

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial (Homo sapiens (Human)) | BDBM50432208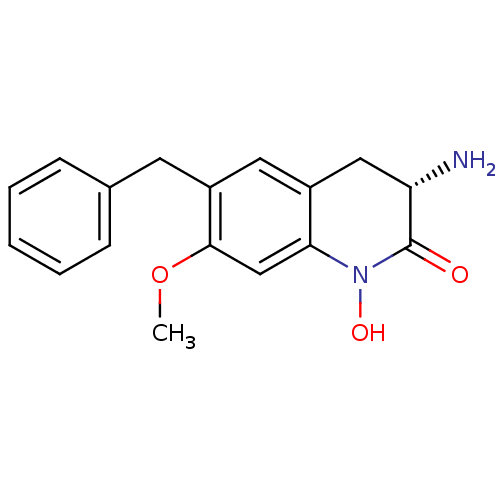 (CHEMBL2347110) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human KAT2 using L-kynurenine as substrate after 15 to 20 hrs by UV-visible spectra analysis | Bioorg Med Chem Lett 23: 1961-6 (2013) Article DOI: 10.1016/j.bmcl.2013.02.039 BindingDB Entry DOI: 10.7270/Q2N87C48 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial (Homo sapiens (Human)) | BDBM50426340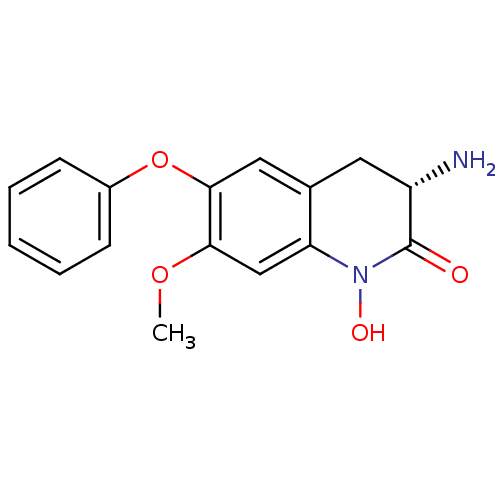 (CHEMBL2321943) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Irreversible inhibition of human KAT2 using L-kynurenine as substrate measured every 5 mins over 16 hrs by SpectraMax plate reader analysis | ACS Med Chem Lett 4: 37-40 (2013) Article DOI: 10.1021/ml300237v BindingDB Entry DOI: 10.7270/Q2KH0PNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial (Homo sapiens (Human)) | BDBM107730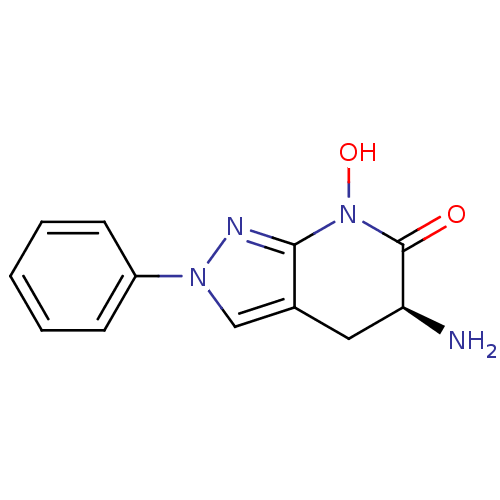 (CHEMBL2347108 | US8933095, 14) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human KAT2 using L-kynurenine as substrate after 15 to 20 hrs by UV-visible spectra analysis | Bioorg Med Chem Lett 23: 1961-6 (2013) Article DOI: 10.1016/j.bmcl.2013.02.039 BindingDB Entry DOI: 10.7270/Q2N87C48 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial (Homo sapiens (Human)) | BDBM50426341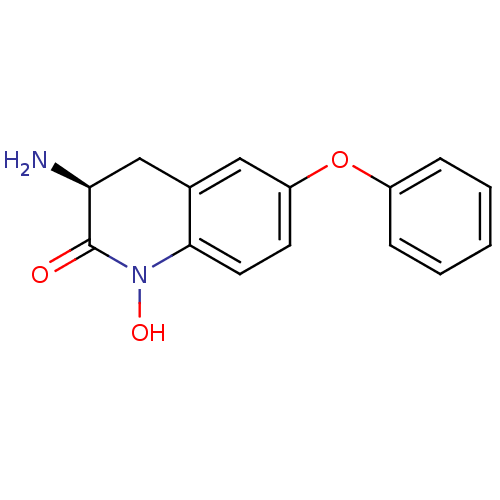 (CHEMBL2321944) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Irreversible inhibition of human KAT2 using L-kynurenine as substrate measured every 5 mins over 16 hrs by SpectraMax plate reader analysis | ACS Med Chem Lett 4: 37-40 (2013) Article DOI: 10.1021/ml300237v BindingDB Entry DOI: 10.7270/Q2KH0PNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial (Homo sapiens (Human)) | BDBM50386310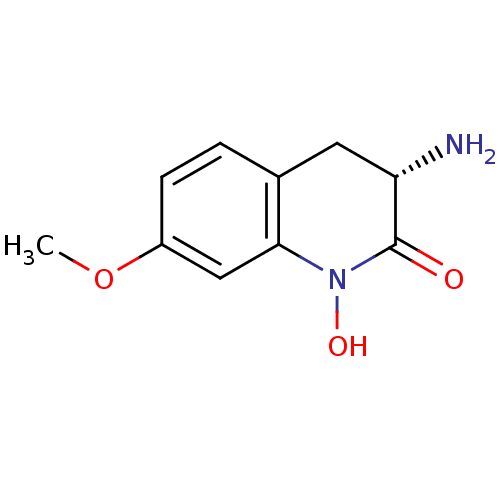 (CHEMBL2049092) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Irreversible inhibition of human KAT2 using L-kynurenine as substrate measured every 5 mins over 16 hrs by SpectraMax plate reader analysis | ACS Med Chem Lett 4: 37-40 (2013) Article DOI: 10.1021/ml300237v BindingDB Entry DOI: 10.7270/Q2KH0PNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial (Homo sapiens (Human)) | BDBM107747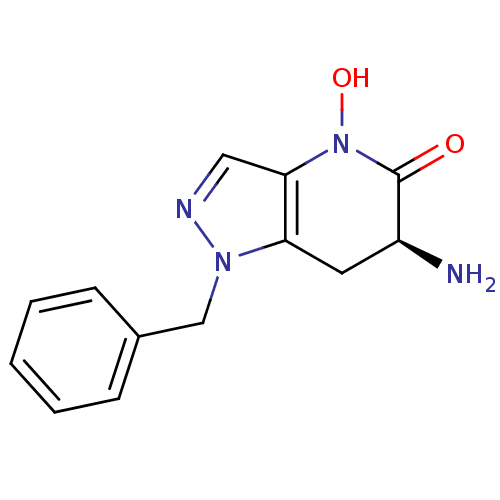 (CHEMBL2347115 | US8933095, 4) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human KAT2 using L-kynurenine as substrate after 15 to 20 hrs by UV-visible spectra analysis | Bioorg Med Chem Lett 23: 1961-6 (2013) Article DOI: 10.1016/j.bmcl.2013.02.039 BindingDB Entry DOI: 10.7270/Q2N87C48 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial (Homo sapiens (Human)) | BDBM50386292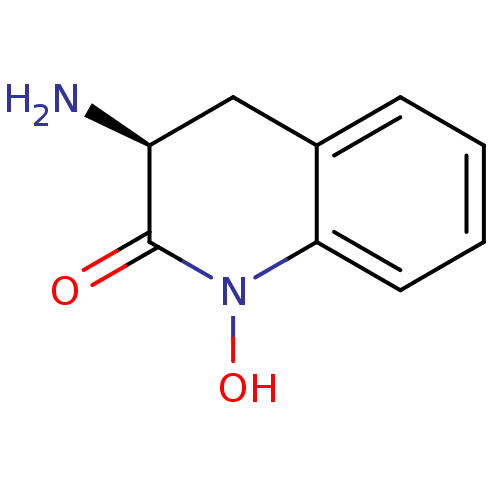 (CHEMBL2047851) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Irreversible inhibition of human KAT2 using L-kynurenine as substrate measured every 5 mins over 16 hrs by SpectraMax plate reader analysis | ACS Med Chem Lett 4: 37-40 (2013) Article DOI: 10.1021/ml300237v BindingDB Entry DOI: 10.7270/Q2KH0PNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial (Homo sapiens (Human)) | BDBM50386292 (CHEMBL2047851) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human KAT2 using L-kynurenine as substrate after 15 to 20 hrs by UV-visible spectra analysis | Bioorg Med Chem Lett 23: 1961-6 (2013) Article DOI: 10.1016/j.bmcl.2013.02.039 BindingDB Entry DOI: 10.7270/Q2N87C48 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial (Homo sapiens (Human)) | BDBM107738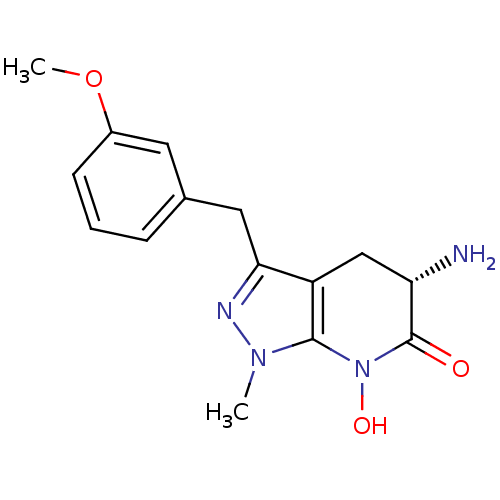 (CHEMBL2347113 | US8933095, 22) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human KAT2 using L-kynurenine as substrate after 15 to 20 hrs by UV-visible spectra analysis | Bioorg Med Chem Lett 23: 1961-6 (2013) Article DOI: 10.1016/j.bmcl.2013.02.039 BindingDB Entry DOI: 10.7270/Q2N87C48 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial (Homo sapiens (Human)) | BDBM107746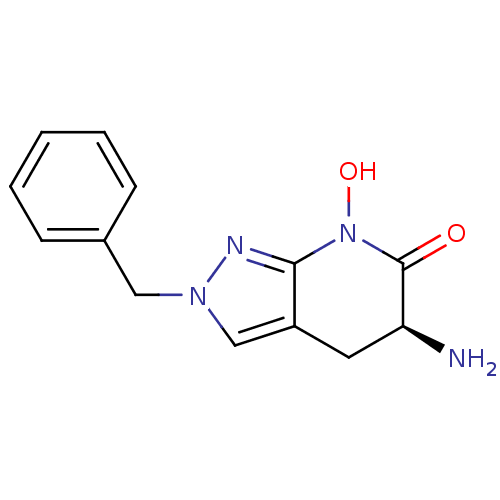 (CHEMBL2347107 | US8933095, 1) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human KAT2 using L-kynurenine as substrate after 15 to 20 hrs by UV-visible spectra analysis | Bioorg Med Chem Lett 23: 1961-6 (2013) Article DOI: 10.1016/j.bmcl.2013.02.039 BindingDB Entry DOI: 10.7270/Q2N87C48 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial (Homo sapiens (Human)) | BDBM107720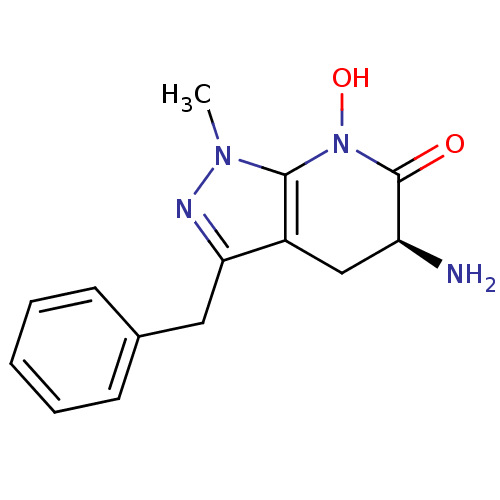 (CHEMBL2347112 | US8598200, 2) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human KAT2 using L-kynurenine as substrate after 15 to 20 hrs by UV-visible spectra analysis | Bioorg Med Chem Lett 23: 1961-6 (2013) Article DOI: 10.1016/j.bmcl.2013.02.039 BindingDB Entry DOI: 10.7270/Q2N87C48 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial (Homo sapiens (Human)) | BDBM50432200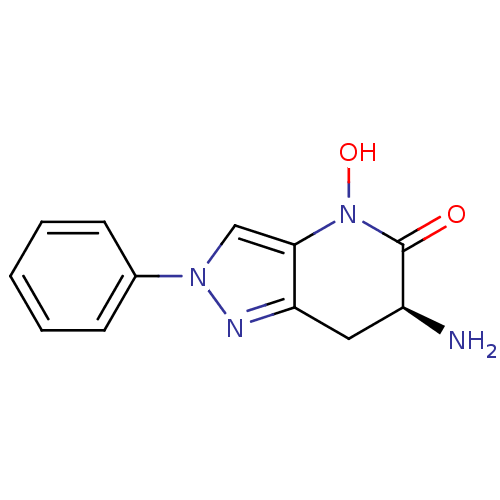 (CHEMBL2347109 | US8933095, 16) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 41 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human KAT2 using L-kynurenine as substrate after 15 to 20 hrs by UV-visible spectra analysis | Bioorg Med Chem Lett 23: 1961-6 (2013) Article DOI: 10.1016/j.bmcl.2013.02.039 BindingDB Entry DOI: 10.7270/Q2N87C48 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial (Homo sapiens (Human)) | BDBM107722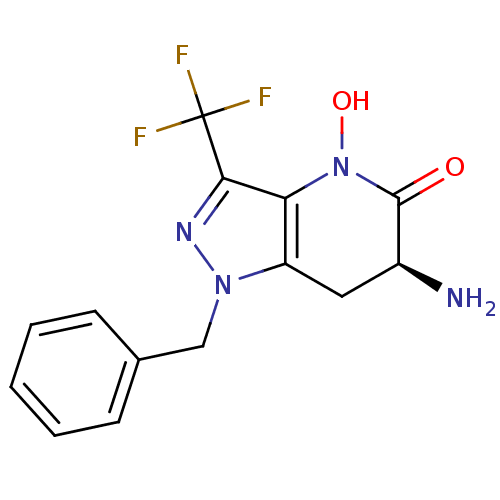 (CHEMBL2347114 | US8933095, 5) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 48 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human KAT2 using L-kynurenine as substrate after 15 to 20 hrs by UV-visible spectra analysis | Bioorg Med Chem Lett 23: 1961-6 (2013) Article DOI: 10.1016/j.bmcl.2013.02.039 BindingDB Entry DOI: 10.7270/Q2N87C48 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial (Homo sapiens (Human)) | BDBM273135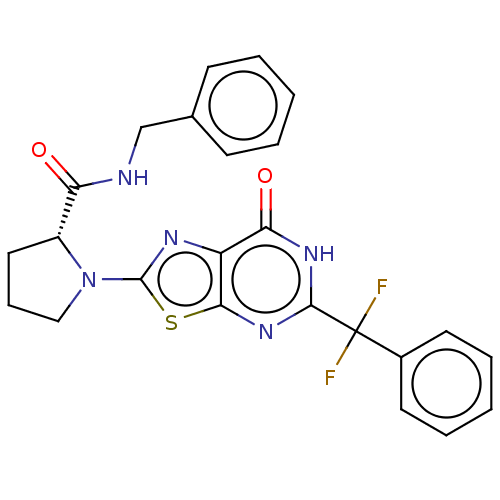 ((R)-N-benzyl-1-{5-[difluoro(phenyl)methyl]-7-oxo-6...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | 8.0 | n/a |
MITSUBISHI TANABE PHARMA CORPORATION US Patent | Assay Description The inhibitory action of the test compound on human recombinant KAT-II was determined by the following method.To a reaction mixture (45 μL) cont... | US Patent US10065972 (2018) BindingDB Entry DOI: 10.7270/Q23X88P5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial (Homo sapiens (Human)) | BDBM273091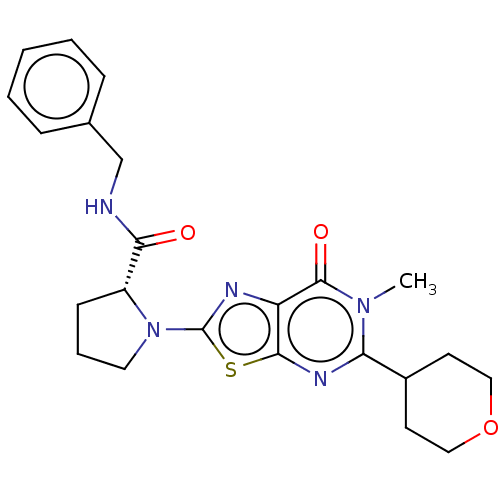 ((R)-N-benzyl-1-[6-methyl-7-oxo-5-(tetrahydro-2H-py...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB US Patent | n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | 8.0 | n/a |
MITSUBISHI TANABE PHARMA CORPORATION US Patent | Assay Description The inhibitory action of the test compound on human recombinant KAT-II was determined by the following method.To a reaction mixture (45 μL) cont... | US Patent US10065972 (2018) BindingDB Entry DOI: 10.7270/Q23X88P5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial (Homo sapiens (Human)) | BDBM273115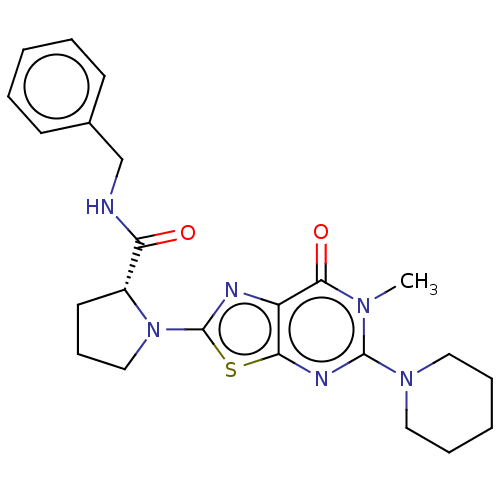 ((R)-N-benzyl-1-[6-methyl-7-oxo-5-(piperidin-1-yl)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | 8.0 | n/a |
MITSUBISHI TANABE PHARMA CORPORATION US Patent | Assay Description The inhibitory action of the test compound on human recombinant KAT-II was determined by the following method.To a reaction mixture (45 μL) cont... | US Patent US10065972 (2018) BindingDB Entry DOI: 10.7270/Q23X88P5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial (Homo sapiens (Human)) | BDBM273041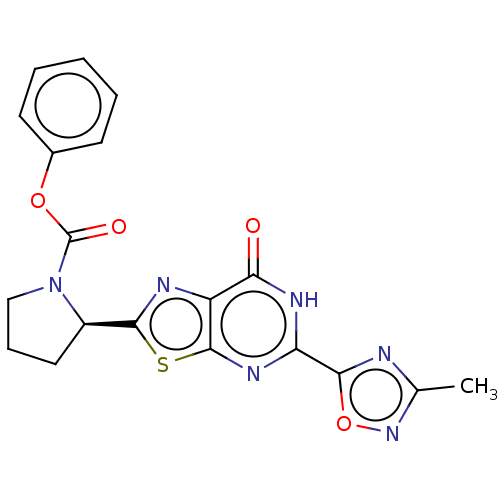 ((R)-2-[5-(3-methyl-[1,2,4]oxadiazol-5-yl)-7-oxo-6,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | 8.0 | n/a |
MITSUBISHI TANABE PHARMA CORPORATION US Patent | Assay Description The inhibitory action of the test compound on human recombinant KAT-II was determined by the following method.To a reaction mixture (45 μL) cont... | US Patent US10065972 (2018) BindingDB Entry DOI: 10.7270/Q23X88P5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial (Homo sapiens (Human)) | BDBM273079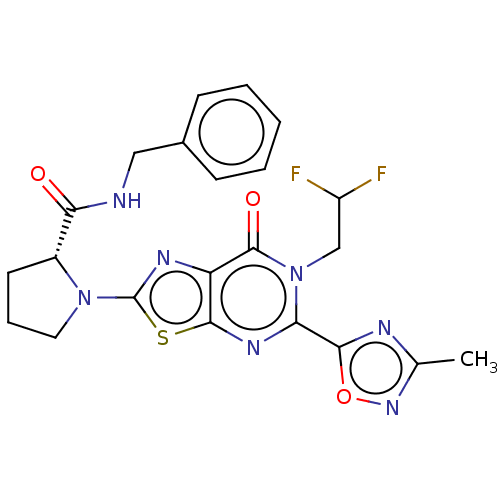 ((R)-N-benzyl-1-[6-(2,2-difluoroethyl)-5-(3-methyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | 8.0 | n/a |
MITSUBISHI TANABE PHARMA CORPORATION US Patent | Assay Description The inhibitory action of the test compound on human recombinant KAT-II was determined by the following method.To a reaction mixture (45 μL) cont... | US Patent US10065972 (2018) BindingDB Entry DOI: 10.7270/Q23X88P5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial (Homo sapiens (Human)) | BDBM273157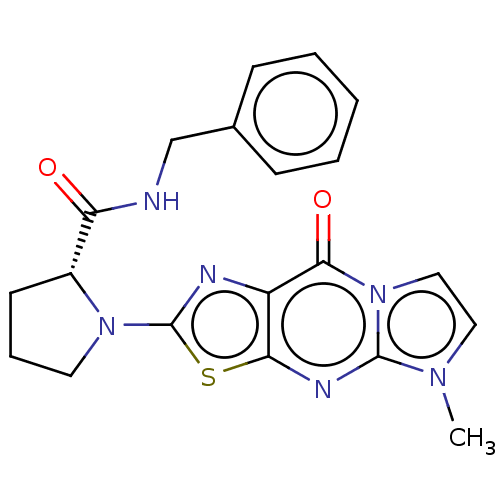 ((R)-N-benzyl-1-(5-methyl-9-oxo-5,9-dihydroimidazo[...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.90 | n/a | n/a | n/a | n/a | 8.0 | n/a |
MITSUBISHI TANABE PHARMA CORPORATION US Patent | Assay Description The inhibitory action of the test compound on human recombinant KAT-II was determined by the following method.To a reaction mixture (45 μL) cont... | US Patent US10065972 (2018) BindingDB Entry DOI: 10.7270/Q23X88P5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial (Homo sapiens (Human)) | BDBM273070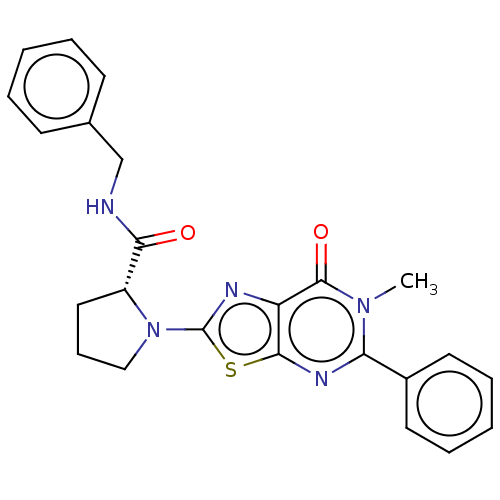 ((R)-N-benzyl-1-(6-methyl-7-oxo-5-phenyl-6,7-dihydr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5.40 | n/a | n/a | n/a | n/a | 8.0 | n/a |
MITSUBISHI TANABE PHARMA CORPORATION US Patent | Assay Description The inhibitory action of the test compound on human recombinant KAT-II was determined by the following method.To a reaction mixture (45 μL) cont... | US Patent US10065972 (2018) BindingDB Entry DOI: 10.7270/Q23X88P5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial (Homo sapiens (Human)) | BDBM273114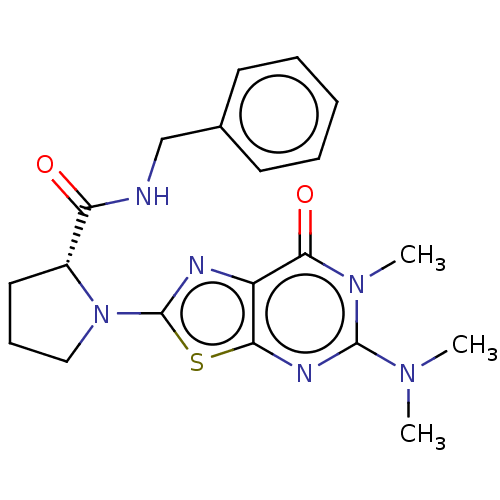 ((R)-N-benzyl-1-[5-(N',N'-dimethylamino)-6-methyl-7...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5.60 | n/a | n/a | n/a | n/a | 8.0 | n/a |
MITSUBISHI TANABE PHARMA CORPORATION US Patent | Assay Description The inhibitory action of the test compound on human recombinant KAT-II was determined by the following method.To a reaction mixture (45 μL) cont... | US Patent US10065972 (2018) BindingDB Entry DOI: 10.7270/Q23X88P5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial (Homo sapiens (Human)) | BDBM273096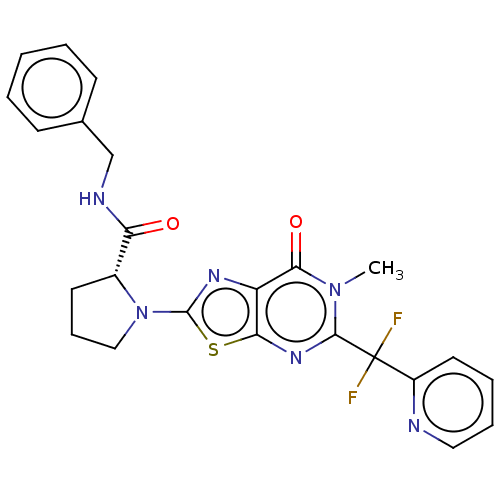 ((R)-N-benzyl-1-{5-[difluoro(pyridin-2-yl)methyl]-6...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5.60 | n/a | n/a | n/a | n/a | 8.0 | n/a |
MITSUBISHI TANABE PHARMA CORPORATION US Patent | Assay Description The inhibitory action of the test compound on human recombinant KAT-II was determined by the following method.To a reaction mixture (45 μL) cont... | US Patent US10065972 (2018) BindingDB Entry DOI: 10.7270/Q23X88P5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial (Homo sapiens (Human)) | BDBM273131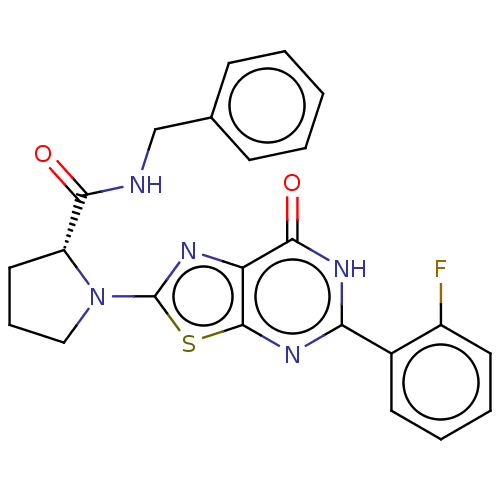 ((R)-N-benzyl-1-[5-(2-fluorophenyl)-7-oxo-6,7-dihyd...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6 | n/a | n/a | n/a | n/a | 8.0 | n/a |
MITSUBISHI TANABE PHARMA CORPORATION US Patent | Assay Description The inhibitory action of the test compound on human recombinant KAT-II was determined by the following method.To a reaction mixture (45 μL) cont... | US Patent US10065972 (2018) BindingDB Entry DOI: 10.7270/Q23X88P5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial (Homo sapiens (Human)) | BDBM273030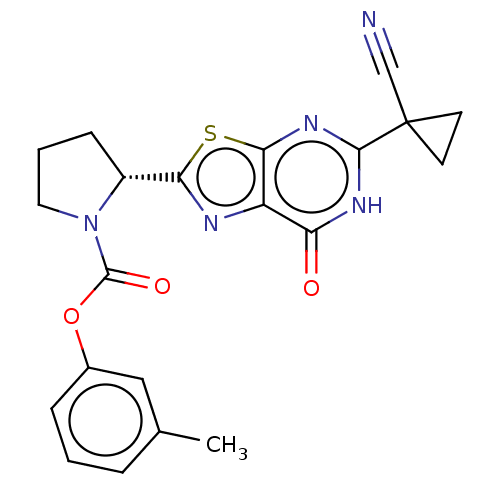 ((R)-2-[5-(1-cyanocyclopropyl)-7-oxo-6,7-dihydro[1,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6.10 | n/a | n/a | n/a | n/a | 8.0 | n/a |
MITSUBISHI TANABE PHARMA CORPORATION US Patent | Assay Description The inhibitory action of the test compound on human recombinant KAT-II was determined by the following method.To a reaction mixture (45 μL) cont... | US Patent US10065972 (2018) BindingDB Entry DOI: 10.7270/Q23X88P5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial (Homo sapiens (Human)) | BDBM273139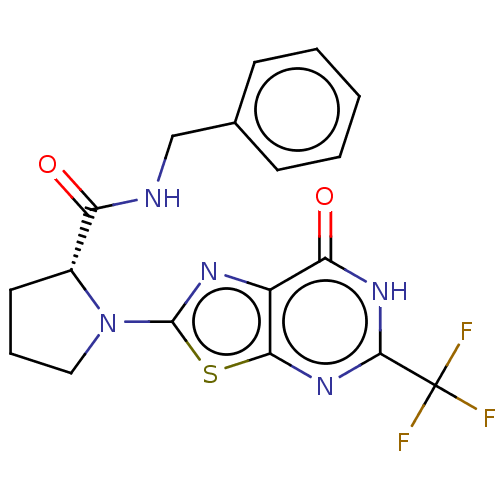 ((R)-N-benzyl-1-(7-oxo-5-trifluoromethyl-6,7-dihydr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6.90 | n/a | n/a | n/a | n/a | 8.0 | n/a |
MITSUBISHI TANABE PHARMA CORPORATION US Patent | Assay Description The inhibitory action of the test compound on human recombinant KAT-II was determined by the following method.To a reaction mixture (45 μL) cont... | US Patent US10065972 (2018) BindingDB Entry DOI: 10.7270/Q23X88P5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial (Homo sapiens (Human)) | BDBM273076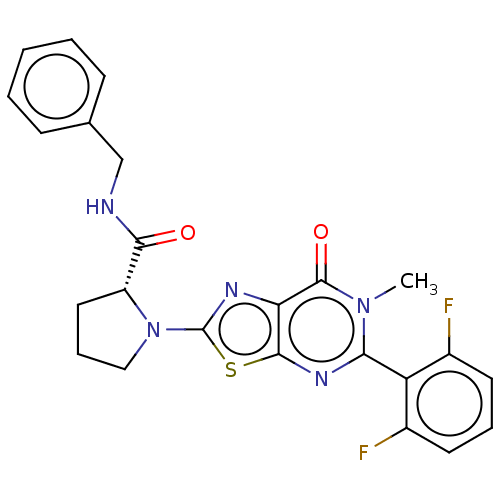 ((R)-N-benzyl-1-[5-(2,6-difluorophenyl)-6-methyl-7-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 7 | n/a | n/a | n/a | n/a | 8.0 | n/a |
MITSUBISHI TANABE PHARMA CORPORATION US Patent | Assay Description The inhibitory action of the test compound on human recombinant KAT-II was determined by the following method.To a reaction mixture (45 μL) cont... | US Patent US10065972 (2018) BindingDB Entry DOI: 10.7270/Q23X88P5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial (Homo sapiens (Human)) | BDBM273132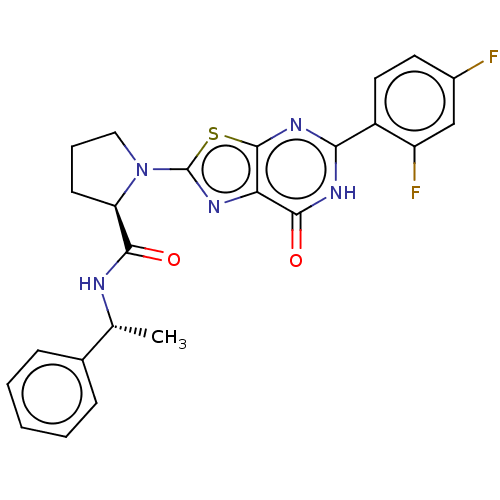 ((R)-1-[5-(2,4-difluorophenyl)-7-oxo-6,7-dihydro[1,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 7.10 | n/a | n/a | n/a | n/a | 8.0 | n/a |
MITSUBISHI TANABE PHARMA CORPORATION US Patent | Assay Description The inhibitory action of the test compound on human recombinant KAT-II was determined by the following method.To a reaction mixture (45 μL) cont... | US Patent US10065972 (2018) BindingDB Entry DOI: 10.7270/Q23X88P5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial (Homo sapiens (Human)) | BDBM273077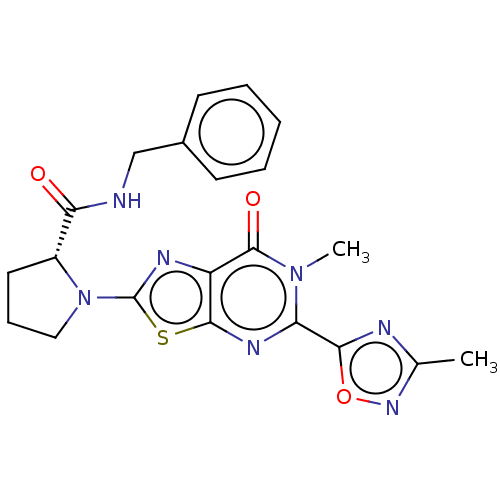 ((R)-N-benzyl-1-[6-methyl-5-(3-methyl-1,2,4-oxadiaz...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 7.30 | n/a | n/a | n/a | n/a | 8.0 | n/a |
MITSUBISHI TANABE PHARMA CORPORATION US Patent | Assay Description The inhibitory action of the test compound on human recombinant KAT-II was determined by the following method.To a reaction mixture (45 μL) cont... | US Patent US10065972 (2018) BindingDB Entry DOI: 10.7270/Q23X88P5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial (Homo sapiens (Human)) | BDBM273159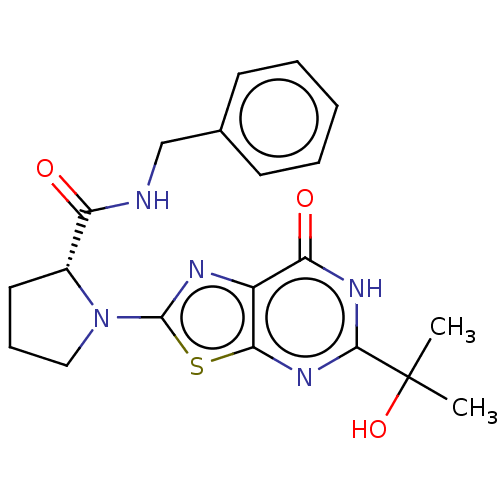 ((R)-N-benzyl-1-[5-(2-hydroxypropan-2-yl)-7-oxo-6,7...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 7.60 | n/a | n/a | n/a | n/a | 8.0 | n/a |
MITSUBISHI TANABE PHARMA CORPORATION US Patent | Assay Description The inhibitory action of the test compound on human recombinant KAT-II was determined by the following method.To a reaction mixture (45 μL) cont... | US Patent US10065972 (2018) BindingDB Entry DOI: 10.7270/Q23X88P5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial (Homo sapiens (Human)) | BDBM273116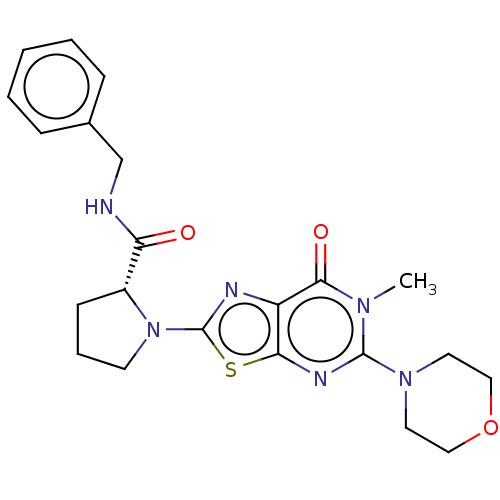 ((R)-N-benzyl-1-[6-methyl-5-(morpholin-4-yl)-7-oxo-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | 8.0 | n/a |
MITSUBISHI TANABE PHARMA CORPORATION US Patent | Assay Description The inhibitory action of the test compound on human recombinant KAT-II was determined by the following method.To a reaction mixture (45 μL) cont... | US Patent US10065972 (2018) BindingDB Entry DOI: 10.7270/Q23X88P5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial (Homo sapiens (Human)) | BDBM273123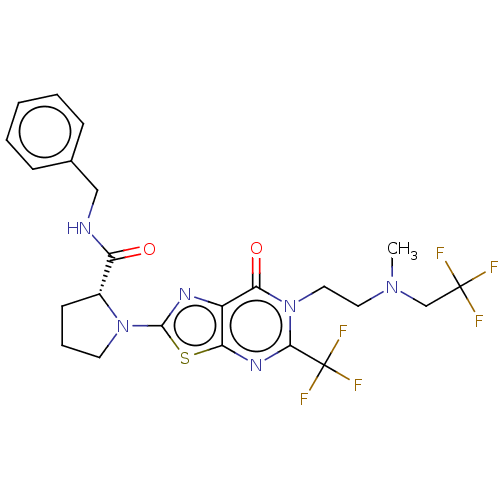 ((R)-N-benzyl-1-[6-{2-[N'-methyl-N'-(2,2,2-trifluor...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 8 | n/a | n/a | n/a | n/a | 8.0 | n/a |
MITSUBISHI TANABE PHARMA CORPORATION US Patent | Assay Description The inhibitory action of the test compound on human recombinant KAT-II was determined by the following method.To a reaction mixture (45 μL) cont... | US Patent US10065972 (2018) BindingDB Entry DOI: 10.7270/Q23X88P5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial (Homo sapiens (Human)) | BDBM273158 ((R)-N-benzyl-1-(5,5-difluoro-10-oxo-5,7,8,10-tetra...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 8.20 | n/a | n/a | n/a | n/a | 8.0 | n/a |
MITSUBISHI TANABE PHARMA CORPORATION US Patent | Assay Description The inhibitory action of the test compound on human recombinant KAT-II was determined by the following method.To a reaction mixture (45 μL) cont... | US Patent US10065972 (2018) BindingDB Entry DOI: 10.7270/Q23X88P5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial (Homo sapiens (Human)) | BDBM273028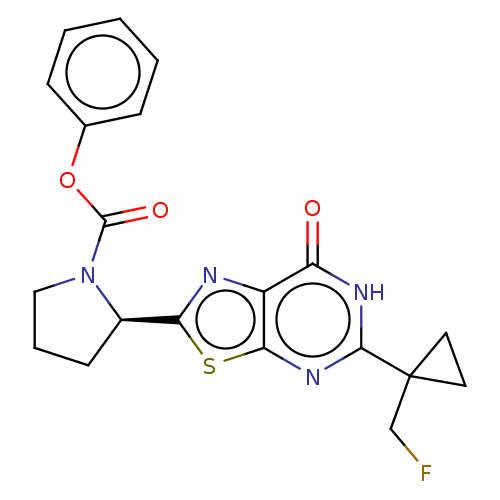 ((R)-2-{5-[1-(fluoromethyl)cyclopropyl]-7-oxo-6,7-d...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 8.60 | n/a | n/a | n/a | n/a | 8.0 | n/a |
MITSUBISHI TANABE PHARMA CORPORATION US Patent | Assay Description The inhibitory action of the test compound on human recombinant KAT-II was determined by the following method.To a reaction mixture (45 μL) cont... | US Patent US10065972 (2018) BindingDB Entry DOI: 10.7270/Q23X88P5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial (Homo sapiens (Human)) | BDBM273118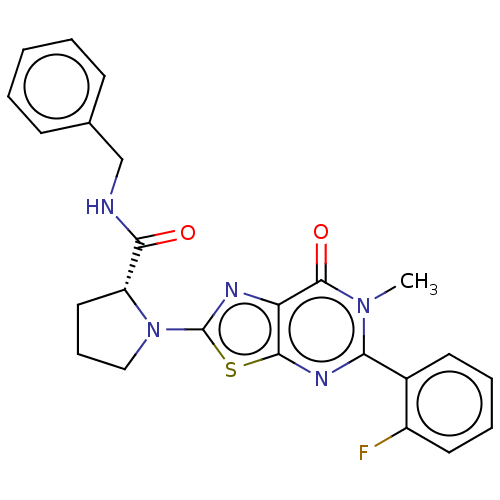 ((R)-N-benzyl-1-[5-(2-fluorophenyl)-6-methyl-7-oxo-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 8.70 | n/a | n/a | n/a | n/a | 8.0 | n/a |
MITSUBISHI TANABE PHARMA CORPORATION US Patent | Assay Description The inhibitory action of the test compound on human recombinant KAT-II was determined by the following method.To a reaction mixture (45 μL) cont... | US Patent US10065972 (2018) BindingDB Entry DOI: 10.7270/Q23X88P5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial (Homo sapiens (Human)) | BDBM273095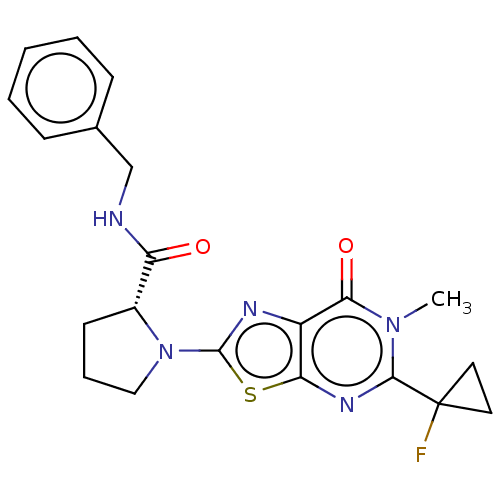 ((R)-N-benzyl-1-[5-(1-fluorocyclopropyl)-6-methyl-7...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 8.80 | n/a | n/a | n/a | n/a | 8.0 | n/a |
MITSUBISHI TANABE PHARMA CORPORATION US Patent | Assay Description The inhibitory action of the test compound on human recombinant KAT-II was determined by the following method.To a reaction mixture (45 μL) cont... | US Patent US10065972 (2018) BindingDB Entry DOI: 10.7270/Q23X88P5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial (Homo sapiens (Human)) | BDBM107727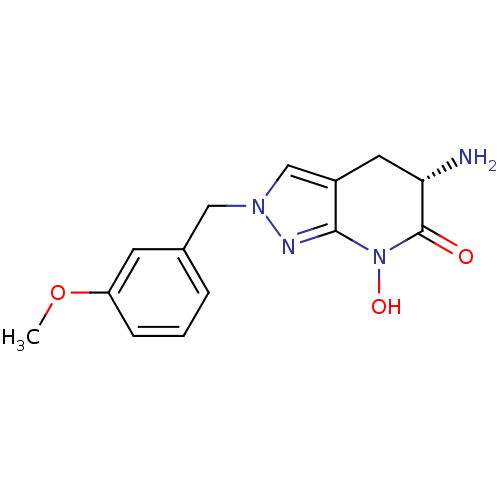 (US8933095, 11) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 8.93 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description Formation of kynurenic acid (KYNA) is indirectly assessed by a decrease in light absorbance at 370 nm (OD370) as the L-kynurenine (KYN) substrate is ... | US Patent US8598200 (2013) BindingDB Entry DOI: 10.7270/Q2G73CBJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial (Homo sapiens (Human)) | BDBM107727 (US8933095, 11) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 8.93 | n/a | n/a | n/a | n/a | n/a | 37 |
Pfizer Inc. US Patent | Assay Description Formation of kynurenic acid (KYNA) is indirectly assessed by a decrease in light absorbance at 370 nm (OD370) as the L-kynurenine (KYN) substrate is ... | US Patent US8933095 (2015) BindingDB Entry DOI: 10.7270/Q2J9653C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial (Homo sapiens (Human)) | BDBM273151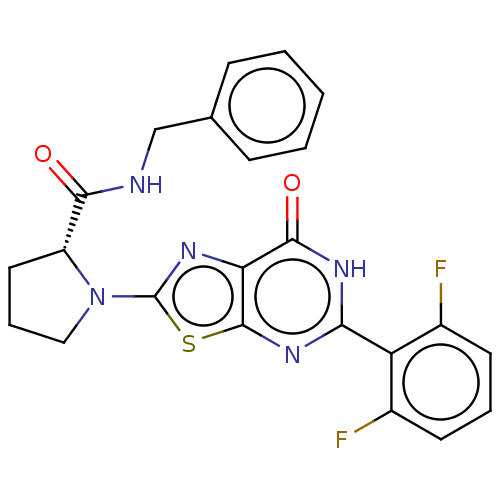 ((R)-N-benzyl-1-[5-(2,6-difluorophenyl)-7-oxo-6,7-d...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 9 | n/a | n/a | n/a | n/a | 8.0 | n/a |
MITSUBISHI TANABE PHARMA CORPORATION US Patent | Assay Description The inhibitory action of the test compound on human recombinant KAT-II was determined by the following method.To a reaction mixture (45 μL) cont... | US Patent US10065972 (2018) BindingDB Entry DOI: 10.7270/Q23X88P5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial (Homo sapiens (Human)) | BDBM273129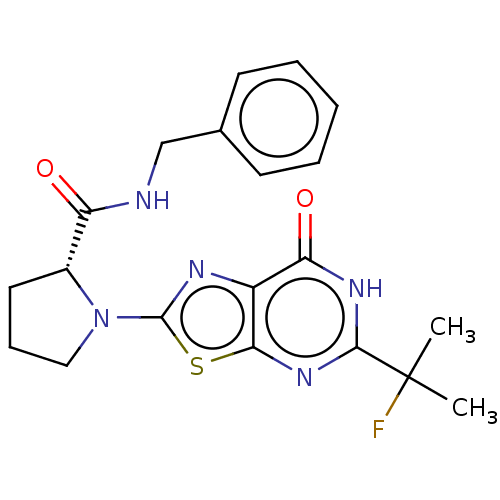 ((R)-N-benzyl-1-[5-(2-fluoropropan-2-yl)-7-oxo-6,7-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 9.70 | n/a | n/a | n/a | n/a | 8.0 | n/a |
MITSUBISHI TANABE PHARMA CORPORATION US Patent | Assay Description The inhibitory action of the test compound on human recombinant KAT-II was determined by the following method.To a reaction mixture (45 μL) cont... | US Patent US10065972 (2018) BindingDB Entry DOI: 10.7270/Q23X88P5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial (Homo sapiens (Human)) | BDBM273145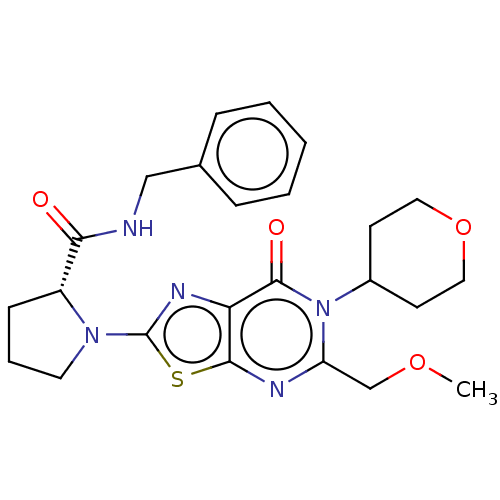 ((R)-N-benzyl-1-[5-methoxymethyl-7-oxo-6-(tetrahydr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 10 | n/a | n/a | n/a | n/a | 8.0 | n/a |
MITSUBISHI TANABE PHARMA CORPORATION US Patent | Assay Description The inhibitory action of the test compound on human recombinant KAT-II was determined by the following method.To a reaction mixture (45 μL) cont... | US Patent US10065972 (2018) BindingDB Entry DOI: 10.7270/Q23X88P5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial (Homo sapiens (Human)) | BDBM273150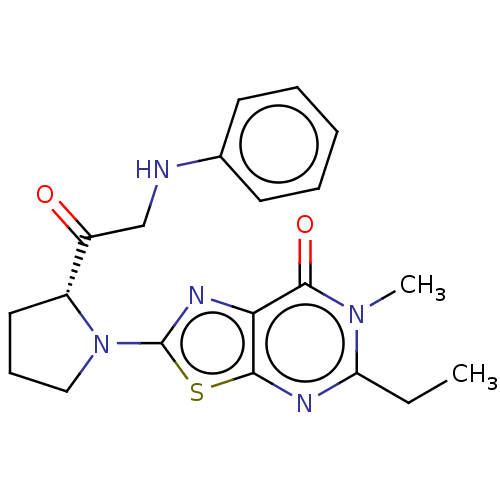 (5-ethyl-6-methyl-2-[(R)-2-(2-phenylaminoacetyl)pyr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 10 | n/a | n/a | n/a | n/a | 8.0 | n/a |
MITSUBISHI TANABE PHARMA CORPORATION US Patent | Assay Description The inhibitory action of the test compound on human recombinant KAT-II was determined by the following method.To a reaction mixture (45 μL) cont... | US Patent US10065972 (2018) BindingDB Entry DOI: 10.7270/Q23X88P5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial (Homo sapiens (Human)) | BDBM273117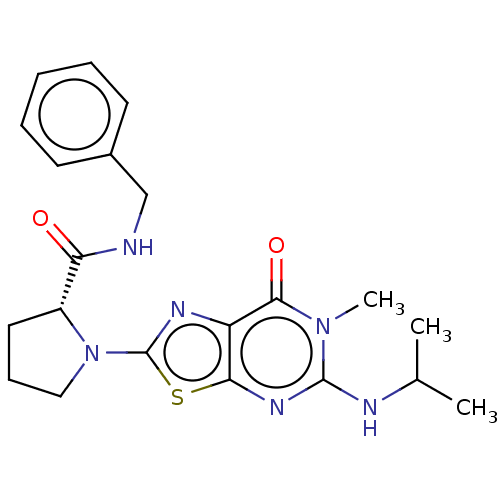 ((R)-N-benzyl-1-{6-methyl-7-oxo-5-[(propan-2-yl)ami...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 11 | n/a | n/a | n/a | n/a | 8.0 | n/a |
MITSUBISHI TANABE PHARMA CORPORATION US Patent | Assay Description The inhibitory action of the test compound on human recombinant KAT-II was determined by the following method.To a reaction mixture (45 μL) cont... | US Patent US10065972 (2018) BindingDB Entry DOI: 10.7270/Q23X88P5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial (Homo sapiens (Human)) | BDBM273070 ((R)-N-benzyl-1-(6-methyl-7-oxo-5-phenyl-6,7-dihydr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 11 | n/a | n/a | n/a | n/a | 8.0 | n/a |
MITSUBISHI TANABE PHARMA CORPORATION US Patent | Assay Description The inhibitory action of the test compound on human recombinant KAT-II was determined by the following method.To a reaction mixture (45 μL) cont... | US Patent US10065972 (2018) BindingDB Entry DOI: 10.7270/Q23X88P5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial (Homo sapiens (Human)) | BDBM273085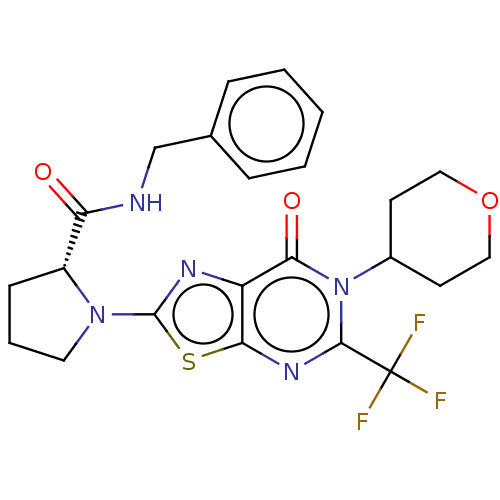 ((R)-N-benzyl-1-[7-oxo-6-(tetrahydro-2H-pyran-4-yl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 11 | n/a | n/a | n/a | n/a | 8.0 | n/a |
MITSUBISHI TANABE PHARMA CORPORATION US Patent | Assay Description The inhibitory action of the test compound on human recombinant KAT-II was determined by the following method.To a reaction mixture (45 μL) cont... | US Patent US10065972 (2018) BindingDB Entry DOI: 10.7270/Q23X88P5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial (Homo sapiens (Human)) | BDBM273089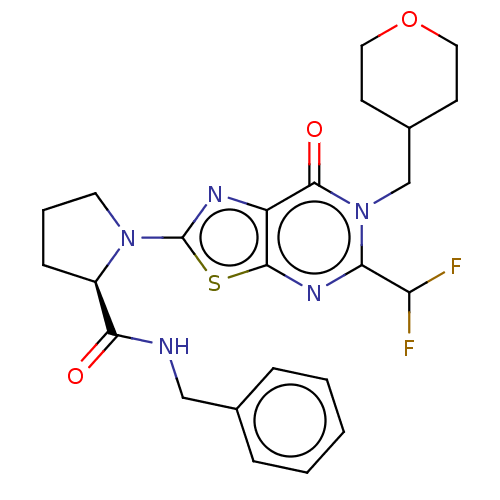 ((R)-N-benzyl-1-{5-difluoromethyl-7-oxo-6-[(tetrahy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 11 | n/a | n/a | n/a | n/a | 8.0 | n/a |
MITSUBISHI TANABE PHARMA CORPORATION US Patent | Assay Description The inhibitory action of the test compound on human recombinant KAT-II was determined by the following method.To a reaction mixture (45 μL) cont... | US Patent US10065972 (2018) BindingDB Entry DOI: 10.7270/Q23X88P5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial (Homo sapiens (Human)) | BDBM107721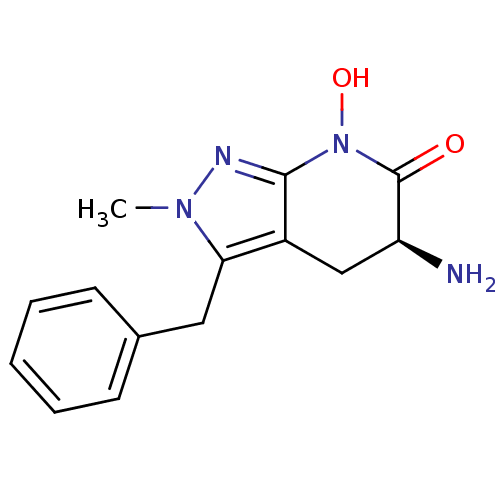 (US8933095, 3) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 11.5 | n/a | n/a | n/a | n/a | n/a | 37 |
Pfizer Inc. US Patent | Assay Description Formation of kynurenic acid (KYNA) is indirectly assessed by a decrease in light absorbance at 370 nm (OD370) as the L-kynurenine (KYN) substrate is ... | US Patent US8933095 (2015) BindingDB Entry DOI: 10.7270/Q2J9653C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial (Homo sapiens (Human)) | BDBM107721 (US8933095, 3) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 11.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description Formation of kynurenic acid (KYNA) is indirectly assessed by a decrease in light absorbance at 370 nm (OD370) as the L-kynurenine (KYN) substrate is ... | US Patent US8598200 (2013) BindingDB Entry DOI: 10.7270/Q2G73CBJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial (Homo sapiens (Human)) | BDBM466252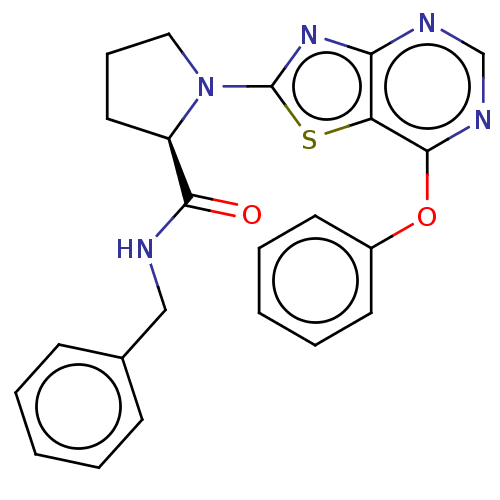 (US10793582, Example 66) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
MITSUBISHI TANABE PHARMA CORPORATION US Patent | Assay Description To a reaction mixture (45 μL) containing 3.0 μmol/L kynurenine, 10 μmol/L pyridoxal phosphate, 2.0 ng/μL human recombinant KAT-II... | US Patent US10793582 (2020) BindingDB Entry DOI: 10.7270/Q27W6G8D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial (Homo sapiens (Human)) | BDBM466253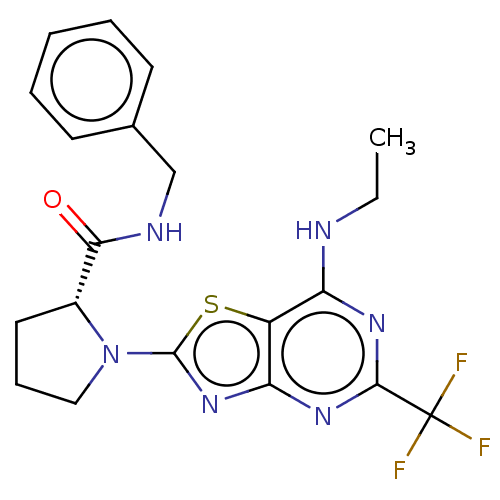 (US10793582, Example 67) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
MITSUBISHI TANABE PHARMA CORPORATION US Patent | Assay Description To a reaction mixture (45 μL) containing 3.0 μmol/L kynurenine, 10 μmol/L pyridoxal phosphate, 2.0 ng/μL human recombinant KAT-II... | US Patent US10793582 (2020) BindingDB Entry DOI: 10.7270/Q27W6G8D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial (Homo sapiens (Human)) | BDBM273152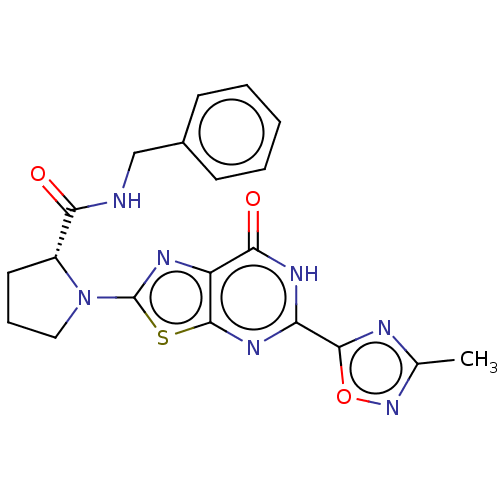 ((R)-N-benzyl-1-[5-(3-methyl-[1,2,4]oxadiazol-5-yl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 12 | n/a | n/a | n/a | n/a | 8.0 | n/a |
MITSUBISHI TANABE PHARMA CORPORATION US Patent | Assay Description The inhibitory action of the test compound on human recombinant KAT-II was determined by the following method.To a reaction mixture (45 μL) cont... | US Patent US10065972 (2018) BindingDB Entry DOI: 10.7270/Q23X88P5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 437 total ) | Next | Last >> |