

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM554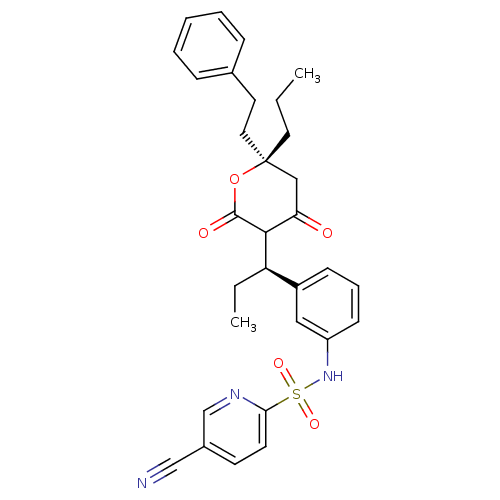 (5-cyano-N-{3-[(1R)-1-[(6R)-4-hydroxy-2-oxo-6-(2-ph...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.00700 | -63.0 | n/a | n/a | n/a | n/a | n/a | 5.0 | 22 |
Upjohn | Assay Description HIV-1 protease was purified and refolded from E. coli inclusion bodies. The substrate used spans the p17-p24 processing site (R-V-S-Q-N-Y-P-I-V-Q-N-K... | J Med Chem 39: 4349-53 (1996) Article DOI: 10.1021/jm960541s BindingDB Entry DOI: 10.7270/Q2HQ3X35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM558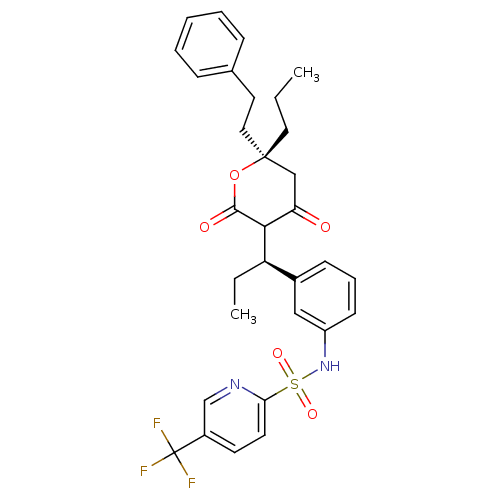 (N-{3-[(1R)-1-[(6R)-4-hydroxy-2-oxo-6-(2-phenylethy...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | 0.00800 | -62.7 | n/a | n/a | n/a | n/a | n/a | 5.0 | 22 |
Upjohn | Assay Description HIV-1 protease was purified and refolded from E. coli inclusion bodies. The substrate used spans the p17-p24 processing site (R-V-S-Q-N-Y-P-I-V-Q-N-K... | J Med Chem 39: 4349-53 (1996) Article DOI: 10.1021/jm960541s BindingDB Entry DOI: 10.7270/Q2HQ3X35 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM557 (N-{3-[(1R)-1-[(6S)-4-hydroxy-2-oxo-6-(2-phenylethy...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0180 | -60.7 | n/a | n/a | n/a | n/a | n/a | 5.0 | 22 |
Upjohn | Assay Description HIV-1 protease was purified and refolded from E. coli inclusion bodies. The substrate used spans the p17-p24 processing site (R-V-S-Q-N-Y-P-I-V-Q-N-K... | J Med Chem 39: 4349-53 (1996) Article DOI: 10.1021/jm960541s BindingDB Entry DOI: 10.7270/Q2HQ3X35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM560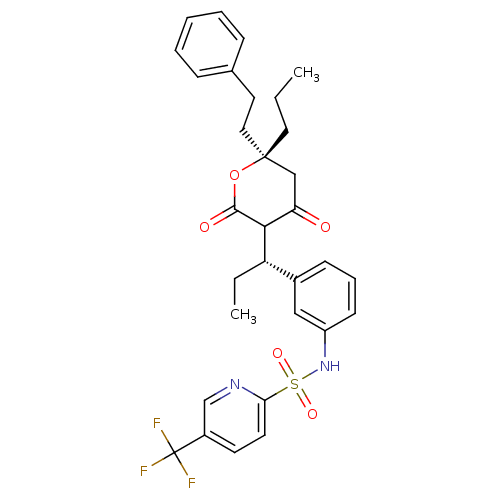 (N-{3-[(1S)-1-[(6R)-4-hydroxy-2-oxo-6-(2-phenylethy...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0320 | -59.3 | n/a | n/a | n/a | n/a | n/a | 5.0 | 22 |
Upjohn | Assay Description HIV-1 protease was purified and refolded from E. coli inclusion bodies. The substrate used spans the p17-p24 processing site (R-V-S-Q-N-Y-P-I-V-Q-N-K... | J Med Chem 39: 4349-53 (1996) Article DOI: 10.1021/jm960541s BindingDB Entry DOI: 10.7270/Q2HQ3X35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM553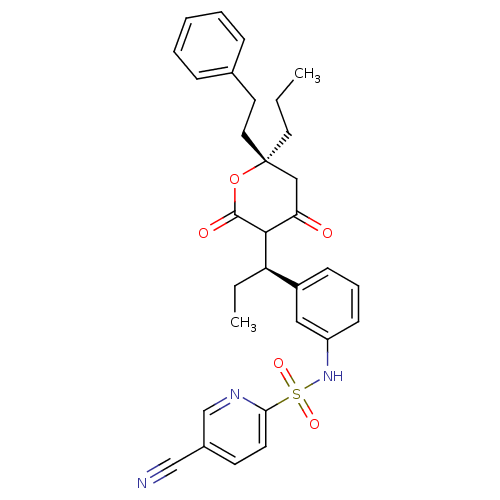 (5-cyano-N-{3-[(1R)-1-[(6S)-4-hydroxy-2-oxo-6-(2-ph...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0400 | -58.8 | n/a | n/a | n/a | n/a | n/a | 5.0 | 22 |
Upjohn | Assay Description HIV-1 protease was purified and refolded from E. coli inclusion bodies. The substrate used spans the p17-p24 processing site (R-V-S-Q-N-Y-P-I-V-Q-N-K... | J Med Chem 39: 4349-53 (1996) Article DOI: 10.1021/jm960541s BindingDB Entry DOI: 10.7270/Q2HQ3X35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM550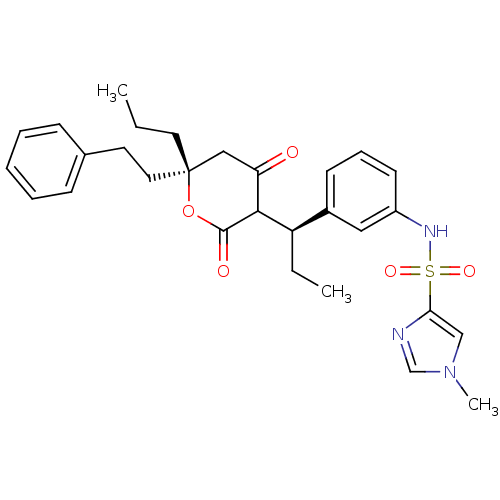 (N-{3-[(1R)-1-[(6R)-4-hydroxy-2-oxo-6-(2-phenylethy...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0600 | -57.8 | n/a | n/a | n/a | n/a | n/a | 5.0 | 22 |
Upjohn | Assay Description HIV-1 protease was purified and refolded from E. coli inclusion bodies. The substrate used spans the p17-p24 processing site (R-V-S-Q-N-Y-P-I-V-Q-N-K... | J Med Chem 39: 4349-53 (1996) Article DOI: 10.1021/jm960541s BindingDB Entry DOI: 10.7270/Q2HQ3X35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM556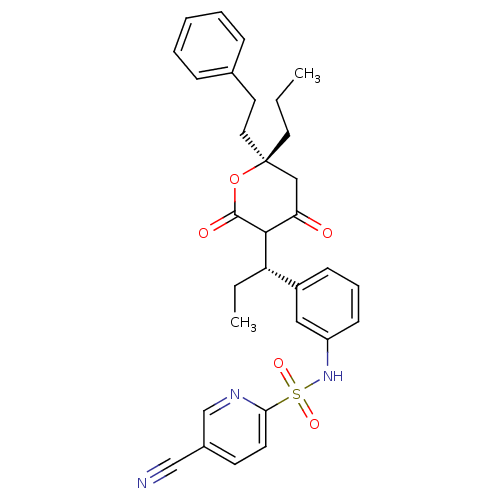 (5-cyano-N-{3-[(1S)-1-[(6R)-4-hydroxy-2-oxo-6-(2-ph...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.100 | -56.5 | n/a | n/a | n/a | n/a | n/a | 5.0 | 22 |
Upjohn | Assay Description HIV-1 protease was purified and refolded from E. coli inclusion bodies. The substrate used spans the p17-p24 processing site (R-V-S-Q-N-Y-P-I-V-Q-N-K... | J Med Chem 39: 4349-53 (1996) Article DOI: 10.1021/jm960541s BindingDB Entry DOI: 10.7270/Q2HQ3X35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM549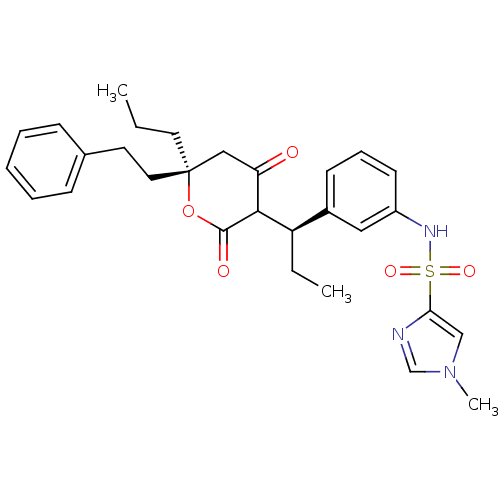 (N-{3-[(1R)-1-[(6S)-4-hydroxy-2-oxo-6-(2-phenylethy...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.120 | -56.1 | n/a | n/a | n/a | n/a | n/a | 5.0 | 22 |
Upjohn | Assay Description HIV-1 protease was purified and refolded from E. coli inclusion bodies. The substrate used spans the p17-p24 processing site (R-V-S-Q-N-Y-P-I-V-Q-N-K... | J Med Chem 39: 4349-53 (1996) Article DOI: 10.1021/jm960541s BindingDB Entry DOI: 10.7270/Q2HQ3X35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM555 (5-cyano-N-{3-[(1S)-1-[(6S)-4-hydroxy-2-oxo-6-(2-ph...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.120 | -56.1 | n/a | n/a | n/a | n/a | n/a | 5.0 | 22 |
Upjohn | Assay Description HIV-1 protease was purified and refolded from E. coli inclusion bodies. The substrate used spans the p17-p24 processing site (R-V-S-Q-N-Y-P-I-V-Q-N-K... | J Med Chem 39: 4349-53 (1996) Article DOI: 10.1021/jm960541s BindingDB Entry DOI: 10.7270/Q2HQ3X35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50072775 (2-((1R,2R,5R)-5-hydroxy-2-(3-hydroxypropyl)cyclohe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Mississippi Curated by ChEMBL | Assay Description Agonist activity at recombinant human full length CB2 receptor expressed in HEK293 cell membranes after 2 hrs by [35S]GTP-gammaS binding assay | Eur J Med Chem 143: 983-996 (2018) Article DOI: 10.1016/j.ejmech.2017.11.043 BindingDB Entry DOI: 10.7270/Q2D50QN8 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM559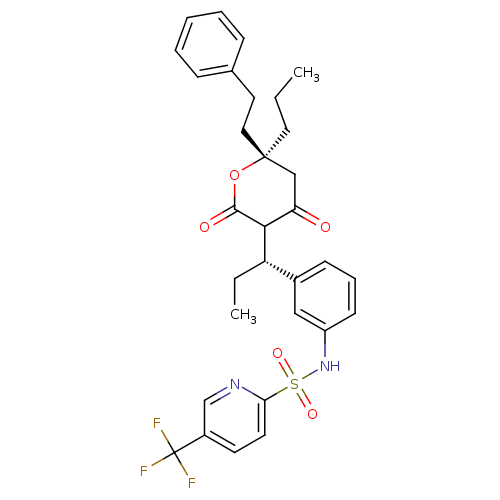 (N-{3-[(1S)-1-[(6S)-4-hydroxy-2-oxo-6-(2-phenylethy...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.220 | -54.6 | n/a | n/a | n/a | n/a | n/a | 5.0 | 22 |
Upjohn | Assay Description HIV-1 protease was purified and refolded from E. coli inclusion bodies. The substrate used spans the p17-p24 processing site (R-V-S-Q-N-Y-P-I-V-Q-N-K... | J Med Chem 39: 4349-53 (1996) Article DOI: 10.1021/jm960541s BindingDB Entry DOI: 10.7270/Q2HQ3X35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM552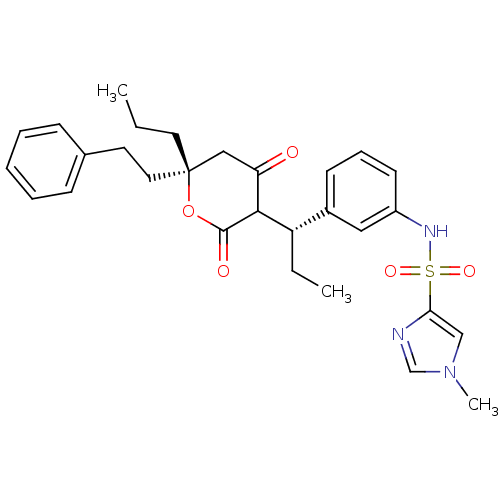 (N-{3-[(1S)-1-[(6R)-4-hydroxy-2-oxo-6-(2-phenylethy...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.300 | -53.8 | n/a | n/a | n/a | n/a | n/a | 5.0 | 22 |
Upjohn | Assay Description HIV-1 protease was purified and refolded from E. coli inclusion bodies. The substrate used spans the p17-p24 processing site (R-V-S-Q-N-Y-P-I-V-Q-N-K... | J Med Chem 39: 4349-53 (1996) Article DOI: 10.1021/jm960541s BindingDB Entry DOI: 10.7270/Q2HQ3X35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50072775 (2-((1R,2R,5R)-5-hydroxy-2-(3-hydroxypropyl)cyclohe...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Mississippi Curated by ChEMBL | Assay Description Displacement of [3H]CP-55,940 from recombinant human full length CB1 receptor expressed in HEK293 cell membranes after 90 mins by topcount method | Eur J Med Chem 143: 983-996 (2018) Article DOI: 10.1016/j.ejmech.2017.11.043 BindingDB Entry DOI: 10.7270/Q2D50QN8 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| 5-hydroxytryptamine receptor 1B (Homo sapiens (Human)) | BDBM50408704 (CHEMBL282229) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity for human 5-hydroxytryptamine 1B receptor | J Med Chem 41: 1218-35 (1998) Article DOI: 10.1021/jm970457s BindingDB Entry DOI: 10.7270/Q2XS5WMN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50072775 (2-((1R,2R,5R)-5-hydroxy-2-(3-hydroxypropyl)cyclohe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.690 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Mississippi Curated by ChEMBL | Assay Description Displacement of [3H]CP-55,940 from recombinant human full length CB2 receptor expressed in HEK293 cell membranes after 90 mins by topcount method | Eur J Med Chem 143: 983-996 (2018) Article DOI: 10.1016/j.ejmech.2017.11.043 BindingDB Entry DOI: 10.7270/Q2D50QN8 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50072775 (2-((1R,2R,5R)-5-hydroxy-2-(3-hydroxypropyl)cyclohe...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Mississippi Curated by ChEMBL | Assay Description Agonist activity at recombinant human full length CB1 receptor expressed in HEK293 cell membranes after 2 hrs by [35S]GTP-gammaS binding assay | Eur J Med Chem 143: 983-996 (2018) Article DOI: 10.1016/j.ejmech.2017.11.043 BindingDB Entry DOI: 10.7270/Q2D50QN8 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM548 (CHEMBL21188 | N-(3-{1-[4-hydroxy-2-oxo-6-(2-phenyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | -50.9 | n/a | n/a | n/a | n/a | n/a | 5.0 | 22 |
Upjohn | Assay Description HIV-1 protease was purified and refolded from E. coli inclusion bodies. The substrate used spans the p17-p24 processing site (R-V-S-Q-N-Y-P-I-V-Q-N-K... | J Med Chem 39: 4349-53 (1996) Article DOI: 10.1021/jm960541s BindingDB Entry DOI: 10.7270/Q2HQ3X35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Homo sapiens (Human)) | BDBM85166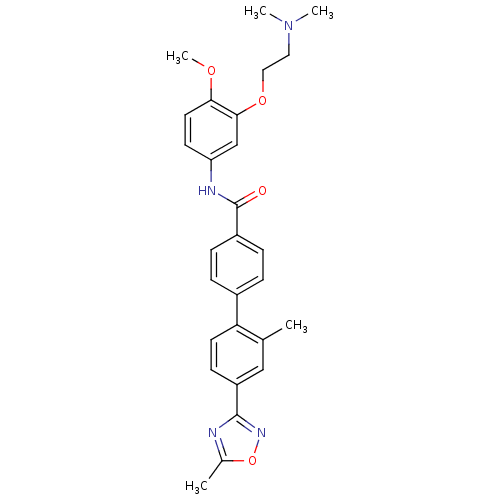 (CAS_3292447 | NSC_3292447 | SB 216641) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity for human 5-hydroxytryptamine 1B receptor | J Med Chem 41: 1218-35 (1998) Article DOI: 10.1021/jm970457s BindingDB Entry DOI: 10.7270/Q2XS5WMN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Homo sapiens (Human)) | BDBM79215 (CHEMBL15928 | GR 127935 | GR 127935 hydrochloride ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity for human 5-hydroxytryptamine 1B receptor | J Med Chem 41: 1218-35 (1998) Article DOI: 10.1021/jm970457s BindingDB Entry DOI: 10.7270/Q2XS5WMN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM551 (N-{3-[(1S)-1-[(6S)-4-hydroxy-2-oxo-6-(2-phenylethy...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1 | -50.9 | n/a | n/a | n/a | n/a | n/a | 5.0 | 22 |
Upjohn | Assay Description HIV-1 protease was purified and refolded from E. coli inclusion bodies. The substrate used spans the p17-p24 processing site (R-V-S-Q-N-Y-P-I-V-Q-N-K... | J Med Chem 39: 4349-53 (1996) Article DOI: 10.1021/jm960541s BindingDB Entry DOI: 10.7270/Q2HQ3X35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Homo sapiens (Human)) | BDBM50408701 (CHEMBL21724) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.58 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity for human 5-hydroxytryptamine 1B receptor | J Med Chem 41: 1218-35 (1998) Article DOI: 10.1021/jm970457s BindingDB Entry DOI: 10.7270/Q2XS5WMN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Homo sapiens (Human)) | BDBM50408705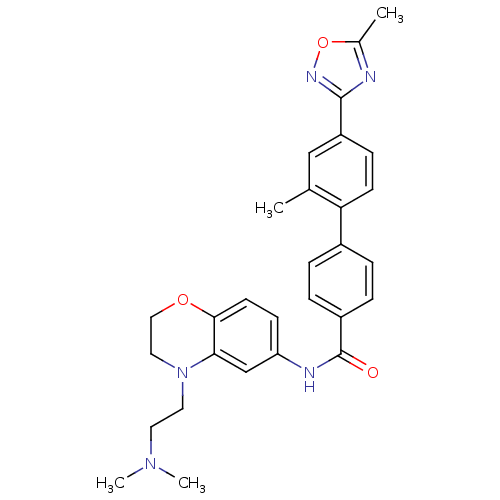 (CHEMBL20791) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.51 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity for human 5-hydroxytryptamine 1B receptor | J Med Chem 41: 1218-35 (1998) Article DOI: 10.1021/jm970457s BindingDB Entry DOI: 10.7270/Q2XS5WMN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Homo sapiens (Human)) | BDBM50408700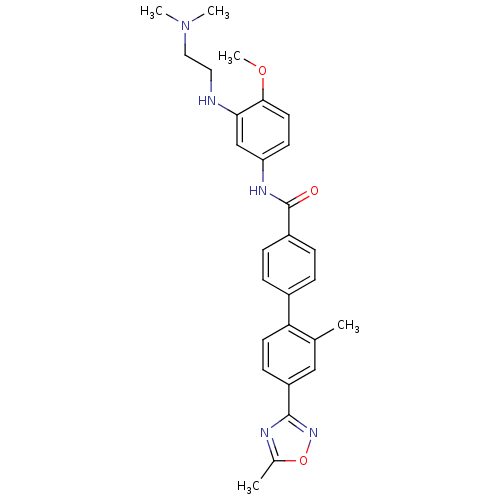 (CHEMBL282693) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.51 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity for human 5-hydroxytryptamine 1B receptor | J Med Chem 41: 1218-35 (1998) Article DOI: 10.1021/jm970457s BindingDB Entry DOI: 10.7270/Q2XS5WMN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM79215 (CHEMBL15928 | GR 127935 | GR 127935 hydrochloride ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 2.51 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity for human 5-hydroxytryptamine 1D receptor | J Med Chem 41: 1218-35 (1998) Article DOI: 10.1021/jm970457s BindingDB Entry DOI: 10.7270/Q2XS5WMN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM547 (CHEMBL20846 | N-(3-{1-[4-hydroxy-2-oxo-6-(2-phenyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3 | -48.2 | n/a | n/a | n/a | n/a | n/a | 5.0 | 22 |
Upjohn | Assay Description HIV-1 protease was purified and refolded from E. coli inclusion bodies. The substrate used spans the p17-p24 processing site (R-V-S-Q-N-Y-P-I-V-Q-N-K... | J Med Chem 39: 4349-53 (1996) Article DOI: 10.1021/jm960541s BindingDB Entry DOI: 10.7270/Q2HQ3X35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Homo sapiens (Human)) | BDBM50408702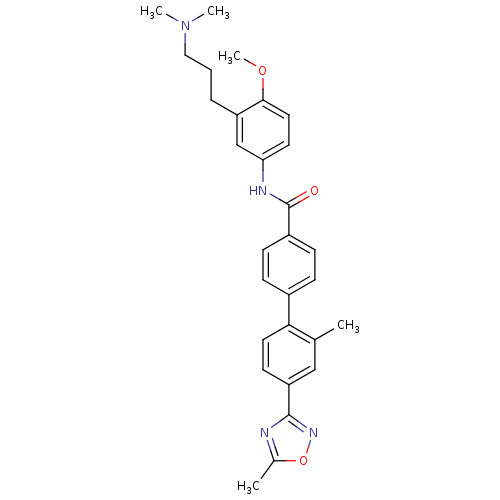 (CHEMBL21790) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity for human 5-hydroxytryptamine 1B receptor | J Med Chem 41: 1218-35 (1998) Article DOI: 10.1021/jm970457s BindingDB Entry DOI: 10.7270/Q2XS5WMN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50408705 (CHEMBL20791) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.98 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity for human 5-hydroxytryptamine 2A receptor | J Med Chem 41: 1218-35 (1998) Article DOI: 10.1021/jm970457s BindingDB Entry DOI: 10.7270/Q2XS5WMN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM50408704 (CHEMBL282229) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 5.01 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity for human 5-hydroxytryptamine 1D receptor | J Med Chem 41: 1218-35 (1998) Article DOI: 10.1021/jm970457s BindingDB Entry DOI: 10.7270/Q2XS5WMN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50408700 (CHEMBL282693) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.01 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity for human 5-hydroxytryptamine 2A receptor | J Med Chem 41: 1218-35 (1998) Article DOI: 10.1021/jm970457s BindingDB Entry DOI: 10.7270/Q2XS5WMN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM546 (4-fluoro-N-(3-{1-[4-hydroxy-2-oxo-6-(2-phenylethyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 6 | -46.5 | n/a | n/a | n/a | n/a | n/a | 5.0 | 22 |
Upjohn | Assay Description HIV-1 protease was purified and refolded from E. coli inclusion bodies. The substrate used spans the p17-p24 processing site (R-V-S-Q-N-Y-P-I-V-Q-N-K... | J Med Chem 39: 4349-53 (1996) Article DOI: 10.1021/jm960541s BindingDB Entry DOI: 10.7270/Q2HQ3X35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Homo sapiens (Human)) | BDBM50408703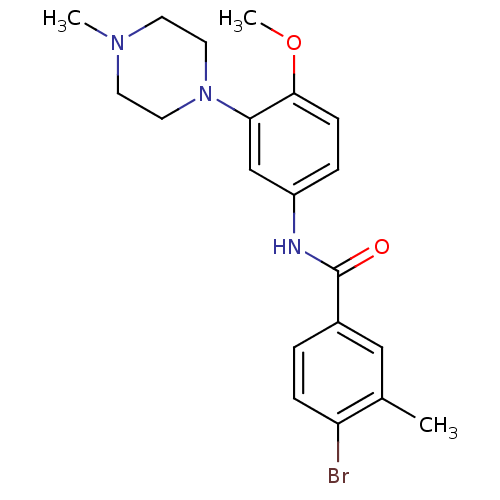 (CHEMBL277467) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 6.31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity for human 5-hydroxytryptamine 1B receptor | J Med Chem 41: 1218-35 (1998) Article DOI: 10.1021/jm970457s BindingDB Entry DOI: 10.7270/Q2XS5WMN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50408702 (CHEMBL21790) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity for human 5-hydroxytryptamine 2A receptor | J Med Chem 41: 1218-35 (1998) Article DOI: 10.1021/jm970457s BindingDB Entry DOI: 10.7270/Q2XS5WMN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Homo sapiens (Human)) | BDBM50084959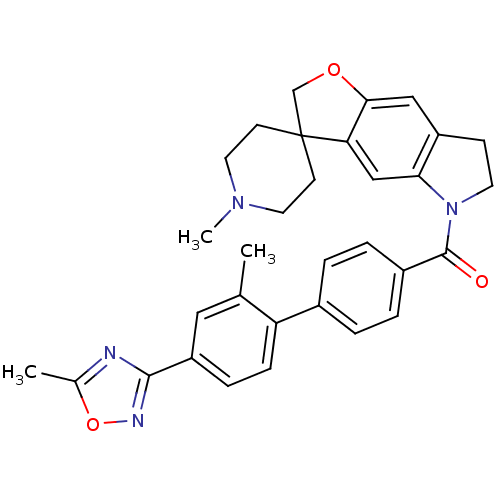 (11'-Methyl-5-[[2'-methyl-4'-(5-methyl-1,2,4-oxadia...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 6.31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity for human 5-hydroxytryptamine 1B receptor | J Med Chem 41: 1218-35 (1998) Article DOI: 10.1021/jm970457s BindingDB Entry DOI: 10.7270/Q2XS5WMN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM545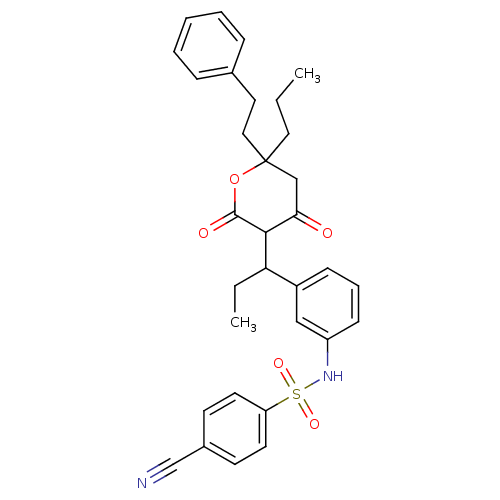 (4-cyano-N-(3-{1-[4-hydroxy-2-oxo-6-(2-phenylethyl)...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8 | -45.7 | n/a | n/a | n/a | n/a | n/a | 5.0 | 22 |
Upjohn | Assay Description HIV-1 protease was purified and refolded from E. coli inclusion bodies. The substrate used spans the p17-p24 processing site (R-V-S-Q-N-Y-P-I-V-Q-N-K... | J Med Chem 39: 4349-53 (1996) Article DOI: 10.1021/jm960541s BindingDB Entry DOI: 10.7270/Q2HQ3X35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50464596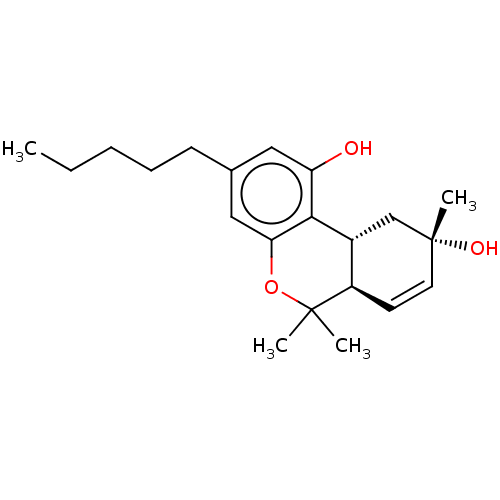 (CHEMBL4282822) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 9.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Mississippi Curated by ChEMBL | Assay Description Displacement of [3H]CP-55,940 from recombinant human full length CB2 receptor expressed in HEK293 cell membranes after 90 mins by topcount method | Eur J Med Chem 143: 983-996 (2018) Article DOI: 10.1016/j.ejmech.2017.11.043 BindingDB Entry DOI: 10.7270/Q2D50QN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 2 [11-552] (Homo sapiens (Human)) | BDBM97666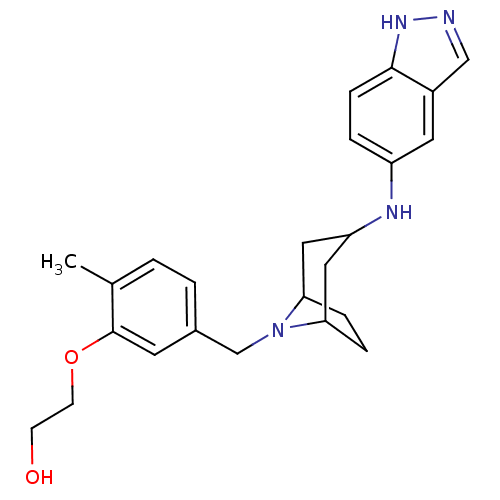 (US8476295, 1.1.04) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 10 | n/a | n/a | n/a | n/a | n/a | n/a | 7.2 | n/a |
Inspire US Patent | Assay Description Inhibition of Rho kinase 2 and Rho kinase I activity was determined using the IMAP Screening Express Kit (Molecular Devices product number #8073). | US Patent US8476295 (2013) BindingDB Entry DOI: 10.7270/Q2H993TS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50067734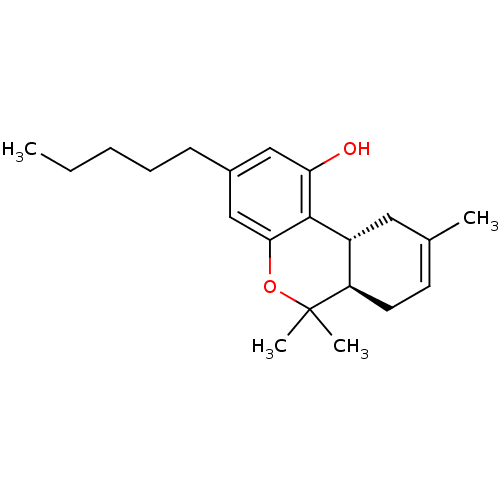 ((-)-(6aR-trans)-6,6,9-trimethyl-3-pentyl-6a,7,10,1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Mississippi Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from full length human recombinant CB2 receptor expressed in HEK293 cells after 90 mins by scintillation counting analysi... | J Nat Prod 78: 1271-6 (2015) Article DOI: 10.1021/acs.jnatprod.5b00065 BindingDB Entry DOI: 10.7270/Q2SQ923K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Homo sapiens (Human)) | BDBM50408706 (CHEMBL20771) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 12.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity for human 5-hydroxytryptamine 1B receptor | J Med Chem 41: 1218-35 (1998) Article DOI: 10.1021/jm970457s BindingDB Entry DOI: 10.7270/Q2XS5WMN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM50408703 (CHEMBL277467) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 12.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity for human 5-hydroxytryptamine 1D receptor | J Med Chem 41: 1218-35 (1998) Article DOI: 10.1021/jm970457s BindingDB Entry DOI: 10.7270/Q2XS5WMN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM79215 (CHEMBL15928 | GR 127935 | GR 127935 hydrochloride ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 15.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity for human 5-hydroxytryptamine 2A receptor | J Med Chem 41: 1218-35 (1998) Article DOI: 10.1021/jm970457s BindingDB Entry DOI: 10.7270/Q2XS5WMN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM544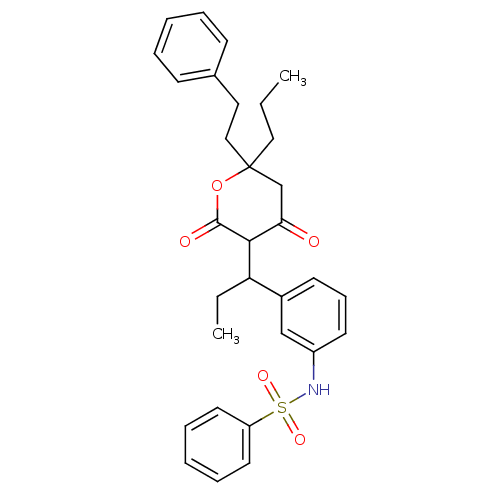 (N-(3-{1-[4-hydroxy-2-oxo-6-(2-phenylethyl)-6-propy...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 17 | -43.9 | n/a | n/a | n/a | n/a | n/a | 5.0 | 22 |
Upjohn | Assay Description HIV-1 protease was purified and refolded from E. coli inclusion bodies. The substrate used spans the p17-p24 processing site (R-V-S-Q-N-Y-P-I-V-Q-N-K... | J Med Chem 39: 4349-53 (1996) Article DOI: 10.1021/jm960541s BindingDB Entry DOI: 10.7270/Q2HQ3X35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM60994 ((10R,10aR)-6,6,9-Trimethyl-3-pentyl-6a,7,8,10a-tet...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL DrugBank KEGG PC cid PC sid PDB UniChem Similars | DrugBank Article PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Mississippi Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from full length human recombinant CB1 receptor expressed in HEK293 cells after 90 mins by scintillation counting analysi... | J Nat Prod 78: 1271-6 (2015) Article DOI: 10.1021/acs.jnatprod.5b00065 BindingDB Entry DOI: 10.7270/Q2SQ923K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM50408705 (CHEMBL20791) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 19.9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity for human 5-hydroxytryptamine 1D receptor | J Med Chem 41: 1218-35 (1998) Article DOI: 10.1021/jm970457s BindingDB Entry DOI: 10.7270/Q2XS5WMN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 2 [11-552] (Homo sapiens (Human)) | BDBM97664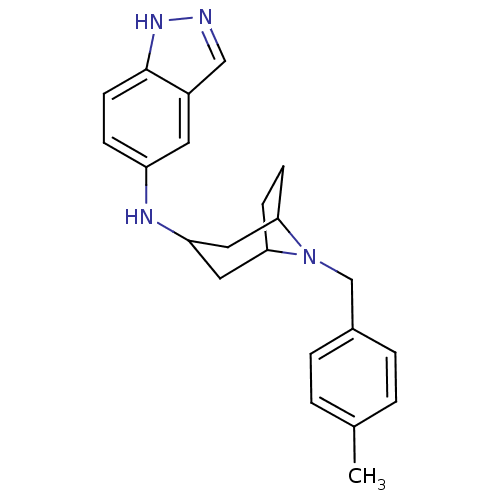 (US8476295, 1.1.02) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 20 | n/a | n/a | n/a | n/a | n/a | n/a | 7.2 | n/a |
Inspire US Patent | Assay Description Inhibition of Rho kinase 2 and Rho kinase I activity was determined using the IMAP Screening Express Kit (Molecular Devices product number #8073). | US Patent US8476295 (2013) BindingDB Entry DOI: 10.7270/Q2H993TS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 2 [11-552] (Homo sapiens (Human)) | BDBM97665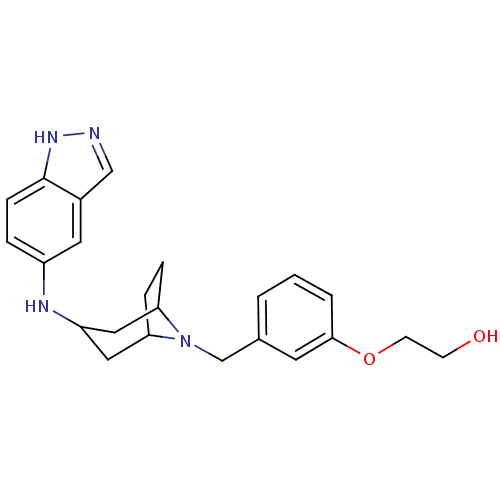 (US8476295, 1.1.03) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 21 | n/a | n/a | n/a | n/a | n/a | n/a | 7.2 | n/a |
Inspire US Patent | Assay Description Inhibition of Rho kinase 2 and Rho kinase I activity was determined using the IMAP Screening Express Kit (Molecular Devices product number #8073). | US Patent US8476295 (2013) BindingDB Entry DOI: 10.7270/Q2H993TS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM50408701 (CHEMBL21724) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 25.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity for human 5-hydroxytryptamine 1D receptor | J Med Chem 41: 1218-35 (1998) Article DOI: 10.1021/jm970457s BindingDB Entry DOI: 10.7270/Q2XS5WMN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 2 [11-552] (Homo sapiens (Human)) | BDBM97663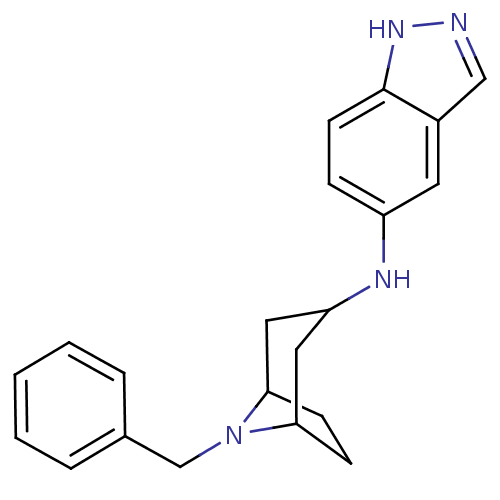 (US8476295, 1.1.01) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 29 | n/a | n/a | n/a | n/a | n/a | n/a | 7.2 | n/a |
Inspire US Patent | Assay Description Inhibition of Rho kinase 2 and Rho kinase I activity was determined using the IMAP Screening Express Kit (Molecular Devices product number #8073). | US Patent US8476295 (2013) BindingDB Entry DOI: 10.7270/Q2H993TS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50092347 (CHEMBL3586106) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Mississippi Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from full length human recombinant CB2 receptor expressed in HEK293 cells after 90 mins by scintillation counting analysi... | J Nat Prod 78: 1271-6 (2015) Article DOI: 10.1021/acs.jnatprod.5b00065 BindingDB Entry DOI: 10.7270/Q2SQ923K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50092347 (CHEMBL3586106) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Mississippi Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from full length human recombinant CB1 receptor expressed in HEK293 cells after 90 mins by scintillation counting analysi... | J Nat Prod 78: 1271-6 (2015) Article DOI: 10.1021/acs.jnatprod.5b00065 BindingDB Entry DOI: 10.7270/Q2SQ923K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Homo sapiens (Human)) | BDBM50086096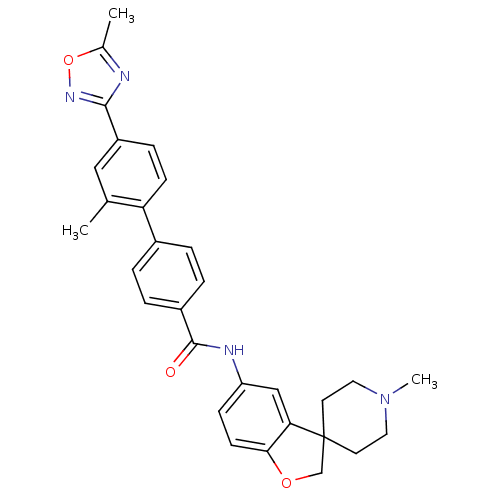 (2'-Methyl-4'-(5-methyl-[1,2,4]oxadiazol-3-yl)-biph...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 31.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity for human 5-hydroxytryptamine 1B receptor | J Med Chem 41: 1218-35 (1998) Article DOI: 10.1021/jm970457s BindingDB Entry DOI: 10.7270/Q2XS5WMN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 324 total ) | Next | Last >> |