

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50121409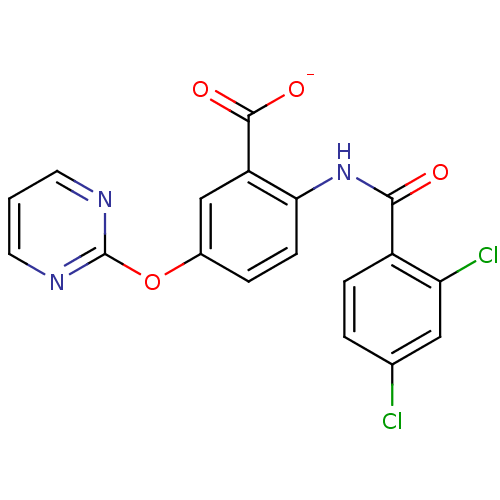 (CHEMBL118206 | Lithium; 2-(2,4-dichloro-benzoylami...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | PubMed | 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biovitrum AB Curated by ChEMBL | Assay Description Ligand binding affinity was determined by displacement of a tritiated tracer by the unlabeled compound to a GST-PPAR fusion protein for Peroxisome pr... | Bioorg Med Chem Lett 12: 3565-7 (2002) BindingDB Entry DOI: 10.7270/Q2Q23ZK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50121424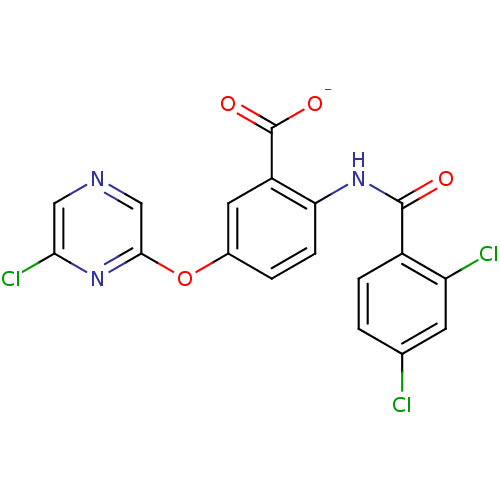 (CHEMBL119111 | Lithium; 5-(6-chloro-pyrazin-2-ylox...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biovitrum AB Curated by ChEMBL | Assay Description Ligand binding affinity was determined by displacement of a tritiated tracer by the unlabeled compound to a GST-PPAR fusion protein for Peroxisome pr... | Bioorg Med Chem Lett 12: 3565-7 (2002) BindingDB Entry DOI: 10.7270/Q2Q23ZK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50121420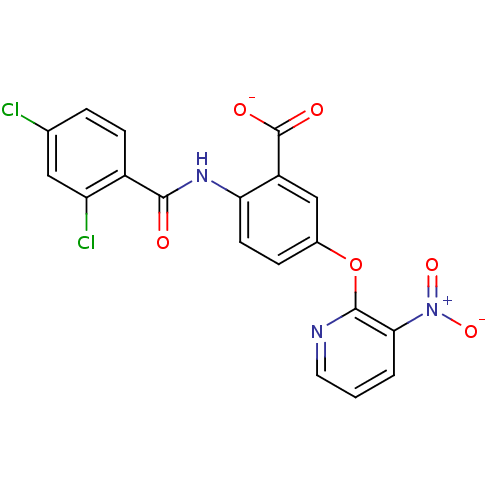 (CHEMBL330938 | Lithium; 2-(2,4-dichloro-benzoylami...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biovitrum AB Curated by ChEMBL | Assay Description Ligand binding affinity was determined by displacement of a tritiated tracer by the unlabeled compound to a GST-PPAR fusion protein for Peroxisome pr... | Bioorg Med Chem Lett 12: 3565-7 (2002) BindingDB Entry DOI: 10.7270/Q2Q23ZK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50121421 (CHEMBL118546 | Lithium; 2-(2,4-dichloro-benzoylami...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biovitrum AB Curated by ChEMBL | Assay Description Ligand binding affinity was determined by displacement of a tritiated tracer by the unlabeled compound to a GST-PPAR fusion protein for Peroxisome pr... | Bioorg Med Chem Lett 12: 3565-7 (2002) BindingDB Entry DOI: 10.7270/Q2Q23ZK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50121422 (CHEMBL119869 | Lithium; 2-(2,4-dichloro-benzoylami...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biovitrum AB Curated by ChEMBL | Assay Description Ligand binding affinity was determined by displacement of a tritiated tracer by the unlabeled compound to a GST-PPAR fusion protein for Peroxisome pr... | Bioorg Med Chem Lett 12: 3565-7 (2002) BindingDB Entry DOI: 10.7270/Q2Q23ZK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50121415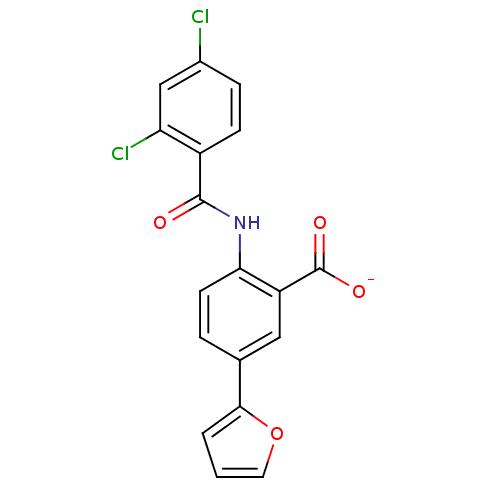 (CHEMBL119030 | Lithium; 2-(2,4-dichloro-benzoylami...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biovitrum AB Curated by ChEMBL | Assay Description Ligand binding affinity was determined by displacement of a tritiated tracer by the unlabeled compound to a GST-PPAR fusion protein for Peroxisome pr... | Bioorg Med Chem Lett 12: 3565-7 (2002) BindingDB Entry DOI: 10.7270/Q2Q23ZK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50121414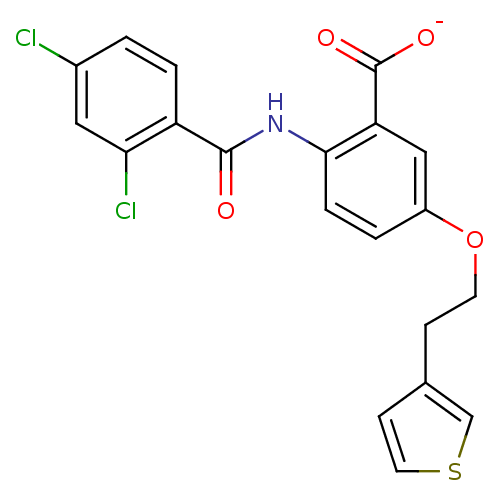 (CHEMBL333890 | Lithium; 2-(2,4-dichloro-benzoylami...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biovitrum AB Curated by ChEMBL | Assay Description Ligand binding affinity was determined by displacement of a tritiated tracer by the unlabeled compound to a GST-PPAR fusion protein for Peroxisome pr... | Bioorg Med Chem Lett 12: 3565-7 (2002) BindingDB Entry DOI: 10.7270/Q2Q23ZK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50121418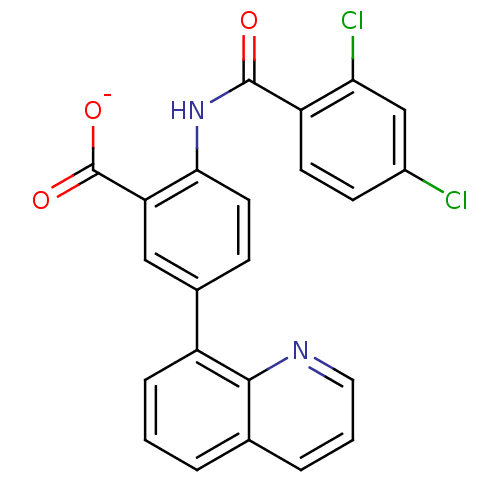 (CHEMBL119078 | Lithium; 2-(2,4-dichloro-benzoylami...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biovitrum AB Curated by ChEMBL | Assay Description Ligand binding affinity was determined by displacement of a tritiated tracer by the unlabeled compound to a GST-PPAR fusion protein for Peroxisome pr... | Bioorg Med Chem Lett 12: 3565-7 (2002) BindingDB Entry DOI: 10.7270/Q2Q23ZK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50121411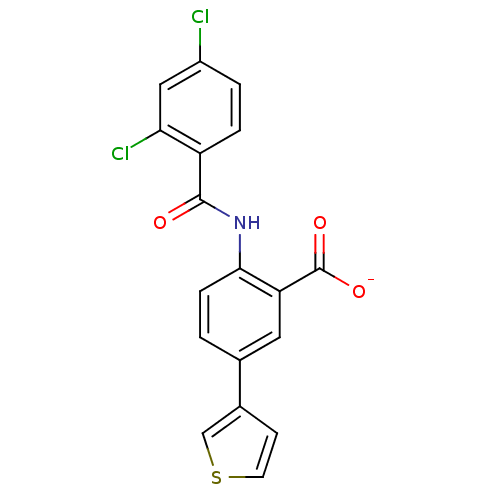 (CHEMBL118617 | Lithium; 2-(2,4-dichloro-benzoylami...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biovitrum AB Curated by ChEMBL | Assay Description Ligand binding affinity was determined by displacement of a tritiated tracer by the unlabeled compound to a GST-PPAR fusion protein for Peroxisome pr... | Bioorg Med Chem Lett 12: 3565-7 (2002) BindingDB Entry DOI: 10.7270/Q2Q23ZK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50121423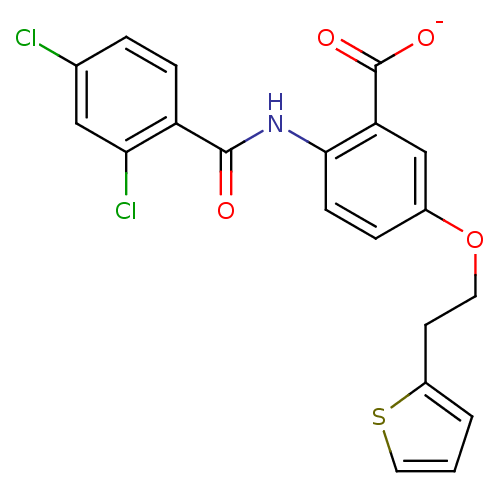 (CHEMBL119278 | Lithium; 2-(2,4-dichloro-benzoylami...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biovitrum AB Curated by ChEMBL | Assay Description Ligand binding affinity was determined by displacement of a tritiated tracer by the unlabeled compound to a GST-PPAR fusion protein for Peroxisome pr... | Bioorg Med Chem Lett 12: 3565-7 (2002) BindingDB Entry DOI: 10.7270/Q2Q23ZK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50121413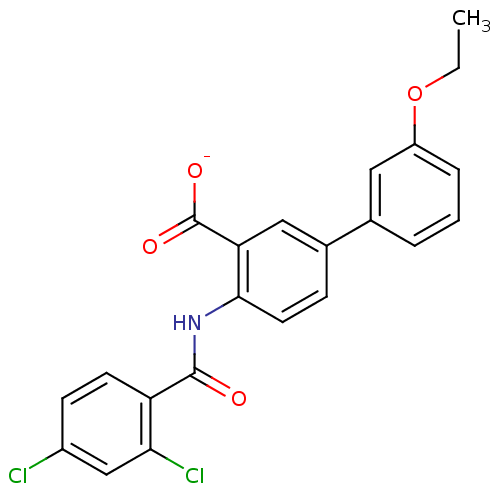 (CHEMBL118852 | Lithium; 4-(2,4-dichloro-benzoylami...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biovitrum AB Curated by ChEMBL | Assay Description Ligand binding affinity was determined by displacement of a tritiated tracer by the unlabeled compound to a GST-PPAR fusion protein for Peroxisome pr... | Bioorg Med Chem Lett 12: 3565-7 (2002) BindingDB Entry DOI: 10.7270/Q2Q23ZK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50121416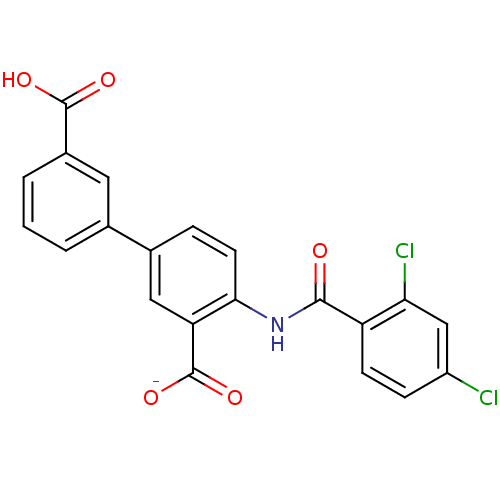 (CHEMBL325172 | Lithium; 3'-carboxy-4-(2,4-dichloro...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biovitrum AB Curated by ChEMBL | Assay Description Transactivation potency was measured by luciferase activity in Caco- 2/TC7 cells transiently co-transfected for the fusion-protein Gal4-PPAR alpha | Bioorg Med Chem Lett 12: 3565-7 (2002) BindingDB Entry DOI: 10.7270/Q2Q23ZK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50121410 (CHEMBL420525 | Lithium; 2-(2,4-dichloro-benzoylami...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biovitrum AB Curated by ChEMBL | Assay Description Ligand binding affinity was determined by displacement of a tritiated tracer by the unlabeled compound to a GST-PPAR fusion protein for Peroxisome pr... | Bioorg Med Chem Lett 12: 3565-7 (2002) BindingDB Entry DOI: 10.7270/Q2Q23ZK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50121417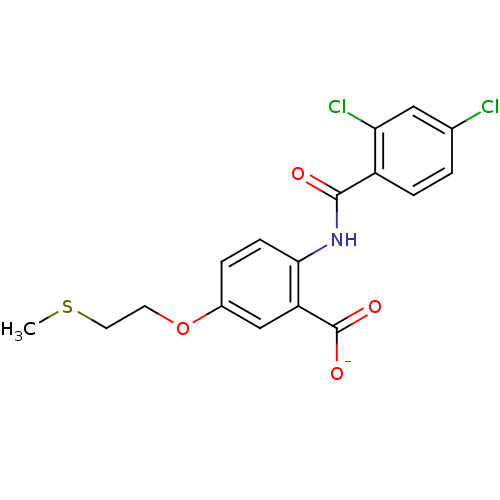 (CHEMBL119646 | Lithium; 2-(2,4-dichloro-benzoylami...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biovitrum AB Curated by ChEMBL | Assay Description Ligand binding affinity was determined by displacement of a tritiated tracer by the unlabeled compound to a GST-PPAR fusion protein for Peroxisome pr... | Bioorg Med Chem Lett 12: 3565-7 (2002) BindingDB Entry DOI: 10.7270/Q2Q23ZK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50121412 (2-(2,4-Dichloro-benzoylamino)-5-methyl-benzoic aci...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biovitrum AB Curated by ChEMBL | Assay Description Ligand binding affinity was determined by displacement of a tritiated tracer by the unlabeled compound to a GST-PPAR fusion protein for Peroxisome pr... | Bioorg Med Chem Lett 12: 3565-7 (2002) BindingDB Entry DOI: 10.7270/Q2Q23ZK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50121419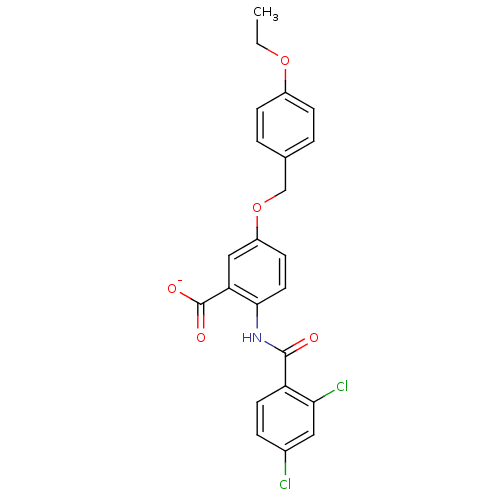 (CHEMBL119798 | Lithium; 2-(2,4-dichloro-benzoylami...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biovitrum AB Curated by ChEMBL | Assay Description Ligand binding affinity was determined by displacement of a tritiated tracer by the unlabeled compound to a GST-PPAR fusion protein for Peroxisome pr... | Bioorg Med Chem Lett 12: 3565-7 (2002) BindingDB Entry DOI: 10.7270/Q2Q23ZK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50121417 (CHEMBL119646 | Lithium; 2-(2,4-dichloro-benzoylami...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biovitrum AB Curated by ChEMBL | Assay Description Ligand binding affinity was determined by displacement of a tritiated tracer by the unlabeled compound to a GST-PPAR fusion protein for Peroxisome pr... | Bioorg Med Chem Lett 12: 3565-7 (2002) BindingDB Entry DOI: 10.7270/Q2Q23ZK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50121414 (CHEMBL333890 | Lithium; 2-(2,4-dichloro-benzoylami...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biovitrum AB Curated by ChEMBL | Assay Description Transactivation potency was measured by luciferase activity in Caco- 2/TC7 cells transiently co-transfected for the fusion-protein Gal4-PPAR alpha | Bioorg Med Chem Lett 12: 3565-7 (2002) BindingDB Entry DOI: 10.7270/Q2Q23ZK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50121424 (CHEMBL119111 | Lithium; 5-(6-chloro-pyrazin-2-ylox...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 2.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biovitrum AB Curated by ChEMBL | Assay Description Ligand binding affinity was determined by displacement of a tritiated tracer by the unlabeled compound to a GST-PPAR fusion protein for Peroxisome pr... | Bioorg Med Chem Lett 12: 3565-7 (2002) BindingDB Entry DOI: 10.7270/Q2Q23ZK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50121415 (CHEMBL119030 | Lithium; 2-(2,4-dichloro-benzoylami...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 2.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biovitrum AB Curated by ChEMBL | Assay Description Ligand binding affinity was determined by displacement of a tritiated tracer by the unlabeled compound to a GST-PPAR fusion protein for Peroxisome pr... | Bioorg Med Chem Lett 12: 3565-7 (2002) BindingDB Entry DOI: 10.7270/Q2Q23ZK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50121422 (CHEMBL119869 | Lithium; 2-(2,4-dichloro-benzoylami...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biovitrum AB Curated by ChEMBL | Assay Description Ligand binding affinity was determined by displacement of a tritiated tracer by the unlabeled compound to a GST-PPAR fusion protein for Peroxisome pr... | Bioorg Med Chem Lett 12: 3565-7 (2002) BindingDB Entry DOI: 10.7270/Q2Q23ZK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50121411 (CHEMBL118617 | Lithium; 2-(2,4-dichloro-benzoylami...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biovitrum AB Curated by ChEMBL | Assay Description Ligand binding affinity was determined by displacement of a tritiated tracer by the unlabeled compound to a GST-PPAR fusion protein for Peroxisome pr... | Bioorg Med Chem Lett 12: 3565-7 (2002) BindingDB Entry DOI: 10.7270/Q2Q23ZK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50121412 (2-(2,4-Dichloro-benzoylamino)-5-methyl-benzoic aci...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biovitrum AB Curated by ChEMBL | Assay Description Ligand binding affinity was determined by displacement of a tritiated tracer by the unlabeled compound to a GST-PPAR fusion protein for Peroxisome pr... | Bioorg Med Chem Lett 12: 3565-7 (2002) BindingDB Entry DOI: 10.7270/Q2Q23ZK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50121416 (CHEMBL325172 | Lithium; 3'-carboxy-4-(2,4-dichloro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 6.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biovitrum AB Curated by ChEMBL | Assay Description Transactivation potency was measured by luciferase activity in Caco- 2/TC7 cells transiently co-transfected for the fusion-protein Gal4-PPAR alpha | Bioorg Med Chem Lett 12: 3565-7 (2002) BindingDB Entry DOI: 10.7270/Q2Q23ZK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50121418 (CHEMBL119078 | Lithium; 2-(2,4-dichloro-benzoylami...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biovitrum AB Curated by ChEMBL | Assay Description Transactivation potency was measured by luciferase activity in Caco- 2/TC7 cells transiently co-transfected for the fusion-protein Gal4-PPAR alpha | Bioorg Med Chem Lett 12: 3565-7 (2002) BindingDB Entry DOI: 10.7270/Q2Q23ZK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50121420 (CHEMBL330938 | Lithium; 2-(2,4-dichloro-benzoylami...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biovitrum AB Curated by ChEMBL | Assay Description Transactivation potency was measured by luciferase activity in Caco- 2/TC7 cells transiently co-transfected for the fusion-protein Gal4-PPAR gamma | Bioorg Med Chem Lett 12: 3565-7 (2002) BindingDB Entry DOI: 10.7270/Q2Q23ZK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50121413 (CHEMBL118852 | Lithium; 4-(2,4-dichloro-benzoylami...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biovitrum AB Curated by ChEMBL | Assay Description Ligand binding affinity was determined by displacement of a tritiated tracer by the unlabeled compound to a GST-PPAR fusion protein for Peroxisome pr... | Bioorg Med Chem Lett 12: 3565-7 (2002) BindingDB Entry DOI: 10.7270/Q2Q23ZK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50121421 (CHEMBL118546 | Lithium; 2-(2,4-dichloro-benzoylami...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biovitrum AB Curated by ChEMBL | Assay Description Transactivation potency was measured by luciferase activity in Caco- 2/TC7 cells transiently co-transfected for the fusion-protein Gal4-PPAR gamma | Bioorg Med Chem Lett 12: 3565-7 (2002) BindingDB Entry DOI: 10.7270/Q2Q23ZK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50121419 (CHEMBL119798 | Lithium; 2-(2,4-dichloro-benzoylami...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biovitrum AB Curated by ChEMBL | Assay Description Ligand binding affinity was determined by displacement of a tritiated tracer by the unlabeled compound to a GST-PPAR fusion protein for Peroxisome pr... | Bioorg Med Chem Lett 12: 3565-7 (2002) BindingDB Entry DOI: 10.7270/Q2Q23ZK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50121410 (CHEMBL420525 | Lithium; 2-(2,4-dichloro-benzoylami...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biovitrum AB Curated by ChEMBL | Assay Description Ligand binding affinity was determined by displacement of a tritiated tracer by the unlabeled compound to a GST-PPAR fusion protein for Peroxisome pr... | Bioorg Med Chem Lett 12: 3565-7 (2002) BindingDB Entry DOI: 10.7270/Q2Q23ZK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50121423 (CHEMBL119278 | Lithium; 2-(2,4-dichloro-benzoylami...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biovitrum AB Curated by ChEMBL | Assay Description Ligand binding affinity was determined by displacement of a tritiated tracer by the unlabeled compound to a GST-PPAR fusion protein for Peroxisome pr... | Bioorg Med Chem Lett 12: 3565-7 (2002) BindingDB Entry DOI: 10.7270/Q2Q23ZK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50121409 (CHEMBL118206 | Lithium; 2-(2,4-dichloro-benzoylami...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biovitrum AB Curated by ChEMBL | Assay Description Ligand binding affinity was determined by displacement of a tritiated tracer by the unlabeled compound to a GST-PPAR fusion protein for Peroxisome pr... | Bioorg Med Chem Lett 12: 3565-7 (2002) BindingDB Entry DOI: 10.7270/Q2Q23ZK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50010904 (6-Cyclohexyl-4-hydroxy-2-isopropyl-5-[2-(8-propyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of human renin | J Med Chem 33: 2335-42 (1990) BindingDB Entry DOI: 10.7270/Q2C8288F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50012938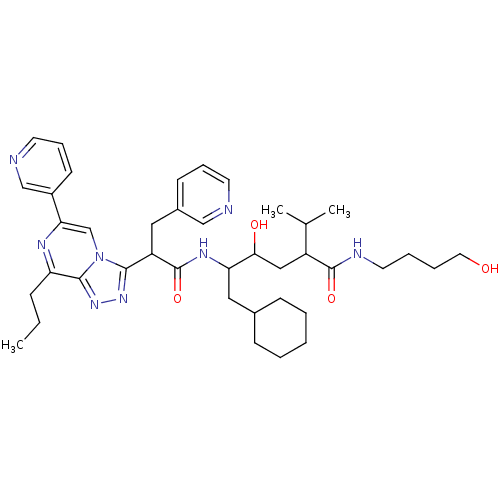 (6-Cyclohexyl-4-hydroxy-2-isopropyl-5-[2-(8-propyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of human renin | J Med Chem 33: 2335-42 (1990) BindingDB Entry DOI: 10.7270/Q2C8288F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50012937 (CHEMBL76697 | Less polar Epimer-6-Cyclohexyl-4-hyd...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of human renin | J Med Chem 33: 2335-42 (1990) BindingDB Entry DOI: 10.7270/Q2C8288F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50012937 (CHEMBL76697 | Less polar Epimer-6-Cyclohexyl-4-hyd...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of human renin | J Med Chem 33: 2335-42 (1990) BindingDB Entry DOI: 10.7270/Q2C8288F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50012946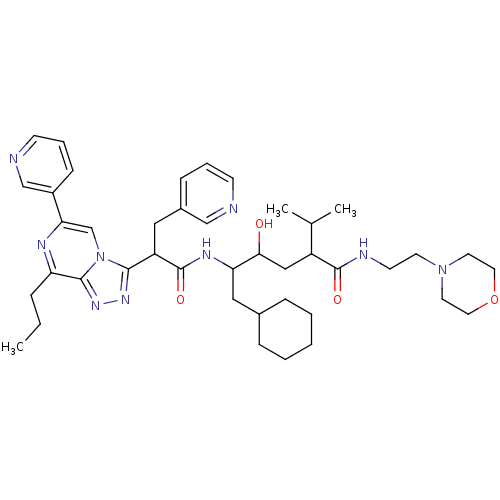 (6-Cyclohexyl-4-hydroxy-2-isopropyl-5-[2-(8-propyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of human renin | J Med Chem 33: 2335-42 (1990) BindingDB Entry DOI: 10.7270/Q2C8288F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50012944 (6-Cyclohexyl-4-hydroxy-2-isopropyl-5-[2-(8-propyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of human renin | J Med Chem 33: 2335-42 (1990) BindingDB Entry DOI: 10.7270/Q2C8288F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50012939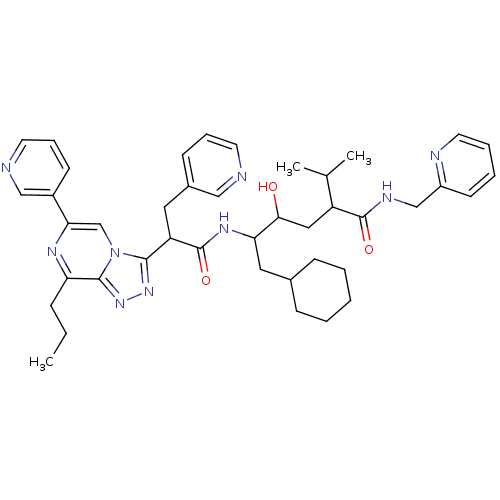 (6-Cyclohexyl-4-hydroxy-2-isopropyl-5-[2-(8-propyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of human renin | J Med Chem 33: 2335-42 (1990) BindingDB Entry DOI: 10.7270/Q2C8288F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50012943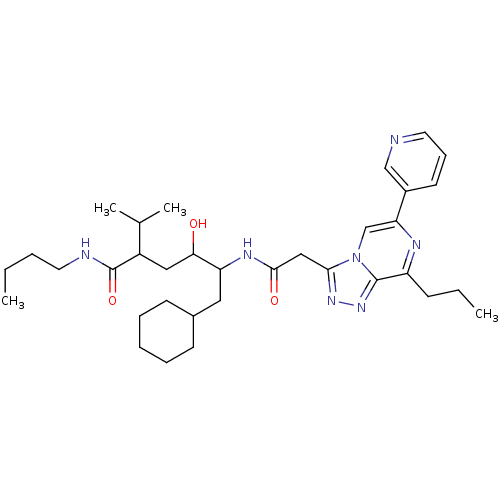 (6-Cyclohexyl-4-hydroxy-2-isopropyl-5-[2-(8-propyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of human renin | J Med Chem 33: 2335-42 (1990) BindingDB Entry DOI: 10.7270/Q2C8288F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50007355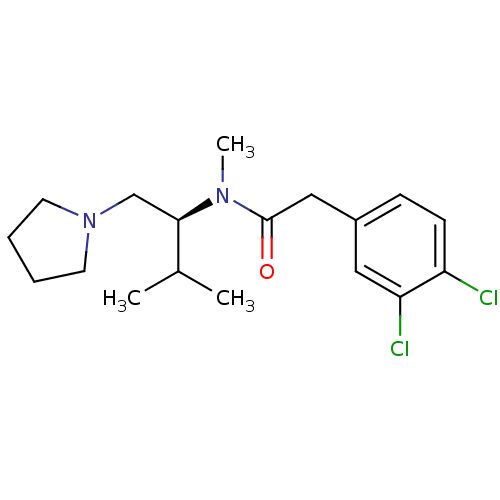 ((S)-2-(3,4-Dichloro-phenyl)-N-methyl-N-(2-methyl-1...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Curated by ChEMBL | Assay Description Compound was evaluated for the Opioid receptor kappa 1 affinity using guinea pig brain membranes. | J Med Chem 34: 181-9 (1991) BindingDB Entry DOI: 10.7270/Q27080CR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50007344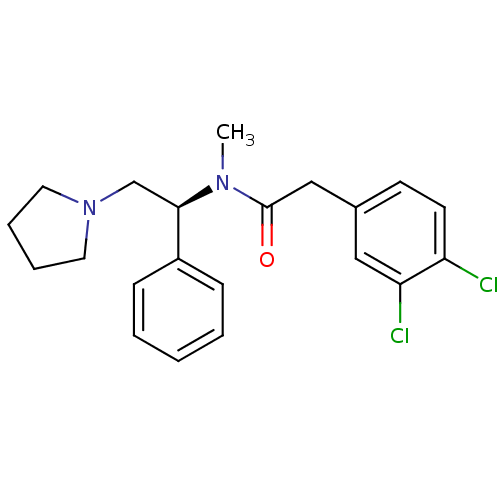 ((S)-2-(3,4-Dichloro-phenyl)-N-methyl-N-(1-phenyl-2...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 6.90 | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Curated by ChEMBL | Assay Description Compound was evaluated for the Opioid receptor kappa 1 affinity using guinea pig brain membranes. | J Med Chem 34: 181-9 (1991) BindingDB Entry DOI: 10.7270/Q27080CR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM50419102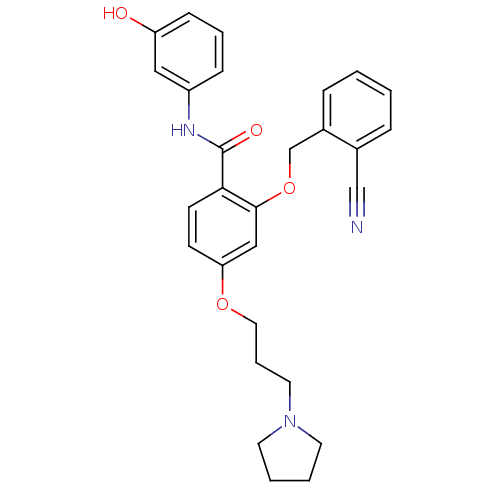 (CHEMBL1824369) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 17.4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of recombinant GST-tagged wild type c-met kinase domain (residues 1056-1371) using biotin-poly EAY as substrate in the presence of ATP aft... | Bioorg Med Chem Lett 21: 5224-9 (2011) Article DOI: 10.1016/j.bmcl.2011.07.047 BindingDB Entry DOI: 10.7270/Q2K35VX8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50008845 (2-(3,4-Dichloro-phenyl)-N-(2,2-dimethyl-1-pyrrolid...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Curated by ChEMBL | Assay Description Compound was evaluated for the opioid receptor kappa affinity using guinea pig brain membranes. | J Med Chem 34: 181-9 (1991) BindingDB Entry DOI: 10.7270/Q27080CR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50012941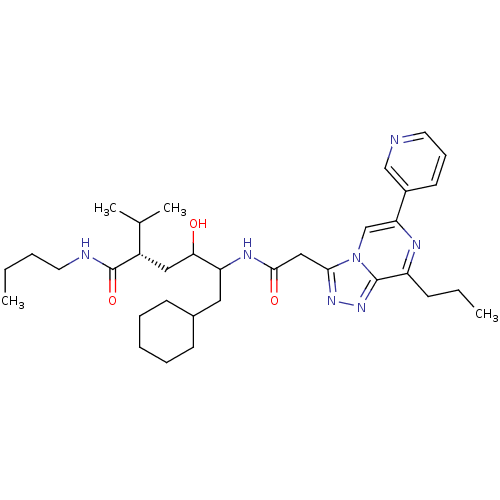 (6-Cyclohexyl-4-hydroxy-2-isopropyl-5-[2-(8-propyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of human renin | J Med Chem 33: 2335-42 (1990) BindingDB Entry DOI: 10.7270/Q2C8288F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase mTOR (Homo sapiens (Human)) | BDBM50384248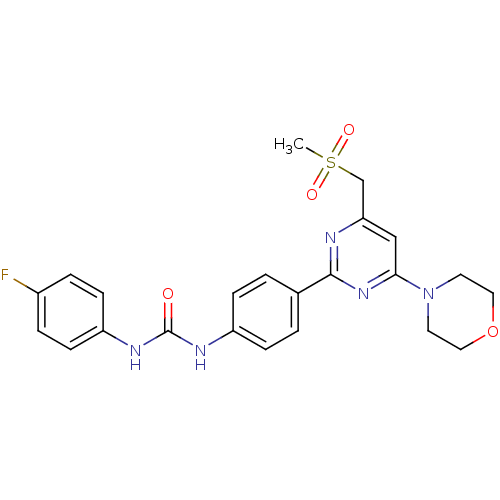 (CHEMBL2030451) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of recombinant FLAG-tagged mTOR kinase expressed in HEK293 cells using Biotin-Ahx-Lys-Lys-Ala-Asn-Gln-Val-Phe-Leu-Gly-Phe-Thr-Tyr-Val-Ala-... | Bioorg Med Chem Lett 22: 4163-8 (2012) Article DOI: 10.1016/j.bmcl.2012.04.036 BindingDB Entry DOI: 10.7270/Q2N017KD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50012940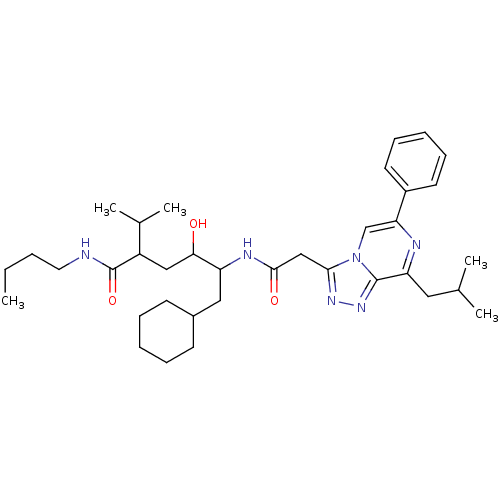 (6-Cyclohexyl-4-hydroxy-5-[2-(8-isobutyl-6-phenyl-[...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of human renin | J Med Chem 33: 2335-42 (1990) BindingDB Entry DOI: 10.7270/Q2C8288F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase mTOR (Homo sapiens (Human)) | BDBM50384249 (CHEMBL2030450) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of recombinant FLAG-tagged mTOR kinase expressed in HEK293 cells using Biotin-Ahx-Lys-Lys-Ala-Asn-Gln-Val-Phe-Leu-Gly-Phe-Thr-Tyr-Val-Ala-... | Bioorg Med Chem Lett 22: 4163-8 (2012) Article DOI: 10.1016/j.bmcl.2012.04.036 BindingDB Entry DOI: 10.7270/Q2N017KD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM50419101 (CHEMBL1824368) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 40.7 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of recombinant GST-tagged wild type c-met kinase domain (residues 1056-1371) using biotin-poly EAY as substrate in the presence of ATP aft... | Bioorg Med Chem Lett 21: 5224-9 (2011) Article DOI: 10.1016/j.bmcl.2011.07.047 BindingDB Entry DOI: 10.7270/Q2K35VX8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50012942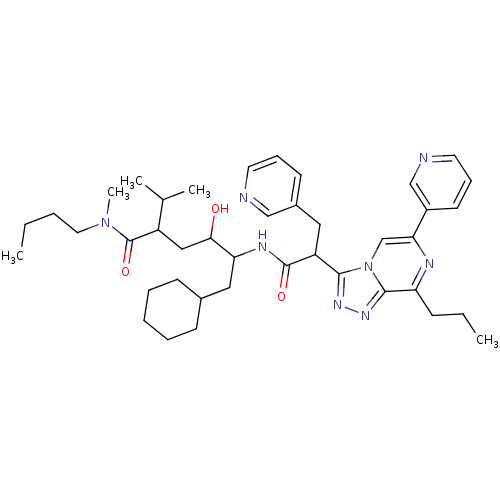 (6-Cyclohexyl-4-hydroxy-2-isopropyl-5-[2-(8-propyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of human renin | J Med Chem 33: 2335-42 (1990) BindingDB Entry DOI: 10.7270/Q2C8288F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 359 total ) | Next | Last >> |