

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Type-1 angiotensin II receptor B (RAT) | BDBM50449917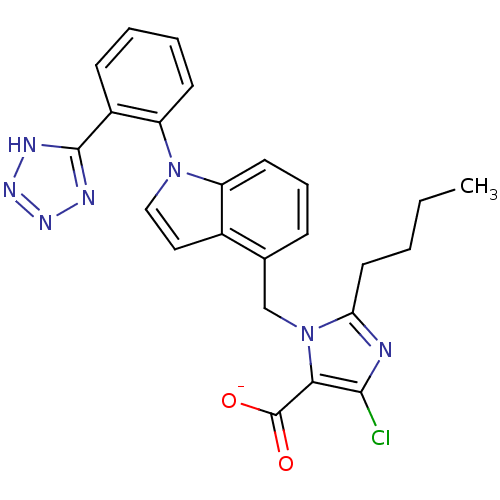 (BMS-180560 | CHEMBL2021417) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity for Angiotensin II receptor, type 1 determined using [125I]- labeled [Sar1,Ile8] angiotensin II in rat adrenocortical membranes | Bioorg Med Chem Lett 4: 145-150 (1994) Article DOI: 10.1016/S0960-894X(01)81137-9 BindingDB Entry DOI: 10.7270/Q2V988KF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor B (RAT) | BDBM50449914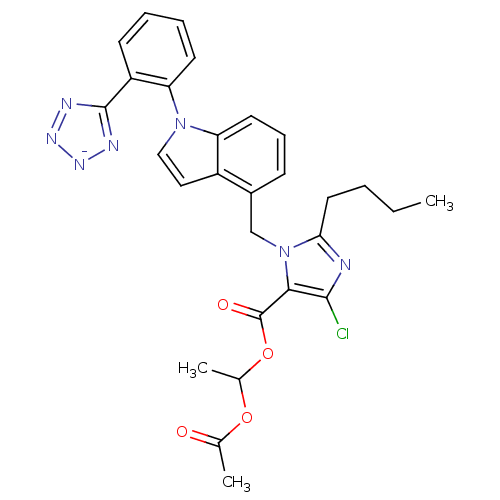 (CHEMBL2079784) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity for Angiotensin II receptor, type 1 determined using [125I]- labeled [Sar1,Ile8] angiotensin II in rat adrenocortical membranes | Bioorg Med Chem Lett 4: 145-150 (1994) Article DOI: 10.1016/S0960-894X(01)81137-9 BindingDB Entry DOI: 10.7270/Q2V988KF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor B (RAT) | BDBM50366331 (CHEMBL1790055) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity for Angiotensin II receptor, type 1 determined using [125I]- labeled [Sar1,Ile8] angiotensin II in rat adrenocortical membranes | Bioorg Med Chem Lett 4: 145-150 (1994) Article DOI: 10.1016/S0960-894X(01)81137-9 BindingDB Entry DOI: 10.7270/Q2V988KF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor B (RAT) | BDBM50449919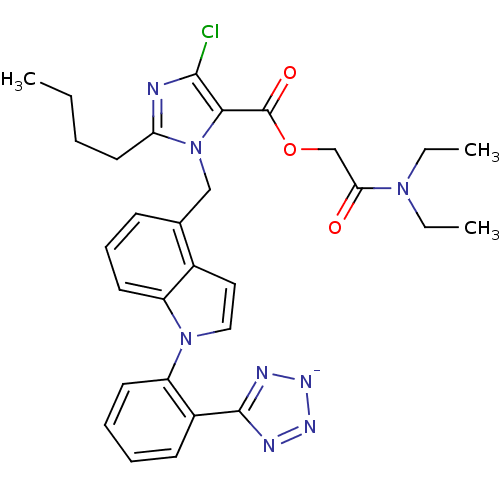 (CHEMBL2021415) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity for Angiotensin II receptor, type 1 determined using [125I]- labeled [Sar1,Ile8] angiotensin II in rat adrenocortical membranes | Bioorg Med Chem Lett 4: 145-150 (1994) Article DOI: 10.1016/S0960-894X(01)81137-9 BindingDB Entry DOI: 10.7270/Q2V988KF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor B (RAT) | BDBM50449910 (CHEMBL2079782) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 7.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity for Angiotensin II receptor, type 1 determined using [125I]- labeled [Sar1,Ile8] angiotensin II in rat adrenocortical membranes | Bioorg Med Chem Lett 4: 145-150 (1994) Article DOI: 10.1016/S0960-894X(01)81137-9 BindingDB Entry DOI: 10.7270/Q2V988KF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor B (RAT) | BDBM50449912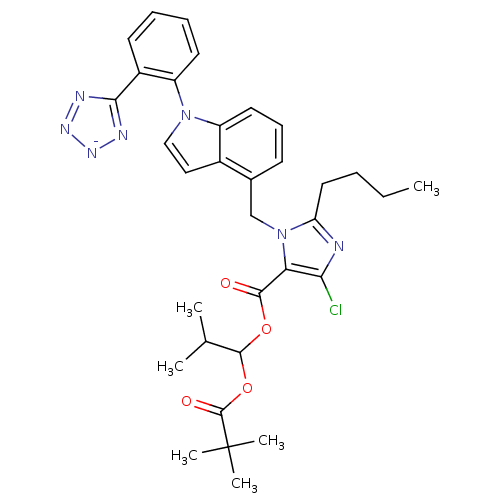 (CHEMBL2079781) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 8.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity for Angiotensin II receptor, type 1 determined using [125I]- labeled [Sar1,Ile8] angiotensin II in rat adrenocortical membranes | Bioorg Med Chem Lett 4: 145-150 (1994) Article DOI: 10.1016/S0960-894X(01)81137-9 BindingDB Entry DOI: 10.7270/Q2V988KF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor B (RAT) | BDBM50449909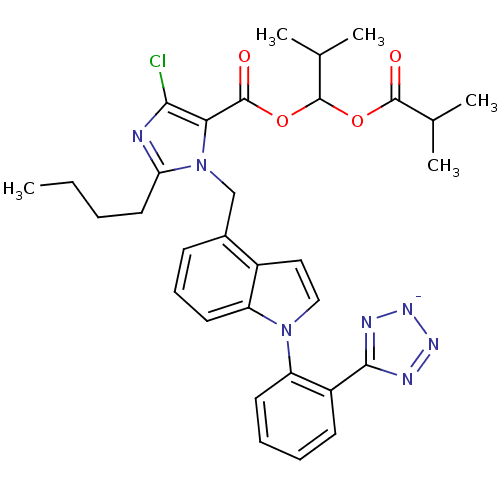 (CHEMBL2079769) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity for Angiotensin II receptor, type 1 determined using [125I]- labeled [Sar1,Ile8] angiotensin II in rat adrenocortical membranes | Bioorg Med Chem Lett 4: 145-150 (1994) Article DOI: 10.1016/S0960-894X(01)81137-9 BindingDB Entry DOI: 10.7270/Q2V988KF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor B (RAT) | BDBM50449918 (BMS-181688 | CHEMBL2021416) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity for Angiotensin II receptor, type 1 determined using [125I]- labeled [Sar1,Ile8] angiotensin II in rat adrenocortical membranes | Bioorg Med Chem Lett 4: 145-150 (1994) Article DOI: 10.1016/S0960-894X(01)81137-9 BindingDB Entry DOI: 10.7270/Q2V988KF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor B (RAT) | BDBM50449920 (CHEMBL2079768) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity for Angiotensin II receptor, type 1 determined using [125I]- labeled [Sar1,Ile8] angiotensin II in rat adrenocortical membranes | Bioorg Med Chem Lett 4: 145-150 (1994) Article DOI: 10.1016/S0960-894X(01)81137-9 BindingDB Entry DOI: 10.7270/Q2V988KF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor B (RAT) | BDBM50449911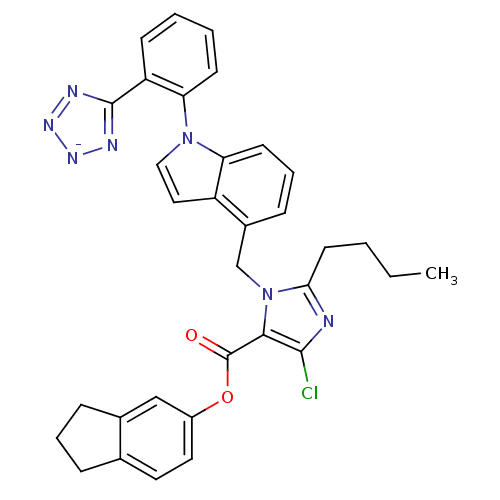 (CHEMBL2079770) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 39 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity for Angiotensin II receptor, type 1 determined using [125I]- labeled [Sar1,Ile8] angiotensin II in rat adrenocortical membranes | Bioorg Med Chem Lett 4: 145-150 (1994) Article DOI: 10.1016/S0960-894X(01)81137-9 BindingDB Entry DOI: 10.7270/Q2V988KF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor B (RAT) | BDBM50282240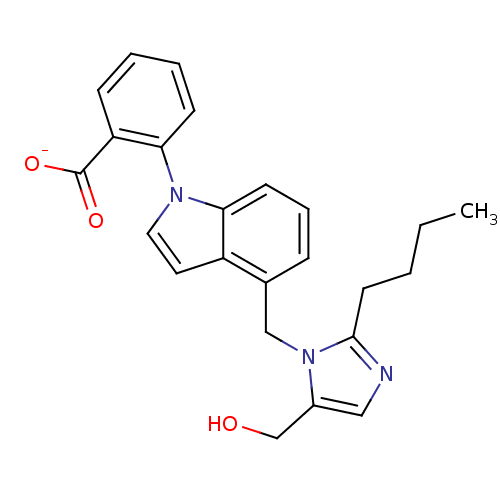 (CHEMBL288691 | Lithium; 2-[4-(2-butyl-5-hydroxymet...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 79 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity for Angiotensin II receptor, type 1 determined using [125I]- labeled [Sar1,Ile8] angiotensin II in rat adrenocortical membranes | Bioorg Med Chem Lett 4: 145-150 (1994) Article DOI: 10.1016/S0960-894X(01)81137-9 BindingDB Entry DOI: 10.7270/Q2V988KF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor B (RAT) | BDBM50449915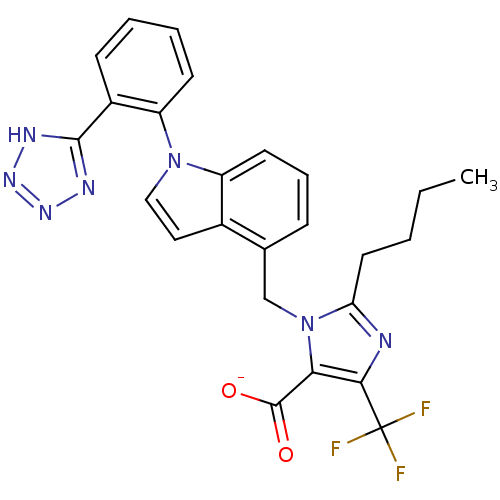 (CHEMBL2021419) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 79 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity for Angiotensin II receptor, type 1 determined using [125I]- labeled [Sar1,Ile8] angiotensin II in rat adrenocortical membranes | Bioorg Med Chem Lett 4: 145-150 (1994) Article DOI: 10.1016/S0960-894X(01)81137-9 BindingDB Entry DOI: 10.7270/Q2V988KF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor B (RAT) | BDBM50282248 (CHEMBL287065 | Lithium; 2-[5-(2-butyl-5-hydroxymet...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 760 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity for Angiotensin II receptor, type 1 determined using [125I]- labeled [Sar1,Ile8] angiotensin II in rat adrenocortical membranes | Bioorg Med Chem Lett 4: 145-150 (1994) Article DOI: 10.1016/S0960-894X(01)81137-9 BindingDB Entry DOI: 10.7270/Q2V988KF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor B (RAT) | BDBM50449916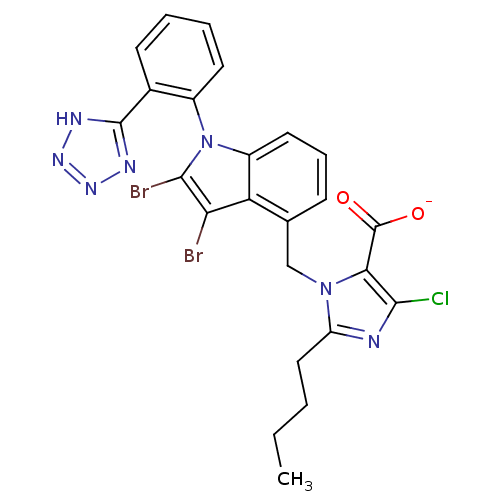 (CHEMBL2021418) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 840 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity for Angiotensin II receptor, type 1 determined using [125I]- labeled [Sar1,Ile8] angiotensin II in rat adrenocortical membranes | Bioorg Med Chem Lett 4: 145-150 (1994) Article DOI: 10.1016/S0960-894X(01)81137-9 BindingDB Entry DOI: 10.7270/Q2V988KF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor B (RAT) | BDBM50282244 (2-Butyl-3-{1-[2-(2H-tetrazol-5-yl)-phenyl]-1H-indo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity for Angiotensin II receptor, type 1 determined using [125I]- labeled [Sar1,Ile8] angiotensin II in rat adrenocortical membranes | Bioorg Med Chem Lett 4: 145-150 (1994) Article DOI: 10.1016/S0960-894X(01)81137-9 BindingDB Entry DOI: 10.7270/Q2V988KF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor B (RAT) | BDBM50282254 (CHEMBL36259 | Potassium; 2-[6-(2-butyl-4-chloro-5-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article | 6.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity for Angiotensin II receptor, type 1 determined using [125I]- labeled [Sar1,Ile8] angiotensin II in rat adrenocortical membranes | Bioorg Med Chem Lett 4: 145-150 (1994) Article DOI: 10.1016/S0960-894X(01)81137-9 BindingDB Entry DOI: 10.7270/Q2V988KF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-2 angiotensin II receptor (RAT) | BDBM50449917 (BMS-180560 | CHEMBL2021417) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 6.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Receptor binding affinity for Angiotensin II receptor, type 2 determined | Bioorg Med Chem Lett 4: 145-150 (1994) Article DOI: 10.1016/S0960-894X(01)81137-9 BindingDB Entry DOI: 10.7270/Q2V988KF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50031845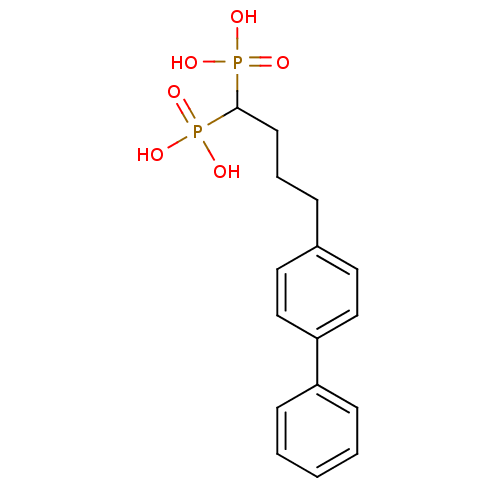 ((4-Biphenyl-4-yl-1-phosphono-butyl)-phosphonic aci...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against rat liver microsomal squalene synthase was determined using [3H]-FPP as radioligand | J Med Chem 39: 657-60 (1996) Article DOI: 10.1021/jm9507340 BindingDB Entry DOI: 10.7270/Q2N29W1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50049232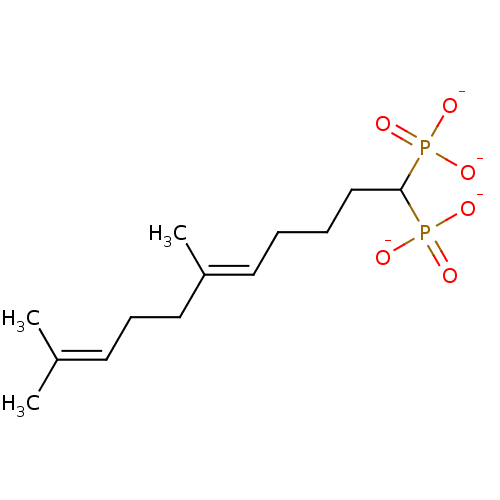 (CHEMBL348349 | Tetrasodium salt of 4-(4'-Methyl-bi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against rat liver microsomal squalene synthase was determined using [3H]-FPP as radioligand | J Med Chem 39: 657-60 (1996) Article DOI: 10.1021/jm9507340 BindingDB Entry DOI: 10.7270/Q2N29W1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Rattus norvegicus (Rat)) | BDBM50282859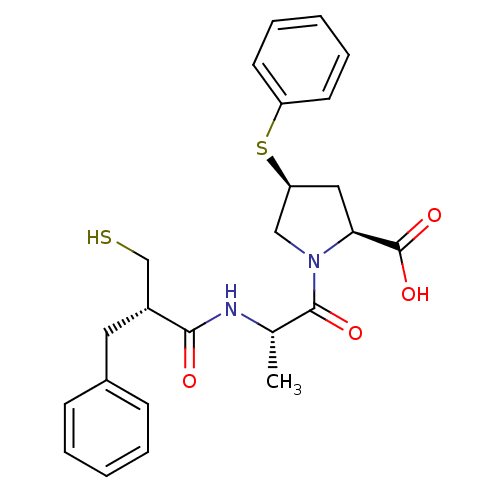 ((2S,4S)-1-[(S)-2-((S)-2-Mercaptomethyl-3-phenyl-pr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibition of rat kidney neutral endopeptidase (NEP) by fluorometric assay using Dansyl-Gly-Phe-Arg as substrate | Bioorg Med Chem Lett 4: 1783-1788 (1994) Article DOI: 10.1016/S0960-894X(01)80371-1 BindingDB Entry DOI: 10.7270/Q2Z3204W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Rattus norvegicus (Rat)) | BDBM50282855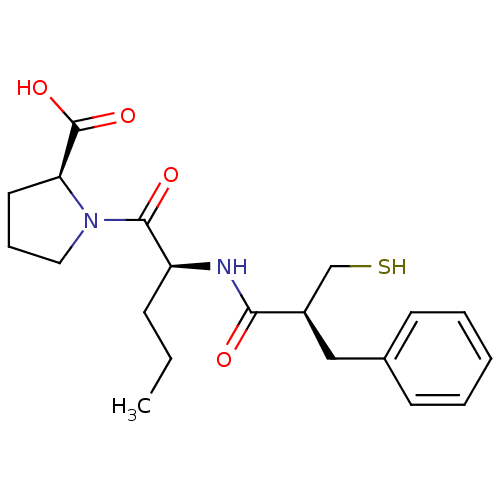 ((S)-1-[(S)-2-((S)-2-Mercaptomethyl-3-phenyl-propio...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibition of rat kidney neutral endopeptidase (NEP) by fluorometric assay using Dansyl-Gly-Phe-Arg as substrate | Bioorg Med Chem Lett 4: 1783-1788 (1994) Article DOI: 10.1016/S0960-894X(01)80371-1 BindingDB Entry DOI: 10.7270/Q2Z3204W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Rattus norvegicus (Rat)) | BDBM50282848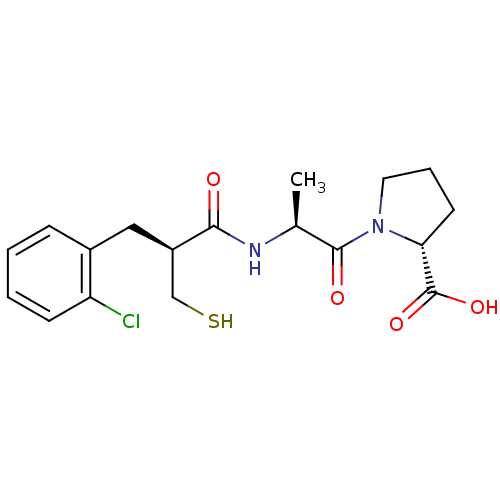 (1-{2-[(S)-3-(2-Chloro-phenyl)-2-mercaptomethyl-pro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibition of rat kidney neutral endopeptidase (NEP) by fluorometric assay using Dansyl-Gly-Phe-Arg as substrate | Bioorg Med Chem Lett 4: 1783-1788 (1994) Article DOI: 10.1016/S0960-894X(01)80371-1 BindingDB Entry DOI: 10.7270/Q2Z3204W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Rattus norvegicus (Rat)) | BDBM50282845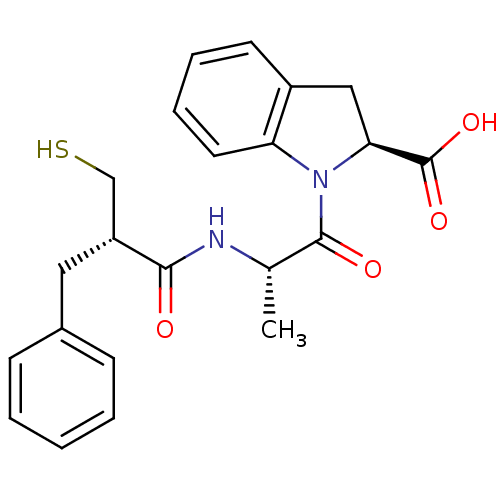 ((S)-1-[(S)-2-((S)-2-Mercaptomethyl-3-phenyl-propio...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibition of rat kidney neutral endopeptidase (NEP) by fluorometric assay using Dansyl-Gly-Phe-Arg as substrate | Bioorg Med Chem Lett 4: 1783-1788 (1994) Article DOI: 10.1016/S0960-894X(01)80371-1 BindingDB Entry DOI: 10.7270/Q2Z3204W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Rattus norvegicus (Rat)) | BDBM50282851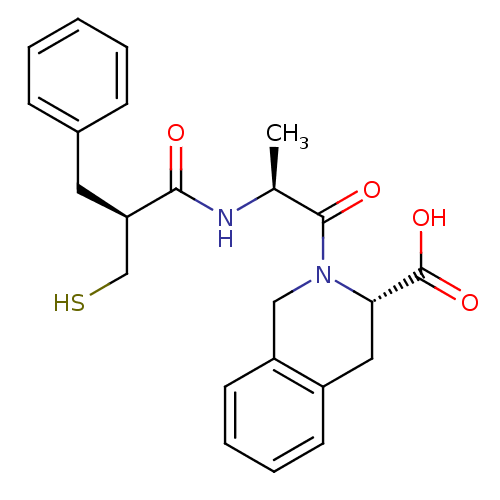 ((S)-2-[(S)-2-((S)-2-Mercaptomethyl-3-phenyl-propio...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibition of rat kidney neutral endopeptidase (NEP) by fluorometric assay using Dansyl-Gly-Phe-Arg as substrate | Bioorg Med Chem Lett 4: 1783-1788 (1994) Article DOI: 10.1016/S0960-894X(01)80371-1 BindingDB Entry DOI: 10.7270/Q2Z3204W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50282861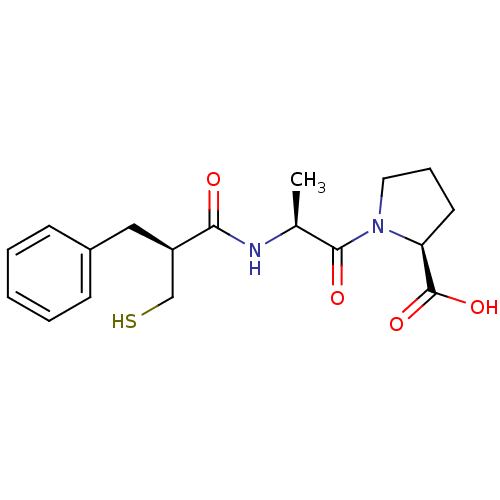 ((S)-1-[(S)-2-((S)-2-Mercaptomethyl-3-phenyl-propio...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibition of rabbit lung Angiotensin I converting enzyme (ACE) using Hippuryl-His-Leu as substrate | Bioorg Med Chem Lett 4: 1783-1788 (1994) Article DOI: 10.1016/S0960-894X(01)80371-1 BindingDB Entry DOI: 10.7270/Q2Z3204W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50073120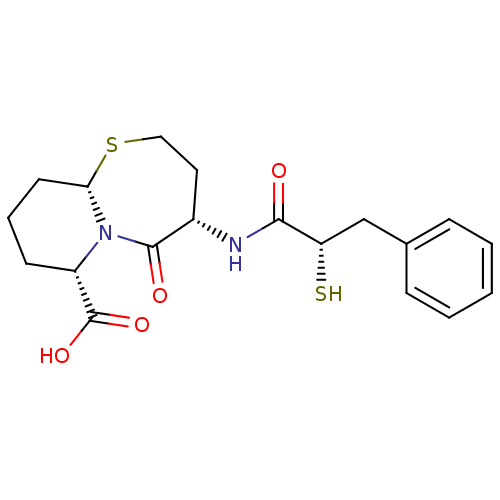 ((4S,6S,9aS)-6-((S)-2-Mercapto-3-phenyl-propionylam...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibitory activity against Angiotensin I converting enzyme (ACE) isolated from rabbit lung extract using hippuryl-L-histidyl-L-leucine (HHL... | J Med Chem 42: 305-11 (1999) Article DOI: 10.1021/jm980542f BindingDB Entry DOI: 10.7270/Q2DV1J2V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Homo sapiens (Human)) | BDBM50403268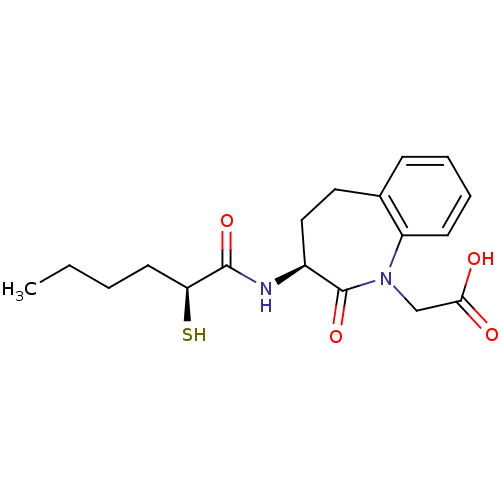 (CHEMBL299875) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article | n/a | n/a | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Angiotensin I converting enzyme | Bioorg Med Chem Lett 4: 1795-1800 (1994) Article DOI: 10.1016/S0960-894X(01)80373-5 BindingDB Entry DOI: 10.7270/Q2Z60Q76 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50049227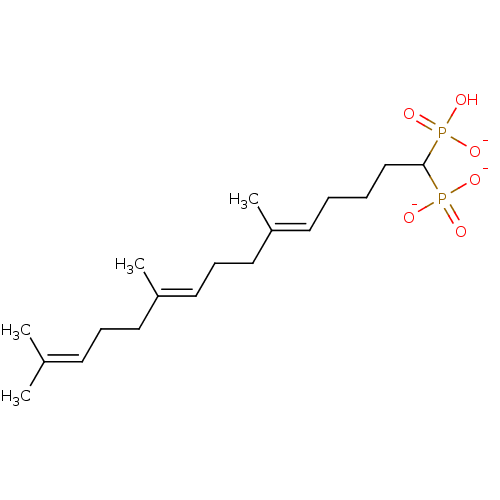 (CHEMBL158707 | Trisodium salt of [1-(Dimethoxy-pho...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against rat liver microsomal squalene synthase was determined using [3H]-FPP as radioligand | J Med Chem 39: 657-60 (1996) Article DOI: 10.1021/jm9507340 BindingDB Entry DOI: 10.7270/Q2N29W1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Rattus norvegicus (Rat)) | BDBM50024101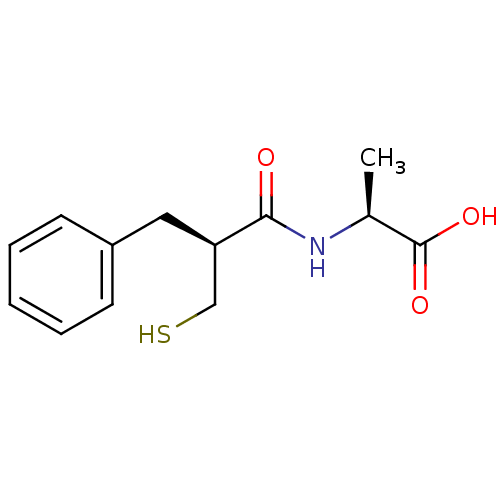 ((S)-2-((S)-2-Mercaptomethyl-3-phenyl-propionylamin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibition of rat kidney neutral endopeptidase (NEP) by fluorometric assay using Dansyl-Gly-Phe-Arg as substrate | Bioorg Med Chem Lett 4: 1783-1788 (1994) Article DOI: 10.1016/S0960-894X(01)80371-1 BindingDB Entry DOI: 10.7270/Q2Z3204W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Homo sapiens (Human)) | BDBM50048512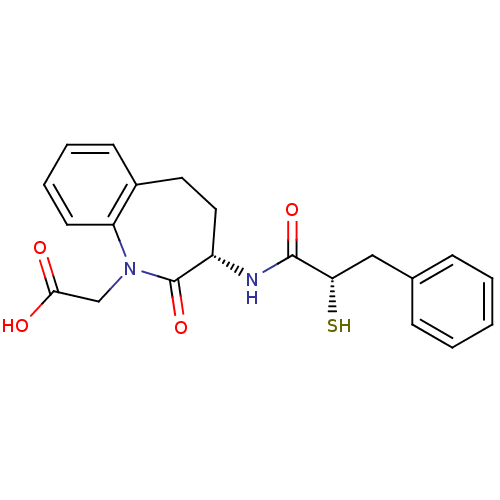 (CHEMBL299639 | [(S)-3-((S)-2-Mercapto-3-phenyl-pro...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Neutral Endopeptidase (NEP) | Bioorg Med Chem Lett 4: 1795-1800 (1994) Article DOI: 10.1016/S0960-894X(01)80373-5 BindingDB Entry DOI: 10.7270/Q2Z60Q76 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50073118 (CHEMBL319237 | [(S)-8-((S)-2-Mercapto-3-phenyl-pro...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibitory activity against Angiotensin I converting enzyme (ACE) isolated from rabbit lung extract using hippuryl-L-histidyl-L-leucine (HHL... | J Med Chem 42: 305-11 (1999) Article DOI: 10.1021/jm980542f BindingDB Entry DOI: 10.7270/Q2DV1J2V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Rattus norvegicus (Rat)) | BDBM50282852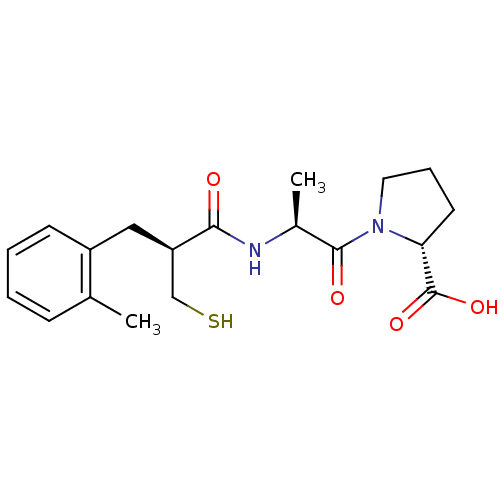 (1-[2-((S)-2-Mercaptomethyl-3-o-tolyl-propionylamin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 6.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibition of rat kidney neutral endopeptidase (NEP) by fluorometric assay using Dansyl-Gly-Phe-Arg as substrate | Bioorg Med Chem Lett 4: 1783-1788 (1994) Article DOI: 10.1016/S0960-894X(01)80371-1 BindingDB Entry DOI: 10.7270/Q2Z3204W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Homo sapiens (Human)) | BDBM50403266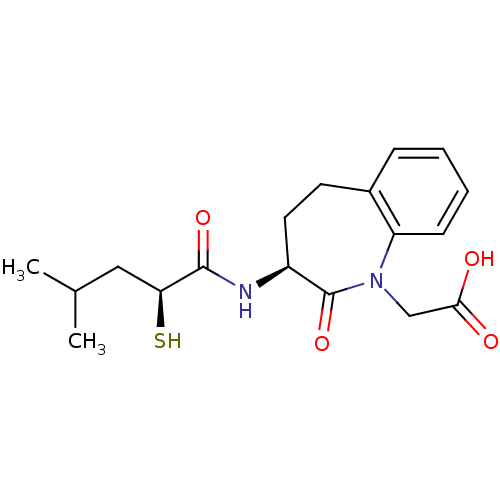 (CHEMBL415932) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article | n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Angiotensin I converting enzyme | Bioorg Med Chem Lett 4: 1795-1800 (1994) Article DOI: 10.1016/S0960-894X(01)80373-5 BindingDB Entry DOI: 10.7270/Q2Z60Q76 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Rattus norvegicus (Rat)) | BDBM50282861 ((S)-1-[(S)-2-((S)-2-Mercaptomethyl-3-phenyl-propio...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 6.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibition of rat kidney neutral endopeptidase (NEP) by fluorometric assay using Dansyl-Gly-Phe-Arg as substrate | Bioorg Med Chem Lett 4: 1783-1788 (1994) Article DOI: 10.1016/S0960-894X(01)80371-1 BindingDB Entry DOI: 10.7270/Q2Z3204W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Rattus norvegicus (Rat)) | BDBM50282847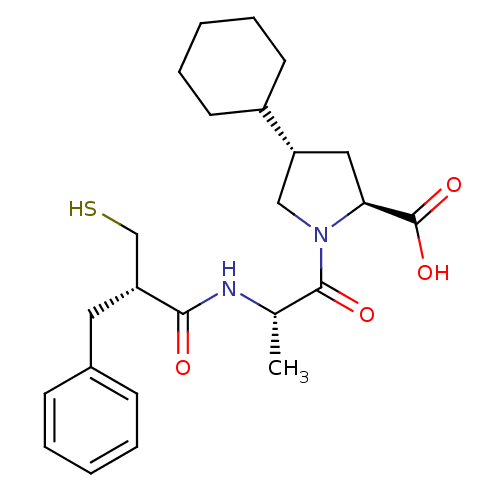 ((2S,4S)-4-Cyclohexyl-1-[(S)-2-((S)-2-mercaptomethy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 6.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibition of rat kidney neutral endopeptidase (NEP) by fluorometric assay using Dansyl-Gly-Phe-Arg as substrate | Bioorg Med Chem Lett 4: 1783-1788 (1994) Article DOI: 10.1016/S0960-894X(01)80371-1 BindingDB Entry DOI: 10.7270/Q2Z3204W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Rattus norvegicus (Rat)) | BDBM50282862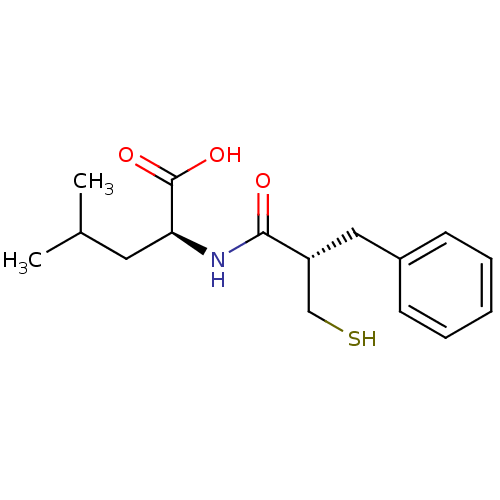 ((S)-2-((S)-2-Mercaptomethyl-3-phenyl-propionylamin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 6.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibition of rat kidney neutral endopeptidase (NEP) by fluorometric assay using Dansyl-Gly-Phe-Arg as substrate | Bioorg Med Chem Lett 4: 1783-1788 (1994) Article DOI: 10.1016/S0960-894X(01)80371-1 BindingDB Entry DOI: 10.7270/Q2Z3204W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Homo sapiens (Human)) | BDBM50403266 (CHEMBL415932) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Neutral Endopeptidase (NEP) | Bioorg Med Chem Lett 4: 1795-1800 (1994) Article DOI: 10.1016/S0960-894X(01)80373-5 BindingDB Entry DOI: 10.7270/Q2Z60Q76 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50282845 ((S)-1-[(S)-2-((S)-2-Mercaptomethyl-3-phenyl-propio...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 7.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibition of rabbit lung Angiotensin I converting enzyme (ACE) using Hippuryl-His-Leu as substrate | Bioorg Med Chem Lett 4: 1783-1788 (1994) Article DOI: 10.1016/S0960-894X(01)80371-1 BindingDB Entry DOI: 10.7270/Q2Z3204W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Rattus norvegicus (Rat)) | BDBM50073120 ((4S,6S,9aS)-6-((S)-2-Mercapto-3-phenyl-propionylam...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibitory activity against purified rat kidney neutral endopeptidase (NEP) using a fluorometric assay with dansyl-Gly-Phe-Arg as the substr... | J Med Chem 42: 305-11 (1999) Article DOI: 10.1021/jm980542f BindingDB Entry DOI: 10.7270/Q2DV1J2V | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50048506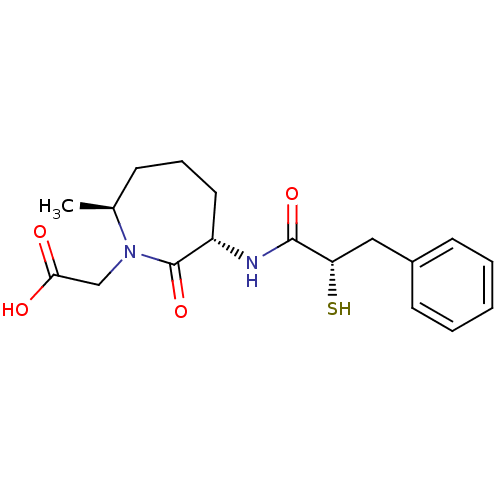 (CHEMBL104054 | [(3S,7S)-3-((S)-2-Mercapto-3-phenyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.20 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibitory activity against Angiotensin I converting enzyme (ACE) isolated from rabbit lung extract using hippuryl-L-histidyl-L-leucine (HHL... | J Med Chem 42: 305-11 (1999) Article DOI: 10.1021/jm980542f BindingDB Entry DOI: 10.7270/Q2DV1J2V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50282859 ((2S,4S)-1-[(S)-2-((S)-2-Mercaptomethyl-3-phenyl-pr...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 8.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibition of rabbit lung Angiotensin I converting enzyme (ACE) using Hippuryl-His-Leu as substrate | Bioorg Med Chem Lett 4: 1783-1788 (1994) Article DOI: 10.1016/S0960-894X(01)80371-1 BindingDB Entry DOI: 10.7270/Q2Z3204W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Rattus norvegicus (Rat)) | BDBM50282858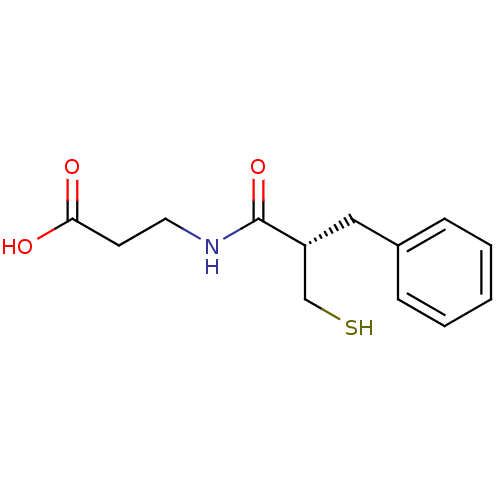 (3-((S)-2-Mercaptomethyl-3-phenyl-propionylamino)-p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article | n/a | n/a | 9.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibition of rat kidney neutral endopeptidase (NEP) by fluorometric assay using Dansyl-Gly-Phe-Arg as substrate | Bioorg Med Chem Lett 4: 1783-1788 (1994) Article DOI: 10.1016/S0960-894X(01)80371-1 BindingDB Entry DOI: 10.7270/Q2Z3204W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50073121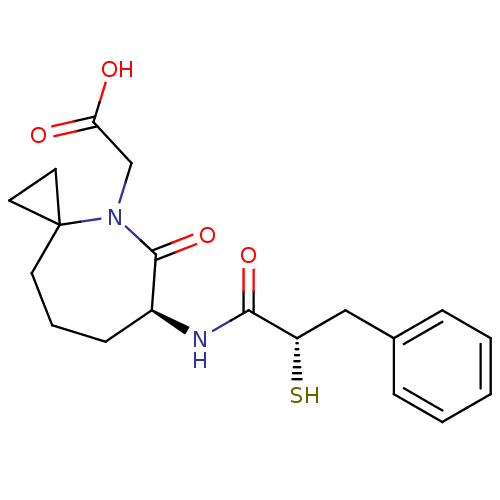 (CHEMBL430484 | [(S)-6-((S)-2-Mercapto-3-phenyl-pro...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.60 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibitory activity against Angiotensin I converting enzyme (ACE) isolated from rabbit lung extract using hippuryl-L-histidyl-L-leucine (HHL... | J Med Chem 42: 305-11 (1999) Article DOI: 10.1021/jm980542f BindingDB Entry DOI: 10.7270/Q2DV1J2V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50282846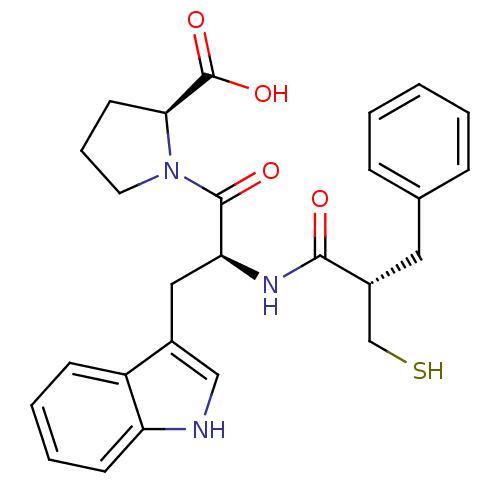 ((S)-1-[(S)-3-(1H-Indol-3-yl)-2-((S)-2-mercaptometh...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibition of rabbit lung Angiotensin I converting enzyme (ACE) using Hippuryl-His-Leu as substrate | Bioorg Med Chem Lett 4: 1783-1788 (1994) Article DOI: 10.1016/S0960-894X(01)80371-1 BindingDB Entry DOI: 10.7270/Q2Z3204W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50282855 ((S)-1-[(S)-2-((S)-2-Mercaptomethyl-3-phenyl-propio...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibition of rabbit lung Angiotensin I converting enzyme (ACE) using Hippuryl-His-Leu as substrate | Bioorg Med Chem Lett 4: 1783-1788 (1994) Article DOI: 10.1016/S0960-894X(01)80371-1 BindingDB Entry DOI: 10.7270/Q2Z3204W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Rattus norvegicus (Rat)) | BDBM50282849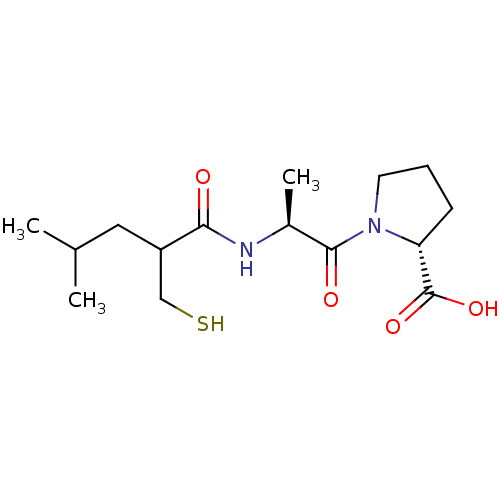 (1-[2-(2-Mercaptomethyl-4-methyl-pentanoylamino)-pr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibition of rat kidney neutral endopeptidase (NEP) by fluorometric assay using Dansyl-Gly-Phe-Arg as substrate | Bioorg Med Chem Lett 4: 1783-1788 (1994) Article DOI: 10.1016/S0960-894X(01)80371-1 BindingDB Entry DOI: 10.7270/Q2Z3204W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50073119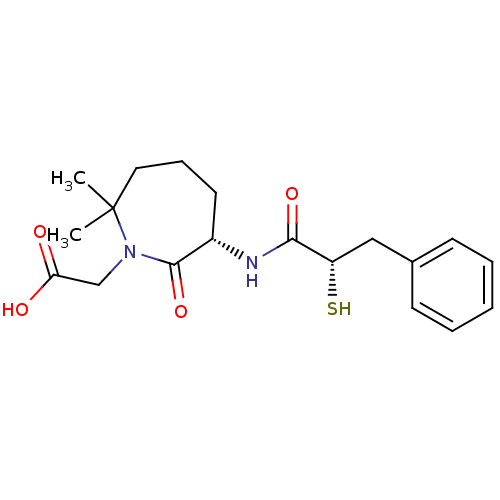 (CHEMBL107747 | Gemopatrilat | [(S)-6-((S)-2-Mercap...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibitory activity against Angiotensin I converting enzyme (ACE) isolated from rabbit lung extract using hippuryl-L-histidyl-L-leucine (HHL... | J Med Chem 42: 305-11 (1999) Article DOI: 10.1021/jm980542f BindingDB Entry DOI: 10.7270/Q2DV1J2V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Homo sapiens (Human)) | BDBM50048512 (CHEMBL299639 | [(S)-3-((S)-2-Mercapto-3-phenyl-pro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Angiotensin I converting enzyme | Bioorg Med Chem Lett 4: 1795-1800 (1994) Article DOI: 10.1016/S0960-894X(01)80373-5 BindingDB Entry DOI: 10.7270/Q2Z60Q76 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50049217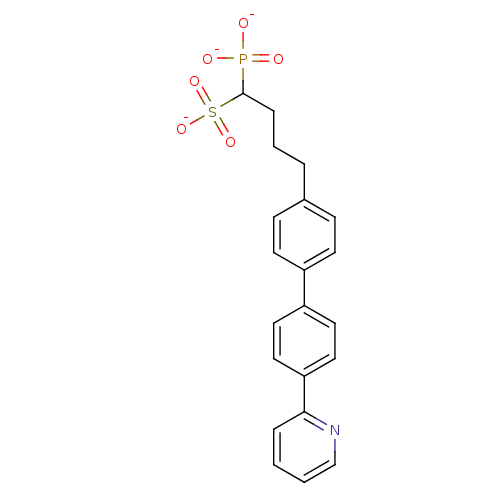 (CHEMBL158517 | Tripotassium salt of 1-Phosphono-4-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against rat liver microsomal squalene synthase was determined using [3H]-FPP as radioligand | J Med Chem 39: 657-60 (1996) Article DOI: 10.1021/jm9507340 BindingDB Entry DOI: 10.7270/Q2N29W1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Homo sapiens (Human)) | BDBM50403267 (CHEMBL422944) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Neutral Endopeptidase (NEP) | Bioorg Med Chem Lett 4: 1795-1800 (1994) Article DOI: 10.1016/S0960-894X(01)80373-5 BindingDB Entry DOI: 10.7270/Q2Z60Q76 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 330 total ) | Next | Last >> |