

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Atrial natriuretic peptide receptor 3 (Homo sapiens (Human)) | BDBM50254108 (CHEMBL4062470) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0540 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd Curated by ChEMBL | Assay Description Displacement of [125I]-human ANP from His-tagged and Fc fragment containing human NPR-3 extracellular domain expressed in FreeStyle 293 cells incubat... | Bioorg Med Chem Lett 27: 3542-3545 (2017) Article DOI: 10.1016/j.bmcl.2017.05.061 BindingDB Entry DOI: 10.7270/Q2F19241 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Atrial natriuretic peptide receptor 3 (Homo sapiens (Human)) | BDBM50254063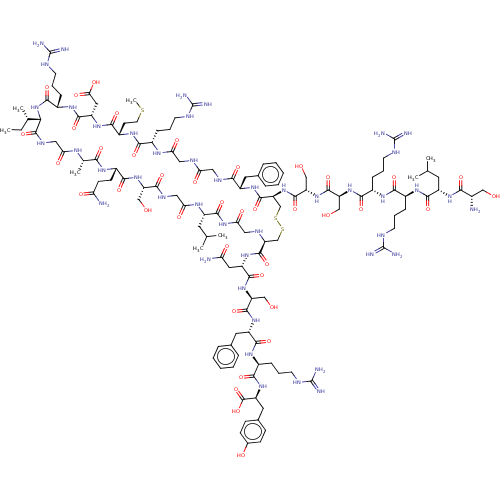 (CHEMBL4102000) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0580 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd Curated by ChEMBL | Assay Description Displacement of [125I]-human ANP from His-tagged and Fc fragment containing human NPR-3 extracellular domain expressed in FreeStyle 293 cells incubat... | Bioorg Med Chem Lett 27: 3542-3545 (2017) Article DOI: 10.1016/j.bmcl.2017.05.061 BindingDB Entry DOI: 10.7270/Q2F19241 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Atrial natriuretic peptide receptor 3 (Homo sapiens (Human)) | BDBM50254062 (CHEMBL4060314) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0790 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd Curated by ChEMBL | Assay Description Displacement of [125I]-human ANP from His-tagged and Fc fragment containing human NPR-3 extracellular domain expressed in FreeStyle 293 cells incubat... | Bioorg Med Chem Lett 27: 3542-3545 (2017) Article DOI: 10.1016/j.bmcl.2017.05.061 BindingDB Entry DOI: 10.7270/Q2F19241 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Atrial natriuretic peptide receptor 3 (Homo sapiens (Human)) | BDBM50254060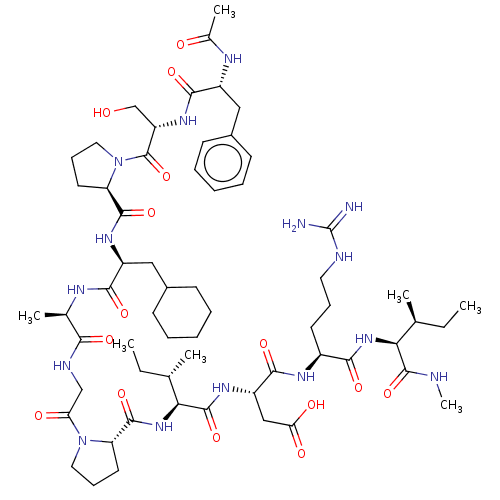 (CHEMBL4061379) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.0870 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd Curated by ChEMBL | Assay Description Displacement of [125I]-human ANP from His-tagged and Fc fragment containing human NPR-3 extracellular domain expressed in FreeStyle 293 cells incubat... | Bioorg Med Chem Lett 27: 3542-3545 (2017) Article DOI: 10.1016/j.bmcl.2017.05.061 BindingDB Entry DOI: 10.7270/Q2F19241 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Atrial natriuretic peptide receptor 1 (Homo sapiens (Human)) | BDBM50254063 (CHEMBL4102000) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0940 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd Curated by ChEMBL | Assay Description Displacement of [125I]-human ANP from human NPR-1 incubated for 2 hrs by top count method | Bioorg Med Chem Lett 27: 3542-3545 (2017) Article DOI: 10.1016/j.bmcl.2017.05.061 BindingDB Entry DOI: 10.7270/Q2F19241 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Atrial natriuretic peptide receptor 3 (Homo sapiens (Human)) | BDBM50254096 (CHEMBL4099663) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0990 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd Curated by ChEMBL | Assay Description Displacement of [125I]-human ANP from His-tagged and Fc fragment containing human NPR-3 extracellular domain expressed in FreeStyle 293 cells incubat... | Bioorg Med Chem Lett 27: 3542-3545 (2017) Article DOI: 10.1016/j.bmcl.2017.05.061 BindingDB Entry DOI: 10.7270/Q2F19241 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Atrial natriuretic peptide receptor 3 (Homo sapiens (Human)) | BDBM50254094 (CHEMBL4076904) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd Curated by ChEMBL | Assay Description Displacement of [125I]-human ANP from His-tagged and Fc fragment containing human NPR-3 extracellular domain expressed in FreeStyle 293 cells incubat... | Bioorg Med Chem Lett 27: 3542-3545 (2017) Article DOI: 10.1016/j.bmcl.2017.05.061 BindingDB Entry DOI: 10.7270/Q2F19241 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Atrial natriuretic peptide receptor 3 (Homo sapiens (Human)) | BDBM50254091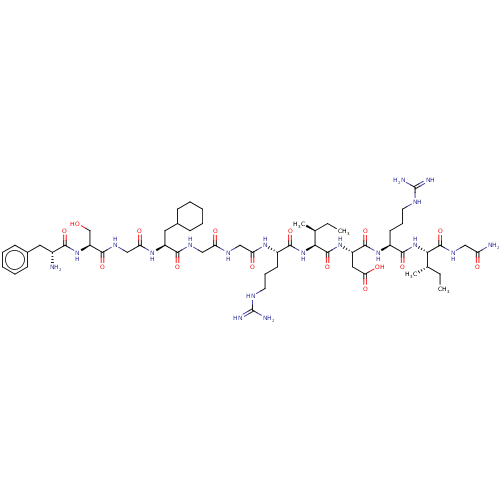 (CHEMBL4061318) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd Curated by ChEMBL | Assay Description Displacement of [125I]-human ANP from His-tagged and Fc fragment containing human NPR-3 extracellular domain expressed in FreeStyle 293 cells incubat... | Bioorg Med Chem Lett 27: 3542-3545 (2017) Article DOI: 10.1016/j.bmcl.2017.05.061 BindingDB Entry DOI: 10.7270/Q2F19241 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Atrial natriuretic peptide receptor 3 (Homo sapiens (Human)) | BDBM50254106 (CHEMBL4084160) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd Curated by ChEMBL | Assay Description Displacement of [125I]-human ANP from His-tagged and Fc fragment containing human NPR-3 extracellular domain expressed in FreeStyle 293 cells incubat... | Bioorg Med Chem Lett 27: 3542-3545 (2017) Article DOI: 10.1016/j.bmcl.2017.05.061 BindingDB Entry DOI: 10.7270/Q2F19241 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Atrial natriuretic peptide receptor 3 (Homo sapiens (Human)) | BDBM50254061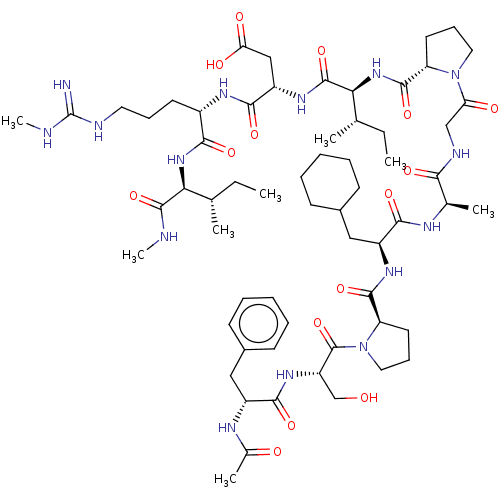 (CHEMBL4089530) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd Curated by ChEMBL | Assay Description Displacement of [125I]-human ANP from His-tagged and Fc fragment containing human NPR-3 extracellular domain expressed in FreeStyle 293 cells incubat... | Bioorg Med Chem Lett 27: 3542-3545 (2017) Article DOI: 10.1016/j.bmcl.2017.05.061 BindingDB Entry DOI: 10.7270/Q2F19241 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Atrial natriuretic peptide receptor 3 (Homo sapiens (Human)) | BDBM50254095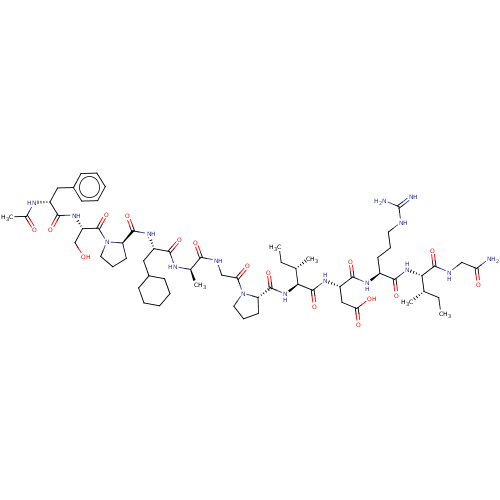 (CHEMBL4084171) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd Curated by ChEMBL | Assay Description Displacement of [125I]-human ANP from His-tagged and Fc fragment containing human NPR-3 extracellular domain expressed in FreeStyle 293 cells incubat... | Bioorg Med Chem Lett 27: 3542-3545 (2017) Article DOI: 10.1016/j.bmcl.2017.05.061 BindingDB Entry DOI: 10.7270/Q2F19241 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Atrial natriuretic peptide receptor 3 (Homo sapiens (Human)) | BDBM50254089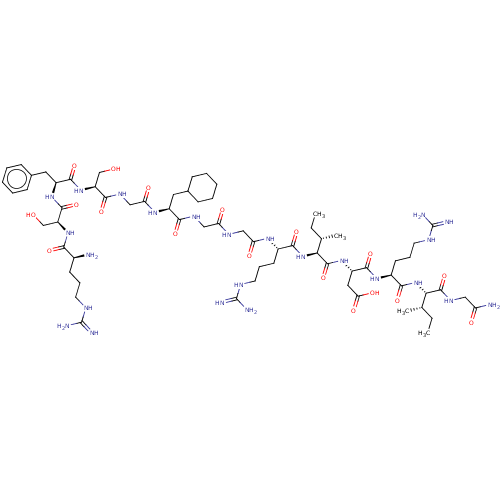 (CHEMBL4081715) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd Curated by ChEMBL | Assay Description Displacement of [125I]-human ANP from His-tagged and Fc fragment containing human NPR-3 extracellular domain expressed in FreeStyle 293 cells incubat... | Bioorg Med Chem Lett 27: 3542-3545 (2017) Article DOI: 10.1016/j.bmcl.2017.05.061 BindingDB Entry DOI: 10.7270/Q2F19241 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Atrial natriuretic peptide receptor 3 (Homo sapiens (Human)) | BDBM50254093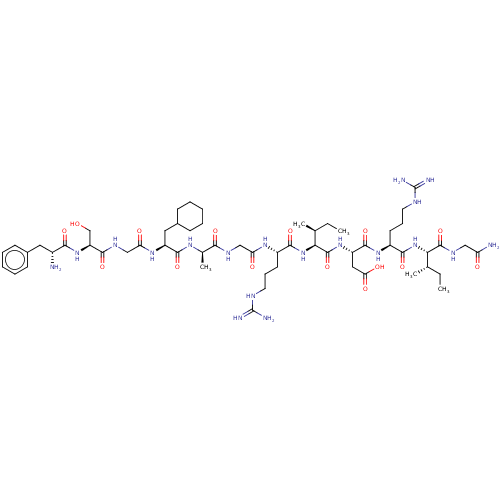 (CHEMBL4071568) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.360 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd Curated by ChEMBL | Assay Description Displacement of [125I]-human ANP from His-tagged and Fc fragment containing human NPR-3 extracellular domain expressed in FreeStyle 293 cells incubat... | Bioorg Med Chem Lett 27: 3542-3545 (2017) Article DOI: 10.1016/j.bmcl.2017.05.061 BindingDB Entry DOI: 10.7270/Q2F19241 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Atrial natriuretic peptide receptor 3 (Homo sapiens (Human)) | BDBM50254081 (CHEMBL4103929) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.510 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd Curated by ChEMBL | Assay Description Displacement of [125I]-human ANP from His-tagged and Fc fragment containing human NPR-3 extracellular domain expressed in FreeStyle 293 cells incubat... | Bioorg Med Chem Lett 27: 3542-3545 (2017) Article DOI: 10.1016/j.bmcl.2017.05.061 BindingDB Entry DOI: 10.7270/Q2F19241 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Atrial natriuretic peptide receptor 3 (Homo sapiens (Human)) | BDBM50254088 (CHEMBL4088351) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.520 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd Curated by ChEMBL | Assay Description Displacement of [125I]-human ANP from His-tagged and Fc fragment containing human NPR-3 extracellular domain expressed in FreeStyle 293 cells incubat... | Bioorg Med Chem Lett 27: 3542-3545 (2017) Article DOI: 10.1016/j.bmcl.2017.05.061 BindingDB Entry DOI: 10.7270/Q2F19241 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 9 subunit alpha/subunit beta-1/subunit beta-2 (Mus musculus) | BDBM50257179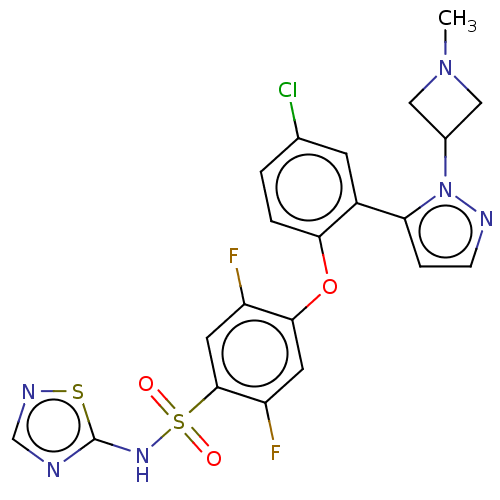 (CHEMBL2325622) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.550 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of mouse NaV1.7/beta1/beta2 expressed in HEK293A cells by Ionworks high-throughput electrophysiology method | J Med Chem 63: 10204-10220 (2020) Article DOI: 10.1021/acs.jmedchem.0c00259 BindingDB Entry DOI: 10.7270/Q2Q52T67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM29995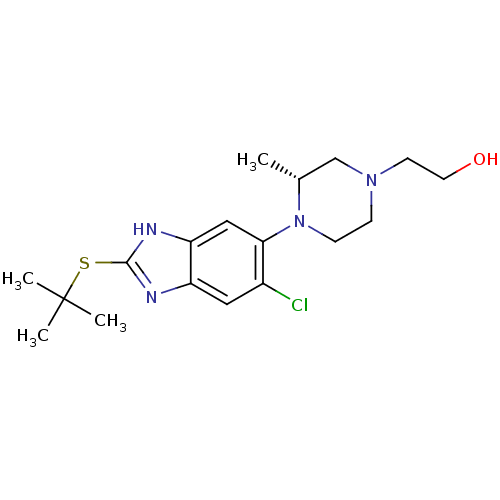 (CHEMBL494350 | benzimidazole-based antagonist, 1) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.650 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute Curated by ChEMBL | Assay Description Antagonist activity at human ORL1 receptor by [35S]GTPgammaS binding assay | Bioorg Med Chem Lett 18: 3282-5 (2008) Article DOI: 10.1016/j.bmcl.2008.04.037 BindingDB Entry DOI: 10.7270/Q2542NDJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Atrial natriuretic peptide receptor 3 (Homo sapiens (Human)) | BDBM50254097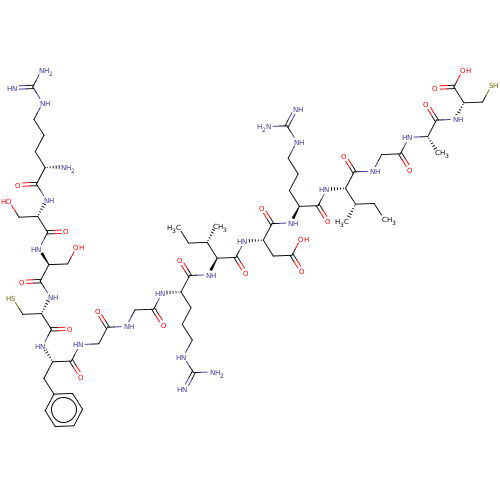 (CHEMBL4082715) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.650 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd Curated by ChEMBL | Assay Description Displacement of [125I]-human ANP from His-tagged and Fc fragment containing human NPR-3 extracellular domain expressed in FreeStyle 293 cells incubat... | Bioorg Med Chem Lett 27: 3542-3545 (2017) Article DOI: 10.1016/j.bmcl.2017.05.061 BindingDB Entry DOI: 10.7270/Q2F19241 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50239749 (2-(4-(6-chloro-2-(3-methylpentan-3-ylthio)-3H-benz...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.660 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute Curated by ChEMBL | Assay Description Antagonist activity at human ORL1 receptor by [35S]GTPgammaS binding assay | Bioorg Med Chem Lett 18: 3282-5 (2008) Article DOI: 10.1016/j.bmcl.2008.04.037 BindingDB Entry DOI: 10.7270/Q2542NDJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50239749 (2-(4-(6-chloro-2-(3-methylpentan-3-ylthio)-3H-benz...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.660 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute Curated by ChEMBL | Assay Description Antagonist activity at human ORL1 receptor expressed in CHO cell membrane by [35S]GTP-gamma-S binding assay | Bioorg Med Chem Lett 18: 3278-81 (2008) Article DOI: 10.1016/j.bmcl.2008.04.054 BindingDB Entry DOI: 10.7270/Q2HT2Q57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM29994 (2-Cyclohexylcarbonylbenzimidazole, 7e) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.800 | n/a | 2.40 | n/a | n/a | 7.4 | 37 |
Banyu Pharmaceutical Co. | Assay Description Compounds were tested for their inhibitory effects on ligand binding to the human ORL1 receptor. Bound and free radioligands are separated by filtra... | Bioorg Med Chem Lett 19: 3096-9 (2009) Article DOI: 10.1016/j.bmcl.2009.04.023 BindingDB Entry DOI: 10.7270/Q24J0CFR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM29988 (benzimidazole analogue, 7h | benzimidazole derivat...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.810 | n/a | 1.30 | n/a | n/a | 7.4 | 37 |
Banyu Pharmaceutical Co. | Assay Description Compounds were tested for their inhibitory effects on ligand binding to the human ORL1 receptor. Bound and free radioligands are separated by filtra... | Bioorg Med Chem Lett 19: 3100-3 (2009) Article DOI: 10.1016/j.bmcl.2009.04.022 BindingDB Entry DOI: 10.7270/Q20R9MRX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM29988 (benzimidazole analogue, 7h | benzimidazole derivat...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.810 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Banyu Pharmaceutical Co. | Assay Description Compounds were tested for their inhibitory effects on ligand binding to the human ORL1 receptor. Bound and free radioligands are separated by filtra... | Bioorg Med Chem Lett 19: 3096-9 (2009) Article DOI: 10.1016/j.bmcl.2009.04.023 BindingDB Entry DOI: 10.7270/Q24J0CFR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Atrial natriuretic peptide receptor 3 (Homo sapiens (Human)) | BDBM50254092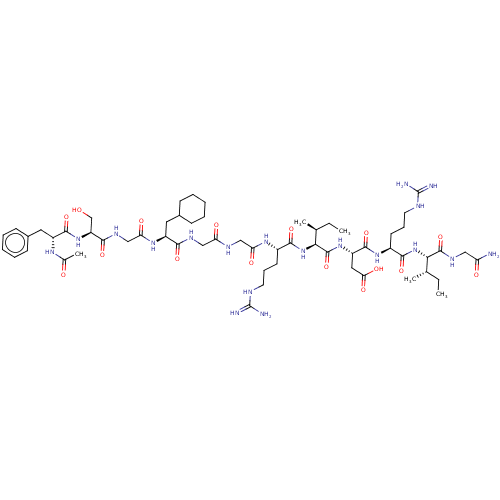 (CHEMBL4062458) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.930 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd Curated by ChEMBL | Assay Description Displacement of [125I]-human ANP from His-tagged and Fc fragment containing human NPR-3 extracellular domain expressed in FreeStyle 293 cells incubat... | Bioorg Med Chem Lett 27: 3542-3545 (2017) Article DOI: 10.1016/j.bmcl.2017.05.061 BindingDB Entry DOI: 10.7270/Q2F19241 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM30012 (benzimidazole analogue, 7k) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | 1.10 | n/a | n/a | 7.4 | 37 |
Banyu Pharmaceutical Co. | Assay Description Compounds were tested for their inhibitory effects on ligand binding to the human ORL1 receptor. Bound and free radioligands are separated by filtra... | Bioorg Med Chem Lett 19: 3100-3 (2009) Article DOI: 10.1016/j.bmcl.2009.04.022 BindingDB Entry DOI: 10.7270/Q20R9MRX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM30017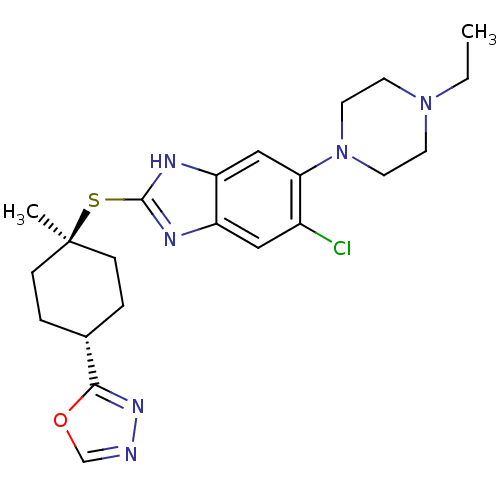 (benzimidazole analogue, 7p) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10 | n/a | 0.590 | n/a | n/a | 7.4 | 37 |
Banyu Pharmaceutical Co. | Assay Description Compounds were tested for their inhibitory effects on ligand binding to the human ORL1 receptor. Bound and free radioligands are separated by filtra... | Bioorg Med Chem Lett 19: 3100-3 (2009) Article DOI: 10.1016/j.bmcl.2009.04.022 BindingDB Entry DOI: 10.7270/Q20R9MRX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50260633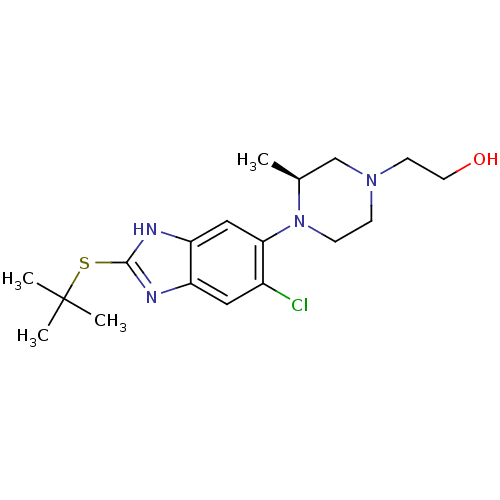 ((S)-2-(4-(2-(tert-butylthio)-6-chloro-3H-benzo[d]i...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]Tyr14-NC/OFQ from human ORL1 receptor | Bioorg Med Chem Lett 18: 3282-5 (2008) Article DOI: 10.1016/j.bmcl.2008.04.037 BindingDB Entry DOI: 10.7270/Q2542NDJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Atrial natriuretic peptide receptor 3 (Homo sapiens (Human)) | BDBM50254087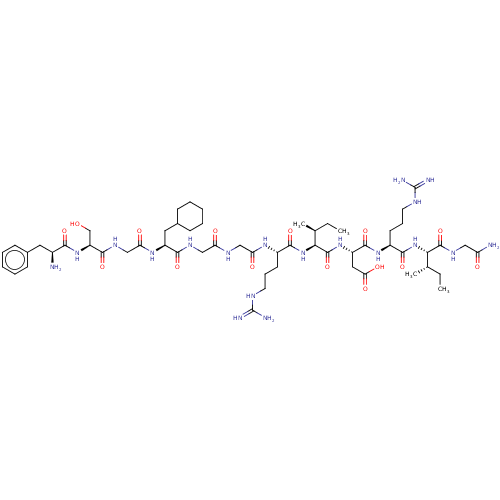 (CHEMBL4075426) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd Curated by ChEMBL | Assay Description Displacement of [125I]-human ANP from His-tagged and Fc fragment containing human NPR-3 extracellular domain expressed in FreeStyle 293 cells incubat... | Bioorg Med Chem Lett 27: 3542-3545 (2017) Article DOI: 10.1016/j.bmcl.2017.05.061 BindingDB Entry DOI: 10.7270/Q2F19241 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50239749 (2-(4-(6-chloro-2-(3-methylpentan-3-ylthio)-3H-benz...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]Tyr14-NC from human ORL1 receptor expressed in CHO cell membrane | Bioorg Med Chem Lett 18: 3278-81 (2008) Article DOI: 10.1016/j.bmcl.2008.04.054 BindingDB Entry DOI: 10.7270/Q2HT2Q57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50239749 (2-(4-(6-chloro-2-(3-methylpentan-3-ylthio)-3H-benz...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]Tyr14-NC/OFQ from human ORL1 receptor | Bioorg Med Chem Lett 18: 3282-5 (2008) Article DOI: 10.1016/j.bmcl.2008.04.037 BindingDB Entry DOI: 10.7270/Q2542NDJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM30011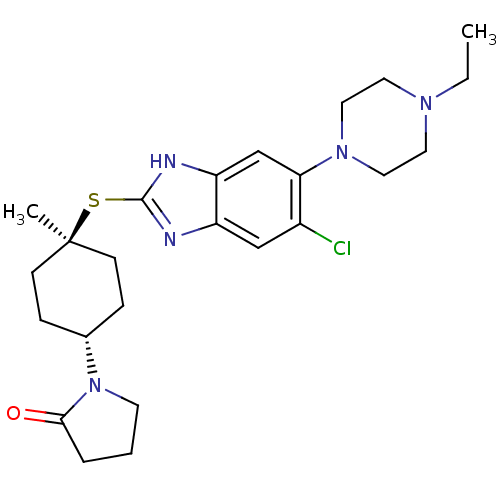 (benzimidazole analogue, 7j) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40 | n/a | 0.550 | n/a | n/a | 7.4 | 37 |
Banyu Pharmaceutical Co. | Assay Description Compounds were tested for their inhibitory effects on ligand binding to the human ORL1 receptor. Bound and free radioligands are separated by filtra... | Bioorg Med Chem Lett 19: 3100-3 (2009) Article DOI: 10.1016/j.bmcl.2009.04.022 BindingDB Entry DOI: 10.7270/Q20R9MRX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM29992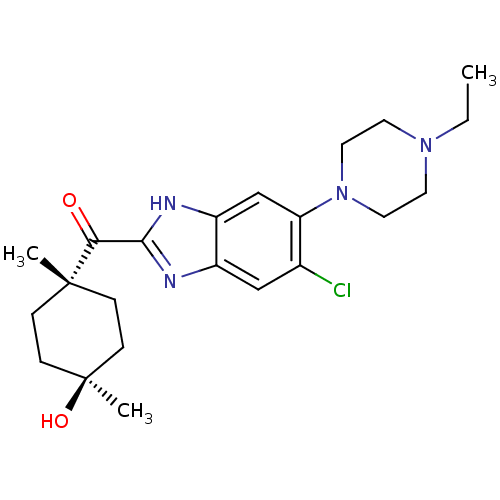 (2-Cyclohexylcarbonylbenzimidazole, 7c) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.40 | n/a | 1.30 | n/a | n/a | 7.4 | 37 |
Banyu Pharmaceutical Co. | Assay Description Compounds were tested for their inhibitory effects on ligand binding to the human ORL1 receptor. Bound and free radioligands are separated by filtra... | Bioorg Med Chem Lett 19: 3096-9 (2009) Article DOI: 10.1016/j.bmcl.2009.04.023 BindingDB Entry DOI: 10.7270/Q24J0CFR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Atrial natriuretic peptide receptor 3 (Homo sapiens (Human)) | BDBM50254098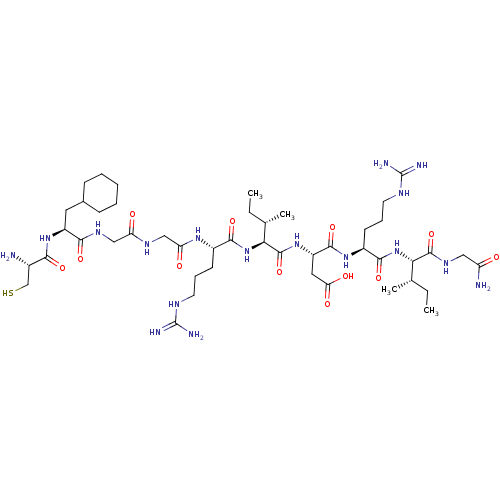 (CHEMBL4098363) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd Curated by ChEMBL | Assay Description Displacement of [125I]-human ANP from His-tagged and Fc fragment containing human NPR-3 extracellular domain expressed in FreeStyle 293 cells incubat... | Bioorg Med Chem Lett 27: 3542-3545 (2017) Article DOI: 10.1016/j.bmcl.2017.05.061 BindingDB Entry DOI: 10.7270/Q2F19241 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM30016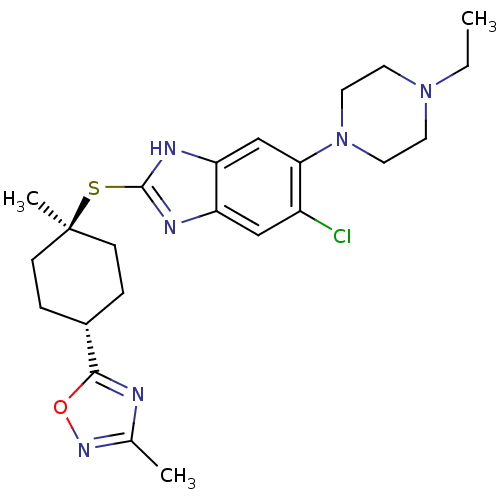 (benzimidazole analogue, 7o) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.70 | n/a | 0.910 | n/a | n/a | 7.4 | 37 |
Banyu Pharmaceutical Co. | Assay Description Compounds were tested for their inhibitory effects on ligand binding to the human ORL1 receptor. Bound and free radioligands are separated by filtra... | Bioorg Med Chem Lett 19: 3100-3 (2009) Article DOI: 10.1016/j.bmcl.2009.04.022 BindingDB Entry DOI: 10.7270/Q20R9MRX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50373360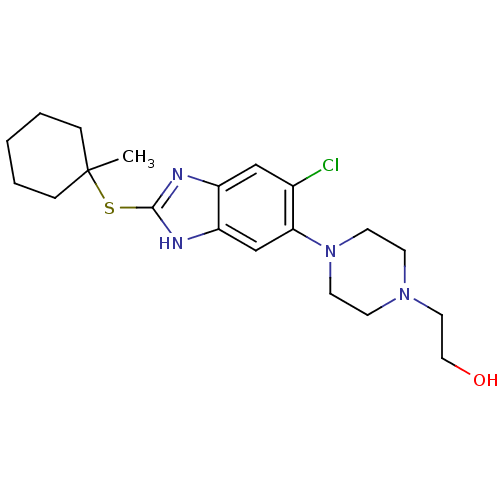 (CHEMBL263917) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute Curated by ChEMBL | Assay Description Antagonist activity at human ORL1 receptor expressed in CHO cell membrane by [35S]GTP-gamma-S binding assay | Bioorg Med Chem Lett 18: 3278-81 (2008) Article DOI: 10.1016/j.bmcl.2008.04.054 BindingDB Entry DOI: 10.7270/Q2HT2Q57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50373360 (CHEMBL263917) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]Tyr14-NC from human ORL1 receptor expressed in CHO cell membrane | Bioorg Med Chem Lett 18: 3278-81 (2008) Article DOI: 10.1016/j.bmcl.2008.04.054 BindingDB Entry DOI: 10.7270/Q2HT2Q57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM30018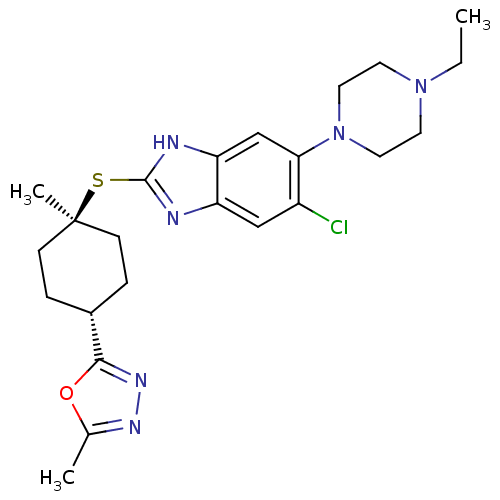 (benzimidazole analogue, 7q) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.80 | n/a | 7.10 | n/a | n/a | 7.4 | 37 |
Banyu Pharmaceutical Co. | Assay Description Compounds were tested for their inhibitory effects on ligand binding to the human ORL1 receptor. Bound and free radioligands are separated by filtra... | Bioorg Med Chem Lett 19: 3100-3 (2009) Article DOI: 10.1016/j.bmcl.2009.04.022 BindingDB Entry DOI: 10.7270/Q20R9MRX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM30008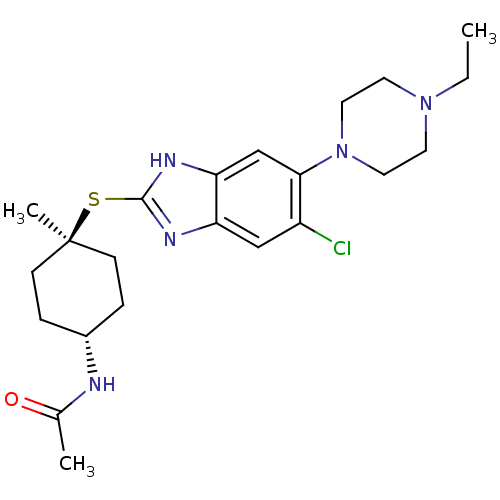 (benzimidazole analogue, 7g) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.90 | n/a | 1.10 | n/a | n/a | 7.4 | 37 |
Banyu Pharmaceutical Co. | Assay Description Compounds were tested for their inhibitory effects on ligand binding to the human ORL1 receptor. Bound and free radioligands are separated by filtra... | Bioorg Med Chem Lett 19: 3100-3 (2009) Article DOI: 10.1016/j.bmcl.2009.04.022 BindingDB Entry DOI: 10.7270/Q20R9MRX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel subunit beta-2 (Homo sapiens) | BDBM50257179 (CHEMBL2325622) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of human NaV1.7/beta1/beta2 expressed in HEK293A cells by Ionworks high-throughput electrophysiology method | J Med Chem 63: 10204-10220 (2020) Article DOI: 10.1021/acs.jmedchem.0c00259 BindingDB Entry DOI: 10.7270/Q2Q52T67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM29990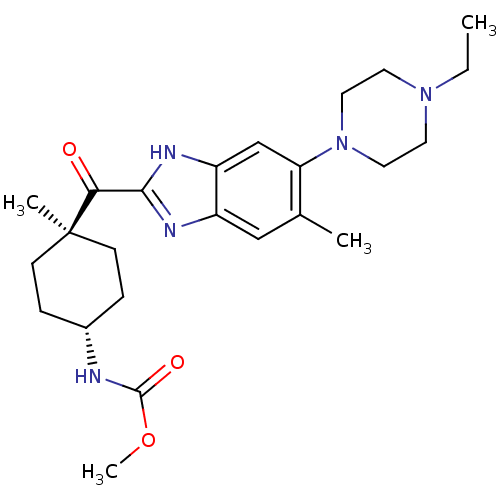 (2-Cyclohexylcarbonylbenzimidazole, 7b) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | 5.30 | n/a | n/a | 7.4 | 37 |
Banyu Pharmaceutical Co. | Assay Description Compounds were tested for their inhibitory effects on ligand binding to the human ORL1 receptor. Bound and free radioligands are separated by filtra... | Bioorg Med Chem Lett 19: 3096-9 (2009) Article DOI: 10.1016/j.bmcl.2009.04.023 BindingDB Entry DOI: 10.7270/Q24J0CFR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50373365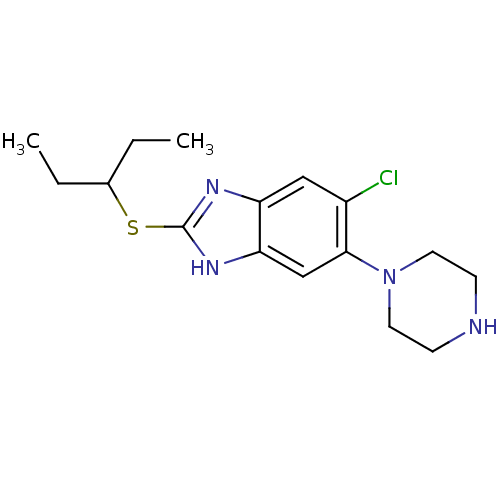 (CHEMBL258710) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]Tyr14-NC from human ORL1 receptor expressed in CHO cell membrane | Bioorg Med Chem Lett 18: 3278-81 (2008) Article DOI: 10.1016/j.bmcl.2008.04.054 BindingDB Entry DOI: 10.7270/Q2HT2Q57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM30010 (benzimidazole analogue, 7i) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.10 | n/a | 1.80 | n/a | n/a | 7.4 | 37 |
Banyu Pharmaceutical Co. | Assay Description Compounds were tested for their inhibitory effects on ligand binding to the human ORL1 receptor. Bound and free radioligands are separated by filtra... | Bioorg Med Chem Lett 19: 3100-3 (2009) Article DOI: 10.1016/j.bmcl.2009.04.022 BindingDB Entry DOI: 10.7270/Q20R9MRX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50260633 ((S)-2-(4-(2-(tert-butylthio)-6-chloro-3H-benzo[d]i...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute Curated by ChEMBL | Assay Description Antagonist activity at human ORL1 receptor by [35S]GTPgammaS binding assay | Bioorg Med Chem Lett 18: 3282-5 (2008) Article DOI: 10.1016/j.bmcl.2008.04.037 BindingDB Entry DOI: 10.7270/Q2542NDJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM30014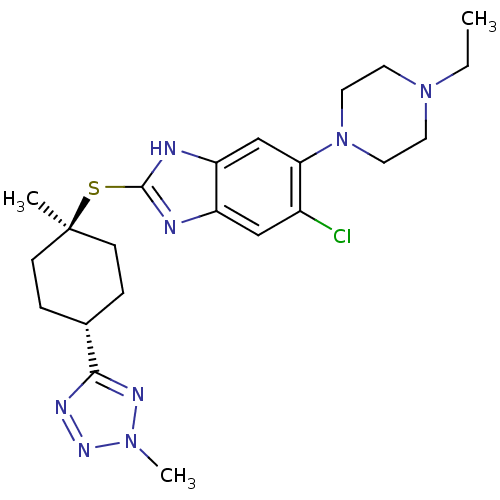 (benzimidazole analogue, 7m) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.20 | n/a | 0.900 | n/a | n/a | 7.4 | 37 |
Banyu Pharmaceutical Co. | Assay Description Compounds were tested for their inhibitory effects on ligand binding to the human ORL1 receptor. Bound and free radioligands are separated by filtra... | Bioorg Med Chem Lett 19: 3100-3 (2009) Article DOI: 10.1016/j.bmcl.2009.04.022 BindingDB Entry DOI: 10.7270/Q20R9MRX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM29987 (benzimidazole analogue, 7e | benzimidazole derivat...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.40 | n/a | 0.720 | n/a | n/a | 7.4 | 37 |
Banyu Pharmaceutical Co. | Assay Description Compounds were tested for their inhibitory effects on ligand binding to the human ORL1 receptor. Bound and free radioligands are separated by filtra... | Bioorg Med Chem Lett 19: 3100-3 (2009) Article DOI: 10.1016/j.bmcl.2009.04.022 BindingDB Entry DOI: 10.7270/Q20R9MRX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM29987 (benzimidazole analogue, 7e | benzimidazole derivat...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.40 | n/a | 0.720 | n/a | n/a | 7.4 | 37 |
Banyu Pharmaceutical Co. | Assay Description Compounds were tested for their inhibitory effects on ligand binding to the human ORL1 receptor. Bound and free radioligands are separated by filtra... | Bioorg Med Chem Lett 19: 3096-9 (2009) Article DOI: 10.1016/j.bmcl.2009.04.023 BindingDB Entry DOI: 10.7270/Q24J0CFR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50373362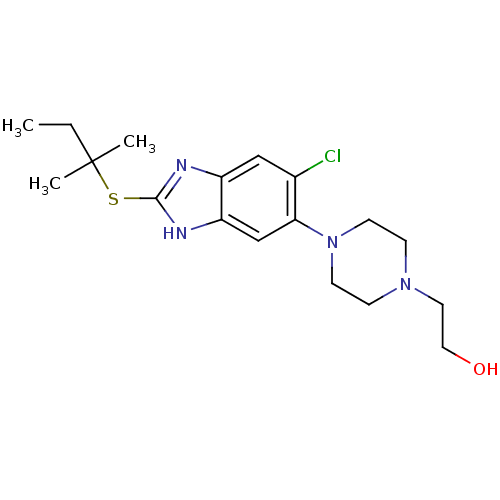 (CHEMBL263919) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute Curated by ChEMBL | Assay Description Antagonist activity at human ORL1 receptor expressed in CHO cell membrane by [35S]GTP-gamma-S binding assay | Bioorg Med Chem Lett 18: 3278-81 (2008) Article DOI: 10.1016/j.bmcl.2008.04.054 BindingDB Entry DOI: 10.7270/Q2HT2Q57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM29995 (CHEMBL494350 | benzimidazole-based antagonist, 1) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.60 | n/a | 0.650 | n/a | n/a | 7.4 | 37 |
Banyu Pharmaceutical Co. | Assay Description Compounds were tested for their inhibitory effects on ligand binding to the human ORL1 receptor. Bound and free radioligands are separated by filtra... | Bioorg Med Chem Lett 19: 3100-3 (2009) Article DOI: 10.1016/j.bmcl.2009.04.022 BindingDB Entry DOI: 10.7270/Q20R9MRX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM29995 (CHEMBL494350 | benzimidazole-based antagonist, 1) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]Tyr14-NC/OFQ from human ORL1 receptor | Bioorg Med Chem Lett 18: 3282-5 (2008) Article DOI: 10.1016/j.bmcl.2008.04.037 BindingDB Entry DOI: 10.7270/Q2542NDJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50239745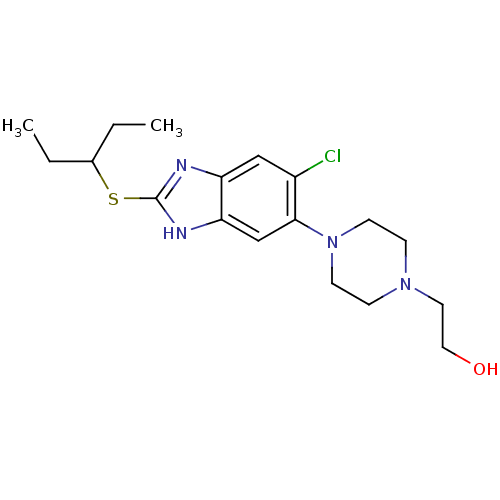 (2-(4-(6-chloro-2-(pentan-3-ylthio)-3H-benzo[d]imid...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute Curated by ChEMBL | Assay Description Antagonist activity at human ORL1 receptor expressed in CHO cell membrane by [35S]GTP-gamma-S binding assay | Bioorg Med Chem Lett 18: 3278-81 (2008) Article DOI: 10.1016/j.bmcl.2008.04.054 BindingDB Entry DOI: 10.7270/Q2HT2Q57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 556 total ) | Next | Last >> |