 Found 1017 hits with Last Name = 'sali' and Initial = 'a'
Found 1017 hits with Last Name = 'sali' and Initial = 'a' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50020217
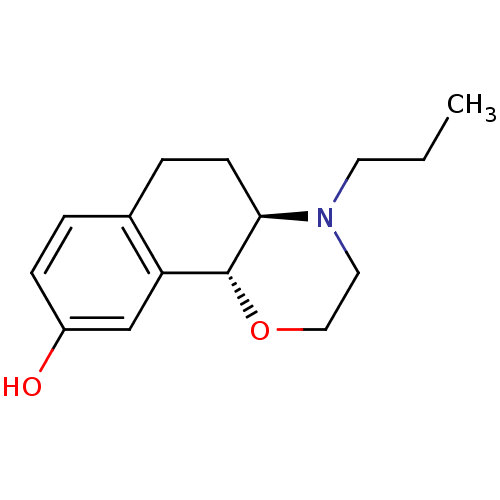
((4aR,10bR)-4-propyl-3,4,4a,5,6,10b-hexahydro-2H-na...)Show InChI InChI=1S/C15H21NO2/c1-2-7-16-8-9-18-15-13-10-12(17)5-3-11(13)4-6-14(15)16/h3,5,10,14-15,17H,2,4,6-9H2,1H3/t14-,15-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Imperial College London
Curated by ChEMBL
| Assay Description
Agonistic activity at DRD3 receptor |
Bioorg Med Chem Lett 19: 5056-9 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.055
BindingDB Entry DOI: 10.7270/Q2PR7X7C |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50414563
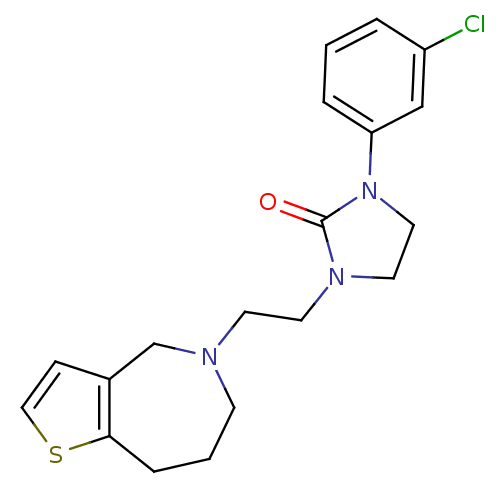
(CHEMBL559873)Show InChI InChI=1S/C19H22ClN3OS/c20-16-3-1-4-17(13-16)23-11-10-22(19(23)24)9-8-21-7-2-5-18-15(14-21)6-12-25-18/h1,3-4,6,12-13H,2,5,7-11,14H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Imperial College London
Curated by ChEMBL
| Assay Description
Binding affinity to human DRD3 receptor by GTPgammaS binding assay |
Bioorg Med Chem Lett 19: 5056-9 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.055
BindingDB Entry DOI: 10.7270/Q2PR7X7C |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50331549
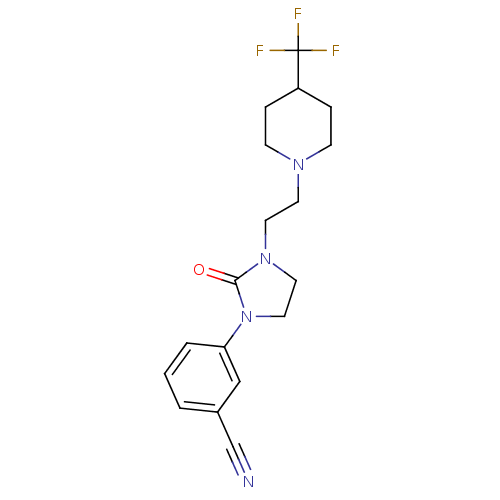
(CHEMBL567286 | [11C]-3-(2-oxo-3-(2-(4-(trifluorome...)Show SMILES FC(F)(F)C1CCN(CCN2CCN(C2=O)c2cccc(c2)C#N)CC1 Show InChI InChI=1S/C18H21F3N4O/c19-18(20,21)15-4-6-23(7-5-15)8-9-24-10-11-25(17(24)26)16-3-1-2-14(12-16)13-22/h1-3,12,15H,4-11H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Imperial College London
Curated by ChEMBL
| Assay Description
Antagonistic activity at human DRD3 receptor by filtration binding assay |
Bioorg Med Chem Lett 19: 5056-9 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.055
BindingDB Entry DOI: 10.7270/Q2PR7X7C |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Rattus norvegicus (Rat)) | BDBM50331549

(CHEMBL567286 | [11C]-3-(2-oxo-3-(2-(4-(trifluorome...)Show SMILES FC(F)(F)C1CCN(CCN2CCN(C2=O)c2cccc(c2)C#N)CC1 Show InChI InChI=1S/C18H21F3N4O/c19-18(20,21)15-4-6-23(7-5-15)8-9-24-10-11-25(17(24)26)16-3-1-2-14(12-16)13-22/h1-3,12,15H,4-11H2 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Imperial College London
Curated by ChEMBL
| Assay Description
Antagonistic activity at rat DRD3 receptor |
Bioorg Med Chem Lett 19: 5056-9 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.055
BindingDB Entry DOI: 10.7270/Q2PR7X7C |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50414574

(CHEMBL563580)Show SMILES Clc1cccc(c1)N1CCN(CCN2[C@H]3CC[C@@H]2CCC3)C1=O |r,TLB:12:13:19.18.20:16.15| Show InChI InChI=1S/C18H24ClN3O/c19-14-3-1-6-17(13-14)22-12-10-20(18(22)23)9-11-21-15-4-2-5-16(21)8-7-15/h1,3,6,13,15-16H,2,4-5,7-12H2/t15-,16+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Imperial College London
Curated by ChEMBL
| Assay Description
Binding affinity to human DRD3 receptor by GTPgammaS binding assay |
Bioorg Med Chem Lett 19: 5056-9 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.055
BindingDB Entry DOI: 10.7270/Q2PR7X7C |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50411402
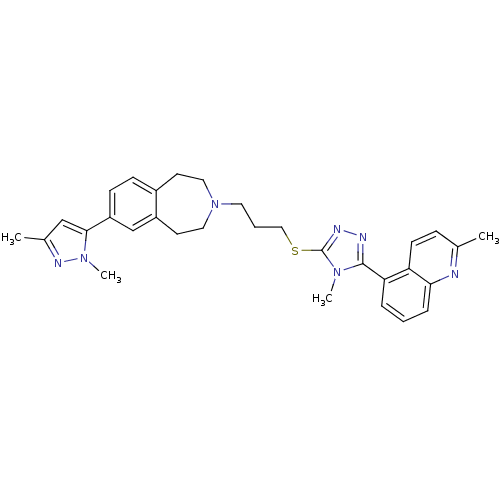
(CHEMBL244946)Show SMILES Cc1cc(-c2ccc3CCN(CCCSc4nnc(-c5cccc6nc(C)ccc56)n4C)CCc3c2)n(C)n1 Show InChI InChI=1S/C31H35N7S/c1-21-9-12-26-27(7-5-8-28(26)32-21)30-33-34-31(36(30)3)39-18-6-15-38-16-13-23-10-11-25(20-24(23)14-17-38)29-19-22(2)35-37(29)4/h5,7-12,19-20H,6,13-18H2,1-4H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.58 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Imperial College London
Curated by ChEMBL
| Assay Description
Binding affinity to human DRD3 receptor by GTPgammaS binding assay |
Bioorg Med Chem Lett 19: 5056-9 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.055
BindingDB Entry DOI: 10.7270/Q2PR7X7C |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50414578
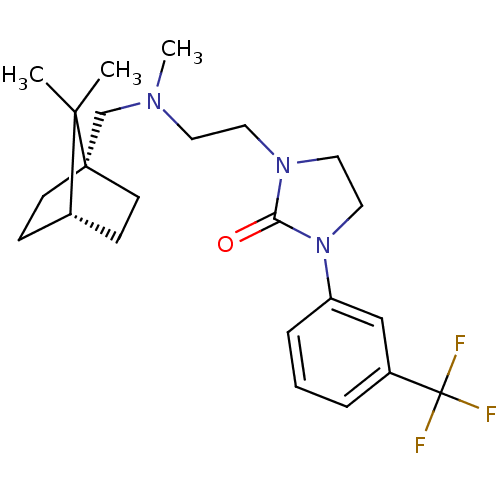
(CHEMBL558058)Show SMILES CN(CCN1CCN(C1=O)c1cccc(c1)C(F)(F)F)C[C@@]12CC[C@@H](CC1)C2(C)C |r| Show InChI InChI=1S/C23H32F3N3O/c1-21(2)17-7-9-22(21,10-8-17)16-27(3)11-12-28-13-14-29(20(28)30)19-6-4-5-18(15-19)23(24,25)26/h4-6,15,17H,7-14,16H2,1-3H3/t17-,22+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.58 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Imperial College London
Curated by ChEMBL
| Assay Description
Binding affinity to human DRD3 receptor by GTPgammaS binding assay |
Bioorg Med Chem Lett 19: 5056-9 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.055
BindingDB Entry DOI: 10.7270/Q2PR7X7C |
More data for this
Ligand-Target Pair | |
Sigma non-opioid intracellular receptor 1
(Homo sapiens (Human)) | BDBM50251208
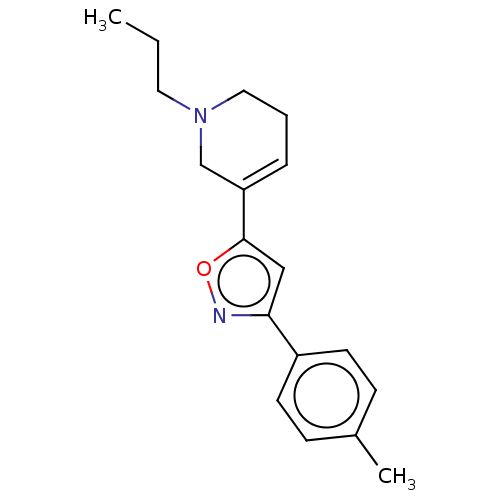
(CHEMBL4088272)Show InChI InChI=1S/C18H22N2O/c1-3-10-20-11-4-5-16(13-20)18-12-17(19-21-18)15-8-6-14(2)7-9-15/h5-9,12H,3-4,10-11,13H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
QBI COVID-19 Research Group (QCRG)
Curated by ChEMBL
| Assay Description
Displacement of [3H]-pentazocin from the Sigma1 receptor |
Nature 583: 459-468 (2020)
Article DOI: 10.1038/s41586-020-2286-9
BindingDB Entry DOI: 10.7270/Q29Z984K |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Sigma non-opioid intracellular receptor 1
(Homo sapiens (Human)) | BDBM50048866

(1-Cyclohexyl-4-[3-(5-methoxy-1,2,3,4-tetrahydro-na...)Show InChI InChI=1S/C24H38N2O/c1-27-24-14-6-12-22-20(8-5-13-23(22)24)9-7-15-25-16-18-26(19-17-25)21-10-3-2-4-11-21/h6,12,14,20-21H,2-5,7-11,13,15-19H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
QBI COVID-19 Research Group (QCRG)
Curated by ChEMBL
| Assay Description
Displacement of [3H]-pentazocin from the Sigma1 receptor |
Nature 583: 459-468 (2020)
Article DOI: 10.1038/s41586-020-2286-9
BindingDB Entry DOI: 10.7270/Q29Z984K |
More data for this
Ligand-Target Pair | |
Sigma intracellular receptor 2
(Homo sapiens (Human)) | BDBM50048866

(1-Cyclohexyl-4-[3-(5-methoxy-1,2,3,4-tetrahydro-na...)Show InChI InChI=1S/C24H38N2O/c1-27-24-14-6-12-22-20(8-5-13-23(22)24)9-7-15-25-16-18-26(19-17-25)21-10-3-2-4-11-21/h6,12,14,20-21H,2-5,7-11,13,15-19H2,1H3 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
QBI COVID-19 Research Group (QCRG)
Curated by ChEMBL
| Assay Description
Displacement of [3H]-DTG from the Sigma2 receptor |
Nature 583: 459-468 (2020)
Article DOI: 10.1038/s41586-020-2286-9
BindingDB Entry DOI: 10.7270/Q29Z984K |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50414403

(CHEMBL560177)Show InChI InChI=1S/C19H20ClN3O/c20-17-6-3-7-18(12-17)23-11-10-22(19(23)24)9-8-21-13-15-4-1-2-5-16(15)14-21/h1-7,12H,8-11,13-14H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.51 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Imperial College London
Curated by ChEMBL
| Assay Description
Binding affinity to human DRD3 receptor by GTPgammaS binding assay |
Bioorg Med Chem Lett 19: 5056-9 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.055
BindingDB Entry DOI: 10.7270/Q2PR7X7C |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50414557

(CHEMBL562464)Show SMILES Clc1cccc(c1)N1CCN(CCN2C[C@@H]3CC[C@H]2Cc2ccccc2C3)C1=O |r| Show InChI InChI=1S/C24H28ClN3O/c25-21-6-3-7-23(16-21)28-13-12-26(24(28)29)10-11-27-17-18-8-9-22(27)15-20-5-2-1-4-19(20)14-18/h1-7,16,18,22H,8-15,17H2/t18-,22+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 2.51 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Imperial College London
Curated by ChEMBL
| Assay Description
Binding affinity to human DRD3 receptor by GTPgammaS binding assay |
Bioorg Med Chem Lett 19: 5056-9 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.055
BindingDB Entry DOI: 10.7270/Q2PR7X7C |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50414570

(CHEMBL563635)Show InChI InChI=1S/C15H22ClN3O/c1-3-17(4-2)8-9-18-10-11-19(15(18)20)14-7-5-6-13(16)12-14/h5-7,12H,3-4,8-11H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.51 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Imperial College London
Curated by ChEMBL
| Assay Description
Binding affinity to human DRD3 receptor by GTPgammaS binding assay |
Bioorg Med Chem Lett 19: 5056-9 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.055
BindingDB Entry DOI: 10.7270/Q2PR7X7C |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50414572
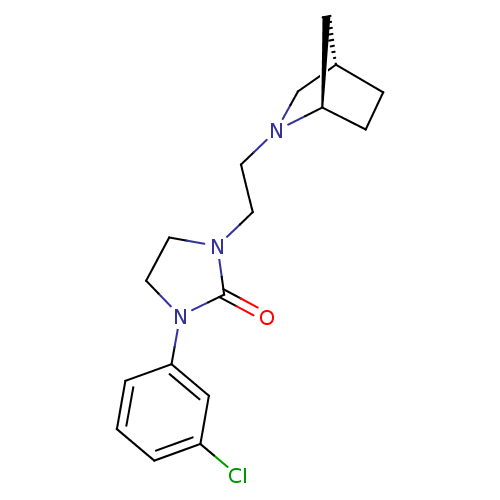
(CHEMBL563860)Show SMILES Clc1cccc(c1)N1CCN(CCN2C[C@H]3CC[C@@H]2C3)C1=O |r,TLB:12:13:17.16:19| Show InChI InChI=1S/C17H22ClN3O/c18-14-2-1-3-16(11-14)21-9-8-19(17(21)22)6-7-20-12-13-4-5-15(20)10-13/h1-3,11,13,15H,4-10,12H2/t13-,15+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.51 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Imperial College London
Curated by ChEMBL
| Assay Description
Binding affinity to human DRD3 receptor by GTPgammaS binding assay |
Bioorg Med Chem Lett 19: 5056-9 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.055
BindingDB Entry DOI: 10.7270/Q2PR7X7C |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50414563

(CHEMBL559873)Show InChI InChI=1S/C19H22ClN3OS/c20-16-3-1-4-17(13-16)23-11-10-22(19(23)24)9-8-21-7-2-5-18-15(14-21)6-12-25-18/h1,3-4,6,12-13H,2,5,7-11,14H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 3.16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Imperial College London
Curated by ChEMBL
| Assay Description
Binding affinity to human DRD2 receptor by GTPgammaS binding assay |
Bioorg Med Chem Lett 19: 5056-9 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.055
BindingDB Entry DOI: 10.7270/Q2PR7X7C |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50414555
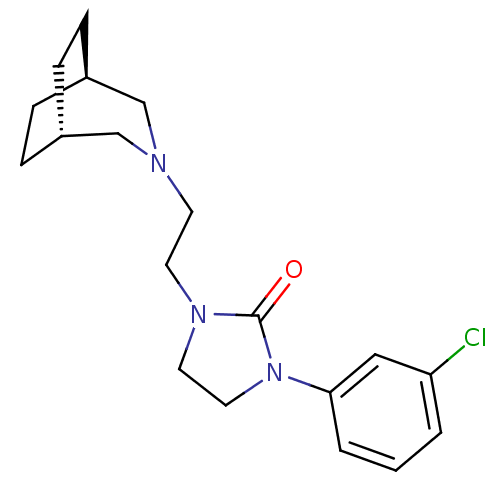
(CHEMBL565075)Show SMILES Clc1cccc(c1)N1CCN(CCN2C[C@H]3CC[C@H](CC3)C2)C1=O |r,wD:15.21,18.19,(12.76,-.71,;12.72,-2.25,;11.37,-2.98,;11.33,-4.53,;12.64,-5.34,;14,-4.6,;14.04,-3.05,;15.44,-5.15,;15.87,-6.63,;17.41,-6.67,;17.92,-5.22,;19.25,-4.44,;20.59,-5.2,;21.92,-4.42,;22.75,-2.96,;24.43,-2.94,;25.71,-3.64,;25.72,-5.11,;24.45,-5.86,;23.16,-5.16,;23.15,-3.69,;22.77,-5.87,;16.7,-4.28,;16.74,-2.74,)| Show InChI InChI=1S/C19H26ClN3O/c20-17-2-1-3-18(12-17)23-11-10-22(19(23)24)9-8-21-13-15-4-5-16(14-21)7-6-15/h1-3,12,15-16H,4-11,13-14H2/t15-,16+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Imperial College London
Curated by ChEMBL
| Assay Description
Binding affinity to human DRD3 receptor by GTPgammaS binding assay |
Bioorg Med Chem Lett 19: 5056-9 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.055
BindingDB Entry DOI: 10.7270/Q2PR7X7C |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50414572

(CHEMBL563860)Show SMILES Clc1cccc(c1)N1CCN(CCN2C[C@H]3CC[C@@H]2C3)C1=O |r,TLB:12:13:17.16:19| Show InChI InChI=1S/C17H22ClN3O/c18-14-2-1-3-16(11-14)21-9-8-19(17(21)22)6-7-20-12-13-4-5-15(20)10-13/h1-3,11,13,15H,4-10,12H2/t13-,15+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.98 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Imperial College London
Curated by ChEMBL
| Assay Description
Binding affinity to human DRD2 receptor by GTPgammaS binding assay |
Bioorg Med Chem Lett 19: 5056-9 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.055
BindingDB Entry DOI: 10.7270/Q2PR7X7C |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50370572

(CHEMBL85606 | SB-277011)Show SMILES O=C(N[C@H]1CC[C@H](CCN2CCc3cc(ccc3C2)C#N)CC1)c1ccnc2ccccc12 |r,wU:3.2,wD:6.6,(-5.17,2.38,;-5.16,.84,;-3.83,.08,;-2.49,.85,;-2.5,2.39,;-1.16,3.16,;.18,2.39,;1.51,3.16,;2.84,2.39,;4.18,3.16,;4.17,4.69,;5.51,5.46,;6.84,4.68,;8.18,5.44,;9.51,4.67,;9.5,3.12,;8.16,2.36,;6.83,3.14,;5.51,2.38,;10.85,5.43,;12.18,6.19,;.17,.85,;-1.15,.08,;-6.49,.07,;-7.83,.83,;-9.16,.06,;-9.16,-1.48,;-7.83,-2.25,;-7.83,-3.79,;-6.49,-4.56,;-5.15,-3.79,;-5.16,-2.25,;-6.49,-1.48,)| Show InChI InChI=1S/C28H30N4O/c29-18-21-5-8-23-19-32(16-13-22(23)17-21)15-12-20-6-9-24(10-7-20)31-28(33)26-11-14-30-27-4-2-1-3-25(26)27/h1-5,8,11,14,17,20,24H,6-7,9-10,12-13,15-16,19H2,(H,31,33)/t20-,24- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.98 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Imperial College London
Curated by ChEMBL
| Assay Description
Binding affinity to human DRD3 receptor by GTPgammaS binding assay |
Bioorg Med Chem Lett 19: 5056-9 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.055
BindingDB Entry DOI: 10.7270/Q2PR7X7C |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Bos taurus) | BDBM50029909
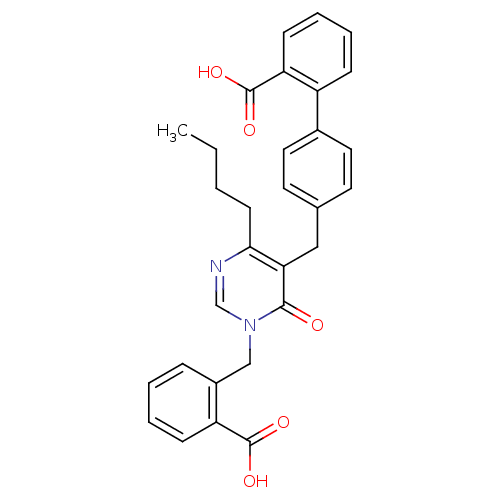
(4'-[4-Butyl-1-(2-carboxy-benzyl)-6-oxo-1,6-dihydro...)Show SMILES CCCCc1ncn(Cc2ccccc2C(O)=O)c(=O)c1Cc1ccc(cc1)-c1ccccc1C(O)=O Show InChI InChI=1S/C30H28N2O5/c1-2-3-12-27-26(28(33)32(19-31-27)18-22-8-4-5-10-24(22)29(34)35)17-20-13-15-21(16-14-20)23-9-6-7-11-25(23)30(36)37/h4-11,13-16,19H,2-3,12,17-18H2,1H3,(H,34,35)(H,36,37) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| >4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto Lusofarmaco
Curated by ChEMBL
| Assay Description
Binding affinity towards Angiotensin II type 2 receptor in bovine cerebellar cortical membranes; Inactive |
J Med Chem 38: 4806-20 (1996)
BindingDB Entry DOI: 10.7270/Q2JQ101N |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Bos taurus) | BDBM50029907
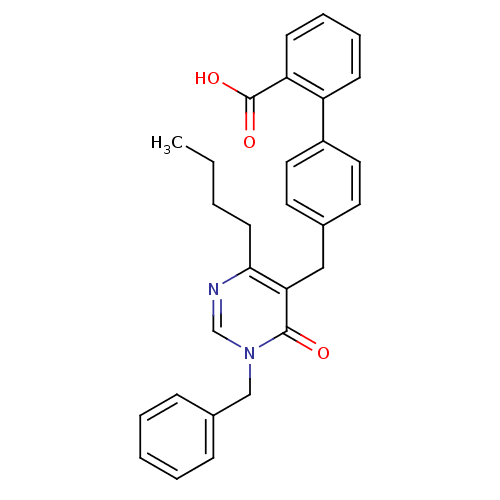
(4'-(1-Benzyl-4-butyl-6-oxo-1,6-dihydro-pyrimidin-5...)Show SMILES CCCCc1ncn(Cc2ccccc2)c(=O)c1Cc1ccc(cc1)-c1ccccc1C(O)=O Show InChI InChI=1S/C29H28N2O3/c1-2-3-13-27-26(28(32)31(20-30-27)19-22-9-5-4-6-10-22)18-21-14-16-23(17-15-21)24-11-7-8-12-25(24)29(33)34/h4-12,14-17,20H,2-3,13,18-19H2,1H3,(H,33,34) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| >4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto Lusofarmaco
Curated by ChEMBL
| Assay Description
Binding affinity towards Angiotensin II type 2 receptor in bovine cerebellar cortical membranes; Inactive |
J Med Chem 38: 4806-20 (1996)
BindingDB Entry DOI: 10.7270/Q2JQ101N |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Bos taurus) | BDBM50029910
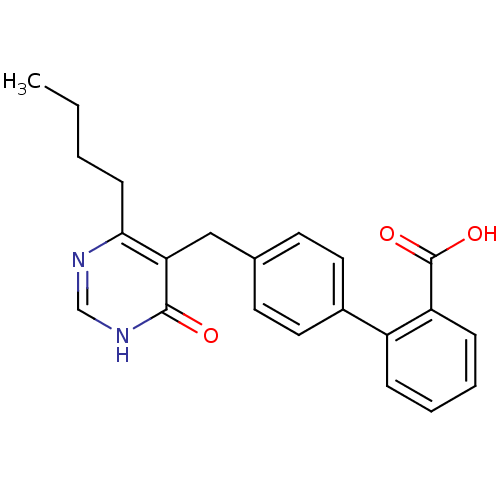
(4'-(4-Butyl-6-oxo-1,6-dihydro-pyrimidin-5-ylmethyl...)Show SMILES CCCCc1nc[nH]c(=O)c1Cc1ccc(cc1)-c1ccccc1C(O)=O Show InChI InChI=1S/C22H22N2O3/c1-2-3-8-20-19(21(25)24-14-23-20)13-15-9-11-16(12-10-15)17-6-4-5-7-18(17)22(26)27/h4-7,9-12,14H,2-3,8,13H2,1H3,(H,26,27)(H,23,24,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| >4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto Lusofarmaco
Curated by ChEMBL
| Assay Description
Binding affinity towards Angiotensin II type 2 receptor in bovine cerebellar cortical membranes; Inactive |
J Med Chem 38: 4806-20 (1996)
BindingDB Entry DOI: 10.7270/Q2JQ101N |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Bos taurus) | BDBM50029911
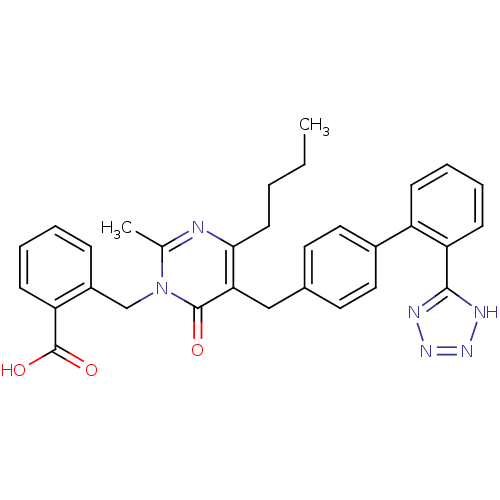
(2-{4-Butyl-2-methyl-6-oxo-5-[2'-(1H-tetrazol-5-yl)...)Show SMILES CCCCc1nc(C)n(Cc2ccccc2C(O)=O)c(=O)c1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C31H30N6O3/c1-3-4-13-28-27(30(38)37(20(2)32-28)19-23-9-5-6-11-25(23)31(39)40)18-21-14-16-22(17-15-21)24-10-7-8-12-26(24)29-33-35-36-34-29/h5-12,14-17H,3-4,13,18-19H2,1-2H3,(H,39,40)(H,33,34,35,36) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| >4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto Lusofarmaco
Curated by ChEMBL
| Assay Description
Binding affinity towards Angiotensin II type 2 receptor in bovine cerebellar cortical membranes; Inactive |
J Med Chem 38: 4806-20 (1996)
BindingDB Entry DOI: 10.7270/Q2JQ101N |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Bos taurus) | BDBM50029903
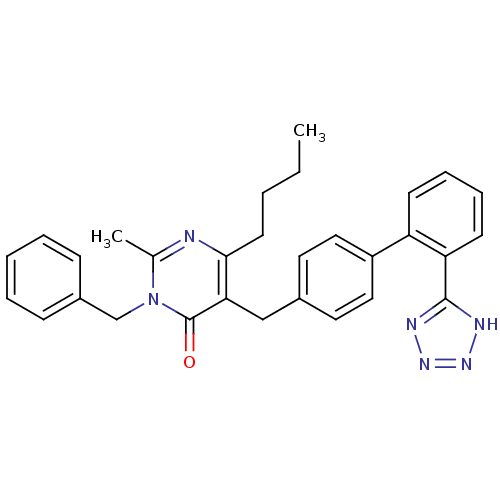
(3-Benzyl-6-butyl-2-methyl-5-[2'-(1H-tetrazol-5-yl)...)Show SMILES CCCCc1nc(C)n(Cc2ccccc2)c(=O)c1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C30H30N6O/c1-3-4-14-28-27(30(37)36(21(2)31-28)20-23-10-6-5-7-11-23)19-22-15-17-24(18-16-22)25-12-8-9-13-26(25)29-32-34-35-33-29/h5-13,15-18H,3-4,14,19-20H2,1-2H3,(H,32,33,34,35) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| >4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto Lusofarmaco
Curated by ChEMBL
| Assay Description
Binding affinity towards Angiotensin II type 2 receptor in bovine cerebellar cortical membranes; Inactive |
J Med Chem 38: 4806-20 (1996)
BindingDB Entry DOI: 10.7270/Q2JQ101N |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Bos taurus) | BDBM50029906

(2-[4-Butyl-5-(2'-carboxy-biphenyl-4-ylmethyl)-2-me...)Show SMILES CCCCc1nc(C)n(Cc2sccc2C(O)=O)c(=O)c1Cc1ccc(cc1)-c1ccccc1C(O)=O Show InChI InChI=1S/C29H28N2O5S/c1-3-4-9-25-24(27(32)31(18(2)30-25)17-26-23(29(35)36)14-15-37-26)16-19-10-12-20(13-11-19)21-7-5-6-8-22(21)28(33)34/h5-8,10-15H,3-4,9,16-17H2,1-2H3,(H,33,34)(H,35,36) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| >4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto Lusofarmaco
Curated by ChEMBL
| Assay Description
Binding affinity towards Angiotensin II type 2 receptor in bovine cerebellar cortical membranes; Inactive |
J Med Chem 38: 4806-20 (1996)
BindingDB Entry DOI: 10.7270/Q2JQ101N |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Bos taurus) | BDBM50029893
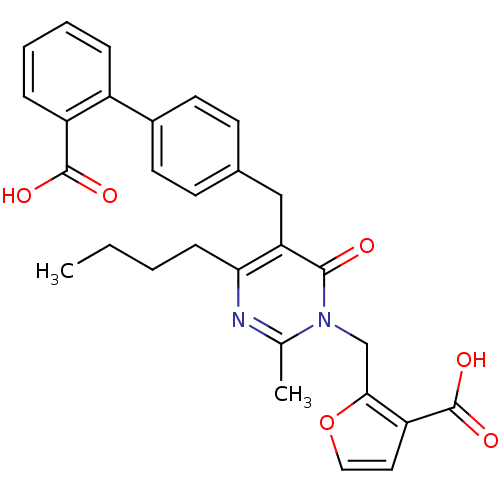
(2-[4-Butyl-5-(2'-carboxy-biphenyl-4-ylmethyl)-2-me...)Show SMILES CCCCc1nc(C)n(Cc2occc2C(O)=O)c(=O)c1Cc1ccc(cc1)-c1ccccc1C(O)=O Show InChI InChI=1S/C29H28N2O6/c1-3-4-9-25-24(27(32)31(18(2)30-25)17-26-23(29(35)36)14-15-37-26)16-19-10-12-20(13-11-19)21-7-5-6-8-22(21)28(33)34/h5-8,10-15H,3-4,9,16-17H2,1-2H3,(H,33,34)(H,35,36) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| >4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto Lusofarmaco
Curated by ChEMBL
| Assay Description
Binding affinity towards Angiotensin II type 2 receptor in bovine cerebellar cortical membranes; Inactive |
J Med Chem 38: 4806-20 (1996)
BindingDB Entry DOI: 10.7270/Q2JQ101N |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Bos taurus) | BDBM50029908

(4'-(4-Butyl-6-oxo-1-thiophen-2-ylmethyl-1,6-dihydr...)Show SMILES CCCCc1ncn(Cc2cccs2)c(=O)c1Cc1ccc(cc1)-c1ccccc1C(O)=O Show InChI InChI=1S/C27H26N2O3S/c1-2-3-10-25-24(26(30)29(18-28-25)17-21-7-6-15-33-21)16-19-11-13-20(14-12-19)22-8-4-5-9-23(22)27(31)32/h4-9,11-15,18H,2-3,10,16-17H2,1H3,(H,31,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| >4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto Lusofarmaco
Curated by ChEMBL
| Assay Description
Binding affinity towards Angiotensin II type 2 receptor in bovine cerebellar cortical membranes; Inactive |
J Med Chem 38: 4806-20 (1996)
BindingDB Entry DOI: 10.7270/Q2JQ101N |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Bos taurus) | BDBM50029899

(3-{4-Butyl-2-methyl-6-oxo-5-[2'-(1H-tetrazol-5-yl)...)Show SMILES CCCCc1nc(C)n(Cc2ccsc2C(=O)OC)c(=O)c1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C30H30N6O3S/c1-4-5-10-26-25(29(37)36(19(2)31-26)18-22-15-16-40-27(22)30(38)39-3)17-20-11-13-21(14-12-20)23-8-6-7-9-24(23)28-32-34-35-33-28/h6-9,11-16H,4-5,10,17-18H2,1-3H3,(H,32,33,34,35) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| >4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto Lusofarmaco
Curated by ChEMBL
| Assay Description
Binding affinity towards Angiotensin II type 2 receptor in bovine cerebellar cortical membranes; Inactive |
J Med Chem 38: 4806-20 (1996)
BindingDB Entry DOI: 10.7270/Q2JQ101N |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Bos taurus) | BDBM50029905

(4'-(2-Butyl-4-methyl-6-oxo-6H-pyrimidin-1-ylmethyl...)Show SMILES CCCCc1nc(C)cc(=O)n1Cc1ccc(cc1)-c1ccccc1C(O)=O Show InChI InChI=1S/C23H24N2O3/c1-3-4-9-21-24-16(2)14-22(26)25(21)15-17-10-12-18(13-11-17)19-7-5-6-8-20(19)23(27)28/h5-8,10-14H,3-4,9,15H2,1-2H3,(H,27,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| >4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto Lusofarmaco
Curated by ChEMBL
| Assay Description
Binding affinity towards Angiotensin II type 2 receptor in bovine cerebellar cortical membranes; Inactive |
J Med Chem 38: 4806-20 (1996)
BindingDB Entry DOI: 10.7270/Q2JQ101N |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Bos taurus) | BDBM50029895
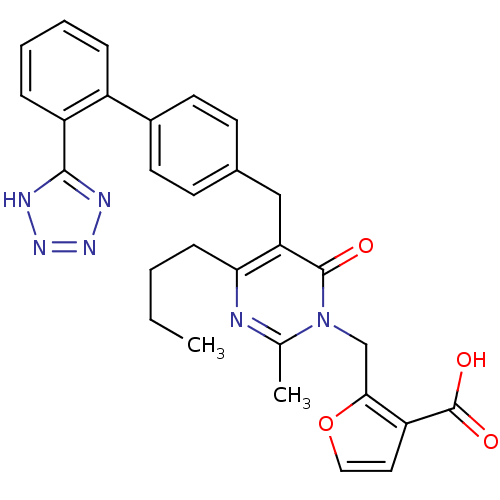
(2-{4-Butyl-2-methyl-6-oxo-5-[2'-(1H-tetrazol-5-yl)...)Show SMILES CCCCc1nc(C)n(Cc2occc2C(O)=O)c(=O)c1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C29H28N6O4/c1-3-4-9-25-24(28(36)35(18(2)30-25)17-26-23(29(37)38)14-15-39-26)16-19-10-12-20(13-11-19)21-7-5-6-8-22(21)27-31-33-34-32-27/h5-8,10-15H,3-4,9,16-17H2,1-2H3,(H,37,38)(H,31,32,33,34) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| >4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto Lusofarmaco
Curated by ChEMBL
| Assay Description
Binding affinity towards Angiotensin II type 2 receptor in bovine cerebellar cortical membranes; Inactive |
J Med Chem 38: 4806-20 (1996)
BindingDB Entry DOI: 10.7270/Q2JQ101N |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Bos taurus) | BDBM50029894

(4'-(4-Butyl-2-methyl-6-oxo-1,6-dihydro-pyrimidin-5...)Show SMILES CCCCc1nc(C)[nH]c(=O)c1Cc1ccc(cc1)-c1ccccc1C(O)=O Show InChI InChI=1S/C23H24N2O3/c1-3-4-9-21-20(22(26)25-15(2)24-21)14-16-10-12-17(13-11-16)18-7-5-6-8-19(18)23(27)28/h5-8,10-13H,3-4,9,14H2,1-2H3,(H,27,28)(H,24,25,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| >4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto Lusofarmaco
Curated by ChEMBL
| Assay Description
Binding affinity towards Angiotensin II type 2 receptor in bovine cerebellar cortical membranes; Inactive |
J Med Chem 38: 4806-20 (1996)
BindingDB Entry DOI: 10.7270/Q2JQ101N |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Bos taurus) | BDBM50029904

(4'-[4-Butyl-1-(2-carboxy-benzyl)-2-methyl-6-oxo-1,...)Show SMILES CCCCc1nc(C)n(Cc2ccccc2C(O)=O)c(=O)c1Cc1ccc(cc1)-c1ccccc1C(O)=O Show InChI InChI=1S/C31H30N2O5/c1-3-4-13-28-27(18-21-14-16-22(17-15-21)24-10-7-8-12-26(24)31(37)38)29(34)33(20(2)32-28)19-23-9-5-6-11-25(23)30(35)36/h5-12,14-17H,3-4,13,18-19H2,1-2H3,(H,35,36)(H,37,38) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| >4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto Lusofarmaco
Curated by ChEMBL
| Assay Description
Binding affinity towards Angiotensin II type 2 receptor in bovine cerebellar cortical membranes; Inactive |
J Med Chem 38: 4806-20 (1996)
BindingDB Entry DOI: 10.7270/Q2JQ101N |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Bos taurus) | BDBM50029902
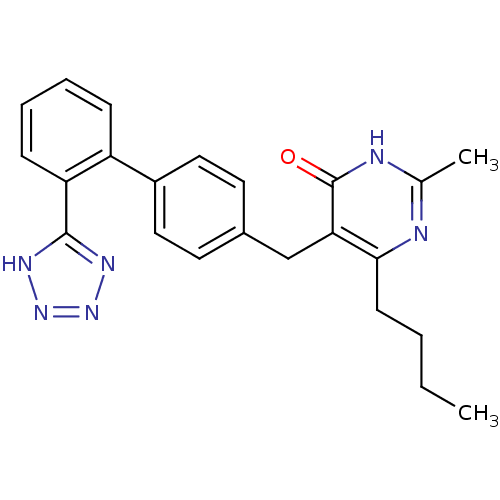
(6-Butyl-2-methyl-5-[2'-(1H-tetrazol-5-yl)-biphenyl...)Show SMILES CCCCc1nc(C)[nH]c(=O)c1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C23H24N6O/c1-3-4-9-21-20(23(30)25-15(2)24-21)14-16-10-12-17(13-11-16)18-7-5-6-8-19(18)22-26-28-29-27-22/h5-8,10-13H,3-4,9,14H2,1-2H3,(H,24,25,30)(H,26,27,28,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| >4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto Lusofarmaco
Curated by ChEMBL
| Assay Description
Binding affinity towards Angiotensin II type 2 receptor in bovine cerebellar cortical membranes; Inactive |
J Med Chem 38: 4806-20 (1996)
BindingDB Entry DOI: 10.7270/Q2JQ101N |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Bos taurus) | BDBM50029897

(4'-[4-Butyl-1-(4-carboxy-benzyl)-6-oxo-1,6-dihydro...)Show SMILES CCCCc1ncn(Cc2ccc(cc2)C(O)=O)c(=O)c1Cc1ccc(cc1)-c1ccccc1C(O)=O Show InChI InChI=1S/C30H28N2O5/c1-2-3-8-27-26(28(33)32(19-31-27)18-21-11-15-23(16-12-21)29(34)35)17-20-9-13-22(14-10-20)24-6-4-5-7-25(24)30(36)37/h4-7,9-16,19H,2-3,8,17-18H2,1H3,(H,34,35)(H,36,37) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| >4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto Lusofarmaco
Curated by ChEMBL
| Assay Description
Binding affinity towards Angiotensin II type 2 receptor in bovine cerebellar cortical membranes; Inactive |
J Med Chem 38: 4806-20 (1996)
BindingDB Entry DOI: 10.7270/Q2JQ101N |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Bos taurus) | BDBM50029901
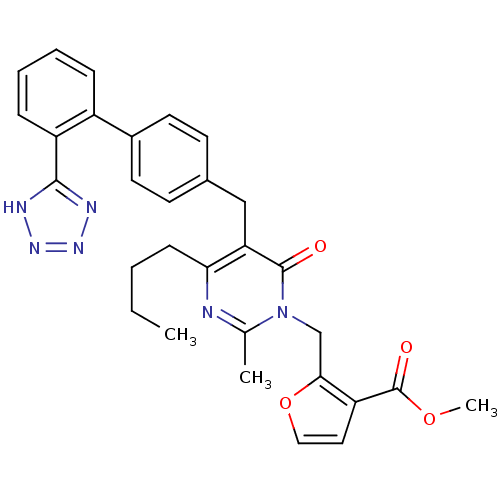
(2-{4-Butyl-2-methyl-6-oxo-5-[2'-(1H-tetrazol-5-yl)...)Show SMILES CCCCc1nc(C)n(Cc2occc2C(=O)OC)c(=O)c1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C30H30N6O4/c1-4-5-10-26-25(29(37)36(19(2)31-26)18-27-24(15-16-40-27)30(38)39-3)17-20-11-13-21(14-12-20)22-8-6-7-9-23(22)28-32-34-35-33-28/h6-9,11-16H,4-5,10,17-18H2,1-3H3,(H,32,33,34,35) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| >4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto Lusofarmaco
Curated by ChEMBL
| Assay Description
Binding affinity towards Angiotensin II type 2 receptor in bovine cerebellar cortical membranes; Inactive |
J Med Chem 38: 4806-20 (1996)
BindingDB Entry DOI: 10.7270/Q2JQ101N |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Bos taurus) | BDBM50241501

(6-Butyl-2-methyl-5-[2'-(1H-tetrazol-5-yl)-biphenyl...)Show SMILES CCCCc1nc(C)n(Cc2cccs2)c(=O)c1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C28H28N6OS/c1-3-4-11-26-25(28(35)34(19(2)29-26)18-22-8-7-16-36-22)17-20-12-14-21(15-13-20)23-9-5-6-10-24(23)27-30-32-33-31-27/h5-10,12-16H,3-4,11,17-18H2,1-2H3,(H,30,31,32,33) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| >4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto Lusofarmaco
Curated by ChEMBL
| Assay Description
Binding affinity towards Angiotensin II type 2 receptor in bovine cerebellar cortical membranes; Inactive |
J Med Chem 38: 4806-20 (1996)
BindingDB Entry DOI: 10.7270/Q2JQ101N |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Bos taurus) | BDBM50241491
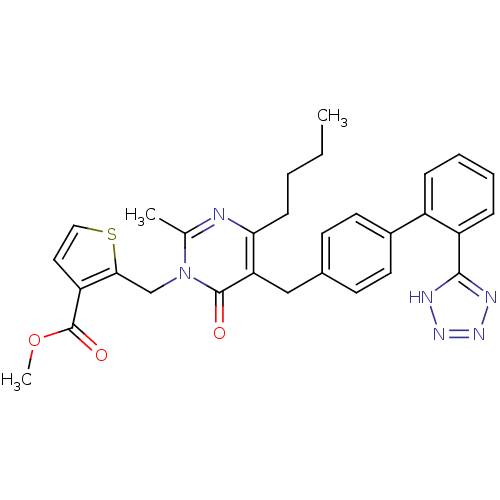
(2-{4-Butyl-2-methyl-6-oxo-5-[2'-(1H-tetrazol-5-yl)...)Show SMILES CCCCc1nc(C)n(Cc2sccc2C(=O)OC)c(=O)c1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C30H30N6O3S/c1-4-5-10-26-25(29(37)36(19(2)31-26)18-27-24(15-16-40-27)30(38)39-3)17-20-11-13-21(14-12-20)22-8-6-7-9-23(22)28-32-34-35-33-28/h6-9,11-16H,4-5,10,17-18H2,1-3H3,(H,32,33,34,35) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| >4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto Lusofarmaco
Curated by ChEMBL
| Assay Description
Binding affinity towards Angiotensin II type 2 receptor in bovine cerebellar cortical membranes; Inactive |
J Med Chem 38: 4806-20 (1996)
BindingDB Entry DOI: 10.7270/Q2JQ101N |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Bos taurus) | BDBM50029896

(4'-(2-Butyl-6-oxo-6H-pyrimidin-1-ylmethyl)-bipheny...)Show InChI InChI=1S/C22H22N2O3/c1-2-3-8-20-23-14-13-21(25)24(20)15-16-9-11-17(12-10-16)18-6-4-5-7-19(18)22(26)27/h4-7,9-14H,2-3,8,15H2,1H3,(H,26,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| >4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto Lusofarmaco
Curated by ChEMBL
| Assay Description
Binding affinity towards Angiotensin II type 2 receptor in bovine cerebellar cortical membranes; Inactive |
J Med Chem 38: 4806-20 (1996)
BindingDB Entry DOI: 10.7270/Q2JQ101N |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Bos taurus) | BDBM50029892

(2-{4-Butyl-2-methyl-6-oxo-5-[2'-(1H-tetrazol-5-yl)...)Show SMILES CCCCc1nc(C)n(Cc2sccc2C(O)=O)c(=O)c1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C29H28N6O3S/c1-3-4-9-25-24(28(36)35(18(2)30-25)17-26-23(29(37)38)14-15-39-26)16-19-10-12-20(13-11-19)21-7-5-6-8-22(21)27-31-33-34-32-27/h5-8,10-15H,3-4,9,16-17H2,1-2H3,(H,37,38)(H,31,32,33,34) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| >4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto Lusofarmaco
Curated by ChEMBL
| Assay Description
Binding affinity towards Angiotensin II type 2 receptor in bovine cerebellar cortical membranes; Inactive |
J Med Chem 38: 4806-20 (1996)
BindingDB Entry DOI: 10.7270/Q2JQ101N |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50414566
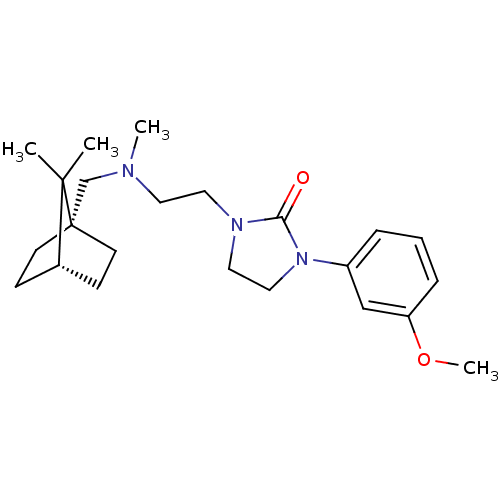
(CHEMBL563435)Show SMILES COc1cccc(c1)N1CCN(CCN(C)C[C@@]23CC[C@@H](CC2)C3(C)C)C1=O |r| Show InChI InChI=1S/C23H35N3O2/c1-22(2)18-8-10-23(22,11-9-18)17-24(3)12-13-25-14-15-26(21(25)27)19-6-5-7-20(16-19)28-4/h5-7,16,18H,8-15,17H2,1-4H3/t18-,23+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.01 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Imperial College London
Curated by ChEMBL
| Assay Description
Binding affinity to human DRD3 receptor by GTPgammaS binding assay |
Bioorg Med Chem Lett 19: 5056-9 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.055
BindingDB Entry DOI: 10.7270/Q2PR7X7C |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50414574

(CHEMBL563580)Show SMILES Clc1cccc(c1)N1CCN(CCN2[C@H]3CC[C@@H]2CCC3)C1=O |r,TLB:12:13:19.18.20:16.15| Show InChI InChI=1S/C18H24ClN3O/c19-14-3-1-6-17(13-14)22-12-10-20(18(22)23)9-11-21-15-4-2-5-16(21)8-7-15/h1,3,6,13,15-16H,2,4-5,7-12H2/t15-,16+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.01 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Imperial College London
Curated by ChEMBL
| Assay Description
Binding affinity to human DRD2 receptor by GTPgammaS binding assay |
Bioorg Med Chem Lett 19: 5056-9 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.055
BindingDB Entry DOI: 10.7270/Q2PR7X7C |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50020217

((4aR,10bR)-4-propyl-3,4,4a,5,6,10b-hexahydro-2H-na...)Show InChI InChI=1S/C15H21NO2/c1-2-7-16-8-9-18-15-13-10-12(17)5-3-11(13)4-6-14(15)16/h3,5,10,14-15,17H,2,4,6-9H2,1H3/t14-,15-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 6.31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Imperial College London
Curated by ChEMBL
| Assay Description
Agonistic activity at DRD2 receptor |
Bioorg Med Chem Lett 19: 5056-9 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.055
BindingDB Entry DOI: 10.7270/Q2PR7X7C |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50414565

(CHEMBL563274)Show SMILES CN(CCN1CCN(C1=O)c1cccc(Cl)c1)C[C@@]12CC[C@@H](CC1)C2(C)C |r| Show InChI InChI=1S/C22H32ClN3O/c1-21(2)17-7-9-22(21,10-8-17)16-24(3)11-12-25-13-14-26(20(25)27)19-6-4-5-18(23)15-19/h4-6,15,17H,7-14,16H2,1-3H3/t17-,22+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Imperial College London
Curated by ChEMBL
| Assay Description
Binding affinity to human DRD3 receptor by GTPgammaS binding assay |
Bioorg Med Chem Lett 19: 5056-9 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.055
BindingDB Entry DOI: 10.7270/Q2PR7X7C |
More data for this
Ligand-Target Pair | |
Sigma non-opioid intracellular receptor 1
(Homo sapiens (Human)) | BDBM50253157

((+)-(2R)-2-(2-(((R)-p-chloro-alpha-methyl-alpha-ph...)Show SMILES CN1CCC[C@@H]1CCO[C@](C)(c1ccccc1)c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C21H26ClNO/c1-21(17-7-4-3-5-8-17,18-10-12-19(22)13-11-18)24-16-14-20-9-6-15-23(20)2/h3-5,7-8,10-13,20H,6,9,14-16H2,1-2H3/t20-,21-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
QBI COVID-19 Research Group (QCRG)
Curated by ChEMBL
| Assay Description
Displacement of [3H]-pentazocin from the Sigma1 receptor |
Nature 583: 459-468 (2020)
Article DOI: 10.1038/s41586-020-2286-9
BindingDB Entry DOI: 10.7270/Q29Z984K |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50414567

(CHEMBL562948)Show SMILES CN(CCN1CCN(C1=O)c1ccccc1)C[C@@]12CC[C@@H](CC1)C2(C)C |r| Show InChI InChI=1S/C22H33N3O/c1-21(2)18-9-11-22(21,12-10-18)17-23(3)13-14-24-15-16-25(20(24)26)19-7-5-4-6-8-19/h4-8,18H,9-17H2,1-3H3/t18-,22+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Imperial College London
Curated by ChEMBL
| Assay Description
Binding affinity to human DRD3 receptor by GTPgammaS binding assay |
Bioorg Med Chem Lett 19: 5056-9 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.055
BindingDB Entry DOI: 10.7270/Q2PR7X7C |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50414571
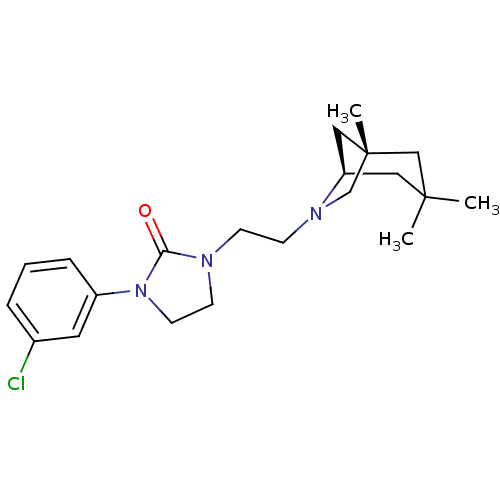
(CHEMBL563563)Show SMILES C[C@@]12C[C@@H](CC(C)(C)C1)N(CCN1CCN(C1=O)c1cccc(Cl)c1)C2 |r| Show InChI InChI=1S/C21H30ClN3O/c1-20(2)12-18-13-21(3,14-20)15-24(18)8-7-23-9-10-25(19(23)26)17-6-4-5-16(22)11-17/h4-6,11,18H,7-10,12-15H2,1-3H3/t18-,21-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 12.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Imperial College London
Curated by ChEMBL
| Assay Description
Binding affinity to human DRD3 receptor by GTPgammaS binding assay |
Bioorg Med Chem Lett 19: 5056-9 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.055
BindingDB Entry DOI: 10.7270/Q2PR7X7C |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50414554

(CHEMBL551927)Show SMILES C[C@]12C[C@H](CC(C1)c1ccccc1)N(CCN1CCN(C1=O)c1cccc(Cl)c1)C2 |r,THB:14:13:2:5.6.4,7:5:2:29.13| Show InChI InChI=1S/C25H30ClN3O/c1-25-16-20(19-6-3-2-4-7-19)14-23(17-25)28(18-25)11-10-27-12-13-29(24(27)30)22-9-5-8-21(26)15-22/h2-9,15,20,23H,10-14,16-18H2,1H3/t20?,23-,25+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 15.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Imperial College London
Curated by ChEMBL
| Assay Description
Binding affinity to human DRD2 receptor by GTPgammaS binding assay |
Bioorg Med Chem Lett 19: 5056-9 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.055
BindingDB Entry DOI: 10.7270/Q2PR7X7C |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50414556
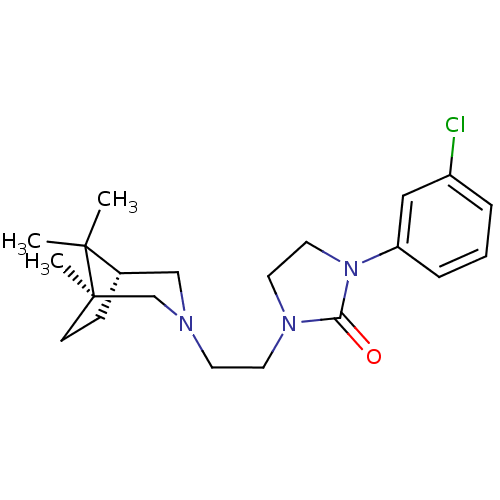
(CHEMBL564930)Show SMILES CC1(C)[C@H]2CC[C@]1(C)CN(CCN1CCN(C1=O)c1cccc(Cl)c1)C2 |r,THB:10:9:1:5.4| Show InChI InChI=1S/C21H30ClN3O/c1-20(2)16-7-8-21(20,3)15-23(14-16)9-10-24-11-12-25(19(24)26)18-6-4-5-17(22)13-18/h4-6,13,16H,7-12,14-15H2,1-3H3/t16-,21+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 15.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Imperial College London
Curated by ChEMBL
| Assay Description
Binding affinity to human DRD3 receptor by GTPgammaS binding assay |
Bioorg Med Chem Lett 19: 5056-9 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.055
BindingDB Entry DOI: 10.7270/Q2PR7X7C |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50414578

(CHEMBL558058)Show SMILES CN(CCN1CCN(C1=O)c1cccc(c1)C(F)(F)F)C[C@@]12CC[C@@H](CC1)C2(C)C |r| Show InChI InChI=1S/C23H32F3N3O/c1-21(2)17-7-9-22(21,10-8-17)16-27(3)11-12-28-13-14-29(20(28)30)19-6-4-5-18(15-19)23(24,25)26/h4-6,15,17H,7-14,16H2,1-3H3/t17-,22+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 19.9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Imperial College London
Curated by ChEMBL
| Assay Description
Binding affinity to human DRD2 receptor by GTPgammaS binding assay |
Bioorg Med Chem Lett 19: 5056-9 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.055
BindingDB Entry DOI: 10.7270/Q2PR7X7C |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50414554

(CHEMBL551927)Show SMILES C[C@]12C[C@H](CC(C1)c1ccccc1)N(CCN1CCN(C1=O)c1cccc(Cl)c1)C2 |r,THB:14:13:2:5.6.4,7:5:2:29.13| Show InChI InChI=1S/C25H30ClN3O/c1-25-16-20(19-6-3-2-4-7-19)14-23(17-25)28(18-25)11-10-27-12-13-29(24(27)30)22-9-5-8-21(26)15-22/h2-9,15,20,23H,10-14,16-18H2,1H3/t20?,23-,25+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 19.9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Imperial College London
Curated by ChEMBL
| Assay Description
Binding affinity to human DRD3 receptor by GTPgammaS binding assay |
Bioorg Med Chem Lett 19: 5056-9 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.055
BindingDB Entry DOI: 10.7270/Q2PR7X7C |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50414581

(CHEMBL560270)Show SMILES CN(CCN1CCN(C1=O)c1cccc(C)c1)C[C@@]12CC[C@@H](CC1)C2(C)C |r| Show InChI InChI=1S/C23H35N3O/c1-18-6-5-7-20(16-18)26-15-14-25(21(26)27)13-12-24(4)17-23-10-8-19(9-11-23)22(23,2)3/h5-7,16,19H,8-15,17H2,1-4H3/t19-,23+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 19.9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Imperial College London
Curated by ChEMBL
| Assay Description
Binding affinity to human DRD3 receptor by GTPgammaS binding assay |
Bioorg Med Chem Lett 19: 5056-9 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.055
BindingDB Entry DOI: 10.7270/Q2PR7X7C |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data