

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Monocarboxylate transporter 1 (Homo sapiens (Human)) | BDBM22000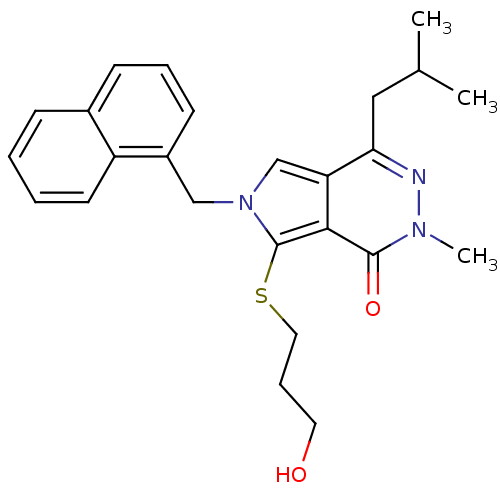 (7-[(3-hydroxypropyl)sulfanyl]-2-methyl-4-(2-methyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.100 | -56.5 | n/a | n/a | n/a | n/a | n/a | 7.8 | 22 |
AstraZeneca | Assay Description Jurkat cell membranes were incubated with [3H]-labeled ligand in the absence or presence of increasing concentrations of test compound. The reagents ... | Bioorg Med Chem Lett 16: 2260-5 (2006) Article DOI: 10.1016/j.bmcl.2006.01.024 BindingDB Entry DOI: 10.7270/Q2C24TQK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monocarboxylate transporter 1 (Homo sapiens (Human)) | BDBM22001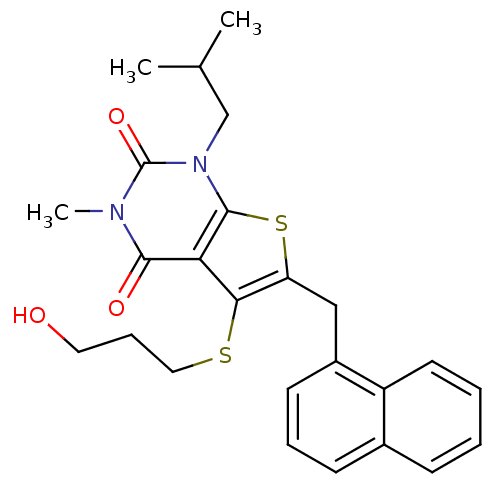 (5-[(3-hydroxypropyl)sulfanyl]-3-methyl-1-(2-methyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | 0.280 | -54.0 | n/a | n/a | n/a | n/a | n/a | 7.8 | 22 |
AstraZeneca | Assay Description Jurkat cell membranes were incubated with [3H]-labeled ligand in the absence or presence of increasing concentrations of test compound. The reagents ... | Bioorg Med Chem Lett 16: 2260-5 (2006) Article DOI: 10.1016/j.bmcl.2006.01.024 BindingDB Entry DOI: 10.7270/Q2C24TQK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monocarboxylate transporter 1 (Homo sapiens (Human)) | BDBM22009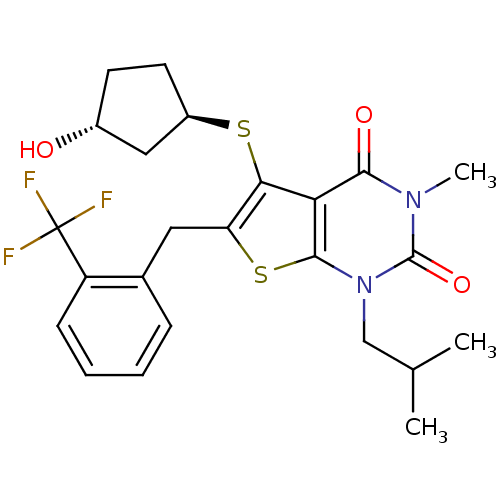 (5-{[(1R,3R)-3-hydroxycyclopentyl]sulfanyl}-3-methy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.290 | -53.9 | n/a | n/a | n/a | n/a | n/a | 7.8 | 22 |
AstraZeneca | Assay Description Jurkat cell membranes were incubated with [3H]-labeled ligand in the absence or presence of increasing concentrations of test compound. The reagents ... | Bioorg Med Chem Lett 16: 2260-5 (2006) Article DOI: 10.1016/j.bmcl.2006.01.024 BindingDB Entry DOI: 10.7270/Q2C24TQK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monocarboxylate transporter 1 (Homo sapiens (Human)) | BDBM21986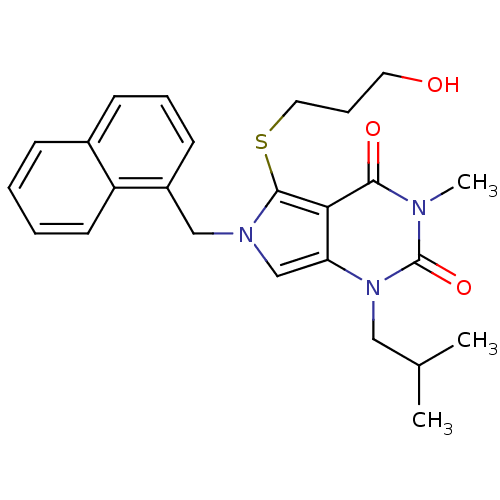 (5-[(3-hydroxypropyl)sulfanyl]-3-methyl-1-(2-methyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.330 | -53.6 | n/a | n/a | n/a | n/a | n/a | 7.8 | 22 |
AstraZeneca | Assay Description Jurkat cell membranes were incubated with [3H]-labeled ligand in the absence or presence of increasing concentrations of test compound. The reagents ... | Bioorg Med Chem Lett 16: 2260-5 (2006) Article DOI: 10.1016/j.bmcl.2006.01.024 BindingDB Entry DOI: 10.7270/Q2C24TQK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monocarboxylate transporter 1 (Homo sapiens (Human)) | BDBM22002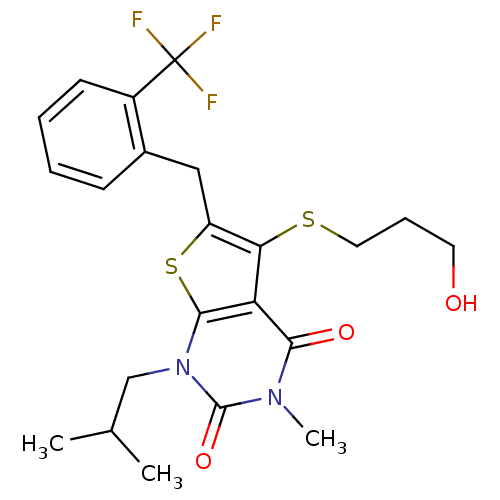 (5-[(3-hydroxypropyl)sulfanyl]-3-methyl-1-(2-methyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.350 | -53.4 | n/a | n/a | n/a | n/a | n/a | 7.8 | 22 |
AstraZeneca | Assay Description Jurkat cell membranes were incubated with [3H]-labeled ligand in the absence or presence of increasing concentrations of test compound. The reagents ... | Bioorg Med Chem Lett 16: 2260-5 (2006) Article DOI: 10.1016/j.bmcl.2006.01.024 BindingDB Entry DOI: 10.7270/Q2C24TQK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monocarboxylate transporter 1 (Homo sapiens (Human)) | BDBM22010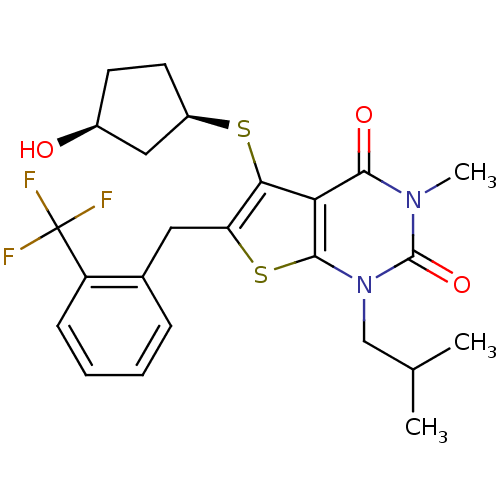 (5-{[(1R,3S)-3-hydroxycyclopentyl]sulfanyl}-3-methy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.420 | -53.0 | n/a | n/a | n/a | n/a | n/a | 7.8 | 22 |
AstraZeneca | Assay Description Jurkat cell membranes were incubated with [3H]-labeled ligand in the absence or presence of increasing concentrations of test compound. The reagents ... | Bioorg Med Chem Lett 16: 2260-5 (2006) Article DOI: 10.1016/j.bmcl.2006.01.024 BindingDB Entry DOI: 10.7270/Q2C24TQK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monocarboxylate transporter 1 (Homo sapiens (Human)) | BDBM22011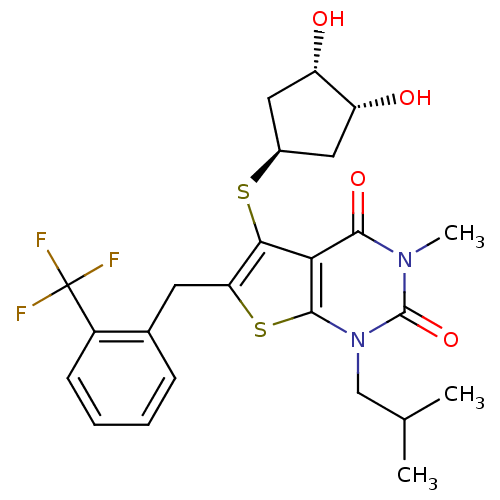 (5-{[(1S,3R,4S)-3,4-dihydroxycyclopentyl]sulfanyl}-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.680 | -51.8 | n/a | n/a | n/a | n/a | n/a | 7.8 | 22 |
AstraZeneca | Assay Description Jurkat cell membranes were incubated with [3H]-labeled ligand in the absence or presence of increasing concentrations of test compound. The reagents ... | Bioorg Med Chem Lett 16: 2260-5 (2006) Article DOI: 10.1016/j.bmcl.2006.01.024 BindingDB Entry DOI: 10.7270/Q2C24TQK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monocarboxylate transporter 1 (Homo sapiens (Human)) | BDBM22025 (5-{[(3R)-3-hydroxypyrrolidin-1-yl]carbonyl}-6-(1H-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.790 | -51.4 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
AstraZeneca | Assay Description A radioligand-binding assay was developed using scintillation proximity assay (SPA) technology. The wheat germ agglutinin SPA beads (Amersham) (0.2 m... | Bioorg Med Chem Lett 16: 2260-5 (2006) Article DOI: 10.1016/j.bmcl.2006.01.024 BindingDB Entry DOI: 10.7270/Q2C24TQK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monocarboxylate transporter 1 (Homo sapiens (Human)) | BDBM22014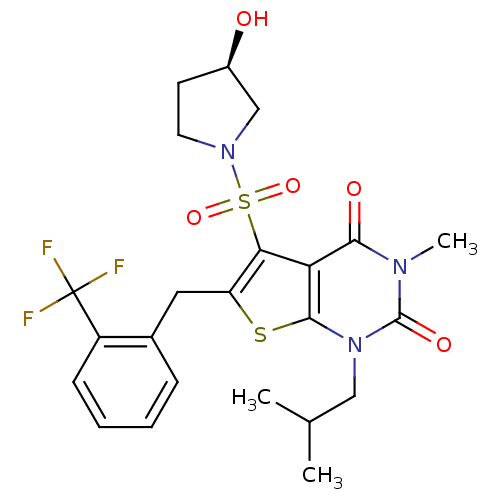 (5-{[(3R)-3-hydroxypyrrolidine-1-]sulfonyl}-3-methy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.10 | -50.6 | n/a | n/a | n/a | n/a | n/a | 7.8 | 22 |
AstraZeneca | Assay Description Jurkat cell membranes were incubated with [3H]-labeled ligand in the absence or presence of increasing concentrations of test compound. The reagents ... | Bioorg Med Chem Lett 16: 2260-5 (2006) Article DOI: 10.1016/j.bmcl.2006.01.024 BindingDB Entry DOI: 10.7270/Q2C24TQK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monocarboxylate transporter 1 (Homo sapiens (Human)) | BDBM22004 (3-methyl-1-(2-methylpropyl)-5-(propan-2-ylsulfanyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.20 | -48.9 | n/a | n/a | n/a | n/a | n/a | 7.8 | 22 |
AstraZeneca | Assay Description Jurkat cell membranes were incubated with [3H]-labeled ligand in the absence or presence of increasing concentrations of test compound. The reagents ... | Bioorg Med Chem Lett 16: 2260-5 (2006) Article DOI: 10.1016/j.bmcl.2006.01.024 BindingDB Entry DOI: 10.7270/Q2C24TQK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monocarboxylate transporter 1 (Homo sapiens (Human)) | BDBM22008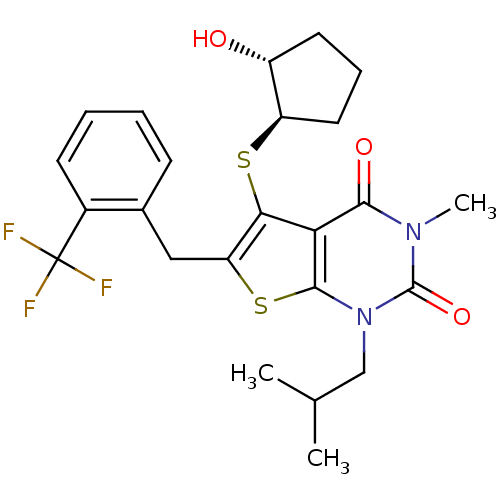 (5-{[(1R,2R)-2-hydroxycyclopentyl]sulfanyl}-3-methy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.70 | -48.4 | n/a | n/a | n/a | n/a | n/a | 7.8 | 22 |
AstraZeneca | Assay Description Jurkat cell membranes were incubated with [3H]-labeled ligand in the absence or presence of increasing concentrations of test compound. The reagents ... | Bioorg Med Chem Lett 16: 2260-5 (2006) Article DOI: 10.1016/j.bmcl.2006.01.024 BindingDB Entry DOI: 10.7270/Q2C24TQK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monocarboxylate transporter 1 (Homo sapiens (Human)) | BDBM22006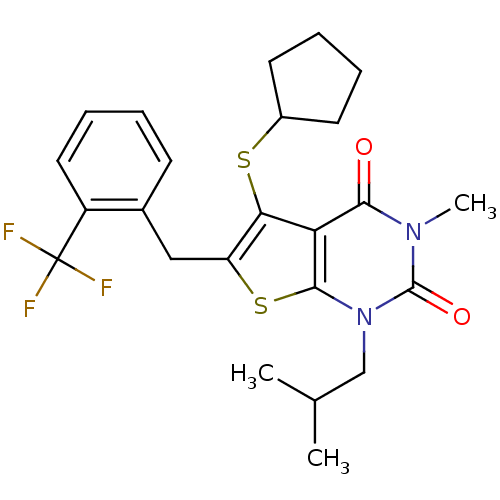 (5-(cyclopentylsulfanyl)-3-methyl-1-(2-methylpropyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3.20 | -48.0 | n/a | n/a | n/a | n/a | n/a | 7.8 | 22 |
AstraZeneca | Assay Description Jurkat cell membranes were incubated with [3H]-labeled ligand in the absence or presence of increasing concentrations of test compound. The reagents ... | Bioorg Med Chem Lett 16: 2260-5 (2006) Article DOI: 10.1016/j.bmcl.2006.01.024 BindingDB Entry DOI: 10.7270/Q2C24TQK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monocarboxylate transporter 1 (Homo sapiens (Human)) | BDBM22020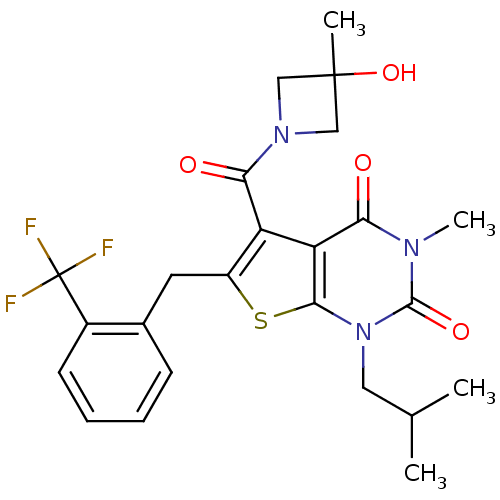 (5-[(3-hydroxy-3-methylazetidin-1-yl)carbonyl]-3-me...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3.5 | -47.8 | n/a | n/a | n/a | n/a | n/a | 7.8 | 22 |
AstraZeneca | Assay Description Jurkat cell membranes were incubated with [3H]-labeled ligand in the absence or presence of increasing concentrations of test compound. The reagents ... | Bioorg Med Chem Lett 16: 2260-5 (2006) Article DOI: 10.1016/j.bmcl.2006.01.024 BindingDB Entry DOI: 10.7270/Q2C24TQK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monocarboxylate transporter 1 (Homo sapiens (Human)) | BDBM22024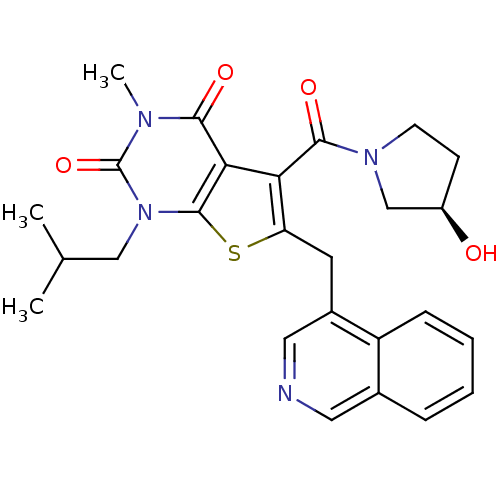 (5-{[(3R)-3-hydroxypyrrolidin-1-yl]carbonyl}-6-(iso...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3.90 | -47.5 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
AstraZeneca | Assay Description A radioligand-binding assay was developed using scintillation proximity assay (SPA) technology. The wheat germ agglutinin SPA beads (Amersham) (0.2 m... | Bioorg Med Chem Lett 16: 2260-5 (2006) Article DOI: 10.1016/j.bmcl.2006.01.024 BindingDB Entry DOI: 10.7270/Q2C24TQK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monocarboxylate transporter 1 (Homo sapiens (Human)) | BDBM22026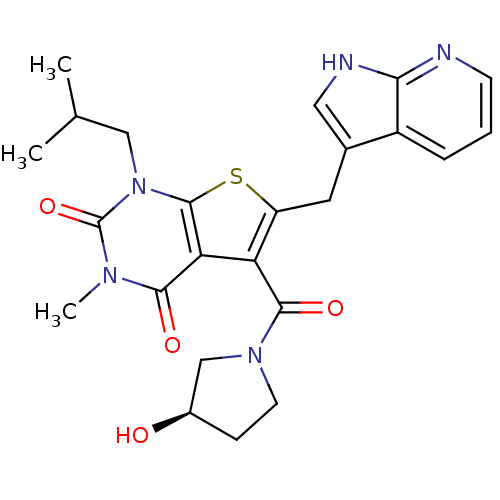 (5-{[(3R)-3-hydroxypyrrolidin-1-yl]carbonyl}-3-meth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 4.70 | -47.1 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
AstraZeneca | Assay Description A radioligand-binding assay was developed using scintillation proximity assay (SPA) technology. The wheat germ agglutinin SPA beads (Amersham) (0.2 m... | Bioorg Med Chem Lett 16: 2260-5 (2006) Article DOI: 10.1016/j.bmcl.2006.01.024 BindingDB Entry DOI: 10.7270/Q2C24TQK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monocarboxylate transporter 1 (Homo sapiens (Human)) | BDBM21985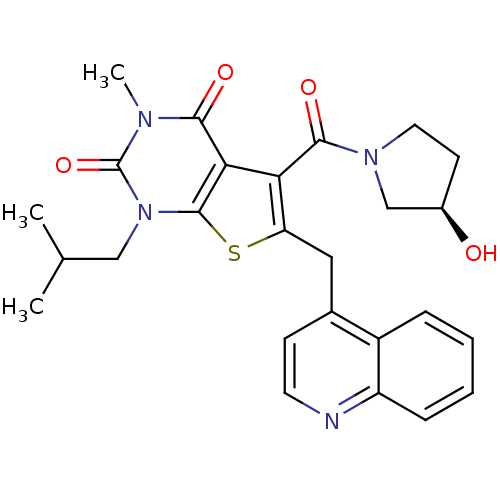 (5-{[(3R)-3-hydroxypyrrolidin-1-yl]carbonyl}-3-meth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | 4.80 | -47.0 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
AstraZeneca | Assay Description A radioligand-binding assay was developed using scintillation proximity assay (SPA) technology. The wheat germ agglutinin SPA beads (Amersham) (0.2 m... | Bioorg Med Chem Lett 16: 2260-5 (2006) Article DOI: 10.1016/j.bmcl.2006.01.024 BindingDB Entry DOI: 10.7270/Q2C24TQK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monocarboxylate transporter 1 (Homo sapiens (Human)) | BDBM22015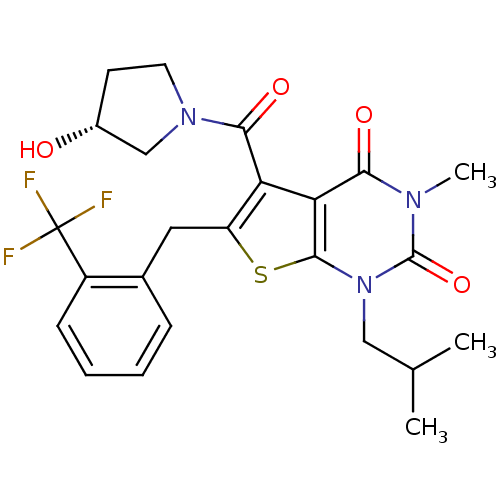 (5-{[(3R)-3-hydroxypyrrolidin-1-yl]carbonyl}-3-meth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 4.90 | -47.0 | n/a | n/a | n/a | n/a | n/a | 7.8 | 22 |
AstraZeneca | Assay Description Jurkat cell membranes were incubated with [3H]-labeled ligand in the absence or presence of increasing concentrations of test compound. The reagents ... | Bioorg Med Chem Lett 16: 2260-5 (2006) Article DOI: 10.1016/j.bmcl.2006.01.024 BindingDB Entry DOI: 10.7270/Q2C24TQK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monocarboxylate transporter 1 (Homo sapiens (Human)) | BDBM22023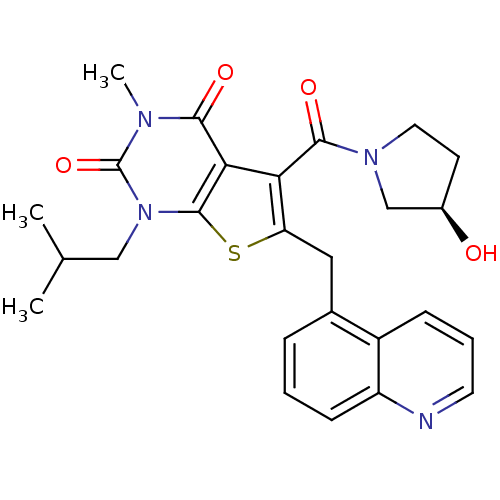 (5-{[(3R)-3-hydroxypyrrolidin-1-yl]carbonyl}-3-meth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 5.30 | -46.8 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
AstraZeneca | Assay Description A radioligand-binding assay was developed using scintillation proximity assay (SPA) technology. The wheat germ agglutinin SPA beads (Amersham) (0.2 m... | Bioorg Med Chem Lett 16: 2260-5 (2006) Article DOI: 10.1016/j.bmcl.2006.01.024 BindingDB Entry DOI: 10.7270/Q2C24TQK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monocarboxylate transporter 1 (Homo sapiens (Human)) | BDBM22017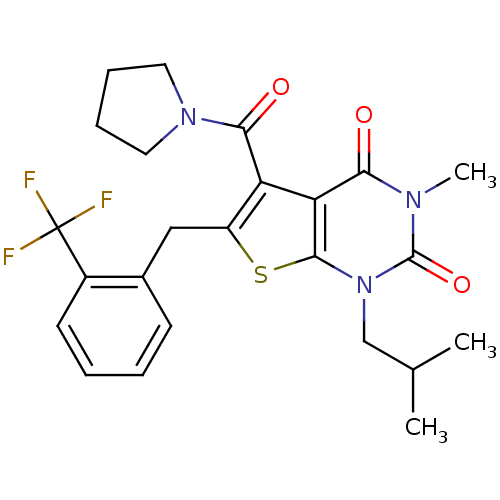 (3-methyl-1-(2-methylpropyl)-5-(pyrrolidin-1-ylcarb...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 5.5 | -46.7 | n/a | n/a | n/a | n/a | n/a | 7.8 | 22 |
AstraZeneca | Assay Description Jurkat cell membranes were incubated with [3H]-labeled ligand in the absence or presence of increasing concentrations of test compound. The reagents ... | Bioorg Med Chem Lett 16: 2260-5 (2006) Article DOI: 10.1016/j.bmcl.2006.01.024 BindingDB Entry DOI: 10.7270/Q2C24TQK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monocarboxylate transporter 1 (Homo sapiens (Human)) | BDBM22003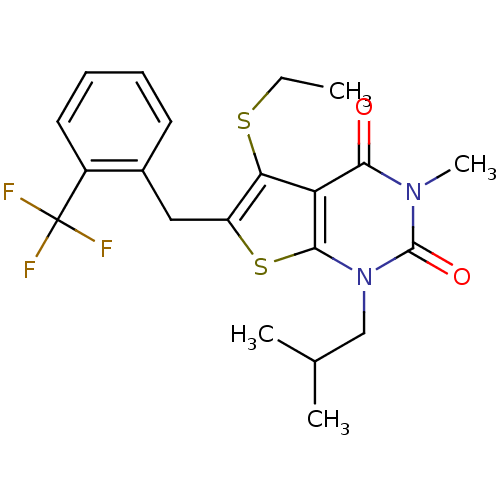 (5-(ethylsulfanyl)-3-methyl-1-(2-methylpropyl)-6-{[...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 6 | -46.5 | n/a | n/a | n/a | n/a | n/a | 7.8 | 22 |
AstraZeneca | Assay Description Jurkat cell membranes were incubated with [3H]-labeled ligand in the absence or presence of increasing concentrations of test compound. The reagents ... | Bioorg Med Chem Lett 16: 2260-5 (2006) Article DOI: 10.1016/j.bmcl.2006.01.024 BindingDB Entry DOI: 10.7270/Q2C24TQK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monocarboxylate transporter 1 (Homo sapiens (Human)) | BDBM22007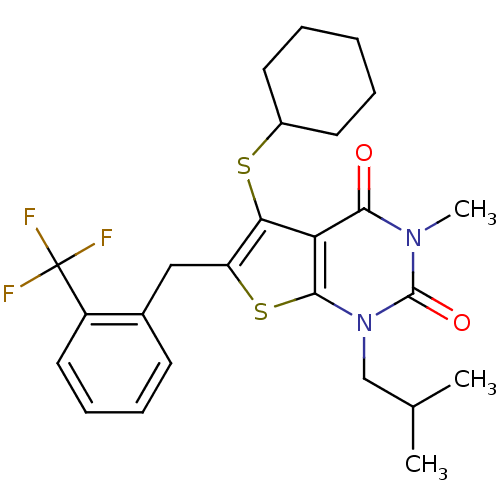 (5-(cyclohexylsulfanyl)-3-methyl-1-(2-methylpropyl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 6 | -46.5 | n/a | n/a | n/a | n/a | n/a | 7.8 | 22 |
AstraZeneca | Assay Description Jurkat cell membranes were incubated with [3H]-labeled ligand in the absence or presence of increasing concentrations of test compound. The reagents ... | Bioorg Med Chem Lett 16: 2260-5 (2006) Article DOI: 10.1016/j.bmcl.2006.01.024 BindingDB Entry DOI: 10.7270/Q2C24TQK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monocarboxylate transporter 1 (Homo sapiens (Human)) | BDBM22012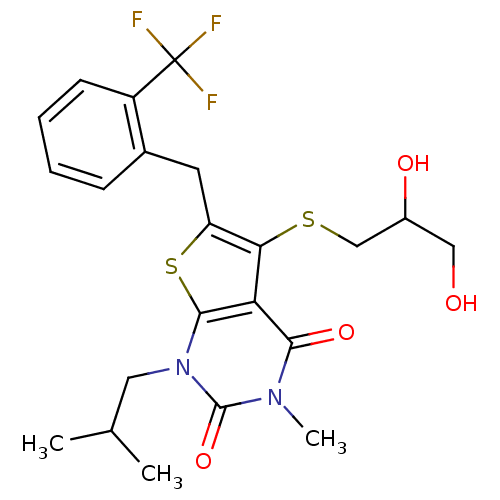 (5-[(2,3-dihydroxypropyl)sulfanyl]-3-methyl-1-(2-me...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 6.5 | -46.3 | n/a | n/a | n/a | n/a | n/a | 7.8 | 22 |
AstraZeneca | Assay Description Jurkat cell membranes were incubated with [3H]-labeled ligand in the absence or presence of increasing concentrations of test compound. The reagents ... | Bioorg Med Chem Lett 16: 2260-5 (2006) Article DOI: 10.1016/j.bmcl.2006.01.024 BindingDB Entry DOI: 10.7270/Q2C24TQK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monocarboxylate transporter 1 (Homo sapiens (Human)) | BDBM22027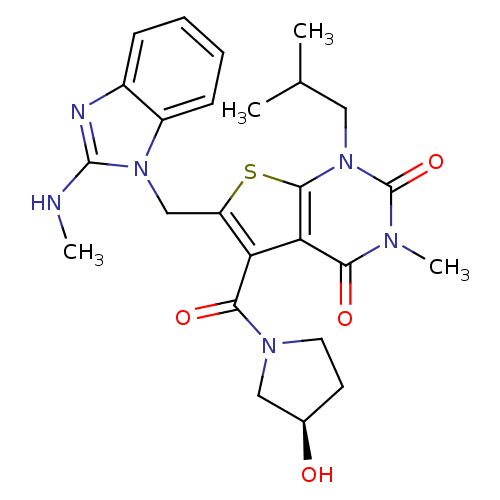 (5-{[(3R)-3-hydroxypyrrolidin-1-yl]carbonyl}-3-meth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 6.60 | -46.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
AstraZeneca | Assay Description A radioligand-binding assay was developed using scintillation proximity assay (SPA) technology. The wheat germ agglutinin SPA beads (Amersham) (0.2 m... | Bioorg Med Chem Lett 16: 2260-5 (2006) Article DOI: 10.1016/j.bmcl.2006.01.024 BindingDB Entry DOI: 10.7270/Q2C24TQK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monocarboxylate transporter 1 (Homo sapiens (Human)) | BDBM22018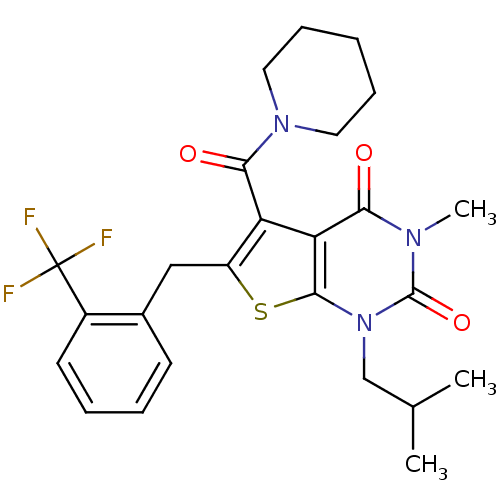 (3-methyl-1-(2-methylpropyl)-5-(piperidin-1-ylcarbo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 8.70 | -45.5 | n/a | n/a | n/a | n/a | n/a | 7.8 | 22 |
AstraZeneca | Assay Description Jurkat cell membranes were incubated with [3H]-labeled ligand in the absence or presence of increasing concentrations of test compound. The reagents ... | Bioorg Med Chem Lett 16: 2260-5 (2006) Article DOI: 10.1016/j.bmcl.2006.01.024 BindingDB Entry DOI: 10.7270/Q2C24TQK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monocarboxylate transporter 1 (Homo sapiens (Human)) | BDBM22016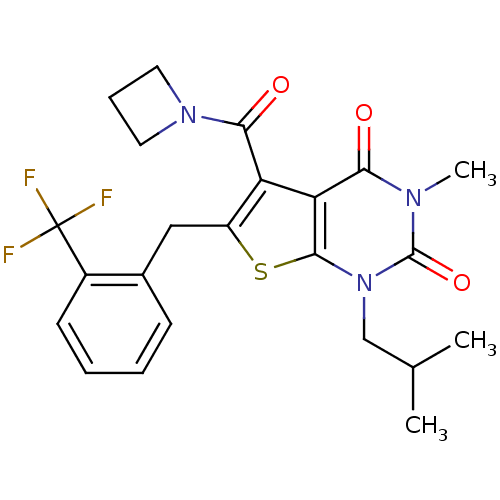 (5-(azetidin-1-ylcarbonyl)-3-methyl-1-(2-methylprop...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 9.20 | -45.4 | n/a | n/a | n/a | n/a | n/a | 7.8 | 22 |
AstraZeneca | Assay Description Jurkat cell membranes were incubated with [3H]-labeled ligand in the absence or presence of increasing concentrations of test compound. The reagents ... | Bioorg Med Chem Lett 16: 2260-5 (2006) Article DOI: 10.1016/j.bmcl.2006.01.024 BindingDB Entry DOI: 10.7270/Q2C24TQK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monocarboxylate transporter 1 (Homo sapiens (Human)) | BDBM22013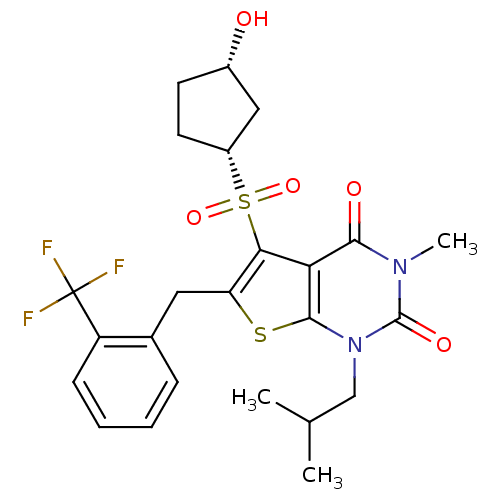 (5-{[(1R,3S)-3-hydroxycyclopentane]sulfonyl}-3-meth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 12 | -44.8 | n/a | n/a | n/a | n/a | n/a | 7.8 | 22 |
AstraZeneca | Assay Description Jurkat cell membranes were incubated with [3H]-labeled ligand in the absence or presence of increasing concentrations of test compound. The reagents ... | Bioorg Med Chem Lett 16: 2260-5 (2006) Article DOI: 10.1016/j.bmcl.2006.01.024 BindingDB Entry DOI: 10.7270/Q2C24TQK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monocarboxylate transporter 1 (Homo sapiens (Human)) | BDBM22005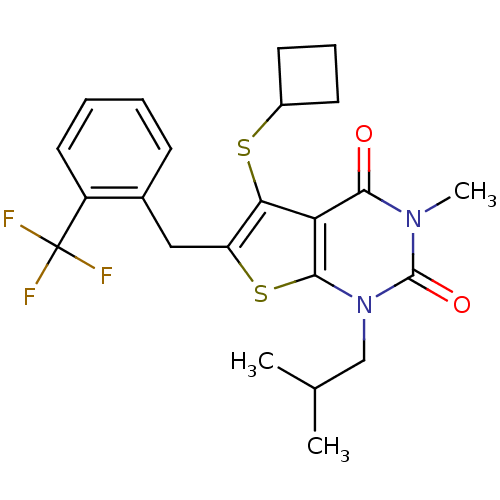 (5-(cyclobutylsulfanyl)-3-methyl-1-(2-methylpropyl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 13 | -44.6 | n/a | n/a | n/a | n/a | n/a | 7.8 | 22 |
AstraZeneca | Assay Description Jurkat cell membranes were incubated with [3H]-labeled ligand in the absence or presence of increasing concentrations of test compound. The reagents ... | Bioorg Med Chem Lett 16: 2260-5 (2006) Article DOI: 10.1016/j.bmcl.2006.01.024 BindingDB Entry DOI: 10.7270/Q2C24TQK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monocarboxylate transporter 1 (Homo sapiens (Human)) | BDBM22028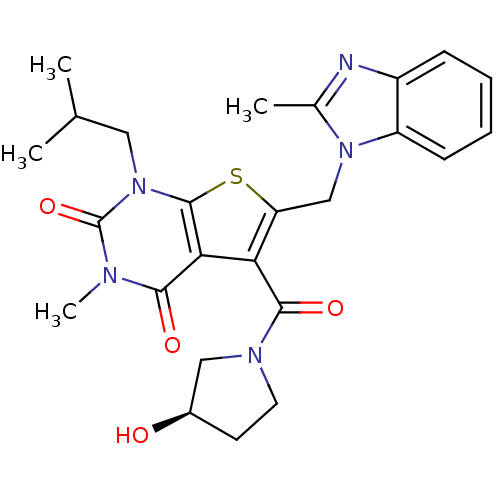 (5-{[(3R)-3-hydroxypyrrolidin-1-yl]carbonyl}-3-meth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 16 | -44.0 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
AstraZeneca | Assay Description A radioligand-binding assay was developed using scintillation proximity assay (SPA) technology. The wheat germ agglutinin SPA beads (Amersham) (0.2 m... | Bioorg Med Chem Lett 16: 2260-5 (2006) Article DOI: 10.1016/j.bmcl.2006.01.024 BindingDB Entry DOI: 10.7270/Q2C24TQK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monocarboxylate transporter 1 (Homo sapiens (Human)) | BDBM22029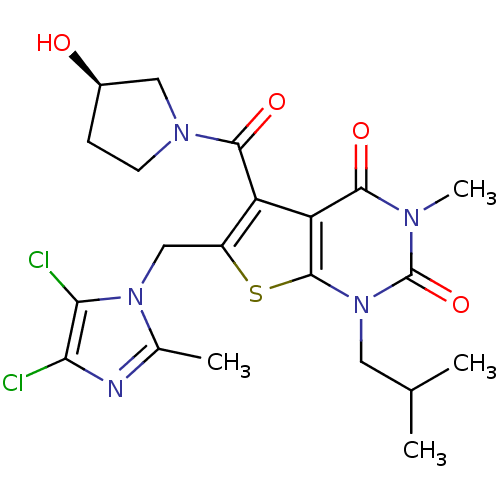 (6-[(4,5-dichloro-2-methyl-1H-imidazol-1-yl)methyl]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 26 | -42.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
AstraZeneca | Assay Description A radioligand-binding assay was developed using scintillation proximity assay (SPA) technology. The wheat germ agglutinin SPA beads (Amersham) (0.2 m... | Bioorg Med Chem Lett 16: 2260-5 (2006) Article DOI: 10.1016/j.bmcl.2006.01.024 BindingDB Entry DOI: 10.7270/Q2C24TQK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monocarboxylate transporter 1 (Homo sapiens (Human)) | BDBM22019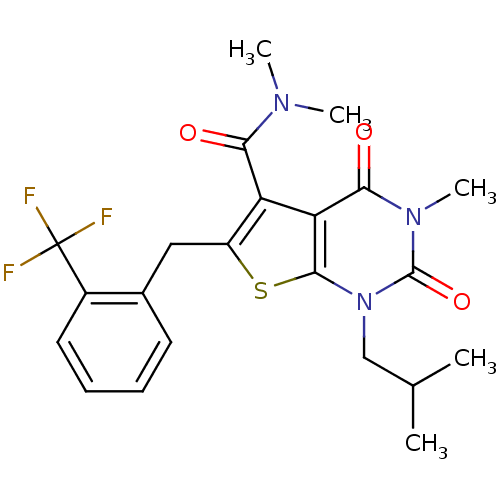 (N,N,3-trimethyl-1-(2-methylpropyl)-2,4-dioxo-6-{[2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 37 | -42.0 | n/a | n/a | n/a | n/a | n/a | 7.8 | 22 |
AstraZeneca | Assay Description Jurkat cell membranes were incubated with [3H]-labeled ligand in the absence or presence of increasing concentrations of test compound. The reagents ... | Bioorg Med Chem Lett 16: 2260-5 (2006) Article DOI: 10.1016/j.bmcl.2006.01.024 BindingDB Entry DOI: 10.7270/Q2C24TQK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (RAT) | BDBM50054959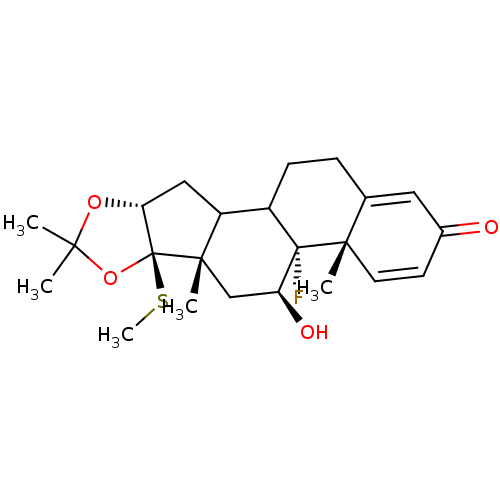 ((4aS,4bR,5S,6aS,6bR,9aR)-4b-Fluoro-5-hydroxy-4a,6a...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Rh£ne-Poulenc Rorer Central Research Curated by ChEMBL | Assay Description Steroid binding against the rat thymus glucocorticoid receptor | J Med Chem 39: 4888-96 (1997) Article DOI: 10.1021/jm9604639 BindingDB Entry DOI: 10.7270/Q23779C8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (RAT) | BDBM50054966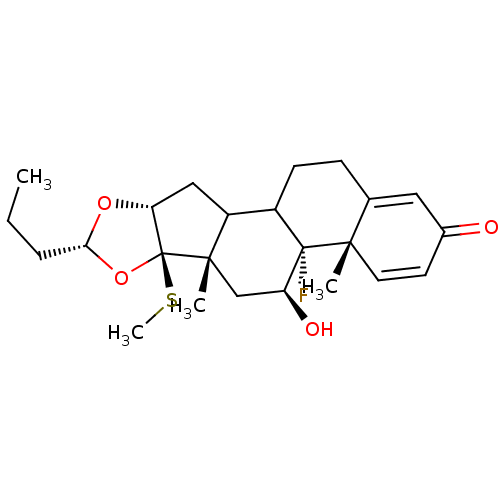 ((4aS,4bR,5S,6aS,6bR,8R,9aR)-4b-Fluoro-5-hydroxy-4a...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Rh£ne-Poulenc Rorer Central Research Curated by ChEMBL | Assay Description Steroid binding against the rat thymus glucocorticoid receptor | J Med Chem 39: 4888-96 (1997) Article DOI: 10.1021/jm9604639 BindingDB Entry DOI: 10.7270/Q23779C8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (RAT) | BDBM50407988 (CHEMBL2112857) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Rh£ne-Poulenc Rorer Central Research Curated by ChEMBL | Assay Description Steroid binding against the rat thymus glucocorticoid receptor | J Med Chem 39: 4888-96 (1997) Article DOI: 10.1021/jm9604639 BindingDB Entry DOI: 10.7270/Q23779C8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (RAT) | BDBM50054953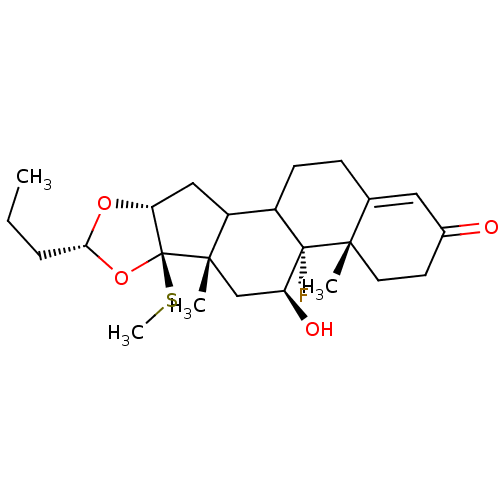 ((4aS,4bR,5S,6aS,6bR,8R,9aR)-4b-Fluoro-5-hydroxy-4a...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Rh£ne-Poulenc Rorer Central Research Curated by ChEMBL | Assay Description Steroid binding against the rat thymus glucocorticoid receptor | J Med Chem 39: 4888-96 (1997) Article DOI: 10.1021/jm9604639 BindingDB Entry DOI: 10.7270/Q23779C8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (RAT) | BDBM50054955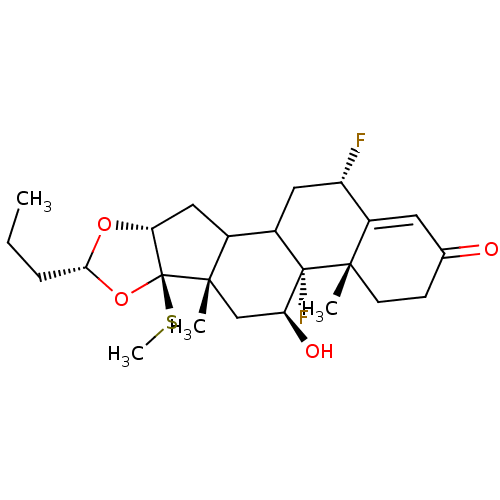 ((4aS,4bR,5S,6aS,6bR,8R,9aR,12S)-4b,12-Difluoro-5-h...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Rh£ne-Poulenc Rorer Central Research Curated by ChEMBL | Assay Description Steroid binding against the rat thymus glucocorticoid receptor | J Med Chem 39: 4888-96 (1997) Article DOI: 10.1021/jm9604639 BindingDB Entry DOI: 10.7270/Q23779C8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (RAT) | BDBM50054961 ((4aS,4bR,5S,6aS,6bR,9aR,12S)-4b,12-Difluoro-5-hydr...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Rh£ne-Poulenc Rorer Central Research Curated by ChEMBL | Assay Description Steroid binding against the rat thymus glucocorticoid receptor | J Med Chem 39: 4888-96 (1997) Article DOI: 10.1021/jm9604639 BindingDB Entry DOI: 10.7270/Q23779C8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (RAT) | BDBM50054964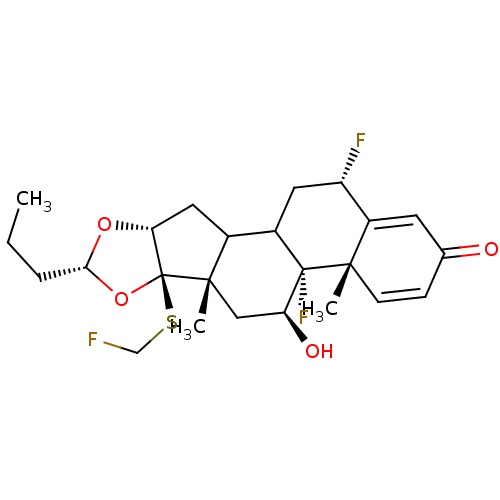 ((4aS,4bR,5S,6aS,6bR,8R,9aR,12S)-4b,12-Difluoro-6b-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Rh£ne-Poulenc Rorer Central Research Curated by ChEMBL | Assay Description Steroid binding against the rat thymus glucocorticoid receptor | J Med Chem 39: 4888-96 (1997) Article DOI: 10.1021/jm9604639 BindingDB Entry DOI: 10.7270/Q23779C8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (RAT) | BDBM50369181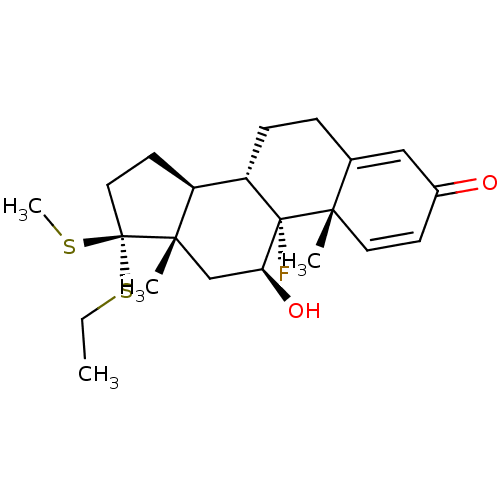 (TIPREDANE) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Rh£ne-Poulenc Rorer Central Research Curated by ChEMBL | Assay Description Steroid binding against the rat thymus glucocorticoid receptor | J Med Chem 39: 4888-96 (1997) Article DOI: 10.1021/jm9604639 BindingDB Entry DOI: 10.7270/Q23779C8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (RAT) | BDBM50054960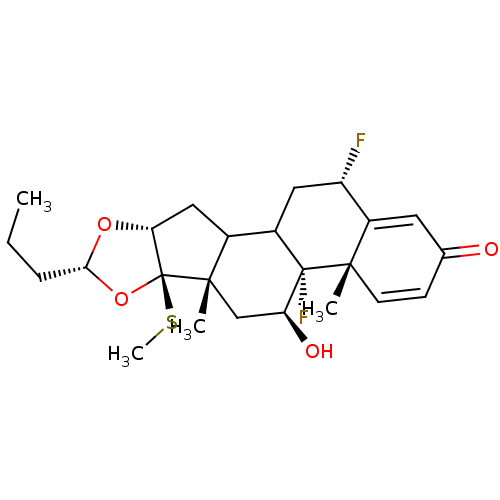 ((4aS,4bR,5S,6aS,6bR,8R,9aR,12S)-4b,12-Difluoro-5-h...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Rh£ne-Poulenc Rorer Central Research Curated by ChEMBL | Assay Description Steroid binding against the rat thymus glucocorticoid receptor | J Med Chem 39: 4888-96 (1997) Article DOI: 10.1021/jm9604639 BindingDB Entry DOI: 10.7270/Q23779C8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (RAT) | BDBM50354850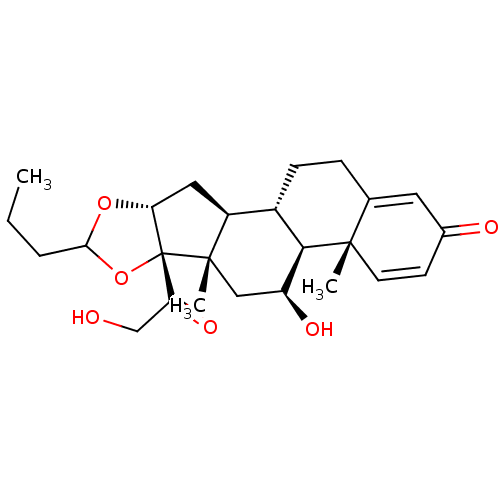 (BUDESONIDE | US10869929, Compound Budesonide | US1...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Rh£ne-Poulenc Rorer Central Research Curated by ChEMBL | Assay Description Steroid binding against the rat thymus glucocorticoid receptor | J Med Chem 39: 4888-96 (1997) Article DOI: 10.1021/jm9604639 BindingDB Entry DOI: 10.7270/Q23779C8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (RAT) | BDBM50054952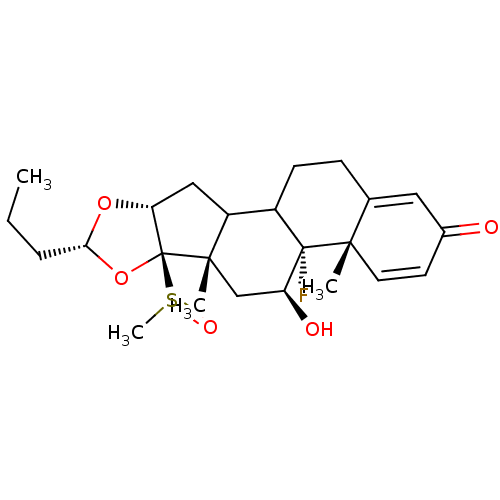 ((4aS,4bR,5S,6aS,6bR,8R,9aR)-4b-Fluoro-5-hydroxy-6b...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Rh£ne-Poulenc Rorer Central Research Curated by ChEMBL | Assay Description Steroid binding against the rat thymus glucocorticoid receptor | J Med Chem 39: 4888-96 (1997) Article DOI: 10.1021/jm9604639 BindingDB Entry DOI: 10.7270/Q23779C8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (RAT) | BDBM50054963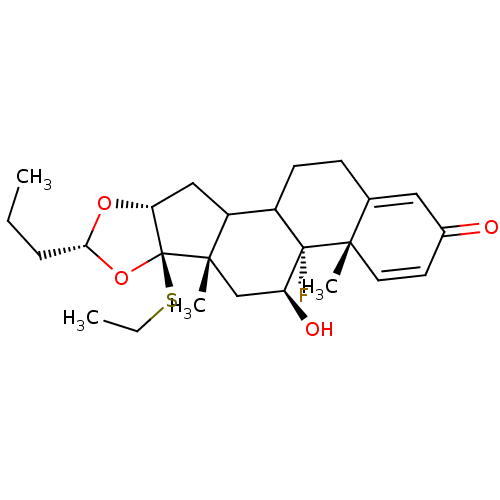 ((4aS,4bR,5S,6aS,6bR,8R,9aR)-6b-Ethylsulfanyl-4b-fl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Rh£ne-Poulenc Rorer Central Research Curated by ChEMBL | Assay Description Steroid binding against the rat thymus glucocorticoid receptor | J Med Chem 39: 4888-96 (1997) Article DOI: 10.1021/jm9604639 BindingDB Entry DOI: 10.7270/Q23779C8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (RAT) | BDBM50054962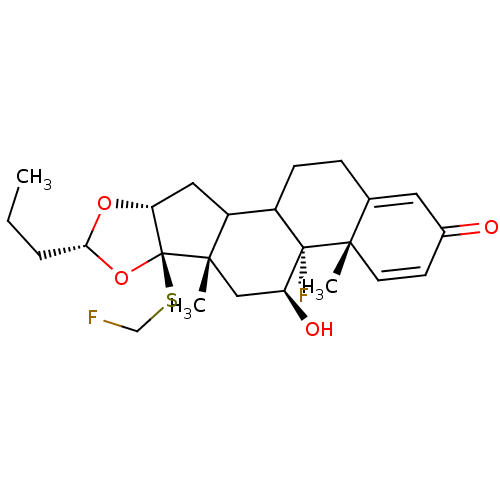 ((4aS,4bR,5S,6aS,6bR,8R,9aR)-4b-Fluoro-6b-fluoromet...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Rh£ne-Poulenc Rorer Central Research Curated by ChEMBL | Assay Description Steroid binding against the rat thymus glucocorticoid receptor | J Med Chem 39: 4888-96 (1997) Article DOI: 10.1021/jm9604639 BindingDB Entry DOI: 10.7270/Q23779C8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytosolic phospholipase A2 beta (Homo sapiens (Human)) | BDBM50110881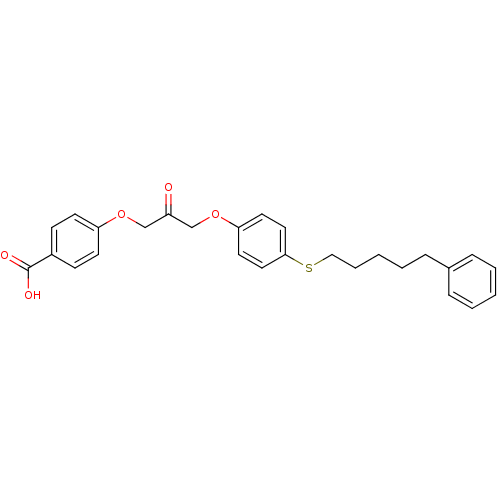 (4-(2-oxo-3-(4-(5-phenylpentylthio)phenoxy)propoxy)...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Charnwood Curated by ChEMBL | Assay Description Inhibitory activity against cytosolic Phospholipase A2 (PLA2) by bilayer assay | J Med Chem 45: 1348-62 (2002) BindingDB Entry DOI: 10.7270/Q2F76BVP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (RAT) | BDBM50407989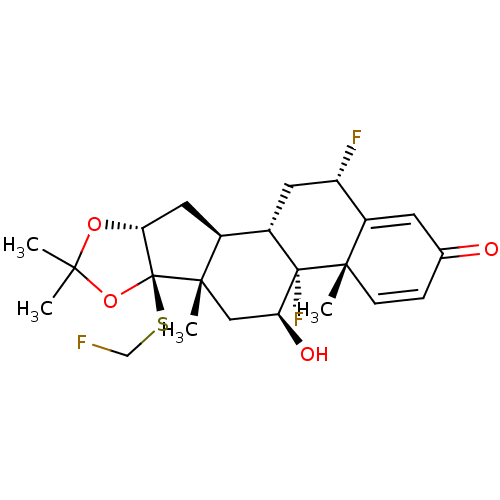 (CHEMBL2112850) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Rh£ne-Poulenc Rorer Central Research Curated by ChEMBL | Assay Description Steroid binding against the rat thymus glucocorticoid receptor | J Med Chem 39: 4888-96 (1997) Article DOI: 10.1021/jm9604639 BindingDB Entry DOI: 10.7270/Q23779C8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (RAT) | BDBM50354849 (CCI-18781 | Cutivate | FLUTICASONE PROPIONATE | Fl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Rh£ne-Poulenc Rorer Central Research Curated by ChEMBL | Assay Description Steroid binding against the rat thymus glucocorticoid receptor | J Med Chem 39: 4888-96 (1997) Article DOI: 10.1021/jm9604639 BindingDB Entry DOI: 10.7270/Q23779C8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (RAT) | BDBM50407990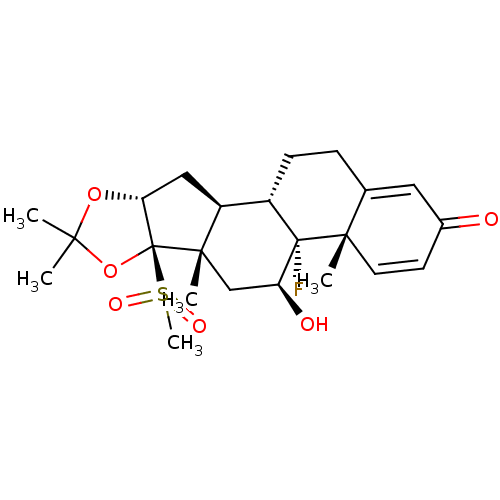 (CHEMBL2112858) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Rh£ne-Poulenc Rorer Central Research Curated by ChEMBL | Assay Description Steroid binding against the rat thymus glucocorticoid receptor | J Med Chem 39: 4888-96 (1997) Article DOI: 10.1021/jm9604639 BindingDB Entry DOI: 10.7270/Q23779C8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (RAT) | BDBM50054967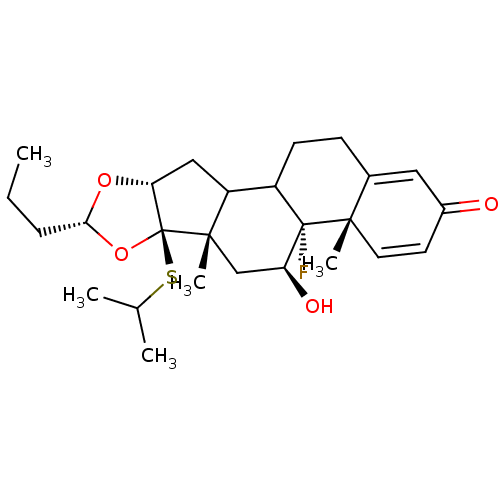 ((4aS,4bR,5S,6aS,6bR,8R,9aR)-4b-Fluoro-5-hydroxy-6b...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Rh£ne-Poulenc Rorer Central Research Curated by ChEMBL | Assay Description Steroid binding against the rat thymus glucocorticoid receptor | J Med Chem 39: 4888-96 (1997) Article DOI: 10.1021/jm9604639 BindingDB Entry DOI: 10.7270/Q23779C8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytosolic phospholipase A2 beta (Homo sapiens (Human)) | BDBM50110846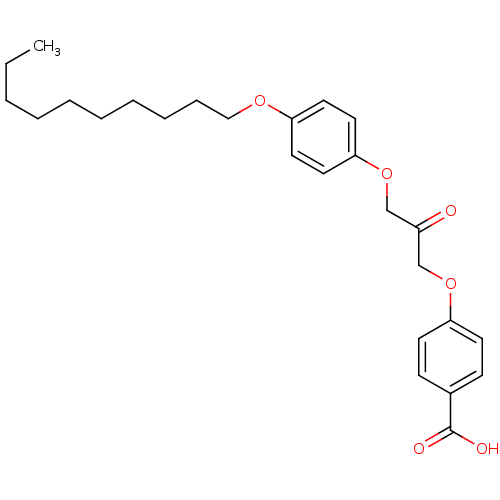 (4-(3-(4-(decyloxy)phenoxy)-2-oxopropoxy)benzoic ac...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Charnwood Curated by ChEMBL | Assay Description Inhibitory activity against cytosolic Phospholipase A2 (PLA2) by bilayer assay | J Med Chem 45: 1348-62 (2002) BindingDB Entry DOI: 10.7270/Q2F76BVP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (RAT) | BDBM50054957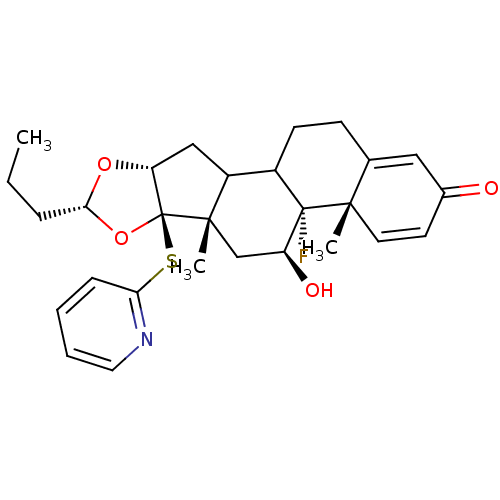 ((4aS,4bR,5S,6aS,6bR,8R,9aR)-4b-Fluoro-5-hydroxy-4a...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Rh£ne-Poulenc Rorer Central Research Curated by ChEMBL | Assay Description Steroid binding against the rat thymus glucocorticoid receptor | J Med Chem 39: 4888-96 (1997) Article DOI: 10.1021/jm9604639 BindingDB Entry DOI: 10.7270/Q23779C8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 116 total ) | Next | Last >> |