

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Chitin synthase 1 (Candida albicans) | BDBM50089536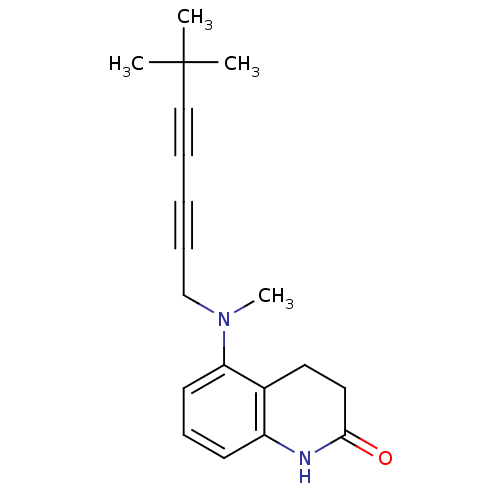 (5-[(6,6-Dimethyl-hepta-2,4-diynyl)-methyl-amino]-3...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a |
Nippon Roche Research Center Curated by ChEMBL | Assay Description Inhibitory of Candida albicans chitin synthase 1 | Bioorg Med Chem Lett 10: 1459-62 (2000) BindingDB Entry DOI: 10.7270/Q2R210MK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 1,3-beta-glucan synthase (Candida albicans (Yeast)) | BDBM50100335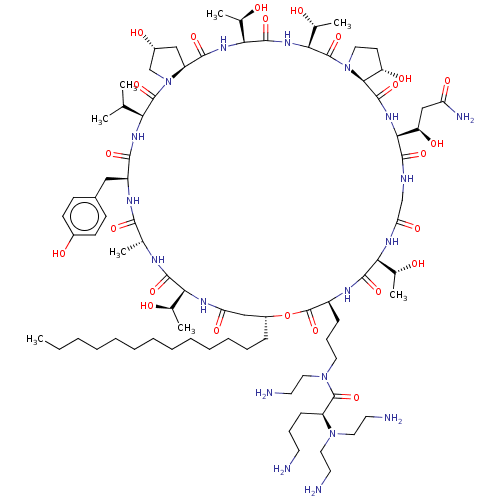 (CHEMBL2371681 | RO-09-3655 derivative) | UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Nippon Roche Research Center Curated by ChEMBL | Assay Description In vitro inhibitory activity against Beta glucan synthase | Bioorg Med Chem Lett 11: 1273-6 (2001) BindingDB Entry DOI: 10.7270/Q2CN74FQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 1,3-beta-glucan synthase (Candida albicans (Yeast)) | BDBM50100338 (CHEMBL267407 | RO-09-3655 derivative) | UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
Nippon Roche Research Center Curated by ChEMBL | Assay Description In vitro inhibitory activity against Beta glucan synthase | Bioorg Med Chem Lett 11: 1273-6 (2001) BindingDB Entry DOI: 10.7270/Q2CN74FQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 1,3-beta-glucan synthase (Candida albicans (Yeast)) | BDBM50100332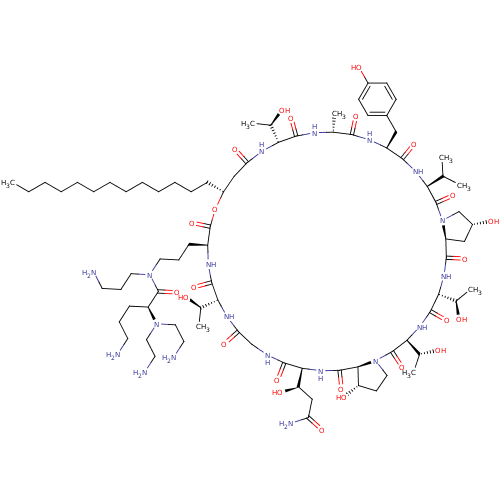 (CHEMBL415843 | RO-09-3655 derivative) | UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a |
Nippon Roche Research Center Curated by ChEMBL | Assay Description In vitro inhibitory activity against Beta glucan synthase | Bioorg Med Chem Lett 11: 1273-6 (2001) BindingDB Entry DOI: 10.7270/Q2CN74FQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chitin synthase 1 (Candida albicans) | BDBM50089557 (5-[Butyl-((E)-6,6-dimethyl-hept-2-en-4-ynyl)-amino...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a |
Nippon Roche Research Center Curated by ChEMBL | Assay Description Inhibitory of Candida albicans chitin synthase 1 | Bioorg Med Chem Lett 10: 1459-62 (2000) BindingDB Entry DOI: 10.7270/Q2R210MK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50335557 (CHEMBL1652605 | N,N-dipropyl-N'-[4-({[(1H-imidazol...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.610 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Infectious Diseases Curated by ChEMBL | Assay Description Displacement of [125I]SDF-1alpha form CXCR4 expressed in CHO cells by scintillation counting | Antimicrob Agents Chemother 53: 2940-8 (2009) Article DOI: 10.1128/AAC.01727-08 BindingDB Entry DOI: 10.7270/Q2GF0TR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 1,3-beta-glucan synthase (Candida albicans (Yeast)) | BDBM50100333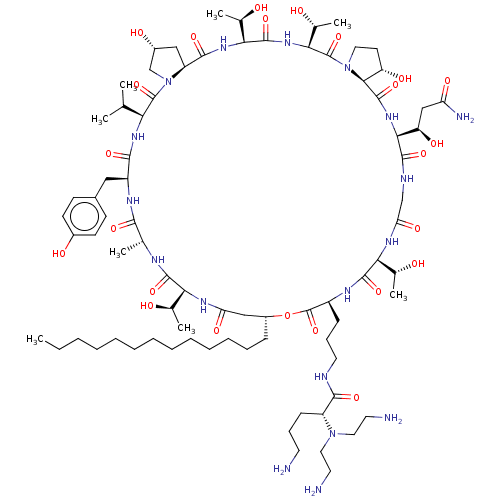 (CHEMBL2371765 | RO-09-3655 derivative) | UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.630 | n/a | n/a | n/a | n/a | n/a | n/a |
Nippon Roche Research Center Curated by ChEMBL | Assay Description In vitro inhibitory activity against Beta glucan synthase | Bioorg Med Chem Lett 11: 1273-6 (2001) BindingDB Entry DOI: 10.7270/Q2CN74FQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chitin synthase 1 (Candida albicans) | BDBM50089551 (5-[((E)-6,6-Dimethyl-hept-2-en-4-ynyl)-propyl-amin...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.680 | n/a | n/a | n/a | n/a | n/a | n/a |
Nippon Roche Research Center Curated by ChEMBL | Assay Description Inhibitory of Candida albicans chitin synthase 1 | Bioorg Med Chem Lett 10: 1459-62 (2000) BindingDB Entry DOI: 10.7270/Q2R210MK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chitin synthase 1 (Candida albicans) | BDBM50089540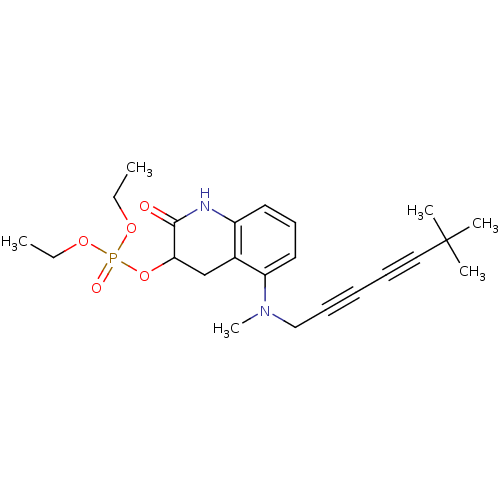 (CHEMBL32125 | Phosphoric acid 5-[(6,6-dimethyl-hep...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 0.760 | n/a | n/a | n/a | n/a | n/a | n/a |
Nippon Roche Research Center Curated by ChEMBL | Assay Description Inhibitory of Candida albicans chitin synthase 1 | Bioorg Med Chem Lett 10: 1459-62 (2000) BindingDB Entry DOI: 10.7270/Q2R210MK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 1,3-beta-glucan synthase (Candida albicans (Yeast)) | BDBM50100331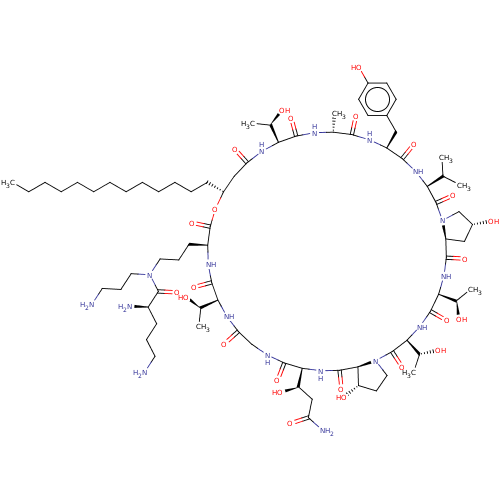 (CHEMBL2371715 | RO-09-3655 derivative) | UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.830 | n/a | n/a | n/a | n/a | n/a | n/a |
Nippon Roche Research Center Curated by ChEMBL | Assay Description In vitro inhibitory activity against Beta glucan synthase | Bioorg Med Chem Lett 11: 1273-6 (2001) BindingDB Entry DOI: 10.7270/Q2CN74FQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 1,3-beta-glucan synthase (Candida albicans (Yeast)) | BDBM50100329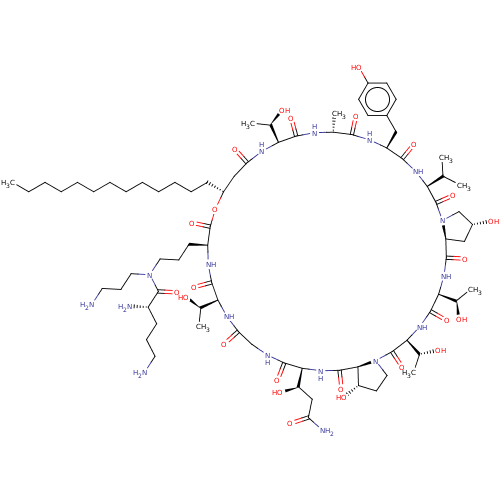 (CHEMBL2371727 | RO-09-3655 derivative) | UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.890 | n/a | n/a | n/a | n/a | n/a | n/a |
Nippon Roche Research Center Curated by ChEMBL | Assay Description In vitro inhibitory activity against Beta glucan synthase | Bioorg Med Chem Lett 11: 1273-6 (2001) BindingDB Entry DOI: 10.7270/Q2CN74FQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 1,3-beta-glucan synthase (Candida albicans (Yeast)) | BDBM50100337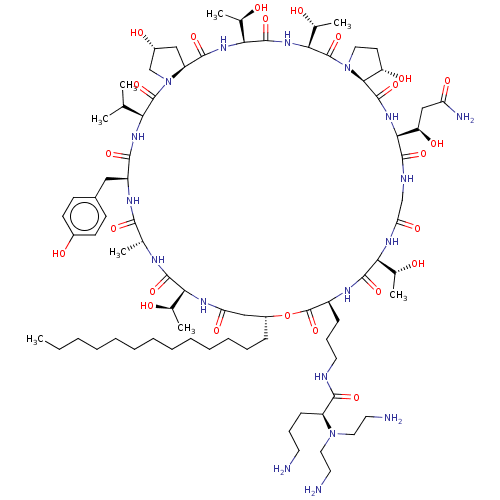 (CHEMBL2371766 | RO-09-3655 derivative) | UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Nippon Roche Research Center Curated by ChEMBL | Assay Description In vitro inhibitory activity against Beta glucan synthase | Bioorg Med Chem Lett 11: 1273-6 (2001) BindingDB Entry DOI: 10.7270/Q2CN74FQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chitin synthase 1 (Candida albicans) | BDBM50089563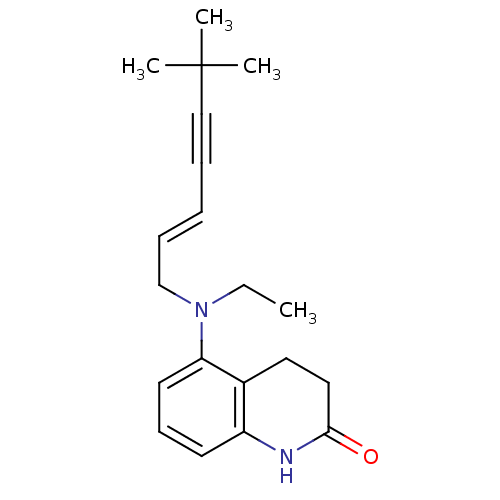 (5-[((E)-6,6-Dimethyl-hept-2-en-4-ynyl)-ethyl-amino...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Nippon Roche Research Center Curated by ChEMBL | Assay Description Inhibitory of Candida albicans chitin synthase 1 | Bioorg Med Chem Lett 10: 1459-62 (2000) BindingDB Entry DOI: 10.7270/Q2R210MK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chitin synthase 1 (Candida albicans) | BDBM50089538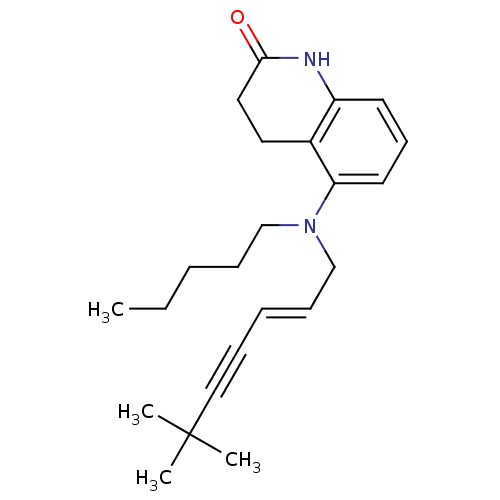 (5-[((E)-6,6-Dimethyl-hept-2-en-4-ynyl)-pentyl-amin...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Nippon Roche Research Center Curated by ChEMBL | Assay Description Inhibitory of Candida albicans chitin synthase 1 | Bioorg Med Chem Lett 10: 1459-62 (2000) BindingDB Entry DOI: 10.7270/Q2R210MK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chitin synthase 1 (Candida albicans) | BDBM50089559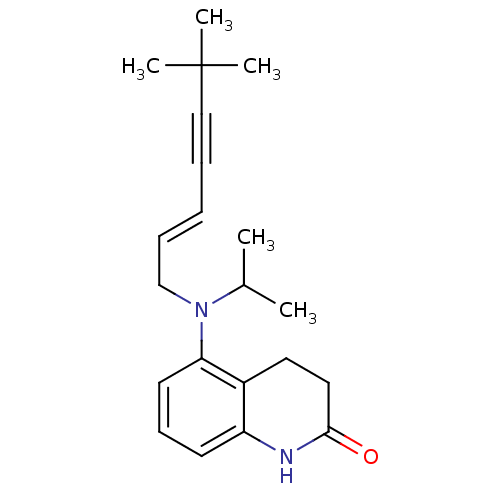 (5-[((E)-6,6-Dimethyl-hept-2-en-4-ynyl)-isopropyl-a...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Nippon Roche Research Center Curated by ChEMBL | Assay Description Inhibitory of Candida albicans chitin synthase 1 | Bioorg Med Chem Lett 10: 1459-62 (2000) BindingDB Entry DOI: 10.7270/Q2R210MK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 1,3-beta-glucan synthase (Candida albicans (Yeast)) | BDBM50366679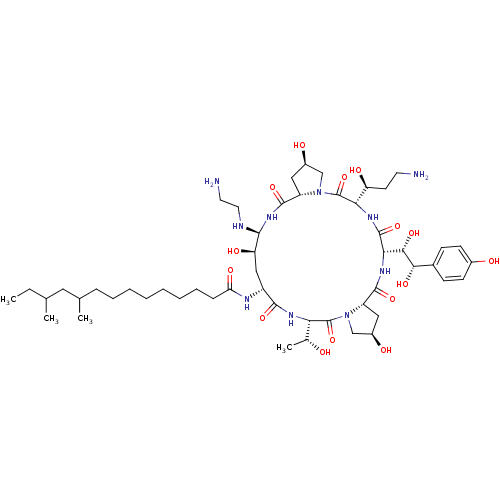 (CHEMBL1793852 | MK-991) | UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Nippon Roche Research Center Curated by ChEMBL | Assay Description In vitro inhibitory activity against Beta glucan synthase | Bioorg Med Chem Lett 11: 1273-6 (2001) BindingDB Entry DOI: 10.7270/Q2CN74FQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 1,3-beta-glucan synthase (Candida albicans (Yeast)) | BDBM50100336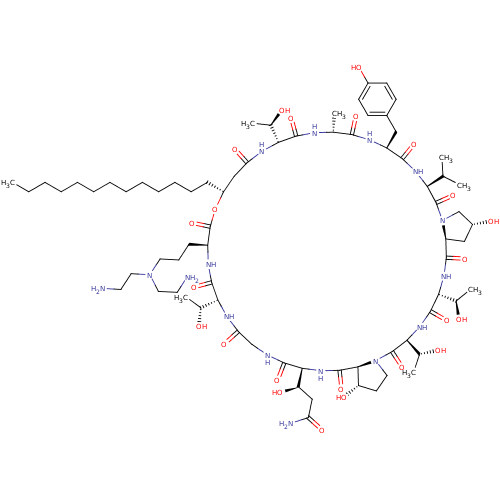 (CHEMBL405487 | RO-09-3655 derivative) | UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Nippon Roche Research Center Curated by ChEMBL | Assay Description In vitro inhibitory activity against Beta glucan synthase | Bioorg Med Chem Lett 11: 1273-6 (2001) BindingDB Entry DOI: 10.7270/Q2CN74FQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-alpha serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM108434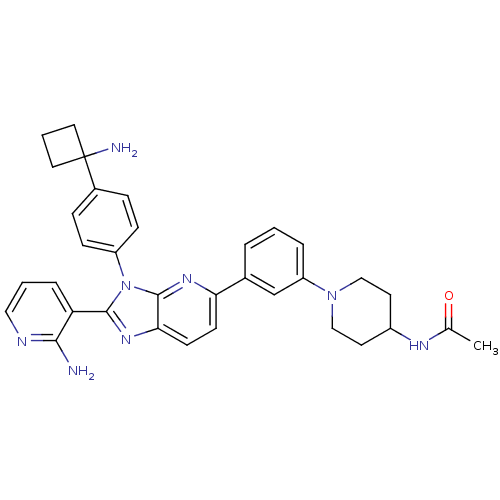 (US8609688, 12) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.44 | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule, Inc. US Patent | Assay Description AKT1 activity was assayed using the GSK3-derived biotinylated peptide substrate, crosstide (biotin-GRPRTSSFAEG), and AlphaScreen (Amplified Luminesce... | US Patent US8962619 (2015) BindingDB Entry DOI: 10.7270/Q2W957W7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-alpha serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM108434 (US8609688, 12) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.44 | n/a | n/a | n/a | n/a | 8.0 | 25 |
ArQule, Inc. US Patent | Assay Description AKT1 activity was assayed using the GSK3-derived biotinylated peptide substrate, crosstide (biotin-GRPRTSSFAEG), and AlphaScreen™ (Amplified Lum... | US Patent US8609688 (2013) BindingDB Entry DOI: 10.7270/Q2H41Q2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-alpha serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM108430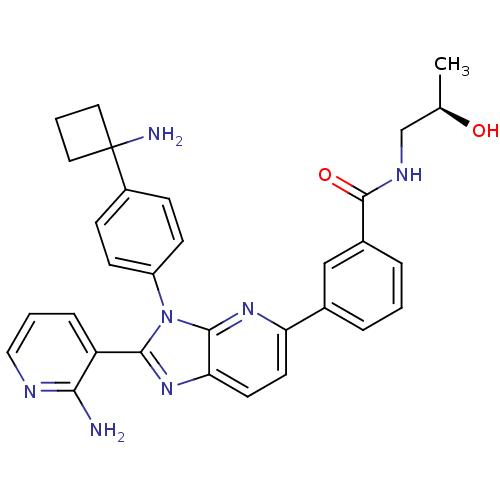 (US8609688, 8) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | 8.0 | 25 |
ArQule, Inc. US Patent | Assay Description AKT1 activity was assayed using the GSK3-derived biotinylated peptide substrate, crosstide (biotin-GRPRTSSFAEG), and AlphaScreen™ (Amplified Lum... | US Patent US8609688 (2013) BindingDB Entry DOI: 10.7270/Q2H41Q2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-alpha serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM108430 (US8609688, 8) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule, Inc. US Patent | Assay Description AKT1 activity was assayed using the GSK3-derived biotinylated peptide substrate, crosstide (biotin-GRPRTSSFAEG), and AlphaScreen (Amplified Luminesce... | US Patent US8962619 (2015) BindingDB Entry DOI: 10.7270/Q2W957W7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-alpha serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM108428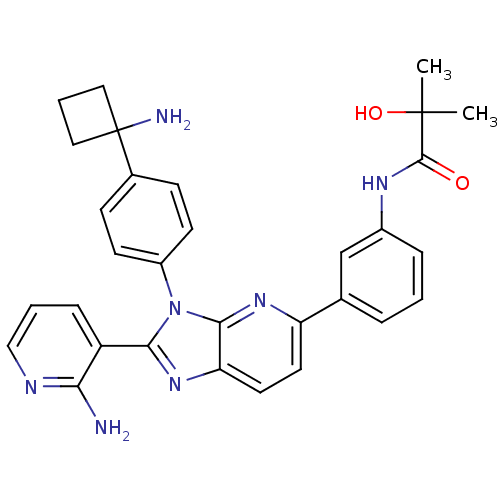 (US8609688, 6) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.63 | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule, Inc. US Patent | Assay Description AKT1 activity was assayed using the GSK3-derived biotinylated peptide substrate, crosstide (biotin-GRPRTSSFAEG), and AlphaScreen (Amplified Luminesce... | US Patent US8962619 (2015) BindingDB Entry DOI: 10.7270/Q2W957W7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-alpha serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM108428 (US8609688, 6) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.63 | n/a | n/a | n/a | n/a | 8.0 | 25 |
ArQule, Inc. US Patent | Assay Description AKT1 activity was assayed using the GSK3-derived biotinylated peptide substrate, crosstide (biotin-GRPRTSSFAEG), and AlphaScreen™ (Amplified Lum... | US Patent US8609688 (2013) BindingDB Entry DOI: 10.7270/Q2H41Q2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-alpha serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM108426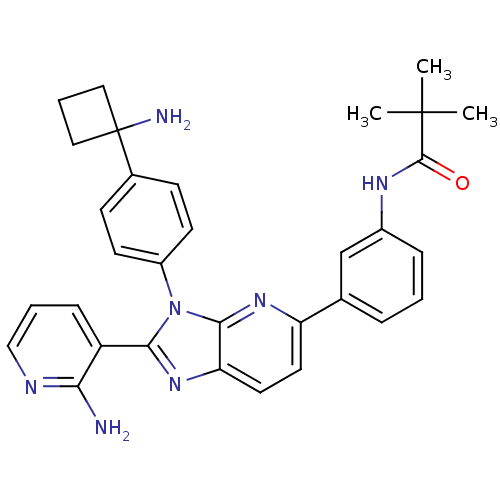 (US8609688, 4) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule, Inc. US Patent | Assay Description AKT1 activity was assayed using the GSK3-derived biotinylated peptide substrate, crosstide (biotin-GRPRTSSFAEG), and AlphaScreen (Amplified Luminesce... | US Patent US8962619 (2015) BindingDB Entry DOI: 10.7270/Q2W957W7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-alpha serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM108426 (US8609688, 4) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | 8.0 | 25 |
ArQule, Inc. US Patent | Assay Description AKT1 activity was assayed using the GSK3-derived biotinylated peptide substrate, crosstide (biotin-GRPRTSSFAEG), and AlphaScreen™ (Amplified Lum... | US Patent US8609688 (2013) BindingDB Entry DOI: 10.7270/Q2H41Q2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-alpha serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM108440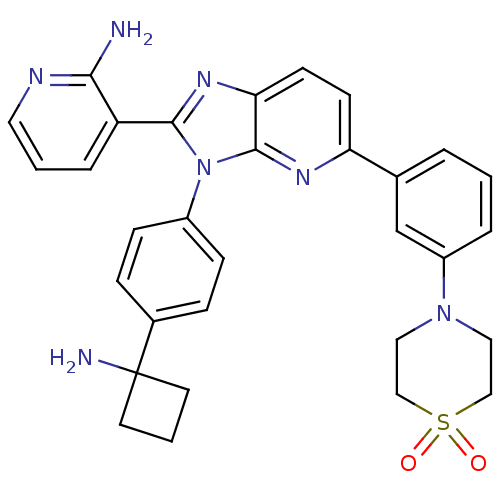 (US8609688, 18) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.79 | n/a | n/a | n/a | n/a | 8.0 | 25 |
ArQule, Inc. US Patent | Assay Description AKT1 activity was assayed using the GSK3-derived biotinylated peptide substrate, crosstide (biotin-GRPRTSSFAEG), and AlphaScreen™ (Amplified Lum... | US Patent US8609688 (2013) BindingDB Entry DOI: 10.7270/Q2H41Q2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-alpha serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM108440 (US8609688, 18) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.79 | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule, Inc. US Patent | Assay Description AKT1 activity was assayed using the GSK3-derived biotinylated peptide substrate, crosstide (biotin-GRPRTSSFAEG), and AlphaScreen (Amplified Luminesce... | US Patent US8962619 (2015) BindingDB Entry DOI: 10.7270/Q2W957W7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50222422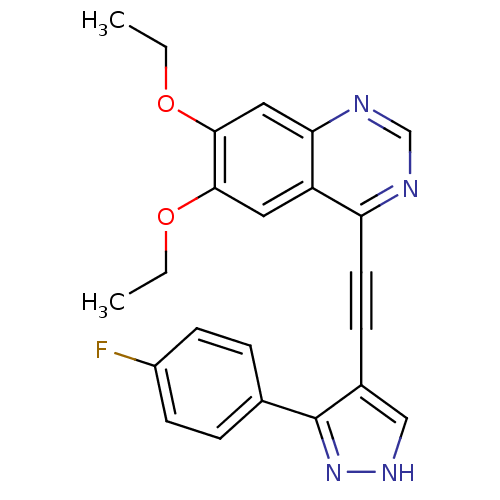 (6,7-diethoxy-4-(2-(3-(4-fluorophenyl)-1H-pyrazol-4...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Pharma Corporation Curated by ChEMBL | Assay Description Inhibition of partially purified EGFR tyrosine kinase from human A431 cells | Bioorg Med Chem Lett 17: 5863-7 (2007) Article DOI: 10.1016/j.bmcl.2007.08.020 BindingDB Entry DOI: 10.7270/Q2H70FJ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-alpha serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM108436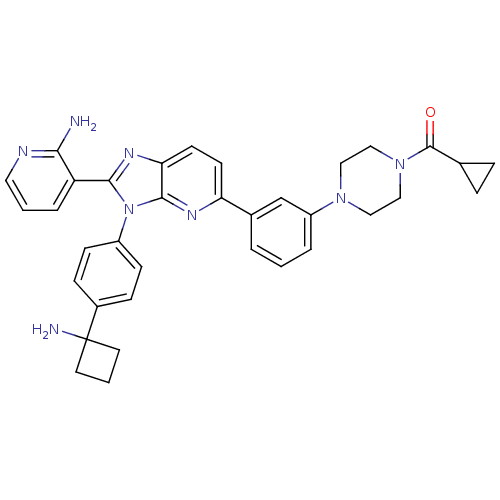 (US8609688, 14) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.84 | n/a | n/a | n/a | n/a | 8.0 | 25 |
ArQule, Inc. US Patent | Assay Description AKT1 activity was assayed using the GSK3-derived biotinylated peptide substrate, crosstide (biotin-GRPRTSSFAEG), and AlphaScreen™ (Amplified Lum... | US Patent US8609688 (2013) BindingDB Entry DOI: 10.7270/Q2H41Q2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-alpha serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM108436 (US8609688, 14) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.84 | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule, Inc. US Patent | Assay Description AKT1 activity was assayed using the GSK3-derived biotinylated peptide substrate, crosstide (biotin-GRPRTSSFAEG), and AlphaScreen (Amplified Luminesce... | US Patent US8962619 (2015) BindingDB Entry DOI: 10.7270/Q2W957W7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 1,3-beta-glucan synthase (Candida albicans (Yeast)) | BDBM50096797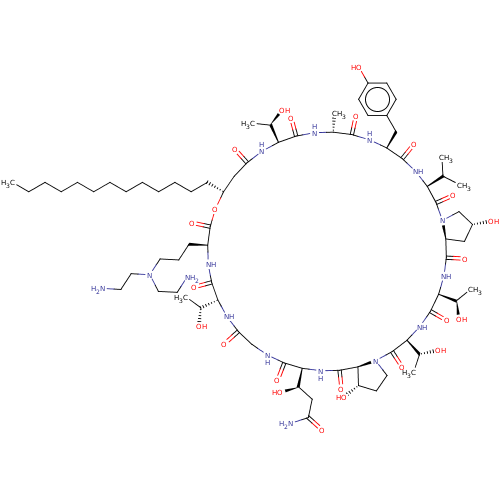 (CHEMBL2370665 | macrocyclic lipopeptidolactone der...) | UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Nippon Roche Research Center Curated by ChEMBL | Assay Description Inhibition of 1,3-beta-D-glucan synthase (GS) from candida albicans | Bioorg Med Chem Lett 11: 395-8 (2001) BindingDB Entry DOI: 10.7270/Q28051W2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-alpha serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM108435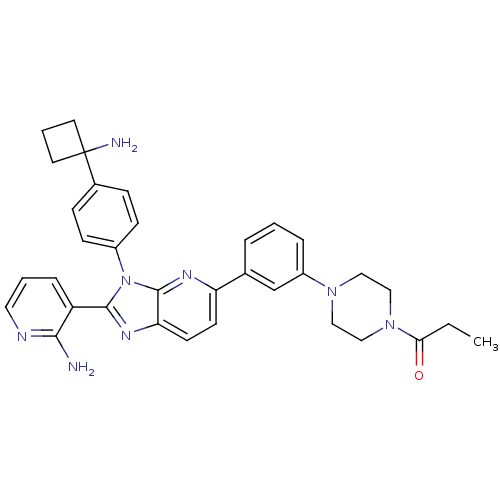 (US8609688, 13) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.15 | n/a | n/a | n/a | n/a | 8.0 | 25 |
ArQule, Inc. US Patent | Assay Description AKT1 activity was assayed using the GSK3-derived biotinylated peptide substrate, crosstide (biotin-GRPRTSSFAEG), and AlphaScreen™ (Amplified Lum... | US Patent US8609688 (2013) BindingDB Entry DOI: 10.7270/Q2H41Q2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-alpha serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM108435 (US8609688, 13) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.15 | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule, Inc. US Patent | Assay Description AKT1 activity was assayed using the GSK3-derived biotinylated peptide substrate, crosstide (biotin-GRPRTSSFAEG), and AlphaScreen (Amplified Luminesce... | US Patent US8962619 (2015) BindingDB Entry DOI: 10.7270/Q2W957W7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-alpha serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM108429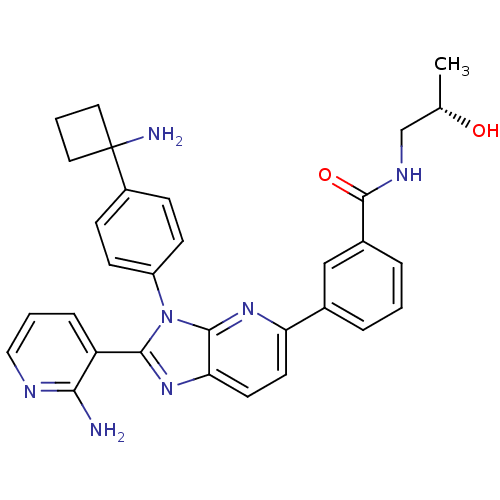 (US8609688, 7) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.45 | n/a | n/a | n/a | n/a | 8.0 | 25 |
ArQule, Inc. US Patent | Assay Description AKT1 activity was assayed using the GSK3-derived biotinylated peptide substrate, crosstide (biotin-GRPRTSSFAEG), and AlphaScreen™ (Amplified Lum... | US Patent US8609688 (2013) BindingDB Entry DOI: 10.7270/Q2H41Q2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-alpha serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM108429 (US8609688, 7) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.45 | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule, Inc. US Patent | Assay Description AKT1 activity was assayed using the GSK3-derived biotinylated peptide substrate, crosstide (biotin-GRPRTSSFAEG), and AlphaScreen (Amplified Luminesce... | US Patent US8962619 (2015) BindingDB Entry DOI: 10.7270/Q2W957W7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-alpha serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM108431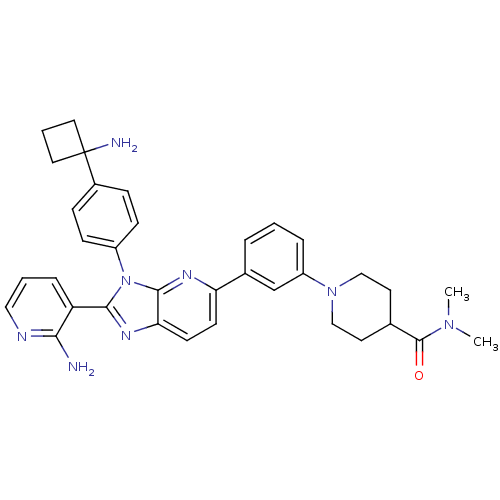 (US8609688, 9) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.51 | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule, Inc. US Patent | Assay Description AKT1 activity was assayed using the GSK3-derived biotinylated peptide substrate, crosstide (biotin-GRPRTSSFAEG), and AlphaScreen (Amplified Luminesce... | US Patent US8962619 (2015) BindingDB Entry DOI: 10.7270/Q2W957W7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-alpha serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM108431 (US8609688, 9) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.51 | n/a | n/a | n/a | n/a | 8.0 | 25 |
ArQule, Inc. US Patent | Assay Description AKT1 activity was assayed using the GSK3-derived biotinylated peptide substrate, crosstide (biotin-GRPRTSSFAEG), and AlphaScreen™ (Amplified Lum... | US Patent US8609688 (2013) BindingDB Entry DOI: 10.7270/Q2H41Q2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50335557 (CHEMBL1652605 | N,N-dipropyl-N'-[4-({[(1H-imidazol...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Infectious Diseases Curated by ChEMBL | Assay Description Inhibition of Mab 12G5 binding to wild type CXCR4 expressed in HEK293 cells | Antimicrob Agents Chemother 53: 2940-8 (2009) Article DOI: 10.1128/AAC.01727-08 BindingDB Entry DOI: 10.7270/Q2GF0TR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chitin synthase 1 (Candida albicans) | BDBM50089543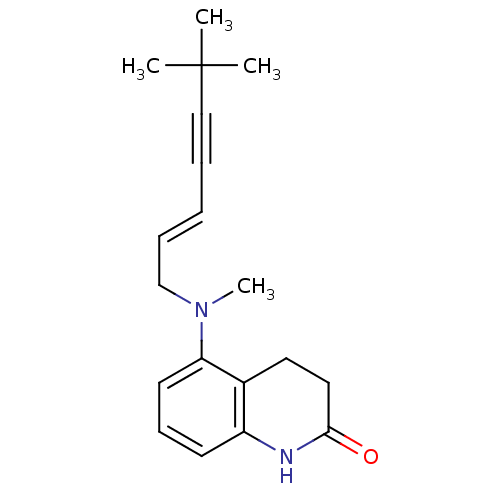 (5-[((E)-6,6-Dimethyl-hept-2-en-4-ynyl)-methyl-amin...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Nippon Roche Research Center Curated by ChEMBL | Assay Description Inhibitory of Candida albicans chitin synthase 1 | Bioorg Med Chem Lett 10: 1459-62 (2000) BindingDB Entry DOI: 10.7270/Q2R210MK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50222428 ((R)-4-(6,7-dimethoxyquinazolin-4-yl)-N,N-diethyl-2...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Pharma Corporation Curated by ChEMBL | Assay Description Inhibition of partially purified EGFR tyrosine kinase from human A431 cells | Bioorg Med Chem Lett 17: 5863-7 (2007) Article DOI: 10.1016/j.bmcl.2007.08.020 BindingDB Entry DOI: 10.7270/Q2H70FJ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B (Homo sapiens (Human)) | BDBM50495787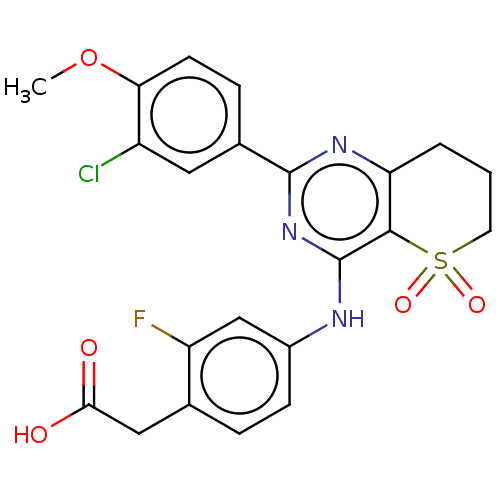 (CHEMBL3114956) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyushu University Curated by ChEMBL | Assay Description Inhibition of full-length human PDE4B2 assessed as cAMP hydrolysis preincubated for 20 mins followed by cAMP addition measured after 30 mins | Bioorg Med Chem Lett 24: 893-9 (2014) Article DOI: 10.1016/j.bmcl.2013.12.076 BindingDB Entry DOI: 10.7270/Q2GX4FHW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chitin synthase 1 (Candida albicans) | BDBM50089547 (5-[(6,6-Dimethyl-hepta-2,4-diynyl)-methyl-amino]-3...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Nippon Roche Research Center Curated by ChEMBL | Assay Description Inhibitory of Candida albicans chitin synthase 1 | Bioorg Med Chem Lett 10: 1459-62 (2000) BindingDB Entry DOI: 10.7270/Q2R210MK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-alpha serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM108425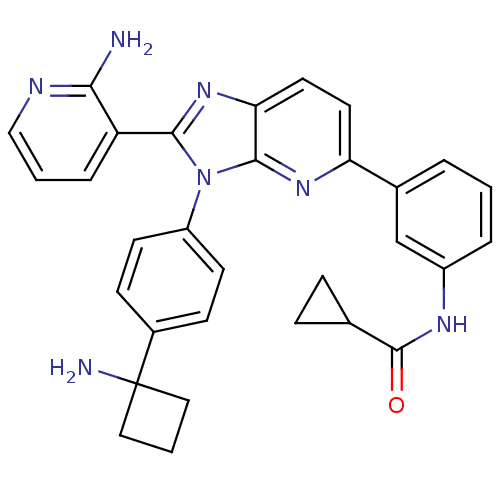 (US8609688, 3) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3.09 | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule, Inc. US Patent | Assay Description AKT1 activity was assayed using the GSK3-derived biotinylated peptide substrate, crosstide (biotin-GRPRTSSFAEG), and AlphaScreen (Amplified Luminesce... | US Patent US8962619 (2015) BindingDB Entry DOI: 10.7270/Q2W957W7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-alpha serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM108425 (US8609688, 3) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3.09 | n/a | n/a | n/a | n/a | 8.0 | 25 |
ArQule, Inc. US Patent | Assay Description AKT1 activity was assayed using the GSK3-derived biotinylated peptide substrate, crosstide (biotin-GRPRTSSFAEG), and AlphaScreen™ (Amplified Lum... | US Patent US8609688 (2013) BindingDB Entry DOI: 10.7270/Q2H41Q2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-alpha serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM108433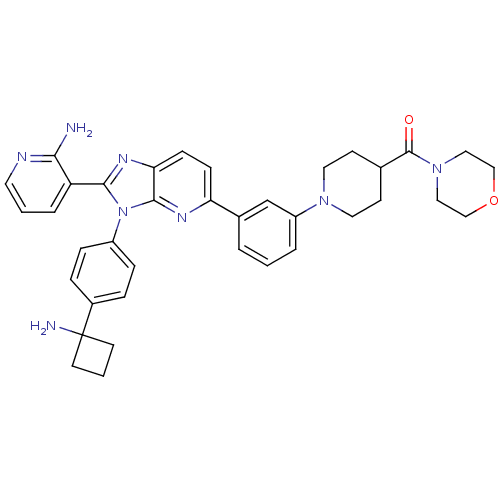 (US8609688, 11) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3.36 | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule, Inc. US Patent | Assay Description AKT1 activity was assayed using the GSK3-derived biotinylated peptide substrate, crosstide (biotin-GRPRTSSFAEG), and AlphaScreen (Amplified Luminesce... | US Patent US8962619 (2015) BindingDB Entry DOI: 10.7270/Q2W957W7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-alpha serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM108433 (US8609688, 11) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3.36 | n/a | n/a | n/a | n/a | 8.0 | 25 |
ArQule, Inc. US Patent | Assay Description AKT1 activity was assayed using the GSK3-derived biotinylated peptide substrate, crosstide (biotin-GRPRTSSFAEG), and AlphaScreen™ (Amplified Lum... | US Patent US8609688 (2013) BindingDB Entry DOI: 10.7270/Q2H41Q2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-alpha serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM108438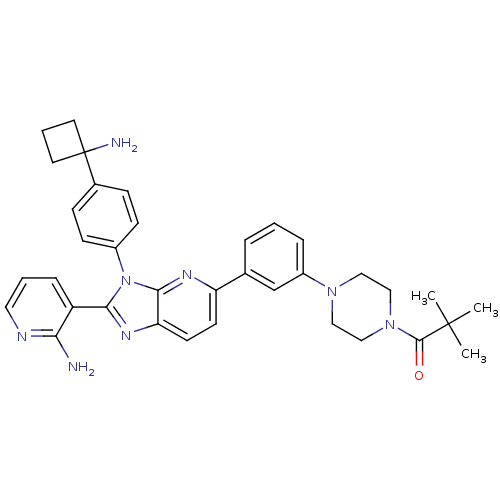 (US8609688, 16) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3.42 | n/a | n/a | n/a | n/a | 8.0 | 25 |
ArQule, Inc. US Patent | Assay Description AKT1 activity was assayed using the GSK3-derived biotinylated peptide substrate, crosstide (biotin-GRPRTSSFAEG), and AlphaScreen™ (Amplified Lum... | US Patent US8609688 (2013) BindingDB Entry DOI: 10.7270/Q2H41Q2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-alpha serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM108438 (US8609688, 16) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3.42 | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule, Inc. US Patent | Assay Description AKT1 activity was assayed using the GSK3-derived biotinylated peptide substrate, crosstide (biotin-GRPRTSSFAEG), and AlphaScreen (Amplified Luminesce... | US Patent US8962619 (2015) BindingDB Entry DOI: 10.7270/Q2W957W7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 1,3-beta-glucan synthase (Candida albicans (Yeast)) | BDBM50100334 (CHEMBL407924 | RO-09-3655 derivative) | UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Nippon Roche Research Center Curated by ChEMBL | Assay Description In vitro inhibitory activity against Beta glucan synthase | Bioorg Med Chem Lett 11: 1273-6 (2001) BindingDB Entry DOI: 10.7270/Q2CN74FQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-alpha serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM108441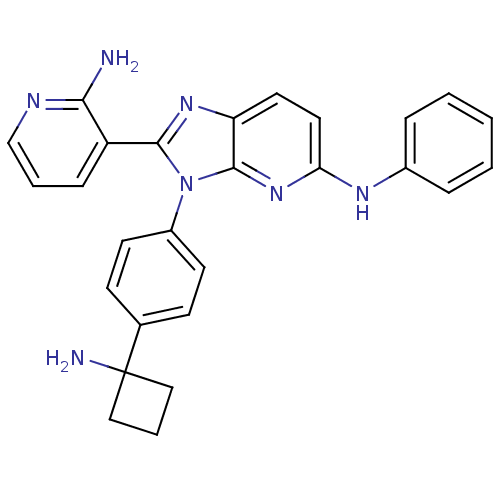 (US8609688, 19) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3.77 | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule, Inc. US Patent | Assay Description AKT1 activity was assayed using the GSK3-derived biotinylated peptide substrate, crosstide (biotin-GRPRTSSFAEG), and AlphaScreen (Amplified Luminesce... | US Patent US8962619 (2015) BindingDB Entry DOI: 10.7270/Q2W957W7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 328 total ) | Next | Last >> |