

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bcl-2-like protein 1 (Homo sapiens (Human)) | BDBM31406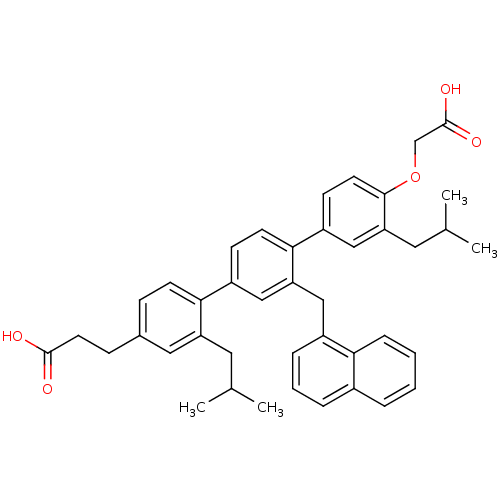 (terphenyl scaffold, 14) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 114 | -39.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Yale University | Assay Description Fluorescence polarization experiments were conducted on a Photon Technology International instrument using a 0.3 cm path length cuvette. Spectra were... | J Am Chem Soc 127: 10191-6 (2005) Article DOI: 10.1021/ja050122x BindingDB Entry DOI: 10.7270/Q2668BHF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM50091160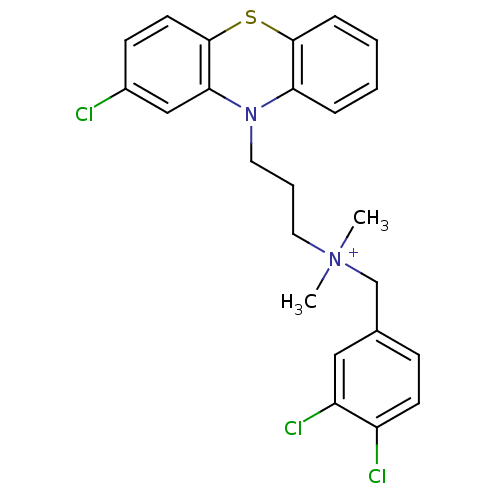 (CHEMBL106127 | [3-(2-Chloro-phenothiazin-10-yl)-pr...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibitory activity against Trypanosoma cruzi trypanothione reductase | J Med Chem 43: 3148-56 (2000) BindingDB Entry DOI: 10.7270/Q2R78FXC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM50091165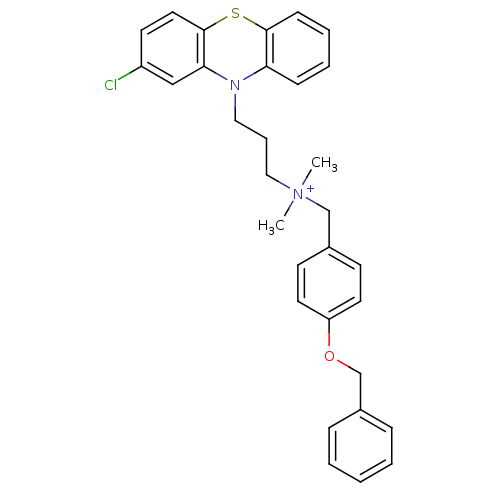 ((4-Benzyloxy-benzyl)-[3-(2-chloro-phenothiazin-10-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibitory activity against Trypanosoma cruzi trypanothione reductase | J Med Chem 43: 3148-56 (2000) BindingDB Entry DOI: 10.7270/Q2R78FXC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM50091158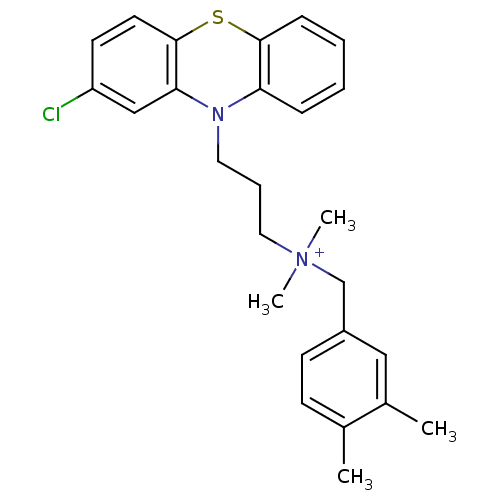 (CHEMBL322826 | [3-(2-Chloro-phenothiazin-10-yl)-pr...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibitory activity against Trypanosoma cruzi trypanothione reductase | J Med Chem 43: 3148-56 (2000) BindingDB Entry DOI: 10.7270/Q2R78FXC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM50091148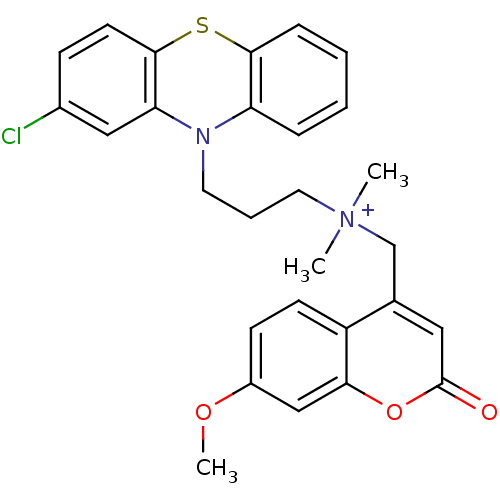 (CHEMBL106769 | [3-(2-Chloro-phenothiazin-10-yl)-pr...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibitory activity against Trypanosoma cruzi trypanothione reductase | J Med Chem 43: 3148-56 (2000) BindingDB Entry DOI: 10.7270/Q2R78FXC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM50091162 ((4-tert-Butyl-benzyl)-[3-(2-chloro-phenothiazin-10...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 680 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibitory activity against Trypanosoma cruzi trypanothione reductase | J Med Chem 43: 3148-56 (2000) BindingDB Entry DOI: 10.7270/Q2R78FXC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM50091163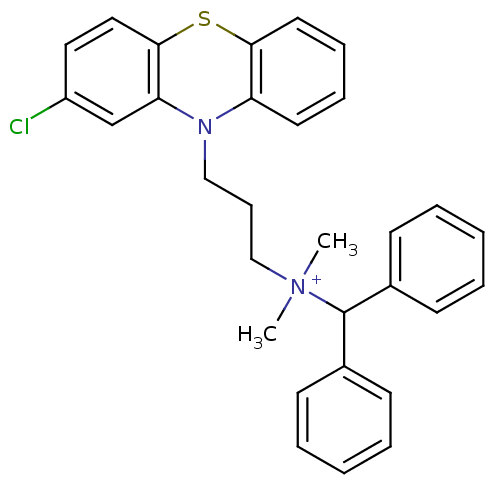 (Benzhydryl-[3-(2-chloro-phenothiazin-10-yl)-propyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 710 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibitory activity against Trypanosoma cruzi trypanothione reductase | J Med Chem 43: 3148-56 (2000) BindingDB Entry DOI: 10.7270/Q2R78FXC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM50091154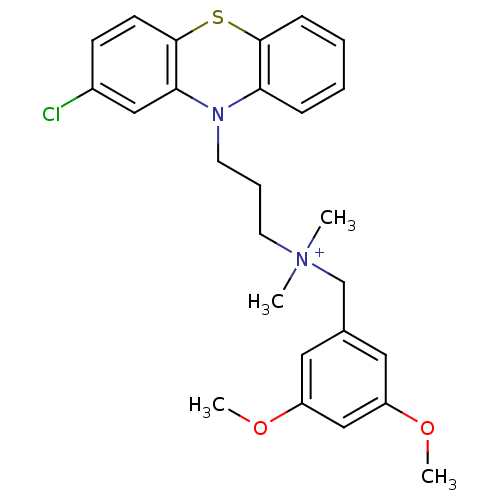 (CHEMBL106901 | [3-(2-Chloro-phenothiazin-10-yl)-pr...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 770 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibitory activity against Trypanosoma cruzi trypanothione reductase | J Med Chem 43: 3148-56 (2000) BindingDB Entry DOI: 10.7270/Q2R78FXC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bcl-2-like protein 1 (Homo sapiens (Human)) | BDBM31373 (CHEMBL28137 | terephthalamide scaffold, 9) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 780 | -34.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Yale University | Assay Description Fluorescence polarization experiments were conducted on a Photon Technology International instrument using a 0.3 cm path length cuvette. Spectra were... | J Am Chem Soc 127: 5463-8 (2005) Article DOI: 10.1021/ja0446404 BindingDB Entry DOI: 10.7270/Q29Z937V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bcl-2-like protein 1 (Homo sapiens (Human)) | BDBM31373 (CHEMBL28137 | terephthalamide scaffold, 9) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 781 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University Curated by ChEMBL | Assay Description Binding affinity for Bcl-xL was assessed by a fluorescence polarizationassay using a fluorescently labeled 16-mer Bak-peptide | Bioorg Med Chem Lett 14: 1375-9 (2004) Article DOI: 10.1016/j.bmcl.2003.09.096 BindingDB Entry DOI: 10.7270/Q2D799V3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bcl-2-like protein 1 (Homo sapiens (Human)) | BDBM31374 (CHEMBL286715 | terephthalamide scaffold, 10) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 839 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University Curated by ChEMBL | Assay Description Binding affinity for Bcl-xL was assessed by a fluorescence polarizationassay using a fluorescently labeled 16-mer Bak-peptide | Bioorg Med Chem Lett 14: 1375-9 (2004) Article DOI: 10.1016/j.bmcl.2003.09.096 BindingDB Entry DOI: 10.7270/Q2D799V3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bcl-2-like protein 1 (Homo sapiens (Human)) | BDBM31374 (CHEMBL286715 | terephthalamide scaffold, 10) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 840 | -34.7 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Yale University | Assay Description Fluorescence polarization experiments were conducted on a Photon Technology International instrument using a 0.3 cm path length cuvette. Spectra were... | J Am Chem Soc 127: 5463-8 (2005) Article DOI: 10.1021/ja0446404 BindingDB Entry DOI: 10.7270/Q29Z937V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Mus musculus (mouse)) | BDBM50296704 (3-{4-[2-Amino-6-(4-benzyl-piperazin-1-yl)-pyrimidi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of mouse liver microsome 11betaHSD1 reductase activity expressed in HEK293 cells by scintillation proximity assay | Bioorg Med Chem 17: 5722-32 (2009) Article DOI: 10.1016/j.bmc.2009.05.082 BindingDB Entry DOI: 10.7270/Q2571CZ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bcl-2-like protein 1 (Homo sapiens (Human)) | BDBM31381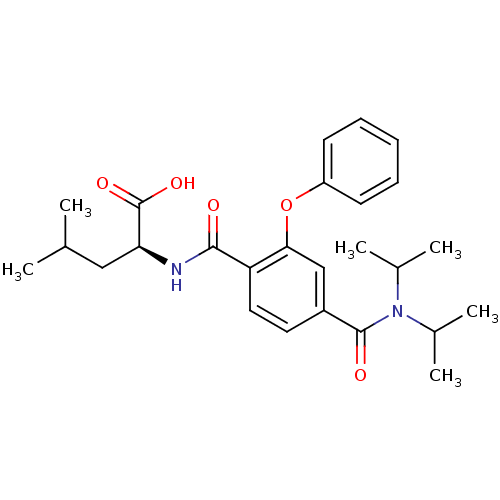 (CHEMBL282624 | terephthalamide scaffold, 29) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.01E+3 | -34.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Yale University | Assay Description Fluorescence polarization experiments were conducted on a Photon Technology International instrument using a 0.3 cm path length cuvette. Spectra were... | J Am Chem Soc 127: 5463-8 (2005) Article DOI: 10.1021/ja0446404 BindingDB Entry DOI: 10.7270/Q29Z937V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bcl-2-like protein 1 (Homo sapiens (Human)) | BDBM31381 (CHEMBL282624 | terephthalamide scaffold, 29) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.01E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University Curated by ChEMBL | Assay Description Binding affinity for Bcl-xL was assessed by a fluorescence polarizationassay using a fluorescently labeled 16-mer Bak-peptide | Bioorg Med Chem Lett 14: 1375-9 (2004) Article DOI: 10.1016/j.bmcl.2003.09.096 BindingDB Entry DOI: 10.7270/Q2D799V3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM50019879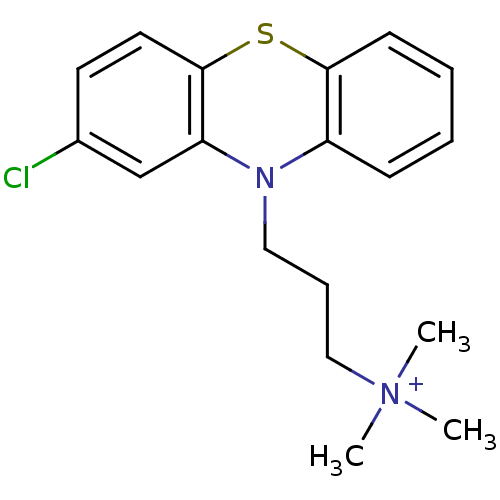 (CHEMBL279905 | [3-(2-Chloro-phenothiazin-10-yl)-pr...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibitory activity against Trypanosoma cruzi trypanothione reductase | J Med Chem 43: 3148-56 (2000) BindingDB Entry DOI: 10.7270/Q2R78FXC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM50091157 ((2-Adamantan-1-yl-2-oxo-ethyl)-[3-(2-chloro-phenot...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.23E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibitory activity against Trypanosoma cruzi trypanothione reductase | J Med Chem 43: 3148-56 (2000) BindingDB Entry DOI: 10.7270/Q2R78FXC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM50091153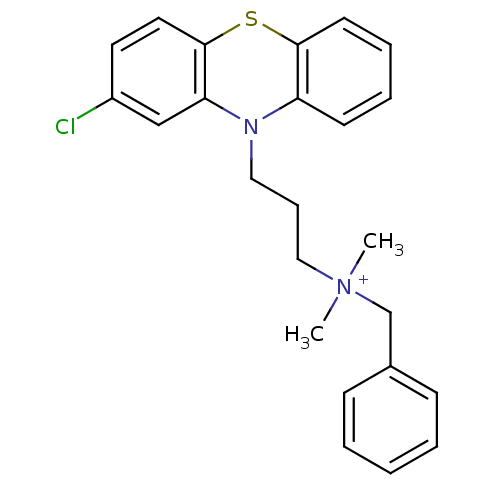 (Benzyl-[3-(2-chloro-phenothiazin-10-yl)-propyl]-di...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | PubMed | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibitory activity against Trypanosoma cruzi trypanothione reductase | J Med Chem 43: 3148-56 (2000) BindingDB Entry DOI: 10.7270/Q2R78FXC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM50091159 (CHEMBL326458 | [2-(4-Chloro-3-methyl-phenyl)-2-oxo...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 1.43E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibitory activity against Trypanosoma cruzi trypanothione reductase | J Med Chem 43: 3148-56 (2000) BindingDB Entry DOI: 10.7270/Q2R78FXC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM50091156 ((4-Bromo-benzyl)-[3-(2-chloro-phenothiazin-10-yl)-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibitory activity against Trypanosoma cruzi trypanothione reductase | J Med Chem 43: 3148-56 (2000) BindingDB Entry DOI: 10.7270/Q2R78FXC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM50091155 (CHEMBL323271 | [3-(2-Chloro-phenothiazin-10-yl)-pr...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.55E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibitory activity against Trypanosoma cruzi trypanothione reductase | J Med Chem 43: 3148-56 (2000) BindingDB Entry DOI: 10.7270/Q2R78FXC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM50091164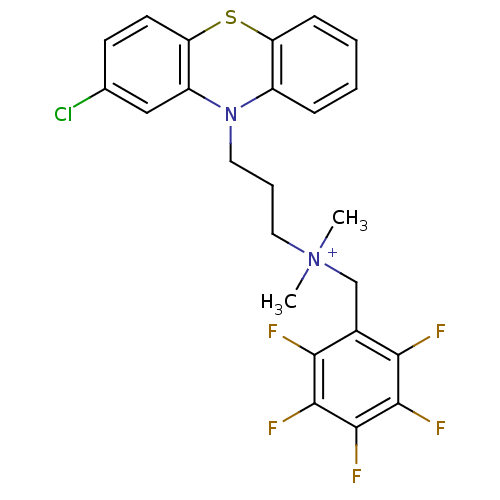 (CHEMBL106236 | [3-(2-Chloro-phenothiazin-10-yl)-pr...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.67E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibitory activity against Trypanosoma cruzi trypanothione reductase | J Med Chem 43: 3148-56 (2000) BindingDB Entry DOI: 10.7270/Q2R78FXC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM50091149 ((4-Chloro-benzyl)-[3-(2-chloro-phenothiazin-10-yl)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibitory activity against Trypanosoma cruzi trypanothione reductase | J Med Chem 43: 3148-56 (2000) BindingDB Entry DOI: 10.7270/Q2R78FXC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bcl-2-like protein 1 (Homo sapiens (Human)) | BDBM31379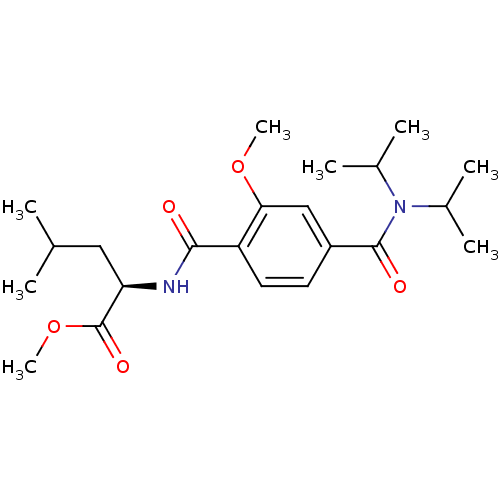 (CHEMBL28872 | terephthalamide scaffold, 27) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.79E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University Curated by ChEMBL | Assay Description Binding affinity for Bcl-xL was assessed by a fluorescence polarizationassay using a fluorescently labeled 16-mer Bak-peptide | Bioorg Med Chem Lett 14: 1375-9 (2004) Article DOI: 10.1016/j.bmcl.2003.09.096 BindingDB Entry DOI: 10.7270/Q2D799V3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bcl-2-like protein 1 (Homo sapiens (Human)) | BDBM31379 (CHEMBL28872 | terephthalamide scaffold, 27) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.79E+3 | -32.8 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Yale University | Assay Description Fluorescence polarization experiments were conducted on a Photon Technology International instrument using a 0.3 cm path length cuvette. Spectra were... | J Am Chem Soc 127: 5463-8 (2005) Article DOI: 10.1021/ja0446404 BindingDB Entry DOI: 10.7270/Q29Z937V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM50091150 (CHEMBL323540 | [3-(2-Chloro-phenothiazin-10-yl)-pr...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibitory activity against Trypanosoma cruzi trypanothione reductase | J Med Chem 43: 3148-56 (2000) BindingDB Entry DOI: 10.7270/Q2R78FXC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bcl-2-like protein 1 (Homo sapiens (Human)) | BDBM31408 (terphenyl scaffold, 16) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.82E+3 | -32.8 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Yale University | Assay Description Fluorescence polarization experiments were conducted on a Photon Technology International instrument using a 0.3 cm path length cuvette. Spectra were... | J Am Chem Soc 127: 10191-6 (2005) Article DOI: 10.1021/ja050122x BindingDB Entry DOI: 10.7270/Q2668BHF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bcl-2-like protein 1 (Homo sapiens (Human)) | BDBM31378 (CHEMBL284686 | terephthalamide scaffold, 26) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.85E+3 | -32.7 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Yale University | Assay Description Fluorescence polarization experiments were conducted on a Photon Technology International instrument using a 0.3 cm path length cuvette. Spectra were... | J Am Chem Soc 127: 5463-8 (2005) Article DOI: 10.1021/ja0446404 BindingDB Entry DOI: 10.7270/Q29Z937V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bcl-2-like protein 1 (Homo sapiens (Human)) | BDBM31378 (CHEMBL284686 | terephthalamide scaffold, 26) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.85E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University Curated by ChEMBL | Assay Description Binding affinity for Bcl-xL was assessed by a fluorescence polarizationassay using a fluorescently labeled 16-mer Bak-peptide | Bioorg Med Chem Lett 14: 1375-9 (2004) Article DOI: 10.1016/j.bmcl.2003.09.096 BindingDB Entry DOI: 10.7270/Q2D799V3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bcl-2-like protein 1 (Homo sapiens (Human)) | BDBM31407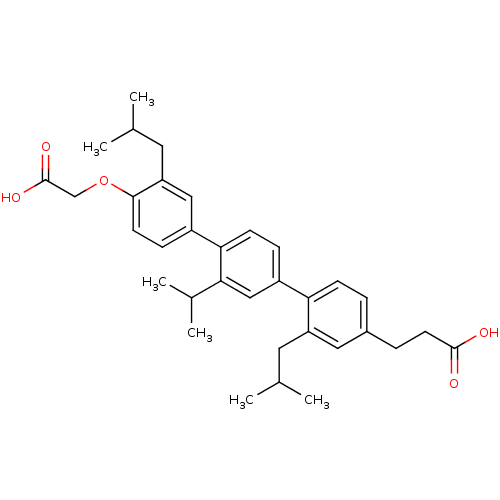 (terphenyl scaffold, 15) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.09E+3 | -32.4 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Yale University | Assay Description Fluorescence polarization experiments were conducted on a Photon Technology International instrument using a 0.3 cm path length cuvette. Spectra were... | J Am Chem Soc 127: 10191-6 (2005) Article DOI: 10.1021/ja050122x BindingDB Entry DOI: 10.7270/Q2668BHF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM50091152 (CHEMBL106108 | [3-(2-Chloro-phenothiazin-10-yl)-pr...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibitory activity against Trypanosoma cruzi trypanothione reductase | J Med Chem 43: 3148-56 (2000) BindingDB Entry DOI: 10.7270/Q2R78FXC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bcl-2-like protein 1 (Homo sapiens (Human)) | BDBM31380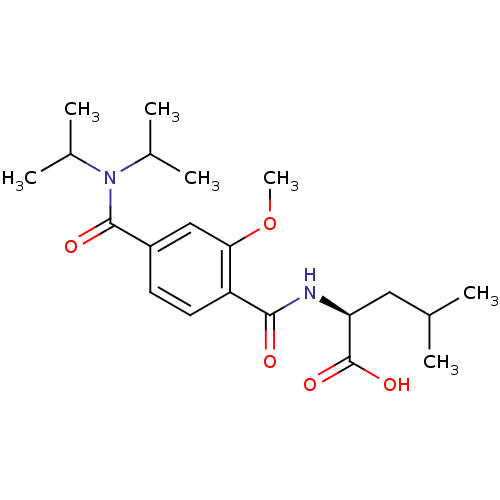 (terephthalamide scaffold, 28) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.31E+3 | -32.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Yale University | Assay Description Fluorescence polarization experiments were conducted on a Photon Technology International instrument using a 0.3 cm path length cuvette. Spectra were... | J Am Chem Soc 127: 5463-8 (2005) Article DOI: 10.1021/ja0446404 BindingDB Entry DOI: 10.7270/Q29Z937V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bcl-2-like protein 1 (Homo sapiens (Human)) | BDBM31412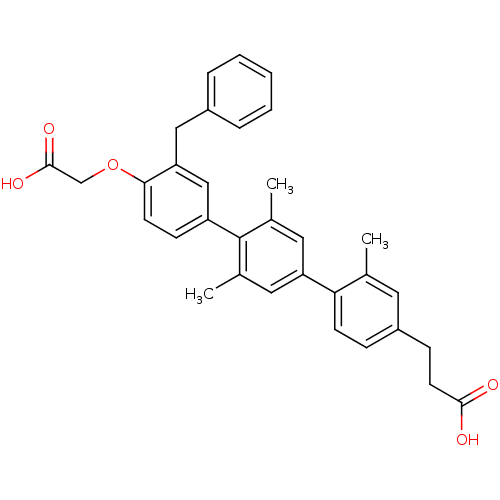 (terphenyl scaffold, 20) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.34E+3 | -32.1 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Yale University | Assay Description Fluorescence polarization experiments were conducted on a Photon Technology International instrument using a 0.3 cm path length cuvette. Spectra were... | J Am Chem Soc 127: 10191-6 (2005) Article DOI: 10.1021/ja050122x BindingDB Entry DOI: 10.7270/Q2668BHF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM50091151 ((3-Chloro-benzyl)-[3-(2-chloro-phenothiazin-10-yl)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibitory activity against Trypanosoma cruzi trypanothione reductase | J Med Chem 43: 3148-56 (2000) BindingDB Entry DOI: 10.7270/Q2R78FXC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bcl-2-like protein 1 (Homo sapiens (Human)) | BDBM31390 (terephthalamide scaffold, 37) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.44E+3 | -32.0 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Yale University | Assay Description Fluorescence polarization experiments were conducted on a Photon Technology International instrument using a 0.3 cm path length cuvette. Spectra were... | J Am Chem Soc 127: 5463-8 (2005) Article DOI: 10.1021/ja0446404 BindingDB Entry DOI: 10.7270/Q29Z937V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bcl-2-like protein 1 (Homo sapiens (Human)) | BDBM31409 (terphenyl scaffold, 17) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.50E+3 | -32.0 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Yale University | Assay Description Fluorescence polarization experiments were conducted on a Photon Technology International instrument using a 0.3 cm path length cuvette. Spectra were... | J Am Chem Soc 127: 10191-6 (2005) Article DOI: 10.1021/ja050122x BindingDB Entry DOI: 10.7270/Q2668BHF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bcl-2-like protein 1 (Homo sapiens (Human)) | BDBM31411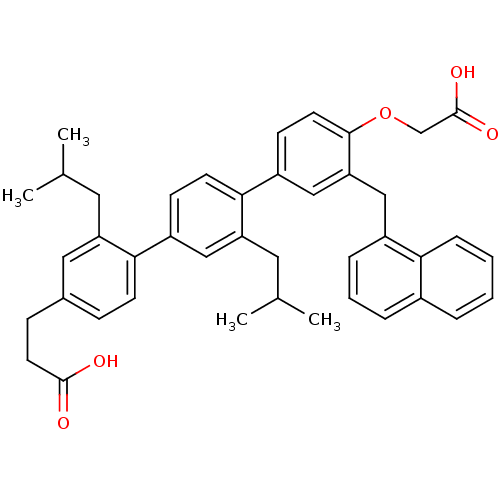 (terphenyl scaffold, 19) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.70E+3 | -31.8 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Yale University | Assay Description Fluorescence polarization experiments were conducted on a Photon Technology International instrument using a 0.3 cm path length cuvette. Spectra were... | J Am Chem Soc 127: 10191-6 (2005) Article DOI: 10.1021/ja050122x BindingDB Entry DOI: 10.7270/Q2668BHF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bcl-2-like protein 1 (Homo sapiens (Human)) | BDBM31410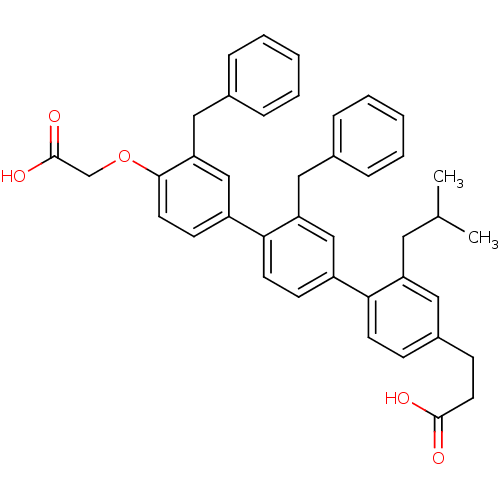 (terphenyl scaffold, 18) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.73E+3 | -31.8 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Yale University | Assay Description Fluorescence polarization experiments were conducted on a Photon Technology International instrument using a 0.3 cm path length cuvette. Spectra were... | J Am Chem Soc 127: 10191-6 (2005) Article DOI: 10.1021/ja050122x BindingDB Entry DOI: 10.7270/Q2668BHF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM50369578 (CHEMBL239370) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.98E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibitory activity against Trypanosoma cruzi trypanothione reductase | J Med Chem 43: 3148-56 (2000) BindingDB Entry DOI: 10.7270/Q2R78FXC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bcl-2-like protein 1 (Homo sapiens (Human)) | BDBM31387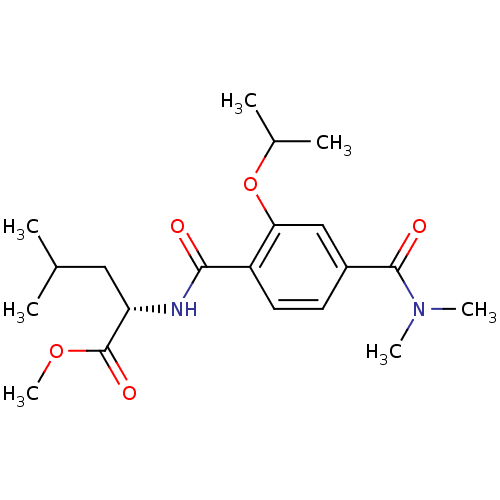 (terephthalamide scaffold, 34) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3.14E+3 | -31.4 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Yale University | Assay Description Fluorescence polarization experiments were conducted on a Photon Technology International instrument using a 0.3 cm path length cuvette. Spectra were... | J Am Chem Soc 127: 5463-8 (2005) Article DOI: 10.1021/ja0446404 BindingDB Entry DOI: 10.7270/Q29Z937V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bcl-2-like protein 1 (Homo sapiens (Human)) | BDBM31420 (terphenyl scaffold, 28) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3.27E+3 | -31.3 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Yale University | Assay Description Fluorescence polarization experiments were conducted on a Photon Technology International instrument using a 0.3 cm path length cuvette. Spectra were... | J Am Chem Soc 127: 10191-6 (2005) Article DOI: 10.1021/ja050122x BindingDB Entry DOI: 10.7270/Q2668BHF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bcl-2-like protein 1 (Homo sapiens (Human)) | BDBM31385 (CHEMBL281240 | terephthalamide scaffold, 17) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 3.31E+3 | -31.3 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Yale University | Assay Description Fluorescence polarization experiments were conducted on a Photon Technology International instrument using a 0.3 cm path length cuvette. Spectra were... | J Am Chem Soc 127: 5463-8 (2005) Article DOI: 10.1021/ja0446404 BindingDB Entry DOI: 10.7270/Q29Z937V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bcl-2-like protein 1 (Homo sapiens (Human)) | BDBM31385 (CHEMBL281240 | terephthalamide scaffold, 17) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 3.31E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University Curated by ChEMBL | Assay Description Binding affinity for Bcl-xL was assessed by a fluorescence polarizationassay using a fluorescently labeled 16-mer Bak-peptide | Bioorg Med Chem Lett 14: 1375-9 (2004) Article DOI: 10.1016/j.bmcl.2003.09.096 BindingDB Entry DOI: 10.7270/Q2D799V3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bcl-2-like protein 1 (Homo sapiens (Human)) | BDBM31419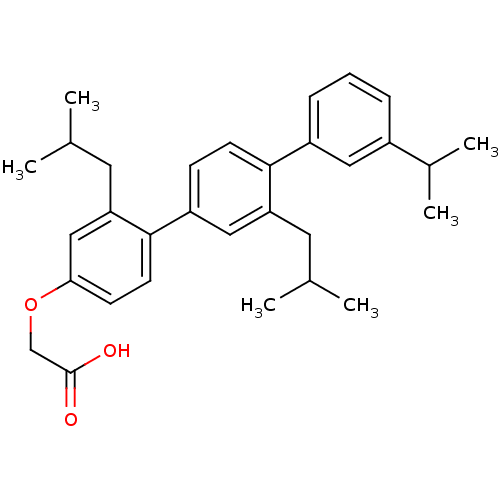 (terphenyl scaffold, 27) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 4.82E+3 | -30.3 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Yale University | Assay Description Fluorescence polarization experiments were conducted on a Photon Technology International instrument using a 0.3 cm path length cuvette. Spectra were... | J Am Chem Soc 127: 10191-6 (2005) Article DOI: 10.1021/ja050122x BindingDB Entry DOI: 10.7270/Q2668BHF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bcl-2-like protein 1 (Homo sapiens (Human)) | BDBM31376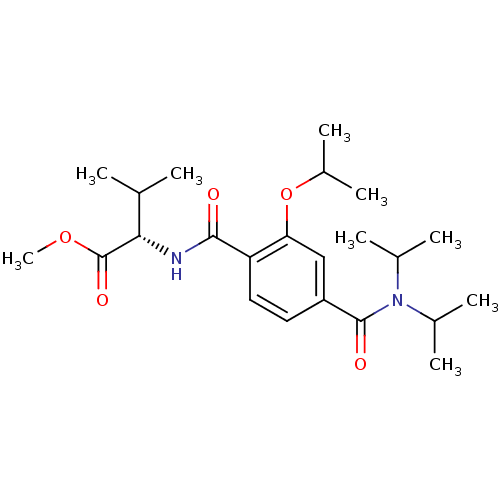 (CHEMBL433422 | terephthalamide scaffold, 24) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.85E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University Curated by ChEMBL | Assay Description Binding affinity for Bcl-xL was assessed by a fluorescence polarizationassay using a fluorescently labeled 16-mer Bak-peptide | Bioorg Med Chem Lett 14: 1375-9 (2004) Article DOI: 10.1016/j.bmcl.2003.09.096 BindingDB Entry DOI: 10.7270/Q2D799V3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bcl-2-like protein 1 (Homo sapiens (Human)) | BDBM31376 (CHEMBL433422 | terephthalamide scaffold, 24) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.85E+3 | -29.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Yale University | Assay Description Fluorescence polarization experiments were conducted on a Photon Technology International instrument using a 0.3 cm path length cuvette. Spectra were... | J Am Chem Soc 127: 5463-8 (2005) Article DOI: 10.1021/ja0446404 BindingDB Entry DOI: 10.7270/Q29Z937V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bcl-2-like protein 1 (Homo sapiens (Human)) | BDBM31421 (CHEMBL43232 | terphenyl scaffold, 29) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.88E+3 | -29.5 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Yale University | Assay Description Fluorescence polarization experiments were conducted on a Photon Technology International instrument using a 0.3 cm path length cuvette. Spectra were... | J Am Chem Soc 127: 10191-6 (2005) Article DOI: 10.1021/ja050122x BindingDB Entry DOI: 10.7270/Q2668BHF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bcl-2-like protein 1 (Homo sapiens (Human)) | BDBM31383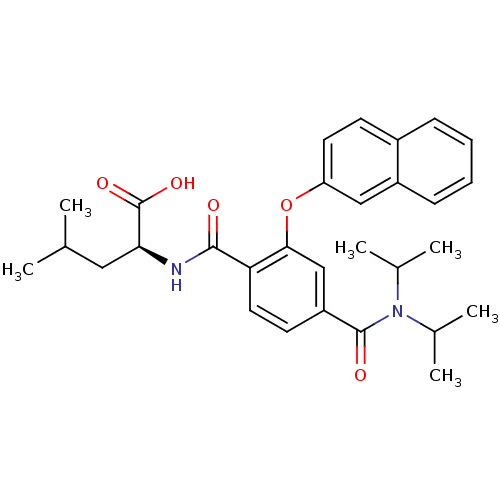 (terephthalamide scaffold, 31) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 7.63E+3 | -29.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Yale University | Assay Description Fluorescence polarization experiments were conducted on a Photon Technology International instrument using a 0.3 cm path length cuvette. Spectra were... | J Am Chem Soc 127: 5463-8 (2005) Article DOI: 10.1021/ja0446404 BindingDB Entry DOI: 10.7270/Q29Z937V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bcl-2-like protein 1 (Homo sapiens (Human)) | BDBM50141117 ((S)-2-[4-Diisopropylcarbamoyl-2-(naphthalen-1-ylox...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.63E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University Curated by ChEMBL | Assay Description Binding affinity for Bcl-xL was assessed by a fluorescence polarizationassay using a fluorescently labeled 16-mer Bak-peptide | Bioorg Med Chem Lett 14: 1375-9 (2004) Article DOI: 10.1016/j.bmcl.2003.09.096 BindingDB Entry DOI: 10.7270/Q2D799V3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bcl-2-like protein 1 (Homo sapiens (Human)) | BDBM31386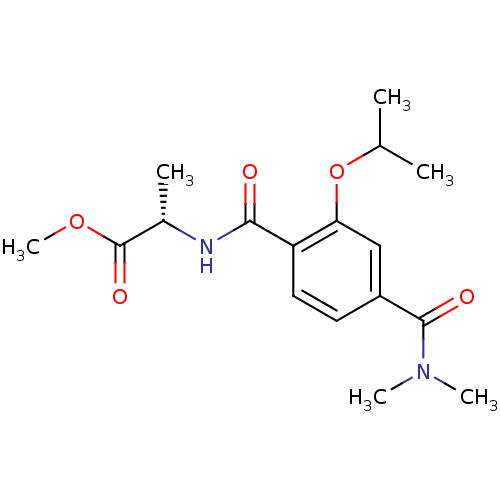 (terephthalamide scaffold, 33) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 8.34E+3 | -29.0 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Yale University | Assay Description Fluorescence polarization experiments were conducted on a Photon Technology International instrument using a 0.3 cm path length cuvette. Spectra were... | J Am Chem Soc 127: 5463-8 (2005) Article DOI: 10.1021/ja0446404 BindingDB Entry DOI: 10.7270/Q29Z937V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1189 total ) | Next | Last >> |