

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Thymidine kinase, cytosolic (Homo sapiens (Human)) | BDBM50118502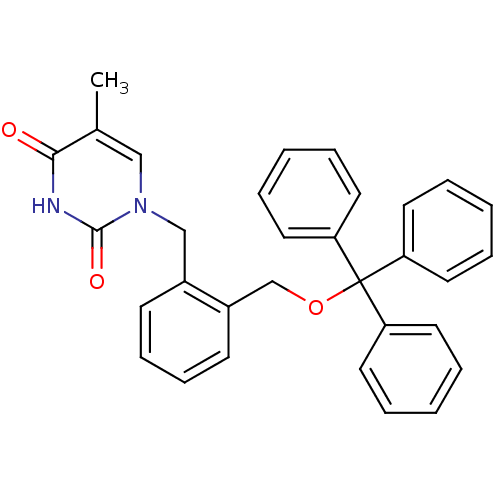 (5-Methyl-1-(2-trityloxymethyl-benzyl)-1H-pyrimidin...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica (CSIC) Curated by ChEMBL | Assay Description Inhibitory activity against HSV-1 Thymidine kinase in OST-TK-/HSV-1 TK+ cell line in combination with gancicclovir | J Med Chem 45: 4254-63 (2002) BindingDB Entry DOI: 10.7270/Q2GQ6ZGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine kinase, cytosolic (Homo sapiens (Human)) | BDBM50118502 (5-Methyl-1-(2-trityloxymethyl-benzyl)-1H-pyrimidin...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica (CSIC) Curated by ChEMBL | Assay Description Inhibitory activity against HSV-1 Thymidine kinase in OST-TK-/HSV-1 TK+ cell line in combination with BVAraU | J Med Chem 45: 4254-63 (2002) BindingDB Entry DOI: 10.7270/Q2GQ6ZGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine kinase, cytosolic (Homo sapiens (Human)) | BDBM1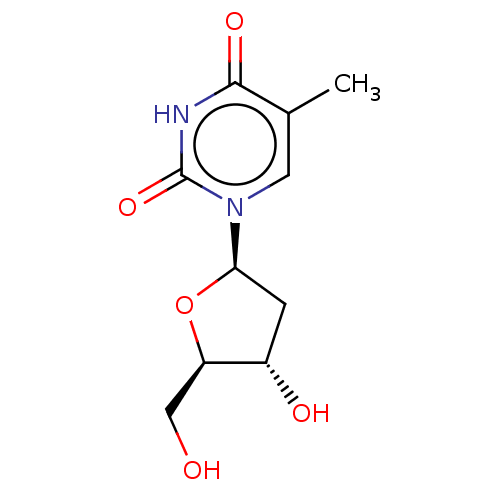 (dT | thymidine) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica (CSIC) Curated by ChEMBL | Assay Description Inhibitory activity (50 uM) against HSV-1 Thymidine kinase in OST-TK-/HSV-1 TK+ cell line in combination with BVAraU | J Med Chem 45: 4254-63 (2002) BindingDB Entry DOI: 10.7270/Q2GQ6ZGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine kinase, cytosolic (Homo sapiens (Human)) | BDBM1 (dT | thymidine) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica (CSIC) Curated by ChEMBL | Assay Description Inhibitory activity (50 uM) against HSV-1 Thymidine kinase in OST-TK-/HSV-1 TK+ cell line in combination with gancicclovir | J Med Chem 45: 4254-63 (2002) BindingDB Entry DOI: 10.7270/Q2GQ6ZGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine kinase, cytosolic (Homo sapiens (Human)) | BDBM50118490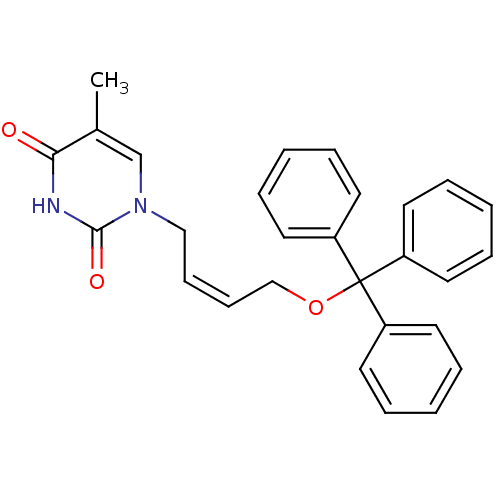 (1-[(Z)-4-(triphenylmethoxy)-2-butenyl]thymine | 5-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica (CSIC) Curated by ChEMBL | Assay Description Inhibitory activity against HSV-1 Thymidine kinase in OST-TK-/HSV-1 TK+ cell line in combination with gancicclovir | J Med Chem 45: 4254-63 (2002) BindingDB Entry DOI: 10.7270/Q2GQ6ZGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine kinase, cytosolic (Homo sapiens (Human)) | BDBM50118491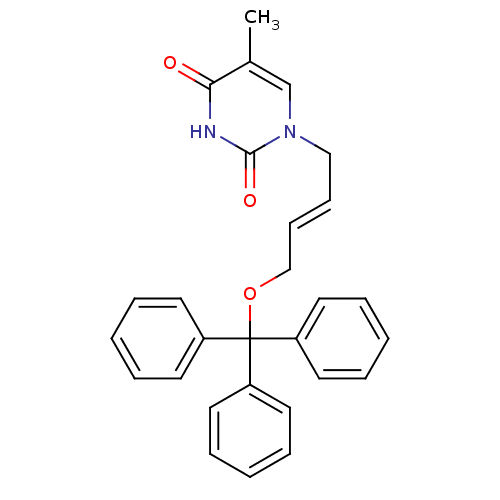 (5-Methyl-1-(4-trityloxy-but-2-enyl)-1H-pyrimidine-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica (CSIC) Curated by ChEMBL | Assay Description Inhibitory activity against HSV-1 Thymidine kinase in OST-TK-/HSV-1 TK+ cell line in combination with gancicclovir | J Med Chem 45: 4254-63 (2002) BindingDB Entry DOI: 10.7270/Q2GQ6ZGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine kinase, cytosolic (Homo sapiens (Human)) | BDBM50118491 (5-Methyl-1-(4-trityloxy-but-2-enyl)-1H-pyrimidine-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 390 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica (CSIC) Curated by ChEMBL | Assay Description Inhibitory concentration against HSV-1 TK (WT) catalyzed [3H]-GCV phosphorylation | J Med Chem 45: 4254-63 (2002) BindingDB Entry DOI: 10.7270/Q2GQ6ZGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine kinase, cytosolic (Homo sapiens (Human)) | BDBM50118501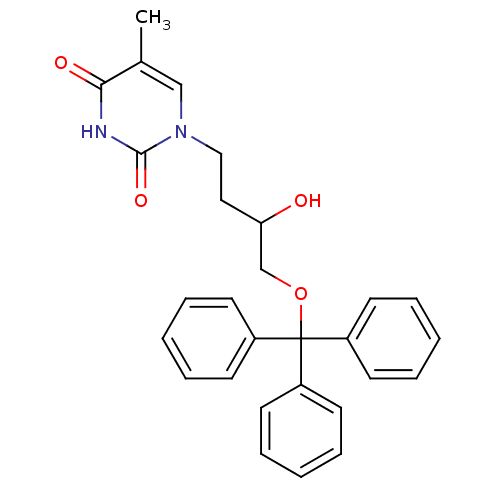 (1-(3-Hydroxy-4-trityloxy-butyl)-5-methyl-1H-pyrimi...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica (CSIC) Curated by ChEMBL | Assay Description Compound was evaluated for inhibitory effect against phosphorylation of [methyl-3H]dThd by HSV-1 Thymidine kinase | J Med Chem 45: 4254-63 (2002) BindingDB Entry DOI: 10.7270/Q2GQ6ZGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine kinase, cytosolic (Homo sapiens (Human)) | BDBM50118490 (1-[(Z)-4-(triphenylmethoxy)-2-butenyl]thymine | 5-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica (CSIC) Curated by ChEMBL | Assay Description Inhibitory effect against phosphorylation of [methyl-3H]dThd by Thymidine Kinase 2 (TK-2) | J Med Chem 45: 4254-63 (2002) BindingDB Entry DOI: 10.7270/Q2GQ6ZGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine kinase, cytosolic (Homo sapiens (Human)) | BDBM50118490 (1-[(Z)-4-(triphenylmethoxy)-2-butenyl]thymine | 5-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Química Médica (CSIC) Curated by ChEMBL | Assay Description Inhibitory concentration of the compound on phosphorylation of [methyl-3H]-dTh by HSV-1 Thymidine Kinase | Bioorg Med Chem Lett 13: 3027-30 (2003) BindingDB Entry DOI: 10.7270/Q2T15310 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine kinase, cytosolic (Homo sapiens (Human)) | BDBM1 (dT | thymidine) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica (CSIC) Curated by ChEMBL | Assay Description Inhibitory concentration against HSV-1 TK (WT) catalyzed [3H]-GCV phosphorylation | J Med Chem 45: 4254-63 (2002) BindingDB Entry DOI: 10.7270/Q2GQ6ZGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine kinase, cytosolic (Homo sapiens (Human)) | BDBM50118490 (1-[(Z)-4-(triphenylmethoxy)-2-butenyl]thymine | 5-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica (CSIC) Curated by ChEMBL | Assay Description Inhibitory activity against HSV-1 Thymidine kinase in OST-TK-/HSV-1 TK+ cell line in combination with BVAraU | J Med Chem 45: 4254-63 (2002) BindingDB Entry DOI: 10.7270/Q2GQ6ZGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine kinase, cytosolic (Homo sapiens (Human)) | BDBM50132253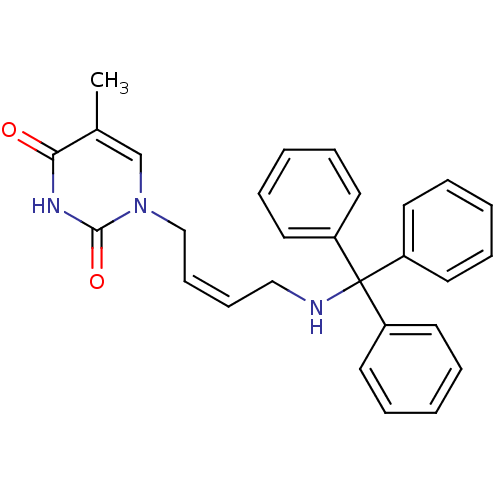 (5-Methyl-1-[4-(trityl-amino)-but-2-enyl]-1H-pyrimi...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Química Médica (CSIC) Curated by ChEMBL | Assay Description Inhibitory concentration of the compound on phosphorylation of [methyl-3H]-dTh by human Thymidine Kinase 2 | Bioorg Med Chem Lett 13: 3027-30 (2003) BindingDB Entry DOI: 10.7270/Q2T15310 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine kinase, cytosolic (Homo sapiens (Human)) | BDBM50002692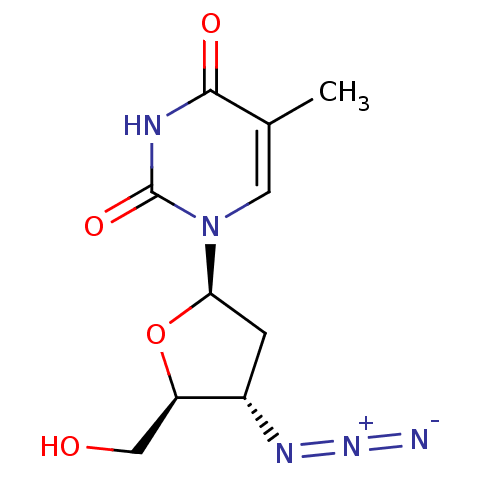 ((AZT) 1-(4-Azido-5-hydroxymethyl-tetrahydro-furan-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Swedish University Agricultural Sciences Curated by ChEMBL | Assay Description Inhibition of human TK1 by liquid scintillation counting | Bioorg Med Chem 18: 3261-9 (2010) Article DOI: 10.1016/j.bmc.2010.03.023 BindingDB Entry DOI: 10.7270/Q2QN66Z0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine kinase, cytosolic (Homo sapiens (Human)) | BDBM50282061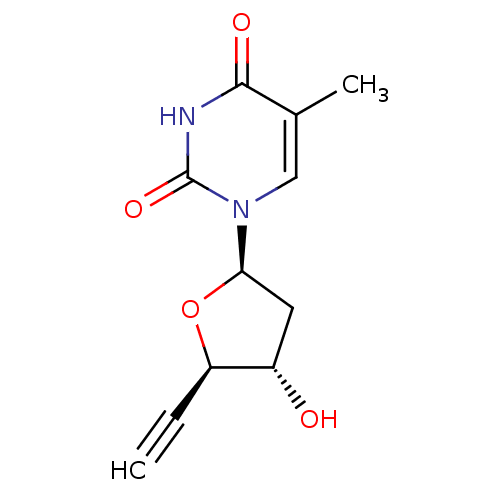 (1-((2R,4S,5R)-5-Ethynyl-4-hydroxy-tetrahydro-furan...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of HSV-1 Thymidine kinase | Bioorg Med Chem Lett 3: 1571-1576 (1993) Article DOI: 10.1016/S0960-894X(00)80020-7 BindingDB Entry DOI: 10.7270/Q28P60F3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine kinase, cytosolic (Homo sapiens (Human)) | BDBM50118491 (5-Methyl-1-(4-trityloxy-but-2-enyl)-1H-pyrimidine-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica (CSIC) Curated by ChEMBL | Assay Description Compound was evaluated for inhibitory effect against phosphorylation of [methyl-3H]dThd by HSV-1 Thymidine kinase | J Med Chem 45: 4254-63 (2002) BindingDB Entry DOI: 10.7270/Q2GQ6ZGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine kinase, cytosolic (Homo sapiens (Human)) | BDBM50118498 (5-Methyl-1-(4-trityloxy-but-2-ynyl)-1H-pyrimidine-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 3.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica (CSIC) Curated by ChEMBL | Assay Description Compound was evaluated for inhibitory effect against phosphorylation of [methyl-3H]dThd by HSV-1 Thymidine kinase | J Med Chem 45: 4254-63 (2002) BindingDB Entry DOI: 10.7270/Q2GQ6ZGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine kinase, cytosolic (Homo sapiens (Human)) | BDBM50118491 (5-Methyl-1-(4-trityloxy-but-2-enyl)-1H-pyrimidine-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica (CSIC) Curated by ChEMBL | Assay Description Inhibitory activity against HSV-1 Thymidine kinase in OST-TK-/HSV-1 TK+ cell line in combination with BVAraU | J Med Chem 45: 4254-63 (2002) BindingDB Entry DOI: 10.7270/Q2GQ6ZGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine kinase, cytosolic (Homo sapiens (Human)) | BDBM50118496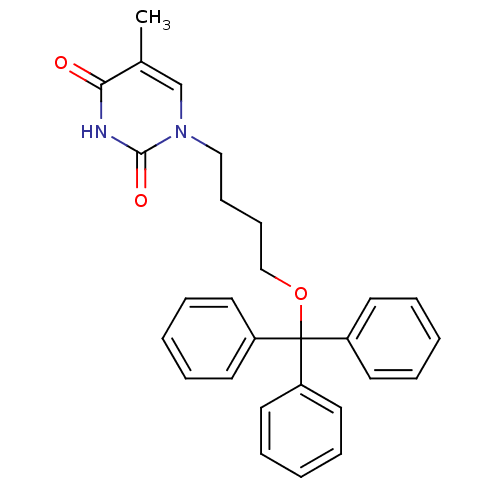 (5-Methyl-1-(4-trityloxy-butyl)-1H-pyrimidine-2,4-d...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica (CSIC) Curated by ChEMBL | Assay Description Inhibitory effect against phosphorylation of [methyl-3H]dThd by Thymidine Kinase 2 (TK-2) | J Med Chem 45: 4254-63 (2002) BindingDB Entry DOI: 10.7270/Q2GQ6ZGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine kinase, cytosolic (Homo sapiens (Human)) | BDBM50132251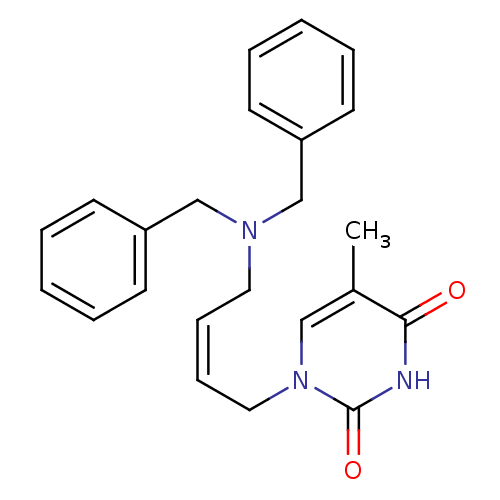 (1-(4-Dibenzylamino-but-2-enyl)-5-methyl-1H-pyrimid...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Química Médica (CSIC) Curated by ChEMBL | Assay Description Inhibitory concentration of the compound on phosphorylation of [methyl-3H]-dTh by human Thymidine Kinase 1 | Bioorg Med Chem Lett 13: 3027-30 (2003) BindingDB Entry DOI: 10.7270/Q2T15310 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine kinase, cytosolic (Homo sapiens (Human)) | BDBM50118501 (1-(3-Hydroxy-4-trityloxy-butyl)-5-methyl-1H-pyrimi...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica (CSIC) Curated by ChEMBL | Assay Description Inhibitory effect against phosphorylation of [methyl-3H]dThd by Thymidine Kinase 2 (TK-2) | J Med Chem 45: 4254-63 (2002) BindingDB Entry DOI: 10.7270/Q2GQ6ZGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine kinase, cytosolic (Homo sapiens (Human)) | BDBM50132252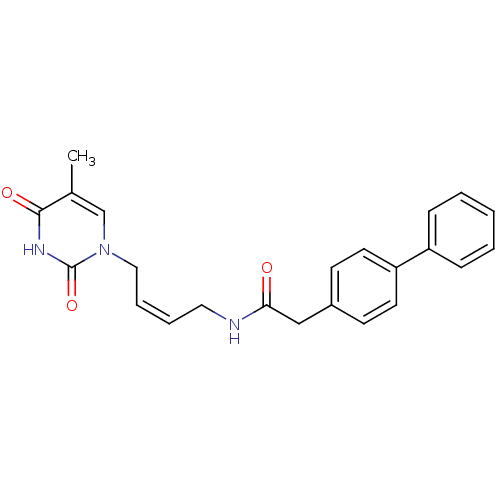 (2-Biphenyl-4-yl-N-[4-(5-methyl-2,4-dioxo-3,4-dihyd...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Química Médica (CSIC) Curated by ChEMBL | Assay Description Inhibitory concentration of the compound on phosphorylation of [methyl-3H]-dTh by human Thymidine Kinase 2 | Bioorg Med Chem Lett 13: 3027-30 (2003) BindingDB Entry DOI: 10.7270/Q2T15310 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine kinase, cytosolic (Homo sapiens (Human)) | BDBM50118506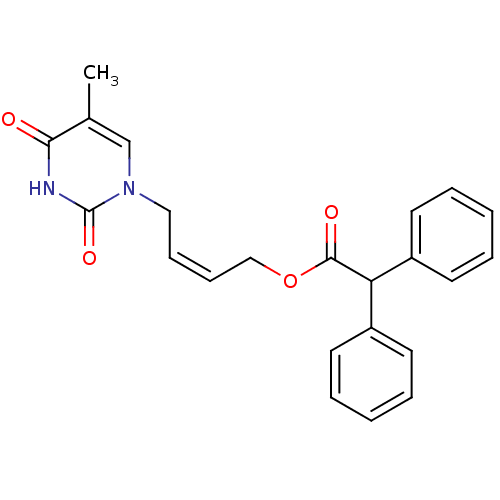 (CHEMBL335866 | Diphenyl-acetic acid 4-(5-methyl-2,...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 4.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica (CSIC) Curated by ChEMBL | Assay Description Inhibitory effect against phosphorylation of [methyl-3H]dThd by Thymidine Kinase 2 (TK-2) | J Med Chem 45: 4254-63 (2002) BindingDB Entry DOI: 10.7270/Q2GQ6ZGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine kinase, cytosolic (Homo sapiens (Human)) | BDBM50118503 (5-Iodo-1-(3-trityloxy-propenyl)-1H-pyrimidine-2,4-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 4.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica (CSIC) Curated by ChEMBL | Assay Description Inhibitory effect against phosphorylation of [methyl-3H]dThd by Thymidine Kinase 2 (TK-2) | J Med Chem 45: 4254-63 (2002) BindingDB Entry DOI: 10.7270/Q2GQ6ZGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine kinase, cytosolic (Homo sapiens (Human)) | BDBM50282067 (1-((2R,4S,5S)-4-Hydroxy-5-methoxy-tetrahydro-furan...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of HSV-1 Thymidine kinase | Bioorg Med Chem Lett 3: 1571-1576 (1993) Article DOI: 10.1016/S0960-894X(00)80020-7 BindingDB Entry DOI: 10.7270/Q28P60F3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine kinase, cytosolic (Homo sapiens (Human)) | BDBM50118489 (1-(4-Hydroxy-5-trityloxymethyl-tetrahydro-furan-2-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica (CSIC) Curated by ChEMBL | Assay Description Compound was evaluated for inhibitory effect against phosphorylation of [methyl-3H]dThd by HSV-1 Thymidine kinase | J Med Chem 45: 4254-63 (2002) BindingDB Entry DOI: 10.7270/Q2GQ6ZGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine kinase, cytosolic (Homo sapiens (Human)) | BDBM50118490 (1-[(Z)-4-(triphenylmethoxy)-2-butenyl]thymine | 5-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 8.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica (CSIC) Curated by ChEMBL | Assay Description Inhibitory concentration against HSV-1 TK (WT) catalyzed [3H]-GCV phosphorylation | J Med Chem 45: 4254-63 (2002) BindingDB Entry DOI: 10.7270/Q2GQ6ZGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine kinase, cytosolic (Homo sapiens (Human)) | BDBM50118494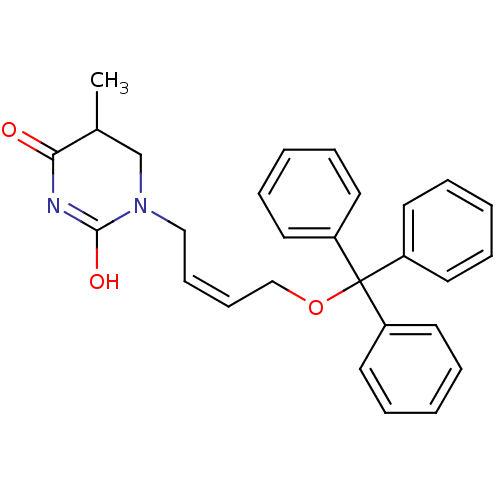 (5-Methyl-1-(4-trityloxy-but-2-enyl)-dihydro-pyrimi...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 9.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica (CSIC) Curated by ChEMBL | Assay Description Inhibitory effect against phosphorylation of [methyl-3H]dThd by Thymidine Kinase 2 (TK-2) | J Med Chem 45: 4254-63 (2002) BindingDB Entry DOI: 10.7270/Q2GQ6ZGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine kinase, cytosolic (Homo sapiens (Human)) | BDBM50243089 (2',3'-Dideoxy-3'-(2-aminoethyldithio)thymidine | C...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Grenoble/CNRS Curated by ChEMBL | Assay Description Inhibition of recombinant TK1 (unknown origin)-mediated phosphorylation of [CH3-3H]deoxythymidine | Bioorg Med Chem 16: 6824-31 (2008) Article DOI: 10.1016/j.bmc.2008.05.065 BindingDB Entry DOI: 10.7270/Q26H4H62 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine kinase, cytosolic (Homo sapiens (Human)) | BDBM50282063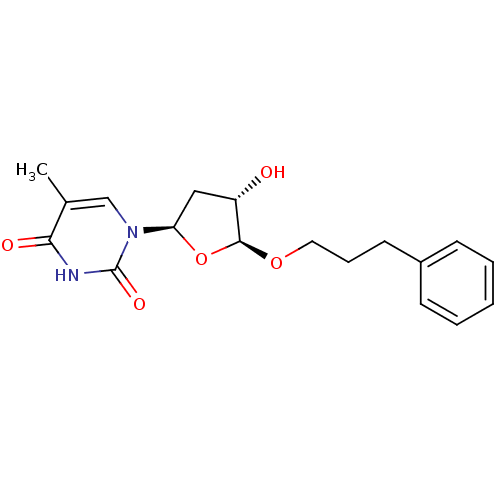 (1-[(2R,4S,5S)-4-Hydroxy-5-(3-phenyl-propoxy)-tetra...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of HSV-1 Thymidine kinase | Bioorg Med Chem Lett 3: 1571-1576 (1993) Article DOI: 10.1016/S0960-894X(00)80020-7 BindingDB Entry DOI: 10.7270/Q28P60F3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine kinase, cytosolic (Homo sapiens (Human)) | BDBM50118496 (5-Methyl-1-(4-trityloxy-butyl)-1H-pyrimidine-2,4-d...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica (CSIC) Curated by ChEMBL | Assay Description Compound was evaluated for inhibitory effect against phosphorylation of [methyl-3H]dThd by HSV-1 Thymidine kinase | J Med Chem 45: 4254-63 (2002) BindingDB Entry DOI: 10.7270/Q2GQ6ZGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine kinase, cytosolic (Homo sapiens (Human)) | BDBM448320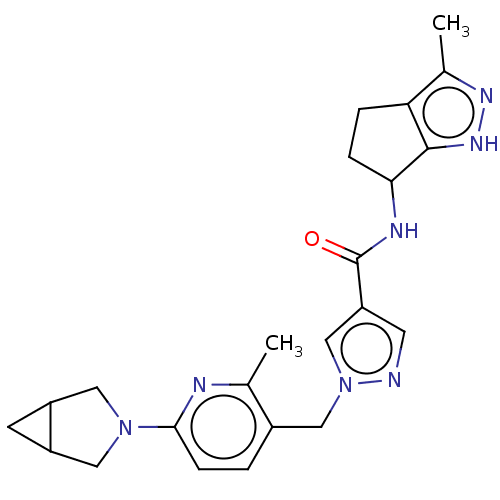 (1-[(6-{3-Azabicyclo[3.1.0]hexan-3-yl}-2-methylpyri...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Prior to the assay, human TK1 (R&D Systems) was activated by incubation with human trypsin (Calbiochem) in a 1:10,000 ratio for 15 min at 37° C. For ... | US Patent US10695334 (2020) BindingDB Entry DOI: 10.7270/Q2VT1W4K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine kinase, cytosolic (Homo sapiens (Human)) | BDBM448376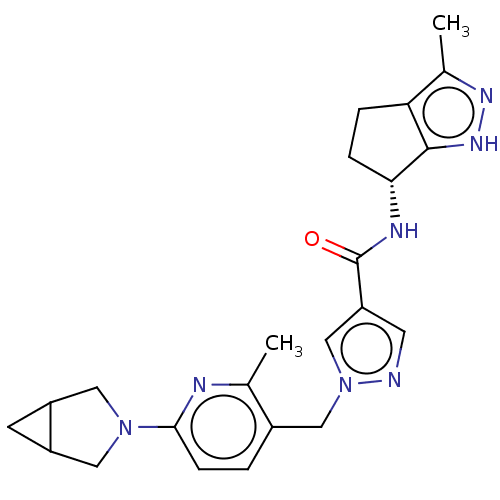 (1-[(6-{3-Azabicyclo[3.1.0]hexan-3-yl}-2-methylpyri...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Prior to the assay, human TK1 (R&D Systems) was activated by incubation with human trypsin (Calbiochem) in a 1:10,000 ratio for 15 min at 37° C. For ... | US Patent US10695334 (2020) BindingDB Entry DOI: 10.7270/Q2VT1W4K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine kinase, cytosolic (Homo sapiens (Human)) | BDBM448377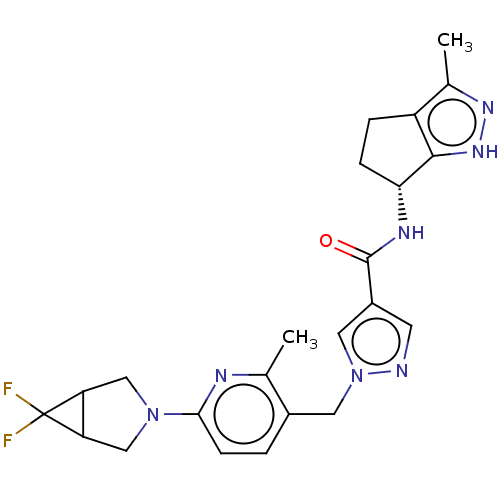 (1-[(6-{6,6-Difluoro-3-azabicyclo[3.1.0]hexan-3-yl}...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Prior to the assay, human TK1 (R&D Systems) was activated by incubation with human trypsin (Calbiochem) in a 1:10,000 ratio for 15 min at 37° C. For ... | US Patent US10695334 (2020) BindingDB Entry DOI: 10.7270/Q2VT1W4K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine kinase, cytosolic (Homo sapiens (Human)) | BDBM448378 (4-[(6-{3-Azabicyclo[3.1.0]hexan-3-yl}-2-methylpyri...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Prior to the assay, human TK1 (R&D Systems) was activated by incubation with human trypsin (Calbiochem) in a 1:10,000 ratio for 15 min at 37° C. For ... | US Patent US10695334 (2020) BindingDB Entry DOI: 10.7270/Q2VT1W4K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine kinase, cytosolic (Homo sapiens (Human)) | BDBM448379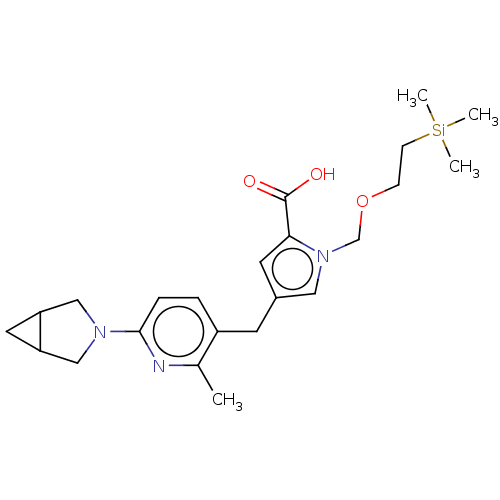 (4-[(6-{3-Azabicyclo[3.1.0]hexan-3-yl}-2-methylpyri...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Prior to the assay, human TK1 (R&D Systems) was activated by incubation with human trypsin (Calbiochem) in a 1:10,000 ratio for 15 min at 37° C. For ... | US Patent US10695334 (2020) BindingDB Entry DOI: 10.7270/Q2VT1W4K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine kinase, cytosolic (Homo sapiens (Human)) | BDBM448380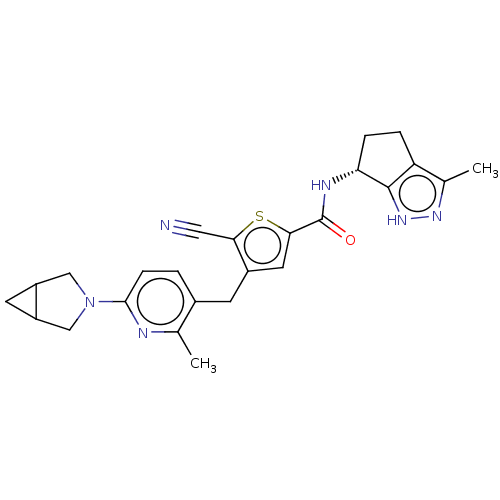 (4-[(6-{3-Azabicyclo[3.1.0]hexan-3-yl}-2-methylpyri...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Prior to the assay, human TK1 (R&D Systems) was activated by incubation with human trypsin (Calbiochem) in a 1:10,000 ratio for 15 min at 37° C. For ... | US Patent US10695334 (2020) BindingDB Entry DOI: 10.7270/Q2VT1W4K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine kinase, cytosolic (Homo sapiens (Human)) | BDBM448381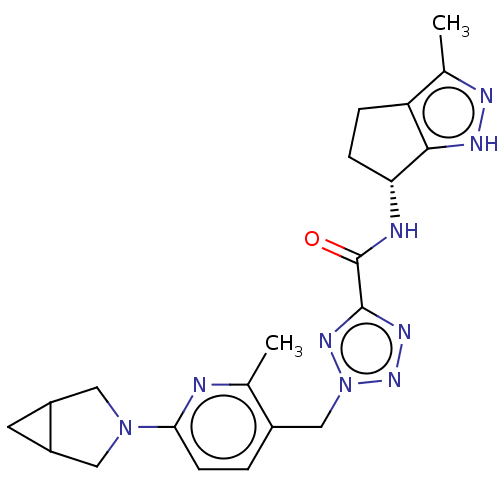 (2-[(6-{3-Azabicyclo[3.1.0]hexan-3-yl}-2-methylpyri...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Prior to the assay, human TK1 (R&D Systems) was activated by incubation with human trypsin (Calbiochem) in a 1:10,000 ratio for 15 min at 37° C. For ... | US Patent US10695334 (2020) BindingDB Entry DOI: 10.7270/Q2VT1W4K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine kinase, cytosolic (Homo sapiens (Human)) | BDBM448382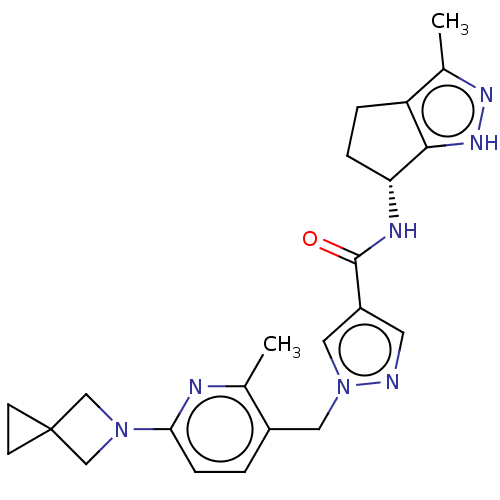 (1-[(6-{5-azaspiro[2.3]hexan-5-yl}-2-methylpyridin-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Prior to the assay, human TK1 (R&D Systems) was activated by incubation with human trypsin (Calbiochem) in a 1:10,000 ratio for 15 min at 37° C. For ... | US Patent US10695334 (2020) BindingDB Entry DOI: 10.7270/Q2VT1W4K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine kinase, cytosolic (Homo sapiens (Human)) | BDBM448383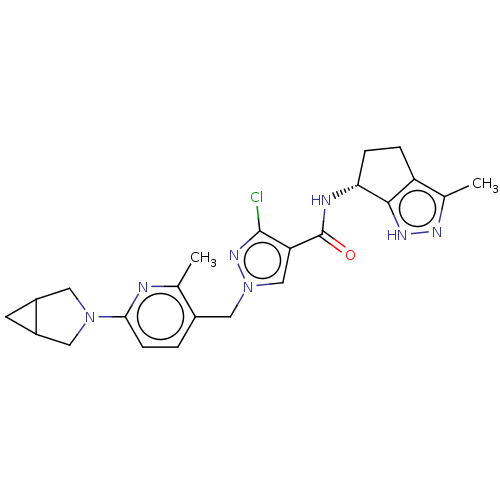 (1-[(6-{3-Azabicyclo[3.1.0]hexan-3-yl}-2-methylpyri...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Prior to the assay, human TK1 (R&D Systems) was activated by incubation with human trypsin (Calbiochem) in a 1:10,000 ratio for 15 min at 37° C. For ... | US Patent US10695334 (2020) BindingDB Entry DOI: 10.7270/Q2VT1W4K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine kinase, cytosolic (Homo sapiens (Human)) | BDBM448384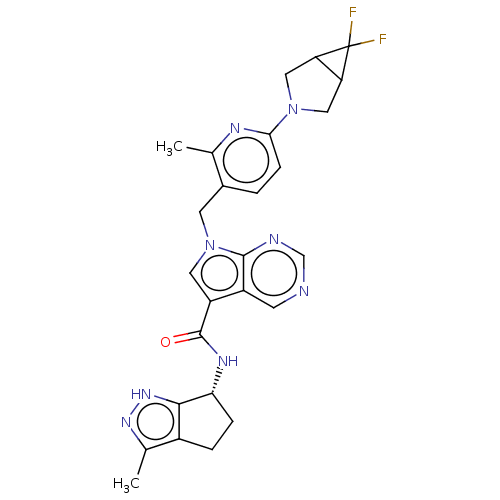 (7-[(6-{6,6-Difluoro-3-azabicyclo[3.1.0]hexan-3-yl}...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Prior to the assay, human TK1 (R&D Systems) was activated by incubation with human trypsin (Calbiochem) in a 1:10,000 ratio for 15 min at 37° C. For ... | US Patent US10695334 (2020) BindingDB Entry DOI: 10.7270/Q2VT1W4K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine kinase, cytosolic (Homo sapiens (Human)) | BDBM448385 (1-[(6-{3-Azabicyclo[3.1.0]hexan-3-yl}-2-methyl- | ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Prior to the assay, human TK1 (R&D Systems) was activated by incubation with human trypsin (Calbiochem) in a 1:10,000 ratio for 15 min at 37° C. For ... | US Patent US10695334 (2020) BindingDB Entry DOI: 10.7270/Q2VT1W4K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine kinase, cytosolic (Homo sapiens (Human)) | BDBM448386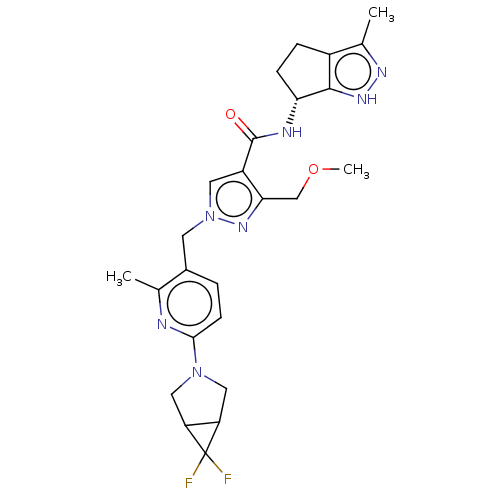 (1-[(6-{6,6-Difluoro-3-azabicyclo[3.1.0]hexan-3-yl}...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Prior to the assay, human TK1 (R&D Systems) was activated by incubation with human trypsin (Calbiochem) in a 1:10,000 ratio for 15 min at 37° C. For ... | US Patent US10695334 (2020) BindingDB Entry DOI: 10.7270/Q2VT1W4K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine kinase, cytosolic (Homo sapiens (Human)) | BDBM448387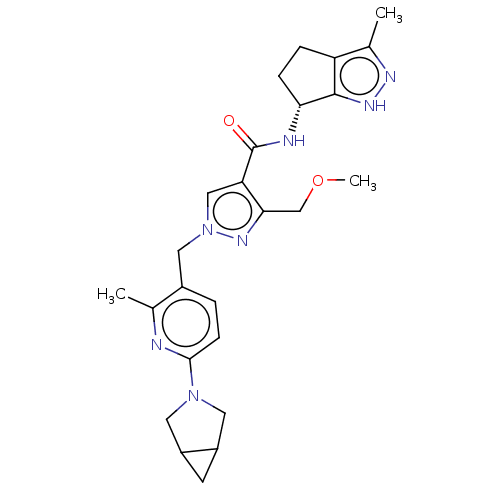 (1-[(6-{3-Azabicyclo[3.1.0]hexan-3-yl}-2-methyl- | ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Prior to the assay, human TK1 (R&D Systems) was activated by incubation with human trypsin (Calbiochem) in a 1:10,000 ratio for 15 min at 37° C. For ... | US Patent US10695334 (2020) BindingDB Entry DOI: 10.7270/Q2VT1W4K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine kinase, cytosolic (Homo sapiens (Human)) | BDBM448388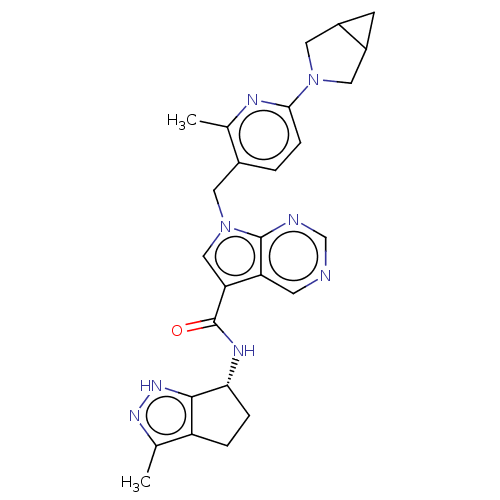 (7-[(6-{3-Azabicyclo[3.1.0]hexan-3-yl}-2-methyl- | ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Prior to the assay, human TK1 (R&D Systems) was activated by incubation with human trypsin (Calbiochem) in a 1:10,000 ratio for 15 min at 37° C. For ... | US Patent US10695334 (2020) BindingDB Entry DOI: 10.7270/Q2VT1W4K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine kinase, cytosolic (Homo sapiens (Human)) | BDBM448390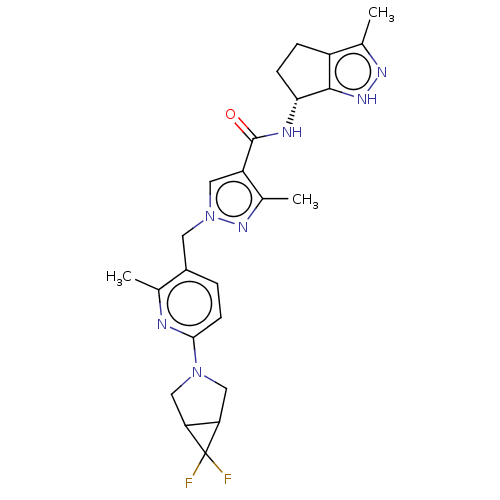 (1-[(6-{6,6-Difluoro-3-azabicyclo[3.1.0]hexan-3-yl}...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Prior to the assay, human TK1 (R&D Systems) was activated by incubation with human trypsin (Calbiochem) in a 1:10,000 ratio for 15 min at 37° C. For ... | US Patent US10695334 (2020) BindingDB Entry DOI: 10.7270/Q2VT1W4K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine kinase, cytosolic (Homo sapiens (Human)) | BDBM448391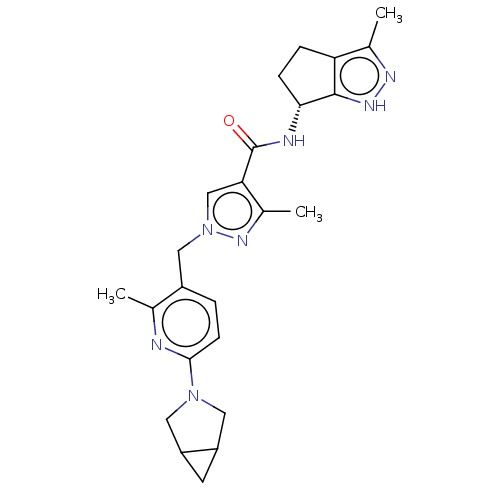 (1-[(6-{3-Azabicyclo[3.1.0]hexan-3-yl}-2-methyl- | ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Prior to the assay, human TK1 (R&D Systems) was activated by incubation with human trypsin (Calbiochem) in a 1:10,000 ratio for 15 min at 37° C. For ... | US Patent US10695334 (2020) BindingDB Entry DOI: 10.7270/Q2VT1W4K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine kinase, cytosolic (Homo sapiens (Human)) | BDBM448392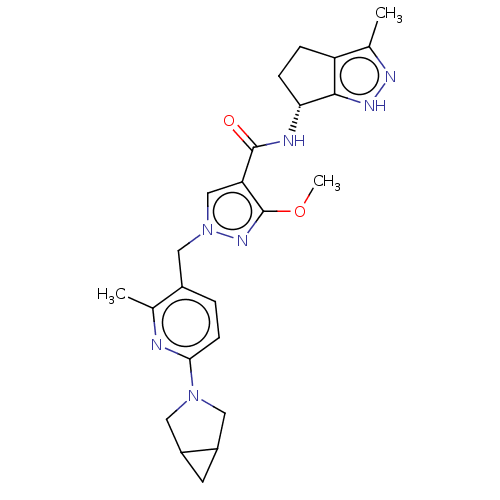 (1-[(6-{3-Azabicyclo[3.1.0]hexan-3-yl}-2-methyl- | ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Prior to the assay, human TK1 (R&D Systems) was activated by incubation with human trypsin (Calbiochem) in a 1:10,000 ratio for 15 min at 37° C. For ... | US Patent US10695334 (2020) BindingDB Entry DOI: 10.7270/Q2VT1W4K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine kinase, cytosolic (Homo sapiens (Human)) | BDBM448393 (7-[(2-{6,6-Difluoro-3-azabicyclo[3.1.0]hexan-3-yl}...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Prior to the assay, human TK1 (R&D Systems) was activated by incubation with human trypsin (Calbiochem) in a 1:10,000 ratio for 15 min at 37° C. For ... | US Patent US10695334 (2020) BindingDB Entry DOI: 10.7270/Q2VT1W4K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine kinase, cytosolic (Homo sapiens (Human)) | BDBM448394 (1-[(6-{5-Azaspiro[2.3]hexan-5-yl}-2-methylpyridin-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Prior to the assay, human TK1 (R&D Systems) was activated by incubation with human trypsin (Calbiochem) in a 1:10,000 ratio for 15 min at 37° C. For ... | US Patent US10695334 (2020) BindingDB Entry DOI: 10.7270/Q2VT1W4K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 209 total ) | Next | Last >> |