002)
BindingDB Entry DOI: 10.7270/Q2CV4H28 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50114254
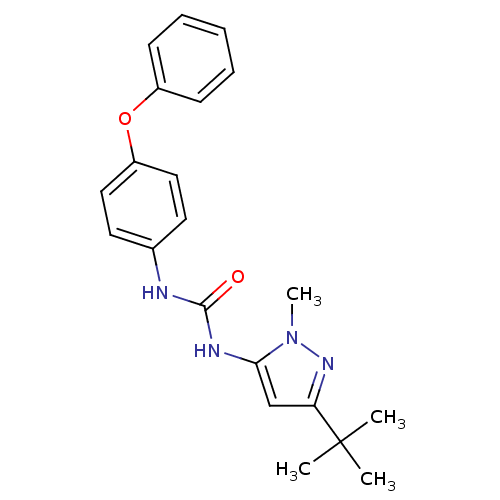
(1-(5-tert-Butyl-2-methyl-2H-pyrazol-3-yl)-3-(4-phe...)Show SMILES Cn1nc(cc1NC(=O)Nc1ccc(Oc2ccccc2)cc1)C(C)(C)C Show InChI InChI=1S/C21H24N4O2/c1-21(2,3)18-14-19(25(4)24-18)23-20(26)22-15-10-12-17(13-11-15)27-16-8-6-5-7-9-16/h5-14H,1-4H3,(H2,22,23,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 270 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Research Center
Curated by ChEMBL
| Assay Description
Inhibitory activity against Mitogen-activated protein kinase p38 alpha 2 |
Bioorg Med Chem Lett 12: 1559-62 (2002)
BindingDB Entry DOI: 10.7270/Q2CV4H28 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50106077

((S)-1-[2-(4-Chloro-phenyl)-2-oxo-ethyl]-piperidine...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@@H]1CCCCN1CC(=O)c1ccc(Cl)cc1)\C=C\c1ccccc1 Show InChI InChI=1S/C27H33ClN2O2/c1-3-20(2)24(17-12-21-9-5-4-6-10-21)29-27(32)25-11-7-8-18-30(25)19-26(31)22-13-15-23(28)16-14-22/h4-6,9-10,12-17,20,24-25H,3,7-8,11,18-19H2,1-2H3,(H,29,32)/b17-12+/t20-,24+,25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 280 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Research Center
Curated by ChEMBL
| Assay Description
Inhibitory activity against cathepsin K |
Bioorg Med Chem Lett 11: 2951-4 (2001)
BindingDB Entry DOI: 10.7270/Q23R0S5W |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50114234
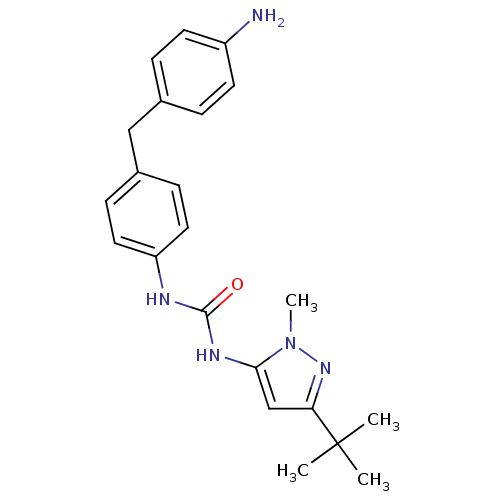
(1-[4-(4-Amino-benzyl)-phenyl]-3-(5-tert-butyl-2-me...)Show SMILES Cn1nc(cc1NC(=O)Nc1ccc(Cc2ccc(N)cc2)cc1)C(C)(C)C Show InChI InChI=1S/C22H27N5O/c1-22(2,3)19-14-20(27(4)26-19)25-21(28)24-18-11-7-16(8-12-18)13-15-5-9-17(23)10-6-15/h5-12,14H,13,23H2,1-4H3,(H2,24,25,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 290 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Research Center
Curated by ChEMBL
| Assay Description
Inhibitory activity against Mitogen-activated protein kinase p38 alpha 2 |
Bioorg Med Chem Lett 12: 1559-62 (2002)
BindingDB Entry DOI: 10.7270/Q2CV4H28 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50114241
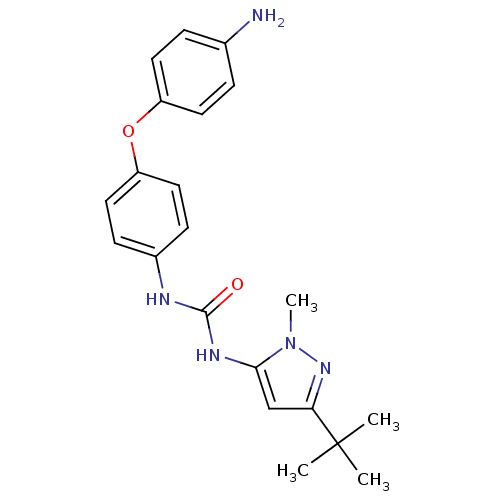
(1-[4-(4-Amino-phenoxy)-phenyl]-3-(5-tert-butyl-2-m...)Show SMILES Cn1nc(cc1NC(=O)Nc1ccc(Oc2ccc(N)cc2)cc1)C(C)(C)C Show InChI InChI=1S/C21H25N5O2/c1-21(2,3)18-13-19(26(4)25-18)24-20(27)23-15-7-11-17(12-8-15)28-16-9-5-14(22)6-10-16/h5-13H,22H2,1-4H3,(H2,23,24,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 310 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Research Center
Curated by ChEMBL
| Assay Description
Inhibitory activity against Mitogen-activated protein kinase p38 alpha 2 |
Bioorg Med Chem Lett 12: 1559-62 (2002)
BindingDB Entry DOI: 10.7270/Q2CV4H28 |
More data for this
Ligand-Target Pair | |
Glucagon receptor
(Homo sapiens (Human)) | BDBM50109331
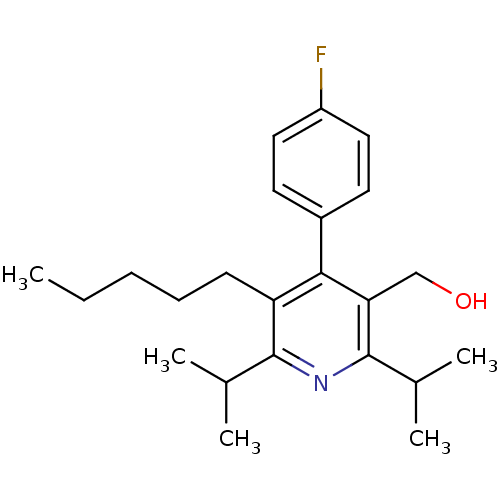
(CHEMBL24616 | [4-(4-Fluoro-phenyl)-2,6-diisopropyl...)Show SMILES CCCCCc1c(nc(C(C)C)c(CO)c1-c1ccc(F)cc1)C(C)C Show InChI InChI=1S/C23H32FNO/c1-6-7-8-9-19-21(17-10-12-18(24)13-11-17)20(14-26)23(16(4)5)25-22(19)15(2)3/h10-13,15-16,26H,6-9,14H2,1-5H3 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Research Center
Curated by ChEMBL
| Assay Description
Tested for its ability to inhibit cAMP production in human glucagon receptor expressed CHO cells |
Bioorg Med Chem Lett 12: 1303-6 (2002)
BindingDB Entry DOI: 10.7270/Q25H7FK0 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data