

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Histidinol dehydrogenase, chloroplastic (Brassica oleracea var. capitata) | BDBM50267976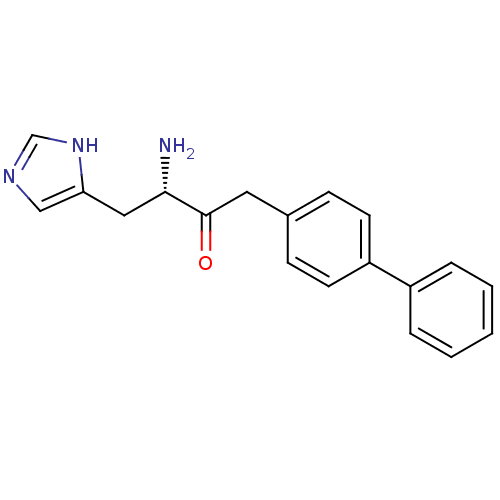 ((S)-3-Amino-1-biphenyl-4-yl-4-(1H-imidazol-4-yl)-b...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Brassica oleracea (cabbage) histidinol dehydrogenase pre-incubated for 10 min before substrate histidinol addition by spectrophotometry | Bioorg Med Chem Lett 6: 2131-2136 (1996) Article DOI: 10.1016/0960-894X(96)00384-8 BindingDB Entry DOI: 10.7270/Q2D79BC7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histidinol dehydrogenase, chloroplastic (Brassica oleracea var. capitata) | BDBM50267975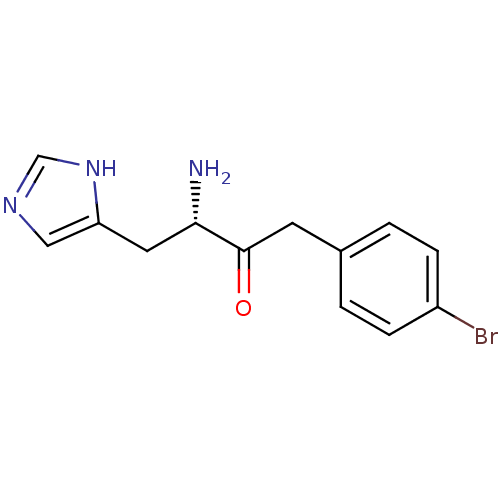 ((S)-3-Amino-1-(4-bromo-phenyl)-4-(1H-imidazol-4-yl...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Brassica oleracea (cabbage) histidinol dehydrogenase pre-incubated for 10 min before substrate histidinol addition by spectrophotometry | Bioorg Med Chem Lett 6: 2131-2136 (1996) Article DOI: 10.1016/0960-894X(96)00384-8 BindingDB Entry DOI: 10.7270/Q2D79BC7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histidinol dehydrogenase, chloroplastic (Brassica oleracea var. capitata) | BDBM50287735 ((S)-3-Amino-1-(3-amino-phenyl)-4-(1H-imidazol-4-yl...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 7.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Brassica oleracea (cabbage) histidinol dehydrogenase pre-incubated for 10 min before substrate histidinol addition by spectrophotometry | Bioorg Med Chem Lett 6: 2131-2136 (1996) Article DOI: 10.1016/0960-894X(96)00384-8 BindingDB Entry DOI: 10.7270/Q2D79BC7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histidinol dehydrogenase, chloroplastic (Brassica oleracea var. capitata) | BDBM50267972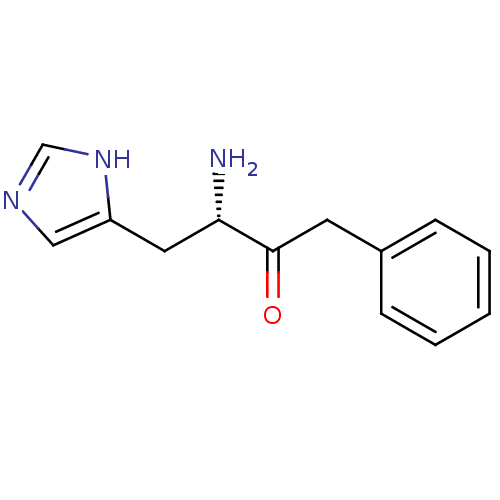 ((S)-3-Amino-4-(1H-imidazol-4-yl)-1-phenyl-butan-2-...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 7.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Brassica oleracea (cabbage) histidinol dehydrogenase pre-incubated for 10 min before substrate histidinol addition by spectrophotometry | Bioorg Med Chem Lett 6: 2131-2136 (1996) Article DOI: 10.1016/0960-894X(96)00384-8 BindingDB Entry DOI: 10.7270/Q2D79BC7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 4 [648-1032] (Homo sapiens (Human)) | BDBM243152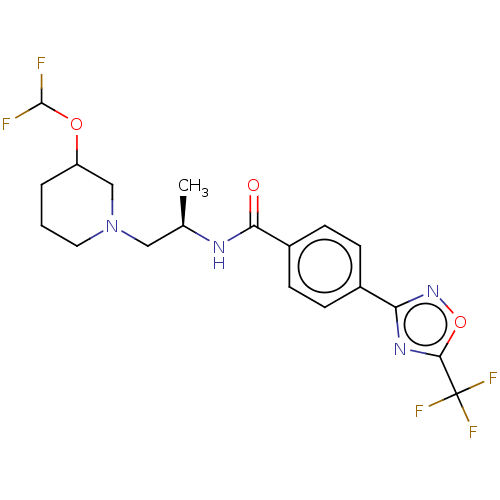 (D1: N-((R)-1-((abs)-3- (Difluoromethoxy)piperidin-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
CHDI FOUNDATION, INC. US Patent | Assay Description 5 μL of each solution of 1:20 diluted compound from above was transferred to a clear bottomed, black, 384-well assay plate using the Bravo or th... | US Patent US10053434 (2018) BindingDB Entry DOI: 10.7270/Q2MG7RJZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 4 (Homo sapiens (Human)) | BDBM228164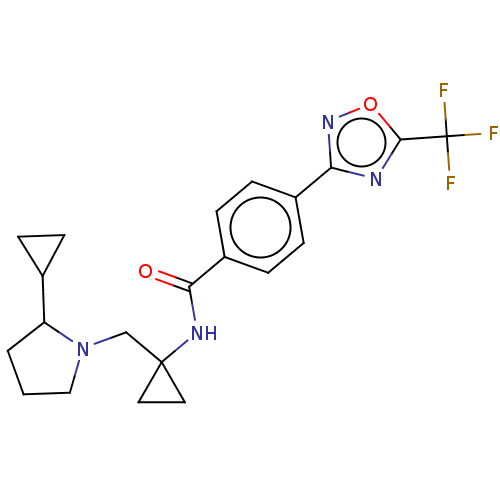 (US10047073, 6 | US10047073, 7) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
CHDI FOUNDATION, INC US Patent | Assay Description The Class I HDAC activity of Class IIa Histone Deacetylase (HDAC) inhibitors was quantified by measuring the cellular histone deacetylase enzymatic a... | US Patent US10047073 (2018) BindingDB Entry DOI: 10.7270/Q2PG1TQC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 4 [648-1032] (Homo sapiens (Human)) | BDBM243173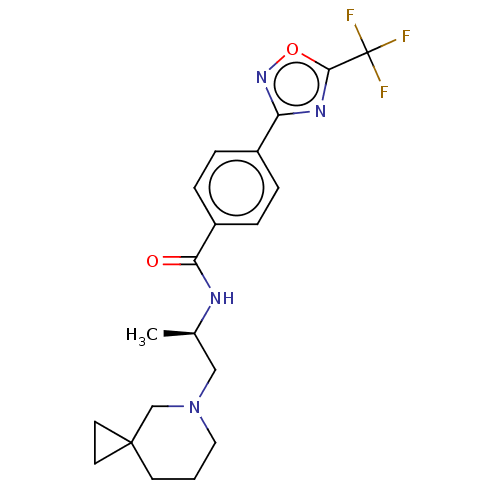 ((R)-N-(1-(5-Azaspiro[2.5]octan-5- yl)propan-2-yl)-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
CHDI FOUNDATION, INC. US Patent | Assay Description 5 μL of each solution of 1:20 diluted compound from above was transferred to a clear bottomed, black, 384-well assay plate using the Bravo or th... | US Patent US10053434 (2018) BindingDB Entry DOI: 10.7270/Q2MG7RJZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 4 [648-1032] (Homo sapiens (Human)) | BDBM243192 (N-((2R)-1-(3-Azabicyclo[3.2.0]heptan- 3-yl)propan-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
CHDI FOUNDATION, INC. US Patent | Assay Description 5 μL of each solution of 1:20 diluted compound from above was transferred to a clear bottomed, black, 384-well assay plate using the Bravo or th... | US Patent US10053434 (2018) BindingDB Entry DOI: 10.7270/Q2MG7RJZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 4 [648-1032] (Homo sapiens (Human)) | BDBM243193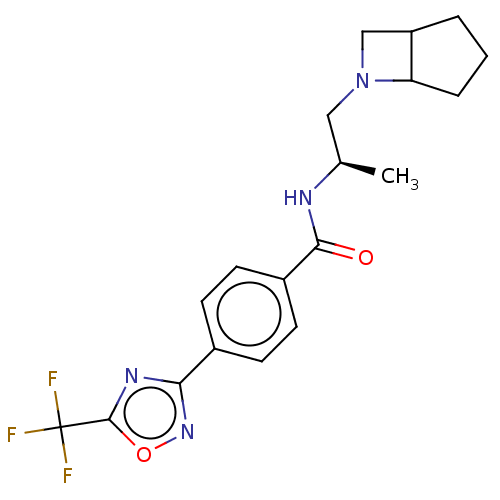 (D1: N-((R)-1-((abs-1,5-cis)-6- Azabicyclo[3.2.0]he...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
CHDI FOUNDATION, INC. US Patent | Assay Description 5 μL of each solution of 1:20 diluted compound from above was transferred to a clear bottomed, black, 384-well assay plate using the Bravo or th... | US Patent US10053434 (2018) BindingDB Entry DOI: 10.7270/Q2MG7RJZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 4 [648-1032] (Homo sapiens (Human)) | BDBM243189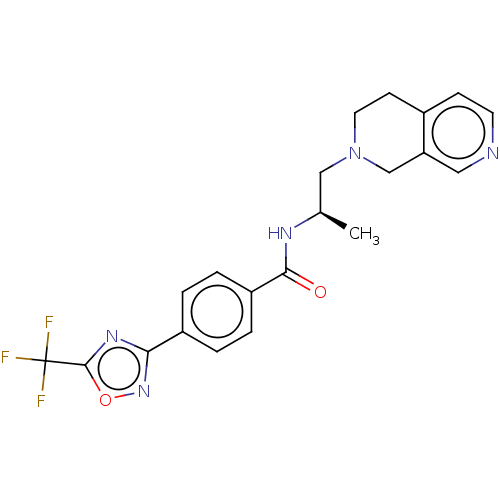 ((R)-N-(1-(3,4-dihydro-2,7-naphthyridin- 2(1H)-yl)p...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
CHDI FOUNDATION, INC. US Patent | Assay Description 5 μL of each solution of 1:20 diluted compound from above was transferred to a clear bottomed, black, 384-well assay plate using the Bravo or th... | US Patent US10053434 (2018) BindingDB Entry DOI: 10.7270/Q2MG7RJZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 4 [648-1032] (Homo sapiens (Human)) | BDBM243183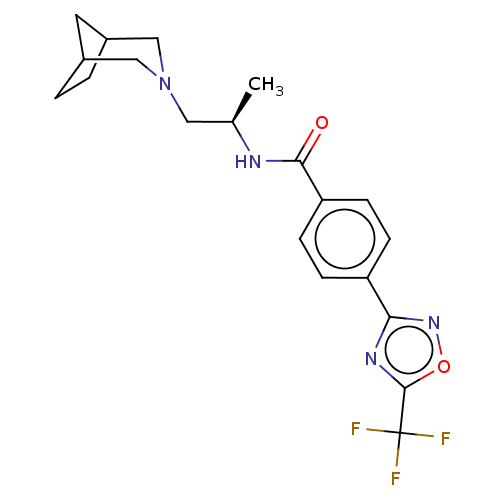 (N-((R)-1-(3-Azabicyclo[3.2.1]octan-3- yl)propan-2-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
CHDI FOUNDATION, INC. US Patent | Assay Description 5 μL of each solution of 1:20 diluted compound from above was transferred to a clear bottomed, black, 384-well assay plate using the Bravo or th... | US Patent US10053434 (2018) BindingDB Entry DOI: 10.7270/Q2MG7RJZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 4 (Homo sapiens (Human)) | BDBM228164 (US10047073, 6 | US10047073, 7) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
CHDI FOUNDATION, INC US Patent | Assay Description The Class I HDAC activity of Class IIa Histone Deacetylase (HDAC) inhibitors was quantified by measuring the cellular histone deacetylase enzymatic a... | US Patent US10047073 (2018) BindingDB Entry DOI: 10.7270/Q2PG1TQC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 4 [648-1032] (Homo sapiens (Human)) | BDBM243091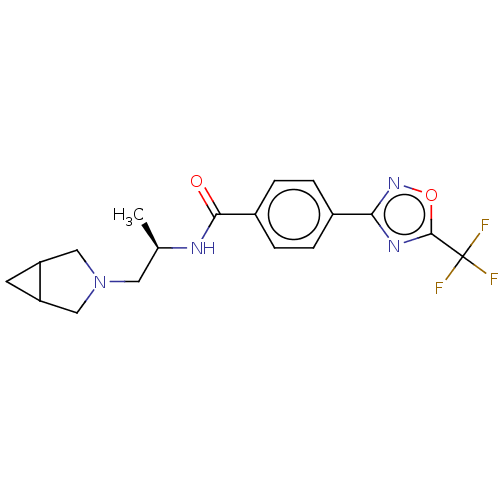 (N-((2R)-1-(3-Azabicyclo[3.1.0]hexan-3- yl)propan-2...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
CHDI FOUNDATION, INC. US Patent | Assay Description 5 μL of each solution of 1:20 diluted compound from above was transferred to a clear bottomed, black, 384-well assay plate using the Bravo or th... | US Patent US10053434 (2018) BindingDB Entry DOI: 10.7270/Q2MG7RJZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 4 [648-1032] (Homo sapiens (Human)) | BDBM243180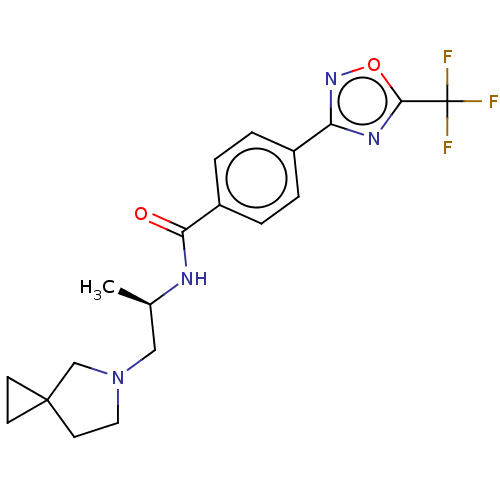 ((R)-N-(1-(5-Azaspiro[2.4]heptan-5- yl)propan-2-yl)...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
CHDI FOUNDATION, INC. US Patent | Assay Description 5 μL of each solution of 1:20 diluted compound from above was transferred to a clear bottomed, black, 384-well assay plate using the Bravo or th... | US Patent US10053434 (2018) BindingDB Entry DOI: 10.7270/Q2MG7RJZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 4 [648-1032] (Homo sapiens (Human)) | BDBM243121 ((R)-N-(1-(3,4-Dihydroisoquinolin- 2(1H)-yl)propan-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
CHDI FOUNDATION, INC. US Patent | Assay Description 5 μL of each solution of 1:20 diluted compound from above was transferred to a clear bottomed, black, 384-well assay plate using the Bravo or th... | US Patent US10053434 (2018) BindingDB Entry DOI: 10.7270/Q2MG7RJZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 4 [648-1032] (Homo sapiens (Human)) | BDBM243202 ((2S)-1-((R)-2-(3-Fluoro-4-(5- (trifluoromethyl)-1,...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
CHDI FOUNDATION, INC. US Patent | Assay Description 5 μL of each solution of 1:20 diluted compound from above was transferred to a clear bottomed, black, 384-well assay plate using the Bravo or th... | US Patent US10053434 (2018) BindingDB Entry DOI: 10.7270/Q2MG7RJZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 4 (Homo sapiens (Human)) | BDBM293911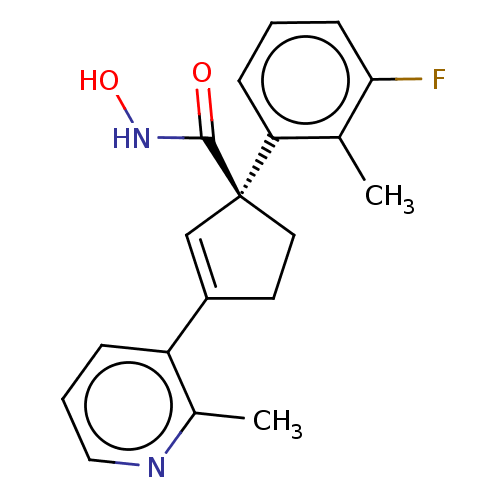 ((S)-1-(3-Fluoro-2- methylphenyl)-N- hydroxy-3-(2- ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
CHDI Foundation, Inc. US Patent | Assay Description 2 μl (200×) of each diluted solution and each control (full activity: 100% DMSO alone or full inhibition 1 mM) is stamped into V-bottomed polypr... | US Patent US9617259 (2017) BindingDB Entry DOI: 10.7270/Q2DN4743 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 4 (Homo sapiens (Human)) | BDBM293912 ((S)-1-(3-Fluoro-2- methylphenyl)-N- hydroxy-3-(1-m...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
CHDI Foundation, Inc. US Patent | Assay Description 2 μl (200×) of each diluted solution and each control (full activity: 100% DMSO alone or full inhibition 1 mM) is stamped into V-bottomed polypr... | US Patent US9617259 (2017) BindingDB Entry DOI: 10.7270/Q2DN4743 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 4 (Homo sapiens (Human)) | BDBM293914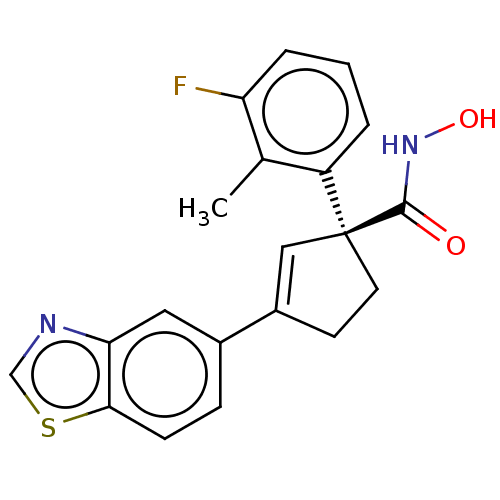 ((S)-3-(Benzo[d]thiazol-5- yl)-1-(3-fluoro-2- methy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
CHDI Foundation, Inc. US Patent | Assay Description 2 μl (200×) of each diluted solution and each control (full activity: 100% DMSO alone or full inhibition 1 mM) is stamped into V-bottomed polypr... | US Patent US9617259 (2017) BindingDB Entry DOI: 10.7270/Q2DN4743 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 4 (Homo sapiens (Human)) | BDBM293916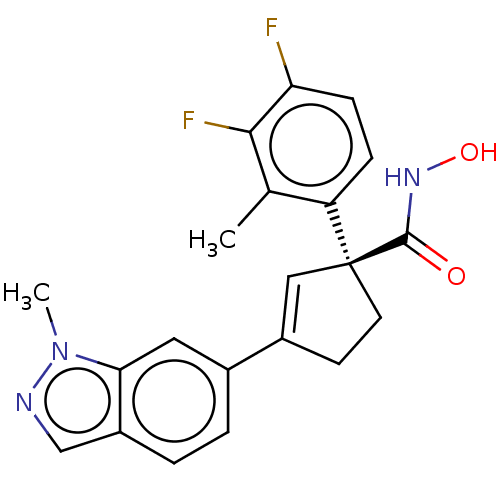 ((S)-1-(3,4-Difluoro-2- methylphenyl)-N- hydroxy-3-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
CHDI Foundation, Inc. US Patent | Assay Description 2 μl (200×) of each diluted solution and each control (full activity: 100% DMSO alone or full inhibition 1 mM) is stamped into V-bottomed polypr... | US Patent US9617259 (2017) BindingDB Entry DOI: 10.7270/Q2DN4743 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 4 (Homo sapiens (Human)) | BDBM293880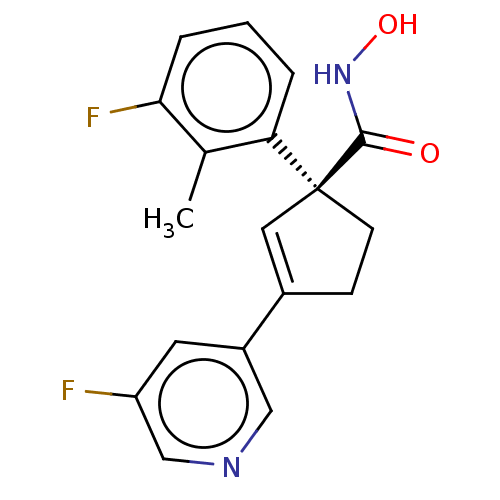 ((S)-1-(3-Fluoro-2- methylphenyl)-3-(5- fluoropyrid...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
CHDI Foundation, Inc. US Patent | Assay Description 2 μl (200×) of each diluted solution and each control (full activity: 100% DMSO alone or full inhibition 1 mM) is stamped into V-bottomed polypr... | US Patent US9617259 (2017) BindingDB Entry DOI: 10.7270/Q2DN4743 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 4 (Homo sapiens (Human)) | BDBM293886 ((S)-1-(3-Fluoro-2- methylphenyl)-N- hydroxy-3-(qui...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
CHDI Foundation, Inc. US Patent | Assay Description 2 μl (200×) of each diluted solution and each control (full activity: 100% DMSO alone or full inhibition 1 mM) is stamped into V-bottomed polypr... | US Patent US9617259 (2017) BindingDB Entry DOI: 10.7270/Q2DN4743 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 4 (Homo sapiens (Human)) | BDBM293895 ((S)-1-(3-Fluoro-2- methylphenyl)-N- hydroxy-3-(imi...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
CHDI Foundation, Inc. US Patent | Assay Description 2 μl (200×) of each diluted solution and each control (full activity: 100% DMSO alone or full inhibition 1 mM) is stamped into V-bottomed polypr... | US Patent US9617259 (2017) BindingDB Entry DOI: 10.7270/Q2DN4743 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 4 (Homo sapiens (Human)) | BDBM293899 ((S)-1-(3-Fluoro-2- methylphenyl)-N- hydroxy-3-(6- ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
CHDI Foundation, Inc. US Patent | Assay Description 2 μl (200×) of each diluted solution and each control (full activity: 100% DMSO alone or full inhibition 1 mM) is stamped into V-bottomed polypr... | US Patent US9617259 (2017) BindingDB Entry DOI: 10.7270/Q2DN4743 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 4 (Homo sapiens (Human)) | BDBM293900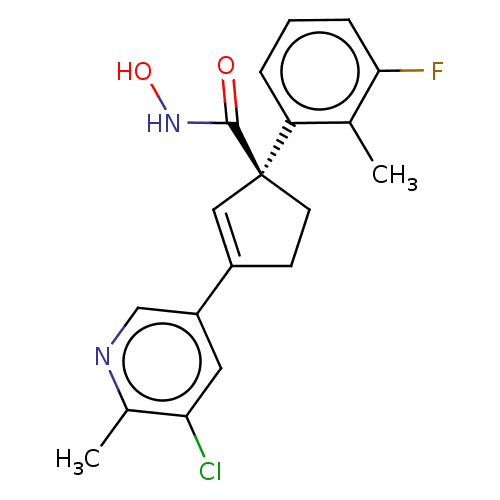 ((S)-3-(5-Chloro-6- methylpyridin-3-yl)-1-(3- fluor...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
CHDI Foundation, Inc. US Patent | Assay Description 2 μl (200×) of each diluted solution and each control (full activity: 100% DMSO alone or full inhibition 1 mM) is stamped into V-bottomed polypr... | US Patent US9617259 (2017) BindingDB Entry DOI: 10.7270/Q2DN4743 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 4 (Homo sapiens (Human)) | BDBM293880 ((S)-1-(3-Fluoro-2- methylphenyl)-3-(5- fluoropyrid...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
CHDI FOUNDATION, INC. US Patent | Assay Description 5 μl of each solution of 1:20 diluted compound from above is transferred to a clear bottomed, black, 384-well assay plate using the Bravo or the... | US Patent US10106535 (2018) BindingDB Entry DOI: 10.7270/Q2F47R6G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 4 (Homo sapiens (Human)) | BDBM293886 ((S)-1-(3-Fluoro-2- methylphenyl)-N- hydroxy-3-(qui...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
CHDI FOUNDATION, INC. US Patent | Assay Description 5 μl of each solution of 1:20 diluted compound from above is transferred to a clear bottomed, black, 384-well assay plate using the Bravo or the... | US Patent US10106535 (2018) BindingDB Entry DOI: 10.7270/Q2F47R6G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 4 (Homo sapiens (Human)) | BDBM293895 ((S)-1-(3-Fluoro-2- methylphenyl)-N- hydroxy-3-(imi...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
CHDI FOUNDATION, INC. US Patent | Assay Description 5 μl of each solution of 1:20 diluted compound from above is transferred to a clear bottomed, black, 384-well assay plate using the Bravo or the... | US Patent US10106535 (2018) BindingDB Entry DOI: 10.7270/Q2F47R6G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 4 (Homo sapiens (Human)) | BDBM293899 ((S)-1-(3-Fluoro-2- methylphenyl)-N- hydroxy-3-(6- ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
CHDI FOUNDATION, INC. US Patent | Assay Description 5 μl of each solution of 1:20 diluted compound from above is transferred to a clear bottomed, black, 384-well assay plate using the Bravo or the... | US Patent US10106535 (2018) BindingDB Entry DOI: 10.7270/Q2F47R6G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 4 (Homo sapiens (Human)) | BDBM293900 ((S)-3-(5-Chloro-6- methylpyridin-3-yl)-1-(3- fluor...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
CHDI FOUNDATION, INC. US Patent | Assay Description 5 μl of each solution of 1:20 diluted compound from above is transferred to a clear bottomed, black, 384-well assay plate using the Bravo or the... | US Patent US10106535 (2018) BindingDB Entry DOI: 10.7270/Q2F47R6G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 4 (Homo sapiens (Human)) | BDBM293911 ((S)-1-(3-Fluoro-2- methylphenyl)-N- hydroxy-3-(2- ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
CHDI FOUNDATION, INC. US Patent | Assay Description 5 μl of each solution of 1:20 diluted compound from above is transferred to a clear bottomed, black, 384-well assay plate using the Bravo or the... | US Patent US10106535 (2018) BindingDB Entry DOI: 10.7270/Q2F47R6G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 4 (Homo sapiens (Human)) | BDBM293912 ((S)-1-(3-Fluoro-2- methylphenyl)-N- hydroxy-3-(1-m...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
CHDI FOUNDATION, INC. US Patent | Assay Description 5 μl of each solution of 1:20 diluted compound from above is transferred to a clear bottomed, black, 384-well assay plate using the Bravo or the... | US Patent US10106535 (2018) BindingDB Entry DOI: 10.7270/Q2F47R6G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 4 (Homo sapiens (Human)) | BDBM293914 ((S)-3-(Benzo[d]thiazol-5- yl)-1-(3-fluoro-2- methy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
CHDI FOUNDATION, INC. US Patent | Assay Description 5 μl of each solution of 1:20 diluted compound from above is transferred to a clear bottomed, black, 384-well assay plate using the Bravo or the... | US Patent US10106535 (2018) BindingDB Entry DOI: 10.7270/Q2F47R6G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 4 (Homo sapiens (Human)) | BDBM293916 ((S)-1-(3,4-Difluoro-2- methylphenyl)-N- hydroxy-3-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
CHDI FOUNDATION, INC. US Patent | Assay Description 5 μl of each solution of 1:20 diluted compound from above is transferred to a clear bottomed, black, 384-well assay plate using the Bravo or the... | US Patent US10106535 (2018) BindingDB Entry DOI: 10.7270/Q2F47R6G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 4 [648-1032] (Homo sapiens (Human)) | BDBM243178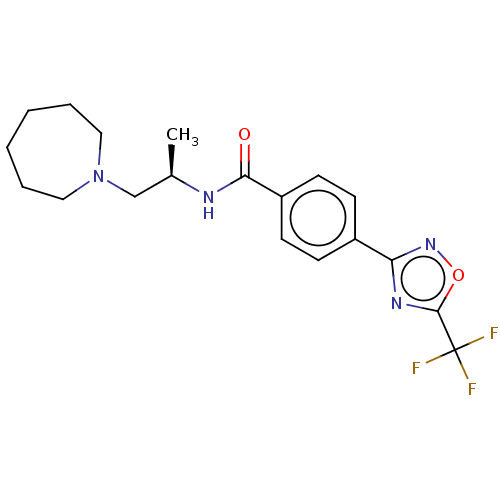 ((R)-N-(1-(Azepan-1-yl)propan-2-yl)-4- (5-(trifluor...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
CHDI FOUNDATION, INC. US Patent | Assay Description 5 μL of each solution of 1:20 diluted compound from above was transferred to a clear bottomed, black, 384-well assay plate using the Bravo or th... | US Patent US10053434 (2018) BindingDB Entry DOI: 10.7270/Q2MG7RJZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 9 (Homo sapiens (Human)) | BDBM272075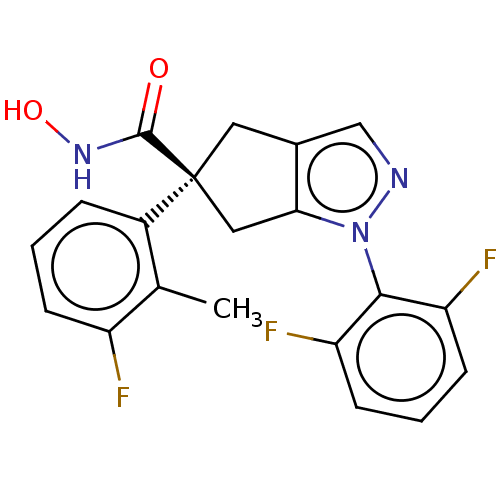 ((S)-1-(2,6-Difluorophenyl)-5-(3-fluoro-2- methylph...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
CHDI Foundation, Inc. US Patent | Assay Description The potency of the compounds is quantified by measuring the Histone Deacetylase 9 (HDAC9) enzymatic activity using the fluorogenic substrate, Boc-Lys... | US Patent US10457675 (2019) BindingDB Entry DOI: 10.7270/Q22J6F7J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 9 (Homo sapiens (Human)) | BDBM272075 ((S)-1-(2,6-Difluorophenyl)-5-(3-fluoro-2- methylph...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
CHDI Foundation, Inc. US Patent | Assay Description The potency of the compounds is quantified by measuring the Histone Deacetylase 9 (HDAC9) enzymatic activity using the fluorogenic substrate, Boc-Lys... | US Patent US10065948 (2018) BindingDB Entry DOI: 10.7270/Q2154K23 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 4 [648-1032] (Homo sapiens (Human)) | BDBM243187 (2-((R)-2-(4-(5-(Trifluoromethyl)-1,2,4- oxadiazol-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
CHDI FOUNDATION, INC. US Patent | Assay Description 5 μL of each solution of 1:20 diluted compound from above was transferred to a clear bottomed, black, 384-well assay plate using the Bravo or th... | US Patent US10053434 (2018) BindingDB Entry DOI: 10.7270/Q2MG7RJZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 4 [648-1032] (Homo sapiens (Human)) | BDBM243123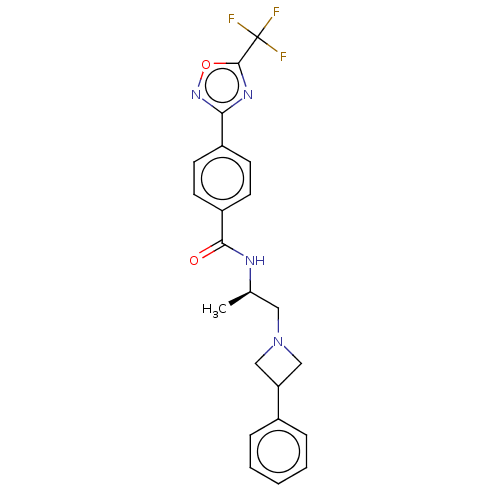 ((R)-N-(1-(3-Phenylazetidin-1-yl)propan- 2-yl)-4-(5...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
CHDI FOUNDATION, INC. US Patent | Assay Description 5 μL of each solution of 1:20 diluted compound from above was transferred to a clear bottomed, black, 384-well assay plate using the Bravo or th... | US Patent US10053434 (2018) BindingDB Entry DOI: 10.7270/Q2MG7RJZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 9 (Homo sapiens (Human)) | BDBM272071 ((S)-1-(2-Chlorophenyl)-5-(3-fluoro-2-methylphenyl)...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
CHDI Foundation, Inc. US Patent | Assay Description The potency of the compounds is quantified by measuring the Histone Deacetylase 9 (HDAC9) enzymatic activity using the fluorogenic substrate, Boc-Lys... | US Patent US10457675 (2019) BindingDB Entry DOI: 10.7270/Q22J6F7J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 9 (Homo sapiens (Human)) | BDBM272078 ((S)-1-(2-Chloro-6-fluorophenyl)-5-(3-fluoro-2- met...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
CHDI Foundation, Inc. US Patent | Assay Description The potency of the compounds is quantified by measuring the Histone Deacetylase 9 (HDAC9) enzymatic activity using the fluorogenic substrate, Boc-Lys... | US Patent US10457675 (2019) BindingDB Entry DOI: 10.7270/Q22J6F7J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 9 (Homo sapiens (Human)) | BDBM272071 ((S)-1-(2-Chlorophenyl)-5-(3-fluoro-2-methylphenyl)...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
CHDI Foundation, Inc. US Patent | Assay Description The potency of the compounds is quantified by measuring the Histone Deacetylase 9 (HDAC9) enzymatic activity using the fluorogenic substrate, Boc-Lys... | US Patent US10065948 (2018) BindingDB Entry DOI: 10.7270/Q2154K23 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 9 (Homo sapiens (Human)) | BDBM272078 ((S)-1-(2-Chloro-6-fluorophenyl)-5-(3-fluoro-2- met...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
CHDI Foundation, Inc. US Patent | Assay Description The potency of the compounds is quantified by measuring the Histone Deacetylase 9 (HDAC9) enzymatic activity using the fluorogenic substrate, Boc-Lys... | US Patent US10065948 (2018) BindingDB Entry DOI: 10.7270/Q2154K23 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 4 [648-1032] (Homo sapiens (Human)) | BDBM243179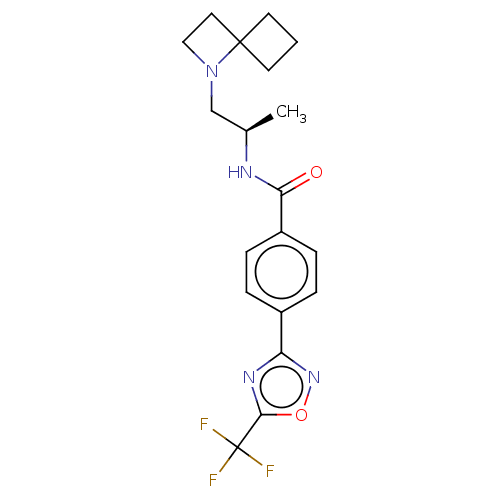 ((R)-N-(1-(1-Azaspiro[3.3]heptan-1- yl)propan-2-yl)...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
CHDI FOUNDATION, INC. US Patent | Assay Description 5 μL of each solution of 1:20 diluted compound from above was transferred to a clear bottomed, black, 384-well assay plate using the Bravo or th... | US Patent US10053434 (2018) BindingDB Entry DOI: 10.7270/Q2MG7RJZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 9 (Homo sapiens (Human)) | BDBM272067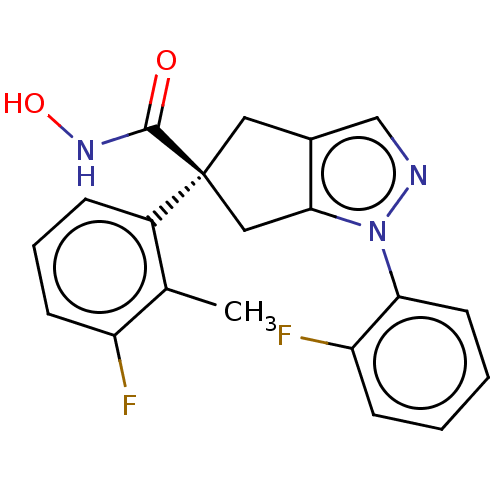 ((S)-5-(3-Fluoro-2-methylphenyl)-l-(2-fluorophenyl)...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
CHDI Foundation, Inc. US Patent | Assay Description The potency of the compounds is quantified by measuring the Histone Deacetylase 9 (HDAC9) enzymatic activity using the fluorogenic substrate, Boc-Lys... | US Patent US10457675 (2019) BindingDB Entry DOI: 10.7270/Q22J6F7J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 9 (Homo sapiens (Human)) | BDBM272074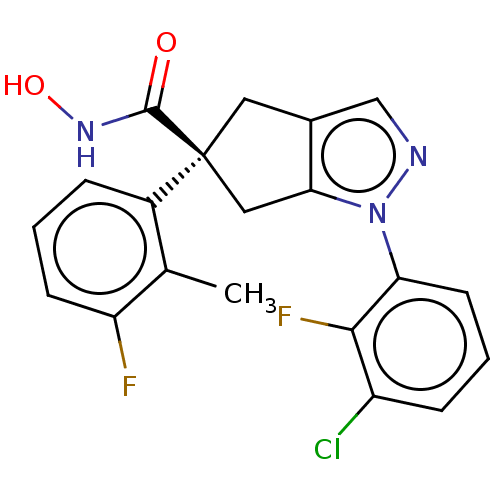 ((S)-1-(3-Chloro-2-fluorophenyl)-5-(3-fluoro-2- met...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
CHDI Foundation, Inc. US Patent | Assay Description The potency of the compounds is quantified by measuring the Histone Deacetylase 9 (HDAC9) enzymatic activity using the fluorogenic substrate, Boc-Lys... | US Patent US10457675 (2019) BindingDB Entry DOI: 10.7270/Q22J6F7J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 9 (Homo sapiens (Human)) | BDBM272067 ((S)-5-(3-Fluoro-2-methylphenyl)-l-(2-fluorophenyl)...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
CHDI Foundation, Inc. US Patent | Assay Description The potency of the compounds is quantified by measuring the Histone Deacetylase 9 (HDAC9) enzymatic activity using the fluorogenic substrate, Boc-Lys... | US Patent US10065948 (2018) BindingDB Entry DOI: 10.7270/Q2154K23 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 9 (Homo sapiens (Human)) | BDBM272074 ((S)-1-(3-Chloro-2-fluorophenyl)-5-(3-fluoro-2- met...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
CHDI Foundation, Inc. US Patent | Assay Description The potency of the compounds is quantified by measuring the Histone Deacetylase 9 (HDAC9) enzymatic activity using the fluorogenic substrate, Boc-Lys... | US Patent US10065948 (2018) BindingDB Entry DOI: 10.7270/Q2154K23 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 9 (Homo sapiens (Human)) | BDBM272079 ((S)-5-(3-Fluoro-2-methylphenyl)-1-(2-fluoro-6- met...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
CHDI Foundation, Inc. US Patent | Assay Description The potency of the compounds is quantified by measuring the Histone Deacetylase 9 (HDAC9) enzymatic activity using the fluorogenic substrate, Boc-Lys... | US Patent US10457675 (2019) BindingDB Entry DOI: 10.7270/Q22J6F7J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 9 (Homo sapiens (Human)) | BDBM272079 ((S)-5-(3-Fluoro-2-methylphenyl)-1-(2-fluoro-6- met...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
CHDI Foundation, Inc. US Patent | Assay Description The potency of the compounds is quantified by measuring the Histone Deacetylase 9 (HDAC9) enzymatic activity using the fluorogenic substrate, Boc-Lys... | US Patent US10065948 (2018) BindingDB Entry DOI: 10.7270/Q2154K23 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 334 total ) | Next | Last >> |