

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50316213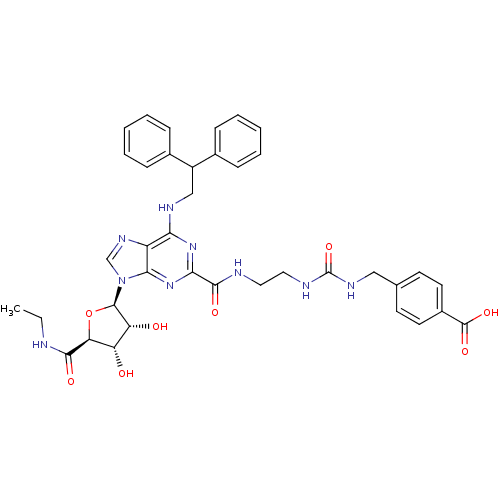 (4-((3-(2-(6-(2,2-diphenylethylamino)-9-((2R,3R,4S,...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Agonist activity at adenosine A2A receptor in fMLP-stimulated human neutrophils assessed as inhibition of superoxide production by colorimetric analy... | Bioorg Med Chem Lett 19: 4471-5 (2009) Article DOI: 10.1016/j.bmcl.2009.05.027 BindingDB Entry DOI: 10.7270/Q2ST7Q0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50276291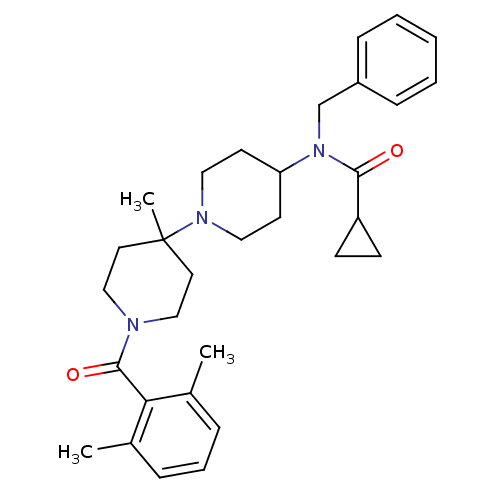 (CHEMBL472464 | N-benzyl-N-(1'-(2,6-dimethylbenzoyl...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Antagonist activity at human recombinant CCR5 expressed in human HeLa-P4 cells assessed as inhibition of human HeLa-P4 cells binding to HIV1 gp160 ex... | Bioorg Med Chem Lett 19: 1084-8 (2009) Article DOI: 10.1016/j.bmcl.2009.01.012 BindingDB Entry DOI: 10.7270/Q2W095SG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50276259 (CHEMBL481068 | exo-N-benzyl-N-((R)-1-(8-(2,6-dimet...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Antagonist activity at human recombinant CCR5 expressed in human HeLa-P4 cells assessed as inhibition of human HeLa-P4 cells binding to HIV1 gp160 ex... | Bioorg Med Chem Lett 19: 1084-8 (2009) Article DOI: 10.1016/j.bmcl.2009.01.012 BindingDB Entry DOI: 10.7270/Q2W095SG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50276260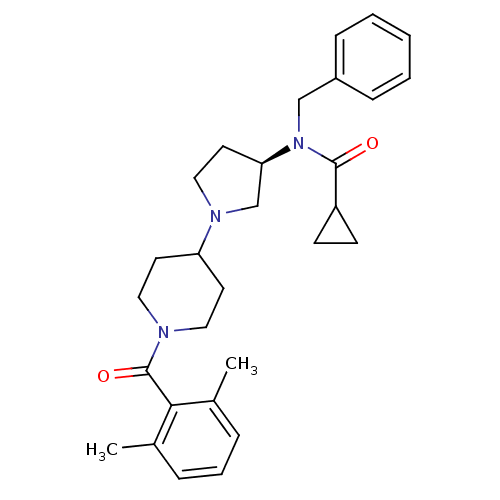 ((R)-N-benzyl-N-(1-(1-(2,6-dimethylbenzoyl)piperidi...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Antagonist activity at human recombinant CCR5 expressed in human HeLa-P4 cells assessed as inhibition of human HeLa-P4 cells binding to HIV1 gp160 ex... | Bioorg Med Chem Lett 19: 1084-8 (2009) Article DOI: 10.1016/j.bmcl.2009.01.012 BindingDB Entry DOI: 10.7270/Q2W095SG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50276290 (CHEMBL472260 | N-benzyl-N-(1'-(2,6-dimethylbenzoyl...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Antagonist activity at human recombinant CCR5 expressed in human HeLa-P4 cells assessed as inhibition of human HeLa-P4 cells binding to HIV1 gp160 ex... | Bioorg Med Chem Lett 19: 1084-8 (2009) Article DOI: 10.1016/j.bmcl.2009.01.012 BindingDB Entry DOI: 10.7270/Q2W095SG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50316210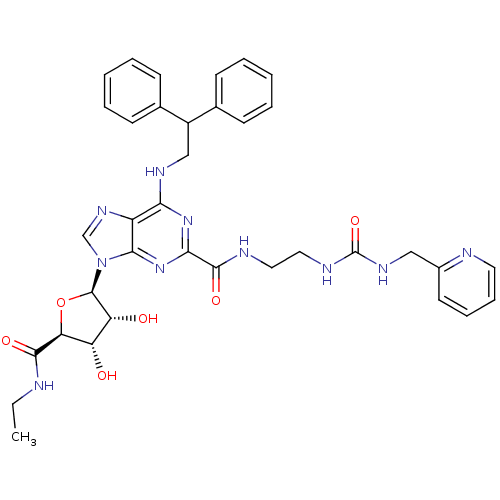 (6-(2,2-diphenylethylamino)-9-((2R,3R,4S,5S)-5-(eth...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Agonist activity at adenosine A2A receptor in fMLP-stimulated human neutrophils assessed as inhibition of superoxide production by colorimetric analy... | Bioorg Med Chem Lett 19: 4471-5 (2009) Article DOI: 10.1016/j.bmcl.2009.05.027 BindingDB Entry DOI: 10.7270/Q2ST7Q0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50316212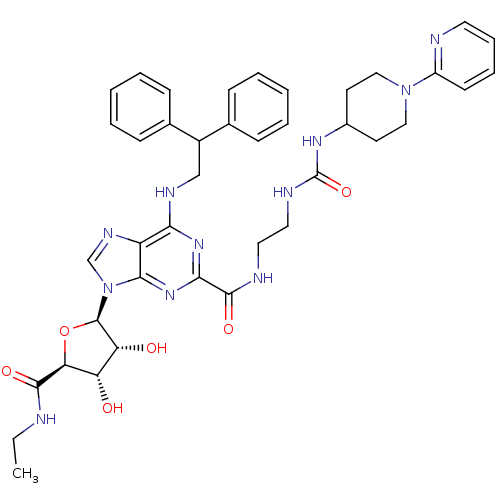 (6-(2,2-diphenylethylamino)-9-((2R,3R,4S,5S)-5-(eth...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Agonist activity at adenosine A2A receptor in fMLP-stimulated human neutrophils assessed as inhibition of superoxide production by colorimetric analy... | Bioorg Med Chem Lett 19: 4471-5 (2009) Article DOI: 10.1016/j.bmcl.2009.05.027 BindingDB Entry DOI: 10.7270/Q2ST7Q0C | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50316211 (CHEMBL1096895 | N-(2-(3-(4-((diethylamino)methyl)b...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Agonist activity at adenosine A2A receptor in fMLP-stimulated human neutrophils assessed as inhibition of superoxide production by colorimetric analy... | Bioorg Med Chem Lett 19: 4471-5 (2009) Article DOI: 10.1016/j.bmcl.2009.05.027 BindingDB Entry DOI: 10.7270/Q2ST7Q0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50316205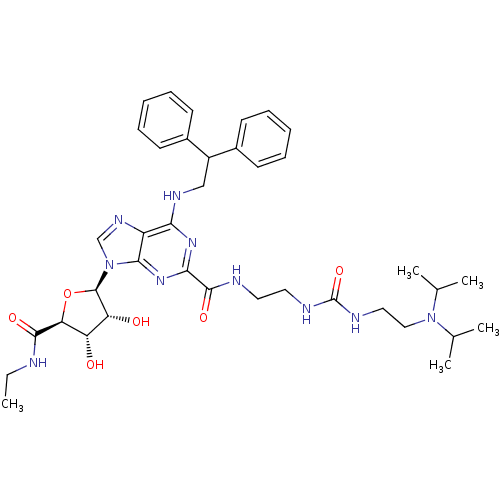 (CHEMBL1096889 | N-(2-(3-(2-(diisopropylamino)ethyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Agonist activity at adenosine A2A receptor in fMLP-stimulated human neutrophils assessed as inhibition of superoxide production by colorimetric analy... | Bioorg Med Chem Lett 19: 4471-5 (2009) Article DOI: 10.1016/j.bmcl.2009.05.027 BindingDB Entry DOI: 10.7270/Q2ST7Q0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50316202 (6-(2,2-diphenylethylamino)-9-((2R,3R,4S,5S)-5-(eth...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Agonist activity at adenosine A2A receptor in fMLP-stimulated human neutrophils assessed as inhibition of superoxide production by colorimetric analy... | Bioorg Med Chem Lett 19: 4471-5 (2009) Article DOI: 10.1016/j.bmcl.2009.05.027 BindingDB Entry DOI: 10.7270/Q2ST7Q0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50233816 ((2S,3S,4R,5R)-5-(2-((3-(2-(cyclopentyl(isopropyl)a...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Agonist activity at adenosine A2A receptor | Bioorg Med Chem Lett 18: 1284-7 (2008) Article DOI: 10.1016/j.bmcl.2008.01.033 BindingDB Entry DOI: 10.7270/Q22808F4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50316209 (CHEMBL1096893 | N-(2-(3-benzylureido)ethyl)-6-(2,2...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Agonist activity at adenosine A2A receptor in fMLP-stimulated human neutrophils assessed as inhibition of superoxide production by colorimetric analy... | Bioorg Med Chem Lett 19: 4471-5 (2009) Article DOI: 10.1016/j.bmcl.2009.05.027 BindingDB Entry DOI: 10.7270/Q2ST7Q0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50233816 ((2S,3S,4R,5R)-5-(2-((3-(2-(cyclopentyl(isopropyl)a...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Agonist activity at adenosine A2A receptor in fMLP-stimulated human neutrophils assessed as inhibition of superoxide production by colorimetric analy... | Bioorg Med Chem Lett 19: 4471-5 (2009) Article DOI: 10.1016/j.bmcl.2009.05.027 BindingDB Entry DOI: 10.7270/Q2ST7Q0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50316204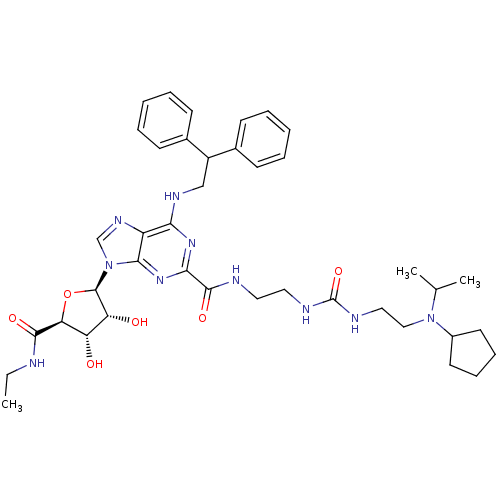 (CHEMBL1096888 | N-(2-(3-(2-(cyclopentyl(isopropyl)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Agonist activity at adenosine A2A receptor in fMLP-stimulated human neutrophils assessed as inhibition of superoxide production by colorimetric analy... | Bioorg Med Chem Lett 19: 4471-5 (2009) Article DOI: 10.1016/j.bmcl.2009.05.027 BindingDB Entry DOI: 10.7270/Q2ST7Q0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50276260 ((R)-N-benzyl-N-(1-(1-(2,6-dimethylbenzoyl)piperidi...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [125]Mip1beta form CCR5 receptor (unknown origin) expressed in MIP34.10 cells | Bioorg Med Chem Lett 19: 1084-8 (2009) Article DOI: 10.1016/j.bmcl.2009.01.012 BindingDB Entry DOI: 10.7270/Q2W095SG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50372023 (CHEMBL403478) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Agonist activity at adenosine A2A receptor | Bioorg Med Chem Lett 18: 1284-7 (2008) Article DOI: 10.1016/j.bmcl.2008.01.033 BindingDB Entry DOI: 10.7270/Q22808F4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50316206 (CHEMBL1096890 | N-(2-(3-(2-(3,4-dihydroisoquinolin...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Agonist activity at adenosine A2A receptor in fMLP-stimulated human neutrophils assessed as inhibition of superoxide production by colorimetric analy... | Bioorg Med Chem Lett 19: 4471-5 (2009) Article DOI: 10.1016/j.bmcl.2009.05.027 BindingDB Entry DOI: 10.7270/Q2ST7Q0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50316208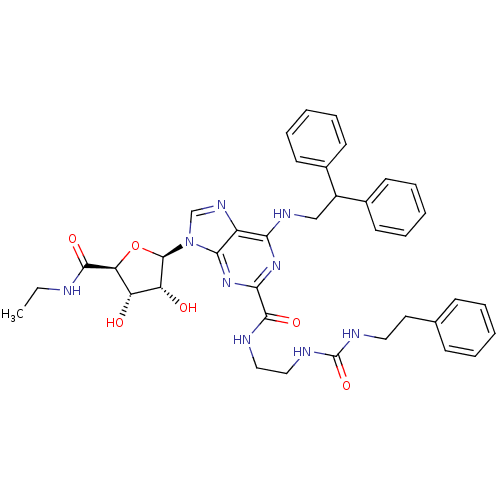 (6-(2,2-diphenylethylamino)-9-((2R,3R,4S,5S)-5-(eth...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Agonist activity at adenosine A2A receptor in fMLP-stimulated human neutrophils assessed as inhibition of superoxide production by colorimetric analy... | Bioorg Med Chem Lett 19: 4471-5 (2009) Article DOI: 10.1016/j.bmcl.2009.05.027 BindingDB Entry DOI: 10.7270/Q2ST7Q0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50372024 (CHEMBL257213) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Agonist activity at adenosine A2A receptor | Bioorg Med Chem Lett 18: 1284-7 (2008) Article DOI: 10.1016/j.bmcl.2008.01.033 BindingDB Entry DOI: 10.7270/Q22808F4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50233809 ((2S,3S,4R,5R)-5-(6-(2,2-diphenylethylamino)-2-((3-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Agonist activity at adenosine A2A receptor | Bioorg Med Chem Lett 18: 1284-7 (2008) Article DOI: 10.1016/j.bmcl.2008.01.033 BindingDB Entry DOI: 10.7270/Q22808F4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50233809 ((2S,3S,4R,5R)-5-(6-(2,2-diphenylethylamino)-2-((3-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Agonist activity at adenosine A2A receptor in fMLP-stimulated human neutrophils assessed as inhibition of superoxide production by colorimetric analy... | Bioorg Med Chem Lett 19: 4471-5 (2009) Article DOI: 10.1016/j.bmcl.2009.05.027 BindingDB Entry DOI: 10.7270/Q2ST7Q0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50276623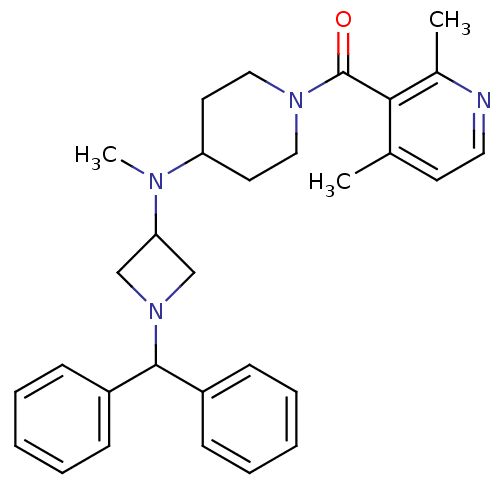 ((4-((1-benzhydrylazetidin-3-yl)(methyl)amino)piper...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Antagonist activity at human recombinant CCR5 expressed in human HeLa-P4 cells assessed as inhibition of human HeLa-P4 cells binding to HIV1 gp160 ex... | Bioorg Med Chem Lett 19: 1084-8 (2009) Article DOI: 10.1016/j.bmcl.2009.01.012 BindingDB Entry DOI: 10.7270/Q2W095SG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50372026 (CHEMBL257429) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Agonist activity at adenosine A2A receptor | Bioorg Med Chem Lett 18: 1284-7 (2008) Article DOI: 10.1016/j.bmcl.2008.01.033 BindingDB Entry DOI: 10.7270/Q22808F4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50372029 (CHEMBL256489) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Agonist activity at adenosine A2A receptor | Bioorg Med Chem Lett 18: 1284-7 (2008) Article DOI: 10.1016/j.bmcl.2008.01.033 BindingDB Entry DOI: 10.7270/Q22808F4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50276672 ((4-(3-benzhydryl-3,8-diazabicyclo[3.2.1]octan-8-yl...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Antagonist activity at human recombinant CCR5 expressed in human HeLa-P4 cells assessed as inhibition of human HeLa-P4 cells binding to HIV1 gp160 ex... | Bioorg Med Chem Lett 19: 1084-8 (2009) Article DOI: 10.1016/j.bmcl.2009.01.012 BindingDB Entry DOI: 10.7270/Q2W095SG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50316203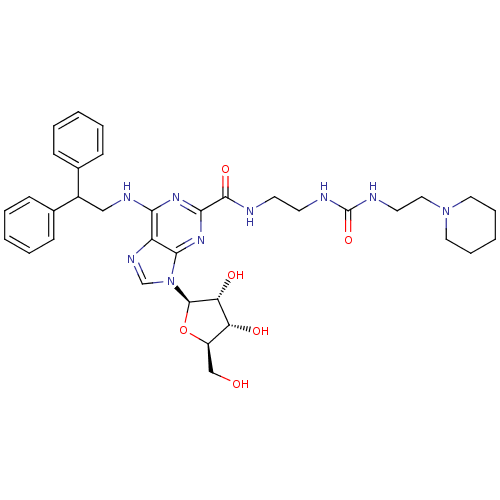 (9-((2R,3R,4S,5R)-3,4-dihydroxy-5-(hydroxymethyl)te...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Agonist activity at adenosine A2A receptor in fMLP-stimulated human neutrophils assessed as inhibition of superoxide production by colorimetric analy... | Bioorg Med Chem Lett 19: 4471-5 (2009) Article DOI: 10.1016/j.bmcl.2009.05.027 BindingDB Entry DOI: 10.7270/Q2ST7Q0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50276793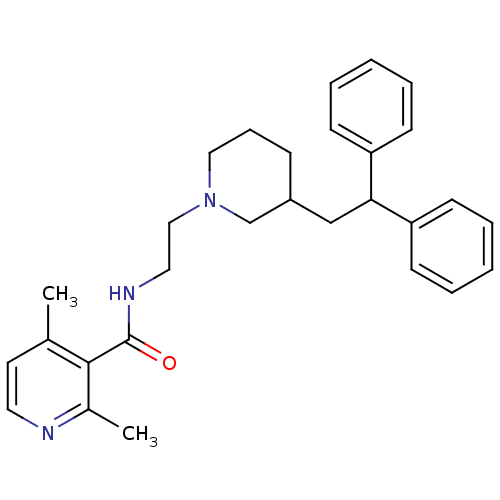 (CHEMBL459986 | rac-N-(2-(3-(2,2-diphenylethyl)pipe...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 56 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Antagonist activity at human recombinant CCR5 expressed in human HeLa-P4 cells assessed as inhibition of human HeLa-P4 cells binding to HIV1 gp160 ex... | Bioorg Med Chem Lett 19: 1084-8 (2009) Article DOI: 10.1016/j.bmcl.2009.01.012 BindingDB Entry DOI: 10.7270/Q2W095SG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50372027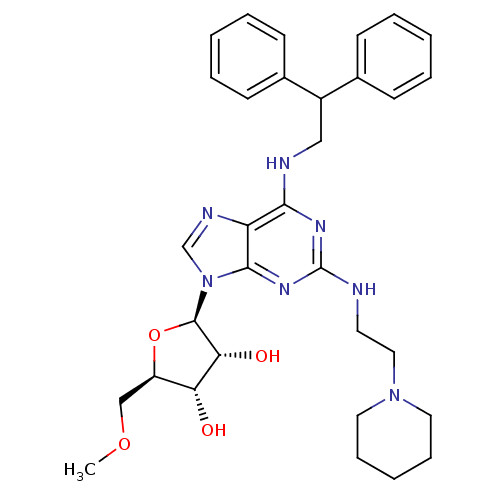 (CHEMBL270378) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Agonist activity at adenosine A2A receptor | Bioorg Med Chem Lett 18: 1284-7 (2008) Article DOI: 10.1016/j.bmcl.2008.01.033 BindingDB Entry DOI: 10.7270/Q22808F4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50233810 (9-((2R,3R,4S,5R)-3,4-dihydroxy-5-(hydroxymethyl)-t...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 65 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Agonist activity at adenosine A2A receptor in fMLP-stimulated human neutrophils assessed as inhibition of superoxide production by colorimetric analy... | Bioorg Med Chem Lett 19: 4471-5 (2009) Article DOI: 10.1016/j.bmcl.2009.05.027 BindingDB Entry DOI: 10.7270/Q2ST7Q0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50233810 (9-((2R,3R,4S,5R)-3,4-dihydroxy-5-(hydroxymethyl)-t...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 65 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Agonist activity at adenosine A2A receptor | Bioorg Med Chem Lett 18: 1284-7 (2008) Article DOI: 10.1016/j.bmcl.2008.01.033 BindingDB Entry DOI: 10.7270/Q22808F4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50372025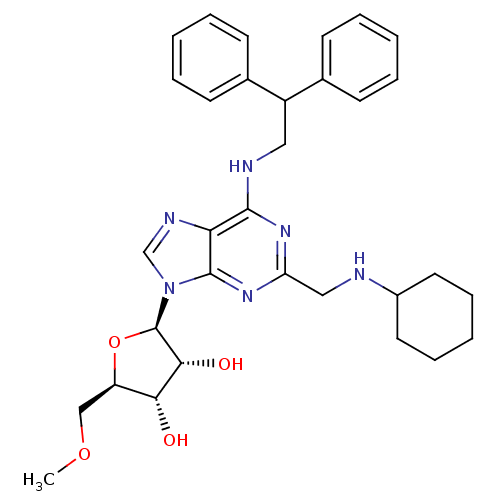 (CHEMBL401571) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Agonist activity at adenosine A2A receptor | Bioorg Med Chem Lett 18: 1284-7 (2008) Article DOI: 10.1016/j.bmcl.2008.01.033 BindingDB Entry DOI: 10.7270/Q22808F4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50276792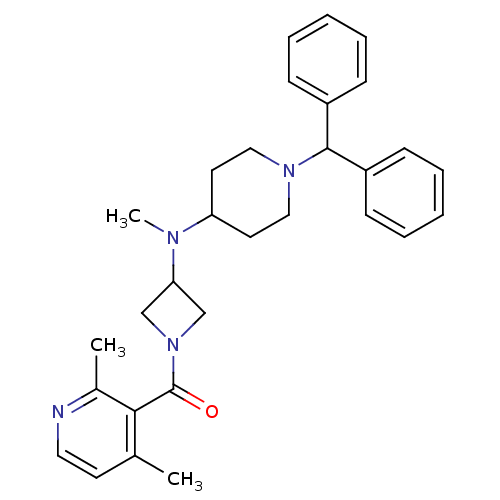 ((3-((1-benzhydrylpiperidin-4-yl)(methyl)amino)azet...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 102 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Antagonist activity at human recombinant CCR5 expressed in human HeLa-P4 cells assessed as inhibition of human HeLa-P4 cells binding to HIV1 gp160 ex... | Bioorg Med Chem Lett 19: 1084-8 (2009) Article DOI: 10.1016/j.bmcl.2009.01.012 BindingDB Entry DOI: 10.7270/Q2W095SG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50276217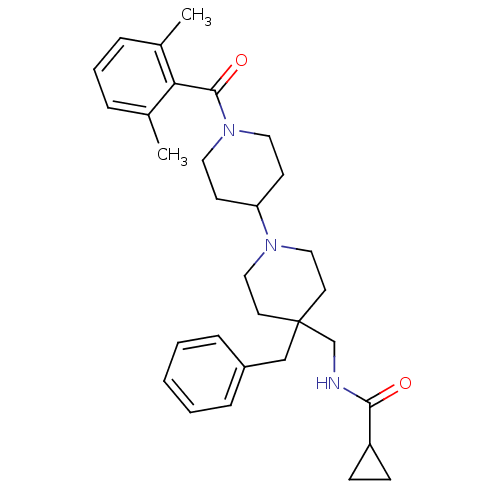 (CHEMBL471403 | N-((4-benzyl-1'-(2,6-dimethylbenzoy...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 145 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Antagonist activity at human recombinant CCR5 expressed in human HeLa-P4 cells assessed as inhibition of human HeLa-P4 cells binding to HIV1 gp160 ex... | Bioorg Med Chem Lett 19: 1084-8 (2009) Article DOI: 10.1016/j.bmcl.2009.01.012 BindingDB Entry DOI: 10.7270/Q2W095SG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50050632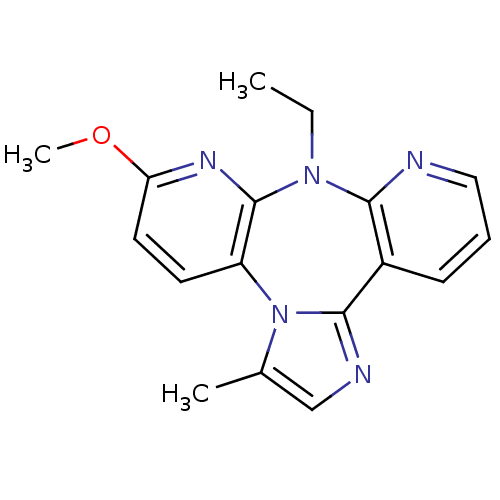 (8-ETHYL-6-METHOXY-3-METHYL-8H-1,3A,7,8,9-PENTAAZA-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 156 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability to inhibit HIV-1 IIIB reverse transcriptase catalyzed incorporation of tritiated thymidine triphosphate onto a biotinylated rA.dT template pr... | Bioorg Med Chem Lett 2: 1745-1750 (1992) Article DOI: 10.1016/S0960-894X(00)80468-0 BindingDB Entry DOI: 10.7270/Q2X63MW9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50050632 (8-ETHYL-6-METHOXY-3-METHYL-8H-1,3A,7,8,9-PENTAAZA-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability to inhibit HIV-1 IIIB reverse transcriptase catalyzed incorporation of tritiated thymidine triphosphate onto a biotinylated rA.dT template pr... | Bioorg Med Chem Lett 2: 1745-1750 (1992) Article DOI: 10.1016/S0960-894X(00)80468-0 BindingDB Entry DOI: 10.7270/Q2X63MW9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50050636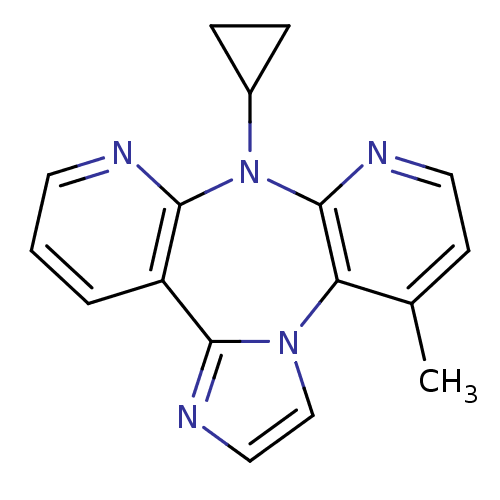 (8-Cyclopropyl-4-methyl-8H-1,3a,7,8,9-pentaaza-dibe...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 217 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability to inhibit HIV-1 IIIB reverse transcriptase catalyzed incorporation of tritiated thymidine triphosphate onto a biotinylated rN.dN template pr... | Bioorg Med Chem Lett 2: 1745-1750 (1992) Article DOI: 10.1016/S0960-894X(00)80468-0 BindingDB Entry DOI: 10.7270/Q2X63MW9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50050633 (8-Ethyl-4-methyl-8H-1,3a,7,8,9-pentaaza-dibenzo[e,...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 245 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability to inhibit HIV-1 IIIB reverse transcriptase catalyzed incorporation of tritiated thymidine triphosphate onto a biotinylated rN.dN template pr... | Bioorg Med Chem Lett 2: 1745-1750 (1992) Article DOI: 10.1016/S0960-894X(00)80468-0 BindingDB Entry DOI: 10.7270/Q2X63MW9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50050628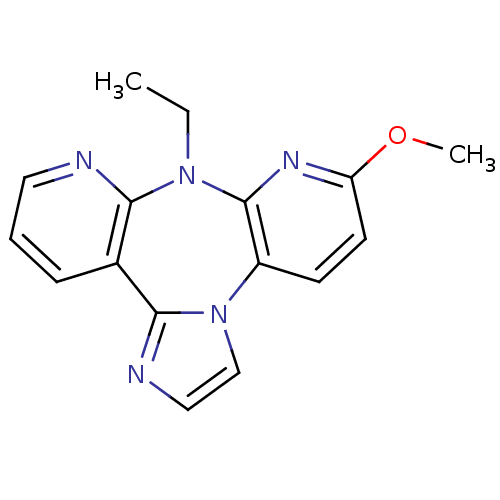 (8-Ethyl-6-methoxy-8H-1,3a,7,8,9-pentaaza-dibenzo[e...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 258 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability to inhibit HIV-1 IIIB reverse transcriptase catalyzed incorporation of tritiated thymidine triphosphate onto a biotinylated rA.dT template pr... | Bioorg Med Chem Lett 2: 1745-1750 (1992) Article DOI: 10.1016/S0960-894X(00)80468-0 BindingDB Entry DOI: 10.7270/Q2X63MW9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50316207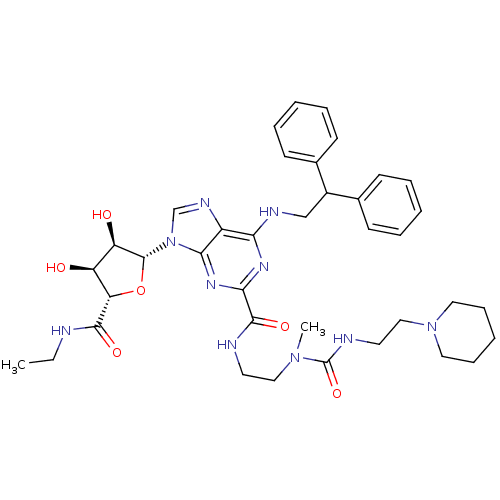 (6-(2,2-diphenylethylamino)-9-((2R,3R,4S,5S)-5-(eth...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 305 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Agonist activity at adenosine A2A receptor in fMLP-stimulated human neutrophils assessed as inhibition of superoxide production by colorimetric analy... | Bioorg Med Chem Lett 19: 4471-5 (2009) Article DOI: 10.1016/j.bmcl.2009.05.027 BindingDB Entry DOI: 10.7270/Q2ST7Q0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50276671 ((4-(8-benzhydryl-3,8-diazabicyclo[3.2.1]octan-3-yl...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 313 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Antagonist activity at human recombinant CCR5 expressed in human HeLa-P4 cells assessed as inhibition of human HeLa-P4 cells binding to HIV1 gp160 ex... | Bioorg Med Chem Lett 19: 1084-8 (2009) Article DOI: 10.1016/j.bmcl.2009.01.012 BindingDB Entry DOI: 10.7270/Q2W095SG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50050640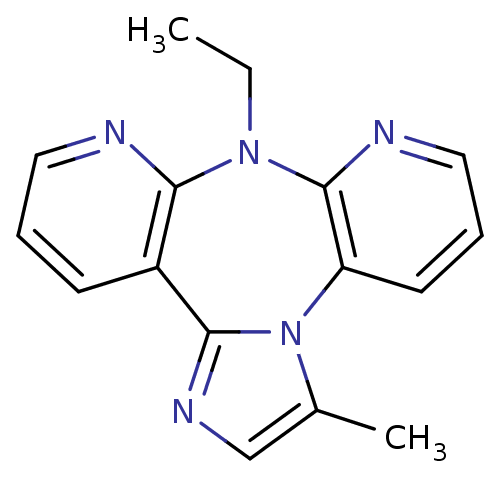 (8-Ethyl-3-methyl-8H-1,3a,7,8,9-pentaaza-dibenzo[e,...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 425 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability to inhibit HIV-1 IIIB reverse transcriptase catalyzed incorporation of tritiated thymidine triphosphate onto a biotinylated rN.dN template pr... | Bioorg Med Chem Lett 2: 1745-1750 (1992) Article DOI: 10.1016/S0960-894X(00)80468-0 BindingDB Entry DOI: 10.7270/Q2X63MW9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50050628 (8-Ethyl-6-methoxy-8H-1,3a,7,8,9-pentaaza-dibenzo[e...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 598 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability to inhibit HIV-1 IIIB reverse transcriptase catalyzed incorporation of tritiated thymidine triphosphate onto a biotinylated rA.dT template pr... | Bioorg Med Chem Lett 2: 1745-1750 (1992) Article DOI: 10.1016/S0960-894X(00)80468-0 BindingDB Entry DOI: 10.7270/Q2X63MW9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50280306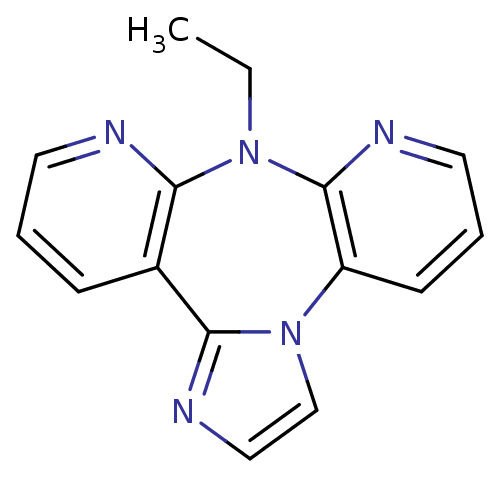 (8-Ethyl-8H-1,3a,7,8,9-pentaaza-dibenzo[e,h]azulene...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 775 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability to inhibit HIV-1 IIIB reverse transcriptase catalyzed incorporation of tritiated thymidine triphosphate onto a biotinylated rN.dN template pr... | Bioorg Med Chem Lett 2: 1745-1750 (1992) Article DOI: 10.1016/S0960-894X(00)80468-0 BindingDB Entry DOI: 10.7270/Q2X63MW9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50372022 (CHEMBL404123) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Agonist activity at adenosine A2A receptor | Bioorg Med Chem Lett 18: 1284-7 (2008) Article DOI: 10.1016/j.bmcl.2008.01.033 BindingDB Entry DOI: 10.7270/Q22808F4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50372028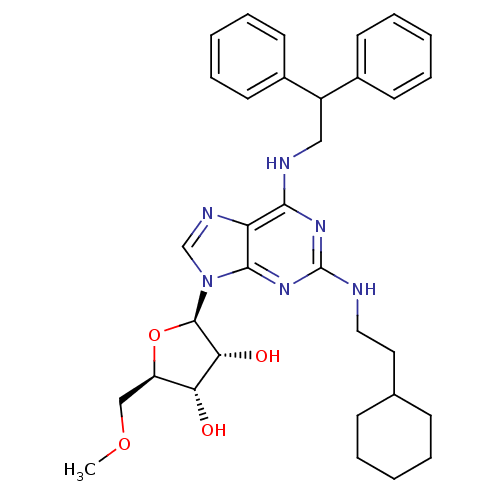 (CHEMBL404521) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.01E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Agonist activity at adenosine A2A receptor | Bioorg Med Chem Lett 18: 1284-7 (2008) Article DOI: 10.1016/j.bmcl.2008.01.033 BindingDB Entry DOI: 10.7270/Q22808F4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50050634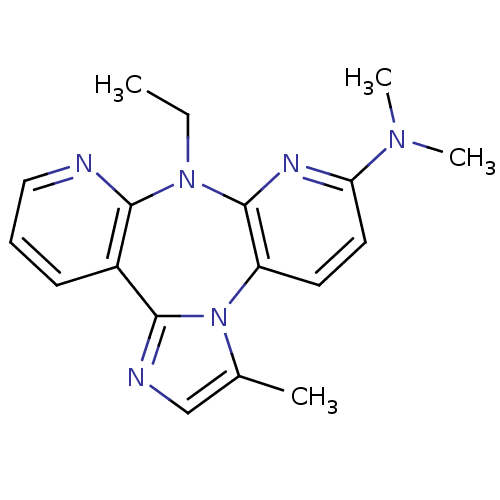 ((8-Ethyl-3-methyl-8H-1,3a,7,8,9-pentaaza-dibenzo[e...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability to inhibit HIV-1 IIIB reverse transcriptase catalyzed incorporation of tritiated thymidine triphosphate onto a biotinylated rN.dN template pr... | Bioorg Med Chem Lett 2: 1745-1750 (1992) Article DOI: 10.1016/S0960-894X(00)80468-0 BindingDB Entry DOI: 10.7270/Q2X63MW9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50280315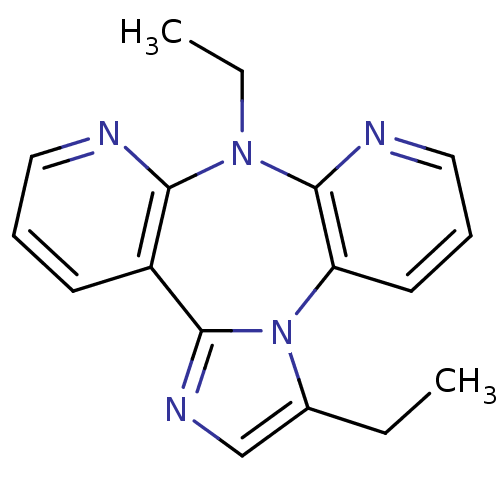 (3,8-Diethyl-8H-1,3a,7,8,9-pentaaza-dibenzo[e,h]azu...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability to inhibit HIV-1 IIIB reverse transcriptase catalyzed incorporation of tritiated thymidine triphosphate onto a biotinylated rN.dN template pr... | Bioorg Med Chem Lett 2: 1745-1750 (1992) Article DOI: 10.1016/S0960-894X(00)80468-0 BindingDB Entry DOI: 10.7270/Q2X63MW9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50050640 (8-Ethyl-3-methyl-8H-1,3a,7,8,9-pentaaza-dibenzo[e,...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 1.22E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability to inhibit HIV-1 IIIB reverse transcriptase catalyzed incorporation of tritiated thymidine triphosphate onto a biotinylated rA.dT template pr... | Bioorg Med Chem Lett 2: 1745-1750 (1992) Article DOI: 10.1016/S0960-894X(00)80468-0 BindingDB Entry DOI: 10.7270/Q2X63MW9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50276261 ((S)-N-benzyl-N-(1-(1-(2,6-dimethylbenzoyl)piperidi...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.23E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Antagonist activity at human recombinant CCR5 expressed in human HeLa-P4 cells assessed as inhibition of human HeLa-P4 cells binding to HIV1 gp160 ex... | Bioorg Med Chem Lett 19: 1084-8 (2009) Article DOI: 10.1016/j.bmcl.2009.01.012 BindingDB Entry DOI: 10.7270/Q2W095SG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM1517 (2-ethyl-7-methyl-2,4,9,15-tetraazatricyclo[9.4.0.0...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability to inhibit HIV-1 IIIB reverse transcriptase catalyzed incorporation of tritiated thymidine triphosphate onto a biotinylated rN.dN template pr... | Bioorg Med Chem Lett 2: 1745-1750 (1992) Article DOI: 10.1016/S0960-894X(00)80468-0 BindingDB Entry DOI: 10.7270/Q2X63MW9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 89 total ) | Next | Last >> |