 Found 10695 hits of ic50 for UniProtKB: P15056
Found 10695 hits of ic50 for UniProtKB: P15056 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Serine/threonine-protein kinase B-raf [V600E]
(Homo sapiens (Human)) | BDBM87369
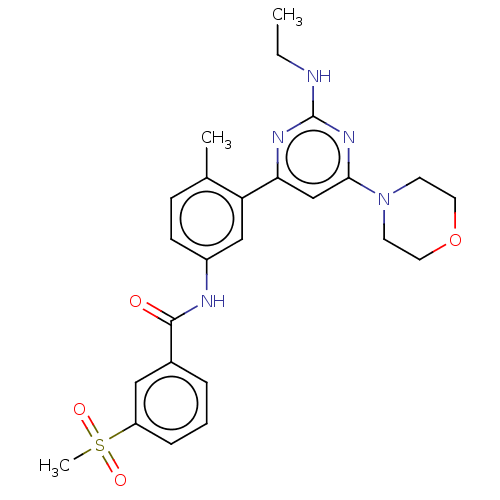
(US10245267, Example 704 | US10709712, Example 704 ...)Show SMILES CCNc1nc(cc(n1)-c1cc(NC(=O)c2cccc(c2)S(C)(=O)=O)ccc1C)N1CCOCC1 Show InChI InChI=1S/C25H29N5O4S/c1-4-26-25-28-22(16-23(29-25)30-10-12-34-13-11-30)21-15-19(9-8-17(21)2)27-24(31)18-6-5-7-20(14-18)35(3,32)33/h5-9,14-16H,4,10-13H2,1-3H3,(H,27,31)(H,26,28,29) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a |
NOVARTIS AG
US Patent
| Assay Description
C-Raf TR refers to a truncated C-Raf protein, a Δ1-324 deletion mutant.C-Raf FL refers to the full-length C-Raf protein.Full length MEK1 with an... |
US Patent US10709712 (2020)
BindingDB Entry DOI: 10.7270/Q2280BP3 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM25619
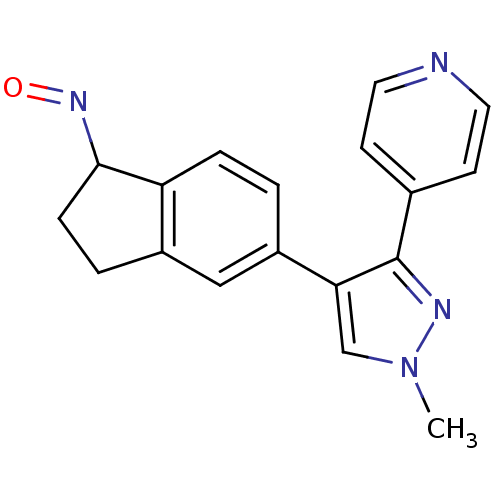
((1Z)-5-[1-methyl-3-(pyridin-4-yl)-1H-pyrazol-4-yl]...)Show InChI InChI=1S/C18H16N4O/c1-22-11-16(18(20-22)12-6-8-19-9-7-12)14-2-4-15-13(10-14)3-5-17(15)21-23/h2,4,6-11,17H,3,5H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0200 | n/a | n/a | n/a | n/a | 7.2 | 22 |
Array BioPharma
| Assay Description
Activity of human recombinant B-Raf protein was assessed in vitro by assay of the incorporation of radiolabeled phosphate to recombinant MAP kinase (... |
Bioorg Med Chem Lett 18: 4692-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.002
BindingDB Entry DOI: 10.7270/Q2NK3CBJ |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf [V600E]
(Homo sapiens (Human)) | BDBM87369

(US10245267, Example 704 | US10709712, Example 704 ...)Show SMILES CCNc1nc(cc(n1)-c1cc(NC(=O)c2cccc(c2)S(C)(=O)=O)ccc1C)N1CCOCC1 Show InChI InChI=1S/C25H29N5O4S/c1-4-26-25-28-22(16-23(29-25)30-10-12-34-13-11-30)21-15-19(9-8-17(21)2)27-24(31)18-6-5-7-20(14-18)35(3,32)33/h5-9,14-16H,4,10-13H2,1-3H3,(H,27,31)(H,26,28,29) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.0200 | n/a | n/a | n/a | n/a | 7.5 | 25 |
NOVARTIS AG
US Patent
| Assay Description
Raf and biotinylated Mek, kinase dead, were combined at 2x final concentrations in assay buffer (50 mM Tris, pH 7.5, 15 mM MgCl2, 0.01% BSA and 1 mM ... |
US Patent US9694016 (2017)
BindingDB Entry DOI: 10.7270/Q2MC8X6W |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf [V600E]
(Homo sapiens (Human)) | BDBM87369

(US10245267, Example 704 | US10709712, Example 704 ...)Show SMILES CCNc1nc(cc(n1)-c1cc(NC(=O)c2cccc(c2)S(C)(=O)=O)ccc1C)N1CCOCC1 Show InChI InChI=1S/C25H29N5O4S/c1-4-26-25-28-22(16-23(29-25)30-10-12-34-13-11-30)21-15-19(9-8-17(21)2)27-24(31)18-6-5-7-20(14-18)35(3,32)33/h5-9,14-16H,4,10-13H2,1-3H3,(H,27,31)(H,26,28,29) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
| Assay Description
Raf and biotinylated Mek, kinase dead, were combined at 2× final concentrations in assay buffer (50 mM Tris, pH 7.5, 15 mM MgCl2, 0.01% BSA and 1 mM ... |
Bioorg Med Chem Lett 17: 5115-20 (2007)
BindingDB Entry DOI: 10.7270/Q2RB76WP |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM25636
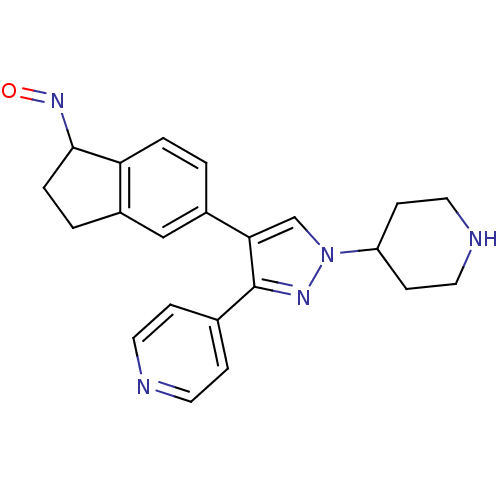
((1Z)-5-[1-(piperidin-4-yl)-3-(pyridin-4-yl)-1H-pyr...)Show SMILES O=NC1CCc2cc(ccc12)-c1cn(nc1-c1ccncc1)C1CCNCC1 Show InChI InChI=1S/C22H23N5O/c28-26-21-4-2-16-13-17(1-3-19(16)21)20-14-27(18-7-11-24-12-8-18)25-22(20)15-5-9-23-10-6-15/h1,3,5-6,9-10,13-14,18,21,24H,2,4,7-8,11-12H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0300 | n/a | n/a | n/a | n/a | 7.2 | 22 |
Array BioPharma
| Assay Description
Activity of human recombinant B-Raf protein was assessed in vitro by assay of the incorporation of radiolabeled phosphate to recombinant MAP kinase (... |
Bioorg Med Chem Lett 18: 4692-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.002
BindingDB Entry DOI: 10.7270/Q2NK3CBJ |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50006650
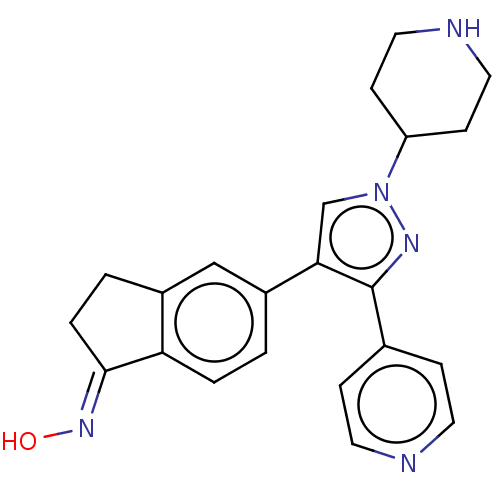
(CHEMBL526479)Show SMILES O\N=C1/CCc2cc(ccc12)-c1cn(nc1-c1ccncc1)C1CCNCC1 Show InChI InChI=1S/C22H23N5O/c28-26-21-4-2-16-13-17(1-3-19(16)21)20-14-27(18-7-11-24-12-8-18)25-22(20)15-5-9-23-10-6-15/h1,3,5-6,9-10,13-14,18,24,28H,2,4,7-8,11-12H2/b26-21+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a |
Cairo University
Curated by ChEMBL
| Assay Description
Inhibition of BRAF (unknown origin) |
Eur J Med Chem 72: 170-205 (2014)
Article DOI: 10.1016/j.ejmech.2013.11.018
BindingDB Entry DOI: 10.7270/Q2F1917Z |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM435718

(US10570155, Compound 2II | US11332479, Compound 2I...)Show SMILES CCN1CCN(CCNc2nccc(n2)-c2c(nc3occn23)-c2cccc(O)c2)S1(=O)=O Show InChI InChI=1S/C21H23N7O4S/c1-2-26-10-11-27(33(26,30)31)9-8-23-20-22-7-6-17(24-20)19-18(15-4-3-5-16(29)14-15)25-21-28(19)12-13-32-21/h3-7,12-14,29H,2,8-11H2,1H3,(H,22,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <0.0400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Reaction biology kinase hotspot service (http://www.reactionbiology.com) was used to measure IC50. In a final reaction volume of 25 μL, kinase (... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2SQ93MQ |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM25625
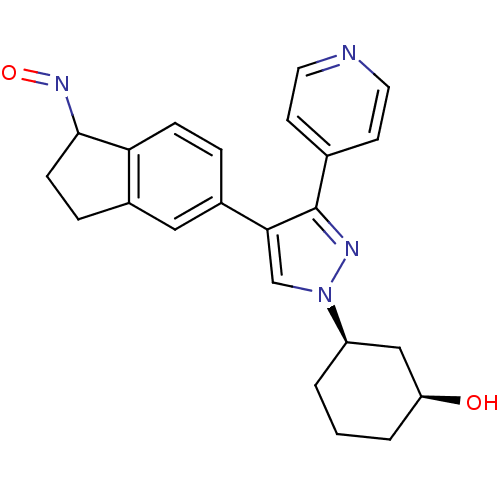
((1S,3R)-3-{4-[(1Z)-1-(hydroxyimino)-2,3-dihydro-1H...)Show SMILES O[C@H]1CCC[C@H](C1)n1cc(c(n1)-c1ccncc1)-c1ccc2C(CCc2c1)N=O |r| Show InChI InChI=1S/C23H24N4O2/c28-19-3-1-2-18(13-19)27-14-21(23(25-27)15-8-10-24-11-9-15)17-4-6-20-16(12-17)5-7-22(20)26-29/h4,6,8-12,14,18-19,22,28H,1-3,5,7,13H2/t18-,19+,22?/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0400 | n/a | n/a | n/a | n/a | 7.2 | 22 |
Array BioPharma
| Assay Description
Activity of human recombinant B-Raf protein was assessed in vitro by assay of the incorporation of radiolabeled phosphate to recombinant MAP kinase (... |
Bioorg Med Chem Lett 18: 4692-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.002
BindingDB Entry DOI: 10.7270/Q2NK3CBJ |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM435718

(US10570155, Compound 2II | US11332479, Compound 2I...)Show SMILES CCN1CCN(CCNc2nccc(n2)-c2c(nc3occn23)-c2cccc(O)c2)S1(=O)=O Show InChI InChI=1S/C21H23N7O4S/c1-2-26-10-11-27(33(26,30)31)9-8-23-20-22-7-6-17(24-20)19-18(15-4-3-5-16(29)14-15)25-21-28(19)12-13-32-21/h3-7,12-14,29H,2,8-11H2,1H3,(H,22,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <0.0400 | n/a | n/a | n/a | n/a | n/a | n/a |
KOREA INSTITUTE OF SCIENCE AND TECHNOLOGY
US Patent
| Assay Description
Reaction biology kinase hotspot service (http://www.reactionbiology.com) was used to measure IC50. In a final reaction volume of 25 μL, kinase (... |
US Patent US10570155 (2020)
BindingDB Entry DOI: 10.7270/Q2N300B1 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM25629

(3-{4-[(1Z)-1-(hydroxyimino)-2,3-dihydro-1H-inden-5...)Show SMILES OCCCn1cc(c(n1)-c1ccncc1)-c1ccc2C(CCc2c1)N=O Show InChI InChI=1S/C20H20N4O2/c25-11-1-10-24-13-18(20(22-24)14-6-8-21-9-7-14)16-2-4-17-15(12-16)3-5-19(17)23-26/h2,4,6-9,12-13,19,25H,1,3,5,10-11H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0500 | n/a | n/a | n/a | n/a | 7.2 | 22 |
Array BioPharma
| Assay Description
Activity of human recombinant B-Raf protein was assessed in vitro by assay of the incorporation of radiolabeled phosphate to recombinant MAP kinase (... |
Bioorg Med Chem Lett 18: 4692-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.002
BindingDB Entry DOI: 10.7270/Q2NK3CBJ |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf [V600E]
(Homo sapiens (Human)) | BDBM554464
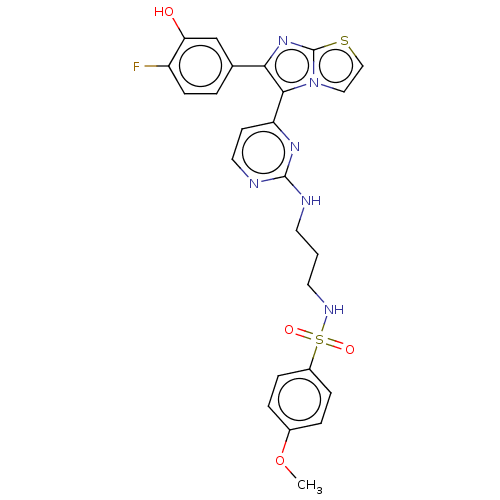
(US11332479, Compound 50III)Show SMILES COc1ccc(cc1)S(=O)(=O)NCCCNc1nccc(n1)-c1c(nc2sccn12)-c1ccc(F)c(O)c1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Reaction biology kinase hotspot service (http://www.reactionbiology.com) was used to measure IC50. In a final reaction volume of 25 μL, kinase (... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2SQ93MQ |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50585092
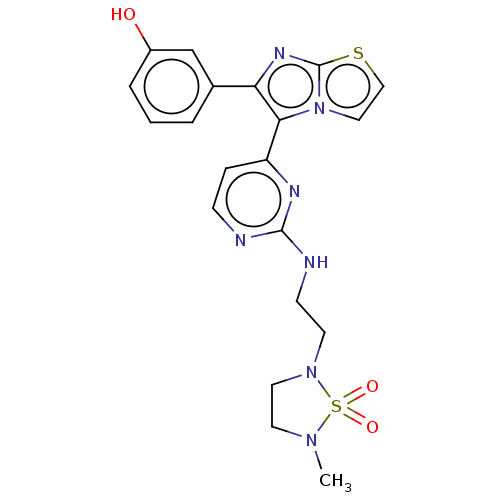
(CHEMBL5087577)Show SMILES CN1CCN(CCNc2nccc(n2)-c2c(nc3sccn23)-c2cccc(O)c2)S1(=O)=O | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human BRAF V600E mutant using myelin basic protein as substrate in presence of [gamma-33P]ATP incubated for 40 mins by scintillation co... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00230
BindingDB Entry DOI: 10.7270/Q2RV0SMN |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf [V600E]
(Homo sapiens (Human)) | BDBM554470
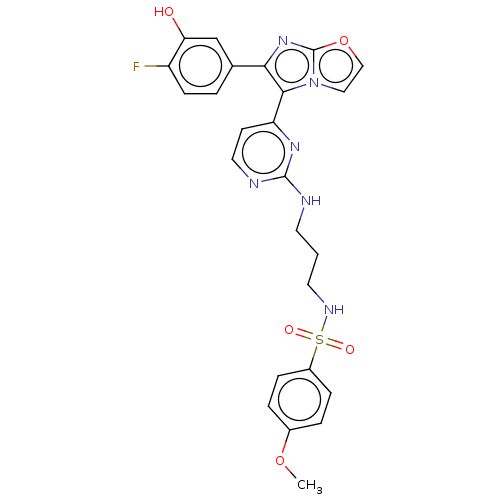
(US11332479, Compound 72IV)Show SMILES COc1ccc(cc1)S(=O)(=O)NCCCNc1nccc(n1)-c1c(nc2occn12)-c1ccc(F)c(O)c1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Reaction biology kinase hotspot service (http://www.reactionbiology.com) was used to measure IC50. In a final reaction volume of 25 μL, kinase (... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2SQ93MQ |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf [V600E]
(Homo sapiens (Human)) | BDBM103702

(US8563553, 127)Show SMILES CS(=O)(=O)Nc1cc(Cl)cc(-c2[nH]c(nc2-c2ccnc(NCCC#N)n2)C2CC2)c1F Show InChI InChI=1S/C20H19ClFN7O2S/c1-32(30,31)29-15-10-12(21)9-13(16(15)22)17-18(28-19(27-17)11-3-4-11)14-5-8-25-20(26-14)24-7-2-6-23/h5,8-11,29H,2-4,7H2,1H3,(H,27,28)(H,24,25,26) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.0800 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Novartis AG; IRM LLC
US Patent
| Assay Description
Raf/Mek amplified luminescence proximity homogeneous assay. |
US Patent US8563553 (2013)
BindingDB Entry DOI: 10.7270/Q2ZP44R7 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf [V600E]
(Homo sapiens (Human)) | BDBM103683
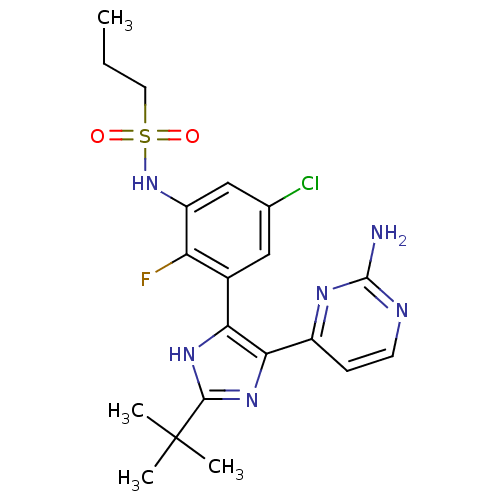
(US8563553, 108)Show SMILES CCCS(=O)(=O)Nc1cc(Cl)cc(-c2[nH]c(nc2-c2ccnc(N)n2)C(C)(C)C)c1F Show InChI InChI=1S/C20H24ClFN6O2S/c1-5-8-31(29,30)28-14-10-11(21)9-12(15(14)22)16-17(13-6-7-24-19(23)25-13)27-18(26-16)20(2,3)4/h6-7,9-10,28H,5,8H2,1-4H3,(H,26,27)(H2,23,24,25) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.0800 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Novartis AG; IRM LLC
US Patent
| Assay Description
Raf/Mek amplified luminescence proximity homogeneous assay. |
US Patent US8563553 (2013)
BindingDB Entry DOI: 10.7270/Q2ZP44R7 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM25626
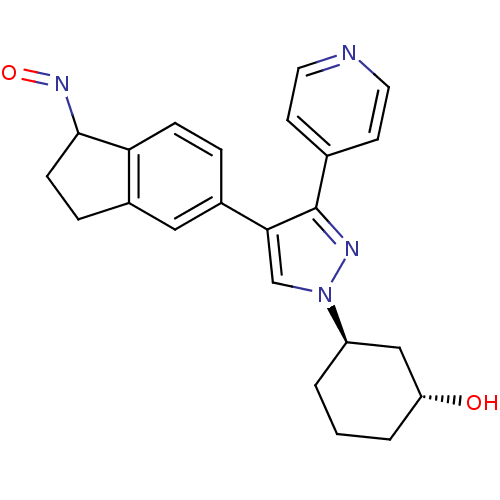
((1R,3R)-3-{4-[(1Z)-1-(hydroxyimino)-2,3-dihydro-1H...)Show SMILES O[C@@H]1CCC[C@H](C1)n1cc(c(n1)-c1ccncc1)-c1ccc2C(CCc2c1)N=O |r| Show InChI InChI=1S/C23H24N4O2/c28-19-3-1-2-18(13-19)27-14-21(23(25-27)15-8-10-24-11-9-15)17-4-6-20-16(12-17)5-7-22(20)26-29/h4,6,8-12,14,18-19,22,28H,1-3,5,7,13H2/t18-,19-,22?/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | 7.2 | 22 |
Array BioPharma
| Assay Description
Activity of human recombinant B-Raf protein was assessed in vitro by assay of the incorporation of radiolabeled phosphate to recombinant MAP kinase (... |
Bioorg Med Chem Lett 18: 4692-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.002
BindingDB Entry DOI: 10.7270/Q2NK3CBJ |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50481593
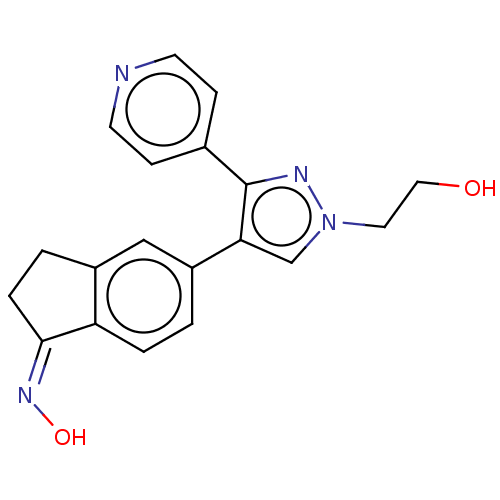
(CHEMBL5287468)Show SMILES COP(F)(=O)CCCn1cc(CNS(=O)(=O)c2ccc3ccc4cccc5ccc2c3c45)nn1 Show InChI InChI=1S/C23H22FN4O4PS/c1-32-33(24,29)13-3-12-28-15-19(26-27-28)14-25-34(30,31)21-11-9-18-7-6-16-4-2-5-17-8-10-20(21)23(18)22(16)17/h2,4-11,15,25H,3,12-14H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50026212
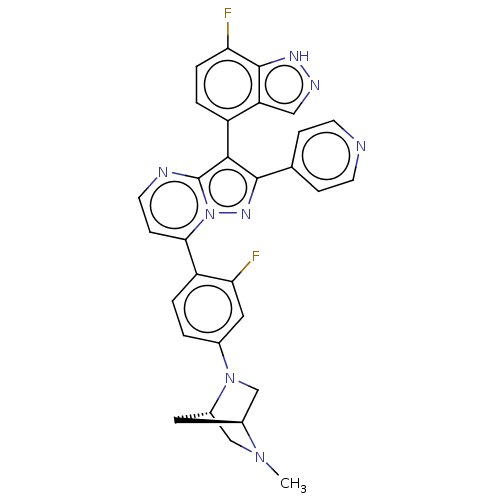
(CHEMBL1276185)Show SMILES [H][C@]12CN(c3ccc(c(F)c3)-c3ccnc4c(c(nn34)-c3ccncc3)-c3ccc(F)c4[nH]ncc34)[C@]([H])(CN1C)C2 |r,wU:36.42,1.0,(5.96,-12.33,;4.87,-13.43,;4.88,-14.97,;3.54,-15.75,;3.55,-17.28,;4.89,-18.05,;4.89,-19.59,;3.56,-20.36,;2.23,-19.6,;.9,-20.38,;2.22,-18.06,;3.57,-21.9,;2.24,-22.67,;2.24,-24.21,;3.57,-24.98,;4.91,-24.21,;6.38,-24.69,;7.29,-23.44,;6.38,-22.18,;4.91,-22.66,;8.83,-23.43,;9.6,-24.77,;11.14,-24.77,;11.91,-23.43,;11.13,-22.09,;9.6,-22.1,;6.86,-26.15,;8.36,-26.47,;8.84,-27.93,;7.81,-29.09,;8.29,-30.55,;6.3,-28.76,;5.05,-29.66,;3.81,-28.75,;4.3,-27.29,;5.83,-27.3,;2.21,-14.99,;.66,-14.98,;2.2,-13.45,;3.53,-12.67,;3.53,-11.12,;3.29,-13.89,)| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of BRAF |
J Med Chem 53: 7874-8 (2010)
Article DOI: 10.1021/jm1007566
BindingDB Entry DOI: 10.7270/Q2HM58Q3 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50026207
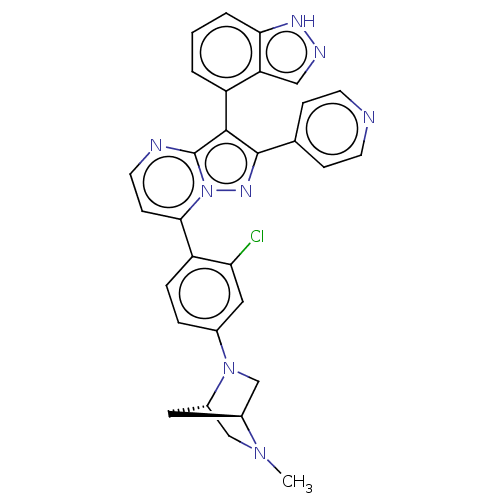
(CHEMBL1276170)Show SMILES [H][C@]12CN(c3ccc(c(Cl)c3)-c3ccnc4c(c(nn34)-c3ccncc3)-c3cccc4[nH]ncc34)[C@]([H])(CN1C)C2 |r,wU:35.41,1.0,(38.98,4.96,;37.9,3.86,;37.9,2.32,;36.57,1.54,;36.58,.01,;37.92,-.76,;37.92,-2.3,;36.58,-3.07,;35.25,-2.31,;33.92,-3.09,;35.24,-.77,;36.59,-4.61,;35.26,-5.38,;35.26,-6.93,;36.6,-7.7,;37.93,-6.92,;39.41,-7.4,;40.32,-6.15,;39.41,-4.89,;37.93,-5.37,;41.86,-6.14,;42.63,-7.48,;44.17,-7.48,;44.94,-6.15,;44.16,-4.81,;42.62,-4.81,;39.88,-8.87,;41.39,-9.18,;41.87,-10.64,;40.83,-11.8,;39.32,-11.48,;38.08,-12.37,;36.84,-11.46,;37.32,-10.01,;38.86,-10.01,;35.24,2.3,;33.69,2.3,;35.22,3.84,;36.56,4.62,;36.55,6.17,;36.32,3.4,)| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of BRAF |
J Med Chem 53: 7874-8 (2010)
Article DOI: 10.1021/jm1007566
BindingDB Entry DOI: 10.7270/Q2HM58Q3 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50139058
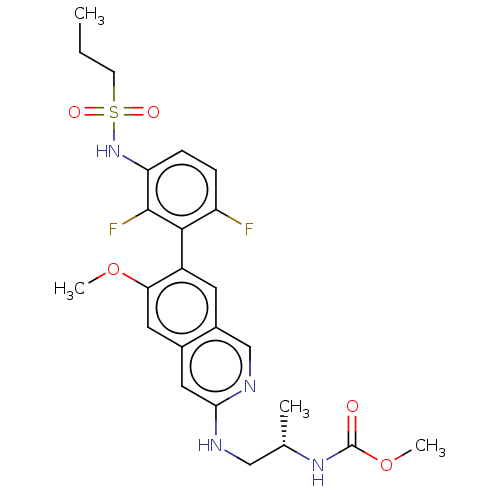
(CHEMBL3752213)Show SMILES CCCS(=O)(=O)Nc1ccc(F)c(c1F)-c1cc2cnc(NC[C@H](C)NC(=O)OC)cc2cc1OC |r,wU:23.24,(-13.09,.93,;-12.02,1.55,;-10.69,.78,;-9.35,1.54,;-9.35,2.78,;-8.28,2.16,;-8.02,.77,;-6.68,1.54,;-6.68,3.08,;-5.35,3.85,;-4.01,3.08,;-2.95,3.7,;-4.01,1.54,;-5.35,.77,;-5.35,-.46,;-2.68,.77,;-1.33,1.54,;,.77,;1.33,1.54,;2.66,.77,;2.66,-.77,;4,-1.54,;5.33,-.76,;6.67,-1.53,;6.67,-2.76,;8,-.76,;9.34,-1.53,;9.34,-2.76,;10.67,-.75,;11.74,-1.37,;1.33,-1.54,;,-.77,;-1.33,-1.54,;-2.68,-.77,;-4.01,-1.54,;-4.01,-2.77,)| Show InChI InChI=1S/C24H28F2N4O5S/c1-5-8-36(32,33)30-19-7-6-18(25)22(23(19)26)17-9-16-13-28-21(11-15(16)10-20(17)34-3)27-12-14(2)29-24(31)35-4/h6-7,9-11,13-14,30H,5,8,12H2,1-4H3,(H,27,28)(H,29,31)/t14-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Hengrui Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of B-Raf V600E mutant (unknown origin) by lantha screen assay |
Bioorg Med Chem Lett 26: 819-23 (2016)
Article DOI: 10.1016/j.bmcl.2015.12.086
BindingDB Entry DOI: 10.7270/Q2W097S9 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf [V600E]
(Homo sapiens (Human)) | BDBM103632
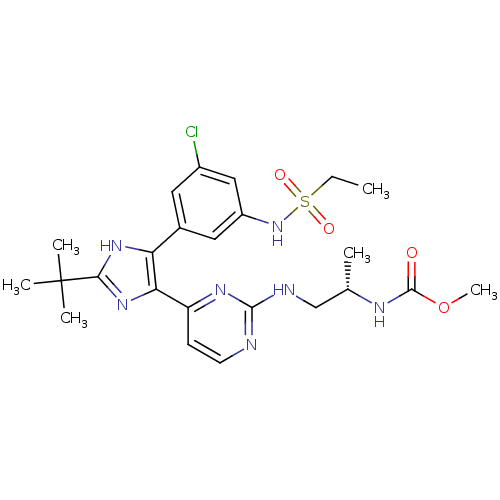
(US8563553, 57)Show SMILES CCS(=O)(=O)Nc1cc(Cl)cc(c1)-c1[nH]c(nc1-c1ccnc(NC[C@H](C)NC(=O)OC)n1)C(C)(C)C |r| Show InChI InChI=1S/C24H32ClN7O4S/c1-7-37(34,35)32-17-11-15(10-16(25)12-17)19-20(31-21(30-19)24(3,4)5)18-8-9-26-22(29-18)27-13-14(2)28-23(33)36-6/h8-12,14,32H,7,13H2,1-6H3,(H,28,33)(H,30,31)(H,26,27,29)/t14-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Novartis AG; IRM LLC
US Patent
| Assay Description
Raf/Mek amplified luminescence proximity homogeneous assay. |
US Patent US8563553 (2013)
BindingDB Entry DOI: 10.7270/Q2ZP44R7 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf [V600E]
(Homo sapiens (Human)) | BDBM103684

(US8563553, 109)Show SMILES COC(=O)N[C@@H](C)CNc1nccc(n1)-c1nc([nH]c1-c1cc(F)cc(NS(=O)(=O)c2c(F)cccc2F)c1Cl)C1CC1 |r| Show InChI InChI=1S/C27H25ClF3N7O4S/c1-13(34-27(39)42-2)12-33-26-32-9-8-19(35-26)23-22(36-25(37-23)14-6-7-14)16-10-15(29)11-20(21(16)28)38-43(40,41)24-17(30)4-3-5-18(24)31/h3-5,8-11,13-14,38H,6-7,12H2,1-2H3,(H,34,39)(H,36,37)(H,32,33,35)/t13-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Novartis AG; IRM LLC
US Patent
| Assay Description
Raf/Mek amplified luminescence proximity homogeneous assay. |
US Patent US8563553 (2013)
BindingDB Entry DOI: 10.7270/Q2ZP44R7 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf [V600E]
(Homo sapiens (Human)) | BDBM103628
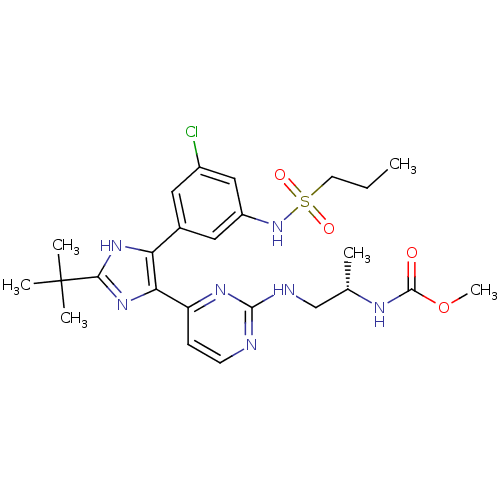
(US8563553, 53)Show SMILES CCCS(=O)(=O)Nc1cc(Cl)cc(c1)-c1[nH]c(nc1-c1ccnc(NC[C@H](C)NC(=O)OC)n1)C(C)(C)C |r| Show InChI InChI=1S/C25H34ClN7O4S/c1-7-10-38(35,36)33-18-12-16(11-17(26)13-18)20-21(32-22(31-20)25(3,4)5)19-8-9-27-23(30-19)28-14-15(2)29-24(34)37-6/h8-9,11-13,15,33H,7,10,14H2,1-6H3,(H,29,34)(H,31,32)(H,27,28,30)/t15-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Novartis AG; IRM LLC
US Patent
| Assay Description
Raf/Mek amplified luminescence proximity homogeneous assay. |
US Patent US8563553 (2013)
BindingDB Entry DOI: 10.7270/Q2ZP44R7 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf [V600E]
(Homo sapiens (Human)) | BDBM103629

(US8563553, 54)Show SMILES COC(=O)N[C@@H](C)CNc1nccc(n1)-c1nc([nH]c1-c1cc(Cl)cc(NS(=O)(=O)C2CC2)c1)C(C)(C)C |r| Show InChI InChI=1S/C25H32ClN7O4S/c1-14(29-24(34)37-5)13-28-23-27-9-8-19(30-23)21-20(31-22(32-21)25(2,3)4)15-10-16(26)12-17(11-15)33-38(35,36)18-6-7-18/h8-12,14,18,33H,6-7,13H2,1-5H3,(H,29,34)(H,31,32)(H,27,28,30)/t14-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Novartis AG; IRM LLC
US Patent
| Assay Description
Raf/Mek amplified luminescence proximity homogeneous assay. |
US Patent US8563553 (2013)
BindingDB Entry DOI: 10.7270/Q2ZP44R7 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf [V600E]
(Homo sapiens (Human)) | BDBM103681

(US8563553, 106)Show SMILES Nc1nccc(n1)-c1nc([nH]c1-c1cc(Cl)cc(NS(=O)(=O)c2c(F)cccc2F)c1F)C1CC1 Show InChI InChI=1S/C22H16ClF3N6O2S/c23-11-8-12(17(26)16(9-11)32-35(33,34)20-13(24)2-1-3-14(20)25)18-19(15-6-7-28-22(27)29-15)31-21(30-18)10-4-5-10/h1-3,6-10,32H,4-5H2,(H,30,31)(H2,27,28,29) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Novartis AG; IRM LLC
US Patent
| Assay Description
Raf/Mek amplified luminescence proximity homogeneous assay. |
US Patent US8563553 (2013)
BindingDB Entry DOI: 10.7270/Q2ZP44R7 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50330925
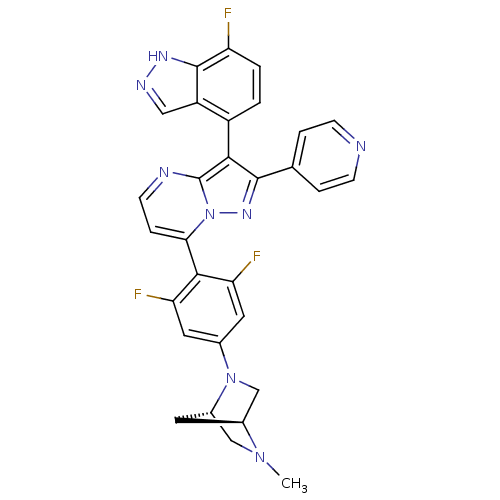
(7-(2,6-difluoro-4-((1S,4S)-5-methyl-2,5-diazabicyc...)Show SMILES CN1C[C@@H]2C[C@H]1CN2c1cc(F)c(c(F)c1)-c1ccnc2c(c(nn12)-c1ccncc1)-c1ccc(F)c2[nH]ncc12 |r,wU:3.3,5.4,(16.85,-11.72,;16.86,-13.27,;15.53,-14.05,;15.54,-15.59,;16.62,-14.49,;18.2,-14.03,;18.21,-15.57,;16.87,-16.35,;16.88,-17.88,;15.54,-18.66,;15.55,-20.2,;14.22,-20.98,;16.89,-20.96,;18.22,-20.19,;19.56,-20.96,;18.22,-18.65,;16.9,-22.5,;15.57,-23.27,;15.57,-24.82,;16.9,-25.59,;18.24,-24.81,;19.71,-25.29,;20.62,-24.04,;19.71,-22.78,;18.23,-23.26,;22.16,-24.03,;22.93,-25.37,;24.47,-25.37,;25.24,-24.04,;24.46,-22.7,;22.92,-22.7,;20.19,-26.76,;21.69,-27.07,;22.17,-28.53,;21.14,-29.69,;21.62,-31.15,;19.63,-29.37,;18.38,-30.26,;17.14,-29.36,;17.62,-27.9,;19.16,-27.9,)| Show InChI InChI=1S/C30H23F3N8/c1-39-14-19-10-18(39)15-40(19)17-11-23(32)27(24(33)12-17)25-6-9-35-30-26(20-2-3-22(31)29-21(20)13-36-37-29)28(38-41(25)30)16-4-7-34-8-5-16/h2-9,11-13,18-19H,10,14-15H2,1H3,(H,36,37)/t18-,19-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of BRAF |
J Med Chem 53: 7874-8 (2010)
Article DOI: 10.1021/jm1007566
BindingDB Entry DOI: 10.7270/Q2HM58Q3 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf [V600E]
(Homo sapiens (Human)) | BDBM103699
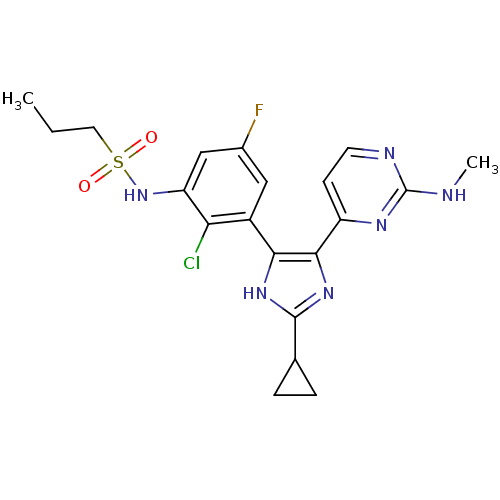
(US8563553, 124)Show SMILES CCCS(=O)(=O)Nc1cc(F)cc(-c2[nH]c(nc2-c2ccnc(NC)n2)C2CC2)c1Cl Show InChI InChI=1S/C20H22ClFN6O2S/c1-3-8-31(29,30)28-15-10-12(22)9-13(16(15)21)17-18(27-19(26-17)11-4-5-11)14-6-7-24-20(23-2)25-14/h6-7,9-11,28H,3-5,8H2,1-2H3,(H,26,27)(H,23,24,25) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.110 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Novartis AG; IRM LLC
US Patent
| Assay Description
Raf/Mek amplified luminescence proximity homogeneous assay. |
US Patent US8563553 (2013)
BindingDB Entry DOI: 10.7270/Q2ZP44R7 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf [V600E]
(Homo sapiens (Human)) | BDBM103677

(US8563553, 102)Show SMILES CC1(CC1)c1nc(c([nH]1)-c1cc(Cl)cc(NS(C)(=O)=O)c1F)-c1ccnc(NCCC#N)n1 Show InChI InChI=1S/C21H21ClFN7O2S/c1-21(5-6-21)19-28-17(13-10-12(22)11-15(16(13)23)30-33(2,31)32)18(29-19)14-4-9-26-20(27-14)25-8-3-7-24/h4,9-11,30H,3,5-6,8H2,1-2H3,(H,28,29)(H,25,26,27) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.110 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Novartis AG; IRM LLC
US Patent
| Assay Description
Raf/Mek amplified luminescence proximity homogeneous assay. |
US Patent US8563553 (2013)
BindingDB Entry DOI: 10.7270/Q2ZP44R7 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf [V600E]
(Homo sapiens (Human)) | BDBM103723

(US8563553, 148)Show SMILES CCCS(=O)(=O)Nc1c(F)ccc(-c2[nH]c(nc2-c2ccnc(NC[C@H](C)NC(=O)OC)n2)C(C)(C)C)c1F |r| Show InChI InChI=1S/C25H33F2N7O4S/c1-7-12-39(36,37)34-20-16(26)9-8-15(18(20)27)19-21(33-22(32-19)25(3,4)5)17-10-11-28-23(31-17)29-13-14(2)30-24(35)38-6/h8-11,14,34H,7,12-13H2,1-6H3,(H,30,35)(H,32,33)(H,28,29,31)/t14-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.120 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Novartis AG; IRM LLC
US Patent
| Assay Description
Raf/Mek amplified luminescence proximity homogeneous assay. |
US Patent US8563553 (2013)
BindingDB Entry DOI: 10.7270/Q2ZP44R7 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM25628
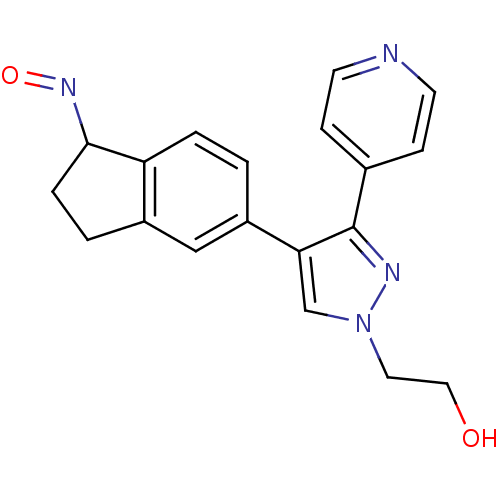
(2-{4-[(1Z)-1-(hydroxyimino)-2,3-dihydro-1H-inden-5...)Show InChI InChI=1S/C19H18N4O2/c24-10-9-23-12-17(19(21-23)13-5-7-20-8-6-13)15-1-3-16-14(11-15)2-4-18(16)22-25/h1,3,5-8,11-12,18,24H,2,4,9-10H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.130 | n/a | n/a | n/a | n/a | 7.2 | 22 |
Array BioPharma
| Assay Description
Activity of human recombinant B-Raf protein was assessed in vitro by assay of the incorporation of radiolabeled phosphate to recombinant MAP kinase (... |
Bioorg Med Chem Lett 18: 4692-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.002
BindingDB Entry DOI: 10.7270/Q2NK3CBJ |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf [V600E]
(Homo sapiens (Human)) | BDBM103721
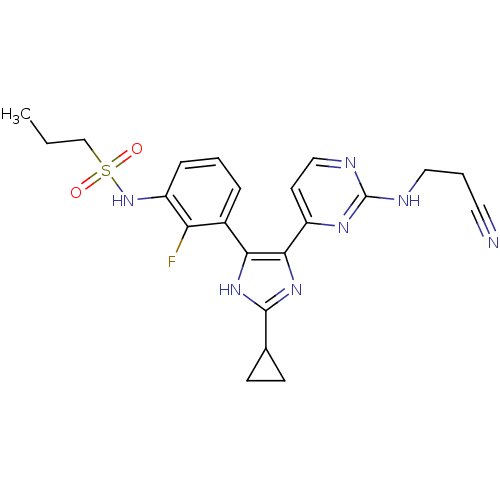
(US8563553, 146)Show SMILES CCCS(=O)(=O)Nc1cccc(-c2[nH]c(nc2-c2ccnc(NCCC#N)n2)C2CC2)c1F Show InChI InChI=1S/C22H24FN7O2S/c1-2-13-33(31,32)30-16-6-3-5-15(18(16)23)19-20(29-21(28-19)14-7-8-14)17-9-12-26-22(27-17)25-11-4-10-24/h3,5-6,9,12,14,30H,2,4,7-8,11,13H2,1H3,(H,28,29)(H,25,26,27) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.130 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Novartis AG; IRM LLC
US Patent
| Assay Description
Raf/Mek amplified luminescence proximity homogeneous assay. |
US Patent US8563553 (2013)
BindingDB Entry DOI: 10.7270/Q2ZP44R7 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf [V600E]
(Homo sapiens (Human)) | BDBM103715

(US8563553, 140)Show SMILES CCCS(=O)(=O)Nc1cccc(-c2[nH]c(nc2-c2ccnc(NC[C@H](C)NC(=O)OC)n2)C2CC2)c1Cl |r| Show InChI InChI=1S/C24H30ClN7O4S/c1-4-12-37(34,35)32-17-7-5-6-16(19(17)25)20-21(31-22(30-20)15-8-9-15)18-10-11-26-23(29-18)27-13-14(2)28-24(33)36-3/h5-7,10-11,14-15,32H,4,8-9,12-13H2,1-3H3,(H,28,33)(H,30,31)(H,26,27,29)/t14-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.130 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Novartis AG; IRM LLC
US Patent
| Assay Description
Raf/Mek amplified luminescence proximity homogeneous assay. |
US Patent US8563553 (2013)
BindingDB Entry DOI: 10.7270/Q2ZP44R7 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf [V600E]
(Homo sapiens (Human)) | BDBM103680

(US8563553, 105)Show SMILES CC(C)(C)c1nc(c([nH]1)-c1cc(Cl)cc(NS(=O)(=O)c2c(F)cccc2F)c1F)-c1ccnc(N)n1 Show InChI InChI=1S/C23H20ClF3N6O2S/c1-23(2,3)21-31-18(19(32-21)15-7-8-29-22(28)30-15)12-9-11(24)10-16(17(12)27)33-36(34,35)20-13(25)5-4-6-14(20)26/h4-10,33H,1-3H3,(H,31,32)(H2,28,29,30) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.130 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Novartis AG; IRM LLC
US Patent
| Assay Description
Raf/Mek amplified luminescence proximity homogeneous assay. |
US Patent US8563553 (2013)
BindingDB Entry DOI: 10.7270/Q2ZP44R7 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf [V600E]
(Homo sapiens (Human)) | BDBM554462
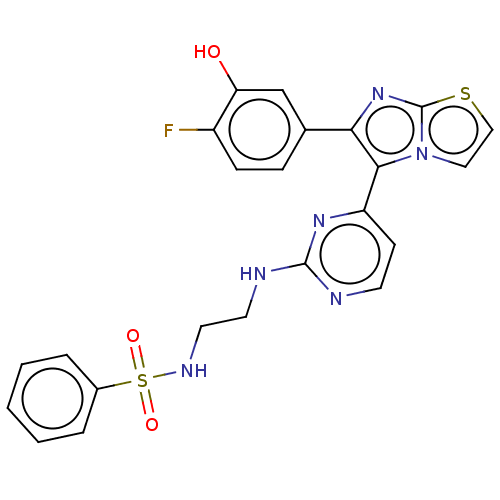
(US11332479, Compound 46III)Show SMILES Oc1cc(ccc1F)-c1nc2sccn2c1-c1ccnc(NCCNS(=O)(=O)c2ccccc2)n1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Reaction biology kinase hotspot service (http://www.reactionbiology.com) was used to measure IC50. In a final reaction volume of 25 μL, kinase (... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2SQ93MQ |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf [V600E]
(Homo sapiens (Human)) | BDBM554469

(US11332479, Compound 70IV)Show SMILES Oc1cc(ccc1F)-c1nc2occn2c1-c1ccnc(NCCCNS(=O)(=O)c2ccc(Cl)cc2)n1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Reaction biology kinase hotspot service (http://www.reactionbiology.com) was used to measure IC50. In a final reaction volume of 25 μL, kinase (... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2SQ93MQ |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM25618
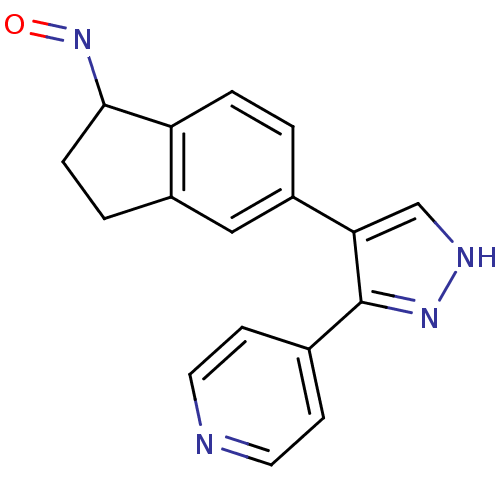
((1Z)-5-[3-(pyridin-4-yl)-1H-pyrazol-4-yl]-2,3-dihy...)Show InChI InChI=1S/C17H14N4O/c22-21-16-4-2-12-9-13(1-3-14(12)16)15-10-19-20-17(15)11-5-7-18-8-6-11/h1,3,5-10,16H,2,4H2,(H,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.150 | n/a | n/a | n/a | n/a | 7.2 | 22 |
Array BioPharma
| Assay Description
Activity of human recombinant B-Raf protein was assessed in vitro by assay of the incorporation of radiolabeled phosphate to recombinant MAP kinase (... |
Bioorg Med Chem Lett 18: 4692-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.002
BindingDB Entry DOI: 10.7270/Q2NK3CBJ |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM202851
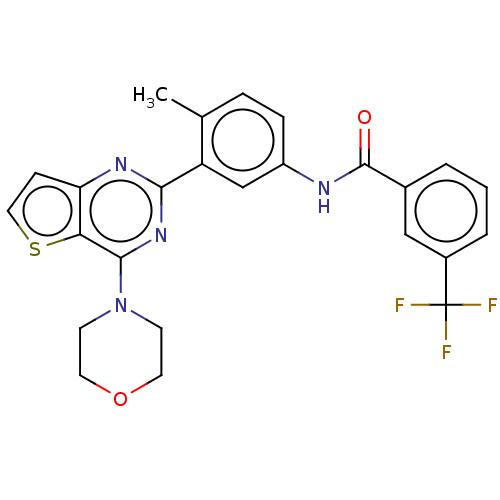
(US10245267, Example 829 | US10709712, Example 204 ...)Show SMILES Cc1ccc(NC(=O)c2cccc(c2)C(F)(F)F)cc1-c1nc(N2CCOCC2)c2sccc2n1 Show InChI InChI=1S/C25H21F3N4O2S/c1-15-5-6-18(29-24(33)16-3-2-4-17(13-16)25(26,27)28)14-19(15)22-30-20-7-12-35-21(20)23(31-22)32-8-10-34-11-9-32/h2-7,12-14H,8-11H2,1H3,(H,29,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.152 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Novartis AG
US Patent
| Assay Description
Final assay conditions were 4 pM cRaf, 3 uM ATP, 10 nM biotin tagged MEK1 kinase dead protein substrate. Reactions were performed in Greiner384 well ... |
US Patent US9242969 (2016)
BindingDB Entry DOI: 10.7270/Q2VT1QXT |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf [V600E]
(Homo sapiens (Human)) | BDBM202851

(US10245267, Example 829 | US10709712, Example 204 ...)Show SMILES Cc1ccc(NC(=O)c2cccc(c2)C(F)(F)F)cc1-c1nc(N2CCOCC2)c2sccc2n1 Show InChI InChI=1S/C25H21F3N4O2S/c1-15-5-6-18(29-24(33)16-3-2-4-17(13-16)25(26,27)28)14-19(15)22-30-20-7-12-35-21(20)23(31-22)32-8-10-34-11-9-32/h2-7,12-14H,8-11H2,1H3,(H,29,33) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.152 | n/a | n/a | n/a | n/a | n/a | n/a |
NOVARTIS AG
US Patent
| Assay Description
C-Raf TR refers to a truncated C-Raf protein, a Δ1-324 deletion mutant.C-Raf FL refers to the full-length C-Raf protein.Full length MEK1 with an... |
US Patent US10709712 (2020)
BindingDB Entry DOI: 10.7270/Q2280BP3 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf [V600E]
(Homo sapiens (Human)) | BDBM554471

(US11332479, Compound 73IV)Show SMILES Oc1cc(ccc1F)-c1nc2occn2c1-c1ccnc(NCCCNS(=O)(=O)c2ccccc2)n1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Reaction biology kinase hotspot service (http://www.reactionbiology.com) was used to measure IC50. In a final reaction volume of 25 μL, kinase (... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2SQ93MQ |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf [V600E]
(Homo sapiens (Human)) | BDBM103717
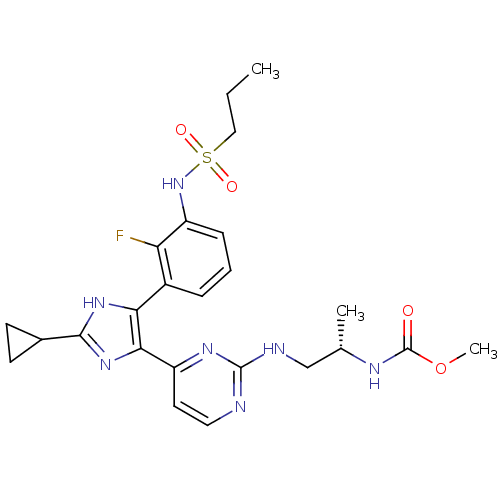
(US8563553, 142)Show SMILES CCCS(=O)(=O)Nc1cccc(-c2[nH]c(nc2-c2ccnc(NC[C@H](C)NC(=O)OC)n2)C2CC2)c1F |r| Show InChI InChI=1S/C24H30FN7O4S/c1-4-12-37(34,35)32-17-7-5-6-16(19(17)25)20-21(31-22(30-20)15-8-9-15)18-10-11-26-23(29-18)27-13-14(2)28-24(33)36-3/h5-7,10-11,14-15,32H,4,8-9,12-13H2,1-3H3,(H,28,33)(H,30,31)(H,26,27,29)/t14-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.160 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Novartis AG; IRM LLC
US Patent
| Assay Description
Raf/Mek amplified luminescence proximity homogeneous assay. |
US Patent US8563553 (2013)
BindingDB Entry DOI: 10.7270/Q2ZP44R7 |
More data for this
Ligand-Target Pair | |
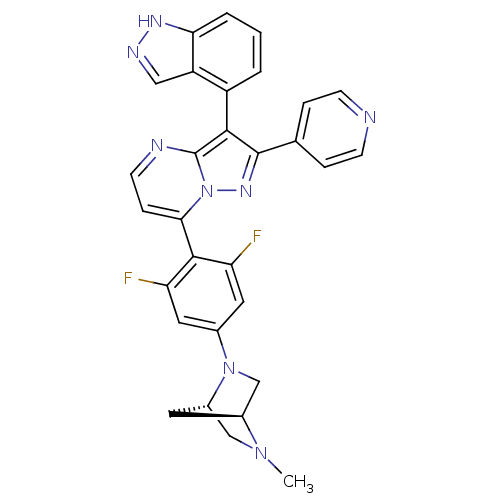
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50330905
(7-(2,6-difluoro-4-((1S,4S)-5-methyl-2,5-diazabicyc...)Show SMILES CN1C[C@@H]2C[C@H]1CN2c1cc(F)c(c(F)c1)-c1ccnc2c(c(nn12)-c1ccncc1)-c1cccc2[nH]ncc12 |r,wU:3.3,5.4,(24.13,-33.61,;24.13,-35.15,;22.8,-35.94,;22.81,-37.47,;23.89,-36.37,;25.47,-35.92,;25.48,-37.46,;24.14,-38.23,;24.15,-39.77,;22.82,-40.55,;22.83,-42.09,;21.5,-42.86,;24.16,-42.85,;25.5,-42.07,;26.83,-42.84,;25.49,-40.53,;24.17,-44.38,;22.84,-45.15,;22.84,-46.7,;24.17,-47.47,;25.51,-46.7,;26.98,-47.18,;27.89,-45.92,;26.98,-44.67,;25.51,-45.15,;29.43,-45.92,;30.2,-47.26,;31.74,-47.26,;32.51,-45.92,;31.73,-44.58,;30.2,-44.59,;27.46,-48.64,;28.96,-48.96,;29.44,-50.42,;28.41,-51.57,;26.9,-51.25,;25.65,-52.15,;24.41,-51.24,;24.9,-49.78,;26.43,-49.79,)| Show InChI InChI=1S/C30H24F2N8/c1-38-15-20-11-19(38)16-39(20)18-12-23(31)28(24(32)13-18)26-7-10-34-30-27(21-3-2-4-25-22(21)14-35-36-25)29(37-40(26)30)17-5-8-33-9-6-17/h2-10,12-14,19-20H,11,15-16H2,1H3,(H,35,36)/t19-,20-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of BRAF |
J Med Chem 53: 7874-8 (2010)
Article DOI: 10.1021/jm1007566
BindingDB Entry DOI: 10.7270/Q2HM58Q3 |
More data for this
Ligand-Target Pair | |
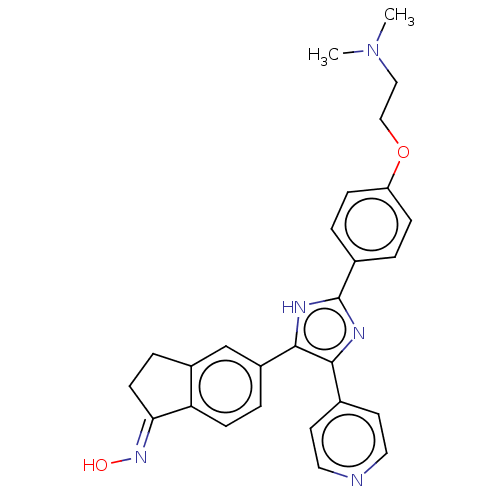
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50457452
(SB-590885 | SB590885)Show SMILES CN(C)CCOc1ccc(cc1)-c1nc(c([nH]1)-c1ccc2\C(CCc2c1)=N\O)-c1ccncc1 Show InChI InChI=1S/C27H27N5O2/c1-32(2)15-16-34-22-7-3-19(4-8-22)27-29-25(18-11-13-28-14-12-18)26(30-27)21-5-9-23-20(17-21)6-10-24(23)31-33/h3-5,7-9,11-14,17,33H,6,10,15-16H2,1-2H3,(H,29,30)/b31-24+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a |
Albert Einstein College of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of full length human 6His-tagged BRAF V600E mutant expressed in baculovirus infected insect cells co-expressing human CDC37 (1 to 378 resi... |
J Med Chem 61: 5775-5793 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01306
BindingDB Entry DOI: 10.7270/Q2J968ZT |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50029085
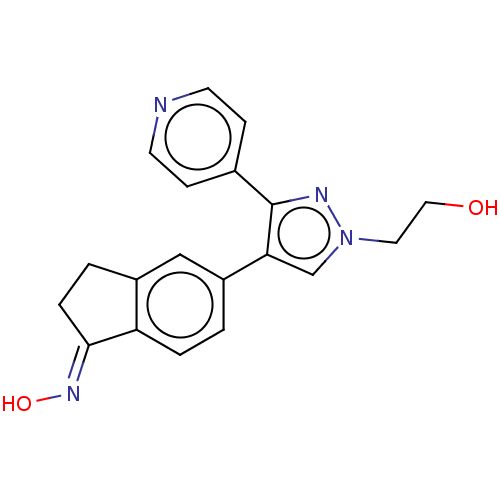
(CHEBI:83405 | CHEMBL525191 | GDC-0879)Show SMILES OCCn1cc(c(n1)-c1ccncc1)-c1ccc2\C(CCc2c1)=N\O Show InChI InChI=1S/C19H18N4O2/c24-10-9-23-12-17(19(21-23)13-5-7-20-8-6-13)15-1-3-16-14(11-15)2-4-18(16)22-25/h1,3,5-8,11-12,24-25H,2,4,9-10H2/b22-18+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a |
Albert Einstein College of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of BRAF V600E mutant in human MALME-3M cells |
J Med Chem 61: 5775-5793 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01306
BindingDB Entry DOI: 10.7270/Q2J968ZT |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Serine/threonine-protein kinase B-raf [V600E]
(Homo sapiens (Human)) | BDBM103648
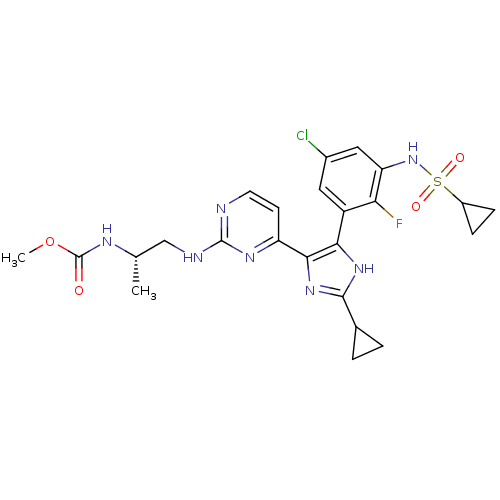
(US8563553, 73)Show SMILES COC(=O)N[C@@H](C)CNc1nccc(n1)-c1nc([nH]c1-c1cc(Cl)cc(NS(=O)(=O)C2CC2)c1F)C1CC1 |r| Show InChI InChI=1S/C24H27ClFN7O4S/c1-12(29-24(34)37-2)11-28-23-27-8-7-17(30-23)21-20(31-22(32-21)13-3-4-13)16-9-14(25)10-18(19(16)26)33-38(35,36)15-5-6-15/h7-10,12-13,15,33H,3-6,11H2,1-2H3,(H,29,34)(H,31,32)(H,27,28,30)/t12-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.180 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Novartis AG; IRM LLC
US Patent
| Assay Description
Raf/Mek amplified luminescence proximity homogeneous assay. |
US Patent US8563553 (2013)
BindingDB Entry DOI: 10.7270/Q2ZP44R7 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf [V600E]
(Homo sapiens (Human)) | BDBM103647

(US8563553, 72)Show SMILES COC(=O)N[C@@H](C)CNc1nccc(n1)-c1nc([nH]c1-c1cc(Cl)cc(NS(C)(=O)=O)c1Cl)C1CC1 |r| Show InChI InChI=1S/C22H25Cl2N7O4S/c1-11(27-22(32)35-2)10-26-21-25-7-6-15(28-21)19-18(29-20(30-19)12-4-5-12)14-8-13(23)9-16(17(14)24)31-36(3,33)34/h6-9,11-12,31H,4-5,10H2,1-3H3,(H,27,32)(H,29,30)(H,25,26,28)/t11-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.180 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Novartis AG; IRM LLC
US Patent
| Assay Description
Raf/Mek amplified luminescence proximity homogeneous assay. |
US Patent US8563553 (2013)
BindingDB Entry DOI: 10.7270/Q2ZP44R7 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf [V600E]
(Homo sapiens (Human)) | BDBM103693

(US8563553, 118)Show SMILES Nc1nccc(n1)-c1nc([nH]c1-c1cc(F)cc(NS(=O)(=O)c2c(F)cccc2F)c1Cl)C1CC1 Show InChI InChI=1S/C22H16ClF3N6O2S/c23-17-12(18-19(15-6-7-28-22(27)29-15)31-21(30-18)10-4-5-10)8-11(24)9-16(17)32-35(33,34)20-13(25)2-1-3-14(20)26/h1-3,6-10,32H,4-5H2,(H,30,31)(H2,27,28,29) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.190 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Novartis AG; IRM LLC
US Patent
| Assay Description
Raf/Mek amplified luminescence proximity homogeneous assay. |
US Patent US8563553 (2013)
BindingDB Entry DOI: 10.7270/Q2ZP44R7 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf [V600E]
(Homo sapiens (Human)) | BDBM221684
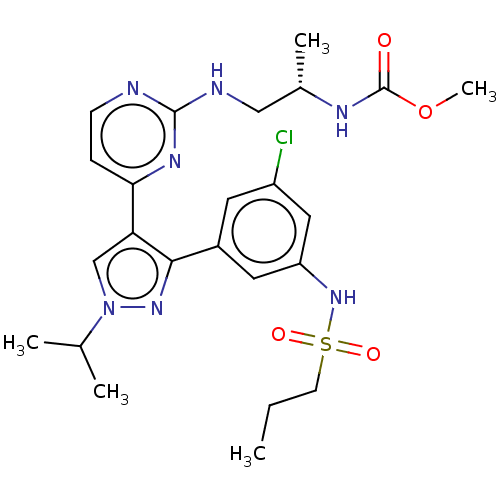
(US10568884, Cpd 5 | US9314464, 5 | US9593100, Comp...)Show SMILES CCCS(=O)(=O)Nc1cc(Cl)cc(c1)-c1nn(cc1-c1ccnc(NC[C@H](C)NC(=O)OC)n1)C(C)C |r| Show InChI InChI=1S/C24H32ClN7O4S/c1-6-9-37(34,35)31-19-11-17(10-18(25)12-19)22-20(14-32(30-22)15(2)3)21-7-8-26-23(29-21)27-13-16(4)28-24(33)36-5/h7-8,10-12,14-16,31H,6,9,13H2,1-5H3,(H,28,33)(H,26,27,29)/t16-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2X63S15 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf [V600E]
(Homo sapiens (Human)) | BDBM103645

(US8563553, 70)Show SMILES COC(=O)N[C@@H](C)CNc1nccc(n1)-c1nc([nH]c1-c1cc(F)cc(NS(C)(=O)=O)c1F)C1CC1 |r| Show InChI InChI=1S/C22H25F2N7O4S/c1-11(27-22(32)35-2)10-26-21-25-7-6-15(28-21)19-18(29-20(30-19)12-4-5-12)14-8-13(23)9-16(17(14)24)31-36(3,33)34/h6-9,11-12,31H,4-5,10H2,1-3H3,(H,27,32)(H,29,30)(H,25,26,28)/t11-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Novartis AG; IRM LLC
US Patent
| Assay Description
Raf/Mek amplified luminescence proximity homogeneous assay. |
US Patent US8563553 (2013)
BindingDB Entry DOI: 10.7270/Q2ZP44R7 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50484212
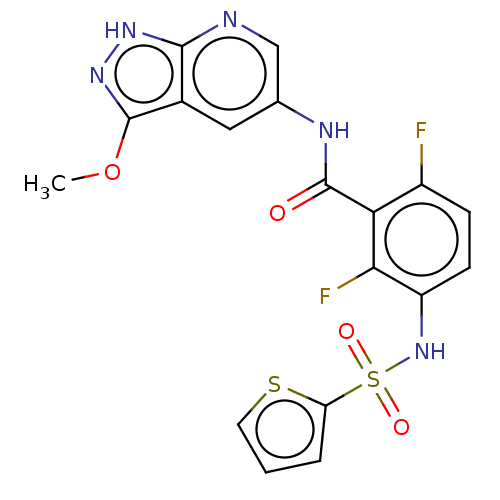
(CHEMBL1822264)Show SMILES COc1n[nH]c2ncc(NC(=O)c3c(F)ccc(NS(=O)(=O)c4cccs4)c3F)cc12 Show InChI InChI=1S/C18H13F2N5O4S2/c1-29-18-10-7-9(8-21-16(10)23-24-18)22-17(26)14-11(19)4-5-12(15(14)20)25-31(27,28)13-3-2-6-30-13/h2-8,25H,1H3,(H,22,26)(H,21,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
ArrayBioPharma
Curated by ChEMBL
| Assay Description
Inhibition of full length B-Raf V600E mutant |
Bioorg Med Chem Lett 21: 5533-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.097
BindingDB Entry DOI: 10.7270/Q28K7CX8 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf [V600E]
(Homo sapiens (Human)) | BDBM87741
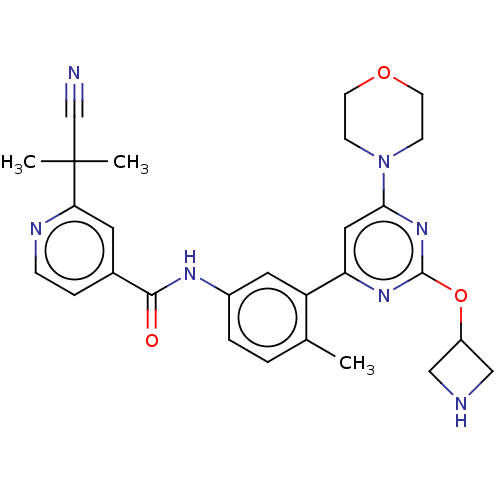
(US10245267, Example 777 | US10709712, Example 777 ...)Show SMILES Cc1ccc(NC(=O)c2ccnc(c2)C(C)(C)C#N)cc1-c1cc(nc(OC2CNC2)n1)N1CCOCC1 Show InChI InChI=1S/C28H31N7O3/c1-18-4-5-20(32-26(36)19-6-7-31-24(12-19)28(2,3)17-29)13-22(18)23-14-25(35-8-10-37-11-9-35)34-27(33-23)38-21-15-30-16-21/h4-7,12-14,21,30H,8-11,15-16H2,1-3H3,(H,32,36) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
NOVARTIS AG
US Patent
| Assay Description
C-Raf TR refers to a truncated C-Raf protein, a Δ1-324 deletion mutant.C-Raf FL refers to the full-length C-Raf protein.Full length MEK1 with an... |
US Patent US10709712 (2020)
BindingDB Entry DOI: 10.7270/Q2280BP3 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data